Abstract
Objectives
Age- and sex-specific rates of dementia are estimated in the U.S. population aged 65 or older in 2000 and 2012 using a large nationally representative dataset, the Health and Retirement Study (HRS), and accounting for mortality selection and specificities of the interview protocol.
Method
A latent cognitive ability model is estimated by maximum simulated likelihood. Prevalence of dementia is identified using HRS cognition measures and the Aging, Demographics and Memory Study (ADAMS), a subset of the HRS (n = 856) with clinical assessment for dementia. Different cognitive measures are collected in self and proxy interviews. From 2006 onward, the HRS collected fewer interviews by proxy. Selection into proxy interviews is modeled as well as survival into the ADAMS sample from the previous HRS interview.
Results
The prevalence of dementia decreased from 12.0% (SE = 0.48%) in 2000 to 10.5% (SE = 0.49%) in 2012 in the 65+ population, a statistically significant decline of 12.6% (p < .01). The percentage change in prevalence was larger among males (16.6% vs 9.5%), and younger individuals.
Discussion
The prevalence of dementia among those 65 or older decreased between 2000 and 2012, although less rapidly than reported in other studies. The difference is primarily due to our modeling selection into proxy interviews.
Keywords: Health outcomes, Measurement, Mortality, Proxy interview
Dementia has a large impact on older adults, their families, and government programs in the United States and many other countries. In 2010, about 4.2 million U.S. adults and more than 36 million around the world had dementia (Hurd, Martorell, Delavande, Mullen, & Langa, 2013; Prince et al., 2013). Its economic impact, including a large burden of unpaid caregiving provided mostly by family members, is estimated at $200 billion per year in the United States (Hurd et al., 2013) and $600 billion worldwide (Wimo, Jonsson, Bond, Prince, & Winblad, 2013).
Assuming no change in age-specific rates of dementia, we can expect prevalence to increase substantially due to population aging—and possibly triple by 2050, when it will affect 130 million worldwide (Prince et al., 2015). However, there is some evidence in the United States and Europe that age-adjusted prevalence of dementia may be decreasing, possibly due to increasing levels of education and better treatment of key cardiovascular risk factors (Langa et al., 2017; Larson, Yaffe, & Langa, 2013; Matthews et al., 2013; Rocca et al., 2011; Satizabal et al., 2016; Schrijvers et al., 2012; Wu et al., 2016). Any change in the trend in age-specific prevalence rates would have a major impact on projected overall prevalence in the population, and consequently, on associated costs, need for care, and other related outcomes.
In the United States, most evidence on trends in the prevalence of dementia comes from community-based cohort studies such as the Framingham Heart Study or the Chicago Health and Aging Project. Rocca and colleagues (2011) reported small and typically not significant decreases in dementia using several such studies. Satizabal and colleagues (2016), however, found a 44% decrease in the 5-year cumulative hazard of dementia incidence from the late 1970s to the late 2000s. These community-based studies typically have very high quality measures of health outcomes, but their comparatively small sample sizes and geographically limited samples, may not generalize to the entire U.S. population.
An alternative approach to measuring trends in the prevalence of dementia is to use survey data, such as the Health and Retirement Study (HRS). The HRS has interviewed about 20,000 individuals per wave since 1992. It is nationally representative, and has a wide range of information about individuals’ health and socioeconomic status. Moreover, 856 sample members were administered a clinical assessment for dementia within the Aging, Demographics and Memory Study (ADAMS). Plassman and colleagues (2007) and Hurd and colleagues (2013) provided the first nationally representative estimates of dementia prevalence and of the costs of dementia in the United States, respectively. Based on HRS data, Langa and colleagues (2017) reported that the prevalence of dementia in the 65+ year old population decreased by about one-fourth from 2000 to 2012, or from 11.6% to 8.8% in 2012.
In this article, we use a latent variable statistical model to estimate on HRS data the prevalence of dementia in 2000 and 2012. The starting point of our model is that cognitive status is not directly observed in the HRS; rather, noisy indicators of cognition are observed. The most important indicators are the HRS cognitive tests (such as word recall), but they only imprecisely establish cognitive status. Additional, observable data such as health indicators, activity limitations, and personal characteristics including education and age provide additional information about cognitive status. The model permits us to address some issues that earlier studies did not address. In particular, we focus on three issues:
The ADAMS interviews followed HRS interviews with a delay of about 1 year, on average, and 12.9% of the ADAMS target sample died before they were contacted and interviewed. If the timing of the ADAMS interviews and mortality are correlated with dementia status, the prevalence estimates of dementia in ADAMS may be biased (Heeringa et al., 2009).
The fraction of interviews by proxy respondents decreased significantly from 2000 to 2012, likely due to changes in the HRS field procedures (Langa et al., 2017). Respondents with low cognitive ability are much more likely to be interviewed by proxy; hence proxy status is a marker of low cognition. It is therefore plausible that some of the measured decrease in dementia from 2000 to 2012 was spurious and due to the falling fraction of proxy interviews. Such changes in interviewing protocols need to be modeled if they are found to be quantitatively important.
ADAMS only included individuals who were 70+ years old at the latest HRS interviews. To measure dementia in the broader 65+ age range in the HRS, a conditional independence assumption is needed: age does not predict dementia status above and beyond the other variables used in the model, such as the cognitive scores. We found, however, that age is a strong predictor of dementia status in ADAMS even when a very detailed set of HRS cognitive measures are controlled (Supplementary Tables D1 and D2.)
To estimate the prevalence of dementia, while accounting for these three issues, we model mortality, interview timing and selection into proxy interviews, and we include a large set of covariates in the estimation, including age itself, so that the conditional independence assumption is more likely satisfied (point 3 above).
Methods
Data
The HRS is a nationally representative, biennial, longitudinal survey of persons aged 51 or older. It is sponsored by the National Institute on Aging (grant number NIA U01AG009740) and is conducted by the University of Michigan. Refreshment cohorts of age 51–56 enter the survey every 6 years. The sample size is about 20,000 per wave, with about 10,000 aged 65+. In most cases, HRS directly interviews the sampled individual (self-interviews), but when the individual is unable to participate (in 5–10% of the cases) the interview is conducted with proxy informants such as spouses, other family members, or friends. Individuals with cognitive limitations are much more likely to be interviewed by proxy. The first interviews are always face-to-face, and subsequent ones are either face-to-face or telephone interviews, depending on many factors.
The HRS is unusually detailed in health and cognition outcomes. To predict dementia status, we used data on a number of risk factors for dementia and on correlates of cognitive difficulties:
Subjectively rated health, which was reported on a 5-point scale: 1 (excellent), 2 (very good), 3 (good), 4 (fair), or 5 (poor). We collapsed these into a 3-point scale by merging categories 1 and 2 as well as categories 4 and 5.
Number of limitations (from 0 to 5) in instrumental activities of daily living (IADL), such as using the phone or dealing with money (see RAND-HRS, 2016, for technical details).
Ever having been diagnosed with (a) hypertension, (b) diabetes, (c) stroke, and (d) psychiatric problems. The first three are known dementia risk factors. The fourth may also correlate with dementia status if, for example, individuals consider cognitive decline a psychiatric problem.
We used an extensive set of cognition measures in the analysis. Both self- and proxy interviews have a 5-point memory scale, varying from 1 (excellent) to 5 (poor). Other cognition variables differ by interview type.
For self-interviews, we use a range of cognitive measures adapted from the Telephone Interview for Cognitive Status (TICS): a 10-word test for immediate recall, a 10-word test for delayed recall, a serial-7 subtraction test (subtracting 7 five times starting from 100), backward counting (from 20 to 10), question on today's date, and day of the week, the name of the president, and being able to name “cactus” and “scissor” after paraphrasing. To simplify the analysis, we collapsed all this information into a composite score based on the sum of the TICS items, subjectively rated memory (reverse coded so that high values mean better memory), and instrumental activity limitations (also reverse coded). The composite score took values from 0 to 43. The Supplementary Appendix shows how cognitive status in ADAMS is related to the individual HRS items included in the score.
For proxy interviews we used four cognition measures. Jorm IQCODE is a 16-item battery about recent improvements/declines in various aspects of memory. All 16 items were first coded into a 1–5 score [1 (memory much improved), 2 (a bit improved), 3 (not much changed), 4 (a bit worse), 5 (much worse)]. We then took the person-specific mean and created a 0–2 score. Those whose mean score was between 1 and 3 (no change or improvement on average) received a value of 0, those whose mean score was between 3 and 4 (some decline on average) were coded as 1, and those whose mean score was above 4 received a value of 2. Similarly, interviewers assessed whether cognitive limitations were a reason for conducting a proxy interview, which we coded on a scale of 0 (no difficulty), 1 (some difficulty), or 2 (could not do interview). The proxies are also asked if the person “ever wanders off,” and whether he or she can be left alone, which were both coded on a 0–1 scale. Again, we created a composite score by taking the sum of all the four measures and adding subjectively rated memory and instrumental activity limitations. This composite score takes values from 0 to 15; it is negatively related to cognition; and the Supplementary Appendix shows how the items of the score predict cognitive status in ADAMS.
The analytic sample is restricted to those aged 65 and older in the 2000 and 2012 HRS, because many cognitive ability measures are only available in this age range.
We used several demographic variables as predictors of dementia status: education (coded as less than high school, high school, some college, or college), gender, age, race (white or nonwhite), ethnicity (Hispanic or non-Hispanic), and marital status. The HRS also follows individuals into nursing homes (and they are part of our estimation sample), and we use nursing home residence in some of our analyses as an indirect marker of cognitive problems.
We use the HRS weights to estimate prevalence of dementia. The handful of missing values in the variables was replaced by their modes, such as being white or being married.
Because the HRS core lacks a direct measure of dementia status, 856 respondents underwent a detailed in-home clinical assessment for dementia as part of ADAMS between 2001 and 2003. The duration of the assessment was 3–4 h. The data from the assessment were studied by an expert panel which assigned a final diagnosis of (a) dementia, (b) cognitive impairment, but not dementia (CIND), or (c) normal cognitive function.
Heeringa and colleagues (2009) discuss the details of the data collection. Originally, a stratified sample of 1,770 individuals aged 70 or older was selected from the 2000 and 2002 HRS based on their performance on the HRS cognitive questions as well as their age and gender. Individuals with lower cognitive status were selected with higher probabilities. Those selected were contacted around a year after their last HRS interview. Of these, 48.4% participated in the ADAMS, 12.9% had died, 28.2% refused to participate, and 10.5% did not participate for other reasons. Because of the stratified sampling and attrition, the ADAMS sample does not represent the U.S. population. The HRS developed weights to deal with the stratified sampling design and nonresponse, but not mortality. A correlation between mortality and cognitive ability, in itself, does not induce a bias in prevalence, it only changes the age of the population the sample represents. However, if both mortality and the timing of the ADAMS interviews varied by cognition, the ADAMS weights may be biased (Heeringa et al., 2009).
Empirical Models
Before we introduce our preferred methodology for estimating dementia from HRS and ADAMS, we discuss an alternative approach.
The cutoff approach to estimate prevalence and trends
Clinical assessment for dementia is expensive and not practical in a large survey like the HRS. An alternative is to use an index of cognitive ability based on HRS survey measures and apply a cutoff point: individuals whose index falls below the cutoff are deemed to have dementia. This method produces both false positives and false negatives, but if the cutoff point is appropriately chosen, prevalence in the survey will be unbiased for population prevalence. The cutoff approach is a simple and transparent research design and is commonly used in dementia research (Crimmins, Kim, Langa, & Weir, 2011; Herzog & Wallace, 1997; Langa et al., 2017).
The cutoff points are normed to particular populations (e.g., countries, age groups) but they may not produce good prevalence estimates in other populations. For example, different cutoff points may be needed for different age and education groups (O’Caoimh et al., 2017); for different countries and languages (Alegret et al., 2013; Yang et al., 2016); and for people with different medical symptoms (Hoops et al., 2009). If populations differ by these or by other relevant dimensions, then different cut-points should be used for each population.
Because there are both self-interviews and proxy interviews, each with its own set of cognitive variables, to apply the cutoff approach in the HRS requires finding cut-points for each type of interview (Langa et al., 2017). Each cut-point is calibrated so that dementia prevalence in the relevant HRS subpopulation (self or proxy interview) aged 71 or older is the same as in the corresponding ADAMS subsample. A weighted average over the two HRS subpopulations yields population prevalence among those 71 or older in 2000. These cut points can then be used to estimate prevalence in the age range 65–70 and in 2012.
There are, however, several potential issues with using the cutoff approach on HRS data age 65+. First, it is not obvious that the cutoff point identified for the 71+ year old population is appropriate for the 65+ year old population. Second, the ADAMS weights did not adjust for mortality and interview timing differences by cognition. Third, because HRS has collected fewer proxy interviews since 2006, the distribution of cognition by proxy status likely differs, making it possible that the cutoff points used for 2000 would not be accurate in later waves. We investigate some of these issues next.
Mortality differences by cognitive status
The target sample of ADAMS was chosen from the 2000 and 2002 HRS, with the ADAMS team approaching these individuals throughout the following 2 years. Among these respondents, however, 12.9% had died by the time they were contacted.
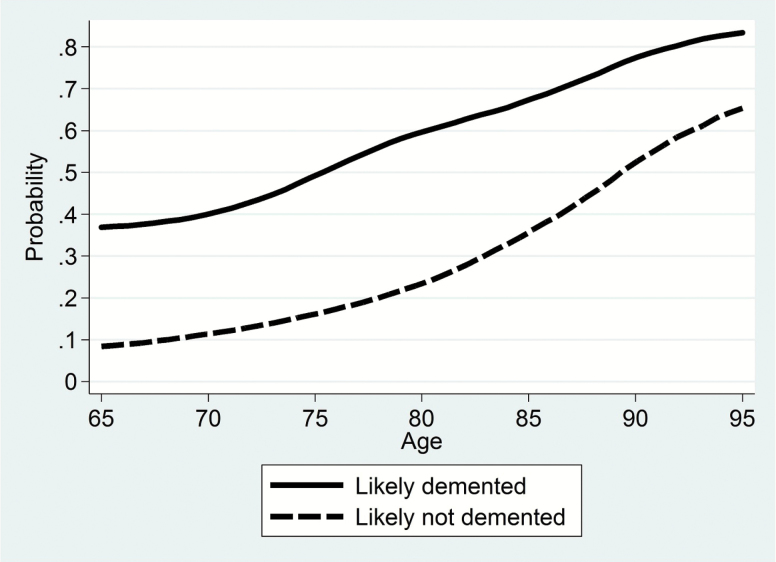
The ADAMS weights were designed to deal with the stratified sampling design and nonresponse, but not with mortality. As Heeringa and colleagues (2009) note, if mortality probabilities differ by cognitive status, as well as with the timing of the ADAMS interviews, then those who survive to participate in ADAMS (about a year after the last HRS interview, on average) would not represent all selected. As Figure 1 shows, there are substantial differences in 5-year mortality rates by imputed dementia status (using a cutoff approach). Individuals with dementia are much more likely to die earlier, and the differences are larger at younger ages. Cognitive ability may also correlate with interview timing, though we should not expect as strong a relationship as with survival. Heeringa and colleagues (2009) show that the two phases of ADAMS differed by cognitive ability and interview timing: the phase 1 interviews that followed the 2000 HRS were scheduled significantly later after HRS interviews than the phase 2 interviews that followed the 2002 HRS. Moreover, the two phases targeted a somewhat different part of the cognitive ability distribution. Furthermore, we also found some evidence (not shown here) that within the phases, cognitively more able individuals tended to provide interviews somewhat later.
Figure 1.
5-Year mortality rates by imputed dementia status, HRS 1998–2008, age 65–95, weighted. The publicly available HRS includes the years and months (but not the days) of the interviews and of deaths. 5-Year mortality rates are estimated by investigating if individuals are alive 5 years and 0 months after their HRS interviews. Dementia status is imputed using the cutoff approach of Langa and colleagues (2017), in which individuals represented by a proxy are imputed to have dementia if they scored 6 or higher on an 11-point scale of cognitive problems, and self respondents are imputed to have dementia if they scored 6 or lower on a 27-point scale of working and episodic memory.
The final ADAMS weights used poststratification to weight the final sample to the general population by age and gender, but that is unlikely to fully correct for mortality and interview timing differences by cognitive ability. A cutoff approach calibrates the cutoff point so that in 2000 the prevalence of dementia in the HRS equals the prevalence of dementia in ADAMS. So any bias in ADAMS is carried over to the 2000 HRS.
As Heeringa and colleagues (2009) discuss, the preferred methodology to account for survival and interview timing would be to model cognition and survival jointly, which is what we do.
Selection into proxy interviews
HRS interviewers are instructed to obtain self-interviews if possible, and to use proxies only when the sampled individual is unable to complete the interview, which, in the older age group, would typically be due to cognitive limitations. Proxy rates have varied across groups and time. For example, the fraction of proxy interviews among respondents aged 65 years or older decreased from 12.2% in 2000 to 6.9% in 2012.
Table 1 shows that the share of proxy interviews could be quantitatively important for the estimates of trends using a simple linear decomposition and a cutoff approach to define dementia. Dementia prevalence decreased slightly in self-interviews but increased among those with proxy interviews. Overall, the fraction of proxies explains all of the estimated trends in dementia. An approximate 5 percentage point fewer proxy interviews and approximately 50 percentage point difference in prevalence leads to a 2.5 percentage point decrease in overall prevalence of dementia, which is close to the total decrease of 2.7 percentage points. This observation does not necessarily mean the decline in prevalence associated with the decline in proxy interviews is incorrect: it means that selection into proxy interviews deserves further investigation.
Table 1.
Imputed Prevalence of Dementia by Proxy Status and Contribution to the Overall Trenda, Age 65+, Dementia Is Imputed Using a Cutoff Methodb
| 2000 | 2012 | Contribution to trend, % | |
|---|---|---|---|
| Prevalence, self-interviews | 0.060 | 0.053 | 26.4 |
| Prevalence, proxy interviews | 0.510 | 0.578 | −32.0 |
| Fraction of proxy interviews | 0.123 | 0.070 | 105.6 |
| Prevalence, total | 0.116 | 0.089 | 100.0 |
aLet denote the fraction of proxy interviews in year ; and denote the prevalence of dementia in proxy and self-interviews respectively; and denotes overall prevalence. The contribution of self interview prevalence to the trend is defined as ; the contribution of proxy interview prevalence is ; and the contribution of the fraction of proxy interviews is defined as .
bDementia status is imputed using the cutoff approach of Langa and colleagues (2017), in which individuals represented by a proxy are imputed to have dementia if they scored 6 or higher on an 11-point scale of cognitive problems, and self respondents are imputed to have dementia if they scored 6 or lower on a 27-point scale of working and episodic memory. The sample only includes individuals with nonmissing scores.
The sharp decrease in the proportion of interviews conducted by proxy may indicate improvements in cognitive status or changes in interviewing protocols. The unadjusted cutoff methodology cannot distinguish between these two explanations. Why did the fraction of proxy interviews decrease? Two reasons are likely. First, the HRS has collected far more face-to-face (FTF) interviews since 2006 so as to collect biomarkers and other items that must be collected directly from the individual participant: among those aged more than 65, the proportion of interviews that were FTF increased from 22% in 2000 to 61% in 2012 (Supplementary Table D3). FTF interviews are more likely to be self-interviews than telephone interviews (Supplementary Table D4). Because the choice of FTF or telephone mode has been randomized since 2006, we can estimate the causal effect of FTF interviews on proxy status. Supplementary Table D5 implements a fixed effect (FE), and an IV model where we instrumented FTF status by the random intended FTF variable. We found that the effect of FTF on the probability of obtaining proxy interviews is negative, as expected, but the magnitude of the effect is not large. Even if we take the larger FE estimate, we find that FTF status could explain only about 1 percentage point [0.027 × (60.6 − 22.3) = 1.03] of the 5.3 percentage point decrease in proxies from 2000 to 2012.
Second, the proportion of proxy interviews may have decreased because of HRS efforts to collect more self-interviews, which are considered to be of higher quality. Moreover, it is likely that the effort to collect more self-interviews was more successful among cognitively able individuals, which would change the distribution of cognitive ability in proxy interviews.
The cutoff methodology estimates dementia prevalence in proxy and self-interviews separately. The cutoff points are calibrated so that the prevalence of dementia in the 2000 HRS is the same as in ADAMS. When these cutoff points are used for a different target population, such as proxy and self-interviews in the 2012 HRS, the prevalence estimates may be biased. In Supplementary Appendix A, we show that there are two cases under which the cutoff method provides valid estimates in a different target population:
If the cutoff predicts dementia status with 100% precision (i.e., there is no classification error).
If the distribution of cognitive abilities is the same in the calibration and the target populations.
If both of these conditions fail, the cutoff points may not be valid in the target population. The Supplementary Appendix shows that under reasonable conditions prevalence is downward biased if the target population has lower cognitive abilities, on average, than the calibration population. Table 2 shows the fraction of interviewees with cognitive limitations in proxy interviews reported by the interviewers. We find a 10 percentage point increase in the fraction of respondents with cognitive limitations. From this, we infer that respondents with higher abilities, who might have been interviewed by proxy in 2000, were given self-interviews in 2012. This evidence, together with the fact the cognitive measures (whether from self-interviews or proxy interviews) are not perfect measures of true cognition, imply that the prevalence of dementia in the 2012 HRS proxy interviews may be downward biased. The Supplementary Appendix shows that the magnitude of the bias can be substantial depending on parameter values.
Table 2.
Number and Fraction of Interviewees With Cognitive Limitation, in Proxy Interviews, Age 65+
| 2000 | 2012 | |||
|---|---|---|---|---|
| N | % | N | % | |
| No cognitive limitation | 550 | 40.4 | 319 | 35.9 |
| Some limitation, could do interview | 175 | 12.9 | 67 | 7.6 |
| Cognitive limitations, cannot do IW | 636 | 46.8 | 502 | 56.5 |
| Total | 1361 | 100.0 | 888 | 100.0 |
Altogether, because of the changing distribution of cognitive ability within proxy and self-interviews, it is likely that unadjusted cutoff points are not accurate in 2012, although the magnitude of the bias is not easy to identify without developing a model that fully accounts for the changes in interviewing protocols, which we will do next.
Our model of dementia prevalence
Based on our findings, we model both selection into proxy interviews, and the additional problems that we have discussed: the relationship between actual (unobserved) cognitive ability and cognitive scores, mortality, ADAMS interview timing, and nonparticipation. Here, we sketch the model; the complete specification is found in the Supplementary Appendix.
Cognitive ability, which is not observed, has a normal distribution in the population; an individual has dementia if cognitive ability falls below a cutpoint which does not change over time. The observed self and proxy scores in the HRS depend on (latent) cognitive ability, but they do not perfectly reveal dementia status.
Whether individuals are interviewed in proxy interviews is modeled as a probit which depends on latent cognitive ability, and the dependence can change over time to permit the apparent change in the HRS protocol for proxy interviews. We modeled invitation to the two phases of ADAMS separately for proxies and nonproxies as functions of the cognition-age-gender strata used by HRS (Heeringa et al., 2009). Mortality is modeled in a Gompertz framework. Of the ADAMS target sample of 1770, 1430 individuals (81%) died by the latest HRS interview in 2014. For them, the time of death is observed, and we measure it to the precision of 0.1 years. The survivors to the 2014 wave are modeled as right censored cases. The lag between the ADAMS interviews and the previous HRS interviews (2000 or 2002) is assumed to follow a lognormal distribution. Conditional on being invited and surviving to ADAMS, actual participation depends on cognition and potentially other covariates.
We added covariates, such as education, age, race, and health, to the equations that specify cognitive ability, proxy selection, observed cognitive scores, mortality, and the timing of and participation in the ADAMS interview so as to increase the precision of the dementia probabilities. All of these variables are interacted with interview waves so that the estimated trends in dementia probabilities are not affected by trends in these variables; they are only affected by trends in the self and proxy scores and the fraction of proxy interviews. We included a cubic polynomial of age in all equations. As the ADAMS did not include 65–70 year old individuals in its sample, our model assumes that the predicted effect of age on cognition identified from the 71+ sample can be extrapolated to the younger 65–70 year old group via the polynomial.
We also experimented with added flexibility to the equations relating latent cognitive ability to observed cognitive scores. Our chosen model includes a cubic polynomial of cognition. We found that this flexibility was important to accurately recover dementia prevalence in the 2000 HRS.
This is a fully specified model, which we estimated by maximum simulated likelihood. After estimating the model, we estimated the probabilities of dementia for each individual in 2000 and 2012, which are functions of the estimated model parameters and all the observable covariates: demographics, health, proxy interview status and, the HRS self and proxy scores. The average of these dementia probabilities provides a consistent estimate of dementia prevalence in 2000 and 2012. The standard errors are estimated by an adapted version of Krinksy and Robb (1986). The Supplementary Appendix provides details.
The main identification assumption of the model is that the distribution of self and proxy scores in the HRS, conditional on latent cognitive ability, is the same in 2000 as in 2012. Any improvements in the HRS self and proxy scores would therefore mark improvements in cognitive ability. This framework overcomes the problem of selection into proxy interviews by modeling it as a function of latent cognitive ability. We tested the performance of the model on a synthetic dataset, and we found that the model accurately recovered the true parameters as well as prevalence and trends.
Results
Trends in Subjectively Rated Memory, IADL Limitations and Nursing Home Residence
Some variables that are known to correlate with cognitive ability are available both in proxy and in self-interviews. These include subjectively rated memory, number of limitations in instrumental activities of daily living (IADL), and whether the respondent lives in a nursing home. As they are available in all interviews, they do not suffer from selection into proxy interviews.
Table 3 shows the trends in those variables. We find little improvement in subjectively rated memory except among older females, for whom the proportion with poor memory decreased by more than 4 percentage points. We find some improvements in IADL: the fraction living with more than three such limitations decreased from 8.0% in 2000 to 7.0% in 2012, with a noticeably larger decrease among males. The overall proportion living in nursing homes also decreased from 4.6% in 2000 to 2.9% in 2012. These trends indicate some moderate improvements in cognitive ability for HRS respondents 65 years of age or older.
Table 3.
Trends in Variables Related to Dementia by Age, Age 65+
| Poor memory | ≥3 IADL limitations | Lives in nursing home | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | 2000 | 2012 | Δ, % | 2000 | 2012 | Δ, % | 2000 | 2012 | Δ, % |
| 65–74 | 4.34 | 4.47 | 2.78 | 3.02 | 2.76 | −8.44 | 1.26 | 0.82 | −35.08 |
| 75–84 | 8.39 | 8.87 | 5.76 | 9.16 | 7.11 | −22.33 | 4.82 | 3.07 | −36.40 |
| 85+ | 19.03 | 15.63 | −17.85 | 27.37 | 24.04 | −12.15 | 18.86 | 10.73 | −43.12 |
| 65+ | 7.48 | 7.36 | −1.56 | 8.01 | 7.01 | −12.48 | 4.56 | 2.87 | −37.07 |
| Males | 2000 | 2012 | Δ, % | 2000 | 2012 | Δ, % | 2000 | 2012 | Δ, % |
| 65–74 | 5.00 | 4.60 | −7.91 | 3.23 | 2.53 | −21.67 | 1.22 | 0.72 | −41.07 |
| 75–84 | 8.51 | 9.72 | 14.23 | 7.56 | 5.48 | −27.57 | 3.34 | 2.36 | −29.39 |
| 85+ | 15.12 | 14.59 | −3.54 | 18.94 | 17.13 | −9.56 | 11.16 | 6.67 | −40.23 |
| 65+ | 7.08 | 7.25 | 2.41 | 6.06 | 4.99 | −17.64 | 2.79 | 1.86 | −33.50 |
| Females | 2000 | 2012 | Δ, % | 2000 | 2012 | Δ, % | 2000 | 2012 | Δ, % |
| 65–74 | 3.82 | 4.35 | 13.79 | 2.85 | 2.96 | 3.99 | 1.29 | 0.90 | −30.15 |
| 75–84 | 8.30 | 8.23 | −0.83 | 10.23 | 8.33 | −18.58 | 5.83 | 3.60 | −38.24 |
| 85+ | 20.72 | 16.17 | −21.95 | 31.01 | 27.60 | −10.99 | 22.19 | 12.82 | −42.23 |
| 65+ | 7.76 | 7.45 | −4.03 | 9.39 | 8.56 | −8.80 | 5.80 | 3.64 | −37.20 |
Results of the Dementia Model
We estimated three alternative specifications of the model. The detailed output is found in Supplementary Table D6. The first specification does not allow changes in selection into proxy interviews and does not model interview timing in ADAMS. Instead, it assumes that selection into proxy interviews was the same in 2000 and 2012, and the timing of ADAMS interview was uncorrelated with cognition. Our second specification models ADAMS interview timing as a function of cognitive ability and other covariates (to deal with the mortality problem), but still ignores changes in selection into proxy interviews. Our third specification models both ADAMS interview timing and changes in selection into proxy interviews by including time varying covariates in the selection equation.
Supplementary Table D6 shows that the model output conforms to expectations: (a) it correctly finds that there were far fewer proxy interviews in 2012 than in 2000; (b) individuals with higher levels of cognitive ability were less likely to have proxy interviews; (c) cognitively more able individuals live longer even when the other demographic controls are applied; (d) cognitively more able individuals were interviewed later in ADAMS, on average; and (e) cognition was a positive predictor of participation in phase 1 of ADAMS and uncorrelated with participation in phase 2.
Table 4 shows the main results: prevalence and trends by age and gender using the output from Supplementary Table D6. The specification without interview timing and proxy selection adjustment estimates that the prevalence of dementia in the 65+ population decreased from 11.9% in 2000 to 9.8% in 2012, a statistically significant decrease of 17.7%. Adjusting for interview timing (mortality) makes very little difference, which is evidence that the ADAMS weights are not biased. (Recall that the ADAMS weights assumed that interview timing was uncorrelated with cognition.)
Table 4.
Estimated Prevalence of Dementia by Age and Gender in 2000 and 2012, Three Model Specifications, HRS, Age 65+
| No adjustment for proxy interviews; or mortality | Adjusting for mortality; but not for proxy interviews | Adjusting for proxy interviews and mortality | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | 2000 | 2012 | Δ | 2000 | 2012 | Δ | 2000 | 2012 | Δ |
| 65–74 | 4.08 | 3.07 | −1.01*** | 4.08 | 3.07 | −1.01*** | 4.07 | 3.47 | −0.6* |
| [0.34] | [0.29] | [0.32] | [0.35] | [0.29] | [0.34] | [0.36] | [0.31] | [0.34] | |
| 75–84 | 14.23 | 10.23 | −4*** | 14.23 | 10.24 | −3.99*** | 14.39 | 11.15 | −3.24*** |
| [0.7] | [0.65] | [0.69] | [0.75] | [0.68] | [0.72] | [0.74] | [0.7] | [0.72] | |
| 85+ | 40.42 | 36.01 | −4.4** | 40.40 | 36.03 | −4.37** | 40.86 | 37.39 | −3.47** |
| [1.57] | [1.59] | [1.72] | [1.58] | [1.63] | [1.71] | [1.58] | [1.59] | [1.7] | |
| 65+ | 11.89 | 9.78 | −2.1*** | 11.89 | 9.79 | −2.1*** | 11.99 | 10.48 | −1.51*** |
| [0.46] | [0.46] | [0.41] | [0.48] | [0.47] | [0.4] | [0.48] | [0.49] | [0.42] | |
| Males | 2000 | 2012 | Δ | 2000 | 2012 | Δ | 2000 | 2012 | Δ |
| 65–74 | 4.20 | 2.27 | −1.93*** | 4.21 | 2.27 | −1.93*** | 4.17 | 2.71 | −1.46*** |
| [0.47] | [0.29] | [0.46] | [0.47] | [0.3] | [0.47] | [0.46] | [0.33] | [0.48] | |
| 75–84 | 11.07 | 7.79 | −3.28*** | 11.07 | 7.80 | −3.27*** | 11.16 | 8.97 | −2.2** |
| [0.87] | [0.71] | [0.95] | [0.87] | [0.74] | [0.97] | [0.91] | [0.73] | [1] | |
| 85+ | 30.01 | 25.84 | −4.17 | 29.99 | 25.85 | −4.14 | 30.29 | 28.11 | −2.19 |
| [2.18] | [2.01] | [2.63] | [2.27] | [2.16] | [2.72] | [2.25] | [2.09] | [2.77] | |
| 65+ | 8.77 | 6.48 | −2.28*** | 8.77 | 6.49 | −2.28*** | 8.81 | 7.34 | −1.46*** |
| [0.53] | [0.46] | [0.52] | [0.54] | [0.48] | [0.53] | [0.54] | [0.47] | [0.55] | |
| Females | 2000 | 2012 | Δ | 2000 | 2012 | Δ | 2000 | 2012 | Δ |
| 65–74 | 3.98 | 3.75 | −0.22 | 3.97 | 3.75 | −0.22 | 3.99 | 4.12 | 0.13 |
| [0.42] | [0.39] | [0.45] | [0.41] | [0.39] | [0.47] | [0.44] | [0.42] | [0.48] | |
| 75–84 | 16.36 | 12.05 | −4.31*** | 16.37 | 12.06 | −4.31*** | 16.57 | 12.78 | −3.79*** |
| [0.92] | [0.86] | [0.95] | [0.96] | [0.86] | [0.96] | [0.96] | [0.92] | [0.99] | |
| 85+ | 44.92 | 41.26 | −3.66* | 44.90 | 41.27 | −3.63* | 45.43 | 42.18 | −3.25 |
| [1.83] | [1.85] | [2.13] | [1.8] | [1.83] | [2.09] | [1.88] | [1.83] | [2.1] | |
| 65+ | 14.08 | 12.31 | −1.77*** | 14.08 | 12.32 | −1.76*** | 14.24 | 12.88 | −1.35** |
| [0.59] | [0.61] | [0.58] | [0.6] | [0.61] | [0.59] | [0.62] | [0.64] | [0.61] | |
Note. Standard errors in brackets. *, **, and *** denote statistical significance at 1%, 5%, and 10%, respectively. The standard errors of the changes in prevalence are about the same as the standard errors of the levels in prevalence because of a positive correlation between the 2000 and 2012 prevalence estimates.
Our preferred third specification shows a decrease in the prevalence of dementia from 12.0% in 2000 to 10.5% in 2012 among those 65 years and older, which is equivalent to a statistically significant (p < .01) 1.5 percentage points, or 12.6%. Thus, accounting for selection into proxy interviews reduced the estimated decline from 17.7% to 12.6%. There was a larger decrease in the prevalence of dementia among 75–84 year old females (22.9%) and 65–74 year old males (35.0%). Estimated prevalence increased among 65–74 year old females by 3.3% (not significant).
In results not shown we found that both in self and in proxy interviews the fraction of individuals with dementia increased; yet population prevalence (weighted average of the two groups) decreased because of the reduced fraction of proxy interviews in 2012. The increase in within group prevalence of dementia is consistent with the hypothesis that in 2012 the more able from the potential pool of proxies were selected into self-interviews, reducing the quality of both the proxy and the self-interview pool.Because of the increasing population size of the 65+ year old group between 2000 and 2012, about 11.6% more people had dementia in 2012 than in 2000 despite the decrease in prevalence rates (Supplementary Appendix).
Discussion
We estimated that, based on the nationally representative HRS, dementia prevalence among those aged 65 or older declined statistically significantly between 2000 and 2012 but that the decline was less than reported in previous literature. Compared with that literature, we addressed several methodological issues. First, the weights provided in ADAMS did not adjust for interview timing and mortality. As long as both mortality and the timing of the ADAMS interviews differed by cognition, the provided weights were biased. Even though we showed evidence that cognition predicted both survival and interview timing, the implied bias was found to be negligible, and the ADAMS weights are practically unbiased. Second, we investigated the changes in selection into proxy interviews (the fact that HRS started collecting fewer interviews by proxies after 2006), and we found that it had a detectable effect on estimated trends in dementia: the adjustment increased dementia prevalence in recent years and, thus, made the estimated negative trend in dementia less steep. Third, we explicitly modeled the dependence of dementia on age.
Our preferred specification shows a decrease in the prevalence of dementia among those at least 65 years of age from 12.0% in 2000 to 10.5% in 2012, or by about 12.6%. A similar estimated decline in dementia prevalence was reported by Matthews and colleagues (2013) using English data, who reported a decline from 8.3% in 1989–1994 to 6.5% in 2008–2011, equivalent to a 1 percentage point or 12% decline per decade. Some researchers found smaller or even zero decline in dementia prevalence (Rocca et al., 2011 using U.S. data; Wu et al., 2016 reporting about Swedish and Dutch data). Langa and colleagues (2017), however, using the same dataset and time period as we did, reported about twice as large a decline. Their methodology was based on the cutoff approach in which adjusting for changes in selection into proxy interviews was not possible. We note, however, that even in the specification that was most similar to the one used by Langa and colleagues we found a smaller decline in dementia prevalence than they did. The cutoff and our modeling frameworks are different in many ways. First, our model included covariates other than the cognitive variables, such as age, education, race, and health. Second, conditional on age, we used a slightly broader sample, about 2% larger, by retaining individuals who had some missing data. Third, the composite cognition scores we used were based on a larger set of variables. Fourth, the modeling techniques were, as discussed, quite different.
We suggest that researchers use a probabilistic approach rather than the cutoff approach and model selection into proxy interviews when they estimate prevalence and trends in the HRS. This is particularly important when the study period includes years both before and after 2006, because of the large change in the fraction of proxy interviews. A probability approach also helps overcome the bias (toward zero) in subgroup differences in prevalence.
Our approach has some shortcomings. It is considerably harder to use than the cutoff approach. Our model uses functional form assumptions that are necessary, but it is unclear how much these assumptions contribute to the estimated trends. We assumed that latent cognition has a normal distribution in the population, and that there is a simple relationship between cognition and the HRS outcome variables; if any of these assumptions are inaccurate, then our model may lead to biased estimates. We found, for example, that we needed to add at least a cubic polynomial of cognition into the equations of the HRS cognitive outcomes to recover prevalence in the 2000 HRS. But we have no direct evidence about whether a higher-order polynomial is required. The HRS is currently collecting a new ADAMS-like study, the HCAP, which will allow testing the accuracy of these modeling assumptions in the future.
Supplementary Material
Supplementary data are available at The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences online.
Funding
This work was supported by the National Institute on Aging (R01AG053972 and P30-AG012846).
Acknowledgments
Author Contributions: All the three authors planned the study and wrote the manuscript together. Hudomiet cleaned the data, derived the statistical models, and performed all data analysis. Hurd and Rohwedder supervised data cleaning, modeling, and analysis.
References
- Alegret M., Espinosa A., Valero S., Vinyes-Junqué G., Ruiz A., Hernández I., … Boada M., (2013). Cut-off scores of a brief neuropsychological battery (NBACE) for Spanish individual adults older than 44 years old. PLoS ONE, 8, e76436. doi:10.1371/journal.pone.0076436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins E. M., Kim J. K., Langa K. M., & Weir D. R (2011). Assessment of cognition using surveys and neuropsychological assessment: The Health and Retirement Study and the Aging, Demographics, and Memory Study. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 66(Suppl 1), i162–i171. doi:10.1093/geronb/gbr048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health and Retirement Study, waves 1992–2014, public use dataset. Produced and distributed by the University of Michigan with funding from the National Institute on Aging (grant number NIA U01AG009740). Ann Arbor, MI (2016).
- Heeringa S., Fisher G., Hurd M., Langa K., Ofstedal M., Plassman B., … Weir D., (2009). ADAMS: Sample design, weighting and analysis for ADAMS. Institute for Social Research, University of Michigan. doi:10.7826/isr-um.06.585031.001.05.0019.2009
- Herzog A. R., & Wallace R. B (1997). Measures of cognitive functioning in the AHEAD Study. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 52 Spec No, 37–48. doi:10.1093/geronb/52B.Special_Issue.37 [DOI] [PubMed] [Google Scholar]
- et al. (2009). Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology, 73, 1738–1745. doi:10.1212/WNL.0b013e3181c34b47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd M. D., Martorell P., Delavande A., Mullen K. J., & Langa K. M (2013). Monetary costs of dementia in the United States. The New England Journal of Medicine, 368, 1326–1334. doi:10.1056/NEJMsa1204629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krinsky I., & Robb A. L (1986). On approximating the statistical properties of elasticities. Review of Economic and Statistics, 68, 715–719. [Google Scholar]
- et al. (2017). A comparison of the prevalence of dementia in the United States in 2000 and 2012. JAMA Internal Medicine, 177, 51–58. doi:10.1001/jamainternmed.2016.6807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson E. B., Yaffe K., & Langa K. M (2013). New insights into the dementia epidemic. The New England Journal of Medicine, 369, 2275–2277. doi:10.1056/NEJMp1311405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews F. E., Arthur A., Barnes L. E., Bond J., Jagger C., Robinson L., … ; on behalf of the Medical Research Council Cognitive Function and Ageing Collaboration. (2013). A two-decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: Results of the Cognitive Function and Ageing Study I and II. The Lancet, 382, 1405–1412. doi:10.3410/f.718043512.793485441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Caoimh R., Gao Y., Svendovski A., Gallagher P., Eustace J., & Molloy D. W (2017). Comparing approaches to optimize cut-off scores for short cognitive screening instruments in mild cognitive impairment and dementia. Journal of Alzheimer’s Disease, 57, 123–133. doi:10.3233/JAD-161204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- et al. (2007). Prevalence of dementia in the United States: The aging, demographics, and memory study. Neuroepidemiology, 29, 125–132. doi:10.1159/000109998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince M., Bryce R., Albanese E., Wimo A., Ribeiro W., & Ferri C. P (2013). The global prevalence of dementia: A systematic review and metaanalysis. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 9, 63.e2–75.e2. doi:10.1016/j.jalz.2012.11.007 [DOI] [PubMed] [Google Scholar]
- Prince M., Wimo A., Guerchet M., Gemma-Claire M., Wu Y., & Prina M. (2015). World Alzheimer Report 2015. The global impact of dementia. An analysis of prevalence, incidence, cost & trends. London: Alzheimer’s Disease International. [Google Scholar]
- RAND HRS Data, Version P. Produced by the RAND Center for the Study of Aging, with funding from the National Institute on Aging and the Social Security Administration. Santa Monica, CA (August 2016).
- Rocca W. A., Petersen R. C., Knopman D. S., Heber L. E., Evans D. A., Hall K. S., … White L. R., (2011). Trends in the incidence and prevalence of Alzheimer’s disease, dementia, and cognitive impairment in the United States. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 7, 80–93. doi:10.1016/j.jalz.2010.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satizabal C. L., Beiser A. S., Chouraki V., Chêne G., Dufouil C., & Seshadri S (2016). Incidence of dementia over three decades in the Framingham Heart Study. The New England Journal of Medicine, 374, 523–532. doi:10.1056/NEJMoa1504327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrijvers E. M., Verhaaren B. F., Koudstaal P. J., Hofman A., Ikram M. A., & Breteler M. M (2012). Is dementia incidence declining? Trends in dementia incidence since 1990 in the Rotterdam Study. Neurology, 78, 1456–1463. doi:10.1212/WNL.0b013e3182553be6 [DOI] [PubMed] [Google Scholar]
- WHO (2012). Dementia: A public health priority.Geneva, Switzerland: WHO Press. [Google Scholar]
- Wimo A., Jönsson L., Bond J., Prince M., & Winblad B; Alzheimer Disease International (2013). The worldwide economic impact of dementia 2010. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 9, 1.e3–11.e3. doi:10.1016/j.jalz.2012.11.006 [DOI] [PubMed] [Google Scholar]
- Wu Y. T., Fratiglioni L., Matthews F. E., Lobo A., Breteler M. M., Skoog I., & Brayne C., (2016). Dementia in western Europe: epidemiological evidence and implications for policy making. The Lancet. Neurology, 15, 116–124. doi:10.1016/S1474-4422(15)00092-7 [DOI] [PubMed] [Google Scholar]
- Yang Z., Holt H. K., Fan J. H., Ma L., Liu Y., Chen W., … Qiao Y. L., (2016). Optimal cutoff scores for Alzheimer’s disease using the Chinese version of Mini-Mental State Examination among Chinese population living in rural areas. American Journal of Alzheimer’s Disease and Other Dementias, 31, 650–657. doi:10.1177/1533317516662336 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.