Abstract
High-elevation lakes in the tropics are subject to extreme environmental fluctuations and microbes may harbor a unique genomic repertoire, but their composition and diversity are largely unknown. Here, we compared the planktonic bacterial community composition (BCC) and diversity of three tropical lakes located in the high Andean plateau (≥4400 m above sea level) during the dry and wet season. Diversity in these lakes was higher in the cool and wet season than in the warm and dry one. Operational taxonomic units (OTUs) composition was significantly different among lakes and between seasons. Members of the class Opitutae, Spartobacteria, Burkholderiales and Actinobacteria were dominant, but only the hgcI clade (Actinobacteria) and the Comamonadaceae family (Burkholderiales) were shared between seasons among the three lakes. In general, a large percentage (up to 42%) of the rare OTUs was unclassified even at the family level. In one lake, a pycnocline and an anoxic water layer with high abundance of Thiocapsa sp. was found in the wet season indicating that the known polymictic thermal condition is not always given. Our study highlights the particular BCC of tropical high-elevation lakes and also how little is known about the variability in physico-chemical conditions of these ecosystems.
Keywords: Bacterial diversity, Thiocapsa sp., 16S rRNA gene, Illumina Miseq, Altiplano
High elevation lakes in the tropics exhibit different bacterial composition patterns, and in one of these polymictic lakes, an anoxic water layer with high abundance of Thiocapsa sp. was found.
INTRODUCTION
High-elevation lakes are very sensitive ecosystems to environmental changes and, thus, ideal sentinels of different global changes (Catalan et al.2006; Adrian et al.2009). Due to many ecological features they share, high-elevation lakes can be considered as one of the best comparable ecosystems across continents (Catalan and Rondón 2016); however, most studies have focused on those lakes located in the temperate zone. Most tropical high-elevation lakes, located above 3000 m, are found in the Andean range (Löffler 1964; Vila and Mühlhauser 1987). The Altiplano south of the tropical Andes is the highest plateau in the world associated with abundant arc magmatism, and it is the second only to Tibet in elevation and extent with an average elevation of 4000 m (Allmendinger et al.1997).
High-elevation tropical lakes have been described as cold polymictic (Hutchinson and Löffler 1956; Mühlhauser et al.1995) with typical holomixis during the night (Wetzel 2001) and weak diurnal stratification taking place only for a few hours (Lewis 1983). However, measurements of physical properties such as water temperature and conductivity at high temporal and spatial resolution are missing in order to support these classifications. For instance, paleolimnological studies in one Altiplano lake (L. Chungará) have revealed important fluctuations in water level causing probably periods with stable stratification when lake water level rise and the wind-driven turbulence is not capable to reach deep-water layers (Sáez et al.2007; Bao et al.2015). High-elevation tropical lakes are naturally meso-eutrophic when considering phosphorus concentrations and are usually limited by nitrogen (Wurtsbaugh et al.1985; Mühlhauser et al.1995). These ecosystems are located in one of the most extreme environments on Earth (Angel, Vila and Herrera 2016) due to the negative water balance, high incident solar UV radiation and extreme daily temperature changes (Dorador et al.2013). The Altiplano climate is characterized by a marked seasonality in precipitation with warm-dry winters and cool-wet summers (Marquez-Garcia et al.2009; Cuyckens et al.2016) suggesting that aquatic ecosystems in this area are extremely sensitive to changes in the hydrological balance and, thus, to climate change (Bao et al.2015).
Bacteria are drivers of most biologically active elements in aquatic ecosystems and changes in community composition, diversity and function are linked to environmental ones (Giorgio and Cole 1998). For instance, thermal stratification patterns can strongly influence the microbial community composition of freshwater systems (Yu et al.2014), because they largely determine the availability of nutrients, light and oxygen in the water column (Rogozin et al.2010; Baatar et al.2016; Shatwell, Adrian and Kirillin 2016). In this sense, it is arguable that the distribution of microbial communities in the water column of cold-polymictic high-elevation tropical lakes will be less affected by temperature or density gradients.
In general, most studies on lake microbial communities have been done in the northern hemisphere mainly in Europe and North America (Logue and Lindström 2008). In contrast, studies of high-elevation lakes in the tropical and subtropical zones are scarce and largely focused on shallow hypersaline lakes (Albarracín, Gärtner and Farias 2016; Rasuk et al.2017). Nevertheless, the bacterial community composition (BCC) in surface waters of several lakes in the Altiplano has been studied but using PCR-DGGE and 16S rDNA clones libraries (Dorador et al. 2009, 2013). A recent study using high throughput sequencing showed that the microbial eukaryotic community composition of lakes in the Chilean Altiplano represent a distinct cluster with little similarity to those from other high mountain lakes of glacial origin in Europe or Africa (Filkner et al.2016).
Here, we compared the BCC among three freshwater-oligohaline high-elevation lakes, located in the same basin during the wet and dry season. We collected composite water samples to obtain a representative picture of the whole water column and of the total diversity, but also at specific depths to test for potential differences. We expected to find a different lake BCC pattern between the wet and dry season, but less differences in the water column when comparing composite samples with those collected from single depths.
MATERIAL AND METHODS
Study sites and sampling
The three lakes are located within the Lauca basin in the Altiplano plateau (17°S). Lakes Chungará and Cotacotani are located ca. 5 km away between them but are connected by groundwater, whereas Lake Piacota is an isolated waterbody located ca. 3 km apart of Cotacotani (Baied and Wheeler 1993; Herrera et al.2006) (Fig. 1). Lake Chungará located at 4520 m above sea level (a.s.l.) is 22.5 km2 large and has a maximum depth between 34 and 40 m (Mühlhauser et al.1995; Herrera et al.2006). Lake Piacota, located at 4400 m a.s.l., is much smaller (0.04 km2) (Dorador et al.2013), whereas Lake Cotacotani, located at 4550 m, has an area of 6 km2 and is formed by four waterbodies that are connected by channels and underground waters (Mladinic, Hrepic and Quintana 1987). Whereas Lake Chungará is oligohaline, the other two ecosystems are typically freshwater.
Figure 1.
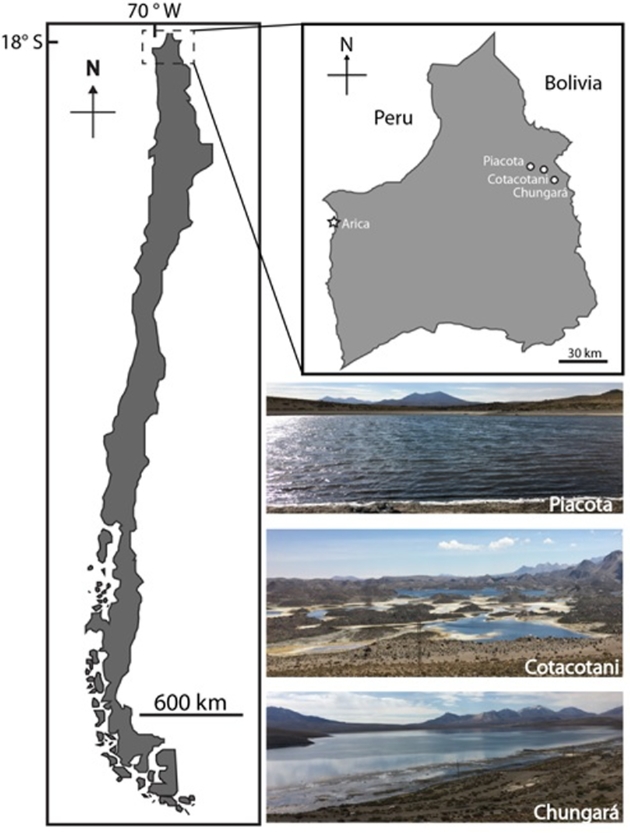
Location of the study sites in the Chilean Altiplano.
Composite (i.e., same volume pooled from every single depth) water samples (Chungará: 0, 1, 2, 4, 6, 8, 10, 12, 15, 20, 25, and 30 m; Cotacotani: 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10 m; and Piacota: 0, 1, 2, 3, and 4 m) were collected in the dry (August 2013) and wet season (March 2014) with a Van Dorn sampler (3 L). Due to unfavorable weather conditions, samples were collected in triplicate only in the wet season. In Lake Chungará, composite samples in the dry season were collected on two consecutive days (August 18 and 19). In addition, on August 19, samples were collected in this lake at 0 and 7 m depth and in Lake Piacota and Cotacotani at 0 m to account for potential differences in BCC. Further, due to a pink hue observed at 8 m depth in Lake Cotacotani in the wet season sampling, a water sample was taken at this depth to compare the results with those obtained from the composite water sample. All samples were collected from a boat placed over the deepest point before midday to avoid typical strong winds in the afternoon. Water samples were kept in cool boxes and afterward (within ca. 2 h) were filtered onto 0.22 μm pore size filters (47 mm, Millipore GPWP), until clogging was observed. Filters were placed in Eppendorf tubes with RNAlater (Qiagen, Germantown, MD) and maintained at −20°C until further analysis.
DNA extraction and Illumina sequencing
Genomic DNA was extracted using a PowerBiofilm DNA Isolation kit (Mo Bio Laboratories Inc., Carlsbad, CA, USA) following the manufacturer's protocol. The concentration and quality of DNA were measured with a Nanodrop spectrophotometer (Nanodrop 8000, Thermo Scientific, Franklin, MA, USA). The total DNA was used as template for the V4 region amplification of the 16S SSU rRNA with the primers 515F (GTGCCAGCMGCCGCGGTAA) and 806R (GGACTACHVGGGTWTCTAAT) (Caporaso et al.2011), and further Illumina sequencing was done at the Research and Testing Laboratory Genomics (Lubbock, Texas, USA) using the Miseq platform. Raw amplicons reads were deposited in the Sequence Read Archive (SRA) of NCBI under accession number SRP076905.
Sequence data processing
A total of 1 024 363 raw reads were found in the 18 samples and analyzed using Mothur (v. 1.35.1) following the standard operating procedure (Schloss, Gevers and Westcott 2011). Briefly, paired end reads obtained from Miseq runs were assembled using the make.contig command. Reads were aligned to the SILVA v.123 database using the align.seqs command. Chimeras were detected and removed using UCHIME. The SILVA123 database was used to classify reads with a confidence threshold of 80%. The remove.lineage command was used to identify and remove mitochondrial, chloroplasts, Archaea, Eukarya, and unknown contaminants. Reads were assigned to operational taxonomic units (OTU) at the 3% level of divergence using the cluster.classic command. Rarefaction curves were made with the rarefaction.single command. Finally, singletons were deleted from the libraries to avoid potential artifacts.
Diversity and statistical analyses
The Shannon diversity index (the exponential of Shannon entropy) of the samples was calculated according with sample completeness instead of equal sample size (Chao and Jost 2012) using the iNEXT package in R, which provides simple functions to compute and plot the seamless rarefaction and extrapolation sampling curves for the most widely used members of the Hill number family (species richness, Shannon diversity and Simpson diversity; Hsieh, Ma and Chao 2016). To test for differences in bacterial composition and relative abundances, all samples were rarified to 26 335 reads, which was the sample with the smallest number of reads. The VEGAN package (Oksanen et al.2013) was used to do the ordinations (metaMDS) based on Bray Curtis distance and using the OTUs relative abundance from the lakes. ANOSIM was used to test for significant differences among lakes in the ordinations. According to the OTUs relative abundance, they were classified as abundant >1%, uncommon >0.1% to <1%, and rare <0.1% (Magurran and Henderson 2003; Pedrós-Alió 2006; Fuhrman 2009). A correspondence analysis was done for the wet season of Lake Cotacotani to test the relationship between the most abundant taxa and the type of samples (i.e., composite and 8 m depth).
Physico-chemical parameters
Profiles in the water column for conductivity, dissolved oxygen and temperature were done with an YSI 6920-M multiprobe (YSI Inc, Yellow Springs, OH, USA). To assess the degree of stratification, water density was calculated based on water temperature and conductivity (Chen and Millero 1986). Oxygen values lower than 1 mg L−1 were considered as a criterion for anoxia (Nürnberg 1995). Anions (nitrate, chloride and sulfate) and cations (potassium, sodium, calcium and magnesium) were measured by ion chromatography (Dionex ICS-1100/1000). Total inorganic carbon (CO32− and HCO3−) was measured by the standard methodology described in APHA (2001). Total nitrogen and phosphorus were measured spectrophotometrically according to Mühlhauser, Soto and Zahradnik (1987). Dissolved organic carbon (DOC) was analyzed in samples collected in pretreated (4 h at 450°C) glass bottles. These samples were filtered through two pre-treated (as for the bottles) GF/F filters (Whatman, England). The filtrate was acidified with HCl to pH 2 and analyzed with a Shimadzu TOC-Vc series (Shimadzu, Kyoto, Japan) equipped with a total nitrogen module. This instrument was calibrated with potassium hydrogen phthalate. Three to five subsamples were analyzed for each sample and for a consensus reference material (CRM) for DOC (batch 5 FS-2005: 0.57 mg L−1; provided by RSMAS/MAC, University of Miami) that was run in parallel on each occasion. Results differed from the CRM given value by 5%, and the coefficient of variation among subsamples was <2%.
RESULTS
General diversity and relative abundance patterns
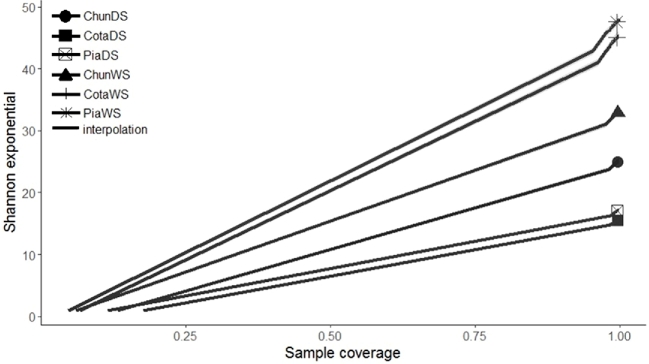
Rarefaction curves showed that all samples reached a plateau suggesting sufficient sequencing depth (Fig. S1, Supporting Information). Shannon diversity was higher in the wet season than in the dry one and in Lake Piacota in the wet season was highest (Fig. 2). Ordination analysis (based on Bray–Curtis dissimilarity) showed that in the wet season, Lake Cotacotani had the most different BCC from all lakes (Fig. 3) and thus, details are presented separately from the other two lakes. Significant differences in OTU relative abundance (ANOSIM; R2 = 0.983, P < 0.01) were found among the lakes. Water temperature and nitrate concentrations were the only parameters showing differences between seasons, both exhibiting a trend to be higher in the dry than in the wet season (Table 1). Lake Piacota was the only system with differences in total nitrogen between seasons.
Figure 2.
Shannon diversity among Altiplano lakes in different seasons. Cota: Lake Cotacotani, Pia: Lake Piacota, Chun: Lake Chungará, DS: dry season, WS: wet season.
Figure 3.
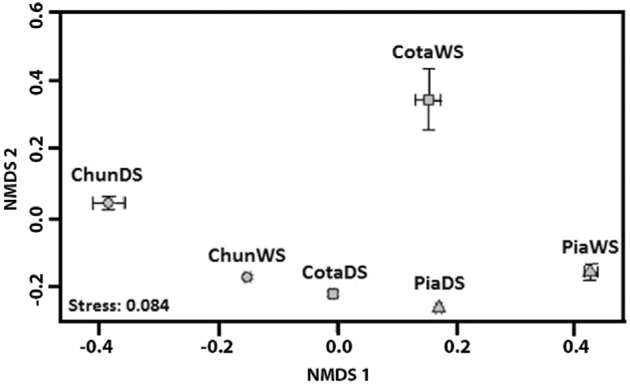
Non-metric multidimensional analysis based on Bray–Curtis dissimilarity showing similarity among lakes communities. Abbreviations as in Fig. 2.
Table 1.
Main physico-chemical parameters of composite samples from Lakes Chungará, Cotacotani and Piacota during the dry and wet season.
| Dry season (August 2013) | Wet season (March 2014) | |||||
|---|---|---|---|---|---|---|
| Chungará | Cotacotani | Piacota | Chungará | Cotacotani | Piacota | |
| Temperature [°C] | 9.9(NA) | 10.5(NA) | 12.6(NA) | 4.8(NA) | 5.7(NA) | 6.4(NA) |
| Total nitrogen [μg L−1] | 1680.7(NA) | 1477.3(NA) | 804.3(NA) | 1663.7(NA) | 1554(NA) | 1482(NA) |
| Total phosphorus [μg L−1] | 959.7(NA) | 469.6(NA) | 96(NA) | 893.4(NA) | 344(NA) | 133(NA) |
| NO3−-N [μg L−1] | 25(±16) | 21.5(±1) | 13(NA) | 5(NA) | 12(NA) | 8(NA) |
| DOC [mg L−1] | 10.5(±0.4) | 6.7(±0.04) | 6.8(±3) | 11(±0.05) | 6.9(±0.5) | 8.6(±0.2) |
| Total alkalinity [mg L−1] | 2.8(NA) | 2.4(NA) | 1.6(NA) | 3.5(NA) | 1.5(NA) | 0.75(NA) |
| CO32− [mg L−1] | 36(NA) | 24(NA) | 36(NA) | 36(NA) | 39(NA) | 42(NA) |
| HCO3− [mg L−1] | 170.8(NA) | 146.4(NA) | 97.6(NA) | 213.5(NA) | 91.5(NA) | 45.8(NA) |
| Conductivity [μS cm−1] | 1506(±7.9) | 848(±5.6) | 633.5(±0.5) | 1400(NA) | 849(NA) | 640(NA) |
| SO42- [mg L−1] | 378.2(±1.4) | 195.3(±0.6) | 135.5(±1) | 383.7(NA) | 186(NA) | 145.5(NA) |
| Cl− [mg L−1] | 63.5(±0.04) | 28.8(±0.1) | 24.1(±0.2) | 62.2(NA) | 27.1(NA) | 26.7(NA) |
| Na+ [mg L−1] | 142.4(±0.4) | 64.2(±1) | 49.7(±0.4) | 143.5(NA) | 62.9(NA) | 55.9(NA) |
| K+ [mg L−1] | 29.2(±0.4) | 8.4(±0.6) | 9.9(±0.9) | 28.9(NA) | 8.9(NA) | 10.7(NA) |
| Mg2+ [mg L−1] | 101.8(±0.4) | 54.3(±0.1) | 34.7(±0.1) | 100.7(NA) | 50.5(NA) | 32.1(NA) |
| Ca2+ [mg L−1] | 57.3(±0.2) | 54.5(±0.5) | 41.4(±0.1) | 54.7(NA) | 47.9(NA) | 33.3(NA) |
Values within brackets are ± 1 standard deviation. NA = not available.
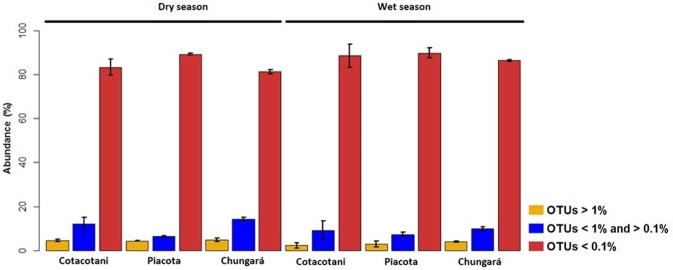
The percentage of abundant OTUs in all lakes was generally low in both seasons, but it was even lower in the wet season than in the dry one. Thus, in Lake Cotacotani, it ranged from 2.7% to 6.1%, in Lake Piacota from 2.8% to 4.3%, and in Lake Chungará from 3.8% to 4.6% (Fig. 4). In consequence, rare OTUs were dominant (80%–98.2% of the total OTU number), particularly in Lake Piacota, where the largest representation was found. In Lake Cotacotani, 23% of the rare OTUs in the dry season and 41.6% in the wet one were unclassified at the family level. Similar unclassified rare OTUs patterns were recorded in Lake Piacota (28.8% in the dry and 36.1% in the wet season) and in Lake Chungará (25% in the dry and 25.9% in the wet season).
Figure 4.
Average relative abundance of abundant (>1%), uncommon (>0.1% to <1%) and rare OTUs (<0.1% of the total number of reads in a given sample).
Comparing the number of shared OTUs between seasons, 45% of the abundant OTUs were shared in Lake Piacota and 28.6% and 66.7% in lakes Cotacotani and Chungará, respectively. In the dry season, the lakes shared 62.5% of abundant OTUs and only 27.8% in the wet one. The percentage of shared uncommon OTUs in all lakes was high (>72%) when composite samples were compared with those from single depths within the dry season, but it was low (<37%) between composite samples from the two seasons (Fig. S2, Supporting Information). Instead, the percentages of shared rare OTUs were low (<53%) in the dry season between single depths and composite samples and between seasons in all lakes (Fig. S3, Supporting Information).
In the dry season, abundant shared OTUs among lakes were classified within hgcl clade (Actinobacteria), unclassified Verrucomicrobia and Comamonadaceae family (Proteobacteria). In the wet season, they were classified within Chitinophagaceae family (Bacteroidetes), hgcl clade and Comamonadaceae family. Therefore, these last two groups were the only bacterial groups shared between seasons.
BCC in lakes Piacota and Chungará
In total, 42 phyla were recorded in these lakes and the most relative abundant ones in the dry season were Verrucomicrobia (22.9%–37.0%), Actinobacteria (29.0%–39.3%), Proteobacteria (16.0%–24.3%) and Bacteroidetes (5.7%–19.8%) (Fig. S4 and S5, Supporting Information). Within Verrucomicrobia, between 90% and 97% of the reads from Lake Piacota in the dry season and Lake Chungará in both seasons were related to unclassified reads at the family level. Furthermore, the occurrence of particular taxa from this phylum changed among lakes. For example, the main class in Lake Piacota was Opitutae, whereas in Lake Chungará it was Spartobacteria. Within Actinobacteria, the main group in these lakes was the hgcI clade. Furthermore, the Candidatus Limnoluna had a high relative abundance in Lake Piacota (14.9%–18.0%). Proteobacteria were mainly dominated by the Comamonadaceae family (Betaproteobacteria) in both lakes. Within Bacteroidetes, the dominant taxa were Flavobacterium sp. in Lake Chungará and Fluviicola sp., Pedobacter sp. and Pseudarcicella sp. in Lake Piacota. In the dry season in Lake Chungará, the same bacterial groups were found between the composite samples collected on two consecutive days and that collected at the surface (Fig. S4, Supporting Information). Similarly, in Lake Piacota, the same bacterial groups were found in surface and composite samples (Fig. S5, Supporting Information).
The same dominant phyla were found in these lakes in the wet season, but their relative abundance changed. For example, Verrucomicrobia was not the most abundant phylum (7.8%–22.4% of relative abundance), and it was mainly dominated by the Opitutae and Verrucomicrobiae class in Lake Chungará and Piacota, respectively. The relative abundance of Actinobacteria was lower in the wet season than in the dry one, but it was dominated by the same groups. Instead, Proteobacteria (Comamonadaceae family) and Bacteroidetes had higher relative abundance in the wet season than in the dry one. Nevertheless, the dominant groups within Bacteroidetes differed between lakes. Thus, in Lake Chungará they were dominated by the Sphingobacteriaceae family and in Lake Piacota by the Flavobacteriales order. Cyanobacteria showed low relative abundance in all lakes (<4%). About 90% of the cyanobacterial OTUs found in the lakes were classified as Synechococcus sp.
BCC in Lake Cotacotani
Different BCC patterns were observed in Lake Cotacotani between the dry and wet season (Fig. 5). In the dry season, the composite and surface samples were dominated by Verrucomicrobia (relative abundance: 44.7%–46.0%), whereas in the wet season this phylum was poorly represented (relative abundance: 0.7%–3.8%). In contrast, the abundant groups in the composite and 8 m depth samples in the wet season were Proteobacteria (relative abundance: 27.2%–45.3%) and Bacteroidetes (relative abundance: 21.2%–26.6%). The uncommon and rare taxa (i.e. with relative abundance <1%) in Lake Cotacotani showed differences in composition (Fig. S6, Supporting Information). For example in the dry season, Chloroflexi, Cyanobacteria, Gemmatimonadetes and Planctomycetes were dominant. By contrast in the wet season, Candidate division SR1, Cyanobacteria, Chloflexi, Cloacimonetes, Hydeogenedentes, Parcubacteria, Planctomycetes and Spirochaetae were dominant.
Figure 5.
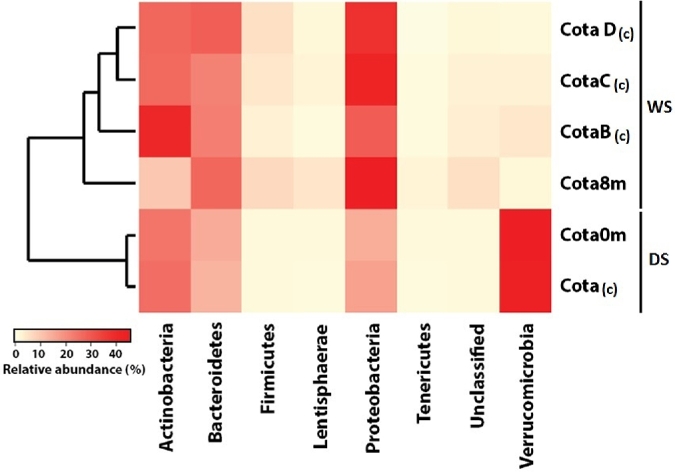
Relative abundance of the main phyla (>1%) in Lake Cotacotani. Clustering of samples represented by a dendrogram based on Bray–Curtis dissimilarity. DS: dry season. WS: wet season. (c): composite simple.
In the wet season and at 8 m depth, the BCC was dominated by Proteobacteria (45.3%), with Gamma- (60.2%) and Delta-Proteobacteria (32%) as the most abundant classes. Although 89.1% of the Deltaproteobacteria were unclassified, the sulfate-reducing bacteria Desulfobacula sp. (2.4%), Desulfocapsa sp. (2.2%) and Desulfomicrobium sp. (2.0%) were present. Within the Gammaproteobacteria, 94.5% were represented by the genus Thiocapsa sp. A comparison with the NCBI database showed a 99% sequence similarity with Thiocapsa pendens (accession number AJ002797). The presence of this bacterium at 8 m depth coincided with the occurrence of anoxic water layers (dissolved oxygen content: 0.48 mg L−1 at 7 m) and with the occurrence of a density gradient (Fig. S7, Supporting Information). Furthermore, at this depth, representatives of other anaerobic taxa such as Microbacter sp., Smithella sp., Fastidiospira sp., Paludibacter sp. and Sporacetigenium sp., and of anaerobic facultative taxa such as Treponema sp., Spirochaeta 2 group and Erysipelothrix sp., as well as sulfate-reducing taxa such as Desulfobacula sp., Desulfomicrobium sp. and Desulfovibrio sp. were found. By contrast, in the composite samples, aerobic taxa such as Mycobacterium sp., Opitutus sp., Owenweeksia sp., Pseudarcicella sp., Sediminibacterium sp., Methylotenera sp., Filimonas sp., Haloferula sp. and Algoriphagus sp. were common (Fig. S8, Supporting Information).
DISCUSSION
We found contrasting BCC patterns among the three lakes during the two typical hydrological seasons in this area, even if the lakes are closely situated. Interestingly, changes in the composition of phytoplankton and zooplankton in aquatic systems of the high Andean plateau has been associated with changes in nitrogen concentration (Márquez-Garcia et al.2009) and here we observed also differences in nitrate concentrations between seasons. As nitrogen has been described as a limiting nutrient in high-elevation tropical lakes (Wurtsbaugh et al.1985), we expected to observe changes in the relative importance of bacteria associated to the cycle of this nutrient. Indeed, we observed that the relative abundance of known denitrifiers taxa such as the members of Comamonadaceae and Peptococcaceae family, Pirellula sp., and Acholeplasma sp. (Fuerst 1995; Chung, Shin and Oh 2009; Ezaki et al. 2009; Willems 2014) was higher in lakes Cotacotani and Piacota during the wet season, when nitrate concentration was lower than in the dry one. Considering the dominance of the Comamonadaceae family in these lakes, its capacity to grow quickly under high nutrient concentrations and balance their vulnerability to grazing in freshwater environments (Newton et al.2011), as well as the known denitrifying capacity of some members, we argue that they could be responsible for causing nitrogen limitation in these tropical lakes.
Further differences in the relative dominance of bacterial groups between seasons were detected when compared with previous studies. In those studies including freshwater and saline aquatic environments of the Altiplano (Dorador et al. 2009, 2013) and Atacama Desert (Demergasso et al. 2004, 2008), Bacteroidetes and Proteobacteria were identified as the most abundant and diverse groups. However, this dominance seems to be characteristic only for the wet season. Similarly, in Lake Chungará, >90% of the sequences found in the dry season of 2003 belong to Bacteroidetes (Dorador et al.2013). In contrast, we found that in the same season the BCC was dominated by Verrucomicrobia and Actinobacteria (Fig. S4, Supporting Information). This difference might be related to interannual changes in BCC or most probably to differences in the methodology used (Illumina vs cloning and DGGE).
In Lake Piacota, we observed the highest diversity and number of unclassified rare groups (wet season) with an average of 36% of unclassified reads at the family level. Although the most abundant phyla were the same in both seasons (Fig. S5, Supporting Information), changes within phyla composition were observed. For example, Verrucomicrobia and Bacteriodetes comprised different dominant taxa between seasons and lakes. Instead, the Comamonadaceae family (Betaproteobacteria) and the hgcI clade (Actinobacteria) were present in high relative abundance in all lakes. Therefore, these groups are probably the ‘core’ representatives in the Altiplano lakes. The hgcI clade, also referred as acI cluster, has been described as the main bacterial group in many freshwater environments (Warnecke, Amann and Pernthaler 2004; Warnecke et al.2005). The first acI sequenced genome suggests a facultative aerobic and actinorhodopsin-based (light-driven) metabolism for these microorganisms (Garcia et al.2013; Ghylin et al.2014). Thus, being less dependent on carbon and other nutrients could explain the high and constant relative abundance of this clade in these variable environments.
Despite several studies have revealed that members of Verrucomicrobia are not abundant in freshwater ecosystems (range: <1% to 6%) (Newton et al.2011; Parveen et al.2012; Kurilkina et al.2016; Schiaffino et al.2016), other studies came to the conclusion that this phylum belongs to the most abundant in aquatic systems (Zhang et al.2014; Schmidt, White and Denef 2016). Previous studies in the Altiplano have found low abundances of Verrucomicrobia in saline aquatic environments (Dorador et al.2013), though they can be one of the most active groups in shallow freshwater ecosystems (Molina et al.2016). Our study, however, confirms that this phylum is an important component in freshwater and oligohaline lakes of the Altiplano. This corresponds also well with the notion that these lakes are naturally meso-eutrophic and that Verrucomicrobia in freshwater lakes seems to be favored by high phosphorus concentrations (Lindström, Vrede and Leskinen 2004). Though knowledge on the ecology of Verrucomicrobia in freshwater ecosystems is limited (Schlesner, Jenkins and Staley 2006), methanotrophy has been identified in strains isolated from sediments surrounded a Solfatara (Pol et al.2007) and more recently mixotrophy in isolates from acidic geothermal sediments (Carere et al.2017).
We found that the rare biosphere was an important component of the bacterial communities in the studied lakes, although this result is prone to be affected by methodological artifacts. While the abundant OTUs were the same for composite and surface samples in the dry season, the rare OTUs were not the same and this was true also when comparing between seasons. The rare biosphere exhibits unique abundance patterns that differ from those of the dominant taxa (Lynch and Neufeld 2015; Liao et al.2017).
One unexpected result of our study was the finding of typical anaerobic bacteria in Lake Cotacotani together with the occurrence of anoxic water layers and a density gradient in the water column (Fig. S7, Supporting Information). High-elevation lakes in the tropics are usually mixed by sporadic strong winds over the year or even on a daily basis in response to strong daily temperature changes (Boehrer and Schultze 2008). They have been classified as polymictic and holomictic (Hutchinson and Löffler 1956; Mühlhauser et al.1995) though this old scheme is based on a low number of measurements and temporal coverage. Although we did not analyze different depths separately, the BCC at 8 m depth differed largely from that of the composite samples, due to the high relative abundance of anaerobic and sulfate-reducing bacteria. The OTUs classified as Thiocapsa sp. were only detected in high abundances in this lake, since in Lake Chungará and Lake Piacota the relative abundance of Thiocapsa sp. was <0.3%. These OTUs showed high similarity with Thiocapsa pendens, which is an obligate phototrophic and strictly anaerobic bacterium that uses sulfide, sulfur, thiosulfate and sulfite as electron donors in the photosynthesis (Pfennig and Trüper 1971).
Previous information of anoxygenic phototrophic bacteria of lakes from the Altiplano is limited and only few sequences have been related to this group in Lake Chungará, where phylotypes classified within Chloroflexi, Betaproteobacteria and Gammaproteobacteria have been reported (Dorador et al.2013). However, Thiocapsa sp. has been found in saline environments of the Altiplano (Dorador, unpublished). These microorganisms are well documented in permanently stratified water bodies and meromictic freshwater lakes (Morana et al.2016), but our finding in this high elevation lake merits further studies.
Despite the indication that at least some of the Altiplano lakes have had apparently periods with stable stratification (Bao et al.2015), water temperature has increased between 1970 and 2010 in tropical and temperate lakes (Kraemer et al.2017). An increase in water temperature together with changes in water levels (e.g., variable precipitation plus increasing demand for water for mining industry) could alter lake stratification patterns, which strongly depend on the lake maximum depth (Kraemer et al.2015). In fact, model projections of future climate change in the tropical Andes indicate that air temperatures will increase 1.5°C at higher elevation by 2030 and 4°C by the end of the century (Vuille et al.2008; Valdivia et al.2010), probably leading to a more stable stratification of the water column. Due to its more wind-protected condition, Lake Cotacotani is probably the most sensitive system to this change in this area.
In summary, lakes in the Andean plateau showed clear seasonal differences in bacterial communities, driven by the strong seasonality of the Altiplano climate. Furthermore, our study highlights the unusual BCC of Lake Cotacotani with high abundances of an anoxygenic phototrophic bacterium. Our results suggest that a reduction of polymixis and the onset of more constant vertical temperature gradients, caused by climate warming, could support a higher diversity of microbial communities.
Supplementary Material
Supplementary data are available at FEMSEC online.
Acknowledgements
We thank Josef Franzoi, Salvador Morales-Gomez, and Gry Larsen for assistance with water chemical analyses, as well as Roland Psenner and Hannes Peter for valuable comments.
SUPPLEMENTARY DATA
Supplementary data are available at FEMSEC online.
AUTHOR CONTRIBUTIONS
RS collected the samples, PA prepared the samples for Illumina sequencing and run the bioinformatic analysis, PA and RS wrote most of the manuscript, and CD and IV contributed with the writing. CD, IV and RS obtained funding for the project. All authors have read and approved this manuscript.
FUNDING
This work was supported by the Austrian Science Fund [FWF, P24442-B25] to R.S. and by the National Commission for Scientific and Technological Research [CONICYT, FONDECYT Nr. 1140179 and 1140543] to C.D. and I.V. PA is supported by a doctoral fellowship from the University of Innsbruck (Austria) and Corporación Cultural Norte Grande (Chile).
Conflict of interest. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
REFERENCES
- Adrian R, O’Reilly CM, Zagarese H et al. Lakes as sentinels of climate change. Limnol Oceanogr 2009;54:2283–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albarracín VH, Gärtner W, Farias ME. Forged under the sun: life and art of extremophiles from Andean lakes. Photochem Photobiol 2016;92:14–28. [DOI] [PubMed] [Google Scholar]
- Allmendinger R, Jordan T, Kay S et al. The evolution of the Altiplano-Puna plateau of the central Andes. Annu Rev Earth Planet Sci 1997;25:139–74. [Google Scholar]
- Angel A, Vila I, Herrera V. Extremophiles: photosynthetic systems in a high-altitude saline basin (Altiplano, Chile). Int Aquat Res 2016;8:91–108. [Google Scholar]
- APHA. In: Clesceri LS, Greenberg AE, Eaton AD (eds). Standard Methods for the Examination of Water and Wastewater. 21th edn Washington DC: American Public Health Association, 2001. [Google Scholar]
- Baatar B, Chiang P, Rogozin D et al. Bacterial communities of three saline meromictic lakes in central Asia. PLoS One 2016;11:e0150847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baied C, Wheeler J. Evolution of high Andean puna ecosystems: environment, climate, and culture change over the last 12000 years in the central Andes. Mt Res Dev 1993;13:145–56. [Google Scholar]
- Bao R, Hernández A, Sáez A et al. Climatic and lacustrine morphometric controls of diatom paleoproductivity in a tropical Andean lake. Quat Sci Rev 2015;129:96–110. [Google Scholar]
- Boehrer B, Schultze M. Stratification of lakes. Rev Geophys 2008;46:620–8. [Google Scholar]
- Caporaso JG, Lauber CL, Walters WA et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Nat Acad Sci 2011;108:4516–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalan J, Camarero L, Felip M et al. High mountain lakes: extreme habitats and witnesses of environmental changes. Limnetica 2006;25:551–84. [Google Scholar]
- Catalan J, Rondón J. Perspectives for an integrated understanding of tropical and temperate high-mountain lakes. J Limnol 2016;75:215–34. [Google Scholar]
- Carere CR, Hards K, Houghton K et al. Mixotrophy drives niche expansion of verrucomicrobial methanotrophs. ISME J 2017;11:2599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao A, Jost L. Coverage-based rarefaction and extrapolation: standardizing samples by completeness rather than size. Ecology 2012;93:2533–47. [DOI] [PubMed] [Google Scholar]
- Chen C, Millero F. Thermodynamic properties for natural waters covering only the limnological range. Limnol Oceanogr 1986;31:657–62. [Google Scholar]
- Chung J, Shin S, Oh J. Characterization of a microbial community capable of reducing perchlorate and nitrate in high salinity. Biotechnol Lett 2009;31:959–66. [DOI] [PubMed] [Google Scholar]
- Cuyckens GAE, Christie DA, Domic AI et al. Climate change and the distribution and conservation of the world's highest elevation woodlands in the South American altiplano. Glob Planet Change 2016;137:79–87. [Google Scholar]
- Demergasso C, Casamayor E, Chong G et al. Distribution of prokaryotic genetic diversity in athalassohaline lakes of the Atacama desert, northern Chile. FEMS Microbiol Ecol 2004;48:57–69. [DOI] [PubMed] [Google Scholar]
- Demergasso C, Escudero L, Casamayor E et al. Novelty and spatio-temporal heterogeneity in the bacterial diversity of hypersaline lake Tebenquiche (Salar de Atacama). Extremophiles 2008;12:491–504. [DOI] [PubMed] [Google Scholar]
- Dorador C, Meneses D, Urtuvia V et al. Diversity of Bacteroidetes in high-altitude saline evaporitic basins in northern Chile. J Geophys Res 2009;114:G00D05. [Google Scholar]
- Dorador C, Vila I, Witzel KP et al. Bacterial and archaeal diversity in high altitude wetlands of the Chilean Altiplano. Fund Appl Limnol 2013;182:135–59. [Google Scholar]
- Ezaki T, De Vos P, Garrity GM et al. Peptococcaceae Bergey's Manual of Systematic Bacteriology. New York: Springer, 2009, 969–1007. [Google Scholar]
- Filker S, Sommaruga R, Vila I et al. Microbial eukaryote plankton communities of high-mountain lakes from three continents exhibit strong biogeographic patterns. Mol Ecol 2016; 25:2286–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst JA. The planctomycetes—emerging models for microbial ecology, evolution and cell biology. Microbiology 1995;141:1493–506. [DOI] [PubMed] [Google Scholar]
- Fuhrman JA. Microbial community structure and its functional implications. Nature 2009;459:193–99. [DOI] [PubMed] [Google Scholar]
- Garcia SL, McMahon KD, Martinez-Garcia M et al. Metabolic potential of a single cell belonging to one of the most abundant lineages in freshwater bacterioplankton. ISME J 2013;7:137–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghylin T, Garcia S, Moya F et al. Comparative single-cell genomics reveals potential ecological niches for the freshwater acI actinobacteria lineage. ISME J 2014;8:2503–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio PA, Cole JJ. Bacterial growth efficiency in natural aquatic systems. Annu Rev Ecol Syst 1998;29:503–41. [Google Scholar]
- Herrera C, Pueyo J, Sáez A et al. Relación de aguas superficiales y subterráneas en el área del lago Chungará y lagunas de Cotacotani. Rev Geol Chile 2006;33:299–325. [Google Scholar]
- Hutchinson GE, Löffler H. The thermal classification of lakes. Proc Nat Acad Sci 1956;24:84–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh TC, Ma KH, Chao A. Interpolation and extrapolation for species diverstiy. 2016, R package version 2.0.12. http://CRAN.R-project.org/package=iNEXT.
- Kraemer BM, Anneville O, Chandra S et al. Morphometry and average temperature affect lake stratification responses to climate change. Geophys Res Lett 2015;42:4981–88. [Google Scholar]
- Kraemer BM, Chandra S, Dell AI et al. Global patterns in lake ecosystem responses to warming based on the temperature dependence of metabolism. Global Change Biol 2017;23:1881–90. [DOI] [PubMed] [Google Scholar]
- Kurilkina M, Zakharova Y, Galachyants Y et al. Bacterial community composition in the water column of the deepest freshwater lake Baikal as determined by next-generation sequencing. FEMS Microbiol Ecol 2016;92, DOI: 10.1093/femsec/fiw094. [DOI] [PubMed] [Google Scholar]
- Lewis W. A revised classification of lakes based on mixing. Can J Fish Aquat Sci 1983;40:1779–87. [Google Scholar]
- Liao J, Cao X, Wang J et al. Similar community assembly mechanisms underlie similar biogeography of rare and abundant bacteria in lakes on Yungui Plateau, China. Limnol Oceanogr 2017;62:723–35. DOI: 10.1002/lno.10455. [DOI] [Google Scholar]
- Lindström E, Vrede K, Leskinen E. Response of a member of the Verrucomicrobia, among the dominating bacteria in a hypolimnion, to increased phosphorus availability. J Plankton Res 2004;26:241–46. [Google Scholar]
- Logue J, Lindström E. Biogeography of bacterioplankton in inland waters. Freshwat Rev 2008;1:99–114. [Google Scholar]
- Löffler H. The limnology of tropical high-mountain lakes. Verh Internat Verein Limnol 1964;15:176–93. [Google Scholar]
- Lynch MDJ, Neufeld JD. Ecology and exploration of the rare biosphere. Nat Rev Micro 2015;13:217–29. [DOI] [PubMed] [Google Scholar]
- Marquez-Garcia M, Vila I, Hinojosa L et al. Distribution and seasonal fluctuations in the aquatic biodiversity of the southern Altiplano. Limnologica 2009;39:314–18. [Google Scholar]
- Magurran AE, Henderson PA. Explaining the excess of rare species in natural species abundance distributions. Nature 2003;422:714–16. [DOI] [PubMed] [Google Scholar]
- Mladinic P, Hrepic N, Quintana E. Caracterizacion física y química de las aguas de los lagos Chungará y Cotacotani. Arch Biol Med Exp 1987;20:89–94. [Google Scholar]
- Molina V, Hernandez K, Dorador C et al. Bacterial active community cycling in response to solar radiation and their influence on nutrient changes in a high-altitude wetland. Front Microbiol 2016;7:1823 DOI: 10.3389/fmicb.2016.01823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morana C, Roland F, Crowe S et al. Chemoautotrophy and anoxygenic photosynthesis within the water column of a large meromictic tropical lake (Lake Kivu, East Africa). Limnol Oceanogr 2016;61:1424–37. [Google Scholar]
- Mühlhauser H, Soto L, Zahradnik P. Improvement of the Kjeldahl method for total nitrogen including acid hydrolizable phosphorus determinations in freshwater ecosystems. Intern J Environ Anal Chem 1987;28:215–26. [Google Scholar]
- Mühlhauser H, Hrepic N, Mladinic P et al. Water quality and limnological features of a high altitude Andean lake, Chungará, in northern Chile. Rev Chil Hist Nat 1995; 68:341–49. [Google Scholar]
- Newton RJ, Jones SE, Eiler A et al. A guide to the natural history of freshwater lake bacteria. Microbiol Mol Biol Rev 2011;75:14–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nürnberg G. Quantifying anoxia in lakes. Limnol Oceanogr 1995;40:1100–11. [Google Scholar]
- Oksanen J, Blanchet FG, Kindt R et al. Vegan: community ecology package. 2013, R package version 2.0-7. http://CRAN.R-project.org/package=vegan.
- Parveen B, Mary I, Vellet A et al. Temporal dynamics and phylogenetic diversity of free-living and particle-associated Verrucomicrobia communities in relation to environmental variables in a mesotrophic lake. FEMS Microbiol Ecol 2012;83:189–201. [DOI] [PubMed] [Google Scholar]
- Pedrós-Alió C. Marine microbial diversity: can it be determined? Trends Microbiol 2006;14:257–63. [DOI] [PubMed] [Google Scholar]
- Pfennig N, Trüper H. New nomenclatural combinations in the phototrophic sulfur bacteria. Int J Syst Evol Microbiol 1971;21:11–4. [Google Scholar]
- Pol A, Heijmans K, Harhangi HR et al. Methanotrophy below pH 1 by a new Verrucomicrobia species. Nature 2007;450:874–78. [DOI] [PubMed] [Google Scholar]
- Rasuk MC, Ferrer GM, Kurth D et al. UV-resistant actinobacteria from high-altitude Andean lakes: isolation, characterization and antagonistic activities. Photochem Photobiol 2017;93:865–80. [DOI] [PubMed] [Google Scholar]
- Rogozin DY, Genova SN, Gulati RD et al. Some generalizations based on stratification and vertical mixing in meromictic Lake Shira, Russia, in the period 2002–2009. Aquat Ecol 2010;44:485–96 [Google Scholar]
- Sáez A, Valero-Garcés B, Moreno A et al. Lacustrine sedimentation in active volcanic settings: the late quaternary depositional evolution of Lake Chungará (northern Chile). Sedimentology 2007; 54:1191–222. [Google Scholar]
- Schlesner H, Jenkins C, Staley JT. The phylum Verrucomicrobia: a phylogenetically heterogeneous bacterial group. Prokaryotes 2006;7:881–96. [Google Scholar]
- Schloss P, Gevers D, Westcott S. Reducing the effects of PCR amplification and sequencing artifacts on 16S rRNA-based studies. PLoS One 2011;6:e27310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiaffino M, Sánchez M, Gerea M et al. Distribution patterns of the abundance of major bacterial and archaeal groups in Patagonian lakes. J Plankton Res 2016;38:64–82. [Google Scholar]
- Schmidt M, White J, Denef V. Phylogenetic conservation of freshwater lake habitat preference varies between abundant bacterioplankton phyla. Environ Microbiol 2016;18:1212–26. [DOI] [PubMed] [Google Scholar]
- Shatwell T, Adrian R, Kirillin G. Planktonic events may cause polymictic-dimictic regime shifts in temperate lakes. Sci Rep 2016;6:24361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivia C, Seth A, Gilles J et al. Adapting to climate change in Andean ecosystems: landscapes, capitals, and perceptions shaping rural livelihood strategies and linking knowledge systems. Ann Assoc Am Geogr 2010;100:818–34. [Google Scholar]
- Vila I, Mühlhauser HA. Dinámica de lagos de altura, perspectivas de investigación. Arch Biol Med Exp 1987; 20:95–103. [Google Scholar]
- Vuille M, Francou B, Wagnon P et al. Climate change and tropical Andean glaciers: past, present and future. Earth-Sci Rev 2008;89:79–96. [Google Scholar]
- Warnecke F, Amann R, Pernthaler J. Actinobacterial 16S rRNA genes from freshwater habitats cluster in four distinct lineages. Environ Microbiol 2004;6:242–53. [DOI] [PubMed] [Google Scholar]
- Warnecke F, Sommaruga R, Sekar R et al. Abundance, identity and growth state of actinobacteria in mountain lakes of different UV transparency. Appl Environ Microbiol 2005;71:5551–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel RG. Limnology, Lake and River Ecosystems, 3th edn London: Academy Press, 2001. [Google Scholar]
- Willems A. The family Comamonadaceae. In: The Prokaryotes. Berlin Heidelberg: Springer, 2014, 777–851. [Google Scholar]
- Wurtsbaugh WA, Vincent WF, Alfaro R et al. Nutrient limitation of algal growth and nitrogen fixation in a Tropical Alpine lake, Lake Titicaca (Peru/Bolivia). Freshwat Biol 1985;15:185–95. [Google Scholar]
- Yu Z, Yang J, Amalfitano S et al. Effects of water stratification and mixing on microbial community structure in a subtropical deep reservoir. Sci Rep 2014;4:5821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhang X, Liu Y et al. Bacterioplankton communities in a high-altitude freshwater wetland. Ann Microbiol 2014;64:1405–11. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data are available at FEMSEC online.