Vaccination of pregnant baboons with a monocomponent pertussis toxoid–only vaccine at the start of the third trimester of gestation is sufficient to protect infant baboons from disease following exposure to Bordetella pertussis at 5 weeks of age.
Keywords: Bordetella pertussis, maternal vaccination, pertussis toxin, vaccine, nonhuman primates
Abstract
Background
Bordetella pertussis is a human pathogen responsible for serious respiratory illness. The disease is most severe in infants too young to be vaccinated with most hospitalizations and deaths occurring within this age group. The Advisory Committee on Immunization Practices recommended immunization of pregnant women to protect infants from birth until their first vaccination at 6–8 weeks of age. We previously demonstrated that maternal vaccination with licensed acellular pertussis vaccines protected newborn baboons from disease. We hypothesized that protection was due to toxin-neutralizing, maternal anti–pertussis toxin antibodies and predicted that maternal vaccination with a pertussis toxoid (PTx)–only vaccine would protect newborns from disease.
Methods
Infant baboons born to unvaccinated mothers or mothers vaccinated with a PTx-only vaccine were challenged with B. pertussis at 5 weeks of age and followed for infection and signs of disease.
Results
Although all challenged infants were heavily colonized, the infant baboons born to mothers vaccinated with PTx-only vaccine were free from clinical disease following exposure to B. pertussis. In contrast, disease was observed in infants born to unvaccinated mothers.
Conclusions
Our results demonstrated that maternal vaccination with a PTx-only vaccine is sufficient to protect newborn baboons from disease following exposure to pertussis.
(See the Editorial Commentary by Edwards, on pages 1177–9.)
Despite high rates of vaccination with acellular pertussis (aP) vaccines, the incidence of Bordetella pertussis infections is on the rise in the United States and other high-income countries [1]. Severe pertussis cases are characterized by intense and paroxysmal cough associated with posttussive vomiting, inspiratory whoop, cyanosis, and leukocytosis [2, 3]. Because the first routine pertussis vaccination occurs at 6–8 weeks of age, infants <2 months of age have the highest rate of serious clinical complications requiring hospitalization and the highest mortality rate [4, 5]. Paroxysmal fits in very young infants are characterized by gagging, gasping, bradycardia, cyanosis, and vomiting [6]. Apneic episodes following paroxysmal fits are common [6]. Severe and fatal pertussis in young infants is associated with extreme leukocytosis, pulmonary hypertension, and pneumonia [6, 7]. Maternal immunization has emerged as the optimal strategy to protect newborns from pertussis illness during the first months of life. Numerous studies have demonstrated that maternal vaccination with licensed aP vaccines during pregnancy results in elevated B. pertussis–specific antibody titers in newborns that are presumed to be protective [8–12]. Based largely on evidence of safety and increased antibody titers in infants, the Advisory Committee on Immunization Practices and the United Kingdom Department of Health recommended that all pregnant women be offered pertussis vaccine to protect newborns from pertussis exposure [13, 14]. Following adoption of maternal vaccination, several studies in the United Kingdom and the United States reported high levels of efficacy of maternal vaccination for the prevention of laboratory-confirmed pertussis in infants [15–19]. Supportive data were provided by a study that used the baboon model of pertussis to demonstrate protection of newborn baboons born to mothers vaccinated during pregnancy with aP vaccine [20].
The baboon model of pertussis developed in our laboratory accurately reproduces severe clinical pertussis [21–23]. In addition to providing an excellent model of pertussis, the baboon has proven to be a relevant model for reproductive studies [24, 25]. Baboons possess the same 4 immunoglobulin G (IgG) subclasses as humans [26] and transplacental transfer of IgG from mother to fetus occurs as in humans [27, 28]. This model provides a unique opportunity to characterize the mechanisms underlying the protection conferred following maternal vaccination and to evaluate the protection conferred by alternative vaccine formulations.
In the previous study, newborn baboons born to vaccinated mothers were protected from clinical disease; however, colonization of the upper respiratory tract was not reduced relative to that in infant baboons born to unvaccinated mothers [20]. The failure of maternal vaccination with aP vaccines to reduce bacterial colonization of the infant airway suggested that maternal antibodies against filamentous hemagglutinin (FHA), pertactin (PRN), and fimbriae (FIM) did not contribute to the observed protection. These bacterial proteins were included in aP vaccines because they are thought to be important for colonization and carriage. We hypothesized that the observed protection of infants from disease was due to the action of maternal, toxin-neutralizing, anti–pertussis toxin (PT) antibodies. If this hypothesis is correct, maternal vaccination with a monocomponent aP vaccine, composed solely of pertussis toxoid (PTx), would be sufficient to protect infants from disease.
In this study, we evaluated the protection conferred to infant baboons by maternal vaccination with a monocomponent PTx vaccine. Infant baboons born to vaccinated mothers and those born to unvaccinated mothers were exposed to B. pertussis at 5–6 weeks of age. There was no significant difference in bacterial colonization of the airway between infants born to vaccinated mothers and those born to unvaccinated mothers. Infant baboons born to unvaccinated mothers developed severe pertussis. In contrast, infants born to mothers vaccinated with the monocomponent PTx vaccine showed no signs of disease, demonstrating that maternal vaccination with PTx alone is sufficient to protect infant baboons from pertussis.
METHODS
Ethics Statement
All animal procedures were performed in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International in accordance with protocols approved by the University of Oklahoma Health Sciences Center Animal Care and Use Committee and in accordance with the principles outlined in the Guide for the Care and Use of Laboratory Animals, produced by the Institute for Laboratory Animal Resources, National Research Council.
Animals
Eight adult female baboons were acquired from a specific-pathogen-free baboon colony (Oklahoma Baboon Research Resource, Oklahoma City) [28]. They had no known exposure to Bordetella species and were free of titers to PT prior to vaccination. Infant baboons were born naturally to their mothers in the colony and transferred to the nonhuman primate nursery on the day of birth to provide more-standardized care and monitoring throughout the studies. Neonates and infants in the nursery were hand-fed a human baby formula (Similac) ad libitum. Once inoculated with B. pertussis, they were housed in animal biosafety level 2+ housing until at least 2 nasopharyngeal washes were B. pertussis negative.
Bacterial Strains and Media
B. pertussis strain D420 was grown on Bordet-Gengou agar plates prepared with Bordet-Gengou agar (Becton Dickinson, Sparks, MD) containing 1% proteose peptone (Becton Dickinson) and 15% defibrinated sheep blood. Regan-Lowe plates were prepared from Regan-Lowe charcoal agar base (Becton Dickinson) with 10% defibrinated sheep blood and 40 µg/mL cephalexin.
Vaccine
Pharmaceutical-grade monocomponent PTx vaccine was provided by MassBiologics (Mattapan, MA). The single-component PTx was produced from PT purified from fermentation cultures of B. pertussis and then inactivated with hydrogen peroxide. The PTx was adsorbed to aluminum hydroxide. The vaccine was formulated at 40 μg/mL of PTx and 1 mg/mL of Alhydrogel. The baboon dose was 0.5 ml administered intramuscularly.
Inoculation
All infant baboons were challenged between 5 and 6 weeks of age. Inocula were prepared to a concentration of 108 bacteria/mL as previously described [21]. Baboons were anesthetized with 10–15 mg/kg ketamine administered intramuscularly. The pharynx was swabbed with 2% lidocaine solution, and animals were intubated using a 2–3-mm (inner diameter) endotracheal tube to deliver 0.5 mL of the inoculum to the proximal trachea. In addition, a 22-gauge, 3.2-cm Teflon intravenous catheter (Abbocath) was used to deliver 0.25 mL of inoculum to both nasal cavities. Animals were placed in a sitting position for 3 minutes, returned to their cages, and observed until they recovered from anesthesia.
Evaluation of Animals
Infants were observed twice daily, and their clinical condition and behavior was noted. Case notes were reviewed by 6 blinded researchers, and an overall clinical score based on the severity and duration of symptoms was assigned to each animal independently by each researcher. For biological sample collection, baboons were anesthetized with ketamine twice per week following inoculation. Whole-blood specimens were collected and evaluated for the number of circulating white blood cells, by complete blood count (CBC). Each nasal cavity was flushed with 0.5 mL of phosphate-buffered saline, using a 22 gauge/3.2-cm intravenous catheter. The recovered nasopharyngeal washes from both nares were combined, and 100 µL of the recovered sample was divided and plated onto 2 Regan-Lowe plates. The number of B. pertussis colony-forming units (CFUs) per plate was recorded after incubation at 37oC for 4–5 days.
Serum Antibody Enzyme-Linked Immunosorbent Assay (ELISA)
Serum IgG for PT, FHA, FIM, and PRN was detected using serum antibody ELISA as described previously [20, 29]. Briefly, Nunc 96-well plates (Fisher Scientific, Rochester, NY) were coated overnight with 2 µg/mL PT, 0.5 µg/mL FHA, 0.2 µg/mL FIM 2/3, or 2 µg/mL PRN (List Biologicals, Campbell, CA). Horseradish peroxidase–conjugated goat anti-monkey IgG polyclonal secondary antibody (AbD Serotec [catalog code AAI42P]; Raleigh, NC) was used to measure serum IgG level for each antigen. The serum antibody was analyzed against a standard curve to assign international units for PT, FHA, and PRN in each sample and relative units for FIM. The standard curve was prepared for each plate by making a serial dilution of the World Health Organization international standard B. pertussis antiserum (NIBSC, Hertfordshire, England).
Statistical Analysis
Statistical analysis was determined using a 2-tailed Student t test assessed by GraphPad Prism software. Results are shown as mean values ± standard deviations for each sample. Antibody data were normalized by log transformation before analysis.
RESULTS
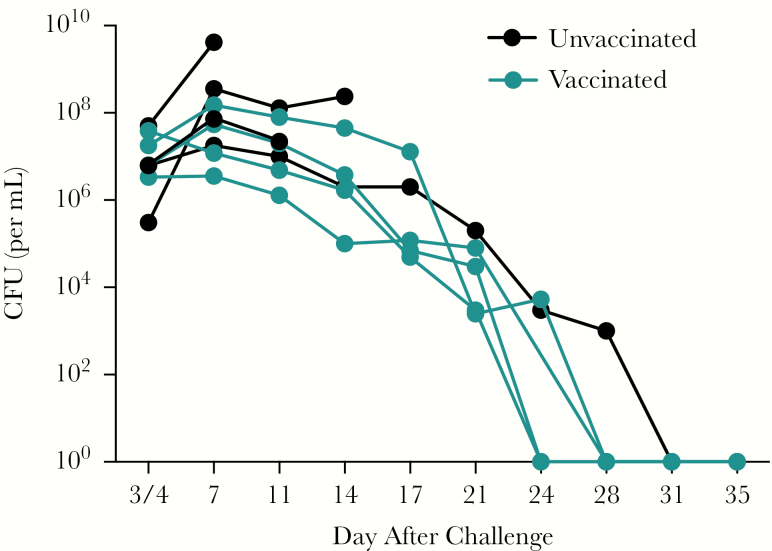
Pregnant baboons were vaccinated with a monocomponent, PTx-only aP vaccine. These baboons were not previously vaccinated with pertussis vaccine and were sourced from a colony with no known Bordetella exposure. Therefore, the response to vaccination in these pregnant animals was a primary response rather than a booster response and was expected to be low. Because of the uncertainty that protection would be achieved with a single vaccination of a naive animal, the first animal was vaccinated twice during pregnancy, on day 60 and day 125 of gestation, and the second animal was vaccinated a single time, on day 125. The infants born to both animals were similarly protected, so the remaining 2 animals in the vaccine group were vaccinated only once, on day 125 of gestation. The 4 infants born to PTx-vaccinated mothers and 4 infants born to unvaccinated mothers were challenged with B. pertussis strain D420 at 5 weeks of age, and bacterial loads in the airway were evaluated by determining the number of CFUs present in nasopharyngeal washes following challenge. Infants born to unvaccinated mothers were heavily colonized, with peak levels between 107 and 1010 CFU/mL (Figure 1). Infants born to the 4 PTx-vaccinated mothers had peak values between 106 and 108 CFU/mL and maintained those high levels for 2 weeks (Figure 1). After day 14, CFUs gradually declined, and the animals subsequently cleared the infection between days 24 and 28 after infection.
Figure 1.
Maternal vaccination with pertussis toxoid (PTx) vaccine does not reduce the bacterial burden in the airway of infants following infection with Bordetella pertussis. Infant baboons born to mothers vaccinated with the monocomponent PTx vaccine during pregnancy (blue; n = 4) or to unvaccinated mothers (black; n = 4) were directly challenged with B. pertussis at 5–6 weeks of age. Colonization was monitored by quantifying B. pertussis colony-forming units (CFUs) in nasopharyngeal washes, with a limit of detection of 10 CFUs/mL. Three of 4 infants born to unvaccinated mothers were euthanized owing to the severity of disease and were lost to follow-up.
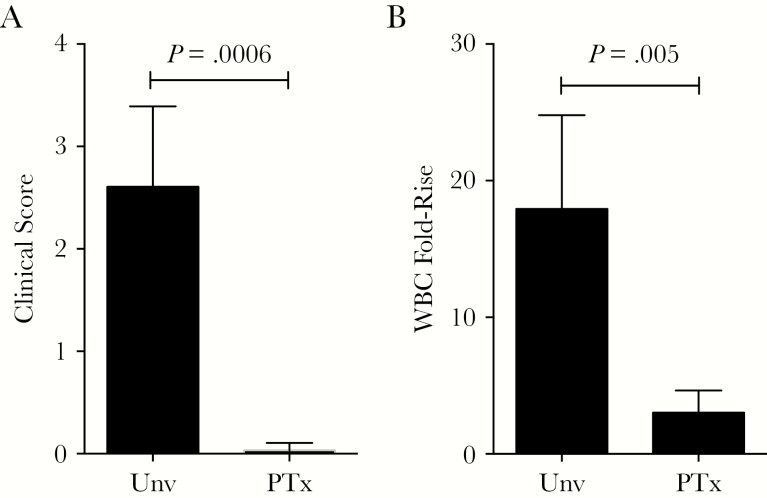
Following challenge, all 4 animals born to unvaccinated mothers developed significant disease. Three were euthanized owing to the severity of their clinical disease. Clinical signs, beginning between days 3 and 7 after exposure, observed in these 3 infants started with infrequent coughing and progressed to frequent paroxysmal coughing that led to extreme lethargy. At the peak of illness, they exhibited tachypnea, dyspnea, tachycardia, and cyanotic mucous membranes. Furthermore, they exhibited anorexia with little to no effort given to obtaining food. The fourth infant in this group exhibited clinical signs of mild disease that included slight anorexia, infrequent coughing, and a mild decrease in activity. This baboon was obviously sick but did not reach the criteria for euthanasia. The significant outward signs of disease observed in these animals were reflected in the high clinical score assigned (Figure 2A). The 3 infants born to unvaccinated mothers with severe clinical symptoms also had highly elevated white blood cell counts, with peak values between 83000 and 110000 cells/µL. The fourth infant, with milder clinical symptoms, had a correspondingly lower peak white blood cell count of 40000 cells/µL. In contrast, all 4 infants born to mothers vaccinated with the monocomponent PTx vaccine remained healthy throughout the 4 weeks following challenge. Despite heavy nasopharyngeal colonization (Figure 1), they remained active and alert. They were assigned clinical scores between 0 and 1, reflecting the lack of outward signs of disease (Figure 2A). The fold change in the number of circulating WBCs relative to preinfection baseline values was low in the infants born to vaccinated mothers relative to the fold change observed in infants born to unvaccinated mothers (Figure 2B).
Figure 2.
Maternal vaccination with pertussis toxoid (PTx) vaccine prevents clinical signs and extreme leukocytosis in newborn baboons following infection with Bordetella pertussis. Infant baboons born to mothers vaccinated with the monocomponent PTx vaccine during pregnancy (PTx) or to unvaccinated mothers (Unv) were challenged with B. pertussis at 5–6 weeks of age. Daily health reports for each animal were reviewed by blinded researchers, and clinical scores were assigned independently, based on the following scale: 0, no disease; 1, mild disease; 2, severe disease; and 3, very severe disease (requiring euthanasia). A, The averages of the assigned clinical scores for each group are shown. B, The peak fold change in circulating white blood cell (WBC) counts relative to the prechallenge baseline counts are shown for each group of animals.
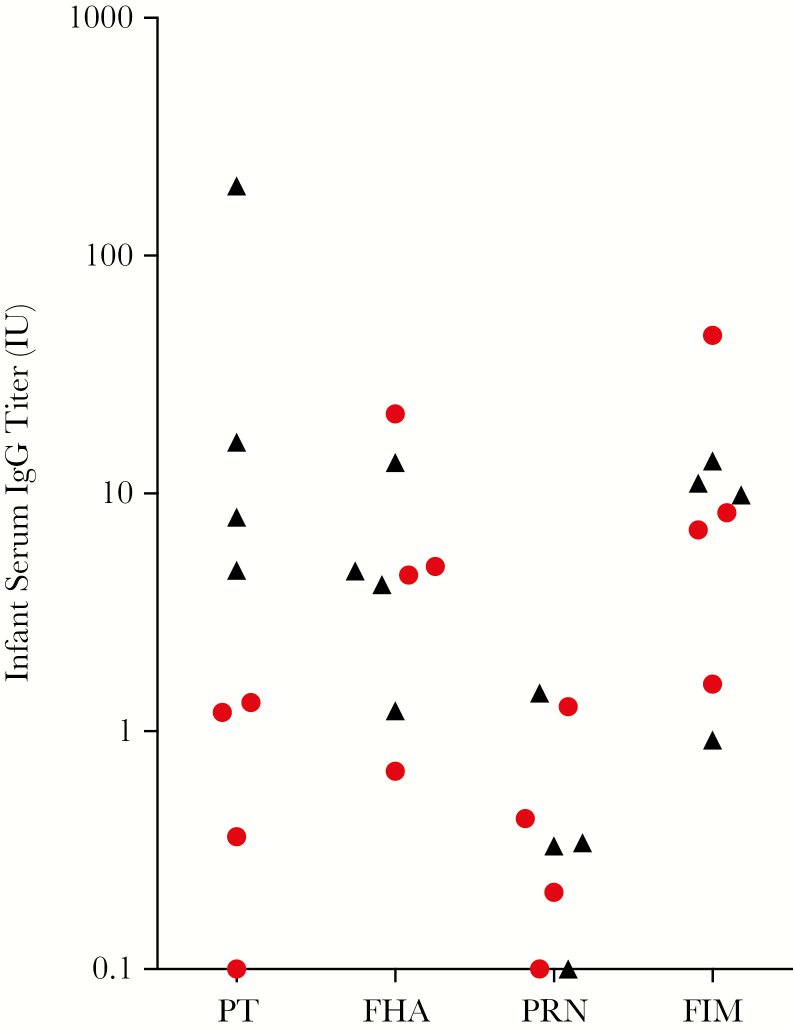
To evaluate the level of immune responses to PT and B. pertussis antigens other than PT, we determined the titers present in the infant prechallenge sera for PT, FHA, PRN, and FIM by ELISA. FHA, PRN, and FIM were selected because our laboratory has established, well-characterized ELISAs for those antigens. We reasoned that a lack of response to these antigens would indicate that the PTx-only vaccine is relatively free of contaminating B. pertussis antigens. All 4 infants born to the vaccinated mothers had elevated anti-PT titers relative to infants born to unvaccinated mothers. One of the 4 had a very high anti-PT titer. This was not the infant whose mother received 2 vaccinations during pregnancy. It is not known why this infant’s mother responded so strongly to PTx. The infant did not exhibit unusually high titers to the other 3 antigens tested, ruling out the concern that this animal’s mother had been previously exposed to Bordetella organisms. All 4 animals born to unvaccinated mothers exhibited very low anti-PT antibody titers (Figure 3). The 4 infants born to vaccinated mothers had low antibody titers to FHA, PRN, and FIM that were comparable to the titers measured in the infants born to unvaccinated mothers (Figure 3).
Figure 3.
Low but measurable pertussis toxin (PT) titers correlate with protection. Serum samples were collected from infants immediately prior to challenge. Immunoglobulin G antibodies to the 4 vaccine antigens present in licensed acellular pertussis vaccines—PT, filamentous hemagglutinin (FHA), pertactin (PRN), and fimbriae (FIM)—were measured by an enzyme-linked immunosorbent assay. Red symbols represent animals with significant clinical signs of disease. Black symbols represent animals without outward signs of disease. Triangles represent animals born to vaccinated mothers. Circles represent animals born to unvaccinated animals.
DISCUSSION
In the previous baboon maternal vaccination study, baboons were vaccinated during pregnancy with US-licensed aP vaccines that contained PT, FHA, and PRN (Infanrix) or PT, FHA, PRN, and FIM (Daptacel) [20]. All infants born to vaccinated mothers were protected from disease, but the magnitude and duration of bacterial colonization of the airway were indistinguishable between the infants born to vaccinated mothers and the infants born to unvaccinated mothers. Similarly, in this study, infants born to mothers vaccinated with the monocomponent PTx vaccine were protected from disease, but the magnitude and duration of infection were not reduced relative to animals born to vaccinated mothers. The magnitude and duration of bacterial colonization of the airway observed in all animals in this study were similar to those observed in unvaccinated and aP-vaccinated baboons in previously published studies. This result is consistent with previous results demonstrating that vaccination with the aP vaccine does not protect against colonization.
The vaccines used in maternal vaccination programs (ie, Tdap vaccines) are reduced-dose adult booster vaccines that contain PT, FHA, and PRN or PT, FHA, PRN, and FIM, depending on the manufacturer. In addition to these B. pertussis antigens, all include Clostridium tetanus toxoid and Corynebacterium diphtheriae toxoid. The protection against pertussis conferred by maternal vaccination with Tdap is presumed to be largely due to the transplacental transfer of IgG to the infant and/or due to secretory immunoglobulin A and IgG transferred in breast milk [30]. It is thought that the role of FHA, FIM, and PRN in pathogenesis is to contribute to bacterial adherence to host tissues and to resist clearance by neutrophils [2, 31]. Antibodies against FHA, FIM, or PRN would most likely confer protection by reducing the magnitude or duration of infection in the airway. As a reduction in colonization was not observed in the previous baboon maternal vaccination study, the observed protection against disease was most likely due to the transfer of maternal toxin-neutralizing antibodies that blocked the actions of PT in the infant. This is consistent with our understanding of B. pertussis pathogenesis. Very-high-level leukocytosis is strongly associated with poor outcome in newborns infected with B. pertussis, and leukocytosis is the result of direct action of PT. In this study, the protection conferred by maternal vaccination with a monocomponent PTx vaccine was evaluated. Despite the relatively low anti-PTx titers in 3 of 4 infants born to mothers vaccinated with the monocomponent PTx vaccine, all 4 infants were protected from disease following exposure to B. pertussis. In contrast, all 4 infants born to unvaccinated mothers experienced significant disease. The possibility that responses to pertussis antigens other than PTx, which were present in trace amounts in the vaccine, contributed to protection cannot be ruled out. To evaluate responses to antigens not included in the PTx vaccine, we evaluated antibody responses to those antigens for which we had well-established ELISAs. In this study, all 4 vaccinated infants had titers for FHA, PRN, and FIM comparable to values observed in the unvaccinated animals indicating that the PTx monocomponent vaccine did not elicit significant responses to antigens other than PT. In contrast, the anti-PT titers in all 4 infants born to vaccinated mothers were higher than the anti-PT titers in the unvaccinated animals. All 4 infants that were protected against disease had PT titers higher than the 4 infants that were not protected. These results suggest that the protection observed in the infants born to vaccinated mothers was due to the provision of toxin-neutralizing (anti-PT) antibodies. The results of this study support the hypothesis that the most severe symptoms associated with pertussis are due to the action of PT and can be blocked by the provision of toxin-neutralizing antibodies.
The global burden of pertussis is difficult to assess. In low- and middle-income countries (LMICs), the surveillance for pertussis is based solely on clinical diagnosis of disease [32]. Pertussis is often misdiagnosed when relying on diagnosis based on symptoms, particularly in young infants, in whom cough is not always observed or is atypical. Pertussis in as many as 90% of infants with cases may be undiagnosed in the absence of active surveillance and laboratory diagnostics [33, 34]. Pertussis deaths in settings where maternal vaccination programs have been introduced have dropped dramatically [15]. Expansion of maternal vaccination to LMICs has the potential to dramatically decrease the infant mortality burden due to pertussis in those countries, but this would only be cost-effective in many LMICs if the vaccine can be obtained at low cost [35]. By reducing the cost of production of the pertussis component of a combined tetanus-pertussis adult booster vaccine, the exclusion of pertussis antigens other than PTx may facilitate the extension of maternal vaccination for pertussis in low-income countries.
Studies evaluating the impact of maternal vaccination on immune responses to the childhood immunizations given at 2, 4, and 6 months of age have produced conflicting results [36]. Some have shown no impact on responses, while others have demonstrated mild blunting of responses to antigens present in the maternal vaccine. In all of those studies, no difference was observed in the responses following the fourth dose, delivered at 12 months. There is no evidence that this blunting is clinically significant. However, one advantage of a maternal vaccine containing only tetanus and PTx would be the avoidance of blunting of responses to other antigens included in the infant vaccination series.
Although it is important to be conservative in interpreting the results of this study and extrapolating them to humans, this study demonstrated, in a highly relevant animal model, that vaccination during pregnancy with a monocomponent PTx vaccine was sufficient to protect newborns from disease. The response to vaccination required to confer protection appears to be easily achievable by a single vaccination in previously unvaccinated adult baboons. It is likely that stronger responses would be observed in human mothers who have a history of childhood pertussis vaccination and/or have had exposure to the disease.
Notes
Acknowledgments. We thank MassBiologics for generously providing the monocomponent PTx vaccine for this study.
Financial support. This work was supported by the Bill and Melinda Gates Foundation, the Food and Drug Administration, and the Office of the Director, National Institutes of Health (grants P40OD010988 and P40OD010431 to the Oklahoma Baboon Research Resource).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Centers for Disease C. 2014 Final Pertussis Surveillance Report. September 18, 2015. [Google Scholar]
- 2. Kilgore PE, Salim AM, Zervos MJ, Schmitt HJ. Pertussis: microbiology, disease, treatment, and prevention. Clin Microbiol Rev 2016; 29:449–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wood N, McIntyre P. Pertussis: review of epidemiology, diagnosis, management and prevention. Paediatr Respir Rev 2008; 9:201–11; quiz 211–2. [DOI] [PubMed] [Google Scholar]
- 4. Chu HY, Englund JA. Maternal immunization. Clin Infect Dis 2014; 59:560–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lindsey B, Kampmann B, Jones C. Maternal immunization as a strategy to decrease susceptibility to infection in newborn infants. Curr Opin Infect Dis 2013; 26:248–53. [DOI] [PubMed] [Google Scholar]
- 6. Cherry JD. Pertussis in young infants throughout the world. Clin Infect Dis 2016; 63:119–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Murray EL, Nieves D, Bradley JS et al. Characteristics of severe Bordetella pertussis infection among infants ≤90 days of age admitted to pediatric intensive care Units - Southern California, September 2009-June 2011. J Pediatric Infect Dis Soc 2013; 2:1–6. [DOI] [PubMed] [Google Scholar]
- 8. Abu Raya B, Srugo I, Kessel A et al. The effect of timing of maternal tetanus, diphtheria, and acellular pertussis (Tdap) immunization during pregnancy on newborn pertussis antibody levels - a prospective study. Vaccine 2014; 32:5787–93. [DOI] [PubMed] [Google Scholar]
- 9. Donegan K, King B, Bryan P. Safety of pertussis vaccination in pregnant women in UK: observational study. BMJ 2014; 349:g4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gall SA, Myers J, Pichichero M. Maternal immunization with tetanus-diphtheria-pertussis vaccine: effect on maternal and neonatal serum antibody levels. Am J Obstet Gynecol 2011; 204:334.e1–5. [DOI] [PubMed] [Google Scholar]
- 11. Kharbanda EO, Vazquez-Benitez G, Lipkind HS et al. Evaluation of the association of maternal pertussis vaccination with obstetric events and birth outcomes. JAMA 2014; 312:1897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Munoz FM, Bond NH, Maccato M et al. Safety and immunogenicity of tetanus diphtheria and acellular pertussis (Tdap) immunization during pregnancy in mothers and infants: a randomized clinical trial. JAMA 2014; 311:1760–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Centers for Disease C, Prevention. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine in adults aged 65 years and older - Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep 2012; 61:468–70. [PubMed] [Google Scholar]
- 14. UK Department of Health and Social Care. Pregnant women to be offered whooping cough vaccination. 28 September 2012 https://www.gov.uk/government/news/pregnant- women-to-be-offered-whooping-cough-vaccination. Accessed 29 January 2018.
- 15. Amirthalingam G, Andrews N, Campbell H et al. Effectiveness of maternal pertussis vaccination in England: an observational study. Lancet 2014; 384:1521–8. [DOI] [PubMed] [Google Scholar]
- 16. Baxter R, Bartlett J, Fireman B, Lewis E, Klein NP. Effectiveness of vaccination during pregnancy to prevent infant pertussis. Pediatrics 2017; 139. [DOI] [PubMed] [Google Scholar]
- 17. Dabrera G, Amirthalingam G, Andrews N et al. A case-control study to estimate the effectiveness of maternal pertussis vaccination in protecting newborn infants in England and Wales, 2012-2013. Clin Infect Dis 2015; 60:333–7. [DOI] [PubMed] [Google Scholar]
- 18. Winter K, Cherry JD, Harriman K. Effectiveness of prenatal tetanus, diphtheria, and acellular pertussis vaccination on pertussis severity in infants. Clin Infect Dis 2017; 64:9–14. [DOI] [PubMed] [Google Scholar]
- 19. Winter K, Nickell S, Powell M, Harriman K. Effectiveness of prenatal versus postpartum tetanus, diphtheria, and acellular pertussis vaccination in preventing infant pertussis. Clin Infect Dis 2017; 64:3–8. [DOI] [PubMed] [Google Scholar]
- 20. Warfel JM, Papin JF, Wolf RF, Zimmerman LI, Merkel TJ. Maternal and neonatal vaccination protects newborn baboons from pertussis infection. J Infect Dis 2014; 210:604–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Warfel JM, Beren J, Kelly VK, Lee G, Merkel TJ. Nonhuman primate model of pertussis. Infect Immun 2012; 80:1530–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Warfel JM, Beren J, Merkel TJ. Airborne transmission of Bordetella pertussis. J Infect Dis 2012; 206:902–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Warfel JM, Merkel TJ. Bordetella pertussis infection induces a mucosal IL-17 response and long-lived Th17 and Th1 immune memory cells in nonhuman primates. Mucosal Immunol 2013; 6:787–96. [DOI] [PubMed] [Google Scholar]
- 24. Carter AM, Mess A. Evolution of the placenta in eutherian mammals. Placenta 2007; 28:259–62. [DOI] [PubMed] [Google Scholar]
- 25. Luckett WP. Comparative development and evolution of the placenta in primates. Contrib Primatol 1974; 3:142–234. [PubMed] [Google Scholar]
- 26. Shearer MH, Dark RD, Chodosh J, Kennedy RC. Comparison and characterization of immunoglobulin G subclasses among primate species. Clin Diagn Lab Immunol 1999; 6:953–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Payton ME, d’Offay JM, Prado ME et al. Comparative transmission of multiple herpesviruses and simian virus 40 in a baboon breeding colony. Comp Med 2004; 54:695–704. [PubMed] [Google Scholar]
- 28. Wolf RF, Eberle R, White GL. Generation of a specific-pathogen-free baboon colony. J Am Assoc Lab Anim Sci 2010; 49:814–20. [PMC free article] [PubMed] [Google Scholar]
- 29. Meade BD, Deforest A, Edwards KM et al. Description and evaluation of serologic assays used in a multicenter trial of acellular pertussis vaccines. Pediatrics 1995; 96:570–5. [PubMed] [Google Scholar]
- 30. Chu HY, Englund JA. Maternal immunization. Birth Defects Res 2017; 109:379–86. [DOI] [PubMed] [Google Scholar]
- 31. Nieves DJ, Heininger U. Bordetella pertussis. Microbiol Spectr 20016; 4. [DOI] [PubMed] [Google Scholar]
- 32. Sobanjo-Ter Meulen A, Duclos P, McIntyre P et al. Assessing the evidence for maternal pertussis immunization: a report from the Bill & Melinda Gates Foundation symposium on pertussis infant disease burden in low- and lower-middle-income Countries. Clin Infect Dis 2016; 63:123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cherry JD. The epidemiology of pertussis and pertussis immunization in the United Kingdom and the United States: a comparative study. Curr Probl Pediatr 1984; 14:1–78. [DOI] [PubMed] [Google Scholar]
- 34. Nicoll A, Gardner A. Whooping cough and unrecognised postperinatal mortality. Arch Dis Child 1988; 63:41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Russell LB, Pentakota SR, Toscano CM, Cosgriff B, Sinha A. What pertussis mortality rates make maternal acellular pertussis immunization cost-effective in low- and middle-income countries? a decision analysis. Clin Infect Dis 2016; 63:227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Abu Raya B, Edwards KM, Scheifele DW, Halperin SA. Pertussis and influenza immunisation during pregnancy: a landscape review. Lancet Infect Dis 2017; 17:e209–22. [DOI] [PubMed] [Google Scholar]