During 5 influenza seasons, 37% of high-risk outpatients with influenza who presented early to care were prescribed antivirals. Early presentation and fever were associated with prescribing; 40% of high-risk outpatients with influenza presented early and 25% were afebrile.
Keywords: influenza, antiviral treatment, outpatient, neuraminidase inhibitors
Abstract
Background
Influenza causes millions of illnesses annually; certain groups are at higher risk for complications. Early antiviral treatment can reduce the risk of complications and is recommended for outpatients at increased risk. We describe antiviral prescribing among high-risk outpatients for 5 influenza seasons and explore factors that may influence prescribing.
Methods
We analyzed antiviral prescription and clinical data for high-risk outpatients aged ≥6 months with an acute respiratory illness (ARI) and enrolled in the US Influenza Vaccine Effectiveness Network during the 2011–2012 through 2015–2016 influenza seasons. We obtained clinical information from interviews and electronic medical records and tested all enrollees for influenza with real-time reverse-transcription polymerase chain reaction (rRT-PCR). We calculated the number of patients with ARI that must be treated to treat 1 patient with influenza.
Results
Among high-risk outpatients with ARI who presented to care within 2 days of symptom onset (early), 15% (718/4861) were prescribed an antiviral medication, including 472 of 1292 (37%) of those with rRT-PCR–confirmed influenza. Forty percent of high-risk outpatients with influenza presented to care early. Earlier presentation was associated with antiviral treatment (odds ratio [OR], 4.1; 95% confidence interval [CI], 3.5–4.8), as was fever (OR, 3.2; 95% CI, 2.7–3.8), although 25% of high-risk outpatients with influenza were afebrile. Empiric treatment of 4 high-risk outpatients with ARI was needed to treat 1 patient with influenza.
Conclusions
Influenza antiviral medications were infrequently prescribed for high-risk outpatients with ARI who would benefit most. Efforts to increase appropriate antiviral prescribing are needed to reduce influenza-associated complications.
(See the Editorial Commentary by Ison on pages 1042–4.)
Influenza is an important cause of morbidity and mortality in the United States [1], with annual seasonal influenza epidemics accounting for a substantial proportion of medically attended outpatient visits each year [2, 3]. Annual influenza vaccination is recommended for everyone aged ≥6 months and is the primary strategy for influenza prevention. The recommended pharmacologic treatment for influenza is a class of antiviral medications called neuraminidase inhibitors (NAIs) [4]. Meta-analyses of randomized controlled trials have found that patients treated with NAIs had fewer subsequent lower respiratory tract infections that required antibiotics [5, 6] and hospital admissions [5] compared with those treated with placebo.
NAIs include oral oseltamivir, inhaled zanamivir, and intravenous peramivir. Administration is recommended as early as possible for any patient with laboratory-confirmed or suspected influenza who is hospitalized; has severe, complicated, or progressive illness; or is at higher risk for influenza-associated complications [4]. However, previous studies suggest that antiviral prescribing in the outpatient setting is low [3, 7–10].
Using an existing study platform, we aimed to (1) describe practices in outpatient antiviral prescribing over 5 influenza seasons, and (2) explore factors associated with prescribing among high-risk outpatients, including timing of presentation to care, symptoms at presentation, and influenza activity throughout the season. In addition, we explored a scenario where all high-risk outpatients with an acute respiratory illness (ARI) were empirically given an antiviral prescription during the influenza season and estimated how many ARI patients a provider would need to empirically treat (number needed to treat [NNT]) to treat 1 patient with laboratory-confirmed influenza.
METHODS
Adults and children seeking care from an outpatient provider for an ARI with cough within 7 days of illness onset were enrolled at 5 geographically diverse research sites, comprising >60 outpatient practices participating in the US Influenza Vaccine Effectiveness (Flu VE) Network during 5 influenza seasons (2011–2012 through 2015–2016). Four sites contributed data during all 5 seasons; 1 site (C) began contributing data during the 2012–2013 season. We obtained clinical information from enrollment interviews and electronic medical record extraction. All enrolled patients were tested for influenza with real-time reverse-transcription polymerase chain reaction (rRT-PCR) for research purposes. At 1 site (B), the clinician was notified of a positive rRT-PCR research test result within 24–48 hours of enrollment. Providers at the other 4 sites received aggregated laboratory results at varying intervals. Data on influenza testing for clinical purposes were inconsistently collected and not analyzed for this study.
High-Risk Status
Patients were considered at high risk for influenza-associated complications if they were aged <2 years or ≥65 years, were pregnant, or had evidence of specific chronic underlying health conditions per the Advisory Committee on Immunization Practices guidelines for antiviral treatment [4]. The presence of a high-risk health condition was ascertained by extraction of electronic diagnosis codes for the 12 months prior to enrollment. High-risk codes were defined using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9) codes for the 2011–2012 through 2014–2015 seasons, and until 1 October for the 2015–2016 season; Tenth Revision (ICD-10) codes were used for the remainder of the 2015–2016 season. Patients who were extremely obese (body mass index [BMI] ≥ 40 kg/m2) or who reported being Native American, Alaska Native, or Native Hawaiian were also considered as high risk [4]. BMI was calculated using height and weight measurements obtained from the medical record.
Antiviral Prescription and Timing of Influenza Season
We examined medical and pharmacy records to determine whether an NAI was prescribed or dispensed within 7 days after enrollment. If a patient was either prescribed or dispensed an NAI, he or she was considered to have been prescribed an antiviral. We identified the timing of the influenza season by plotting the percentage of all research-protocol rRT-PCR tests that were positive for influenza each week. We descriptively compared seasonal trends in influenza-positive test results to antiviral prescribing among high-risk outpatients who were influenza positive and presented early by epidemiologic week during each influenza season. We defined the peak of the season as the week with the highest proportion of outpatients testing positive for influenza. “Early” presentation to care was defined as ≤2 days between symptom onset and date of outpatient enrollment, which is the time frame in which antivirals are the most effective [11–13].
Illness Onset and Symptoms
Illness onset date and limited symptom information were obtained through the enrollment interview. Most sites during most seasons collected either a measured temperature during the enrollment visit or documented self-report of fever or feverishness since illness onset. A patient was considered to have a fever if they either reported feeling feverish during the course of the illness or had a measured temperature at enrollment of ≥37.8°C (≥100°F). Because clinicians may use the syndrome of influenza-like illness (ie, cough plus fever or fever plus sore throat) to make decisions about prescribing, we explored the association of fever and cough with laboratory-confirmed influenza and antiviral use.
Number Needed to Empirically Treat
NNT generally refers to the average number of patients who need to be treated to prevent 1 additional adverse outcome [14]. We applied this concept to estimate the number of high-risk outpatients who present with ARI within 2 days of symptom onset that a provider would need to empirically treat with an NAI to administer effective therapy to 1 patient with laboratory-confirmed influenza. We divided the percentage of high-risk patients who presented early with PCR-confirmed influenza by 100 to find the NNT (NNT = percentage positive / 100). As the NNT decreases, the efficiency of empiric treatment increases. We stratified this analysis by fever status and season.
Statistical Analysis
Comparisons of categorical data were analyzed using a χ2 test. Logistic regression was used to develop a model with predictors of receipt of antiviral medications. Variables in the model included age group, sex, race, site, season, presence of a high-risk medical condition, timing of presentation to care relative to symptom onset, presence of fever, and influenza test results. We selected variables based on scientific plausibility and used an Akaike information criterion to evaluate model selection [15]. All data were analyzed using SAS version 9.3 software (SAS Institute, Cary, North Carolina).
RESULTS
Influenza Antiviral Prescriptions and Timing of Presentation to Care
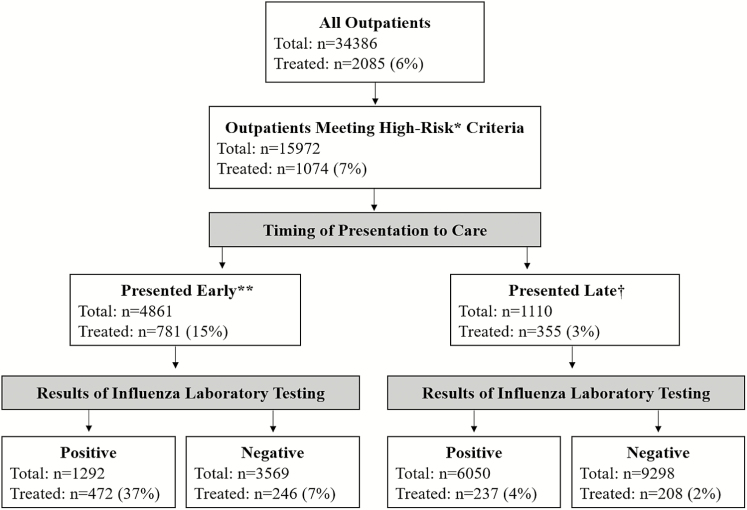
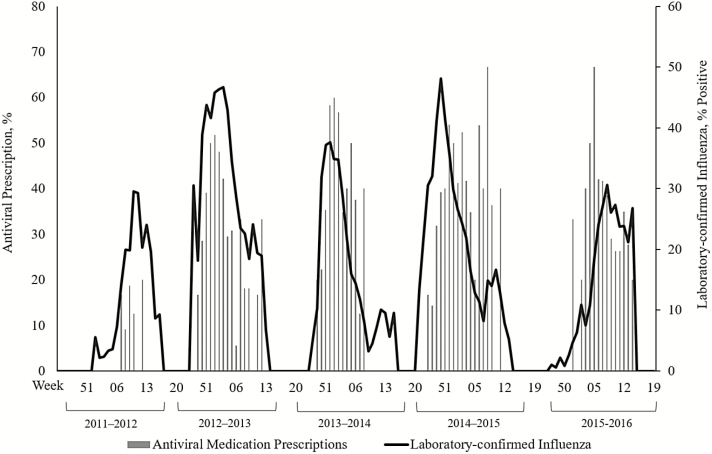
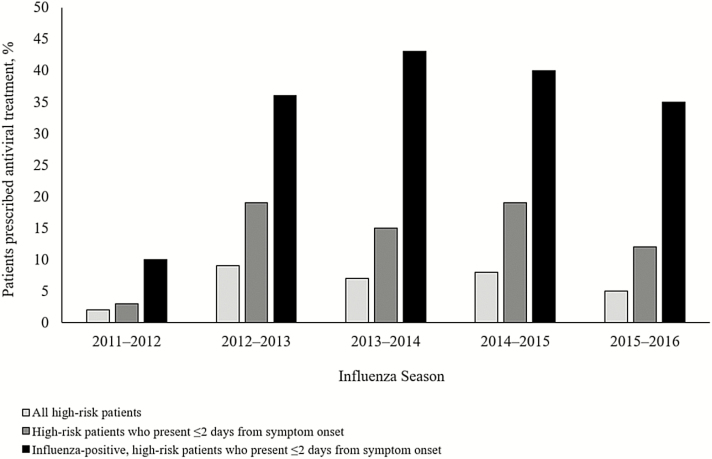
During 5 influenza seasons, we enrolled 15972 high-risk outpatients (Table 1) seeking care for an ARI, of whom 3196 (20%) had laboratory-confirmed influenza (Table 2). Forty percent (1292/3196) of high-risk outpatients with laboratory-confirmed influenza presented to care early; 37% (472/1292) of these patients received a prescription for an antiviral medication (Figure 1). Older adults with influenza were the least likely to present within 2 days (38%) compared to all other age groups (P < .01) and the most likely to present >4 days after symptom onset (26%; P < .01). There was no significant difference in timing of presentation to care across the 5 seasons (P = .15). Trends in antiviral prescribing roughly followed trends in seasonal influenza activity (Figure 2), although increases in antiviral prescribing lagged behind the season onset and declined before the end of the season in some years. Other than the first season these data were collected (2011–2012), we did not identify any change in antiviral prescriptions from 2012–2013 to 2015–2016 (Figure 3).
Table 1.
Demographic Characteristics and Influenza Antiviral Prescriptions Among Enrolled Outpatients With an Acute Respiratory Infection at High Risk for an Influenza-Associated Complication
| Characteristic | All High-Riska Patients Prescribed NAI | P Value | High-Risk Patients Presenting Earlyb | P Value | High-Risk Patients Presenting Earlyb With Laboratory- Confirmed Influenza | P Value |
|---|---|---|---|---|---|---|
| NAI Prescription/ Total No. (%) | NAI Prescription/Total No. (%) | NAI Prescription/Total No. (%) | ||||
| All | 1074/15972 (6.7) | 718/4861 (15) | 472/1292 (37) | |||
| Season | ||||||
| 2011–2012 | 33/1674 (2.0) | 16/530 (3.0) | 8/81 (9.9) | |||
| 2012–2013 | 261/2936 (8.9) | 194/1045 (19) | 137/377 (36) | |||
| 2013–2014 | 201/2785 (7.2) | 127/838 (15) | 84/194 (43) | |||
| 2014–2015 | 403/4799 (8.4) | 256/1381 (19) | 170/431 (39) | |||
| 2015–2016 | 176/3778 (4.7) | <.01 | 125/1067 (12) | <.01 | 73/209 (35) | <.01 |
| Study site | ||||||
| A | 150/4504 (3.3) | 83/899 (9.2) | 58/216 (27) | |||
| B | 419/3280 (12.8) | 284/1109 (26) | 201/362 (56) | |||
| C | 115/2672 (4.3) | 95/1064 (8.9) | 63/222 (28) | |||
| D | 143/2687 (5.3) | 89/862 (10) | 51/222 (23) | |||
| E | 247/2829 (8.7) | <.01 | 167/927 (18) | <.01 | 99/270 (37) | <.01 |
| Age group, y | ||||||
| <2 | 67/2129 (3.2) | 51/792 (6.7) | 24/87 (28) | |||
| 2–17 | 119/2850 (4.2) | 92/1110 (8.3) | 56/244 (23) | |||
| 18–64 | 581/7219 (8.0) | 401/2071 (19) | 264/643 (41) | |||
| ≥65 | 307/3774 (8.1) | <.01 | 174/918 (19) | <.01 | 128/318 (40) | <.01 |
| Sex | ||||||
| Male | 453/6738 (6.7) | 295/2126 (14) | 197/561 (35) | |||
| Female | 621/9234 (6.7) | 1.0 | 423/2735 (16) | .12 | 275/731 (38) | .35 |
| Race | ||||||
| White | 915/12308 (7.4) | 618/3678 (16) | 408/1016 (40) | |||
| Black | 64/1527 (4.2) | 41/509 (8.1) | 30/130 (23) | |||
| Asian | 24/457 (5.3) | 13/114 (11) | 8/26 (31) | |||
| NH/AN | 29/619 (4.7) | 16/166 (9.6) | 5/35 (14) | |||
| Other/mixed race/ unknown | 42/1061 (4.0) | <.01 | 30/394 (7.6) | <.01 | 21/85 (25) | <.01 |
| Symptoms | ||||||
| Cough and fever | 856/8606 (10) | 577/2801 (21) | 409/985 (42) | |||
| Cough without fever | 204/6954 (2.9) | <.01 | 132/1935 (6.8) | <.01 | 60/290 (21) | <.01 |
Abbreviations: NAI, neuraminidase inhibitor; NH/AN, Native Hawaiian/Alaska Native.
aHigh-risk patients are those aged <2 years or ≥65 years; pregnant women; those with extreme obesity (body mass index≥ 40 kg/m2); those with documentation of chronic underlying health condition(s) that increase the risk of influenza-associated complications; and Native Americans, Alaska Natives, and Native Hawaiians.
bEarly presentation to care was defined as ≤2 days between symptom onset and date of outpatient enrollment.
Table 2.
Laboratory-Confirmed Influenza Infection Among Outpatients at High Riska for an Influenza-Associated Complication, by Age Group and Fever Status
| Age Group, y | rRT-PCR–Confirmed Influenza Infection/Total No. (%) | ||
|---|---|---|---|
| All ARI | Cough Without Fever | Cough With Fever | |
| <2 | 224/2129 (11) | 33/534 (6.2) | 190/1546 (12) |
| 2–17 | 573/2850 (20) | 76/1063 (7.2) | 488/1713 (29) |
| 18–64 | 1558/7219 (22) | 408/3294 (12) | 1127/3735 (30) |
| ≥65 | 841/3774 (22) | 280/2063 (14) | 549/1612 (34) |
| Total | 3196/15972 (20) | 797/6954 (12) | 2354/8606 (27) |
Abbreviations: ARI, acute respiratory illness; rRT-PCR, real-time reverse-transcription polymerase chain reaction.
aPatients at high risk for influenza-associated complications are those aged <2 years or ≥ 65 years; pregnant women; those with extreme obesity (body mass index≥40 kg/m2); those with documentation of chronic underlying health condition(s) that increase the risk of influenza-associated complications; and Native Americans, Alaska Natives, and Native Hawaiians.
Figure 1.
Flow diagram of influenza antiviral medication prescriptions among high-risk outpatients by timing of presentation to care and results of influenza laboratory testing.
*Patients at high risk for influenza-associated complications are those aged <2 years or ≥65 years; pregnant women; those with extreme obesity (body mass index≥40 kg/m2); those with documentation of chronic underlying health condition(s) that increase the risk of influenza-associated complications; and Native Americans, Alaska Natives, and Native Hawaiians. **Early presentation to care was defined as ≤2 days between symptom onset and date of outpatient enrollment. †Late presentation to care was defined as >2 days between symptom onset and date of outpatient enrollment.
Figure 2.
Seasonal trends in laboratory-confirmed influenza and influenza antiviral prescribing among high-risk outpatients with laboratory-confirmed influenza who presented to care ≤2 days from symptom onset. Patients at high risk for influenza-associated complications are those aged <2 years or ≥65 years; pregnant women; those with extreme obesity (body mass index ≥40 kg/m2); those with documentation of chronic underlying health condition(s) that increase the risk of influenza-associated complications; and Native Americans, Alaska Natives, and Native Hawaiians.
Figure 3.
Proportion of high-risk outpatients with an acute respiratory illness prescribed antiviral treatment, influenza seasons 2011–2012 through 2015–2016. Patients at high risk for influenza-associated complications are those aged <2 years or ≥65 years; pregnant women; those with extreme obesity (body mass index ≥40 kg/m2); those with documentation of chronic underlying health condition(s) that increase the risk of influenza-associated complications; and Native Americans, Alaska Natives, and Native Hawaiians.
Factors Associated With Influenza Positivity and Antiviral Use
Across 5 seasons, laboratory-confirmed influenza accounted for 20% (3196/15972) of outpatient visits by high-risk patients for acute respiratory infections (Table 2). The proportion of ARI associated with laboratory-confirmed influenza among high-risk outpatients varied by season (2011–2012: 12%; 2012–2013: 31%; 2013–2014: 16%; 2014–2015: 22%; 2015–2016: 15%). Among the 15560 (97%) high-risk outpatients with information regarding fever status, 8606 (55%) had an acute cough and a fever, whereas the remaining 6954 (45%) had an acute cough but did not have a fever. High-risk outpatients with a cough and fever were more likely to have an influenza infection (27% [2354/8606]) than those without a fever (12% [797/6954]) (P < .01; Table 2); however, one-quarter of high-risk outpatients with influenza did not have a fever (25% [797/3151]). Patients with cough and fever were more likely to be prescribed antiviral treatment; among patients who presented early with a cough and fever, 21% (577/2801) received an antiviral prescription vs 7% of those who presented early with a cough but no fever (132/1935) (P < .01; Table 1).
In a multivariable logistic regression model, adjusted for research site, several factors were associated with receipt of an antiviral prescription among outpatients with ARI, including early presentation to care (odds ratio [OR], 4.1; 95% confidence interval [CI], 3.5–4.8), presence of fever (OR, 3.2; 95% CI, 2.7–3.8), positive influenza test result (OR, 4.4; 95% CI, 3.8–5.1), presenting within the 2013–2014 (OR, 2.0; 95% CI, 1.5–2.5) and 2014–2015 (OR, 1.8; 95% CI, 1.4–2.2) seasons, as well as age, sex, and presence of a chronic underlying medical condition (Supplementary Table 1).
Number Needed to Empirically Treat
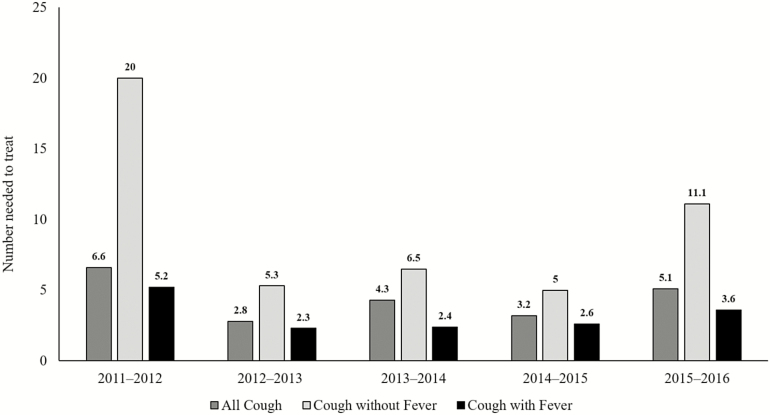
To treat 1 outpatient with a laboratory-confirmed influenza infection among all high-risk patients who presented early in all 5 seasons, a provider needed to empirically treat 3.8 patients, 2.8 patients who presented with a cough and fever, and 6.7 patients who presented with a cough but no fever. NNT also varied by season (Figure 4). Empiric treatment was most efficient during 2012–2013 and 2014–2015, when 1 in 3 patients with an ARI and almost 1 in 2 patients with an ARI and fever had influenza. At the season peak when combining all years of data, a provider needed to empirically treat 1.9 high-risk patients with cough and fever and 4.3 patients with cough but no fever to treat 1 outpatient with laboratory-confirmed influenza (data not shown).
Figure 4.
Number of high-risk patients who present within 2 days of symptom onset who must be empirically treated with influenza antiviral medications in order to treat 1 patient with laboratory-confirmed influenza, stratified by fever status and season. Patients at high risk for influenza-associated complications are those aged <2 years and ≥65 years; pregnant women; those with extreme obesity (body mass index ≥40 kg/m2); those with documentation of chronic underlying health condition(s) that increase the risk of influenza-associated complications; and Native Americans, Alaska Natives, and Native Hawaiians.
DISCUSSION
In 5 recent influenza seasons, influenza antiviral treatment was infrequently prescribed in the outpatient settings involved with the US Flu VE Network. Among study rRT-PCR–confirmed influenza-positive high-risk outpatients who presented early to care, a group highly likely to benefit from outpatient antiviral treatment, 37% of patients received a prescription for an antiviral medication. Although early presentation to care was associated with receipt of an antiviral prescription, less than half (40%) of high-risk outpatients with laboratory-confirmed influenza presented to care within 2 days of symptom onset.
Many viruses can cause an ARI [16], which may partially explain why providers are hesitant to treat for influenza among patients with ARI-associated symptoms. Also, sensitive diagnostic assays, such as those based on nucleic acid detection, are expensive and infrequently used in outpatient settings. The US Flu VE Network used a broad and sensitive definition for enrollment of patients with ARI, which allowed for inclusion of patients with influenza virus infection who did not meet the definition of influenza-like illness (ie, cough plus fever or fever plus sore throat) often used in surveillance and sometimes applied to clinical care. High-risk outpatients with a cough and fever were more likely to have rRT-PCR–confirmed influenza than those without a fever. In most seasons, empiric antiviral prescriptions for all high-risk outpatients presenting with a cough and fever appeared to be the most efficient method to assure early treatment of patients with influenza. However, targeting only patients with cough and fever would miss 25% of high-risk outpatients with laboratory-confirmed influenza. To address this issue, we introduced a new application of the NNT concept. While NNT usually refers to the number of patients a provider would need to treat in order to prevent 1 illness, here we presented a method to determine the number of patients requiring empiric treatment during the influenza season in order to treat 1 patient with laboratory-confirmed influenza. This method may help providers make treatment decisions when timely sensitive diagnostic assays are not available and empiric treatment decisions must be made. While NNT is higher (and therefore less efficient) among high-risk patients without a fever than those with a fever, it is lower in both groups at the peak of the influenza season, a finding that may help guide treatment decisions, especially when weighing the risks, benefits, and cost of treatment [4, 17]. Additionally, during seasons with higher influenza attack rates, empirically treating all ARI patients throughout the season, regardless of fever, was also relatively efficient. In addition to local resources, providers can track influenza activity on the Centers for Disease Control and Prevention’s (CDC) influenza website, FLUVIEW, which provides weekly updates of national influenza surveillance data (https://www.cdc.gov/flu/weekly/index.htm).
In our study, timing of presentation to care had a significant impact on antiviral prescribing. This finding is consistent with results from other studies [3, 9, 10, 18, 19]. Unfortunately, many high-risk patients delay presenting to care, which reduces the opportunity for optimal NAI treatment. This finding provides an area for intervention that could improve patient care and potentially decrease morbidity from influenza. Providers should encourage high-risk patients to seek care early for a respiratory illness during the influenza season and consider additional measures to provide timely antiviral prescriptions for high-risk patients, such as providing phone consultations or remote electronic visits [20]. Previous studies have highlighted the underuse of influenza antiviral medications; other studies from the US Flu VE Network have shown low antiviral prescribing among all outpatients during the 2012–2013 season [10] and low prescribing among high-risk patients who presented early in their illness during the 2013–2014 season [9]. Another study using patient self-report described a low rate of antiviral prescriptions (34%) among adult patients who reported an influenza diagnosis during the first postpandemic season of 2010–2011 [8]. Our study adds to the growing body of evidence demonstrating low antiviral use nationwide, even among high-risk outpatients with laboratory-confirmed influenza who could potentially benefit most from treatment. Unlike previous studies, our study extracted data from multiple influenza seasons from >60 outpatient practices and allowed us to examine changes in antiviral prescribing practices over several years. The lack of any consistent increase in antiviral prescribing despite consistent guidance [17, 21] on the topic is concerning, suggesting that a need for both a deeper understanding of the barriers to prescribing and new approaches for both patients and clinicians.
Our study prospectively enrolled thousands of outpatients over multiple influenza seasons, tested all patients for influenza with a sensitive assay, and confirmed high-risk status and prescribing practices through medical record review. However, our conclusions are still subject to limitations. The study sites have been involved in influenza-related research for multiple years, are large managed care or academic organizations, and care for populations in which a majority of patients have insurance, thereby potentially limiting the generalizability of our results to other US outpatient practices. We did not consistently capture symptoms beyond cough and fever, nor did we have consistent data on influenza testing ordered for clinical purposes or other factors, such as disease severity illness progression, or severity of underlying conditions, all of which may have influenced clinical decisions.
In conclusion, in this large prospective study performed over multiple seasons, influenza antiviral medications were infrequently prescribed to high-risk patients with laboratory-confirmed influenza who presented within 2 days of symptom onset. Further work is warranted to understand additional factors that influence outpatient antiviral prescribing and to educate providers about the benefits of appropriate treatment for high-risk patients. CDC recommends that treatment with antivirals be given as early as possible to high-risk outpatients with suspected or confirmed influenza; in practice, this usually means initiating empiric treatment before or without the results of sensitive diagnostic testing. Strategies to improve the timeliness of care-seeking among high-risk patients could make a substantial impact on optimal antiviral use, as could reminders to providers about local influenza activity and the varied presentations of influenza.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Lois Lamerato, Heather Lipkovich, Joshua G. Petrie, Rachel Truscon, Anne Kaniclides, Ryan E. Malosh, Caroline K. Cheng, E. J. McSpadden, Emileigh Johnson, Richard Evans, and all of the US Flu VE Network staff and study participants.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official views of CDC (Centers for Disease Control and Prevention).
Financial support. This work was supported by CDC (cooperative agreement IP11-003). At the University of Pittsburgh, the project was also supported by the National Institutes of Health (grant numbers UL1 RR024153 and UL1TR000005).
Potential conflicts of interest. E. A. B. has received research support from MedImmune. R. Z. has received research support from Merck, Pfizer, and Sanofi. M. P. N. has received research funding from Pfizer and Merck. M. G. has received grants from MedImmune and Astra Zeneca. L. J. has received grants from Novartis and Takeda. A. S. M. reports grants and personal fees from Sanofi Pasteur, Novartis, and Protein Sciences. E. T. M. reports grants from Merck, Pfizer, and Mugas Foundation. H. M. has received grants from MedImmune. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Reed C, Chaves SS, Daily Kirley P et al. Estimating influenza disease burden from population-based surveillance data in the United States. PLoS One 2015; 10:e0118369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fowlkes A, Dasgupta S, Chao E et al. Estimating influenza incidence and rates of influenza-like illness in the outpatient setting. Influenza Other Respir Viruses 2013; 7:694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fowlkes A, Steffens A, Temte J et al. ; Influenza Incidence Surveillance Project Working Group Incidence of medically attended influenza during pandemic and post-pandemic seasons through the Influenza Incidence Surveillance Project, 2009-13. Lancet Respir Med 2015; 3:709–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fiore AE, Fry A, Shay D, Gubareva L, Bresee JS, Uyeki TM; Centers for Disease Control and Prevention (CDC) Antiviral agents for the treatment and chemoprophylaxis of influenza—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011; 60:1–24. [PubMed] [Google Scholar]
- 5. Dobson J, Whitley RJ, Pocock S, Monto AS. Oseltamivir treatment for influenza in adults: a meta-analysis of randomised controlled trials. Lancet 2015; 385:1729–37. [DOI] [PubMed] [Google Scholar]
- 6. Hernán MA, Lipsitch M. Oseltamivir and risk of lower respiratory tract complications in patients with flu symptoms: a meta-analysis of eleven randomized clinical trials. Clin Infect Dis 2011; 53:277–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Biggerstaff M, Jhung M, Kamimoto L, Balluz L, Finelli L. Self-reported influenza-like illness and receipt of influenza antiviral drugs during the 2009 pandemic, United States, 2009–2010. Am J Public Health 2012; 102:e21–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Biggerstaff M, Jhung MA, Reed C, Fry AM, Balluz L, Finelli L. Influenza-like illness, the time to seek healthcare, and influenza antiviral receipt during the 2010–2011 influenza season—United States. J Infect Dis 2014; 210:535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Havers F, Flannery B, Clippard JR et al. Use of influenza antiviral medications among outpatients at high risk for influenza-associated complications during the 2013–2014 influenza season. Clin Infect Dis 2015; 60:1677–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Havers F, Thaker S, Clippard JR et al. Use of influenza antiviral agents by ambulatory care clinicians during the 2012–2013 influenza season. Clin Infect Dis 2014; 59:774–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee N, Chan PK, Choi KW et al. Factors associated with early hospital discharge of adult influenza patients. Antivir Ther 2007; 12:501–8. [PubMed] [Google Scholar]
- 12. Lee N, Choi KW, Chan PK et al. Outcomes of adults hospitalised with severe influenza. Thorax 2010; 65:510–5. [DOI] [PubMed] [Google Scholar]
- 13. Yu H, Liao Q, Yuan Y et al. Effectiveness of oseltamivir on disease progression and viral RNA shedding in patients with mild pandemic 2009 influenza A H1N1: opportunistic retrospective study of medical charts in China. BMJ 2010; 341:c4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chou R, Dana T, Blazina I et al. U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews. Statin use for the prevention of cardiovascular disease in adults: a systematic review for the US Preventive Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality, 2016. [PubMed] [Google Scholar]
- 15. deLeeuw J. Introduction to Akaike (1973) information theory and an extension of the maximum likelihood principle. In: Kotz S, Johnson NL, eds. Breakthroughs in statistics. New York: Springer, 1992. [Google Scholar]
- 16. Fowlkes A, Giorgi A, Erdman D et al. Viruses associated with acute respiratory infections and influenza-like illness among outpatients from the Influenza Incidence Surveillance Project, 2010–2011. J Infect Dis 2014; 209:1715–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Centers for Disease Control and Prevention. Influenza antiviral medications: summary for clinicians Available at: https://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm. Accessed 10 February 2017.
- 18. Rothberg MB, Bonner AB, Rajab MH, Kim HS, Stechenberg BW, Rose DN. Effects of local variation, specialty, and beliefs on antiviral prescribing for influenza. Clin Infect Dis 2006; 42:95–9. [DOI] [PubMed] [Google Scholar]
- 19. Williams LO, Kupka NJ, Schmaltz SP, Barrett S, Uyeki TM, Jernigan DB. Rapid influenza diagnostic test use and antiviral prescriptions in outpatient settings pre- and post-2009 H1N1 pandemic. J Clin Virol 2014; 60:27–33. [DOI] [PubMed] [Google Scholar]
- 20. Centers for Disease Control and Prevention. Medical office telephone evaluation of patients with possible influenza Available at: https://www.cdc.gov/flu/professionals/antivirals/office-evaluation.htm. Accessed 10 February 2017.
- 21. American Academy of Pediatrics. Recommendations for prevention and control of influenza in children, 2016–2017. Pediatrics 2016; 138(4):e20162527. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.