Drought-induced stomatal closure correlates with JA accumulation, but was unaffected in reciprocal grafts of wild-type and def-1 mutant (does not accumulate JA) plants. Only stomata of def-1 self-grafts were relatively drought insensitive.
Keywords: Drought stress, JA, ABA, grafting, Solanum lycopersicum
Abstract
To determine whether drought-induced root jasmonate [jasmonic acid (JA) and jasmonic acid-isoleucine (JA-Ile)] accumulation affected shoot responses to drying soil, near-isogenic wild-type (WT) tomato (Solanum lycopersicum cv. Castlemart) and the def-1 mutant (which fails to accumulate jasmonates during water deficit) were self- and reciprocally grafted. Rootstock hydraulic conductance was entirely rootstock dependent and significantly lower in def-1, yet def-1 scions maintained a higher leaf water potential as the soil dried due to their lower stomatal conductance (gs). Stomatal sensitivity to drying soil (the slope of gsversus soil water content) was low in def-1 self-grafts but was normalized by grafting onto WT rootstocks. Although soil drying increased 12-oxo-phytodienoic acid (OPDA; a JA precursor and putative antitranspirant) concentrations in def-1 scions, foliar JA accumulation was negligible and foliar ABA accumulation reduced compared with WT scions. A WT rootstock increased drought-induced ABA and JA accumulation in def-1 scions, but decreased OPDA accumulation. Xylem-borne jasmonates were biologically active, since supplying exogenous JA via the transpiration stream to detached leaves decreased transpiration of WT seedlings but had the opposite effect in def-1. Thus foliar accumulation of both ABA and JA at WT levels is required for both maximum (well-watered) gs and stomatal sensitivity to drying soil.
Introduction
Jasmonic acid (JA) and other members of the oxylipin pathway such as 12-oxo-phytodienoic acid (OPDA) and the bioactive jasmonic acid-isoleucine (JA-Ile; Katsir et al., 2008; Fonseca et al., 2009) have been associated with tolerance to biotic stresses (Wasternack and Hause, 2013) by mediating defence against herbivores and necrotrophic pathogens. JA and its derived molecules (mainly JA-Ile as the bioactive interactor with the COI1 receptor) are known as jasmonates, a subgroup of oxylipins derived from OPDA also associated with the systemic response to physical stresses such as wounding (Sun et al., 2011). Nevertheless, they can also be involved in tolerance to other abiotic stresses such as drought and salinity (Cheng et al., 2013; Alam et al., 2014; Savchenko et al., 2014; Kazan, 2015). Water deficit increases tissue JA concentrations in several species (Creelman and Mullet, 1995; Mahouachi et al., 2007; Brossa et al., 2011; Chen et al., 2016), as well as JA-Ile (De Ollas et al., 2015), while mutations in several steps of the JA biosynthesis and signalling pathways alter tolerance to water deficit (Harb et al., 2010; Grebner et al., 2013; Riemann et al., 2015). Although JA and abscisic acid (ABA) seem to influence each other’s biosynthesis (Bandurska et al., 2003; Kim et al., 2009; De Ollas et al., 2013; Fragoso et al., 2014), few studies have assessed their relative importance in mediating physiological responses to water deficit.
Although ABA plays a central role in regulating stomatal closure in response to soil drying (Raghavendra et al., 2010; Lee and Luan, 2012), other phytohormones can also influence this response. Exogenous application of either ABA or methyl jasmonate (MeJA) elicited similar stomatal closure using the same second messengers (Munemasa et al., 2011). The oxylipin OPDA is claimed to promote stomatal closure more effectively and independently from JA and in co-operation with ABA (Savchenko et al., 2014). JA/JA-Ile-dependent stomatal regulation may also be important since applying ABA or MeJA to the Arabidopsis jar1-1 mutant (which is defective in conjugating isoleucine to JA) elicited less stomatal closure than in wild-type (WT) plants (Suhita et al., 2004). Interestingly, the Arabidopsis aos (JA/JA-Ile and OPDA deficient) and the opr3 (deficient in JA/JA-Ile but not OPDA) mutants had higher and lower stomatal aperture than WT plants, respectively (Savchenko et al., 2014). Thus various oxylipins and ABA may be involved in regulating stomatal responses of plants in drying soil.
While these studies explored stomatal responses of isolated epidermal peels (reviewed in De Ollas and Dodd, 2016), few studies have examined JA/JA-Ile effects on whole-plant transpiration. While leaf scorching increased JA concentration concurrent with stomatal closure in distal leaves (Hlaváčková et al., 2006), withholding water from the WT and the Arabidopsis aos mutant (JA/JA-Ile and OPDA deficient) elicited similar stomatal closure (Brossa et al., 2011). Similar drought-induced stomatal closure in genotypes varying in oxylipin status is consistent with a model where stomatal behaviour is primarily determined by plant hydraulics (Tardieu, 2016). In this context, exogenous application of ABA (reviewed in Dodd, 2013) or MeJA (Sánchez-Romera et al., 2014) increased root hydraulic conductivity (Lpr) along with gene expression of several aquaporins (Sánchez-Romera et al., 2014). Moreover, a tomato (Solanum lycopersicum) mutant (def-1) defective in stress-induced JA biosynthesis had lower Lpr, which was restored by MeJA treatment (Sánchez-Romera et al., 2014). Although phytohormone-mediated variation in Lpr may influence leaf water relations, whether this regulates stomatal responses to soil drying is less clear. Reciprocal grafting with different root and scion genotypes determined the role of long-distance JA/JA-Ile signalling in wound responses (Fragoso et al., 2014), but the impact of variation in root and shoot jasmonates on plant water relations responses to soil drying has not been assessed.
Since both ABA and JA can regulate both Lpr and stomatal conductance (gs), JA may alter physiological responses to drying soil. Initially, ungrafted plants of two tomato mutants compromised in the oxylipin pathway [spr2, constitutively deficient in the biosynthesis of OPDA, JA, and the bioactive JA-Ile (Li et al., 2003); and def-1, with diminished stress-induced JA/JA-Ile accumulation (Howe et al., 1996)] were grown under water deficit to characterize root and shoot phytohormonal responses. Then, to better understand the involvement of both scion- and root-sourced jasmonates in physiological responses to water deficit, self- and reciprocally grafted WT and def-1 mutant (with attenuated JA accumulation in drying soil) plants were grown in well-watered and drying soil. Physiological responses such as whole-plant transpiration, leaf and root water potential, Lpr, and gs were correlated with leaf and xylem sap ABA and oxylipin (OPDA and JA/JA-Ile) concentrations. To determine whether xylem-supplied phytohormones regulate stomatal responses, detached leaves were supplied with JA or ABA via the transpiration stream (Zhang and Davies, 1991). We tested the hypothesis that jasmonates can regulate transpiration via local (foliar jasmonates accumulation) but also long-distance (root to shoot transport through the transpiration stream) processes.
Materials and methods
Plant material
To characterize jasmonate effects on plant responses to water deficit, two tomato (S. lycopersicum) mutants (def-1 and spr2) were grown under well-watered and water-limited conditions. The (unidentified) def-1 mutation (originally described as JL5; Howe et al., 1996) prevents JA (and hence JA-Ile) accumulation under wounding and biotic stress, but basal JA/JA-Ile concentrations are similar to those of its near-isogenic line Castlemart. This genetic lesion is not related to any identified biosynthetic gene and this mutant is not compromised in OPDA biosynthesis (Howe et al., 2000). Thus def-1 has attenuated stress-induced JA/JA-Ile accumulation.
In the spr2 (suppressor of prosystemin-mediated responses 2; Howe et al., 1996) mutant, the lack of a chloroplast fatty acid desaturase in the octadecanoid pathway compromises constitutive JA biosynthesis, severely decreasing leaf (Li et al., 2003) and root (Tejeda-Sartorius et al., 2008) JA levels. Wound-induced JA biosynthesis is also impaired (Li et al., 2003) as is OPDA biosynthesis (Wasternack et al., 2012). Thus spr2 is more generally deficient in both jasmonates and OPDA biosynthesis.
Experiments with ungrafted plants
Seeds of the WT, spr2, and def-1 were sown individually in 1 cm diameter plastic pots filled with a mixture of peat/perlite (80/20, v/v) and covered with black plastic to ensure high humidity and darkness to promote germination. After 4–6 d, the plastic was removed to prevent seedling etiolation. After a further week, seedlings were potted into individual 216 cm3 pots and watered daily for a further 2 weeks, then transplanted to 4000 cm3 pots filled with the same substrate. After another week under well-watered conditions, pots were covered with plastic bags to limit soil evaporation. Randomly selected plants of each genotype were divided into well-watered (daily replacement of evapotranspiration) and water deficit [soil water content (SWC) allowed to drop to <0.2–0.15 cm3 cm–3 before re-watering] groups. After 5 d of soil drying when all genotypes were at a similar SWC, whole-plant transpiration and water potential of the youngest fully expanded leaf (Ψleaf) were measured as described below, with xylem sap collected from this leaf. Prior to measuring Ψleaf, the next youngest leaf was excised and placed in liquid N2. Then the roots were carefully washed from the substrate, and placed in liquid N2.
Experiments with grafted plants
Seeds of WT and def-1 tomato were sown individually in 1 cm diameter plastic pots filled with Levington M3 compost (Scotts Company Ltd, Ipswich, UK) and covered with black plastic to ensure high humidity and darkness to promote germination. After 4–6 d, the plastic was removed to prevent seedling etiolation. After a further week, seedlings were potted into individual 500 cm3 pots and watered daily for a further 2 weeks prior to grafting. Graft unions were established just below the cotyledonary node as previously described (Dodd et al., 2009). Two weeks after grafting, plants were transplanted to pots containing an organic loam (John Innes No. 2, UK), for which a moisture release curve has been previously published (Dodd et al., 2010). Pots were cylinders, 6.5cm in diameter and 23cm in height (750cm3 volume), with stainless steel mesh (0.7mm aperture) at the base to assist drainage, and designed to fit tightly in a pressure chamber of the same volume (Soil Moisture Equipment Corp., Santa Barbara, CA, USA). The tops of the pots were taped (Advance Tapes, Leicester, UK) to reduce evaporation from the soil surface. Plants were watered immediately following transplanting and daily thereafter, and allowed to establish for 1 week. Plants were harvested 23–27d post-grafting. All plants were watered to drained capacity every day until 2d before measurements. Pots were placed on a saucer in a walk-in controlled environment room with a day/night temperature of 22/16 °C and a 12 h photoperiod (06.00–18.00 h). Day/night relative humidity was 42/54%, CO2 concentration was 440/390 ppm, and light intensity at plant height was between 400 µmol m−2 s−1 and 640 µmol m−2 s−1 photosynthetic photon flux density (PPFD). The day before harvesting, some randomly chosen plants were watered to drained capacity while others were not watered to impose a soil drying treatment. Preliminary trials showed that transpiration reduced SWC from drained capacity (0.5 cm3 cm–3) to low soil moisture (0.2 cm3 cm–3) within 24–48 h depending on the graft combination. Plants were harvested (09.00–14.00 h) in three blocks with one plant of each graft combination per block.
Physiological measurements
Transpiration was calculated by weighing the pots daily (09.00–10.00 h), with evaporation from the soil surface ignored. Transpiration was normalized to whole-plant leaf area, which was measured with a Li-3100 Area Meter (Li-Cor Inc., Lincoln, NE, USA).
Immediately prior to harvest, stomatal conductance (gs) was measured on the middle leaflet of a fully expanded leaf (fourth or fifth leaf from the top of the plant) using infra-red gas analysis (6400xt Li-Cor Portable Photosynthesis System, Lincoln, NE, USA). Cuvette conditions approximately matched the environmental conditions within the controlled-environment room: CO2 at ambient levels (390 ppm), 600 μmol m−2 s−1 PPFD, cuvette temperature of 22 °C, and ambient humidity typically 40–50%.
Immediately after measuring gs, leaf water potential (Ψleaf) was measured on the same leaf using a pressure chamber (Model 3000F01 Plant Water Status Console; Soil Moisture Equipment Corp.). Detached leaves were transported to the laboratory and placed in the pressure chamber within 60 s of excision. Once in the chamber, the cut petiole surface was cleaned with deionized water and filter paper to remove cellular debris. Pressure was raised in the chamber at a rate of 0.02 MPa s−1, and Ψleaf was recorded when xylem sap collected on the cut surface. An overpressure of 0.4 MPa was then applied to each leaf to collect xylem sap. The initial droplets of sap were discarded, then sap was sampled for 2–3 min using a pipette. Xylem sap samples were stored in a previously weighed 1.5 ml microfuge tube.
Root water potential (Ψroot) was measured after Ψleaf by de-topping the plants with a razor blade 2 cm below the graft union and introducing the whole pot into the pressure chamber. The cut stem was cleaned with deionized water and filter paper to remove cellular debris. Pressure was raised in the chamber at a rate of 0.01 MPa s−1, and Ψroot was recorded when xylem sap collected on the cut surface. An overpressure of 0.2 MPa was then applied to collect xylem sap from the roots. In case natural exudation occurred following shoot removal, Ψroot was considered equal to zero. After measuring Ψroot, the soil was weighed and then placed in a tray in a drying oven. SWC was calculated with the formula [SWC=(mwet–mdry)/mdry] where mwet is the soil fresh weight when harvested, and mdry is the weight of the dry soil after 3 d in an oven at 80 °C.
Root hydraulic conductivity measurements
Three plants of each graft combination were randomly selected to measure root hydraulic conductivity (Lpr). After shoot removal, a series of overpressures (from 0.1 MPa to 0.6 MPa in 0.1 MPa increments) were applied and the sap flow rate determined at each pressure. Roots were washed from the pots and kept moistened with wet paper, before scanning (Epson perfection v700 photo), with the scanner connected to a computer running the WinRHIZO™ software (Regent Instruments, Canada). Root hydraulic conductivity quantifies root permeability to the flow of water, by applying increasing pneumatic pressure to the root zone. Applied pressure and water flow are linearly correlated, with the slope of the relationship defined as the root hydraulic conductance (when data are normalized to root surface area).
Detached leaf transpiration assays
Seeds of the def-1 and spr2 mutants and their corresponding WT (cv. Castlemart) were sown as described above, and then potted into individual 350 cm3 pots filled with a mixture of peat/perlite (80/20, v/v). These were placed in a controlled-environmental chamber with a day/night temperature of 25/16 °C and a 16 h photoperiod (07.00–23.00 h), and watered every 2–3 d. Day/night relative humidity was 42/54%, CO2 concentration was 450/380 ppm, and light intensity at plant height between 400 µmol m−2 s−1 and 500 µmol m−2 s−1 PPFD. Plants were kept in those conditions for a further 3 weeks prior to the experiments.
Well-watered plants (6 weeks old, irrigated to drained capacity) were kept in the dark overnight. A razor blade severed fully expanded leaves at the petiole–stem junction, which were then recut (5 mm from the initial excision site) under deionized water to prevent xylem embolism. The leaves were immediately transferred to a 20 ml glass vial, containing 15 ml of artificial xylem sap (the same composition as in Dodd et al., 2003), and placed in a dark room for 2 h. The top of the glass vial was sealed by parafilm, with a small hole to insert the petiole in the artificial xylem sap (whilst reducing evaporative losses). After 2 h, all leaves were randomly transferred to glass vials containing 15 ml of artificial xylem sap with different ABA or JA concentrations (0, 10, 100, and 1000 nM). After these preliminary dose–response curves, further experiments used 1000 nM as the minimum concentration of JA that significantly decreased transpiration of WT plants.
Leaves were then randomized within the controlled-environment room with conditions described in the previous paragraph. Each vial was weighed initially by a four-point analytical balance, then re-weighed hourly for 5 h. Leaves that showed any (wilting) symptoms of embolism (<10% of the total) were discarded. Finally, leaf area was recorded using a scanner and the Easy Leaf Area software to normalize the transpiration rate for leaf area. After the assay, petioles were gently rinsed with distilled water to remove any of the feeding solution, the leaf was placed in a pressure chamber, and xylem sap (100 µl per sample) was collected at a fixed overpressure (0.1–0.2 MPa) above balancing pressure. Leaves were then frozen in liquid N2 to analyse the hormone concentrations.
To determine possible wounding-related hormonal changes caused by excising leaves for use in the transpiration assay, comparable leaves were collected from intact plants grown under the same conditions, both before (pre-excision control) and after the transpiration assay (post-excision control). These leaves were immediately placed in the pressure chamber to collect xylem sap as described above, then frozen in liquid N2 to analyse hormone concentration.
Phytohormone quantification
ABA, JA, OPDA, and JA-Ile were extracted and analysed essentially as previously described (De Ollas et al., 2013) with slight modifications. Briefly, 0.2 g of dry plant material was extracted in 2 ml of distilled H2O after spiking with 25 μl of a 2 mg l−1 solution of d6-ABA and dihydrojasmonic acid (DHJA) as internal standards. After centrifugation (10 000 g at 4 °C), supernatants were recovered and the pH was adjusted to 3.0 with 30% acetic acid. The acidified water extract was partitioned twice against 3 ml of di-ethyl ether. The organic layer was recovered and evaporated under vacuum in a centrifuge concentrator (Speed Vac, Jouan, Saint Herblain Cedex, France). The dry residue was then re-suspended in a 9:1 H2O:MeOH solution by sonication. The resulting solution was filtered and directly injected into a UPLC system (Waters Acquity SDS, Waters Corp., Milford, MA, USA) interfaced to a TQD triple quadrupole (Micromass Ltd, Manchester, UK) mass spectrometer through an orthogonal Z-spray electrospray ion source. Xylem sap (75 µl) was spiked with 10 µl of internal standard solution and diluted with deionized water to a total volume of 200 µl; the solution was filtered and injected into the UPLC system using the same conditions as used for tissue extraction.
Separations were carried out on a Gravity C18 column (50 × 2.1 mm, 1.8 μm, Macherey-Nagel GmbH, Germany) using a linear gradient of MeOH and H2O supplemented with 0.1% acetic acid at a flow rate of 300 μl min−1. Transitions for ABA/d6-ABA (263>153/269>159), JA/DHJA (209>59/211>59), OPDA (291>165), and JA-Ile (322>130) were monitored in negative ionization mode. Quantitation of plant hormones was achieved by external calibration with known amounts of pure standards using Masslynx v4.1 software.
Stomatal density and size analysis
Epidermal imprints were taken from fully expanded leaves following a similar protocol to that of Scalschi et al. (2015). Leaflets were placed on glass slides with the abaxial epidermis in contact with dental resin. For stomatal analysis, images of randomly selected regions were taken using a Leica IRB microscope equipped with a Leica DC300F camera (Leica Microsystems CMS GmbH, Wetzlar, Germany). Stomatal number and size of at least 30 random sections per genotype were calculated with the ImageJ software. Size or stomatal area was calculated assuming the area of an ellipse.
Statistical analysis
In ungrafted plants (Table 1) and the detached leaf experiments (Figs 8, 9), genotype and treatment effects (and their interaction) were determined using two-way ANOVA, with Duncan’s HSD (P<0.05) used to determine significant differences between genotype/treatment combinations. In grafted plants, rootstock and scion effects (and their interaction) were determined using two-way ANOVA (e.g. Fig. 1), with significant effects of graft combinations on the relationships between soil and plant variables determined by analysis of covariance (ANCOVA; e.g. Figs 2–7). Differences in detached leaf transpiration and foliar phytohormone concentrations (Figs 8, 9) between genotype/treatment combinations were determined using one-way ANOVA, with significant (P<0.05) differences determined using Duncan’s HSD.
Table 1.
Whole-plant transpiration, leaf area, leaf water potential, and ABA, OPDA, JA, and JA-Ile concentrations in leaves, xylem sap, and roots of the wild type (WT), def-1, and spr2 under well-watered (WW) and water deficit (WD) conditions
| Wild type (WT) | def-1 | spr2 | P-values from two-way ANOVA | ||||||
|---|---|---|---|---|---|---|---|---|---|
| WW | WD | WW | WD | WW | WD | Genotype | Treatment | G×T | |
| Whole-plant transpiration (mmol m–2 s–1) | 2.18 ± 0.06 a | 0.70 ± 0.06 c | 1.41 ± 0.05 b | 0.45 ± 0.12 c | 2.50 ± 0.15 a | 0.77 ± 0.45 c | <0.001 | <0.001 | 0.006 |
| Leaf area (cm2) | 430 ± 32 a | 416 ± 25 a | 378 ± 24 a | 385 ± 15 a | 330 ± 36 b | 325 ± 19 b | 0.003 | 0.771 | 0.802 |
| Leaf water potential (MPa) | –0.30 ± 0.004 a | –0.97 ± 0.12 c | –0.27 ± 0.007 a | –0.70 ± 0.005 b | –0.32 ± 0.05 a | –1.08 ± 0.09 c | 0.02 | <0.001 | 0.096 |
| Leaf [ABA] (ng g–1 DW) | 243 ± 9 b | 547 ± 30 a | 252 ± 5 b | 602 ± 59 a | 228 ± 59 a | 660 ± 55 a | 0.301 | <0.001 | 0.234 |
| Leaf [OPDA] (ng g–1 DW) | 27 ± 4 cd | 34 ± 5 c | 54 ± 2 b | 83 ± 7 a | 14 ± 2 de | 11 ± 5 e | 0.007 | <0.001 | 0.009 |
| Leaf [JA] (ng g–1 DW) | 13 ± 1 bc | 49 ± 3 a | 15.5 ± 1.6 b | 17 ± 2 b | 5.1 ± 0.5 d | 8.1 ± 0.9 cd | <0.001 | <0.001 | <0.001 |
| Leaf [JA-Ile] (ng g–1 DW) | 2.7 ± 0.2 b | 15 ± 1 a | 2.1 ± 0.1 bc | 3.5 ± 0.6 b | 0.4 ± 0.1 c | 0.62 ± 0.01 c | <0.001 | <0.001 | <0.001 |
| Xylem sap [ABA] (nM) | 189 ± 15 c | 2775 ± 386 a | 193 ± 15 c | 2178 ± 284 ab | 197 ± 32 c | 1894 ± 409 b | 0.253 | <0.001 | 0.242 |
| Xylem sap [OPDA] (nM) | 58 ± 7 cd | 85 ± 7 bc | 96 ± 78 b | 164 ± 14 a | 30 ± 13 d | 54 ± 7 d | <0.001 | <0.001 | 0.081 |
| Xylem sap [JA] (nM) | 95 ± 8 cd | 385 ± 20 a | 119 ± 17 b | 152 ± 21 b | 31 ± 5 de | 58 ± 11 de | <0.001 | <0.001 | <0.001 |
| Xylem sap [JA-Ile] (nM) | 1.9 ± 0.2 bc | 3.7 ± 0.7 a | 1.4 ± 0.2 c | 2.5 ± 0.2 b | 0.11 ± 0.04 d | 1.0 ± 0.2 cd | <0.001 | <0.001 | 0.328 |
| Root [ABA] (ng g–1 DW) | 247 ± 17 c | 1825 ± 320 a | 238 ± 320 a | 2422 ± 227 a | 240 ± 18 c | 2025 ± 240 a | 0.333 | <0.001 | 0.314 |
| Root [OPDA] (ng g–1 DW) | 26 ± 3 d | 57 ± 3 b | 46 ± 3 c | 68 ± 7 a | 9 ± 1 e | 16 ± 2 de | <0.001 | <0.001 | 0.016 |
| Root [JA] (ng g–1 DW) | 86 ± 13 b | 209 ± 29 a | 62 ± 3 bc | 40 ± 4 cd | 8 ± 2 d | 13 ± 3 de | <0.001 | 0.005 | <0.001 |
| Root [JA-Ile] (ng g–1 DW) | 13 ± 2 b | 23 ± 3 a | 8 ± 1 bc | 11 ± 1 b | 1.3 ± 0.6 d | 3.6 ± 0.6 cd | <0.001 | 0.002 | 0.074 |
Values are the mean ±SE of four replicates, while different letters denote significant (P<0.05) differences across genotypes and treatments. The table summarizes significance (P-values) of genotype (G), treatment (T), and their interaction (G×T) after ANOVA
Fig. 8.
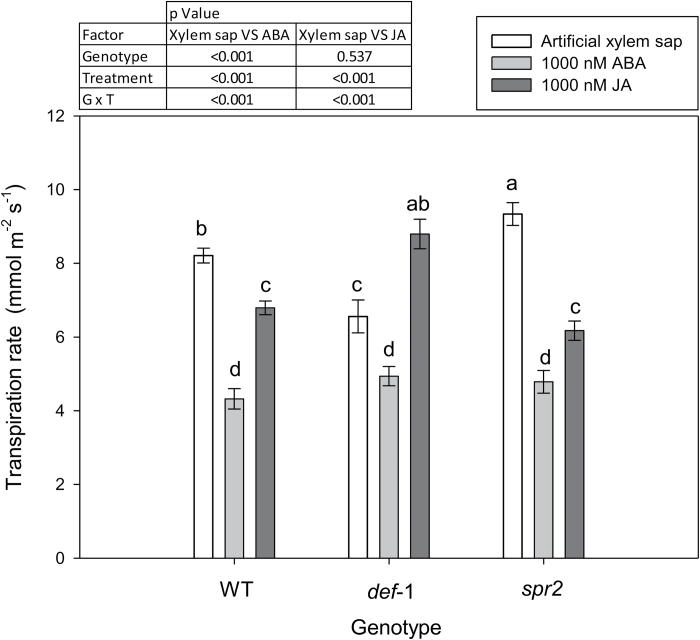
Transpiration rate of detached wild-type (WT), def-1, and spr2 leaves fed with artificial xylem sap (open bars), 1000 nM ABA (light grey bars), or 1000 nM JA (dark grey bars). Symbols are the mean ±SE of four replicates, with different letters denoting significant differences between all genotype/treatment combinations. The table summarizes the significance (P-values) of genotype, treatment, and their interaction after ANOVA of pairwise treatments (artificial xylem sap versus 1000 nM ABA and artificial xylem sap versus 1000 nM JA).
Fig. 9.
ABA (A, B) OPDA (C, D), JA (E, F), and JA-Ile (G, H) concentrations of WT, def-1, and spr2 leaves after 5 h of xylem feeding with artificial xylem sap (open bars), 1000 nM ABA (light grey bars), or 1000 nM JA (dark grey bars) (left panels) or freshly detached leaves before (filled bars) and 5 h after (grey bars) starting the transpiration assay (right panels). Bars are the mean ±SE of four replicates, with different letters denoting significant differences between treatments after Duncan’s HSD, comparing across both panels. The tables summarize the significance (P-values) of genotype, treatment, and their interactions after ANOVA of pairwise treatments (artificial xylem sap versus 1000 nM JA and artificial xylem sap versus 1000 nM ABA).
Fig. 1.
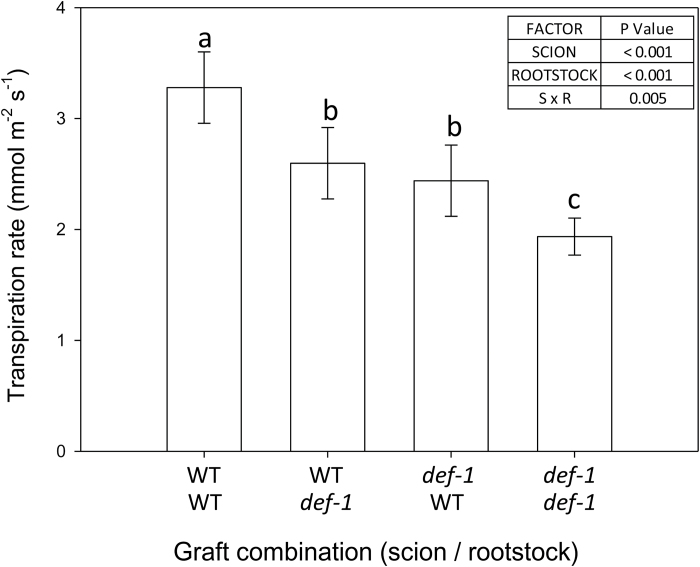
Transpiration rate of wild-type (WT) and def-1 self- and reciprocal grafts (top scion, bottom rootstock), normalized to whole-plant leaf area. Bars represent the mean ±SE of eight replicates per graft combination; different letters denote significant (P<0.05) differences between graft combinations. The table summarizes the significance (P-values) of scion, rootstock, and their interaction (S×R) after ANOVA.
Fig. 2.
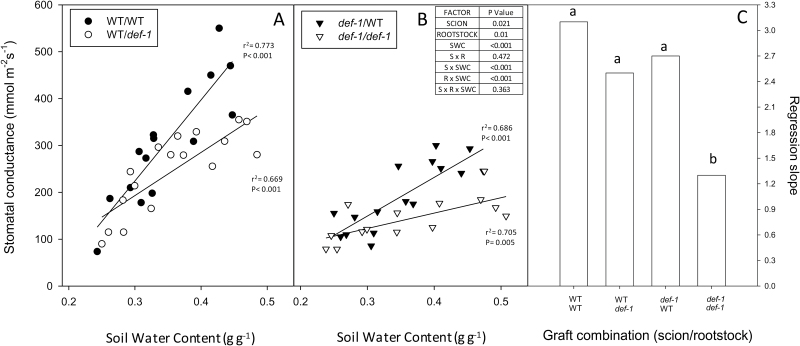
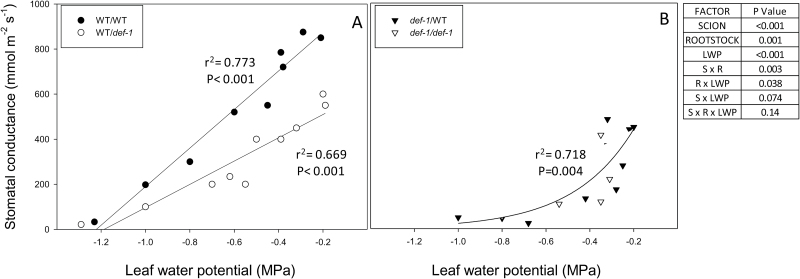
Stomatal conductance (gs) versus soil water content (SWC) of (A) Self-grafted wild-type (WT) plants (filled circles) and WT scions grafted onto def-1 rootstocks (open circles) and (B) self-grafted def-1 plants (open triangles) and def-1 scions grafted onto WT rootstocks (filled triangles). (C) Slope of the regression line between relative gs (normalized to maximum gs) and SWC of the four graft combinations, with different letters showing significant differences in slope. In (A) and (B), each point is an individual plant with linear regression lines fitted for each graft combination with r2 and P-values reported. The table summarizes the significance (P-values) of scion, rootstock, SWC, and their interactions after ANOVA.
Fig. 3.
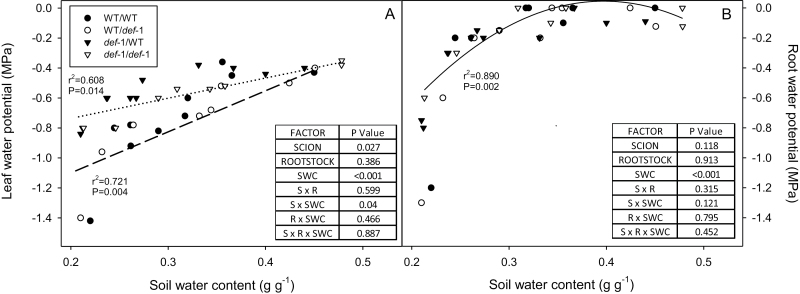
(A) Leaf and (B) root water potential versus soil water content (SWC) of self-grafted wild-type (WT) plants (filled circles), def-1 self-grafts (open triangles) and reciprocal graft (scion/rootstock) combinations (WT/def-1, open circles; and def-1/WT, filled triangles). Each point is an individual plant, with lines defining significant correlations of (A) WT (dashed line) and def-1 (dotted line) scions (linear regressions) and (B) all plants (second-order regression) with r2 and P-values reported. The tables summarize the significance (P-values) of scion, rootstock, SWC, and their interactions after ANOVA.
Fig. 4.
Stomatal conductance versus leaf water potential (LWP) of (A) self-grafted wild-type (WT) plants (filled circles) and WT scions grafted onto def-1 rootstocks (open circles) and (B) self-grafted def-1 plants (open triangles) and def-1 scions grafted onto WT rootstocks (filled triangles). Each point is an individual plant, with lines defining significant correlations of (A) WT self-grafts and WT/def-1 plants (linear regression) and (B) def-1 scions [exponential growth model (y=axb) with r2 and P-values reported. The table summarizes the significance (P-values) of scion, rootstock, LWP, and their interactions after ANOVA.
Fig. 5.
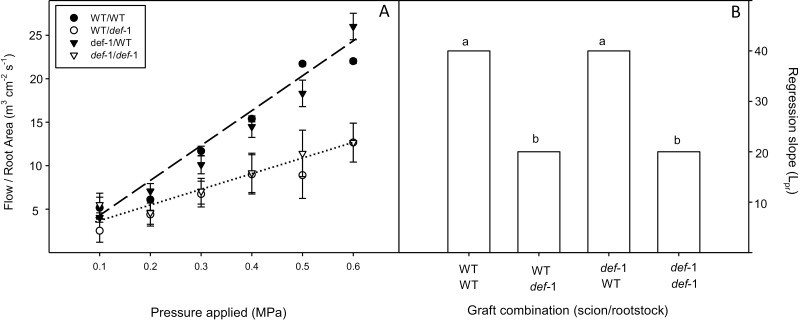
(A) Root xylem sap flow rate (normalized to root area) versus applied pressure for detached roots of self-grafted wild-type (WT) plants (filled circles), def-1 self-grafts (open triangles), and reciprocal graft (scion/rootstock) combinations (WT/def-1, open circles; and def-1/WT, filled triangles). Symbols are the mean ±SE of three replicates. Lines denote a significant correlation between WT (dashed line) and def-1 (dotted line) rootstocks. (B) Root hydraulic conductance (the slope of flow rate versus applied pressure) of the different graft combinations. Letters denote a significant (P<0.05) difference between graft combinations after Duncan’s HSD.
Fig. 6.
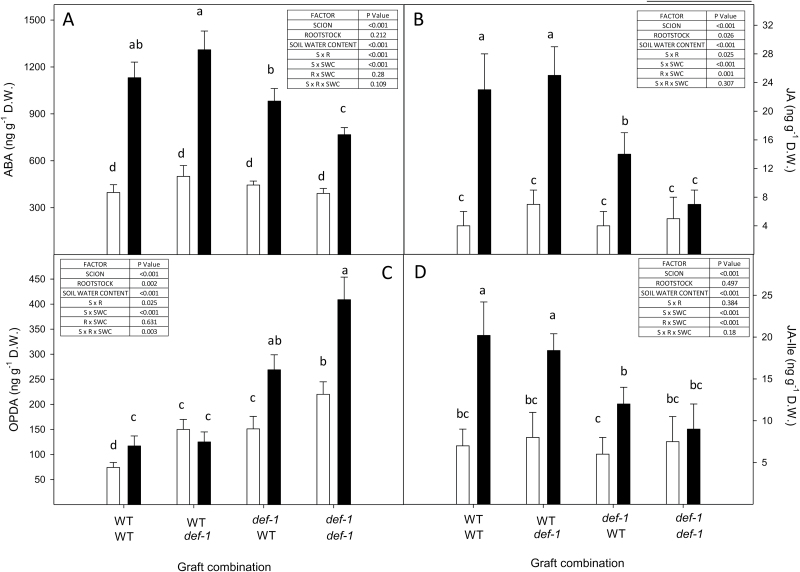
Foliar ABA (A), JA (B), OPDA (C), and JA-Ile (D) concentrations of wild-type (WT) and def-1 self-grafts and reciprocal graft (scion/rootstock) combinations under high (0.3<SWC<0.5 cm3 cm–3) and low (0.2<SWC<0.3 cm3 cm–3) soil water content (SWC), represented by open and filled bars, respectively. Bars are the mean ±SE of four replicates, with different letters denoting significant (P<0.05) differences between graft combinations after Duncan’s HSD within a panel. The tables summarize the significance (P-values) of scion, rootstock, SWC, and their interactions after ANOVA.
Fig. 7.
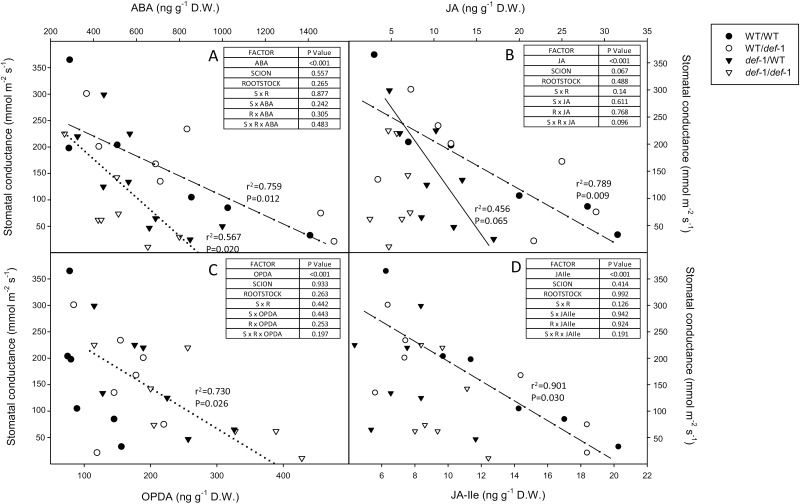
Stomatal conductance versus foliar ABA (A), JA (B), OPDA (C), and JA-Ile (D) concentrations in wild-type (WT) self-grafts (filled circles), def-1 self-grafts (open triangles), and reciprocal graft (scion/rootstock) combinations (WT/def-1, open circles; and def-1/WT, filled triangles). Correlations between hormone concentration and stomatal conductance reported (r2 and P-values) for WT scions (dashed lines), def-1 scions (dotted lines), and def-1/WT plants (plain line). The tables summarize the significance (P-values) of hormones, scion, rootstock, and their interactions after ANOVA.
Results
Ungrafted plants
Under well-watered (WW) conditions, transpiration rates of WT and spr2 plants were statistically similar, while the def-1 transpiration rate was 35% lower (Table 1). Under water deficit (WD) conditions, all three genotypes had a similar transpiration rate. Stomatal density and size were inversely correlated, with stomatal density of WT plants 32% and 42% higher than that of def-1 and spr2 plants, respectively, while stomatal size of WT plants was 36% and 52% lower than that of def-1 and spr2 plants (see Supplementary Fig. S1 at JXB online). Under WW conditions, all genotypes had a similar leaf water potential (Ψleaf), but, under WD conditions, Ψleaf of def-1 plants was higher than in the WT and spr2 (–0.7, –0.97, and –1.08 MPa, respectively). These differences in shoot water relations were not due to differences in SWC (data not shown).
WT, def-1, and spr2 plants had similar tissue ABA concentrations under both WW and WD conditions. Water deficit increased root and leaf ABA concentrations by 8.7- and 2.5-fold, respectively (averaged across all genotypes). Although leaf xylem sap ABA concentrations under WW conditions did not differ between genotypes, under WD conditions xylem ABA concentrations of def-1 and spr2 plants were 22% and 32% lower than that of WT plants, respectively. This genetic variation in ABA status did not affect transpiration.
OPDA concentrations differed significantly between treatments and genotypes. Under WW conditions, root OPDA concentrations of def-1 and spr2 were 70% higher and 70% lower than those of the WT, respectively. Water deficit stimulated root OPDA accumulation by 1.8-fold (averaged across all genotypes). Likewise, water deficit stimulated foliar OPDA accumulation in WT and def-1 plants, but not in spr2 plants. Xylem sap OPDA concentrations showed similar responses, which were 78% higher in def-1 but 42% lower in spr2 (averaged across water treatments). Water deficit increased xylem OPDA concentrations by 65% (averaged across genotypes).
Under WW conditions, the WT and def-1 had similar tissue and xylem sap JA concentrations, whereas spr2 had significantly lower (by 60–80%) JA concentrations than the WT. In WT plants, water deficit increased root, leaf xylem sap, and leaf JA concentrations by 2.4-, 4-, and 3.8-fold, respectively. However, water deficit did not significantly increase JA accumulation in def-1 and spr2 plants. JA-Ile concentrations showed similar genotypic and treatment differences to JA, except that xylem JA-Ile concentration significantly increased in all genotypes, but with lower absolute values in both mutants.
Since def-1 plants had similar leaf area but different transpiration responses to WT plants (Table 1), the role of differential oxylipin accumulation and root and shoot regulation of plant water relations was investigated by reciprocally grafting WT and def-1 plants. Moreover, these graft combinations allowed the physiological effects of attenuated water deficit-induced JA accumulation to be assessed independently of constitutively low oxylipin status.
Grafted plants
Under WW conditions, hormonal imbalance of def-1 self-grafts decreased whole-plant transpiration by 43% compared with WT self-grafts (Fig. 1). Both reciprocal graft combinations had intermediate, similar whole-plant transpiration rates, which were 25% lower than that of WT self-grafts. Thus hormonal imbalance in either scion or rootstock significantly (P<0.001 for rootstock and scion main effects) decreased whole-plant transpiration.
Stomatal conductance (gs) declined linearly with SWC in all graft combinations (Fig. 2A, B). Sensitivity to soil drying (defined as the slope of gsversus SWC) was generally similar in all graft combinations (except def-1 self-grafts) once gs values were normalized according to the maximum gs of each graft combination (Fig. 2C). Although gs of def-1 self-grafts was less sensitive to drying soil, all graft combinations had similar absolute gs values when the soil profile was depleted of soil moisture. Thus the def-1 genotype as either scion or rootstock significantly decreased maximum gs, but only def-1 self-grafts had diminished stomatal sensitivity to drying soil.
Under WW conditions, all graft combinations had similar values of leaf water potential [Ψleaf ranged between –0.4 MPa and –0.55 MPa (Fig. 3A)] and root water potential [Ψroot ranged between –0.01 (with some plants showing spontaneous exudation with no applied pressure) and –0.19 MPa]. Root water potential declined exponentially as the soil dried, but similarly in all graft combinations (Fig. 3B). Leaf water potential declined linearly with SWC (Fig. 3A), but more so in WT scions (significant scion×SWC interaction). Taken together, this implies that drought-induced stomatal closure in def-1 scions (Fig. 2B) was more effective at regulating Ψleaf than in WT scions (Fig. 3A).
Stomatal conductance decreased linearly with Ψleaf in WT scions (Fig. 4A), but exponentially in def-1 scions (Fig. 4B). The rootstock had no impact on the sensitivity of gs to Ψleaf in either def-1 or WT scions (once gs values were normalized according to the maximum gs of each graft combination).
Root hydraulic conductance (Lpr), calculated as the slope of sap flow from de-topped root systems versus applied pressures and normalized to root surface area, was entirely rootstock dependent (Fig 5A). Lpr of WT rootstocks was approximately double that of def-1 rootstocks (Fig. 5B).
Foliar ABA concentration increased with soil drying in all graft combinations (Fig. 6A), but WT scions accumulated significantly more ABA in leaves of plants at low SWC (significant scion×SWC interaction). When exposed to drying soil, ABA accumulation in WT scions was 24% and 50% higher than in the def-1/WT (scion/rootstock) and def-1/def-1 graft combinations, respectively. Although a def-1 rootstock had no significant impact on drought-induced ABA accumulation in WT scions, a WT rootstock enhanced ABA accumulation in def-1 scions (significant scion×rootstock interaction). Stomatal closure was correlated with foliar ABA accumulation (Fig. 7A), but was more sensitive in def-1 scions, which accumulated half as much ABA at maximal stomatal closure.
Foliar JA concentration also increased with soil drying in all graft combinations except def-1 self-grafts (Fig. 6B). JA accumulation in WT scions was double that of the def-1/WT graft combination, while def-1 self-grafts did not accumulate JA when exposed to drying soil. Rootstock genotype did not significantly affect JA accumulation in WT scions, but a WT rootstock significantly increased drought-induced JA accumulation in def-1 scions (significant rootstock×SWC interaction). JA concentration increased with stomatal closure in WT scions (Fig. 7B), whereas this trend neared significance (P=0.065) in the def-1/WT graft combination. Although absolute JA/JA-Ile concentrations were ~5-fold lower in all graft combinations compared with the experiments with ungrafted plants (cf. Table 1), these were probably due to differences in analytical (instrument) sensitivity, and both experiments showed similar treatment and genotypic relative differences.
Soil drying induced a similar pattern of JA-Ile accumulation in the graft combinations as JA (Fig. 6D). JA-Ile accumulation in WT scions was 38% higher than that of the def-1/WT graft combination, while def-1 self-grafts did not accumulate JA-Ile when exposed to drying soil. However, rootstock genotype did not affect JA-Ile accumulation in WT or def-1 scions. Only in WT scions did JA-Ile concentrations increase with stomatal closure (Fig. 7D).
Whereas soil drying induced only limited (36% increase) foliar OPDA accumulation in WT self-grafts and had no effect in WT/def-1 plants (Fig. 6C), soil drying substantially increased OPDA concentrations of def-1 scions (significant scion×SWC interaction). WT rootstocks decreased OPDA accumulation in def-1 scions (significant scion×rootstock×SWC interaction), such that foliar OPDA concentrations were 44% lower than in def-1 self-grafts. Stomatal conductance was not correlated with OPDA concentration in WT scions, but leaf OPDA concentrations increased with stomatal closure in def-1 scions (Fig. 7C).
Water deficit decreased sap flow of all graft combinations (Table 2); however, plants with a def-1 scion had significantly lower flow rates than plants with a WT scion (the significance, P-values, of yhr flow rate and hormone delivery are summarized in Table 3). All graft combinations had similar root xylem ABA concentrations under both WW and WD conditions, with water deficit increasing xylem ABA concentrations by 11.5-fold from 37 nM to 426 nM (averaged across graft combinations). Xylem JA concentrations were similar in all graft combinations under WW conditions (48 nM); however, water deficit significantly increased JA concentrations of graft combinations with a WT rootstock (to 1030 nM). JA-Ile followed a similar trend, with low concentrations under WW conditions but higher JA-Ile concentrations when graft combinations with a WT rootstock dried the soil.
Table 2.
Xylem sap flow rate, root xylem sap hormone concentrations (ABA/JA/JA-Ile), and hormone delivery (the product of sap flow rate and hormone concentration) under well-watered (WW) and water deficit (WD) conditions in the wild type (WT/WT) and def-1 (def-1/def-1) self-grafts and reciprocal grafts (WT/def-1, def-1/WT)
| WT/WT | WT/def-1 | def-1/WT | def-1/def-1 | |||||
|---|---|---|---|---|---|---|---|---|
| WW | WD | WW | WD | WW | WD | WW | WD | |
| Flow (nmol s–1)×100 | 4.8 ± 0.4 a | 1.8 ± 0.3 d | 3.4 ± 0.9 b | 1.6 ± 0.3 de | 3.0 ± 0.5 b | 1.4 ± 0.2 ef | 2.6 ± 0.4 c | 1.1 ± 0.1 f |
| [ABA] (nM) | 44 ± 5 b | 427 ± 35 a | 34 ± 4b | 378 ± 44 a | 31 ± 2 b | 475 ± 52 a | 38 ± 2 b | 538 ± 48 a |
| [JA] (nM) | 52 ± 11 bc | 1047 ± 154 a | 57 ± 9 bc | 598 ± 68 b | 43 ± 7 bc | 1014 ± 69 a | 42 ± 5 c | 426 ± 42 bc |
| [JA-Ile] (nM) | 4.1 ± 0.2 c | 73 ± 9 a | 2.7 ± 0.9 c | 45 ± 13 ab | 3.9 ± 0.6 c | 66 ± 15 a | 6.4 ± 1.4 c | 26 ± 11 b |
| ABA delivery (nmol s–1)×105 | 3.8 ± 0.2 b | 14.2 ± 0.1 a | 2.1 ± 0.2 b | 11 ± 1 a | 1.7 ± 0.2 b | 11.9 ± 0.2 a | 1.4 ± 0.1 b | 10.7 ± 0.9 a |
| JA delivery (nmol s–1)×105 | 4.5 ± 0.6 d | 35 ± 5 a | 3.5 ± 0.9 d | 18 ± 3 b | 5.4 ± 0.7 d | 25 ± 4 b | 4.0 ± 0.4 d | 8.5 ± 2.3 c |
| JA delivery (nmol s–1)×105 | 0.3 ± 0.1 b | 2.4 ± 0.4 a | 0.2 ± 0.1 b | 1.3 ± 0.5 a | 0.2 ± 0.1 b | 1.6 ± 0.4 a | 0.2 ± 0.1 b | 0.5 ± 0.2 b |
Values are the mean ± SE of four replicates. Letters denote significant (P<0.05) differences across genotype and treatment after a post-hoc (Duncan’s HSD).
Table 3.
A summary of the significance (P-values) of rootstock genotype (R), scion genotype (S), treatment (T), and interactions on sap flow and hormone delivery after ANOVA
| Flow | ABA delivery | JA delivery | JA-Ile delivery | |
|---|---|---|---|---|
| Treatment | <0.001 | <0.001 | <0.001 | <0.001 |
| Root | <0.001 | 0.042 | <0.001 | <0.001 |
| Shoot | <0.001 | 0.062 | 0.004 | 0.001 |
| T×R | 0.05 | 0.68 | <0.001 | <0.001 |
| T×S | 0.06 | 0.2 | 0.026 | 0.01 |
| R×S | 0.326 | 0.45 | 0.44 | 0.63 |
| T×R×S | 0.804 | 0.26 | 0.51 | 0.55 |
Hormone delivery from the root system was quantified by multiplying hormone concentration and pressure-induced xylem flow (Table 2). Although water deficit increased ABA delivery by an order of magnitude, all graft combinations had similar ABA delivery. In contrast, both treatment and root/shoot genotype affected JA delivery. Although all graft combinations had similar root JA export under WW conditions, water deficit increased root JA export of WT self-grafts, both reciprocal grafts, and def-1 self-grafts by 7-, 5-, and 2.6-fold, respectively. Root JA-Ile export followed a similar pattern. Thus water deficit always increased root JA/JA-Ile export, even if it had no measurable effect on foliar JA/JA-Ile concentrations (as in def-1 self-grafts).
Detached leaf transpiration assays
To determine whether xylem-supplied phytohormones could regulate transpiration, ABA or JA were supplied via the transpiration stream (to emulate root to shoot hormone delivery) to detached WT, def-1, and spr2 leaves. Detached leaf transpiration was more sensitive to xylem-supplied ABA than xylem-supplied JA, with significant reductions at 100 nM and 1000 nM, respectively (Supplementary Fig. S2). As in whole plant measurements, the transpiration rate of detached def-1 leaves was (21%) lower than that of WT leaves when supplied with an artificial xylem solution, while spr2 transpiration was significantly (9.6%) higher (Fig. 8). Xylem-supplied JA (1000 nM) decreased transpiration of WT and spr2 leaves by 16% and 24%, respectively, but increased transpiration of def-1 leaves by 23%. Xylem-supplied ABA (1000 nM) decreased transpiration in all genotypes, but more effectively in WT and spr2 (50% reduction) than in def-1 (26% reduction).
To determine whether xylem-supplied ABA or JA influenced synthesis or metabolism of each other, foliar (Fig. 9) and xylem sap (Supplementary Fig. S3) phytohormone concentrations were quantified at the end of the transpiration assay, after supplying artificial xylem sap via the transpiration stream for 5 h. All three genotypes had similar ABA and JA/JA-Ile concentrations to freshly harvested leaves (pre- and post-excision controls). Leaf OPDA concentrations of WT and spr2 plants were similar in detached leaves supplied with artificial xylem sap and in freshly harvested leaves. After 5 h of feeding def-1 leaves with artificial xylem sap, their OPDA concentrations were 4.7-fold higher than those of the other genotypes fed artificial xylem sap, and 2.4-fold higher than freshly harvested def-1 leaves. Taken together, these data imply a limited effect of leaf excision and placing the cut petiole in an artificial xylem sap for 5 h, as foliar phytohormone concentrations were generally similar to those of freshly harvested leaves that were immediately placed in liquid nitrogen post-excision.
Xylem-supplied ABA more than doubled leaf ABA concentrations of WT and def-1 plants, but had a much smaller effect in spr2 leaves (Fig. 9A). Xylem-supplied ABA had no effect on JA and OPDA levels in WT and spr2 plants, but decreased OPDA concentrations (by 33%) while increasing JA concentrations (2.4-fold) in def-1 plants (Fig. 9C, E). Xylem-supplied ABA increased JA-Ile concentrations by 5.3-fold, averaged across both genotypes (Fig. 9G). Taken together, xylem-supplied ABA significantly increased JA-Ile concentrations in WT and mutant genotypes, while significantly decreasing OPDA concentrations in def-1.
Xylem-supplied JA had no effect on leaf ABA concentration in all genotypes (Fig. 9A), but substantially increased JA and JA-Ile concentrations (Fig. 9E, G). These increases were much greater in WT plants (5.5-fold averaging both JA and JA-Ile) than in def-1 (3.4-fold) and spr2 (4-fold). Xylem-supplied JA had no effect on leaf OPDA concentrations in WT and spr2 plants (Fig. 9C), but decreased OPDA concentrations of detached def-1 leaves by 51% compared with plants supplied with artificial xylem sap (Fig. 9C, D). Genotypic variation in oxylipin status in leaves supplied with JA via the transpiration stream was not explained by genotypic differences in JA uptake.
Taken together, supplying ABA or JA via the transpiration stream to detached leaves had the intended effects on foliar concentrations of the hormone supplied, but ABA also increased JA-Ile concentrations in all genotypes, and decreased OPDA concentrations in def-1 plants.
Discussion
Although exogenous JA induces stomatal closure (Herde et al., 1997; Hossain et al., 2011; Yin et al., 2016), and water deficit stimulates root (De Ollas et al., 2013; 2015) and shoot (Muñoz-Espinoza et al., 2015) JA and JA-Ile (the bioactive form) accumulation, this study uniquely addresses the physiological significance of JA as a long-distance signal of water deficit. Two complementary approaches were used: allowing reciprocally grafted WT and def-1 tomato plants (which fail to accumulate JA and JA-Ile during water deficit) to dry the soil and supplying detached leaves with xylem-borne JA at the concentrations detected in WT plants grown in drying soil. Although xylem JA concentration of WT plants exposed to water deficit was sufficient to elicit partial stomatal closure when supplied via the xylem to detached WT leaves, attenuating drought-induced root JA export (in WT/def-1 and def-1/WT plants) did not alter stomatal sensitivity to drying soil (Fig. 2C). Only def-1 self-grafts, with the lowest stomatal conductance under well-watered conditions, showed an attenuated stomatal response to drying soil, consistent with their lowest root JA export. Paradoxically, increasing the supply of xylem-borne JA to def-1 shoots (in either detached leaves or def-1/WT plants) increased transpiration, probably by limiting OPDA (a JA precursor) accumulation, which is a more potent antitranspirant than JA (Savchenko et al., 2014). Although spatio-temporal patterns of JA accumulation differed between detached leaf transpiration assays and grafted plants exposed to drying soil, variation in both foliar and xylem sap oxylipin status mediated both constitutive stomatal conductance and stomatal sensitivity to drying soil.
Stomatal responses under well-watered conditions
Well-watered, ungrafted def-1 plants had constitutively lower transpiration rates than either spr2 or WT plants (Table 1). Since all genotypes had similar leaf water potential and xylem and leaf ABA concentration, stomatal morphology and oxylipin status were measured. Decreased stomatal density of the mutants was compensated by increased stomatal size (Supplementary Fig. S1), making it difficult to attribute variation in transpiration rate to altered stomatal morphology. Alternatively, while spr2 had lower OPDA, JA, and JA-Ile concentrations than WT plants, foliar OPDA concentrations were almost doubled in def-1 plants, supporting the suggestion that OPDA is a more potent antitranspirant than JA/JA-Ile (Savchenko et al., 2014).
Although both mutants failed to accumulate JA as the soil dried (Table 1), def-1 was preferred in reciprocal grafting studies since it had similar JA/JA-Ile concentrations to WT plants under WW conditions. Moreover, def-1 plants had similar vegetative vigour to WT plants, unlike spr2 (Table 1; Supplementary Fig. S4), eliminating the need for a developmental control in soil drying experiments. Although the grafting process itself probably induced transient differences in endogenous JA concentrations, which could inhibit leaf expansion (Moore et al., 2003) of WT scions, it was possible to select reciprocally grafted def-1 and WT plants of similar leaf area to measure transpiration.
Nevertheless, hormonal imbalance in either rootstock or scion decreased transpiration of well-watered plants (Fig. 1) such that grafting WT scions onto def-1 rootstocks decreased their gs compared with WT self-grafts (Fig. 2), while grafting def-1 scions onto WT rootstocks increased their gs compared with def-1 self-grafts. These rootstock-mediated changes in gs were correlated with decreased root hydraulic conductivity of def-1 rootstocks (Fig. 5) as previously reported (Sánchez-Romera et al., 2014), yet well-watered plants of all graft combinations maintained a similar leaf water potential (Ψleaf) (Fig. 3A). While homeostatic regulation of Ψleaf by gs may co-ordinate root and shoot conductance (Jones, 1998; Ripullone et al., 2007), it seems unlikely that variation in plant hydraulics caused genotypic differences in transpiration of well-watered plants.
Stomatal responses to drying soil were independent of leaf water status
Genetic variation in stomatal sensitivity to drying soil was assessed by normalizing gs to the maximum (well-watered) value achieved by each graft combination (Fig. 2C). Only def-1 self-grafts (which failed to accumulate JA and its bioactive conjugate JA-Ile as the soil dried) were relatively insensitive to soil drying. Thus WT levels of JA/JA-Ile are required (in either rootstock or scion) to maximize stomatal sensitivity to drying soil, suggesting an antitranspirant effect of JA (Herde et al., 1997). Nevertheless, perturbed leaf hydraulics has been suggested as the most likely mechanism causing stomatal closure (Tardieu, 2016). Although soil drying decreased Ψleaf in all graft combinations, def-1 scions maintained a higher Ψleaf than WT scions (Fig. 3A), independent of root hydraulic conductivity (lower in def-1 rootstocks, irrespective of scion; Fig. 5). This implies that stomatal conductance regulates Ψleaf (rather than vice versa), as in previous tomato experiments where higher gs was associated with decreased Ψleaf in WT plants exposed to various irrigation treatments (Kudoyarova et al., 2007) and in reciprocally grafted, well-watered WT and ABA-deficient plants (Dodd et al., 2009). Taken together, these data imply that phytohormones regulate stomatal closure in response to soil drying (Fig. 10).
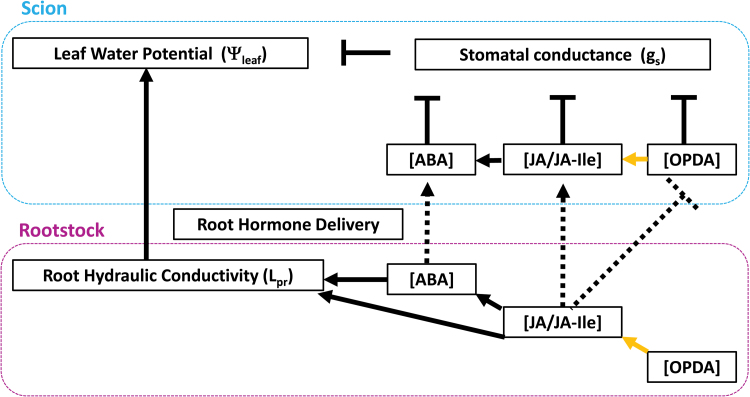
Fig. 10.
Model of plant hydraulics and phytohormone impacts on stomatal conductance of grafted plants. Lines ending in arrowheads indicate a positive impact, while lines ending in a bar indicate negative impacts. Root hormone delivery is indicated by dashed lines, while relationships within a biosynthetic pathway are indicated by a yellow arrow.
Phytohormonal (ABA and JA/JA-Ile) regulation of stomatal response to drying soil
Although an increased foliar and xylem sap ABA concentration has been correlated with stomatal closure in multiple species including tomato (Dodd, 2007; Thompson et al., 2007), def-1 scions required half the foliar ABA concentration to induce maximal stomatal closure (Fig. 7A). Possibly def-1 scions were more sensitive to ABA, but similar stomatal closure of def-1 and WT leaves in response to xylem-borne ABA (Fig. 8; Supplementary Fig. S2) disproves this hypothesis. Stomatal conductance responds more sensitively to xylem sap ABA concentration than foliar ABA accumulation (Geilfus et al., 2015; Tardieu et al., 2015), as much of the latter is compartmentalized in mesophyll cells and is unavailable to the guard cells (Wilkinson et al., 1998). While grafting def-1 scions onto WT rootstocks increased foliar ABA accumulation compared with def-1 self-grafts (Fig. 6A), xylem ABA concentration of def-1 scions was rootstock independent and similar to that of WT scions (Table 2). Taken together, the similar increases in xylem ABA concentration in all graft combinations, yet attenuated stomatal response to drying soil in def-1 self-grafts (Fig. 2C), suggests that other phytohormones were involved in regulating this response.
Restoring normal (WT) stomatal sensitivity to drying soil by grafting def-1 scions onto WT rootstocks was correlated with greater root JA export (Table 2) and partial restoration of foliar JA and JA-Ile status (Fig. 6B, D). Nevertheless, supplemental JA feeding to detached def-1 leaves actually increased their transpiration (Fig. 8), consistent with the higher transpiration of def-1/WT plants than def-1 self-grafts (Fig. 1). Thus stomatal regulation by JA seems dependent on tissue water status, since normal JA levels are required for maximal stomatal conductance in well-watered plants, while drought-induced JA accumulation enhances stomatal closure. Future experiments should establish whether this foliar JA accumulation is a local (induced by decreased Ψleaf) or long-distance (increased root export of JA; Table 2) signal, especially since the xylem JA concentrations detected in WT plants induced partial stomatal closure when fed via the xylem to detached leaves (Fig. 8).
OPDA affects stomatal responses of plants to drying soil
Endogenous OPDA concentrations are rarely monitored in plants exposed to water deficit, although they increase even in WT self-grafts (Fig. 6C), and by up to an order of magnitude (Seo et al., 2001; Arbona et al., 2010; Grebner et al., 2013). With soil drying, OPDA accumulation was inversely related to JA accumulation across all graft combinations (cf. Fig. 6B, C), with maximal OPDA levels in def-1 self-grafts. Grafting def-1 scions onto a WT rootstock increased root JA export (Table 2) and decreased foliar OPDA concentrations (Fig. 6C), consistent with suggestions that JA treatment or transport from distal tissues decreases OPDA concentration (Scalschi et al., 2015) by negatively regulating jasmonate biosynthesis or metabolism (Kazan and Manners, 2008). Thus root to shoot JA transport may directly affect transpiration of WT plants (Fig. 1), but may also indirectly regulate transpiration of def-1 scions by decreasing foliar OPDA concentrations (Fig. 6C).
To test the hypothesis that xylem-borne JA/JA-Ile mediates transpiration by limiting foliar OPDA accumulation, JA was supplied via the xylem to detached def-1 and WT leaves. Xylem-supplied JA had opposite effects on both transpiration and foliar OPDA concentrations in the two genotypes. Although xylem-borne JA decreased transpiration of WT leaves, it was significantly less effective than ABA supplied at the same concentrations. In contrast, xylem-supplied JA increased transpiration of def-1 leaves (Fig. 8), consistent with the partial rescue of transpiration in def-1 scions grafted to WT roots (Fig. 1). Whereas xylem-borne JA had no effect on OPDA concentrations in WT leaves, in def-1 leaves increased transpiration was associated with decreased OPDA (high antitranspirant effect) concentrations and increased JA and in planta synthetized JA-Ile (low antitranspirant effect) concentrations (Fig. 9). Taken together, this supports a model in which xylem-transported JA significantly influences gs by regulating accumulation of the JA precursor OPDA (Fig. 10).
Conclusions
Although spatio-temporal patterns of JA accumulation differed between detached leaf transpiration assays and grafted plants exposed to drying soil, additional xylem-borne JA consistently decreased stomatal conductance of WT shoots (by activating JA signalling through the COI receptor) while increasing stomatal conductance of def-1 shoots (by limiting OPDA accumulation which acts via a COI1-independent pathway). Further work is required to establish the relative importance of these two signalling pathways in WT plants and the distal physiological effects of root to shoot jasmonate transport in plants exposed to water deficit.
Supplementary Material
Acknowledgements
We thank the EU ROOTOPOWER (contract no. 289365) project and the Ministerio de Economia of Spain (MINECO) no. AGL2016-76574-R for supporting the work, and Mike Roberts for supplying def-1 and WT seed.
References
- Alam MM, Nahar K, Hasanuzzaman M, Fujita M. 2014. Exogenous jasmonic acid modulates the physiology, antioxidant defense and glyoxalase systems in imparting drought stress tolerance in different Brassica species. Plant Biotechnology Reports 8, 279–293. [Google Scholar]
- Arbona V, Argamasilla R, Gómez-Cadenas A. 2010. Common and divergent physiological, hormonal and metabolic responses of Arabidopsis thaliana and Thellungiella halophila to water and salt stress. Journal of Plant Physiology 167, 1342–1350. [DOI] [PubMed] [Google Scholar]
- Bandurska H, Stroiński A, Kubiś J. 2003. The effect of jasmonic acid on the accumulation of ABA, proline and spermidine and its influence on membrane injury under water deficit in two barley genotypes. Acta Physiologiae Plantarum 25, 279–285. [Google Scholar]
- Brossa R, Lopez-Carbonell M, Jubany-Mari T, Alegre L. 2011. Interplay between abscisic acid and jasmonic acid and its role in water-oxidative stress in wild-type, ABA-deficient, JA-deficient, and ascorbate-deficient Arabidopsis plants. Journal of Plant Growth Regulation 30, 322–333. [Google Scholar]
- Chen HY, Hsieh EJ, Cheng MC, Chen CY, Hwang SY, Lin TP. 2016. ORA47 (octadecanoid-responsive AP2/ERF-domain transcription factor 47) regulates jasmonic acid and abscisic acid biosynthesis and signaling through binding to a novel cis-element. New Phytologist 211, 599–613. [DOI] [PubMed] [Google Scholar]
- Cheng MC, Liao PM, Kuo WW, Lin TP. 2013. The Arabidopsis ETHYLENE RESPONSE FACTOR1 regulates abiotic stress-responsive gene expression by binding to different cis-acting elements in response to different stress signals. Plant Physiology 162, 1566–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creelman RA, Mullet JE. 1995. Jasmonic acid distribution and action in plants: regulation during development and response to biotic and abiotic stress. Proceedings of the National Academy of Sciences, USA 92, 4114–4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ollas C, Arbona V, Gómez-Cadenas A. 2015. Jasmonoyl isoleucine accumulation is needed for abscisic acid build-up in roots of Arabidopsis under water stress conditions. Plant, Cell and Environment 38, 2157–2170. [DOI] [PubMed] [Google Scholar]
- de Ollas C, Dodd IC. 2016. Physiological impacts of ABA–JA interactions under water-limitation. Plant Molecular Biology 91, 641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ollas C, Hernando B, Arbona V, Gómez-Cadenas A. 2013. Jasmonic acid transient accumulation is needed for abscisic acid increase in citrus roots under drought stress conditions. Physiologia Plantarum 147, 296–306. [DOI] [PubMed] [Google Scholar]
- Dodd IC. 2007. Soil moisture heterogeneity during deficit irrigation alters root-to-shoot signalling of abscisic acid. Functional Plant Biology 34, 439–448. [DOI] [PubMed] [Google Scholar]
- Dodd IC. 2013. Abscisic acid and stomatal closure: a hydraulic conductance conundrum?New Phytologist 197, 6–8. [DOI] [PubMed] [Google Scholar]
- Dodd IC, Egea G, Watts CW, Whalley WR. 2010. Root water potential integrates discrete soil physical properties to influence ABA signalling during partial rootzone drying. Journal of Experimental Botany 61, 3543–3551. [DOI] [PubMed] [Google Scholar]
- Dodd IC, Tan LP, He J. 2003. Do increases in xylem sap pH and/or ABA concentration mediate stomatal closure following nitrate deprivation?Journal of Experimental Botany 54, 1281–1288. [DOI] [PubMed] [Google Scholar]
- Dodd IC, Theobald JC, Richer SK, Davies WJ. 2009. Partial phenotypic reversion of ABA-deficient flacca tomato (Solanum lycopersicum) scions by a wild-type rootstock: normalizing shoot ethylene relations promotes leaf area but does not diminish whole plant transpiration rate. Journal of Experimental Botany 60, 4029–4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca S, Chini A, Hamberg M, Adie B, Porzel A, Kramell R, Miersch O, Wasternack C, Solano R. 2009. (+)-7-iso-Jasmonoyl-l-isoleucine is the endogenous bioactive jasmonate. Nature Chemical Biology 5, 344–350. [DOI] [PubMed] [Google Scholar]
- Fragoso V, Rothe E, Baldwin IT, Kim SG. 2014. Root jasmonic acid synthesis and perception regulate folivore-induced shoot metabolites and increase Nicotiana attenuata resistance. New Phytologist 202, 1335–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geilfus CM, Mithöfer A, Ludwig-Müller J, Zörb C, Muehling KH. 2015. Chloride-inducible transient apoplastic alkalinizations induce stomata closure by controlling abscisic acid distribution between leaf apoplast and guard cells in salt-stressed Vicia faba. New Phytologist 208, 803–816. [DOI] [PubMed] [Google Scholar]
- Grebner W, Stingl NE, Oenel A, Mueller MJ, Berger S. 2013. Lipoxygenase6-dependent oxylipin synthesis in roots is required for abiotic and biotic stress resistance of Arabidopsis. Plant Physiology 161, 2159–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harb A, Krishnan A, Ambavaram MM, Pereira A. 2010. Molecular and physiological analysis of drought stress in Arabidopsis reveals early responses leading to acclimation in plant growth. Plant Physiology 154, 1254–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herde O, Peña-CortéS H, Willmitzer L, Fisahn J. 1997. Stomatal responses to jasmonic acid, linolenic acid and abscisic acid in wild-type and ABA-deficient tomato plants. Plant, Cell and Environment 20, 136–141. [Google Scholar]
- Hlavácková V, Krchnák P, Naus J, Novák O, Spundová M, Strnad M. 2006. Electrical and chemical signals involved in short-term systemic photosynthetic responses of tobacco plants to local burning. Planta 225, 235–244. [DOI] [PubMed] [Google Scholar]
- Hossain MA, Munemasa S, Uraji M, Nakamura Y, Mori IC, Murata Y. 2011. Involvement of endogenous abscisic acid in methyl jasmonate-induced stomatal closure in Arabidopsis. Plant Physiology 156, 430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe GA, Lee GI, Itoh A, Li L, DeRocher AE. 2000. Cytochrome P450-dependent metabolism of oxylipins in tomato. Cloning and expression of allene oxide synthase and fatty acid hydroperoxide lyase. Plant Physiology 123, 711–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe GA, Lightner J, Browse J, Ryan CA. 1996. An octadecanoid pathway mutant (JL5) of tomato is compromised in signaling for defense against insect attack. The Plant Cell 8, 2067–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HG. 1998. Stomatal control of photosynthesis and transpiration. Journal of Experimental Botany 49, 387–398. [Google Scholar]
- Katsir L, Chung HS, Koo AJ, Howe GA. 2008. Jasmonate signaling: a conserved mechanism of hormone sensing. Current Opinion in Plant Biology 11, 428–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazan K. 2015. Diverse roles of jasmonates and ethylene in abiotic stress tolerance. Trends in Plant Science 20, 219–229. [DOI] [PubMed] [Google Scholar]
- Kazan K, Manners JM. 2008. Jasmonate signaling: toward an integrated view. Plant Physiology 146, 1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SK, Sohn EY, Joo GJ, Lee IJ. 2009. Influence of jasmonic acid on endogenous gibberellin and abscisic acid in salt-stressed chard plant. Journal of Environmental Biology 30, 333–338. [PubMed] [Google Scholar]
- Kudoyarova GR, Vysotskaya LB, Cherkozyanova A, Dodd IC. 2007. Effect of partial rootzone drying on the concentration of zeatin-type cytokinins in tomato (Solanum lycopersicum L.) xylem sap and leaves. Journal of Experimental Botany 58, 161–168. [DOI] [PubMed] [Google Scholar]
- Lee SC, Luan S. 2012. ABA signal transduction at the crossroad of biotic and abiotic stress responses. Plant, Cell and Environment 35, 53–60. [DOI] [PubMed] [Google Scholar]
- Li C, Liu G, Xu C, Lee GI, Bauer P, Ling HQ, Ganal MW, Howe GA. 2003. The tomato suppressor of prosystemin-mediated responses2 gene encodes a fatty acid desaturase required for the biosynthesis of jasmonic acid and the production of a systemic wound signal for defense gene expression. The Plant Cell 15, 1646–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahouachi J, Arbona V, Gómez-Cadenas A. 2007. Hormonal changes in papaya seedlings subjected to progressive water stress and re-watering. Plant Growth Regulation 53, 43–51. [Google Scholar]
- Moore JP, Paul ND, Whittaker JB, Taylor JE. 2003. Exogenous jasmonic acid mimics herbivore-induced systemic increase in cell wall bound peroxidase activity and reduction in leaf expansion. Functional Ecology 17, 549–554. [Google Scholar]
- Munemasa S, Mori IC, Murata Y. 2011. Methyl jasmonate signaling and signal crosstalk between methyl jasmonate and abscisic acid in guard cells. Plant Signaling and Behavior 6, 939–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Espinoza VA, López-Climent MF, Casaretto JA, Gómez-Cadenas A. 2015. Water stress responses of tomato mutants impaired in hormone biosynthesis reveal abscisic acid, jasmonic acid and salicylic acid interactions. Frontiers in Plant Science 6, 997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra AS, Gonugunta VK, Christmann A, Grill E. 2010. ABA perception and signalling. Trends in Plant Science 15, 395–401. [DOI] [PubMed] [Google Scholar]
- Riemann M, Dhakarey R, Hazman M, Miro B, Kohli A, Nick P. 2015. Exploring jasmonates in the hormonal network of drought and salinity responses. Frontiers in Plant Science 6, 1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripullone F, Guerrieri MR, Nole’ A, Magnani F, Borghetti M. 2007. Stomatal conductance and leaf water potential responses to hydraulic conductance variation in Pinus pinaster seedlings. Trees - Structure and Function 21, 371–378. [Google Scholar]
- Sánchez-Romera B, Ruiz-Lozano JM, Li G et al. 2014. Enhancement of root hydraulic conductivity by methyl jasmonate and the role of calcium and abscisic acid in this process. Plant, Cell and Environment 37, 995–1008. [DOI] [PubMed] [Google Scholar]
- Savchenko T, Kolla VA, Wang CQ et al. 2014. Functional convergence of oxylipin and abscisic acid pathways controls stomatal closure in response to drought. Plant Physiology 164, 1151–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalschi L, Sanmartín M, Camañes G, Troncho P, Sánchez-Serrano JJ, García-Agustín P, Vicedo B. 2015. Silencing of OPR3 in tomato reveals the role of OPDA in callose deposition during the activation of defense responses against Botrytis cinerea. The Plant Journal 81, 304–315. [DOI] [PubMed] [Google Scholar]
- Seo S, Seto H, Yamakawa H, Ohashi Y. 2001. Transient accumulation of jasmonic acid during the synchronized hypersensitive cell death in Tobacco mosaic virus-infected tobacco leaves. Molecular Plant-Microbe Interactions 14, 261–264. [DOI] [PubMed] [Google Scholar]
- Suhita D, Raghavendra AS, Kwak JM, Vavasseur A. 2004. Cytoplasmic alkalization precedes reactive oxygen species production during methyl jasmonate- and abscisic acid-induced stomatal closure. Plant Physiology 134, 1536–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JQ, Jiang HL, Li CY. 2011. Systemin/jasmonate-mediated systemic defense signaling in tomato. Molecular Plant 4, 607–615. [DOI] [PubMed] [Google Scholar]
- Tardieu F. 2016. Too many partners in root–shoot signals. Does hydraulics qualify as the only signal that feeds back over time for reliable stomatal control?New Phytologist 212, 802–804. [DOI] [PubMed] [Google Scholar]
- Tardieu F, Simonneau T, Parent B. 2015. Modelling the coordination of the controls of stomatal aperture, transpiration, leaf growth, and abscisic acid: update and extension of the Tardieu–Davies model. Journal of Experimental Botany 66, 2227–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejeda-Sartorius M, Martínez de la Vega O, Délano-Frier JP. 2008. Jasmonic acid influences mycorrhizal colonization in tomato plants by modifying the expression of genes involved in carbohydrate partitioning. Physiologia Plantarum 133, 339–353. [DOI] [PubMed] [Google Scholar]
- Thompson AJ, Andrews J, Mulholland BJ et al. 2007. Overproduction of abscisic acid in tomato increases transpiration efficiency and root hydraulic conductivity and influences leaf expansion. Plant Physiology 143, 1905–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasternack C, Goetz S, Hellwege A, Forner S, Strnad M, Hause B. 2012. Another JA/COI1-independent role of OPDA detected in tomato embryo development. Plant Signaling and Behavior 7, 1349–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasternack C, Hause B. 2013. Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Annals of Botany 111, 1021–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson S, Corlett JE, Oger L, Davies WJ. 1998. Effects of xylem pH on transpiration from wild-type and flacca tomato leaves. A vital role for abscisic acid in preventing excessive water loss even from well-watered plants. Plant Physiology 117, 703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Adachi Y, Nakamura Y, Munemasa S, Mori IC, Murata Y. 2016. Involvement of OST1 protein kinase and PYR/PYL/RCAR receptors in methyl jasmonate-induced stomatal closure in Arabidopsis guard cells. Plant and Cell Physiology 57, 1779–1790. [DOI] [PubMed] [Google Scholar]
- Zhang J, Davies WJ. 1991. Antitranspirant activity in xylem sap of maize plants. Journal of Experimental Botany 42, 317–321. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.