Abstract
This article comments on:
McAdam EL, Reid JB, Foo E. 2018. Gibberellins promote nodule organogenesis but inhibit the infection stages of nodulation. Journal of Experimental Botany 69, 2117–2130.
Keywords: DELLA, GA3, gibberellin, nodulation, pea, Pisum, Rhizobium
The role of gibberellin (GA) in legume nodulation is controversial, being reported to both enhance and inhibit the process. Now, in an elegant, multi-faceted investigation on peas McAdam et al. (2018) clearly show that GA is needed for nodule formation but inhibits the initiation of infection by Rhizobium. The investigators used genotypes varying in GA biosynthesis and signal transduction, combined with GA and GA-biosynthesis inhibitor applications, as well as an ethylene receptor mutation. Extensive measurements include developmental morphology and anatomy, gene expression, hormone levels and nitrogen fixation.
Nodulation at the whole-root level consists of at least two spatially separate programs: infection at the epidermis, and nodule organogenesis originating in the inner cortex. The process commences with the exchange of chemical signals between the epidermal root hair and the Rhizobium bacteria in the soil (Nelson and Sadowsky, 2015; Ibáñez et al., 2017). The perception of compatible rhizobia-produced NOD-factors by the plant host induces physical changes that enable colonization. This includes root hair curling and infection-thread formation, with transmission of the bacteria from cell to cell in a membrane-bounded infection-thread. The bacteria finally take up residence in membrane-bounded vesicles in the cortex, leading to establishment of the nodule through cell division in the inner-cortical cell layers of the root. Nodule development and nitrogen fixation, like many plant processes, are influenced by plant hormones (Ferguson and Mathesius, 2014). Auxin and cytokinin (CK) are involved in nodule initiation, growth, differentiation and positioning. Auxin accumulation at the site of nodule initiation, regulated by auxin transporters in the cell membranes, is crucial to nodule development (Kohlen et al., 2018). Rhizobium infection rapidly induces the up-regulation of several CK biosynthesis genes, leading to CK accumulation and response in the region of the root where nodulation takes place (Gamas et al., 2017). Ethylene is generally considered a negative regulator of nodulation (Guinel, 2015).
The role of gibberellins (GAs) in legume nodulation is controversial. Rather like Janus (the Roman god of duality who is often presented with two opposite-facing faces), GAs are reported to both enhance and inhibit nodulation. The paper by Erin McAdam and colleagues takes another look at the regulation of nodulation in pea by GAs, using an innovative series of GA biosynthesis and signal-transduction mutants (McAdam et al., 2018). Reid’s group has for forty years been a leader in the use of genetics to elucidate the hormonal regulation of growth in peas, especially with regard to GAs, and they were the first to demonstrate that the growth-active GA in peas is GA1 (Reid et al., 2010). One notable advantage of pea is that it is the only legume for which an extensive array of GA-biosynthesis mutants and signal-transduction mutants is available (see Box 1), most generated by Reid’s group. These include mutants with blocks at known points of the GA-biosynthesis pathway, as well as signal- transduction mutants (Weston et al., 2008), so that both the GA levels and the signal-transduction pathway can be manipulated separately or together. This allows the examination of the effects of both GA levels and signalling on the full range of processes from infection through to mature nodules. Notable amongst the GA-biosynthesis mutants is a tiny pea mutant named nana (gene na) with internodes only a few millimetres long; nana has a block in the three-step conversion of ent-kaurenoic acid to GA12 (Reid et al., 2010). The block in the DELLA negative-signal transduction pathway occasioned by the mutant genes la and cry-s results in an ultra-tall, skinny, light-green pea plant nicknamed ‘slender’, regardless of the presence of any mutations in the GA-biosynthesis pathway or the endogenous levels of GAs (DELLA signal-transduction proteins are so-named because they have a conserved N-terminal domain DELLA, i.e. Aspartate-Glutamate-Leucine-Leucine-Alanine). The root growth in na is only about 40% of wild type, yet the root growth of the DELLA mutants is similar to wild type regardless of the presence of na (Silva and Davies, 2007).
Box 1. The role of pea in plant hormone research: a historical perspective
Pea (Pisum sativum) has been central to much hormone research, including that of auxin and GAs, and has been used in bioassays for auxin, gibberellins and ethylene (Yopp et al., 1986). The discovery of auxin was aided by structurally simple oat coleoptiles before work progressed to whole plants such as pea. [A wonderful historical account of initial efforts to determine the chemical nature of auxin has been told by Sam Wildman (1997).] A problem came when auxin applications to intact stems could not replicate the positive effects on elongation obtained with pea stem segments, leading to the erroneous concept that auxin was inactive in the growth of intact stems. It turns out that in a complex tissue applied auxin can have both positive and negative competing effects, determined by its location and concentration; intact stems do respond to exogenous auxin if the auxin is supplied in a continuous low dose, mimicking the natural transport from the stem apex (Yang et al., 1993).
In the case of GAs the early findings derived from spraying whole plants with gibberellic acid (GA3) from fungal cultures (Phinney, 1983). GAs are now known to be a family of over 100 compounds, although most of these are inactive, being part of the biosynthetic or deactivation pathway (Sponsel and Hedden, 2010). An experimental advantage for GAs is that plants that are in any way GA-deficient, including dwarf peas, respond strongly to commercially available GA3, and plants that make their own GAs can be dwarfed by GA-biosynthesis inhibitors such as paclobutrazol. Some initially puzzling results using the slender pea mutant (genotype la cry-s) (Weston et al., 2008) were the first indications of the DELLA proteins, transcriptional regulators that repress GA responses. Mutations in these proteins can produce positive or negative growth effects depending on whether the mutation is located in the regulatory or functional domain (Sun, 2010).
In 1980 Jonathan Goldthwaite suggested to me over a beer that all plant physiologists should concentrate on pea. Of course Arabidopsis was later chosen for its clear advantages, yet pea endures despite its large genome and transformation difficulties. Pea actually has significant advantages: its larger size and cauline structure permits experimental manipulation, and its genetics, starting of course with Gregor Mendel, are well known. Mendel’s tallness gene turned out to encode GA-3β-hydroxylase (oxidase), which is involved in the conversion of inactive GA20 to growth-active GA1 (Lester et al., 1997). Being a legume pea also nodulates in association with Rhizobium bacteria to enable nitrogen fixation; legumes such as pea are a crucial lynchpin of many agricultural systems (Smýkal et al., 2012; Smýkal et al., 2016; see also Considine et al., 2017, introducing the Journal of Experimental Botany special issue ‘Legumes, food security and climate change’). Indeed, when it comes to investigating the role of plant hormones in nodulation, pea has the largest and best characterized range of plant hormone mutants of any legume species. While the pea genome has yet to be fully sequenced (Kulaeva et al., 2017) it is the subject of a sequencing effort (see www.france-genomique.org). Nonetheless the homologue of almost any Arabidopsis gene can now be isolated and characterized in pea, and the use of related genomes such as Medicago have overcome many of the limitations.
In light of such initial confusion in how plant hormones operate it is not surprising that there has been some controversy on the role of hormones in legume nodulation involving the interaction of two different organisms in a complex tissue, including several steps leading to a brand new plant organ encasing a complex mutualistic biochemistry. Pea is again an ideal plant for these investigations.
Gibberellins in nodulation
Previous legume mutant/GA application studies have clearly suggested both a positive and a negative role for GA in nodulation. Pea mutants possessing root systems deficient in GAs exhibited a reduction in nodule organogenesis, and application of GA to the roots of GA-deficient na plants completely restored their number of nodules to that of the wild type (Ferguson et al., 2005). Grafting studies also revealed that a wild-type shoot or root also restored the nodule number of a GA-deficient mutant. The role of GA does not, however, seem straightforward. Double mutants with na and a supernodulating allele still nodulated, but the nodule structures were aberrant; this indicated that severely reduced GA concentrations are not entirely inhibitory to nodule initiation, but that higher GA concentrations are required for proper nodule development. However, constitutive GA signalling mutants (la cry-s), whether with NA or na, also formed fewer nodules than wild-type plants, suggesting that an optimum degree of GA signalling is needed for nodule formation and that the GA signal, rather than the concentration of GA, is important for nodulation (Ferguson et al., 2011). These findings appeared to be in conflict with recent findings in Lotus and Medicago using DELLA mutants and protein studies that suggested only a negative role for GA in nodule formation (Fonouni-Farde et al., 2016; Jin et al., 2016). GA application to wild-type plants in these species also suppressed nodule number. NOD-factor-activated expression of transcription factors and downstream early nodulation genes was suppressed by pre-treatment of wild-type Lotus and Medicago with GA, and also in the absence of GA treatment in Medicago della mutant lines. These studies provided molecular and physical evidence that GA inhibits nodulation events occurring at the epidermis and/or early in the nodulation process such as infection-thread formation.
McAdam et al. (2018) have resolved this paradox using an elegant series of pea GA mutants, with attention to the different stages and locations of the Rhizobium infection and nodule formation processes. It is now evident that GA has an opposing role in different cell layers of the root, suppressing events leading to infection-thread formation in the epidermis, but promoting nodule organogenesis in the inner cortex. In order to visualize the infection process the investigators used lacZ-labelled Rhizobium, which showed a dramatic increase in the number of infection-threads formed in GA-deficient na mutants. Yet despite increased root infection, na mutants often formed no nodules. Another striking feature of na mutants was that a proportion of infection-threads went on to form ramified structures within the root cortex. These highly ramified infection-threads were never associated with cell division or differentiation characteristic of nodules, showing that GA plays an important role in the checkpoint between infection and nodule organogenesis. All these differences in na plants were substantially reversed by the addition of exogenous GA3 to na plants. On the other side of the coin infection-thread formation was reduced in DELLA-deficient la cry-s GA-signalling pea mutants compared with wild-type plants. Moreover the number of infection-threads and ramification structures formed in GA- and DELLA-deficient na la cry-s plants was no different from DELLA-deficient la cry-s mutants, showing that the important factor is the GA-action pathway rather than the level of GA itself.
Nodule organogenesis involves de-differentiation, division, expansion and re-differentiation of inner cortical cells, into which the bacteria from infection-threads enter and ultimately fix nitrogen. The suppression of nodule size in na plants could be mimicked in wild-type peas by the addition of the GA-biosynthesis inhibitor paclobutrazol, and partially rescued in na mutants by addition of GA3. The expression of several key genes was significantly lower in the nodules of na plants, which have small or inactive nodules, compared to wild-type plants. Nitrogen fixation (estimated using the acetylene-reductase assay) was reduced by about 80% in the presence of na, showing that GA is required not only for nodule development but also for nodules to develop into nitrogen-fixing organs. Although the la cry-s (della) mutants produce fewer nodules than wild-type plants, the acetylene reductase rate of these nodules was not significantly different from nodules on wild-type plants, regardless of the presence or absence of na. This also shows that the effect of na on nodule function is entirely mediated through the DELLA proteins.
As ethylene is implicated as a possible intermediate in the action of GA in nodule formation (Foo et al., 2016), McAdam et al. generated plants with a block in GA biosynthesis combined with defective ethylene perception. GA-deficient na mutants produce more ethylene and this appears to contribute at least in part to the low nodule number in this mutant. Disruption of ethylene perception, through the ein2 mutation, elevated nodule number in na ein2 plants to the same extent as that seen in NA plants. The expression of many of the genes was different in na ein2 nodules compared with wild-type or ein2 nodules. It appears that GA suppresses infection-thread formation relatively independently of ethylene, but acts partly through ethylene to suppress the transition from infection-thread to nodule initiation.
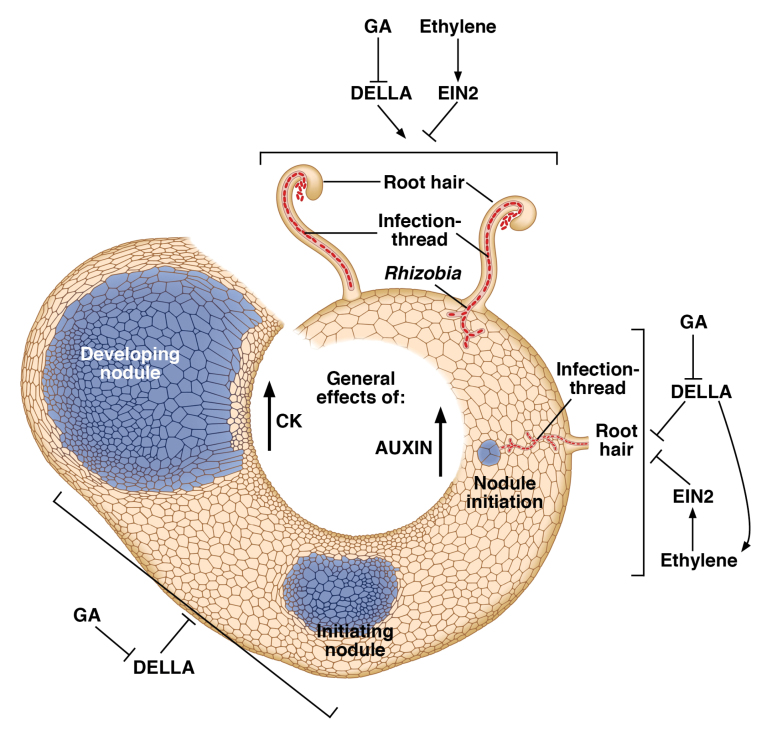
Overall we can conclude that GA, acting through DELLA proteins, suppresses infection-thread formation, but also acts through DELLAs to promote nodule organogenesis and the ultimate function of nodules as nitrogen-fixing organs. A diagrammatic scheme for the action of GA in pea nodulation is shown in Box 2.
Box 2. Stages of nodulation and the roles of GA
A diagrammatic cross section of pea root is shown with decreasing magnification moving clockwise to highlight different stages of nodulation and the roles of GA and other hormones in the process.
Future directions
Researchers now have a sophisticated understanding of the early signalling events triggered by perception of rhizobial signals at the epidermis, and know that much of this pathway overlaps with signalling required for mycorrhizal symbioses (Geurts et al., 2016). However, we know much less about the downstream genes and signals specifically involved in nodule organogenesis, and whether the differences between the different stages of nodulation are regulated by position or functional stage. Indeed GA clearly plays a negative role in mycorrhizal infection (e.g. Foo et al., 2013), indicating GA as a potential differential regulator of the two symbioses in legumes. The results of McAdam et al. highlight several clear areas for future nodulation research. It would be interesting to examine the role of GA in different forms of nodulation (determinate versus indeterminate nodules) and actinorhizal species (non-legumes that form symbioses with N-fixing Frankia bacteria). Indeed, recent studies have shown that the Rhizobia that associate with determinate, but not indeterminate, nodulators themselves synthesize GA (Tatsukami and Ueda, 2016). The connection between GA, auxin and CKs during nodule organogenesis is also worthy of attention, especially given that GA and auxin interact in other processes such as stem elongation (Yang et al., 1996). Future work may explore targets of these three key hormones and potential interactions. Related to this, the nature of the signal(s) that co-ordinates the (initially) spatially separated events of infection at the epidermis and concomitant activation of cell division and differentiation in the inner cortex is still unclear. It is possible that hormones, including GA, play a role in this communication process.
In conclusion I am reminded of a song on photosynthesis from the ‘Biochemists’ Songbook’ [by Harold Baum (1982), to the tune of ‘Auld Lang Syne’ no less; audio link at http://web.csulb.edu/~cohlberg/songbook.html], when it gets to the Calvin cycle: ‘And now occurs a jolly dance’. Clearly the hormonal regulation of nodule formation represents a jolly dance, and this one has two partners!
References
- Considine MJ,Siddique KHM,Foyer CH.. 2017Nature’s pulse power: legumes, food security and climate change.Journal of Experimental Botany 68,1815–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson BJ,Foo E,Ross JJ,Reid JB.. 2011Relationship between gibberellin, ethylene and nodulation in Pisum sativum.New Phytologist 189,829–842. [DOI] [PubMed] [Google Scholar]
- Ferguson BJ,Mathesius U.. 2014Phytohormone regulation of legume-rhizobia interactions.Journal of Chemical Ecology 40,770–790. [DOI] [PubMed] [Google Scholar]
- Ferguson BJ,Ross JJ,Reid JB.. 2005Nodulation phenotypes of gibberellin and brassinosteroid mutants of pea.Plant Physiology 138,2396–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonouni-Farde C,Tan S,Baudin M,Brault M,Wen J,Mysore KS,Niebel A,Frugier F,Diet A.. 2016DELLA-mediated gibberellin signalling regulates Nod factor signalling and rhizobial infection.Nature Communications 7,12636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo E,McAdam EL,Weller JL,Reid JB.. 2016Interactions between ethylene, gibberellins, and brassinosteroids in the development of rhizobial and mycorrhizal symbioses of pea.Journal of Experimental Botany 67,2413–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo E,Ross JJ,Jones WT,Reid JB.. 2013Plant hormones in arbuscular mycorrhizal symbioses: an emerging role for gibberellins.Annals of Botany 111,769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamas P,Brault M,Jardinaud MF,Frugier F.. 2017Cytokinins in symbiotic nodulation: when, where, what for?Trends in Plant Science 22,792–802. [DOI] [PubMed] [Google Scholar]
- Geurts R,Xiao TT,Reinhold-Hurek B.. 2016What does it take to evolve a nitrogen-fixing endosymbiosis?Trends in Plant Science 21,199–208. [DOI] [PubMed] [Google Scholar]
- Guinel FC. 2015Ethylene, a hormone at the center-stage of nodulation.Frontiers in Plant Science 6,1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibáñez F,Wall L,Fabra A.. 2017Starting points in plant-bacteria nitrogen-fixing symbioses: intercellular invasion of the roots.Journal of Experimental Botany 68,1905–1918. [DOI] [PubMed] [Google Scholar]
- Jin Y,Liu H,Luo D et al. . 2016DELLA proteins are common components of symbiotic rhizobial and mycorrhizal signalling pathways.Nature Communications 7,12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlen W,Ng JLP,Deinum EE,Mathesius U.. 2018Auxin transport, metabolism, and signalling during nodule initiation: indeterminate and determinate nodules.Journal of Experimental Botany 69,229–244. [DOI] [PubMed] [Google Scholar]
- Kulaeva OA,Zhernakov AI,Afonin AM,Boikov SS,Sulima AS,Tikhonovich IA,Zhukov VA.. 2017Pea Marker Database (PMD) - A new online database combining known pea (Pisum sativum L.) gene-based markers.PLoS One 12,e0186713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester DR,Ross JJ,Davies PJ,Reid JB.. 1997Mendel’s stem length gene (Le) encodes a gibberellin 3 beta-hydroxylase.The Plant Cell 9,1435–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam EL,Reid JB,Foo E.. 2018Gibberellins promote nodule organogenesis but inhibit the infection stages of nodulation.Journal of Experimental Botany 69,2117–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MS,Sadowsky MJ.. 2015Secretion systems and signal exchange between nitrogen-fixing rhizobia and legumes.Frontiers in Plant Science 6,491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phinney BO. 1983. The history of gibberellins.In:Crozier A, ed.The Biochemistry and physiology of gibberellins,Vol. 1New York:Praeger,15–52. [Google Scholar]
- Reid JB,Symons GM,Ross JJ.. 2010Regulation of gibberellin and brassinosteroid biosynthesis by genetic, environmental and hormonal factors.In:Davies PJ, ed.Plant hormones: biosynthesis, signal transduction, action!Revised 3rd ednDordrecht, The Netherlands:Springer,179–201. [Google Scholar]
- Silva T,Davies PJ.. 2007Elongation rates and endogenous indoleacetic acid levels in roots of pea mutants differing in internode length.Physiologia Plantarum 129,804–812. [Google Scholar]
- Smýkal P,Aubert G,Burstin J et al. . 2012Pea (Pisum sativum L.) in the genomic era.Agronomy 2,74–115. [Google Scholar]
- Smýkal P,K Varshney R,K Singh V,Coyne CJ,Domoney C,Kejnovský E,Warkentin T.. 2016From Mendel’s discovery on pea to today’s plant genetics and breeding: Commemorating the 150th anniversary of the reading of Mendel’s discovery.Theoretical and Applied Genetics 129,2267–2280. [DOI] [PubMed] [Google Scholar]
- Sponsel VM,Hedden P.. 2010Gibberellin biosynthesis and inactivation.In:Davies PJ, ed.Plant hormones: biosynthesis, signal transduction, action!Revised 3rd ednDordrecht, The Netherlands:Springer,63–94. [Google Scholar]
- Sun TP. 2010Gibberellin signal transduction in stem elongation & leaf growth.In:Davies PJ, ed.Plant hormones: biosynthesis, signal transduction, action!Revised 3rd ednDordrecht, The Netherlands:Springer,308–328. [Google Scholar]
- Tatsukami Y,Ueda M.. 2016Rhizobial gibberellin negatively regulates host nodule number.Scientific Reports 6,27998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston DE,Elliott RC,Lester DR,Rameau C,Reid JB,Murfet IC,Ross JJ.. 2008The Pea DELLA proteins LA and CRY are important regulators of gibberellin synthesis and root growth.Plant Physiology 147,199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildman SG. 1997The auxin-A, B enigma: Scientific fraud or scientific ineptitude?Plant Growth Regulation 22,37–68. [Google Scholar]
- Yang T,Davies PJ,Reid JB.. 1996Genetic dissection of the relative roles of auxin and gibberellin in the regulation of stem elongation in intact light-grown peas.Plant Physiology 110,1029–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T,Law DM,Davies PJ.. 1993Magnitude and kinetics of stem elongation induced by exogenous indole-3-acetic acid in intact light-grown pea seedlings.Plant Physiology 102,717–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yopp JH,Aung LH,Steffens GL,eds1986Bioassays and other special techniques for plant hormones and plant growth regulators.Lake Alfred, FL, USA:Plant Growth Regulator Society of America,208. [Google Scholar]