Ethylene-mediated flavonoid biosynthesis is controlled by a GhmiR157–GhSPL10 regulatory module necessary for initial cellular dedifferentiation and callus proliferation during cotton somatic embryogenesis.
Keywords: Callus proliferation, cotton, ethylene, flavonoids, GhmiR157a–GhSPL10, somatic embryogenesis
Abstract
MicroRNAs (miRNAs) modulate many biological processes through inactivation of specific mRNA targets such as those encoding transcription factors. A delicate spatial/temporal balance between specific miRNAs and their targets is central to achieving the appropriate biological outcomes. Somatic embryogenesis in cotton (Gossypium hirsutum), which goes through initial cellular dedifferentiation, callus proliferation, and somatic embryo development, is of great importance for both fundamental research and biotechnological applications. In this study, we characterize the function of the GhmiR157a–GhSPL10 miRNA–transcription factor module during somatic embryogenesis in cotton. We show that overexpression of GhSPL10, a target of GhmiR157a, increases free auxin and ethylene content and expression of associated signaling pathways, activates the flavonoid biosynthesis pathway, and promotes initial cellular dedifferentiation and callus proliferation. Inhibition of expression of the flavonoid synthesis gene F3H in GhSPL10 overexpression lines (35S:rSPL10-7) blocked callus initiation, while exogenous application of several types of flavonol promoted callus proliferation, associated with cell cycle-related gene expression. Inhibition of ethylene synthesis by aminoethoxyvinylglycine treatment in the 35S:rSPL10-7 line severely inhibited callus initiation, while activation of ethylene signaling through 1-aminocyclopropane 1-carboxylic acid treatment, EIN2 overexpression, or inhibition of the ethylene negative regulator CTR1 by RNA interference promoted flavonoid-related gene expression and flavonol accumulation. These results show that an up-regulation of ethylene signaling and the activation of flavonoid biosynthesis in GhSPL10 overexpression lines were associated with initial cellular dedifferentiation and callus proliferation. Our results demonstrate the importance of a GhmiR157a–GhSPL10 gene module in regulating somatic embryogenesis via hormonal and flavonoid pathways.
Introduction
Plant cells exhibit developmental plasticity manifested by diverse tissue or organ regeneration pathways, which can be classified into two main types, de novo organogenesis and somatic embryogenesis (SE) (Ikeuchi et al., 2016).
Ethylene plays an important hormonal role during plant growth and development. The ethylene signaling pathway is initiated from a receptor complex that includes ETHYLENE RESISTANT1 (ETR1), which in the presence of ethylene inactivates the negative regulator CONSTITUTIVE TRIPLE RESPONSE1 (CTR1), leading to ethylene responses via ETHYLENE INSENSITIVE2 (EIN2), EIN3 and ETHYLENE RESPONSE FACTOR1 (ERF1), with the assistance of the F-box proteins EBF1 and EBF2 (Yu and Huang, 2017). Several pieces of evidence implicate ethylene as an endogenous stimulator of cell division. For example, ethylene can stimulate root stem cell division (Ortega-Martínez et al., 2007) and reunion of disconnected stem tissue by stimulating pith cell division in Arabidopsis (Asahina et al., 2011). Ethylene also promotes cambium cell division in Populus during tension wood production (Love et al., 2009).
Ethylene has been found to induce callus formation and plant regeneration in several species. Ethylene promoted callus formation in citrus bud explants in culture (Goren et al. 1979). During apple callus proliferation, endogenous ethylene concentrations rose rapidly before declining (Lieberman et al., 1979). In barley, endogenous ethylene content was closely related to regeneration competency, and application of the ethylene precursor 1-aminocyclopropane 1-carboxylic acid (ACC) promoted regeneration (Jha et al., 2007). In Arabidopsis, inhibition of ethylene responses by Ag2S2SO3 in wild type and ethylene-insensitive mutants etr1-1, ein2-1, ein4, and ein7 reduced the rate of shoot regeneration, while the constitutive ethylene response mutants ctr1-1 and ctr1-12 and the ethylene overproduction mutant eto1 showed increased shoot regeneration (Chatfield and Raizada, 2008). Ethylene improved SE in soybean, whereby overexpression of GmAGL15 promoted embryogenesis by activating expression of ACS, ACO and ERF; and in wild type, 25 µM ACC treatment promoted SE, while 10 µM aminoethoxyvinylglycine (AVG), 100 µM CoCl2, or 10 µM Ag2S2SO3 significantly inhibited SE (Zheng et al., 2013a). Endogenous ethylene content has been found to be positively related to SE competence in soybean (Zheng et al., 2013b).
Flavonoid biosynthesis is a branched secondary metabolic pathway of phenylpropanoid metabolism, and involves the key enzymes chalcone synthase (CHS), chalcone isomerase (CHI), flavanone 3-hydroxylase (F3H), flavonoid 3′-hydroxylase (F3′H), and flavonol synthase (FLS) for flavonol biosynthesis, and dihydroflavonol 4-reductase (DFR), anthocyanidin synthase, and anthocyanidin reductase for anthocyanin biosynthesis (Liu et al., 2015). The flavonoid biosynthesis pathway was up-regulated in a Medicago truncatula line exhibiting a 500-fold increased capacity to regenerate plants by SE compared with a parent line (Imin et al., 2008), suggesting that flavonoids might have a positive effect on plant regeneration. Recently, polyphenol and flavonoid accumulation was demonstrated during callus proliferation in Psyllium, implying that the antioxidant activity of flavonoids might contribute to callus induction and SE (Talukder et al., 2016). Evidence has also been found for the involvement of flavonoids in somatic embryo maturation in cacao (Maximova et al., 2014).
Ethylene and auxin are reported to induce flavonol accumulation through MYB12, which is a positive regulator of flavonol biosynthetic enzyme genes (Lewis et al., 2011). Expression levels of CHS and FLS were down-regulated in the ethylene-insensitive ein2 mutant. The relatively high expression of ethylene- and flavonoid-related genes during somatic embryo maturation in cacao also links ethylene with flavonoid biosynthesis and SE (Maximova et al., 2014).
Auxin homeostasis is also crucial for SE in both Arabidopsis and cotton. In Arabidopsis the transcription factor genes LEAFY COTYLEDON1 (LEC1) and LEC2 are necessary and sufficient for SE, as ectopic expression of either transcription factor promotes SE and lec mutants have defects in SE (Lotan et al., 1998; Stone et al., 2001; Gaj et al., 2005). Activation of the auxin biosynthesis-related YUC genes by LEC2 may regulate auxin supply during SE (Stone et al., 2008). In cotton, endogenous auxin is also elevated in embryonic callus compared with non-embryonic callus (Yang et al., 2012). Disruption of auxin homoestasis by overexpression of GhCK1, which acts downstream of GhLEC1, resulted in defective embryogenesis (Min et al., 2015).
MicroRNAs (miRNAs) are ~20–25 nt non-coding RNAs that are essential components of the gene silencing machinery in most eukaryotic organisms and direct the post-transcriptional silencing of target mRNA or transcriptional silencing through DNA methylation during development (Rogers and Chen, 2013; Achkar et al., 2016). For example, miR156-targeted SQUAMOSA-PROMOTER BINDING PROTEIN-LIKE (SPL) transcription factor RNAs have been demonstrated as key regulators participating in multiple biological processes in plants, such as reproductive phase change, leaf development, tillering and branching, panicle or tassel architecture, fertility and fruit ripening, and responses to biotic and antibiotic stresses (Wang and Wang, 2015). Moreover, miR156-regulated SPL RNAs accumulate with ageing and result in the gradual reduction in shoot regenerative capacity through genetic interaction with the B-type ARABIDOPSIS RESPONSE REGULATORs, and attenuate the cytokinin signaling pathway (Zhang et al., 2015).
The research reported here aims to understand the biological events modulated by GhmiR157a–GhSPL10 interaction during SE in cotton. We show that GhmiR157a and its target GhSPL10 had opposite expression patterns during the embryonic transition stage in the process of SE in cotton, and overexpression of GhSPL10 promotes callus proliferation by activating hormone signaling pathways, particularly ethylene-mediated flavonol biosynthesis.
Materials and methods
Plant material and growth conditions
G. hirsutum cv. YZ1 was used as wild type, and transgenic plants were all in the YZ1 background. Seeds of wild type and transgenic plants were germinated for 5 d on 1/2 Murashige and Skoog (MS) medium under dark, as described previously (Yang et al., 2012). Hypocotyls of etiolated seedlings were sampled at 0 h, or cut into 5–7 mm sections as explants to induce callus on MSB medium for different time points or subculture for embryogenic callus and somatic embryos as described previously (Yang et al., 2012). Five-day-old etiolated seedlings were photographed and hypocotyl lengths were measured.
Plasmid construction and genetic transformation
A GhmiR157a overexpression vector was generated previously (Liu et al., 2017). The full-length coding sequence (stop codon removed, 1059 bp) together with a 29 bp upstream fragment of GhSPL10 (Gh_A12G0866) was cloned from cotton embryo cDNA and ligated to pGEM-T easy vector. From this the GhmiR157a-resistant GhSPL10 (rSPL10) was generated by introducing seven mutations into the predicted GhmiR157a binding site using recombinant PCR. It was cloned into a Gateway entry vector, and subsequently recombined into a C-terminal 6xhistidine fusion vector, pGWB408, which harbors a 35S promoter using Gateway LR clonase II enzyme mix (Invitrogen), for overexpression studies. The full-length coding sequence of EIN2 was cloned from Arabidopsis (AT5G03280) and ligated to pCAMBIUM2300 to generate an overexpression vector. The RNA interference (RNAi) region of GhCTR1 (coding sequence of Gh_D09G1340 from 2162 to 2556 bp, plus a 83 bp fragment of the 3′-untranslated region) was cloned into the RNAi vector pHellsgate4 by recombination. The primers for vector construction described above are listed in Supplementary Table S1 at JXB online. All overexpression and RNAi vectors were introduced into cotton (YZ1) plants by Agrobacterium tumefaciens (strain EHA105)-mediated transformation as previously described (Jin et al., 2006). Segregating wild type plants harboring no transgenes from self-pollinated 35S:rSPL10-7 overexpression line hemizygotes were used as null controls. A F3H RNAi line was generated and termed F3H-Ri3 as previously described (Tan et al., 2013).
ACC, AVG, CoCl2, Ag2S2SO3, indole-3-acetic acid, and flavonoid treatments during callus induction
For ethylene-related treatments during in vitro culture, a final concentration of 10 μM ACC, 5 μM AVG, 100 μM CoCl2, or 10 μM Ag2S2SO3 was added to the MSB medium, and hypocotyl explants of etiolated seedlings grown under standard conditions were cultured for 2 weeks. Callus morphology was monitored and callus proliferation rate (CPR; see below) was calculated. At 3, 6, and 9 h, flavonoid biosynthesis-related gene expression was analysed, and at 1, 3, and 5 d, flavonols were quantified. For the F3H qRT-PCR assay, 25 μM ACC was added to the MSB medium and explants were cultured for 9 h before analysis.
For auxin treatment during in vitro culture, 1 μM indole-3-acetic acid (IAA) was added to the MSB medium, on which hypocotyl explants of normally grown etiolated seedlings were cultured for 2, 4, and 6 d for flavonoid-related gene expression analysis. For flavonoid treatments during in vitro culture, 10 μM dihydroquercetin (DHQ), kaempferol (K), or quercetin (Q) was added to the MSB medium, on which the hypocotyl explants from etiolated seedlings grown under standard conditions were cultured for 1, 2, and 3 weeks for callus morphological observation and CPR calculation, or for 2, 4, and 6 days for cell cycle-related gene expression analysis. MSB medium supplemented with 2.5, 5, or 10 μM Q, or 2.5 μM dihydrokaempferol (DHK), DHQ, K, and Q (abbreviated as DDKQ), was used for explant culture and CPR calculation post 2 weeks.
All chemicals were filter sterilized, and medium with no supplemental chemicals was defined as mock.
Southern blotting, qRT-PCR, RT-PCR, and RNA ligase-mediated rapid amplification of 5′-cDNA ends
Total DNA was extracted using the Plant Genomic DNA Kit (Tiangen, China). Southern blotting was performed following enzyme digestion of genomic DNA, electrophoresis and hybridization. The PCR fragments of NPTII were used as a probe. The detailed methods were reported previously (Li et al., 2010).
Total RNA was extracted using a modified guanidine thiocyanate method (Zhu et al., 2005) or the RNAprep Pure Plant Kit (Tiangen, China). Reverse transcription of RNA for miRNA qRT-PCR was performed using stem–loop primers as described (Varkonyi-Gasic et al., 2007). qRT-PCR and RT-PCR were performed as described previously (Jin et al., 2014). Relative expression levels of genes were determined by 2−ΔCt with UBQ7 as endogenous reference (Tu et al., 2007). Rapid amplification of 5′-cDNA ends (5′-RACE) of GhSPL10 was performed using a GeneRacer kit (Invitrogen). Briefly, RNA fragments were ligated to RNA adapters and transcribed using the GeneRacer Oligo(dT) primer. Nested PCR amplifications were performed with 5′ adaptor primers and 3′ gene-specific primers according to the manufacturer’s instructions. Finally, the PCR products were cloned to the pGEM-T easy vector, and 12 positive E. coli clones were sequenced. All primers used are listed in Supplementary Table S1.
Callus proliferation rate calculation and histocytological analysis
CPR was calculated as the fold change of the weight gained by explants, i.e. change in weight per unit time divided by the initial weight of the explants.
Excised hypocotyls were cultured on MSB medium for 5 d, and somatic embryos were fixed in 50% FAA [10% formalin, 5% acetic acid, and 50% ethanol (v/v)], dehydrated, cleared, and embedded as described previously (Jackson, 1991). Histological sections of 10 µm thickness were stained with toluidine blue (0.05%) and mounted in DPX resin (Sigma-Aldrich). For whole mount in situ hybridization, a locked nucleic acid probe with both ends with the digoxigenin modification was used as a negative control (scramble-miR, 5′-GTGTAACACGTCTATACGCCCA-3′, Exiqon), and 10 pmol probe was used for each slide. Probes for GhSPL10 were generated by amplifying the 403 bp upstream, the 372 bp middle, and the 436 bp downstream fragments calculated from the start codon of GhSPL10 transcript, ligated to pGEM-T vector (Promega) for sequencing to select the plasmids possessing the target sequences in sense orientation. Linearized plasmids from the end of T7 promoter were used as templates for the antisense probe synthesis using SP6 polymerase (Roche). Then the probes were hydrolysed in 200 mM carbonate buffer (pH 10.2) to be about 150 bp and mixed together for hybridization. Whole mount in situ hybridization was performed as described (Rosen and Beddington, 1993). Hybridization and washing steps were performed at 55 °C. Sections were observed by light microscopy. Primers used for probe amplification are listed in Supplementary Table S1.
RNA sequencing and data analysis
Total RNA was isolated from hypocotyls of 5-day-old seedlings of null and 35S:rSPL10-7 lines growing under dark on 1/2 MS medium, at ~29 °C using a modified guanidine thiocyanate method (Zhu et al., 2005), using two biological replicates. RNA libraries were constructed and sequenced with an Illumina HiSeqTM 2000 at the Beijing Genomics Institute (BGI, Shenzhen, China). High throughput data processing and determination of differentially expressed genes was as described (Liu et al., 2017).
Quantification of endogenous IAA and ethylene content
For measurement of IAA content, ~0.2 g hypocotyl sections of 4-day-old etiolated seedlings were ground in liquid nitrogen and homogenized in 750 μl of 80% (v/v) methanol containing 10 ng ml−1 [2H5]IAA (OIChemlm Ltd, CAS: 76937-78-5) as internal standard and then shaken at 4 °C overnight. The supernatant was collected and another 450 μl of 80% methanol was added to the pellet for another 3 h shaking. The supernatant was twice extracted, mixed, and evaporated, and redissolved in 300 μl 50% (v/v) methanol and then filtered through nylon membranes with 0.22 μm aperture. The quantification of endogenous IAA was performed as described previously (Liu et al., 2012).
For ethylene quantification, about five seeds germinated for 24 h in the dark on 1/2 MS medium were collected and sealed in a 12 ml vial with an air-tight cap at room temperature in the dark for 12 h; subsequently 1 ml gas was sampled from the vial by an air-tight syringe (Agilent) and injected into a gas chromatograph (7890A-5975C, Agilent). The standard curve was created with standard concentrations of ethylene: 1, 2, 3, and 5 ul/L, with the equation y=12.51x−0.914; r2=0.998.
Flavonol measurements
Around 0.2 g hypocotyls or explants of tested lines, cultured for specific time periods, were ground in liquid nitrogen, and flavonols were extracted in 1 ml of 80% methanol and shaken at 4 °C overnight. The supernatant was evaporated and redissolved in 300 μl 50% methanol, which was further filtered through a 0.22 μm nylon membrane. The quantification of flavonols was as previously described (Tan et al., 2013).
Statistical analysis
All statistical analysis was based on at least two biological and three technical replicates, and the significance was determined by multiple comparisons using Statistix software (version 8.0).
Results
GhSPL10 phylogeny and expression pattern during cotton SE
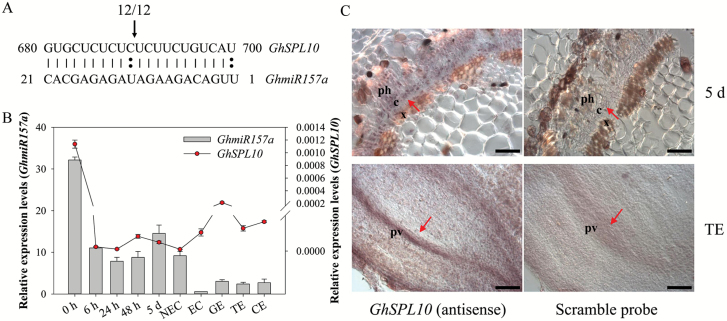
GhmiR156 and -157 and their target, GhSPL9, were hypothesized to be involved in SE, based on previous expression data (Yang et al., 2013). AtSPL10 and AtSPL11 are involved in zygotic embryogenesis in Arabidopsis (Nodine and Bartel, 2010), and we identified four other cotton homologues (Gh_A12G0866, Gh_D12G0947, Gh_A11G0706, and Gh_D11G0821) that exhibit strong similarity to AtSPL10/11; all harbor GhmiR157a target sites (Supplementary Fig. S1). We previously confirmed the cleavage sites of one homologue (Gh_A12G0866) by 5′-RACE (Yang et al., 2013), and found that all tested clones displayed cleavage between 11 and 12 nucleotide along the GhmiR157a strand (Fig. 1A). This gene hereafter was referred to as GhSPL10.
Fig. 1.
5′-RACE verification of GhmiR157a-guided cleavage site on GhSPL10 mRNA and expression patterns of GhmiR157a and GhSPL10 during cotton SE. (A) Cleavage site from the 5′ end for GhSPL10 by GhmiR157a. The top strand depicts the mRNA fragment of GhSPL10 complementary with GhmiR157a, and the bottom strand depicts GhmiR157a nucleotide sequence. Watson–Crick pairing (vertical dashes) and mismatches (double circles) are indicated. Cleavage site is indicated by the arrow, and the proportion of cloned 5′-RACE products corresponding to the cleavage site is indicated above the arrow. (B) qRT-PCR of GhmiR157a and GhSPL10 during cotton SE. The left y-axis refers to the relative expression levels of GhmiR157a shown as grey bars, and the right y-axis refers to the relative expression levels of GhSPL10 shown as red circles. Relative expression values are normalized to UBQ7. Values represent the mean and standard error (n=3). (C) Whole mount in situ localization of GhSPL10 transcripts in explants cultured for 5 d (top) and torpedo-stage embryo (bottom) detected with GhSPL10 antisense probe (left) or scramble DNA probe (right) as negative control. The red arrows indicate the cambium cells in 5 d explants (top) and provascular tissue in torpedo-stage embryo (bottom). Scale bars=50 µm. c: cambium cells; ph: phloem cells; pv: provascular tissue; x: xylem cells. (This figure is available in color at JXB online.)
Since GhmiR157a is the most abundant member of miR156 family (Yang et al., 2013), we investigated the expression patterns of GhmiR157a and GhSPL10 by qRT-PCR using RNA isolated from samples representing different stages of cotton SE. GhmiR157a and GhSPL10 were dynamically expressed during cotton SE (Fig. 1B). Both were expressed at relatively high levels in hypocotyls, and decreased sharply during dedifferentiation and embryogenesis. Interestingly, GhmiR157a and GhSPL10 exhibited inverse expression pattern in the embryonic transition stage from non-embryonic callus to embryonic callus. GhmiR157a showed low transcript levels during embryo formation while GhSPL10 expression was stably low. Whole mount in situ hybridization was performed to determine the tissue specificity of GhSPL10 expression; it was detected in vascular cambium of hypocotyls induced for 5 d and provascular cells of torpedo-stage embryos (Fig. 1C).
Overexpression of GhSPL10 promotes initial cellular dedifferentiation and callus proliferation during cotton SE
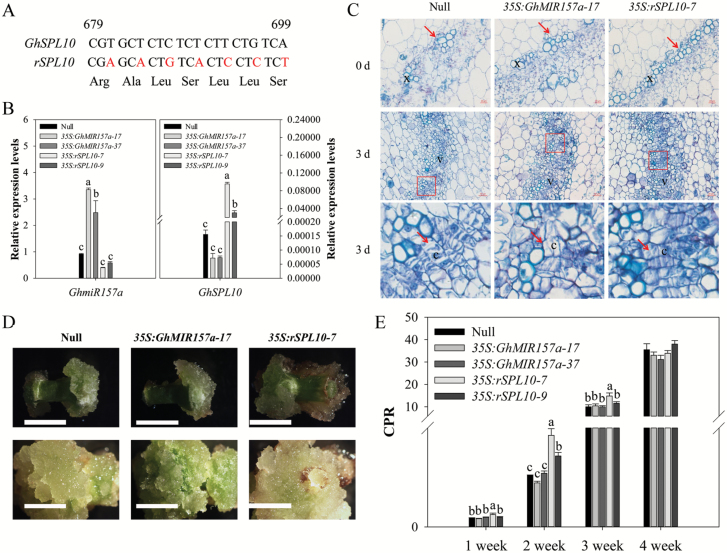
To determine whether GhmiR157a and GhSPL10 are involved in the regulation of cotton SE, a GhmiR157a overexpression vector was constructed as previously described (Liu et al., 2017). A GhmiR157a-resistant GhSPL10 overexpression vector also was constructed, with seven synonymous codon mutations at the GhmiR157a targeting site (Fig. 2A). Both vectors were transformed to G. hirsutum YZ1. In total, two GhmiR157a overexpression lines (GhMIR157a-17 and GhMIR157a-37), two GhSPL10 overexpression lines (35S:rSPL10-7 and 35S:rSPL10-9), and a null line (a negative plant line isolated from GhSPL10 overexpression lines) were selected for further analysis (Supplementary Fig. S2A, B).
Fig. 2.
Phenotype characterizations of GhmiR157a and GhSPL10 overexpression lines during cell dedifferentiation. (A) Schematic representation of GhmiR157a-resistant GhSPL10 (rSPL10) vector constructed by synonymous mutation. The top strand depicts the original GhmiR157a target site of GhSPL10 from 679 to 699 nt calculated from the start codon, and the bottom strand shows the sequence post-mutation; bases in red indicate the replaced nucleotides. (B) qRT-PCR of GhmiR157a and GhSPL10 in hypocotyls of null, GhmiR157a, and GhSPL10 overexpression lines. Bars represent means and standard error of three biological replicates (n=3). (C) Toluidine-blue stained transverse sections of explants in null, 35S:GhMIR157a-17, and 35S:rSPL10-7 lines before culture (0 d, upper panels) and cultured for 3 d (middle and lower panels). Red arrows in the upper panels indicate xylem cells; red rectangles indicate the magnified cambium cells shown in the bottom panel indicated by the red arrows. Scale bars=20 µm for 0 d, 50 µm for 3 d. c: cambium cells; v: vascular tissue; x: xylem cells. (D) Non-embryonic callus induction phenotype in null, 35S:GhMIR157a-17, and 35S:rSPL10-7 lines cultured for 2 (upper panels) and 4 weeks (lower panels). Scale bars=1 cm. (E) CPR in null, GhmiR157a, and GhSPL10 overexpression lines cultured for 1, 2, 3, and 4 weeks. Bars represent means and standard error of five biological replicates (n=5). Different lower-case letters in (B, E) denote statistical differences of pairwise comparisons among each group of bars according to LSD test (P<0.05). (This figure is available in color at JXB online.)
qRT-PCR was performed using RNA extracted from etiolated hypocotyls of 4-day-old seedlings, to test the expression of GhmiR157a and GhSPL10 in transgenic plants (Fig. 2B). The results show that a weak reduction in the abundance of GhSPL10 transcripts was observed in GhmiR157a overexpression lines, while high GhSPL10 expression levels were observed with no alteration of GhmiR157a expression in GhSPL10 overexpression lines.
The growth of hypocotyls was significantly retarded in GhSPL10 overexpression lines, which were 1–2 cm shorter (8.38 ± 0.21 cm for 35S:rSPL10-7 and 8.99 ± 0.23 cm for 35S:rSPL10-9) than null plants (9.99 ± 0.22 cm). In contrast, longer hypocotyls were observed in GhmiR157a overexpression lines, which were about 12.14 ± 0.18 cm for line 17 and 10.52 ± 0.18 cm for line 37 (Supplementary Fig. S2C, D). The concentration of endogenous IAA was measured in the different lines by HPLC-MS, and the results showed that free IAA concentration increased in the hypocotyls of 35S:rSPL10-7, while the most prevalent IAA conjugate, IAA–Asp, decreased, implying that the IAA signaling pathway might be altered in GhSPL10 overexpression lines. No obvious change in IAA content was observed in 35S:GhMIR157a-17 (Supplementary Fig. S2E). The production of the gaseous hormone ethylene was also found to increase significantly in 35S:rSPL10-7, and to decrease in 35S:GhMIR157a-17, compared with nulls (Supplementary Fig. S2E).
To determine the effects of overexpressing GhmiR157a and GhSPL10 during cotton SE, calluses were induced for each transgenic line on MS medium, as described previously (Yang et al., 2012). All transgenic explants showed little morphological differences in comparison with null explants cultured for 3 d. However, histological observation showed more and continuous xylem cells in the vascular system of hypocotyls in 35S:rSPL10-7 at 0 d, and even more xylem cells and more cambial cell division at 3 d, while no obvious difference was observed in vascular tissues between 35S:GhmiR157a-17 and null lines at either 0 d or 3 d (Fig. 2C).
Callus proliferation rate (CPR) was calculated among the transgenic explants during the induction period. It showed that overexpression of GhSPL10 promoted callus proliferation at both ends of the hypocotyls during callus induction (Fig. 2D), with increasing CPR in the first 2 weeks, and thereafter a similar CPR compared with other lines (Fig. 2E). No significant change in CPR was observed in GhmiR157a overexpression lines compared with null at all tested time points. The CPR was higher in line 35S:rSPL10-7 compared with 35S:rSPL10-9 during the first 3 weeks post-induction, consistent with the higher expression level of GhSPL10 in 35S:rSPL10-7 than in 35S:rSPL10-9 (Fig. 2B, E). Therefore, overexpression of GhSPL10 accelerated callus proliferation through promoting cell division of cambium cells while GhmiR157a overexpression led to no obvious effects on callus formation.
Flavonoid biosynthesis and hormonal signaling pathways induced by GhSPL10 overexpression
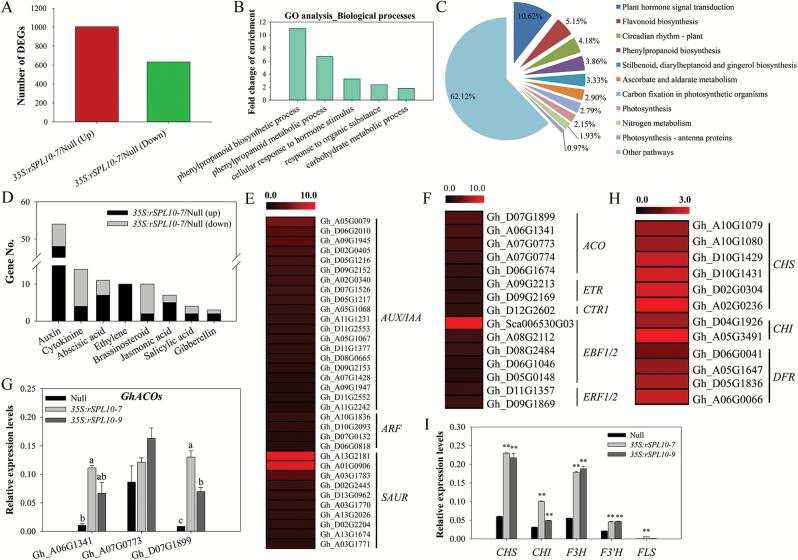
To explore how overexpressing GhSPL10 regulates SE in cotton, we performed genome-wide transcript profiling of hypocotyl explants of a null line and the 35S:rSPL10-7 line. After removing adaptor contaminations and low quality tags, a total of ~12 million clean reads were generated from each library, and about 87% of the reads were mapped to cotton TM-1 genome sequences, with an ~70% gene map rate, and more than 50 000 gene transcripts were identified (Supplementary Table S2). After normalizing raw reads of each gene as fragments per kilobase of transcript per million mapped reads (FPKM), differentially expressed genes (DEGs) were identified by calculating the log2 value of the fold change comparing the FPKM of each gene in the transgenic and null line, with a threshold of 1 and probability of 0.8 of combining two biological replicates. The correlations of gene expression levels between two biological repeats were more than 0.97, indicating high uniformity between repeats (Supplementary Fig. S3A).
Compared with the null control, overexpression of GhSPL10 in 35S:rSPL10-7 resulted in 1005 up-regulated genes and 633 down-regulated genes (Fig. 3A). We identified six up-regulated SPLs, other than GhSPL10 (Supplementary Fig. S3B), among which Gh_D12G1504 also had GhmiR157a targeting sites while the others did not (Supplementary Fig. S3C). Gene ontology (GO) analysis of differentially expressed genes revealed that genes associated with phenylpropanoid biosynthesis and cellular response to hormone stimulus were most enriched (Fig. 3B). Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis further confirmed that plant hormone signaling transduction-related genes and flavonoid biosynthesis-related genes accounted for 10.62% and 5.15%, respectively, of the 932 DEGs with pathway ID (Fig. 3C). Auxin and ethylene signaling-related genes in particular were activated in GhSPL10 overexpression lines, with the ethylene-signaling pathway genes ETR1, CTR1, EBF1/2, and ERF1/2, five ACO biosynthetic genes, and the auxin signal pathway ARF and SAUR genes also up-regulated in the 35S:rSPL10-7 compared with the null line (Fig. 3D–F). qRT-PCR confirmed that 2 ACO genes Gh_A06G1341 and Gh_D07G1899 were induced by GhSPL10 overexpression (Fig. 3G).
Fig. 3.
Overview of RNA sequencing data using hypocotyls of null and 35S:rSPL10-7 lines, two replicates for each line. (A) Statistics of differentially expressed gene (DEG) number between 35S:rSPL10-7 and control. The red bar represents number of up-regulated genes; the green bar represents the number of down-regulated genes. (B) GO enrichment analyses were conducted using WEGO software (Ye et al., 2006), including molecular function (MF), biological process (BP), and cellular component (CC). Enrichment fold changes of genes with different BP terms were used to plot the histogram. Pearson’s chi-square test was applied to indicate significant relationships of the enrichment of each GO term, with the P value <0.05. (C) Proportions of DEGs among all DEGs with KEGG annotation, which include 10 significantly enriched KEGG pathways (Q value <0.05). (D) Number of DEGs involved in diverse hormone signaling pathways. (E) Heat map of auxin-responsive DEGs shown as log2 (35S:rSPL10-7/Null) values. (F) Heat map of DEGs involved in ethylene biosynthesis and signaling pathway, shown as log2 (35S:rSPL10-7/Null) values. (G) qRT-PCR of ACOs in hypocotyls of null and GhSPL10 overexpression lines. Bars represent means and standard error of three biological replicates (n=3). Different lower-case letters in each group denote statistical differences of pairwise comparisons among the three lines according to LSD test (P<0.05). (H) Heat map of flavonoid biosynthesis-related genes shown as log2 (35S:rSPL10-7/Null) values. (I) qRT-PCR of flavonol biosynthesis-related genes in null and GhSPL10 overexpression lines. Bars represented the mean and standard error of two biological replicates and three technical replicates, and statistical significance was determined by multiple comparison; **P<0.01. (This figure is available in color at JXB online.)
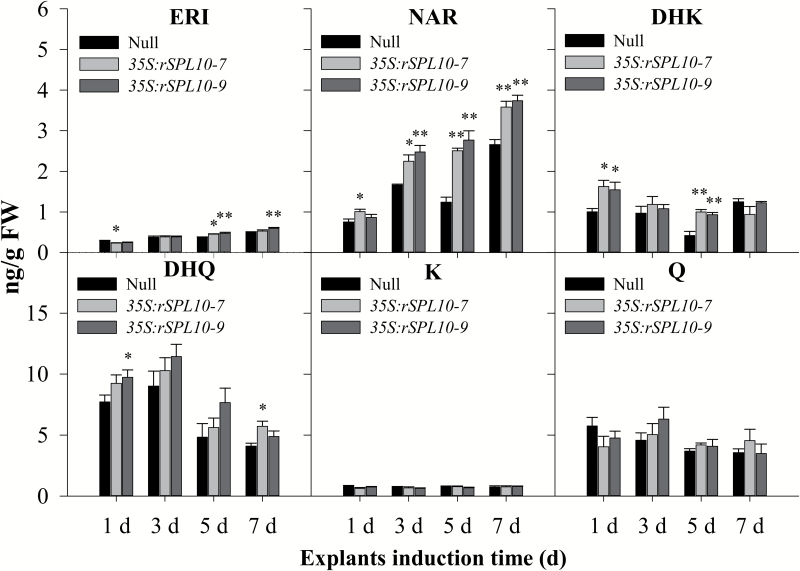
Transcripts representing CHS, CHI, and DFR were activated in GhSPL10 overexpression lines (Fig. 3H), and up-regulation of the flavonoid synthesis genes CHS, CHI, F3H, F3′H, and FLS was validated by qRT-PCR in GhSPL10 overexpression lines (Fig. 3I). Flavonol assays confirmed that the contents of eriodictyol (ERI), naringenin (NAR), dihydrokaempferol (DHK), and dihydroquercetin (DHQ) were increased in GhSPL10 overexpression lines compared with null, but no difference was observed for kaempferol (K) and quercetin (Q) (Fig. 4).
Fig. 4.
Flavonol accumulation in explants of null and GhSPL10 overexpression lines. Quantification of ERI, NAR, DHK, DHQ, K, and Q in explants of null and GhSPL10 overexpression lines cultured for 1, 3, 5, and 7 d. Bars are mean and standard error (n=4). Statistical significance was determined by multiple comparison with null as control; *P<0.05, **P<0.01.
Flavonoids promote initial cellular dedifferentiation and early cell division in cultured explants
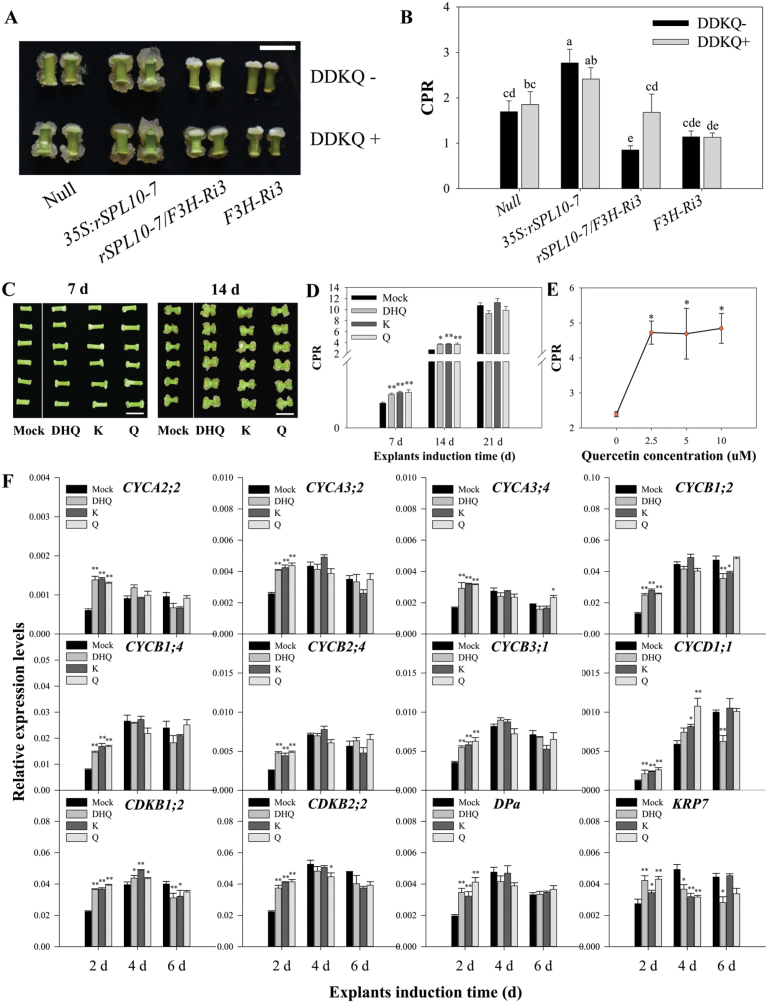
Since the flavonoid biosynthesis pathway was up-regulated by GhSPL10 overexpression, we explored the link between flavonoids and the phenotypes observed in GhSPL10 overexpression lines. To this end, we constructed a F1 generation hybrid line, rSPL10-7/F3H-Ri3, using 35S:rSPL10-7 as female parent, pollinated with pollen from an F3H-Ri3 RNAi line that was previously shown to accumulate reduced levels of flavonols, including DHK, DHQ, K, and Q (Tan et al., 2013). The hypocotyl length of the etiolated seedlings of rSPL10-7/F3H-Ri3 was 9.23 ± 0.20 cm, significantly longer than the hypocotyls of the 35S:rSPL10-7 line (6.75 ± 0.21 cm; Supplementary Fig. S4A, B), and similar to the wild type length (9.94 ± 0.20 cm). Inhibition of F3H activity in 35S:rSPL10-7 resulted in severely reduced callus proliferation, which could be reversed to wild type levels by the in vitro application of DDKQ (2.5 µM DHK, DHQ, K and Q) (Fig. 5A, B). These results indicate that flavonoid overaccumulation contributes to the seedling and callus phenotypes in GhSPL10 overexpression lines.
Fig. 5.
Increased flavonol accumulation in GhSPL10 overexpression lines contributes to the higher callus proliferation rates. Callus phenotype (A) and CPR (B) of null, 35S:rSPL10-7, rSPL10-7/F3H-Ri3, and F3H-Ri3 lines with explants cultured on MSB medium supplemented without or with DDKQ (2.5 μM DHK, DHQ, K, and Q). Scale bar=1 cm. Bars represent means and standard error of four biological replicates (n=4). Different lower-case letters denote statistical differences of pairwise comparisons among all the lines and both treatments according to LSD test (P<0.05). (C) Callus phenotype of YZ1 explants cultured on MSB medium supplemented without (mock) or with 10 μM DHQ, K, or Q for 7 d (left) and 14 d (right). Scale bars=1 cm. (D) CPR of YZ1 explants cultured on MSB medium supplemented without (mock) or with 10 μM DHQ, K, or Q for 7, 14, and 21 d. Bars are mean and standard error (n=3–7). (E) CPR of YZ1 explants cultured on MSB medium supplemented with 0, 2.5, 5, and 10 μM quercetin for 2 weeks. Bars are mean and standard error (n=3). (F) qRT-PCR of cell cycle-related genes in YZ1 explants cultured on MSB medium supplemented without (mock) or with 10 μM DHQ, K, or Q for 2, 4, and 6 d. Bars are mean and standard error (n=3). Statistical significance in (D–F) was determined by multiple comparison with null as control; *P<0.05, **P<0.01. (This figure is available in color at JXB online.)
To test the effect of flavonols on callus induction, we treated YZ1 explants with 10 μM DHQ, K, or Q. The results show that all tested flavonols could promote callus proliferation and increase CPR at both 1 and 2 weeks (Fig. 5C, D). Further analysis using gradient concentrations of Q on MSB medium indicated that 2.5 μM Q is enough to promote callus induction, while higher levels of Q did not promote callus induction further (Fig. 5E).
The expression of cell cycle-related genes was analysed in explants cultured on MSB medium supplemented with 10 μM DHQ, K, or Q for 2, 4, and 6 days (Fig. 5F). The relative expression levels of all A, B, and D types of CYCLIN genes, together with cyclin dependent kinases (CDKs) and DPa, were activated by DHQ, K, and Q in explants cultured for 2 d, with either no effect or an inhibitory effect observed at 4 and 6 d. These results indicated that some flavonols can promote cell division during callus initiation, by up-regulating the transcription of cell cycle-related genes, and suggest that activated flavonol biosynthesis positively contributes to the enhanced callus phenotype in GhSPL10 overexpression lines.
Activation of ethylene signaling accelerates initial cellular dedifferentiation and mediates flavonoid biosynthesis during cotton SE
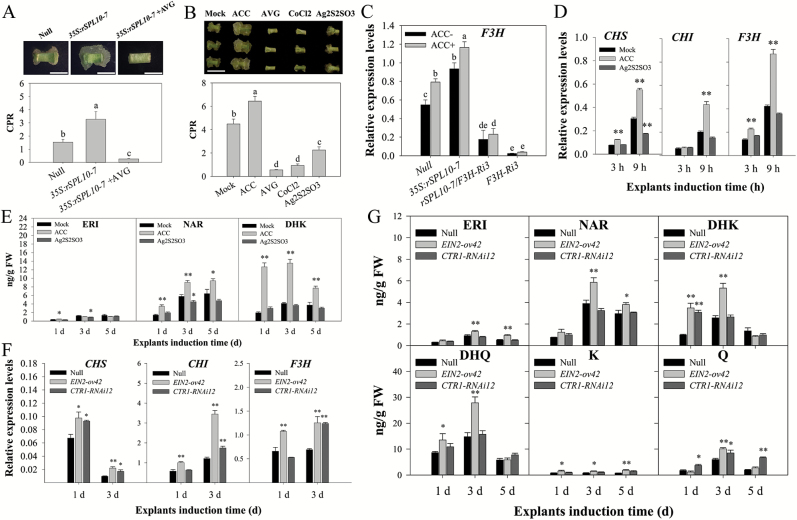
Since ethylene content and signaling were increased in GhSPL10 overexpression lines, we tested whether this was related to the GhSPL10 overexpression phenotype during SE. Inhibition of ethylene biosynthesis by AVG (an ACS inhibitor) treatment for 2 weeks during callus growth completely inhibited callus initiation in 35S:rSPL10-7, seen as a very low CPR (Fig. 6A). This indicates that the up-regulation of ethylene biosynthesis and signaling mediates the rapid initiation of dedifferentiation and callus proliferation following GhSPL10 overexpression.
Fig. 6.
Ethylene-mediated flavonoid biosynthesis linked to callus phenotype in 35S:rSPL10-7 overexpression lines. (A) AVG treatment reversed callus phenotype (upper panel) and CPR (lower panel) in 35S:rSPL10-7. Explants were cultured on MSB medium supplemented with or without 10 μM AVG. Scale bars=1 cm. (B) Callus phenotype (upper panel) and CPR (lower panel) for YZ1 explants treated with 10 μM ACC, 5 μM AVG, 100 μM CoCl2, or 10 μM Ag2S2SO3. Scale bar=1 cm. (C) qRT-PCR of F3H in explants cultured for 9 h on MSB medium supplemented with or without 25 μM ACC. (D) qRT-PCR of CHS, CHI and F3H in YZ1 explants cultured on MSB medium supplemented with 10 μM ACC or 10 μM Ag2S2SO3 for 3 and 9 h. (E) Flavonol quantification in YZ1 explants cultured on MSB medium supplemented with 10 μM ACC or 10 μM Ag2S2SO3 for 1, 3, and 5 d. (F) qRT-PCR of CHS, CHI, and F3H in explants of EIN2-ov42 or CTR1-RNAi12 cultured for 1 and 3 d. (G) Flavonol quantification in EIN2-ov42 and CTR1-RNAi12 explants cultured for 1, 3, and 5 d. Bars in (A, B, E, G) represent means and standard error of four biological replicates (n=4); Bars in (C, F) represent means and standard error of three biological replicates (n=3). Bars in (D) represent mean and standard error of two biological and three technical replicates. In (A–C) different lower-case letters on each bar graph denote statistical differences of pairwise comparisons among all bars according to LSD test (P<0.05). In (D–G), *P<0.05, **P<0.01. (This figure is available in color at JXB online.)
The role of ethylene in callus proliferation was further tested in wild type explants by ACC treatment or inhibition of ethylene biosynthesis by AVG, CoCl2 (an ACO inhibitor), or Ag2S2SO3 (an inhibitor of ethylene signaling response at the receptor). It was found that ACC treatment promoted callus proliferation, while AVG, CoCl2, or Ag2S2SO3 inhibited callus initiation and proliferation (Fig. 6B).
Treatment of 9-h-cultured explants of null, 35S:rSPL10-7, rSPL10-7/F3H-Ri3, and F3H-Ri3 lines without or with 25 μM ACC treatment showed that ACC promotes F3H expression in both null and 35S:rSPL10-7 lines, but no significant enhancement of F3H expression levels was seen in rSPL10-7/F3H-Ri3 and F3H-Ri3 lines, presumably due to the RNAi effects on F3H (Fig. 6C). Expression levels of flavonoid biosynthesis-related genes were analysed by qRT-PCR in hypocotyls cultured for 3 and 9 h on MSB medium supplemented with ACC or Ag2S2SO3. The results show that all early flavonoid biosynthesis-related genes were up-regulated by ACC treatment, but little effect was seen when explants were treated with the ethylene response inhibitor (Fig. 6D). ACC treatment significantly increased ERI, NAR, and DHK production in callus cultured for 1, 3, and 5 d, while Ag2S2SO3 treatment had negligible or no significant effect on flavonol production (Fig. 6E). Enhancement of ethylene signaling using an EIN2 overexpression line (EIN2-ov42) and a CTR1 RNAi line (CTR1-RNAi12) (Supplementary Fig. S4C, D) led to enhanced transcription of flavonoid biosynthesis-related genes CHS, CHI, and F3H (Fig. 6F) and, variously, enhanced accumulation of flavonols (Fig. 6G). These results confirm the ability of the ethylene signaling pathway to activate flavonol biosynthesis.
Discussion
Complicated relationship of miR157-SPLs in cotton
The cleavage site of GhSPL10 was unambiguously verified to be between nucleotides 11 and 12 in this study (Fig. 1), because the 5′-RACE cDNA samples we used here were exactly the samples reported previously (Yang et al., 2013). Although the canonical cleavage site was between 10 and 11, one- or two-nucleotide shifts were also detected previously in cotton (Liu et al., 2017). Besides, this phenomenon is also frequently identified through degradome sequencing, though it cannot always be detected by 5′-RACE (Yang et al., 2013). Thus, multiple recognition mechanisms might exist in different tissues or developmental stages.
A positive feedback loop between miR156 and SPL9/10 was reported previously (Wu et al., 2009), whereby elevated expression of the miR156a precursor was seen in AtSPL9- and AtSPL10-overexpressing transgenic Arabidopsis plants. However we show in the current study that overexpression of GhSPL10 does not alter the expression of GhmiR157a; on the contrary, GhSPL10 triggers the up-regulation of one GhmiR157a-targeted SPL gene (Gh_D12G1504) and several non-targeted SPLs (Supplementary Fig. S3B, C). This phenomenon, revealed by RNA sequencing, needs further experimental validation, and more evidence is needed to illustrate the regulation mechanisms in the future.
Flavonoids might provide antioxidant protection to modulate cell division during callus proliferation
Previous studies have shown that flavonoids can inhibit CDK activity in human cancer cells in the nanomolar concentration range and structural studies show that CDK2 can form complexes with flavonoids (De Azevedo et al., 1996; Kaur et al., 1992). Flavonoids extracted from various plants may be useful as human cancer therapies by promoting apoptosis and cell cycle arrest (Chen and Chen, 2013; Yasuda et al., 2017). While in plants flavonoids are well known for their ecological and developmental functions, including pigmentation, defense against pathogens, signaling, and auxin transport regulation (Winkel-Shirley, 2001), their potential role in cell cycle regulation and differentiation is less obvious.
In this study, we show that overexpression of the transcription factor gene GhSPL10 induces flavonoid biosynthesis during callus induction and cell dedifferentiation, associated with improved activity of cambium cell division. Exogenous flavonols (DHQ, K, and Q) also led to both higher CPR and higher expression levels of cell cycle-related genes compared with controls, all of which indicated a positive effect of some flavonols on cell division, which is in contrast to the roles of flavonoids in animals. However, we only tested the effect of three flavonols on cell dedifferentiation, and others may have different effects. Vigorous callus proliferation in GhSPL10 overexpression lines is likely to be a consequence of the accumulation of one or more flavonols, and effects on cell signaling, including ethylene and auxin responses (see below). Longer exposure to high concentrations of DHQ, K, or Q may have a negative impact on cell cycle-related gene expression, consistent with the effect of such flavonols on callus induction, since higher concentrations of Q could not further promote callus induction. It is well documented that excessive accumulation of phenolics including flavonoids is correlated with tissue browning and necrosis during in vitro callus culture (Michael and John, 1986; Lee et al., 1990; Dubravina et al., 2005), which is due to the formation of quinones that inhibit callus growth in chickpea (Iqbal et al., 1991), date palm (Daayf et al., 2003), and cotton (Ozyigit et al., 2007). Hence, flavonoids may stimulate cell division in a dose- or developmental stage-dependent manner during callus proliferation, which is associated with the modulation of cell cycle-related genes (Kosugi and Ohashi, 2003; Anzola et al., 2010).
Both auxin and ethylene signaling are modulated by GhSPL10 during cotton SE
Multiple hormonal signaling pathways were changed by GhSPL10 overexpression (Fig. 3), and both auxin and ethylene signaling pathways were up-regulated, implying a regulatory role for GhSPL10 in hormonal control during cotton SE. We also found an enrichment of genes associated with circadian rhythms (Fig. 3), which themselves control multiple hormonal signaling pathways (Atamian and Harmer, 2016). It is therefore possible that photoperiod-related regulators mediate the effects of GhSPL10 during cotton SE, but this requires further work.
Activation of flavonoid biosynthesis by ethylene and auxin
Studies in Arabidopsis have demonstrated that SPL9 and SPL15 inhibit anthocyanin biosynthesis by competitively binding to PRODUCTION OF ANTHOCYANIN PIGMENTS1 (PAP1), which is normally bound by TRANSPARENT TESTA8 (TT8), leading to the destabilization of the MYB–BHLH–WD40 complex that activates anthocyanin biosynthesis (Gou et al., 2011). These authors also found that miR156 overexpression leads to down-regulation of CHS and CHI genes, while overexpression of MIM156 promotes their expression. It is, however, unknown whether SPL genes directly regulate flavonol biosynthesis-related gene expression. In this study we showed that GhSPL10 overexpression promotes ethylene responses, and mediates flavonol biosynthesis, suggesting that GhSPL10 might influence flavonoid biosynthesis indirectly through hormone signaling. Consistent with this, GhSPL10 overexpression increased the conversion of auxin from the conjugated form to the free form, and several flavonoid biosynthesis-related genes were up-regulated by IAA treatment during the cell dedifferentiation stage at 2–6 d of culture (Supplementary Fig. S5). Evidence also showed that ethylene and auxin regulate flavonol biosynthesis through TIR1 and EIN2/ETR1 Lewis et al. (2011).
The evidence that the inhibition of ethylene biosynthesis by AVG treatment inhibited callus initiation in GhSPL10-overexpressing explants, and ACC treatment increased expression of flavonoid biosynthesis-related genes in wild type in very early stages of culture (3 and 9 h) (Fig. 6) further supports the importance of ethylene signaling in callus initiation. It is also possible that ethylene signaling might contribute to callus initiation and proliferation through pathways other than flavonoid biosynthesis, because inhibition of ethylene by AVG showed far more severe callus inhibition than inhibition of flavonoid biosynthesis in 35S:rSPL10-7 (Figs 5 and 6).
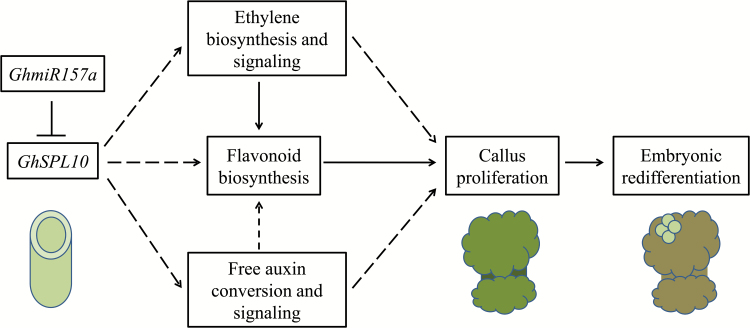
In summary, we show that overexpression of GhSPL10 activates ethylene, auxin and flavonoid pathways to regulate cell division during callus proliferation in cotton (Fig. 7).
Fig. 7.
Model for the role of GhmiR157a–GhSPL10 during cotton SE. During callus induction from hypocotyl explants, GhmiR157a negatively regulates GhSPL10 and promotes callus induction by inducing ethylene and auxin responses, which further activate flavonoid biosynthesis to promote callus proliferation. Flavonoid accumulation in callus might also provide an antioxidant environment to facilitate embryonic fate acquisition and embryonic redifferentiation. T-shapes represent inhibition; arrows and dashed arrows indicate confirmed and probable promotion effects, respectively. The cylinder-shape represents hypocotyl explant, the dumb-bell-shapes represent explants with callus at both ends, and the circles on the callus represent regenerated globular embryos. (This figure is available in color at JXB online.)
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Phylogeny of SPL proteins based on alignment of 42 SPL protein sequences in Gossypium hirsutum TM-1 and 15 SPLs in Arabidopsis.
Fig. S2. Molecular and seedling phenotype characterization of GhmiR157a and GhSPL10 overexpression lines.
Fig. S3. Correlation analysis and identified differentially expressed SPLs by RNA-SEQ.
Fig. S4. Hypocotyl phenotype restoration in 35S:rSPL10-7 by inhibiting F3H activity, and transcription detection in AtEIN2 overexpression and CTR1 RNAi cotton plants.
Fig. S5. IAA treatment promotes flavonoid biosynthesis-related gene expression.
Table S1. List of primers used in this study.
Table S2. Summary statistics of sequencing and mapping.
Acknowledgements
We thank Jiafu Tan for providing the F3H-Ri3 transgenic cotton seeds; Hongbo Liu (National Key Laboratory of Crop Genetic Improvement, Huazhong Agricultural University) for his technical assistance with the determination of flavonol content; Hongyan Zhang (Key Laboratory of Horticultural Plant Biology, Huazhong Agricultural University) for technical support in ethylene quantification, and Professor David Jackson from Cold Spring Harbor Laboratory, USA for the guidance on whole mount in situ hybridization. Funding by the Fundamental Research Funds for the Central Universities (2662014PY028) and Hubei Chenguang Talented Youth Development Foundation is greatly appreciated. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Achkar NP, Cambiagno DA, Manavella PA. 2016. miRNA biogenesis: a dynamic pathway. Trends in Plant Science 21, 1034–1044. [DOI] [PubMed] [Google Scholar]
- Anzola JM, Sieberer T, Ortbauer M, Butt H, Korbei B, Weinhofer I, Mullner AE, Luschnig C. 2010. Putative Arabidopsis transcriptional adaptor protein (PROPORZ1) is required to modulate histone acetylation in response to auxin. Proceedings of the National Academy of Sciences, USA 107, 10308–10313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahina M, Azuma K, Pitaksaringkarn W et al. 2011. Spatially selective hormonal control of RAP2.6L and ANAC071 transcription factors involved in tissue reunion in Arabidopsis. Proceedings of the National Academy of Sciences, USA 108, 16128–16132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atamian HS, Harmer SL. 2016. Circadian regulation of hormone signaling and plant physiology. Plant Molecular Biology 91, 691–702. [DOI] [PubMed] [Google Scholar]
- Chatfield SP, Raizada MN. 2008. Ethylene and shoot regeneration: hookless1 modulates de novo shoot organogenesis in Arabidopsis thaliana. Plant Cell Reports 27, 655–666. [DOI] [PubMed] [Google Scholar]
- Chen AY, Chen YC. 2013. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chemistry 138, 2099–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daayf F, El Bellaj M, El Hassni M, J’Aiti F, El Hadrami I. 2003. Elicitation of soluble phenolics in date palm (Phoenix dactylifera) callus by Fusarium oxysporum f. sp albedinis culture medium. Environmental and Experimental Botany 49, 41–47. [Google Scholar]
- De Azevedo WF Jr, Mueller-Dieckmann HJ, Schulze-Gahmen U, Worland PJ, Sausville E, Kim SH. 1996. Structural basis for specificity and potency of a flavonoid inhibitor of human CDK2, a cell cycle kinase. Proceedings of the National Academy of Sciences, USA 93, 2735–2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubravina GA, Zaytseva SM, Zagoskina NV. 2005. Changes in formation and localization of phenolic compounds in the tissues of European and Canadian yew during dedifferentiation in vitro. Russian Journal of Plant Physiology 52, 672–678. [Google Scholar]
- Gaj MD, Zhang S, Harada JJ, Lemaux PG. 2005. Leafy cotyledon genes are essential for induction of somatic embryogenesis of Arabidopsis. Planta 222, 977–988. [DOI] [PubMed] [Google Scholar]
- Goren R, Altman A, Giladi I. 1979. Role of ethylene in abscisic acid-induced callus formation in citrus bud cultures. Plant Physiology 63, 280–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gou JY, Felippes FF, Liu CJ, Weigel D, Wang JW. 2011. Negative regulation of anthocyanin biosynthesis in Arabidopsis by a miR156-targeted SPL transcription factor. The Plant Cell 23, 1512–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeuchi M, Ogawa Y, Iwase A, Sugimoto K. 2016. Plant regeneration: cellular origins and molecular mechanisms. Development 143, 1442–1451. [DOI] [PubMed] [Google Scholar]
- Imin N, Goffard N, Nizamidin M, Rolfe BG. 2008. Genome-wide transcriptional analysis of super-embryogenic Medicago truncatula explant cultures. BMC Plant Biology 8, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal J, Naz S, Nazir S, Aftab F, Ahmed MS. 1991. Total phenolics, phenylalanine ammonialyase and polyphenol oxidases in in vitro calli of chick pea. Pakistan Journal of Botany 23, 227–235. [Google Scholar]
- Jackson DP. 1991. In situ hybridisation in plants. In: Bowles DJ, Gurr SJ, McPherson M, eds. Molecular plant pathology: A practical approach. Oxford: Oxford University Press, 163–174. [Google Scholar]
- Jha AK, Dahleen LS, Suttle JC. 2007. Ethylene influences green plant regeneration from barley callus. Plant Cell Reports 26, 285–290. [DOI] [PubMed] [Google Scholar]
- Jin F, Hu L, Yuan D, Xu J, Gao W, He L, Yang X, Zhang X. 2014. Comparative transcriptome analysis between somatic embryos (SEs) and zygotic embryos in cotton: evidence for stress response functions in SE development. Plant Biotechnology Journal 12, 161–173. [DOI] [PubMed] [Google Scholar]
- Jin S, Zhang X, Nie Y, Guo X, Liang S, Zhu H. 2006. Identification of a novel elite genotype for in vitro culture and genetic transformation of cotton. Biologia Plantarum 50, 519–524. [Google Scholar]
- Kaur G, Stetler-Stevenson M, Sebers S, Worland P, Sedlacek H, Myers C, Czech J, Naik R, Sausville E. 1992. Growth inhibition with reversible cell cycle arrest of carcinoma cells by flavone L86-8275. Journal of the National Cancer Institute 84, 1736–1740. [DOI] [PubMed] [Google Scholar]
- Kosugi S, Ohashi Y. 2003. Constitutive E2F expression in tobacco plants exhibits altered cell cycle control and morphological change in a cell type-specific manner. Plant Physiology 132, 2012–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CY, Kegan V, Jaworski AW, Brows SK. 1990. Enzymatic browning in relation to phenolic compounds and polyphenol oxidase activity among various peach cultivars. Journal of Agricultural and Food Chemistry 38, 99–101. [Google Scholar]
- Lewis DR, Ramirez MV, Miller ND, Vallabhaneni P, Ray WK, Helm RF, Winkel BS, Muday GK. 2011. Auxin and ethylene induce flavonol accumulation through distinct transcriptional networks. Plant Physiology 156, 144–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YJ, Liu DQ, Tu LL, Zhang XL, Wang L, Zhu LF, Tan J, Deng F. 2010. Suppression of GhAGP4 gene expression repressed the initiation and elongation of cotton fiber. Plant Cell Reports 29, 193–202. [DOI] [PubMed] [Google Scholar]
- Lieberman M, Wang SY, Owens LD. 1979. Ethylene production by callus and suspension cells from cortex tissue of postclimacteric apples. Plant Physiology 63, 811–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Li X, Xiao J, Wang S. 2012. A convenient method for simultaneous quantification of multiple phytohormones and metabolites: application in study of rice-bacterium interaction. Plant Methods 8, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Osbourn A, Ma P. 2015. MYB transcription factors as regulators of phenylpropanoid metabolism in plants. Molecular Plant 8, 689–708. [DOI] [PubMed] [Google Scholar]
- Liu N, Tu L, Wang L, Hu H, Xu J, Zhang X. 2017. MicroRNA 157-targeted SPL genes regulate floral organ size and ovule production in cotton. BMC Plant Biology 17, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotan T, Ohto M, Yee KM, West MA, Lo R, Kwong RW, Yamagishi K, Fischer RL, Goldberg RB, Harada JJ. 1998. Arabidopsis LEAFY COTYLEDON1 is sufficient to induce embryo development in vegetative cells. Cell 93, 1195–1205. [DOI] [PubMed] [Google Scholar]
- Love J, Bjorklund S, Vahala J, Hertzberg M, Kangasjarvi J, Sundberg B. 2009. Ethylene is an endogenous stimulator of cell division in the cambial meristem of Populus. Proceedings of the National Academy of Sciences, USA 106, 5984–5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximova SN, Florez S, Shen X, Niemenak N, Zhang Y, Curtis W, Guiltinan MJ. 2014. Genome-wide analysis reveals divergent patterns of gene expression during zygotic and somatic embryo maturation of Theobroma cacao L., the chocolate tree. BMC Plant Biology 14, 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael EC, John EP. 1986. Exudation and explants establishment. IAPTC Newsletter 50, 9–18. [Google Scholar]
- Min L, Hu Q, Li Y, Xu J, Ma Y, Zhu L, Yang X, Zhang X. 2015. LEAFY COTYLEDON1-CASEIN KINASE I-TCP15-PHYTOCHROME INTERACTING FACTOR4 network regulates somatic embryogenesis by regulating auxin homeostasis. Plant Physiology 169, 2805–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nodine MD, Bartel DP. 2010. MicroRNAs prevent precocious gene expression and enable pattern formation during plant embryogenesis. Genes & Development 24, 2678–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Martínez O, Pernas M, Carol RJ, Dolan L. 2007. Ethylene modulates stem cell division in the Arabidopsis thaliana root. Science 317, 507–510. [DOI] [PubMed] [Google Scholar]
- Ozyigit II, Kahraman MV, Ercan O. 2007. Relation between explant age, total phenols and regeneration response in tissue cultured cotton (Gossypium hirsutum L.). African Journal of Biotechnology 6, 3–8. [Google Scholar]
- Rogers K, Chen X. 2013. Biogenesis, turnover, and mode of action of plant microRNAs. The Plant Cell 25, 2383–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen B, Beddington RS. 1993. Whole-mount in situ hybridization in the mouse embryo: gene expression in three dimensions. Trends in Genetics 9, 162–167. [DOI] [PubMed] [Google Scholar]
- Stone SL, Braybrook SA, Paula SL, Kwong LW, Meuser J, Pelletier J, Hsieh TF, Fischer RL, Goldberg RB, Harada JJ. 2008. Arabidopsis LEAFY COTYLEDON2 induces maturation traits and auxin activity: Implications for somatic embryogenesis. Proceedings of the National Academy of Sciences, USA 105, 3151–3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone SL, Kwong LW, Yee KM, Pelletier J, Lepiniec L, Fischer RL, Goldberg RB, Harada JJ. 2001. LEAFY COTYLEDON2 encodes a B3 domain transcription factor that induces embryo development. Proceedings of the National Academy of Sciences, USA 98, 11806–11811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talukder P, Talapatra S, Ghoshal N, Sen Raychaudhuri S. 2016. Antioxidant activity and high-performance liquid chromatographic analysis of phenolic compounds during in vitro callus culture of Plantago ovata Forsk. and effect of exogenous additives on accumulation of phenolic compounds. Journal of the Science of Food and Agriculture 96, 232–244. [DOI] [PubMed] [Google Scholar]
- Tan J, Tu L, Deng F, Hu H, Nie Y, Zhang X. 2013. A genetic and metabolic analysis revealed that cotton fiber cell development was retarded by flavonoid naringenin. Plant Physiology 162, 86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu L, Zhang X, Liu D, Jin S, Cao J, Zhu L, Deng F, Tan J, Zhang C. 2007. Suitable internal control genes for qRT-PCR normalization in cotton fiber development and somatic embryogenesis. Chinese Science Bulletin 52, 3110–3117. [Google Scholar]
- Varkonyi-Gasic E, Wu R, Wood M, Walton EF, Hellens RP. 2007. Protocol: a highly sensitive RT-PCR method for detection and quantification of microRNAs. Plant Methods 3, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wang H. 2015. The miR156/SPL module, a regulatory hub and versatile toolbox, gears up crops for enhanced agronomic traits. Molecular Plant 8, 677–688. [DOI] [PubMed] [Google Scholar]
- Winkel-Shirley B. 2001. It takes a garden. How work on diverse plant species has contributed to an understanding of flavonoid metabolism. Plant Physiology 127, 1399–1404. [PMC free article] [PubMed] [Google Scholar]
- Wu G, Park MY, Conway SR, Wang JW, Weigel D, Poethig RS. 2009. The sequential action of miR156 and miR172 regulates developmental timing in Arabidopsis. Cell 138, 750–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Wang L, Yuan D, Lindsey K, Zhang X. 2013. Small RNA and degradome sequencing reveal complex miRNA regulation during cotton somatic embryogenesis. Journal of Experimental Botany 64, 1521–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Zhang X, Yuan D, Jin F, Zhang Y, Xu J. 2012. Transcript profiling reveals complex auxin signalling pathway and transcription regulation involved in dedifferentiation and redifferentiation during somatic embryogenesis in cotton. BMC Plant Biology 12, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda MT, Sakakibara H, Shimoi K. 2017. Estrogen- and stress-induced DNA damage in breast cancer and chemoprevention with dietary flavonoid. Genes and Environment 39, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Fang L, Zheng H et al. 2006. WEGO: a web tool for plotting GO annotations. Nucleic Acids Research 34, W293–W297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Huang R. 2017. Integration of ethylene and light signaling affects hypocotyl growth in Arabidopsis. Frontiers in Plant Science 8, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang TQ, Lian H, Tang H et al. 2015. An intrinsic microRNA timer regulates progressive decline in shoot regenerative capacity in plants. The Plant Cell 27, 349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q, Zheng Y, Perry SE. 2013a AGAMOUS-Like15 promotes somatic embryogenesis in Arabidopsis and soybean in part by the control of ethylene biosynthesis and response. Plant Physiology 161, 2113–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q, Zheng Y, Perry SE. 2013b Decreased GmAGL15 expression and reduced ethylene synthesis may contribute to reduced somatic embryogenesis in a poorly embryogenic cultivar of Glycine max. Plant Signaling & Behavior 8, e25422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu LF, Tu LL, Zeng FC, Liu DQ, Zhang XL. 2005. An improved simple protocol for isolation of high quality RNA from Gossypium spp. suitable for cDNA library construction. Acta Agronomica Sinica 31, 1657–1659. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.