Abstract
Background
Inflammation is a hallmark of chronic kidney disease (CKD) and stimulates glomerular expression of vascular adhesion molecules (VCAMs). We investigated in a general population whether estimated glomerular filtration rate (eGFR) is associated with circulating adhesion molecules, inflammation markers or both.
Methods
We measured serum levels of five adhesion molecules [VCAM-1, intracellular adhesion molecule-1 (ICAM-1), P-selectin, E-selectin and monocyte chemoattractant protein-1 (MCP-1)] and seven inflammation markers [C-reactive protein (CRP), neutrophil gelatinase-associated lipocalin (NGAL), tumour necrosis factor receptor 1 (TNF-R1), TNF-α, interleukin 6 (IL-6), IL-8 and vascular endothelial growth factor] in 1338 randomly recruited people (50.8% women, mean age 51.7 years, eGFR 79.9 mL/min/1.73 m2).
Results
In multivariable-adjusted analyses, eGFR decreased (P ≤ 0.004) with higher VCAM-1 (association size expressed in mL/min/1.73 m2 for a doubling of the marker, −2.99), MCP-1 (−1.19), NGAL (−1.19), TNF receptor 1 (−2.78), TNF-α (−2.28) and IL-6 (−0.94). The odds ratios of having eGFR <60 versus ≥60 mL/min/1.73 m2 (n = 138 versus 1200) were significant (P ≤ 0.001) for VCAM-1 (1.77), MCP-1 (1.32), NGAL (1.26), TNF-R1 (1.49), TNF-α (1.45) and IL-6 (1.20). Compared with 24-h albuminuria, VCAM-1 increased (P <0.0001) the area under the curve from 0.57 to 0.65, MCP-1 to 0.67 and TNF-R1 to 0.79, but TNF-R1 outperformed both adhesion molecules (P < 0.0001).
Conclusions
In a general population, eGFR is inversely associated with circulating adhesion molecules VCAM-1 and MCP-1 and several inflammation markers, but inflammation markers, in particular TNF-R1 and TNF-α, identify patients with eGFR <60 mL/min/1.73 m2 more accurately.
Keywords: adhesion molecules, eGFR, inflammation, population science, renal function
INTRODUCTION
The Global Burden of Disease Study 2010 estimated that 0.40 million of nearly 50 million deaths occurring annually worldwide, were attributable to chronic kidney disease (CKD) in 1990 and 0.74 million in 2010, representing an increase of 82.3% [1]. CKD is therefore a major health problem affecting the quality of life of millions of people and draining health care resources [1–3]. The discovery of biomarkers that allow screening for asymptomatic renal disease or predict a decline in the estimated glomerular filtration rate (eGFR) is mainstream in current CKD research with the goal to curtail the epidemic [1, 2].
Inflammation is a hallmark of deteriorating renal function [4–6]. In activated glomerular endothelial cells, inflammation induces expression of adhesion molecules, which in turn add to the renal injury [7]. Vascular adhesion molecule-1 (VCAM-1) is constitutively expressed in Bowman’s capsule and in proximal tubules [8–10]. Inflammation stimuli, such as tumour necrosis factor α (TNF-α) and advanced glycation products enhance its expression [8–10]. Likewise, in glomeruli of diabetic mice, monocyte chemoattractant protein-1 (MCP-1) is upregulated and enhances disruption of glomerular membranes, thereby leading to albuminuria [11, 12]. Few human studies have addressed the question of whether adhesion molecules are associated with glomerular dysfunction or predict its decline. These studies mainly enrolled patients with hypertension, diabetes mellitus [6], vascular disease [13] or glomerulonephritis [14] and involved measurement of a relatively limited number of biomarkers, often including C-reactive protein (CRP). Building on experimental studies [8–12] and research in patients [6, 13, 14], we analyzed the database of the Flemish Study on Environment, Genes and Health Outcomes (FLEMENGHO) [15, 16]. Our objective was to investigate in cross-sectional and longitudinal analyses whether glomerular dysfunction is differentially associated with circulating adhesion molecules, inflammation markers or both.
MATERIALS AND METHODS
Study population
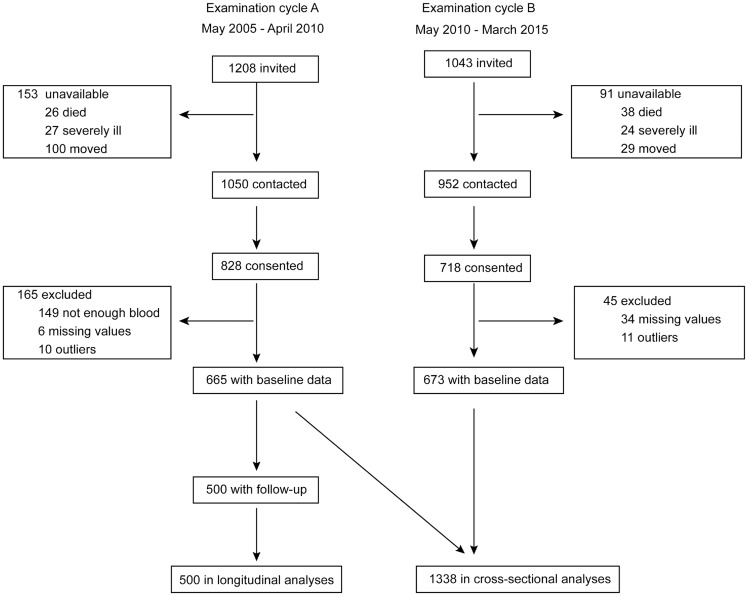
FLEMENGHO complies with the Helsinki Declaration [17] for research in human subjects. The Ethics Committee of the University of Leuven approved the study [18]. Recruitment started in 1985 and continued until 2004. FLEMENGHO participants represent a family-based population sample randomly selected from a geographically defined area in Northern Belgium [15, 16]. The initial participation rate was 78.0%. The participants were repeatedly followed up at the field centre in the catchment area (North Limburg, Belgium). From 2005 to 2010 (examination cycle A) and from 2010 to 2014 (examination cycle B), we mailed an invitation letter to 1208 and 1043 former participants, respectively, for a follow-up examination ( Figure 1), including an assessment of renal function. However, of the individuals invited for examination cycles A and B, 153 and 91 were unavailable because they had died (n = 26 and 38), had been institutionalized or were too ill (n = 27 and 24) or had moved out of the area or did not respond (n = 100 and 29). Of the remaining 1050 and 952 former participants, 828 and 718 renewed informed consent (participation rates 78.5% and 75.4%, respectively). Of examination cycle A and B participants, we excluded 165 and 45, because the biobank of serum samples was exhausted so that the circulating biomarkers could not be measured (n = 149 and 0) or because the circulating biomarkers were either missing (n = 6 and 34) or exceeded the mean by ≥3 standard deviations (SDs) (n = 10 and 11). The number of examination cycle A (n = 665) and B (n = 673) participants available for the cross-sectional analyses therefore totalled 1338. Of 665 examination cycle A participants, 500 took part in a follow-up assessment of their renal function and were included in the longitudinal analyses. At each contact participants renewed their informed written consent.
FIGURE 1.
Flowchart of participants included in the cross-sectional and longitudinal analyses.
Assessment of renal function
We measured the concentration of creatinine in serum using Jaffe’s method [19] with modifications described elsewhere [20, 21] on automated analysers in a single certified laboratory that applied isotope-dilution mass spectrometry for calibration of the serum creatinine measurements. We derived eGFR from serum creatinine by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) [22] equation. We staged eGFR according to the National Kidney Foundation [Kidney Disease Outcomes Quality Initiative (KDOQI)] guideline [23] as eGFR ≥90, 60–89, 45–59, 30–44, 15–29 and <15 mL/min/1.73 m2 for Stages 1, 2, 3, 4 and 5, respectively. Participants collected a timed 24-h urine sample for the measurement of albumin. Micro- and macro-albuminuria were 24-h excretions ranging from 30 to 300 mg or >300 mg, respectively. Guideline-based staging of CKD [23] requires repeat measurement of eGFR or albuminuria or additional evidence for renal disease. However, as this is impracticable in the context of population studies due to multiple visits comprising the participation rate, staging of CKD in our current study, as done in landmark epidemiological research [24, 25], relies on a single serum sample to determine eGFR and a single urine sample collection to determine albuminuria at baseline and follow-up.
Serum biomarkers
The Evidence Investigator system (Randox, Belfast, UK) uses Biochip Array Technology to detect several analytes from a single sample. For this study, the following arrays were employed Adhesion [VCAM-1, intracellular adhesion molecule-1 (ICAM-1), P-selectin, E-selectin], Cerebral II (CRP, NGAL, TNF-R1) and Cytokine High Sensitivity [interleukin 6 (IL-6), IL-8, vascular endothelial growth factor (VEGF), MCP-1, TNF-α] utilizing the sandwich assay format [26–29]. Prior to analysis, serum samples were defrosted and preserved on ice. A single serum aliquot was provided for all arrays. Therefore all panels were commenced within 1 h of defrosting to minimize degradation. Concentrations of individual analytes were determined by Biochip Array Technology according to the manufacturer’s instructions (Adhesion Molecule Array EV3519, Cerebral Array II EV3637 and Cytokine and Growth Factors Array (High Sensitivity) EV3623]. The Supplementary data gives detailed information on the processing of the samples.
Other measurements
At baseline, nurses administered a questionnaire to collect detailed information on each participant’s medical history, smoking and drinking habits and intake of medications. The conventional blood pressure was the average of five consecutive auscultatory readings obtained with the subject in a seated position. Mean arterial pressure was diastolic blood pressure plus one-third of the difference between systolic and diastolic blood pressure. Hypertension was a blood pressure of at least 140 mmHg systolic or 90 mmHg diastolic or use of antihypertensive drugs. Body mass index was weight in kilograms divided by the square of height in metres. A venous blood sample was obtained after the participants had been fasting for 6–8 h for measurement of plasma glucose, serum total and high-density lipoprotein (HDL) cholesterol and serum γ-glutamyltransferase as an index of alcohol intake. Diabetes mellitus was a self-reported diagnosis, a fasting glucose level of at least 126 mg/dL or the use of antidiabetic agents [30].
Statistical analyses
For database management and statistical analysis we used SAS version 9.4 (SAS Institute, Cary, NC, USA). Means were compared using the large-sample z-test and proportions by Fisher’s exact test. We normalized the distributions of γ-glutamyltransferase, 24-h microalbuminuria and all circulating biomarkers by a logarithmic transformation. We identified baseline covariables to be retained in the analyses by a stepwise regression procedure with P-values for covariables to enter and stay in the models set at 0.15. We considered as potential covariables mean arterial pressure, body mass index, waist:hip ratio, smoking, plasma glucose, serum γ-glutamyltransferase, the total:HDL cholesterol ratio, 24-h microalbuminuria and the use of diuretics, inhibitors of the renin–angiotensin system [β-blockers, angiotensin-converting enzyme (ACE) inhibitors and angiotensin type 1 receptor blockers), vasodilators (calcium channel blockers and α-blockers) and lipid-lowering drugs (fibrates and statins). Using principal component analysis, we also combined adhesion molecules and inflammation markers of interest into a single normally distributed variable.
In continuous analyses, we standardized eGFR to the average in the whole study population (mean or ratio) of the covariables measured at baseline as identified by stepwise regression. We did not adjust eGFR for sex and age, because these variables are already included in the CKD-EPI formula [22]. Using linear regression, standardized eGFR at baseline (n = 1338) and standardized eGFR at follow-up (n = 500) were regressed on the biomarkers measured at baseline. Models with eGFR at follow-up, as a dependent variable, were additionally adjusted for follow-up duration. The relative risks of having eGFR <60 versus ≥60 mL/min/1.73 m2 at baseline (n = 138 of 1338) or to experience over follow-up a decline in eGFR from ≥60 to <60 mL/min/1.73 m2 (n = 55 of 500) were modelled in relation to the biomarkers and covariables measured at baseline using logistic or Cox regression, respectively. These models accounted for the same baseline covariables as mentioned earlier. The results of these cross-sectional and longitudinal analyses of continuous (eGFR) and categorical (eGFR stage) outcomes were summarized in −log10 probability plots. Finally, we evaluated the potential of 24-h albuminuria and circulating VCAM-1, MCP-1 and TNF-R1, as measured at baseline to discriminate between participants whose eGFR declined from ≥60 mL/min/1.73 m2 at baseline to <60 mL/min/1.73 m2 at follow-up by constructing receiver operating characteristic (ROC) curves and by calculating the area under the curve (AUC). The 95% confidence interval (CI) of the AUC was calculated by the DeLong method.
RESULTS
Characteristics of participants
Participant age averaged 51.7 years (range 15–90) and the proportion of women was 50.8%. Of 1338 participants, 426 (31.8%) had hypertension, of whom 345 (25.8%) were on antihypertensive drug treatment, 222 (16.6%) were taking lipid-lowering drugs and 55 (4.1%) had diabetes (Supplementary data, Table S1). Among 345 patients on antihypertensive drug treatment, 127 (36.8%) took diuretics, 277 (80.3%) inhibitors of the renin system, 89 (25.8%) vasodilators and 153 (44.3%) were on combination therapy with more than one drug class. Of 222 patients on lipid-lowering treatment, 13 (5.9%) took fibrates and 210 (94.6%) used statins. Compared with examination cycle A participants (Supplementary data, Table S1), people examined in examination cycle B were 3.9 years older (49.7 versus 53.6 years) and therefore had a higher waist:hip ratio (0.87 versus 0.89), elevated systolic/diastolic blood pressure (128.1/79.6 versus 132.7/82.0 mmHg) and a higher prevalence of hypertension (25.5% versus 38.6%), treated hypertension (22.9% versus 27.9%) and use of lipid-lowering drugs (14.0% versus 19.0%) but lower glomerular filtration rate (eGFR 81.1 versus 78.8 mL/min/1.73 m2) and smoking prevalence (20.0 versus 13.5%).
Tables 1 and 2 summarize the characteristics of participants and the circulating markers of eGFR by quartiles, respectively. Supplementary data, Table S2 lists the characteristics of participants by quartiles of the distribution of VCAM-1, the adhesion molecule reported in several experimental studies [8–10]. Age, body mass index, the waist:hip ratio, the proportion of participants on antihypertensive drug treatment, serum creatinine and total cholesterol and plasma glucose increased (0.062 ≤ P ≤0.001) with a higher category of VCAM-1, whereas the proportion of women (P = 0.019) and eGFR (P = 0.051) decreased. The serum levels of ICAM-1, P-selectin, TNF-R1, TNF-α and IL-6 increased (0.0018 ≤ P ≤ 0.087) across quartiles of the VCAM-1 distribution (Supplementary data, Table S3).
Table 1.
Characteristics of 1338 participants by quartiles of eGFR
| Characteristic | Low | Medium low | Medium high | High | P-value |
|---|---|---|---|---|---|
| Limits (mL/min/1.73 m2) | <69.89 | 69.89–79.73 | 79.73–90.77 | ≥90.77 | |
| Participants in category, n | 335 | 334 | 334 | 334 | |
| Women, n (%) | 200 (59.7) | 172 (51.5)* | 176 (52.5) | 132 (39.5)‡ | 0.08 |
| Smokers, n (%) | 34 (10.2) | 50 (15.0) | 58 (17.3)* | 81 (24.3)§ | 0.02 |
| Hypertension, n (%) | 180 (53.7) | 107 (32.0)§ | 94 (28.1) | 62 (18.6)† | 0.050 |
| Antihypertensive treatment, n (%) | 163 (48.7) | 76 (22.8)§ | 61 (18.2) | 24 (7.2)§ | 0.052 |
| Lipid-lowering treatment, n (%) | 101 (30.2) | 65 (19.5)† | 40 (11.9)† | 16 (4.8)‡ | 0.01 |
| Diabetes mellitus, n (%) | 23 (6.9) | 13 (3.9) | 12 (3.6) | 7 (2.1) | 0.056 |
| Age (years), mean (SD) | 66.0 (10.9) | 55.4 (12.3)§ | 48.2 (12.6)§ | 37.0 (12.5)§ | 0.003 |
| Body mass index (kg/m2), mean (SD) | 28.0 (4.6) | 26.9 (4.5)† | 26.2 (4.2)* | 25.2 (4.0)† | 0.003 |
| Waist:hip ratio, mean (SD) | 0.90 (0.08) | 0.89 (0.08) | 0.87 (0.08)† | 0.85 (0.08)† | 0.001 |
| Office blood pressure (mmHg), mean (SD) | |||||
| Systolic pressure | 139.1 (18.3) | 131.7 (15.7)§ | 128.2 (16.7)† | 122.9 (14.2)§ | 0.01 |
| Diastolic pressure | 81.0 (9.8) | 82.2 (9.3) | 82.3 (9.6) | 77.8 (9.6)§ | 0.42 |
| Mean arterial pressure | 100.3 (10.2) | 98.7 (10.0)* | 97.6 (11.0) | 92.8 (10.1)§ | 0.057 |
| Heart rate (beats/min), mean (SD) | 63.7 (9.3) | 63.1 (9.4) | 64.7 (9.8)* | 63.9 (9.3) | 0.57 |
| Biochemical data, mean (SD) | |||||
| Serum creatinine (μmol/L) | 98.3 (16.8) | 87.4 (11.4)§ | 82.1 (11.0)§ | 78.1 (10.3)§ | 0.03 |
| 24-h microalbuminuria (mg) | 6.0 (4.5–8.3) | 6.2 (4.5–8.1) | 5.7 (4.2–8.1) | 5.6 (3.9–7.6) | 0.20 |
| Total cholesterol (mmol/L) | 5.14 (0.99) | 5.17 (0.93) | 5.07 (0.91) | 4.81 (0.95)† | 0.14 |
| HDL cholesterol (mmol/L) | 1.45 (0.40) | 1.47 (0.40) | 1.50 (0.39) | 1.45 (0.35) | 0.84 |
| Total:HDL cholesterol ratio | 3.75 (1.06) | 3.71 (1.03) | 3.59 (1.07) | 3.49 (1.03) | 0.02 |
| Plasma glucose (mmol/L) | 5.04 (0.90) | 4.85 (0.81)† | 4.80 (0.67) | 4.64 (0.52)‡ | 0.02 |
| γ-glutamyltransferase (units/L) | 22 (15–31) | 21 (15–33) | 19 (13–28)* | 18 (13–27) | 0.01 |
eGFR according to the CKD-EPI formula [22]. Office blood pressure was the average of five consecutive auscultatory readings. Hypertension was a blood pressure ≥ 140 mmHg systolic or ≥ 90 mmHg diastolic or the use of antihypertensive drugs. For 24-h microalbuminuria and γ-glutamyltransferase, values are geometric mean (interquartile range). P-values are for linear trend across eGFR categories. Significance of the difference with the adjacent lower fourth: *P ≤ 0.05, †P ≤ 0.01, ‡P ≤ 0.001, §P ≤ 0.0001.
Table 2.
Circulating biomarkers by quartiles of eGFR
| Characteristic | Low | Medium low | Medium high | High | P-value |
|---|---|---|---|---|---|
| Limits (mL/min/1.73 m2) | <69.89 | 69.89–79.73 | 79.73–90.77 | ≥90.77 | |
| Participants in category, n | 335 | 334 | 335 | 334 | |
| Adhesion molecules | |||||
| VCAM-1 (ng/mL) | 592 (469–731) | 524 (433–666)‡ | 496 (417–626) | 507 (410–642) | 0.15 |
| ICAM-1 (ng/mL) | 251 (209–310) | 244 (201–295) | 235 (196–285) | 239 (198–297) | 0.16 |
| E-selectin (ng/mL) | 15 (11–21) | 15 (11–20) | 15 (12–21) | 16 (12–20) | 0.23 |
| P-selectin (ng/mL) | 145 (113–180) | 141 (106–169) | 132 (103–164) | 131 (103–164) | 0.039 |
| MCP-1 (pg/mL) | 187 (127–239) | 161 (110–231)* | 212 (148–212) | 136 (97–192) | 0.60 |
| Inflammation markers | |||||
| CRP (ng/mL) | 1.52 (1.03–2.77) | 1.32 (0.89–2.48) | 1.22 (0.85–2.23) | 1.24 (0.85–2.59) | 0.11 |
| NGAL (ng/mL) | 387 (278–573) | 359 (241–522) | 342 (232–518) | 308 (217–491) | 0.007 |
| TNF-R1 (ng/mL) | 0.82 (0.69–1.01) | 0.70 (0.60–0.84)† | 0.67 (0.55–0.78) | 0.64 (0.53–0.75) | 0.067 |
| TNF-α (pg/mL) | 8.2 (6.9–9.7) | 7.4 (6.1–8.7)§ | 6.8 (5.8–8.4) | 6.7 (5.6–8.03) | 0.046 |
| IL-6 (pg/mL) | 2.00 (1.28–3.31) | 1.61 (1.06–2.50)† | 1.48 (0.99–2.46) | 1.43 (0.93–2.44) | 0.080 |
| IL-8 (pg/mL) | 9.1 (6.2–12.9) | 8.4 (6.1–11.4) | 8.2 (5.8–12.2) | 7.6 (5.4–11.0) | 0.019 |
| VEGF (pg/mL) | 64 (37–112) | 53 (31–98)† | 54 (36–101) | 51 (31–91) | 0.15 |
Values are geometric means (interquartile range). P-values are for the linear trend across eGFR categories. Significance of the difference with the adjacent lower fourth: *P ≤ 0.05, †P ≤ 0.01, ‡P ≤ 0.001, §P ≤ 0.0001.
Across quartiles of the MCP-1 [11, 12] distribution (Supplementary data, Table S4), blood pressure, the prevalence of hypertension and treated hypertension (0.057 ≤ P ≤ 0.004), heart rate (P = 0.095), serum creatinine (P = 0.079) and the risk of diabetes mellitus (P = 0.032) increased, whereas eGFR decreased (P = 0.013). Among all participants, 47 (3.5%) had micro-albuminuria and 5 (0.4%) had macro-albuminuria, but trends across VCAM-1 (Supplementary data, Table S2) and MCP-1 (Supplementary data, Table S4) distributions did not reach significance. Along similar lines, across quartiles of the MCP-1 distribution, VCAM-1 (P = 0.037) and E-selectin (P = 0.025) and all inflammation markers (0.0001 < P ≤ 0.0789) increased (Supplementary data, Table S5).
eGFR stage at baseline and follow-up
At baseline, of 1338 participants, 346 (25.9%) were in eGFR Stage 1, 854 (63.8%) in Stage 2, 112 (8.4%) in Stage 3 and 26 (1.9%) in Stage 4. The median interval from baseline to follow-up in 500 participants with a second eGFR assessment available was 4.7 years (5th–95th percentile interval 3.7–5.2). In these 500 participants, eGFR decreased by 1.04 mL/min/1.73 m2/year. Of 145 participants with eGFR Stage 1 at baseline, 71 (49.0%) maintained Stage 1 and 74 (51.0%) progressed to Stage 2. Of 322 participants with Stage 2 at baseline, 22 (6.8%) regressed to Stage 1, 267 (82.9%) remained in Stage 2 and 33 (10.3%) progressed to Stage 3. Of 33 participants with Stage 3 at baseline, 3 (9.1%) regressed to Stage 2 and 30 (90.9%) stayed in Stage 3. At follow-up, none of the 500 participants had progressed to Stage 4 or 5. Compared with the 500 examination cycle A participants with follow-up, the 165 without follow-up had similar baseline characteristics (Supplementary data, Table S1; P ≥ 0.18).
Cross-sectional analyses of the baseline data in 1338 participants
Based on the stepwise regression procedure (Supplementary data, Table S6), we adjusted the cross-sectional associations between eGFR and the biomarkers under study for mean arterial pressure, waist:hip ratio, smoking, plasma glucose, γ-glutamyltransferase, total:HDL cholesterol ratio, 24-h microalbuminuria and use of diuretics, inhibitors of the renin–angiotensin system (β-blockers, ACE inhibitors and angiotensin type 1 receptor blockers) and vasodilators (calcium channel blockers and α-blockers) and lipid- lowering drugs.
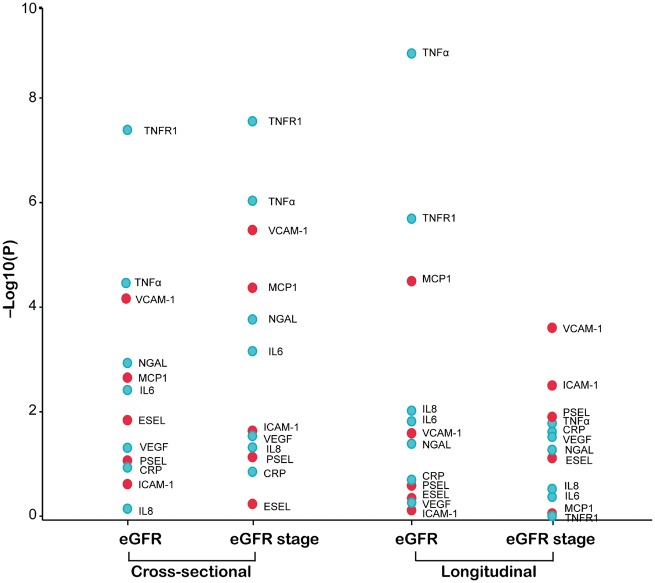
In the cross-sectional analysis of the baseline data (Table 3), with adjustments applied for covariables (Supplementary data, Table S6), standardized eGFR was inversely correlated with circulating VCAM-1 (P < 0.0001), MCP-1 (P = 0.002), neutrophil gelatinase-associated lipocalin (NGAL; P = 0.002), TNF receptor 1 (TNF-R1; P < 0.0001), TNF-α (P < 0.0001) and IL-6 (P = 0.004). The association sizes (expressed in mL/min/1.73 m2) for a doubling of the biomarker were −2.99 for VCAM-1, −1.19 for MCP-1, −1.19 for NGAL, −2.78 for TNF-R1, −2.28 for TNF-α and −0.94 for IL-6. In the categorical analysis of the baseline data, the multivariable-adjusted odds ratios expressing the risk of having eGFR <60 mL/min/1.73 m2 (n = 138) versus ≥60 mL/min/1.73 m2 (n = 1200) for a doubling of the biomarkers were 1.77 (P < 0.0001) for VCAM-1, 1.32 (P < 0.0001) for MCP-1, 1.26 (P = 0.0002) for NGAL, 1.49 (P < 0.0001) for TNF-R1, 1.45 (P < 0.0001) for TNF-α, 1.20 (P < 0.0008) for IL-6 and 1.12 (P = 0.036) for VEGF. Figure 2 shows the −log10(P) probability plot of the multivariable-adjusted association of various markers with eGFR (continuous) or eGFR stage (<60 versus ≥60 mL/min/1.73 m2) in the cross-sectional analyses of the baseline data.
Table 3.
Multivariable-adjusted associations of eGFR with the biomarkers measured at baseline
| Biomarker (baseline) | eGFR at baseline (n = 1338), estimate (95% CI) | eGFR at follow-up (n = 500), estimate (95% CI) |
|---|---|---|
| Adhesion molecules | ||
| VCAM-1 (ng/mL) | –2.99 (–4.49 to –1.50)§ | –2.76 (–5.30 to –0.22)* |
| ICAM-1 (ng/mL) | –1.05 (–2.68 – 0.57) | –0.38 (–2.92 – 2.15) |
| E-selectin (ng/mL) | 1.43 (0.27–2.59)* | 0.44 (–1.26 – 2.14) |
| P-selectin (ng/mL) | –1.25 (–2.65 – 0.15) | –1.40 (–3.66 – 0.86) |
| MCP-1 (pg/mL) | –1.19 (–1.95 to –0.42)† | –2.90 (–4.28 to –1.52)§ |
| Inflammation markers | ||
| CRP (ng/mL) | –0.45 (–1.01 – 0.10) | –0.63 (–1.61 – 0.35) |
| NGAL (ng/mL) | –1.19 (–1.93 to –0.45)† | –1.28 (–2.51 to –0.06)* |
| TNF-R1 (ng/mL) | –2.78 (–3.79 – 1.77)§ | –5.34 (–7.52 to –3.16)§ |
| TNF-α (pg/mL) | –2.28 (–3.35 – 1.20)§ | –6.99 (–9.26 to –4.72)§ |
| IL-6 (pg/mL) | –0.94 (–1.57 to –0.30)† | –1.31 (–2.39 to –0.22)* |
| IL-8 (pg/mL) | –0.31 (–1.06 – 0.44) | –1.62 (–2.88 to –0.35)* |
| VEGF (pg/mL) | –0.63 (–1.24 to –0.01) | –0.84 (–1.93 – 0.25) |
eGFR calculated according to the CKD-EPI formula [22]; Estimates, given with 95% CI, express the difference in eGFR associated with a doubling of the marker. The analyses were adjusted for baseline covariables, including mean arterial pressure, waist:hip ratio, smoking, plasma glucose, γ-glutamyltransferase, total:HDL cholesterol ratio, 24-h microalbuminuria and use of diuretics, inhibitors of the renin–angiotensin system (β-blockers, ACE inhibitors and angiotensin type 1 receptor blockers), vasodilators (calcium channel blockers and α-blockers). Models with eGFR at follow-up as a dependent variable were additionally adjusted for follow-up duration. Significance of the associations: *P ≤ 0.05, †P ≤ 0.01, ‡P ≤ 0.001, §P ≤ 0.0001.
FIGURE 2.
–Log10(P) probability plot of the multivariable-adjusted associations of eGFR (continuous or categorical) with the baseline biomarkers. In categorical analyses, eGFR <60 and ≥60 mL/min/1.73 m2 were contrasted. All analyses were adjusted for mean arterial pressure, waist:hip ratio, smoking, plasma glucose, γ-glutamyltransferase, total:HDL cholesterol ratio, 24-h microalbuminuria and use of diuretics, inhibitors of the renin–angiotensin system (β-blockers, ACE inhibitors and angiotensin type 1 receptor blockers), vasodilators (calcium channel blockers and α-blockers), lipid-lowering drugs and biomarker at baseline. The longitudinal analyses were additionally adjusted for follow-up duration.
Figure 3 shows the AUC for the adhesion molecules and inflammation markers in the discrimination between eGFR <60 versus ≥60 mL/min/1.73 m2 at baseline. Compared with 24 h-microalbuminuria, the AUC was significantly greater for adhesion molecules MCP-1 (P = 0.003) and VCAM-1 (P = 0.024) and for the inflammation markers TNF-R1 (P < 0.0001), TNF-α (P < 0.0001), IL-6 (P = 0.016) and NGAL (P = 0.028). Using 24-h albuminuria as a reference (Figure 4), VCAM-1 increased the AUC from 0.57 (95% CI 0.52–0.62; P = 0.024) to 0.65 (95% CI 0.60–0.70; P = 0.024), MCP-1 to 0.67 (95% CI 0.63–0.71; P = 0.003), TNF-R1 to 0.79 (95% CI, 0.75–0.83; P <0.0001) and TNF-α to 0.73 (95% CI 0.69–0.77; P <0.0001). There was no difference between the AUC associated with the two adhesion molecules, but TNF-R1 outperformed VCAM-1 (P < 0.0001) and MCP-1 (P < 0.0001). TNF-α (P ≤ 0.013), but not NGAL (P ≥ 0.57) and IL-6 (P ≥ 0.39), outperformed the two adhesion molecules.
FIGURE 3.
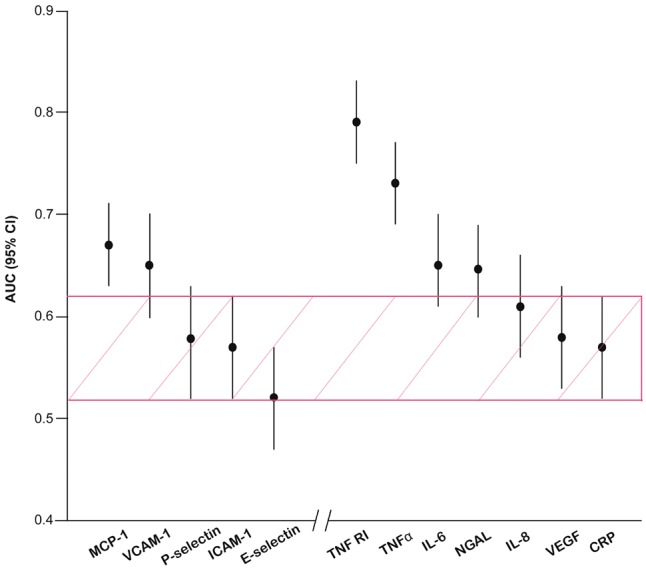
AUC for the adhesion molecules and inflammation markers in the discrimination between eGFR Stage ≥3 versus Stage ≤2 in the baseline study. Vertical bars denote the 95% CI. The shaded area represents the 95% CI of the AUC for 24-h microalbumiuria. AUCs for the biomarkers were ordered by magnitude.
FIGURE 4.
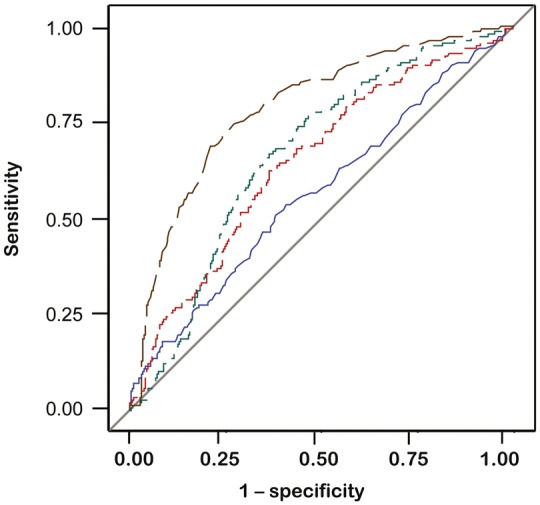
ROC curves for prediction of eGFR decline from ≥60 to <60 mL/min/1.73 m2. Blue, green, red and black lines identify 24-h microalbuminuria and circulating VCAM-1, MCP-1 and TNF-R1 at baseline, respectively.
A single variable derived by principle component analysis from VCAM-1, MCP-1, TNF-R1 and TNF-α, which were highly intercorrelated (0.14 ≤ r ≤ 0.79; P < 0.0001) yielded an AUC of 0.78 (95% CI 0.75–0.83), which was not greater than the AUC of TNF-R1 considered alone (P = 0.68).
Longitudinal analyses in 500 participants with follow-up
In the longitudinal analyses, eGFR (continuous) or eGFR stage at follow-up were related to the biomarkers measured at baseline. The models were adjusted for the same baseline co-variables as in the cross-sectional analyses (Supplementary data, Table S6) and additionally included follow-up duration. Consistent with the cross-sectional analysis, eGFR at follow-up was inversely associated with the baseline biomarkers, with association size of −2.76 for VCAM-1, −2.90 for MCP-1, −1.28 for NGAL, −5.34 for TNF-R1, −6.99 for TNF-α and −1.31 for IL-6 (Table 3 and Figure 2). The hazard ratios expressing the risk of having eGFR <60 mL/min/1.73 m2 (n = 33) at follow-up in participants with eGFR ≥60 mL/min/1.73 m2 at baseline (n = 412) were significant for baseline VCAM-1, TNF-α and VEGF, with estimates of 1.30 (P = 0.0004), 1.15 (P = 0.027) and 1.07 (P = 0.044), respectively. Finally, additional adjustment for sex and age of both the cross-sectional and longitudinal analyses did not materially change the results reported in Table 4 and Figure 2.
Table 4.
Multivariable-adjusted associations of eGFR stage with the biomarkers measured at baseline
| Biomarker | eGFR (≤2 versus ≥3) at baseline (1200 versus 138), odds ratio (95% CI) | ΔeGFR (≤2 → ≥3) from baseline to follow-up (412 versus 33), hazard ratio (95% CI) |
|---|---|---|
| Adhesion molecules | ||
| VCAM-1 (ng/mL) | 1.77 (1.39–2.26)§ | 1.30 (1.12–1.49)‡ |
| ICAM-1 (ng/mL) | 1.37 (0.96–1.75) | 1.23 (1.06–1.42)† |
| E-selectin (ng/mL) | 0.96 (0.77–1.19) | 1.10 (0.99–1.22) |
| P-selectin (ng/mL) | 1.28 (0.99–1.64) | 1.17 (1.02–1.32)† |
| MCP-1 (pg/mL) | 1.32 (1.16–1.51)§ | 0.99 (0.92–1.08) |
| Inflammation markers | ||
| CRP (ng/mL) | 1.08 (0.98–1.20) | 1.06 (1.01–1.12)* |
| NGAL (ng/mL) | 1.26 (1.12–1.43)‡ | 1.07 (1.00–1.16) |
| TNF-R1 (ng/mL) | 1.49 (1.31–1.71)§ | 1.01 (0.92–1.08) |
| TNF-α (pg/mL) | 1.45 (1.25–1.69)§ | 1.15 (1.02–1.31)* |
| IL-6 (pg/mL) | 1.20 (1.08–1.34)‡ | 1.03 (0.96–1.09) |
| IL-8 (pg/mL) | 1.14 (1.00–1.30) | 0.96 (0.89–1.04) |
| VEGF (pg/mL) | 1.12 (1.01–1.25)* | 1.07 (1.01–1.13)* |
ΔCKD, change in CKD stage from baseline to follow-up. eGFR calculated according to the CKD-EPI formula [22]. Stages of CKD were defined according to the National Kidney Foundation KDOQI guideline [23]. Hazard ratios were computed excluding 55 participants who from baseline to follow-up regressed from CKD Stage 2 to 1 or from Stage 3 to 2 or who maintained Stage 3. Of the remaining 445 participants, 33 (7.4%) progressed from Stage ≤2 to Stage ≥3. Estimates, given with 95% CI, express the relative risk associated with a doubling of the marker. All analyses were adjusted for baseline variables, including mean arterial pressure, waist:hip ratio, smoking, plasma glucose, γ-glutamyltransferase, total:HDL cholesterol ratio, 24-h microalbuminuria and use of diuretics, inhibitors of the renin–angiotensin system (β-blockers, ACE inhibitors and angiotensin type 1 receptor blockers), vasodilators (calcium channel blockers and α-blockers). Significance of the associations: *P ≤ 0.05, †P ≤ 0.01, ‡P ≤ 0.001, §P ≤ 0.0001.
DISCUSSION
To our knowledge, our study is the first to assess in a general population [31] the association of eGFR as continuous and categorical variables with circulating inflammation markers and adhesion molecules. The key findings can be summarized as follows: (i) in cross-sectional analyses, eGFR evaluated on a continuous scale was inversely associated with adhesion molecules VCAM-1 and MCP-1 and the inflammation markers TNF-R1 and TNF–α; (ii) the odds of having eGFR <60 mL/min/1.73 m2 at baseline as compared with ≥60 mL/min/1.73 m2 increased with higher serum levels of VCAM-1, MCP-1, TNF-R1 and TNF–α; (iii) TNF-R1, compared with 24-h albuminuria, VCAM-1 and MCP-1 best discriminated eGFR <60 versus ≥60 mL/min/1.73 m2 and combining these highly intercorrelated serum markers into a single variable did not enhance discrimination and (iv) prospective analyses covering ∼5 years of follow-up in about one-third of the study population were confirmatory.
Experimental studies in vitro [8–10] and in mice [11, 12] justified the hypothesis that we set out to test in the current population study. Our observations are in keeping with the large body of evidence showing that TNF-α, also called TNF, is a cell signalling cytokine that plays a pivotal role in mediating inflammation. Binding of TNF-α to TNF-R1 stimulates the transcriptional activity of nuclear factor kappa B (NF-κB), which in turn leads to increased expression of adhesion molecules, including VCAM-1 and MCP-1, in endothelial cells [32, 33]. This pathway (Supplementary data, Figure S1) and the high correlations between the four circulating markers explain why a linear combination of TNF-R1, TNF-α, VCAM-1 and MCP-1 did not enhance the discrimination of eGFR <60 versus ≥60 mL/min/1.73 m2. We did not adjust significance levels for multiple testing. The theoretical basis for applying a correction for multiple testing is that chance serves as the first-order explanation for observed associations [34]. However, if, as in the present study, the biomarkers are highly intercorrelated, each new test does not provide a completely independent chance for a type 1 error, making adjustment for multiple testing inappropriate [34].
Our current study moves beyond the state of the art by translating findings from experimental studies [8–12] and knowledge about molecular pathways to people representative of a general population [32, 33]. Moreover, we searched PubMed for relevant publications without limitation of publication date or language, using terms ‘eGFR, biomarkers, human’ or ‘renal function, biomarkers, human’. We screened papers by title or abstract to identify full-text reports that might be relevant to our hypothesis. We did a full-text review of 29 articles. None of these studies analysed the association of renal function with a set of circulating adhesion molecules in a general population. Thus our study provides the first evidence that circulating adhesion molecules, in particular VCAM-1, are inversely associated with eGFR and predict the incidence of eGFR decline in the general population.
In keeping with recent recommendations [35] and previous studies [24, 36], in our study we applied an early renal endpoint defined as an eGFR <60 mL/min/1.73 m2 without short-term confirmation or association with other manifestations of renal disease. The Framingham investigators used a similar outcome measure [24, 25]. Among 2585 participants free of pre-existing renal disease, 244 (9.4%) developed clinically overt kidney disease during a mean follow-up of 18.5 years. Furthermore, Matsushita et al. [36] conducted a meta-analysis to investigate the association of eGFR with all-cause and cardiovascular mortality in 21 general population cohorts. They reported that the adjusted hazard ratios for all-cause mortality at an eGFR of 60 compared with 95 mL/min/1.73 m2 was 1.18 (95% CI 1.05–1.32).
In line with our current observations, cross-sectional [37] or longitudinal [38] analyses of several population studies identified circulating NGAL and TNF receptors as circulating biomarkers inversely associated with eGFR [37, 38] or predictive of eGFR decline [38]. Similar findings were obtained in a case–control study in patients with CKD Stages 2–4 or end-stage renal disease [5]. In the Chronic Renal Insufficiency Cohort study [39], over a median follow-up time of 6.3 years, 899 of 3430 patients reached the composite endpoint of a ≥50% decline in eGFR or onset of end-stage renal disease. In multivariable-adjusted analyses, accounting for baseline eGFR and other co-variables, the hazard ratios for the composite outcome were greater for patients in the highest quartile of IL-6 [1.44 (95% CI 1.17–1.77)] and TNF-α [1.94 (95% CI 1.52–2.47)] compared with those in the respective lowest quartiles. CRP is a widely used inflammatory biomarker. However, the association of eGFR with CRP in the current literature is inconsistent [32, 33]. In line with these reports [32, 33], our study did not identify CRP as being associated with eGFR or predictive of eGFR decline.
Whereas the available literature frequently addresses the association of renal dysfunction with circulating inflammation markers, few studies have focused on adhesion molecules [31, 40]. Among 4128 older people enrolled in the Cardiovascular Health Study (mean age 72 years), 1059 (25.7%) had an annual decline in eGFR derived from cystatin C exceeding >3 mL/min/1.73 m2. Over 7 years of follow-up, only serum albumin predicted a rapid decline of eGFR, with a multivariable-adjusted odds ratio per 1 SD increment of 1.14 (95% CI 1.06–1.23). CRP (n = 4113), IL-6 (n = 3813), ICAM–1 (n = 1409) and six other inflammatory markers (1628 < n < 4125) were not predictive. In a cross-sectional study of 1950 Asians with type 2 diabetes [40], eGFR declined and the urinary albumin:creatinine ratio increased with higher plasma VCAM-1 levels, whereas the corresponding associations with plasma ICAM-1 levels were not significant. In our current study, why eGFR is inversely associated with VCAM-1 but not ICAM-1 remains to be elucidated. One possible explanation is the differential expression or kinetics of VCAM-1 and ICAM-1 in response to inflammatory stimuli according to the underlying disease or anatomical location [41, 42]. For instance, in atherosclerosis, VCAM-1 is mainly expressed in lesions, whereas ICAM-1 expression extends into lesion-protected and predisposed sites [41]. Our literature search identified one cross-sectional Japanese study [43] involving 860 residents of a fishing community in which, with adjustments applied for sex and age, eGFR declined with MCP-1. A similar inverse association between eGFR and serum MCP-1 was reported among 479 African Americans with type 2 diabetes with a more complete adjustment including covariables of sex, age, body mass index, smoking, hemoglobin A1c and low-density lipoprotein cholesterol [44]. However, the only inflammation biomarker considered in these two MCP-1 studies [43, 44] was the white blood cell count [43].
The present study must be interpreted within the context of some potential limitations. First, circulating biomarkers only provide a snapshot of a state that involves the whole body and are not necessarily representative of a process that is specific to the kidneys. Observational studies can also not infer causality. However, studying the local expression of these biomarkers in relation to histopathological lesions in human kidneys is not practicable. Only experimental studies can clarify how adhesion molecules are involved in glomerular disease either as mediators or bystanders. Second, only 500 of our 1338 participants were followed up, so our longitudinal analyses might have been underpowered. At the time of writing of this article, follow-up of examination cycle B participants was too short for a reassessment of their renal function. Nonetheless, examination cycle A participants with and without follow-up had similar baseline characteristics. Third, we did not measure the serum biomarkers at the time of reassessment of eGFR. Finally, in contrast to previous studies in CKD patients, we did not measure NGAL in urine, but only in serum, which might have led to an underestimation of the association of renal dysfunction with this biomarker.
CONCLUSIONS
Our data demonstrate that eGFR is inversely associated with the serum levels of adhesion molecules VCAM-1 and MCP-1 and the inflammation biomarkers TNF-R1 and TNF-α. Binding of TNF-α with its cellular receptor initiates a signalling pathway leading to expression of the adhesion molecules (Supplementary data, Figure S1). The proximal role of the inflammation markers in this pathway might explain why TNF-R1 and TNF-α outperformed VCAM-1 and MCP-1 in differentiating between eGFR Stage ≤2 and ≥3. Further studies are required to confirm our findings in other populations and to establish whether the circulating biomarkers reflect a systemic inflammatory condition or are specific forerunners of glomerular impairment.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
Supplementary Material
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the contribution of the nurses working at the examination center (Linda Custers, Marie-Jeanne Jehoul, Daisy Thijs and Hanne Truyens) and the clerical staff at the Studies Coordinating Centre (Vera De Leebeeck Yvette Piccart, Renilde Wolfs).
FUNDING
The European Union (HEALTH-FP7-278249-EUMASCARA, HEALTH-F7-305507 HOMAGE) and the European Research Council (Advanced Researcher Grant 2011-294713-EPLORE and Proof-of-Concept Grant 713601-uPROPHET) and the Fonds voor Wetenschappelijk Onderzoek Vlaanderen, Ministry of the Flemish Community, Brussels, Belgium (G.0881.13, G.088013 and 11Z0916N) currently support the Studies Coordinating Centre in Leuven. National Science Funding in China (grant numbers 81470566 and 81670765) supports collaboration between Lu He Hospital and the Studies Coordinating Center.
CONFLICT OF INTEREST STATEMENT
A.-M.J. and R.L. are employees of Randox. The other authors declare no conflict of interest.
REFERENCES
- 1. Wang H, Dwyer-Lindgren L, Lofgren KT. et al. Age-specific and sex-specific mortality in 187 countries, 1970-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2071–2094 [DOI] [PubMed] [Google Scholar]
- 2. Coresh J, Selvin E, Stevens LA. et al. Prevalence of chronic kidney disease in the United States. JAMA 2007; 298: 2038–2047 [DOI] [PubMed] [Google Scholar]
- 3. Kim SH, Jo MW, Go DS. et al. Economic burden of chronic kidney disease in Korea using a national sample cohort. J Nephrol 2017 [DOI] [PubMed] [Google Scholar]
- 4. van Ree RM, Oterdoom LH, de Vries AP. et al. Elevated levels of C-reactive protein independently predict accelerated deterioration of graft function in renal transplant recipients. Nephrol Dial Transplant 2007; 22: 246–253 [DOI] [PubMed] [Google Scholar]
- 5. Sharain K, Hoppensteadt D, Bansal V. et al. Progressive increase of inflammatory biomarkers in chronic kidney disease and end-stage renal disease. Clin Appl Thromb Hemost 2013; 19: 303–308 [DOI] [PubMed] [Google Scholar]
- 6. Perlman AS, Chevalier JM, Wilkinson P. et al. Serum inflammatory and immune mediators are elevated in early stage diabetic nephropathy. Ann Clin Lab Sci 2015; 45: 256–263 [PubMed] [Google Scholar]
- 7. Stinghen AEM, Gonçalves SM, Martines EG. et al. Increased plasma and endothelial cell expression of chemokines and adhesion molecules in chronic kidney disease. Nephron Clin Pract 2009; 111: c117–c126 [DOI] [PubMed] [Google Scholar]
- 8. Park SK, Yang WS, Lee SK. et al. TGF-β1 down-regulates inflammatory cytokine-induced VCAM-1 expression in cultured human glomerular endothelial cells. Nephrol Dial Transplant 2000; 15: 596–604 [DOI] [PubMed] [Google Scholar]
- 9. Matsui T, Nishino Y, Maeda S. et al. Irbesartan inhibits advanced glycation end product (AGE)-induced up-regulation of vascular cell adhesion molecule-1 (VCAM-1) mRNA levels in glomerular endothelial cells. Microvasc Res 2011; 81: 269–273 [DOI] [PubMed] [Google Scholar]
- 10. Jia Z, Nallasamy P, Liu D. et al. Luteolin protects against vascular inflammation in mice and TNF-alpha-induced monocyte adhesion to endothelial cells via suppressing IKBa/NK-κB signaling pathway. J Nutr Biochem 2015; 26: 293–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tarabra E, Giunti S, Barutta F. et al. Effect of the monocyte chemoattractant protein-1/CC chemokine receptor 2 system on nephrin expression in streptozotocin-treated mice and human cultured podocytes. Diabetes 2009; 58: 2109–2118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wiggins JE, Patel SR, Shedden KA. et al. NFκB promotes inflammation, coagulation, and fibrosis in the aging glomerulus. J Am Soc Nephrol 2010; 21: 587–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marouga A, Dalamaga M, Kastania AN. et al. Correlates of serum resistin in elderly, non-diabetic patients with chronic kidney disease. Clin Lab 2013; 59: 1121–1128 [DOI] [PubMed] [Google Scholar]
- 14. Tofik R, Ohlsson S, Bakoush O.. Urinary concentration of monocyte chemoattractant protein-1 in idiopathic glomerulonephritis: a long-term follow-up study. PLoS One 2014; 9: e87857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li Y, Zagato L, Kuznetsova T. et al. Angiotensin-converting enzyme I/D and a-adducin Gly460Trp polymorphisms: from angiotensin-converting enzyme activity to cardiovascular outcome. Hypertension 2007; 49: 1291–1297 [DOI] [PubMed] [Google Scholar]
- 16. Gu YM, Thijs L, Liu YP. et al. The urinary proteome as correlate and predictor of renal function in a population study. Nephrol Dial Transplant 2014; 29: 2260–2268 [DOI] [PubMed] [Google Scholar]
- 17. World Medical Association. Declaration of Helsinki. JAMA 2013; 227: 184–189 [Google Scholar]
- 18. Zhang Z, Staessen JA, Thijs L. et al. Left ventricular diastolic dysfunction in relation to the urinary proteome: a proof-of-concept study in a general population. Int J Cardiol 2014; 176: 158–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jaffe M. Über den Niederschlag, welchen Pikrinsäure in normalen Harn erzeugt und über eine neue Reaction des Kreatinins. Z Physiol Chem 1886; 10: 391–400 [Google Scholar]
- 20. Peake M, Whiting M.. Measurement of serum creatinine — current status and future goals. Clin Biochem Rev 2006; 27: 173–182 [PMC free article] [PubMed] [Google Scholar]
- 21. Myers GL, Miller WG, Coresh J. et al. Recommendations for improving serum creatinine measurement: a report from the laboratory working group of the National Kidney Disease Education Program. Clin Chem 2006; 52: 5–18 [DOI] [PubMed] [Google Scholar]
- 22. Levey AS, Stevens LA, Schmid CH. et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Levey AS, Eckaerdt KU, Tsukamoto Y. et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2005; 67: 2089–2100 [DOI] [PubMed] [Google Scholar]
- 24. Fox CS, Larson MG, Leip EP. et al. Predictors of new-onset kidney disease in a community-based population. JAMA 2004; 291: 844–850 [DOI] [PubMed] [Google Scholar]
- 25. Fox CS, Gona P, Larson MG. et al. A multi-marker approach to predict incident CKD and microalbuminuria. J Am Soc Nephrol 2010; 21: 2143–2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Abogunrin F, O'Kane HF, Ruddock MW. et al. The impact of biomarkers in multivariate algorithms for bladder cancer diagnosis in patients with hematuria. Cancer 2012; 118: 2641–2650 [DOI] [PubMed] [Google Scholar]
- 27. Emmert-Streib F, Abogunrin F, de Matos Simoes R. et al. Collectives of diagnostic biomarkers identify high-risk subpopulations of hematuria patients: exploiting heterogeneity in large-scale biomarker data. BMC Med 2013; 11: 12–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Richards J, Bansal V, Iqbal O. et al. Immunoenzymatic and biochip array profiling of the biomarkers of inflammation and hemostatic activation processes in ESRD. Clin Appl Thromb Hemost 2015; 21: 405–411 [DOI] [PubMed] [Google Scholar]
- 29. Masiha S, Sundström J, Lind L.. Inflammatory markers are associated with left ventricular hypertrophy and diastolic dysfunction in a population-based sample of elderly men and women. J Hum Hypertens 2013; 27: 13–17 [DOI] [PubMed] [Google Scholar]
- 30. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabet Care 2003; 26(Suppl 1): S5–S20 [DOI] [PubMed] [Google Scholar]
- 31. Keller C, Katz R, Sarnak MJ. et al. Inflammatory biomarkers and decline in kidney function in the elderly: the Cardiovascular Health Study. Nephrol Dial Transplant 2010; 25: 119–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mentz RJ, Kelly JP, von Lueder TG. et al. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol 2014; 64: 2281–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pouleur AC. Which biomarkers do clinicians need for diagnosis and management of heart failure with reduced ejection fraction? Clin Chim Acta 2015; 443: 9–16 [DOI] [PubMed] [Google Scholar]
- 34. Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990; 1: 43–46 [PubMed] [Google Scholar]
- 35. European Medicines Agency. Guideline on the clinical investigation of medicinal products to prevent development/slow progression of chronic renal insufficiency. EMA/CHMP/500825/2016. 2016. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2016/10/WC500214980.pdf
- 36. Matsushita K, van der VM, Astor BC. et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality: a collaborative meta-analysis of general population cohorts. Lancet 2010; 375: 2073–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Keller CR, Odden MC, Fried LF. et al. Kidney function and markers of inflammation in elderly persons without chronic kidney disease: the health, aging, and body composition study. Kidney Int 2007; 71: 239–244 [DOI] [PubMed] [Google Scholar]
- 38. Hiramoto JS, Katz R, Peralta CA. et al. Inflammation and coagulation markers and kidney function decline: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis 2012; 60: 225–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Amdur RL, Feldman HI, Gupta J. et al. Inflammation and progression of CKD: the CRIC study. Clin J Am Soc Nephrol 2016; 11: 1546–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu JJ, Yeoh LY, Sum CF. et al. Vascular cell adhesion molecule-1, but not intercellular adhesion molecule-1, is associated with kidney disease in Asians with type 2 diabetes. J Diabetes Complicat 2015; 29: 707–712 [DOI] [PubMed] [Google Scholar]
- 41. Cybulsky MI, Iiyama K, Li H. et al. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J Clin Invest 2001; 107: 1255–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Haubner F, Lehle K, Munzel D. et al. Hyperglycemia increases the levels of vascular cellular adhesion molecule-1 and monocyte-chemoattractant-protein-1 in the diabetic endothelial cell. Biochem Biophys Res Commun 2007; 360: 560–565 [DOI] [PubMed] [Google Scholar]
- 43. Olivi L, Vandenbriele C, Gu YM. et al. PEAR1 is not a human hypertension-susceptibility gene. Blood Press 2015; 24: 61–64 [DOI] [PubMed] [Google Scholar]
- 44. Murea M, Register TC, Divers J. et al. Relationships between serum MCP-1 and subclinical kidney disease: African American-Diabetes Heart Study. BMC Nephrol 2012; 14: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.