Abstract
Background
The independent contributions of microbial translocation and liver fibrosis to immune activation in human immunodeficiency virus (HIV) and/or hepatitis C virus (HCV)–infected persons are unclear.
Methods
Multivariable linear regression was used to evaluate whether intestinal fatty acid binding protein (I-FABP: a marker of gut epithelial integrity) and transient elastography-measured liver fibrosis might mediate the association of HIV and HCV with the soluble CD14 (sCD14) level in 120 individuals with HIV and HCV coinfection, 262 with HIV monoinfection, 72 with HCV monoinfection, and 170 without infection.
Results
Coinfected individuals, HIV-monoinfected individuals, and HCV-monoinfected individuals had 37%, 21%, and 12% higher sCD14 levels, respectively, than uninfected individuals, after multivariable adjustment. Additional adjustment for I-FABP level modestly attenuated the association of HIV infection, but attenuation occurred to a lesser extent in the HCV-monoinfected group. Adjustment for liver fibrosis substantially attenuated the association of HCV infection, but attenuation occurred to a lesser extent in the HIV-monoinfected group. Relative to the uninfected group, the primary mediator of the sCD14 level was the I-FABP level in the HIV-infected groups and liver fibrosis in the HCV-monoinfected group.
Conclusion
HIV and HCV are independently and additively associated with higher a sCD14 level. Our findings suggest that microbial translocation contributes to an increased sCD14 level during HIV infection, whereas liver fibrosis plays a stronger role during HCV monoinfection. Coinfected persons may be at greatest risk for progression, because of the independent effects of microbial translocation and liver fibrosis on immune activation.
Keywords: Liver fibrosis, microbial translocation, immune activation, HCV, HIV
HIV and HCV are independently and additively associated with the immune activation marker, sCD14. HIV/HCV-coinfected persons may be at greatest risk for disease progression, because of the independent effects of microbial translocation and HCV-associated liver fibrosis with immune activation.
Both human immunodeficiency virus (HIV) and hepatitis C virus (HCV) infection have been associated with markers of gut microbial translocation, which, in turn, is thought to lead to immune activation and disease progression [1]. In HIV infection, disruption of gut epithelial integrity has been hypothesized to lead to translocation of microbial products and, thus, sustained immune activation and inflammation, even in patients receiving antiretroviral therapy [1–3]. Elevated levels of markers of gut microbial translocation and immune activation have been associated with mortality in the setting of HIV infection [4–7]. In HCV infection, translocation of gut microbial products is also hypothesized to occur when there is increasing liver insufficiency [6] and portal hypertension [8], with greater translocation occurring with advanced cirrhosis [9]. Few studies, however, have examined the independent contribution of HIV and HCV infection to markers of gut microbial translocation and immune activation in the setting of HIV/HCV coinfection.
A prospective study of HIV/HCV-coinfected women who did not have cirrhosis found that elevated levels of intestinal fatty acid binding protein (I-FABP; a marker of intestinal epithelial integrity and correlate of microbial translocation [10, 11]) was associated with progression to liver fibrosis and cirrhosis [12]. That study, however, was not able to discern whether higher levels of I-FABP were a result of HIV and/or HCV infection. We also recently demonstrated that HCV-associated liver fibrosis was associated with increased systemic inflammation [13], but we could not determine whether microbial translocation might mediate this association. Understanding the complex relationship of HIV, HCV, liver fibrosis, and microbial translocation with systemic inflammation is important because it might help prioritize interventions to reduce the deleterious sequelae of chronic systemic inflammation.
We therefore investigated the relationship of HIV, HCV, liver fibrosis, and I-FABP with immune activation, measured on the basis of the level of soluble CD14 (sCD14; a marker of monocyte activation that is a coreceptor for lipopolysaccharide, which is a major component of the gram-negative bacterial cell wall) [6], using data from 2 ethnically diverse cohorts of women and men with HIV monoinfection, HCV monoinfection, HIV/HCV coinfection, or neither HIV nor HCV infection. We hypothesized that HIV and HCV infection would be independently associated with higher sCD14 levels and that I-FABP levels and liver fibrosis would attenuate the association.
METHODS
Study Population
The Women’s Interagency HIV Study (WIHS) is a multicenter prospective cohort study that was established in 1994 to investigate the progression of HIV infection in women with and at risk for HIV infection. A total of 4982 women (3678 with and 1304 without HIV infection) were enrolled between 1994 and 2015 from 10 sites in the United States (Atlanta, Birmingham, Bronx, Brooklyn, Chapel Hill, Chicago, Jackson, Los Angeles, Miami, San Francisco, and Washington, DC). Baseline sociodemographic characteristics and HIV risk factors were similar between women with and those without HIV infection. Every 6 months, participants complete a comprehensive physical examination, provide biological specimens for CD4+ T-cell count and HIV RNA load determination, and complete an interviewer-administered questionnaire, which collects information on sociodemographic characteristics, disease characteristics, and specific antiretroviral therapy use. The study design and characteristics have been described elsewhere [14, 15].
From December 2003 through July 2015, WIHS participants from 3 sites (Chicago, San Francisco, and Washington, DC) participated in the cross-sectional WIHS Fibroscan substudy [16, 17] and/or the San Francisco WIHS site–only magnetic resonance spectroscopy steatosis substudy [18]; the substudies were designed to investigate the contribution of HIV and HCV and their metabolic and inflammatory consequences to liver fibrosis, estimated using transient elastography (TE; Fibroscan; Echosens, Paris, France), and steatosis, measured by magnetic resonance spectroscopy, respectively. Of the 520 WIHS participants enrolled in these 2 substudies, 434 had stored plasma specimens available within 1 year of liver imaging; of these, 430 were included in the analysis, because monocyte activation data (the outcome measure of our analysis) were available.
From October 2010 through June 2014, the Study of Visceral Adiposity, HIV, and HCV: Biologic Mediators of Hepatic Steatosis (VAHH) enrolled 224 participants (of whom 98% were men) with HIV monoinfection (64 participants), HIV/HCV coinfection (27), HCV monoinfection (55), and neither HIV nor HCV infection (78) from the San Francisco Bay Area. The VAHH was designed to allow for pooled analysis with data from the WIHS, because an aim of the VAHH was to examine sex differences in the factors associated with hepatic steatosis. The VAHH used similar data collection instruments as the WIHS, including the same smoking and alcohol use questionnaires and anthropometry protocol, and enrolled participants in the same age range (35–70 years) as women enrolled in WIHS liver substudies. Liver fibrosis was also measured using TE, and markers of microbial translocation and immune activation were measured using the same assays and in the same laboratory as in the WIHS. The VAHH was a cross-sectional study; TE and phlebotomy were performed at the same visit. Of the 224 participants, 194 had stored plasma specimens available for testing of markers of microbial translocation and monocyte activation and were included in the analysis. VAHH recruitment, study design, and study characteristics have been described elsewhere [18]. For both the WIHS and the VAHH, an institutional review board approved study protocols and consent forms, and each study participant gave written informed consent.
Outcomes
We used a commercially available enzyme-linked immunosorbent assay (ELISA) to measure sCD14 levels (R & D Systems, Minneapolis, MN) in frozen plasma specimens collected from WIHS and VAHH participants at the same time as their study visit and stored at −70oC. Samples from both cohorts were tested centrally at the same laboratory. The assays were performed in duplicate and in accordance with manufacturers’ protocols.
Covariates
Our primary predictors were HIV and HCV infection status. Chronic HCV infection was confirmed by detectable HCV RNA following a positive anti–HCV antibody test result. Participants were classified as HCV uninfected if results of the HCV antibody test were negative or, among those with positive HCV antibody test result, if the HCV RNA level was undetectable. The HCV RNA level was unknown in 3 participants in whom anti–HCV antibody was detected. These participants were coded as HCV infected since spontaneous clearance of HCV is uncommon. We confirmed HIV status with a Food and Drug Administration–approved ELISA and reconfirmed with a Western blot assay when results of immunosorbent assays were positive.
I-FABP levels were measured in frozen plasma specimens stored at −70oC, using a commercially available ELISA (Hycult Biotech, Plymouth Meeting, PA). TE-measured liver stiffness in kiloPascals was available in a subset of participants (556 [89% of the study sample]) and examined as a continuous measure in analysis. Cirrhosis was defined using the American Gastroenterological Association recommended cutoff of ≥12.5kPa for HCV-infected patients, because the majority of our patients with high TE-measured liver stiffness values were HCV infected [19].
Other candidate covariates included demographic characteristics (ie, age, sex, and ethnicity) and lifestyle factors (ie, alcohol use, defined as none, light drinking [1–15 g/day], moderate drinking [15–30 g/day], and heavy drinking [>30 g/day]; and smoking status, defined as none, current, and past), anthropometric measurements (ie, waist circumference and body mass index), and laboratory parameters, including estimated glomerular filtration rate. In HIV-infected participants, HIV-related risk factors included current and nadir CD4+ T-cell count, current HIV RNA level, history of clinical AIDS, and current use of highly active antiretroviral therapy.
Statistical Analysis
We compared sociodemographic and clinical characteristics among 4 groups—HIV-monoinfected participants, HCV-monoinfected participants, coinfected participants, and uninfected participants—using the Kruskal-Wallis test, for continuous variables, and χ2 analysis and the Fisher exact test, for categorical variables. Grouped data were then analyzed using analysis of variance, for multiple-group comparisons, and t tests, for comparisons between 2 groups.
TE-measured liver stiffness values, I-FABP levels, and sCD14 levels were found to be right skewed and were therefore log transformed to normalize their distributions. We then calculated within-group marginal mean values for sCD14 levels for each disease category, adjusted for age, sex, and race/ethnicity.
To determine whether disease status was independently associated with sCD14 level and whether gut epithelial damage and/or liver fibrosis mediated this association, a series of multivariable linear regression models were sequentially adjusted for (1) demographic characteristics, lifestyle factors, and body composition, (2) I-FABP level, (3) liver stiffness, and (4) I-FABP level and liver stiffness. In a separate analysis, restricted to HIV-infected participants, we modeled the association of coinfection with sCD14 level while controlling for HIV-specific variables (ie, nadir CD4+ T-cell count, current HIV RNA level [log transformed], history of clinical AIDS, and cumulative use of antiretroviral therapy), demographic characteristics, lifestyle factors, body composition, I-FABP level, and liver stiffness. In a sensitivity analysis, we replaced TE-measured liver stiffness with the aspartate aminotransferase to platelet ratio index in models of sCD14 level, to compare the differential effects of alternative liver fibrosis measures. The regression coefficients and their confidence intervals were exponentiated to calculate the percentage differences attributable to each factor.
Finally, we tested this series of models under one theoretical framework, known as path analysis, which uses maximum likelihood estimation, to estimate the direct effect of disease status and the indirect effects of I-FABP levels and liver stiffness on sCD14 levels and to further decompose the proportion of direct and indirect effects [20]. This analytic approach provides a means to determine the relationship of the correlation coefficients between I-FABP level, liver stiffness, and sCD14 level in different infection groups simultaneously. To account for missing data, we used the full information maximum likelihood approach in the setting of path analysis, because of its greater efficiency as compared to a multiple imputation approach and its consistency in calculating path coefficients, which the multiple imputation approach lacks because of multiple imputed data sets [21]. Notably, missing data were presumed to be independent of liver fibrosis, and therefore the underlying assumption for using the full information maximum likelihood approach (ie, that data were missing in a random fashion) was met. For comparison purposes, we performed additional analysis restricted to those with complete data.
All analyses were conducted using the SAS system, version 9.4 (SAS Institute, Cary, NC).
RESULTS
Population Characteristics
Table 1 shows the demographic and clinical characteristics of the 624 participants included in the analysis, stratified by HIV and HCV status. The median age was 51 years, 30% (190) were men, and over half were African American. HCV-infected participants (ie, those with and those without HIV coinfection) were older, more likely to report current smoking, and more likely to have cirrhosis as compared to those in the HIV-monoinfected and uninfected groups. HIV-infected participants (ie, those with and those without HCV coinfection) had a lower body mass index and waist circumference than those in the HCV-monoinfected and uninfected groups. Serum I-FABP levels were also highest in coinfected participants, compared with the other infection groups. Furthermore, among HIV-infected participants, those with HCV coinfection had a lower median CD4+ T-cell count and a lower median nadir CD4+ T-cell count and were more likely to have a history of clinical AIDS than those with HIV monoinfection. Coinfected participants were slightly more likely to be receiving antiretroviral therapy but less likely to have undetectable HIV RNA.
Table 1.
Demographic and Clinical Characteristics of 624 Study Participants Stratified by Human Immunodeficiency Virus (HIV) and Hepatitis C Virus (HCV) Status
| Characteristic | HIV/HCV Coinfected (n=120) | HIV Monoinfected (n=262) |
HCV Monoinfected (n=72) |
Uninfected (n=170) |
P a |
|---|---|---|---|---|---|
| Demographic | |||||
| Age, y | 54 (50–58) | 48 (42–55) | 57 (54–60) | 49 (40–55) | <.001 |
| Male sex | 20 | 18 | 65 | 42 | <.001 |
| Race/ethnicity | |||||
| White | 18 | 25 | 38 | 24 | .27 |
| African American | 65 | 56 | 44 | 56 | |
| Hispanic | 15 | 13 | 14 | 11 | |
| Otherb | 2 | 6 | 4 | 9 | |
| Lifestyle | |||||
| Current smoker | 63 | 34 | 49 | 43 | <.001 |
| Alcohol consumption, drinks/wk | |||||
| None | 48 | 41 | 33 | 41 | .067 |
| 0–7 | 36 | 42 | 38 | 36 | |
| 8–12 | 2 | 6 | 8 | 5 | |
| >12 | 13 | 10 | 19 | 18 | |
| Metabolic | |||||
| Body mass indexc | 24 (22–28) | 26 (23–29) | 27 (23–30) | 28 (25–32) | <.001 |
| Waist circumference, cm | 87 (80–99) | 90 (82–100) | 96 (84–108) | 96 (86–110) | <.001 |
| Immune marker | |||||
| I-FABP level, pg/mL | 1140 (613–1784) | 813 (531–1390) | 706 (487–1209) | 496 (332–764) | <.001 |
| Liver related | |||||
| Liver stiffness, kPa | 6.8 (5.4–11.1) | 4.4 (3.8–6.0) | 6.3 (4.8–9.5) | 4.6 (3.8–5.6) | <.001 |
| Cirrhosisd | 27 | 3 | 18 | 1 | <.001 |
| HIV related | |||||
| CD4+ T-cell count, cells/mm3 | |||||
| Current | 502 (285–678) | 584 (379–800) | … | … | .006 |
| Nadir | 175 (101–274) | 248 (107–352) | … | … | .004 |
| History of AIDS | 56 | 37 | … | … | .001 |
| Current HAART use | 84 | 88 | … | … | .33 |
| Duration of ART, y | 8.0 (3.0–12.5) | 8.3 (3.1–11.7) | … | … | .94 |
| Undetectable viral load | 60 | 68 | … | … | .13 |
Data are median value (interquartile range) or % of participants. Of the total sample, 3 individuals (0.5%) were missing data on smoking status, 14 (2.2%) were missing data on waist circumference, 68 (10.9%) were missing data on live stiffness, and 2 (0.3%) were missing data on HCV RNA level. Of the HIV-infected subset, 3 individuals (0.8%) were missing data on nadir CD4+ T-cell count, 13 (3.4%) were missing data on ART duration, 2 (0.5%) were missing data on HCV RNA level, and 1 (0.3%) was missing data on HIV load.
Abbreviations: ART, antiretroviral therapy; HAART, highly active antiretroviral therapy; I-FABP, intestinal fatty acid binding protein.
aBy the Pearson χ2 test, Wilcoxon rank sum test, or Fischer exact test.
bIncludes Asian, Pacific Islander, Native American, Alaskan, and other study participants.
cCalculated as the weight in kilograms divided by the height in meters squared.
dDefined as a Fibroscan value of ≥12.5kPa.
Association of HIV and/or HCV Infection with Plasma sCD14 Concentration
Coinfected and HIV-monoinfected participants had higher sCD14 levels as compared to HCV-monoinfected and uninfected participants (mean value adjusted by demographic characteristics, 1646 ng/mL [95% confidence interval {CI}, 1620–1924 ng/mL] and 1441 ng/mL [95% CI, 1419–1675] vs 1328 ng/mL [95% CI, 1307–1560] and 1178 ng/mL [95% CI, 1162–1354], respectively; Table 2). After further adjustment for demographic characteristics, lifestyle factors, and body composition, coinfected, HIV-monoinfected, and HCV-monoinfected participants had 37%, 21%, and 12% higher sCD14 levels, respectively, than uninfected participants (Table 2).
Table 2.
Adjusted Associations of Human Immunodeficiency Virus (HIV) and Hepatitis C Virus (HCV) Infection With Soluble CD14 (sCD14) Level
| Model, Infection Status | Adjusted sCD14 Level,a ng/mL, Mean (95% CI) | Adjusted Association Between Infection Status and sCD14 Levelb | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No Additional Adjustment | Also Adjusted for I-FABP Level | Also Adjusted for Liver Stiffness | Also Adjusted for I-FABP Level and Liver Stiffness | ||||||
| Estimate,c % (95% CI) | P | Estimate,c % (95% CI) | P | Estimate,c % (95% CI) | P | Estimate,c % (95% CI) | P | ||
| Model 1 | |||||||||
| Uninfected (n=170) | 1178 (1162–1354) | Reference | Reference | Reference | Reference | ||||
| Coinfected (n=120) | 1646 (1620–1924) | 37 (28–47) | <.001 | 33 (23–43) | <.001 | 29 (19–39) | <.001 | 24 (14–34) | <.001 |
| HIV monoinfected (n=262) | 1441 (1419–1675) | 21 (15–29) | <.001 | 18 (11–25) | <.001 | 21 (14–28) | <.001 | 17 (11–25) | <.001 |
| HCV monoinfected (n=72) | 1328 (1307–1560) | 12 (3–21) | .008 | 10 (2–20) | .017 | 6 (−2–15) | .154 | 5 (−3–14) | .254 |
| Model 2 | |||||||||
| HIV monoinfectedd (n=262) | 1441 (1419–1675) | Reference | Reference | Reference | Reference | ||||
| Coinfected (n=120) | 1646 (1620–1924) | 13 (6–22) | <.001 | 13 (5–21) | .001 | 7 (−1–16) | .101 | 6 (−3–15) | .177 |
Full information maximum likelihood analysis was used, to account for missing data.
Abbreviations: CI, confidence interval; I-FABP, intestinal fatty acid binding protein.
aAdjusted for demographic characteristics (age, sex, and race).
bAdjusted for demographic characteristics (age, sex, and race), smoking status, alcohol use, and waist circumference.
cDenotes difference in levels relative to uninfected controls and, in the HIV-infected models, relative to the HIV-monoinfected group.
dAlso adjusted for HIV load, history of clinical AIDS, nadir CD4+ T-cell count, and antiretroviral therapy duration.
Additional adjustment for I-FABP level modestly attenuated the associations in both HIV-infected groups (from 37% to 33% higher sCD14 levels in the coinfected group and from 21% to 18% higher levels in the monoinfected group), compared with the uninfected group. The attenuation in HCV-monoinfected participants was minimal (from 12% to 10% higher sCD14 levels).
By contrast, additional adjustment for liver stiffness substantially attenuated the association in both HCV-infected groups (from 37% to 29% higher sCD14 levels in the coinfected group and from 12% to 6% higher levels the monoinfected group). The attenuation in the HIV-monoinfected participants was minimal (21% higher sCD14 levels in both models).
Adjustment for I-FABP level and liver stiffness had an additive attenuating effect on sCD14 levels, which was most marked in both HCV-infected groups (from 37% to 24% higher sCD14 levels in the coinfected group and from 12% to 5% higher levels in the monoinfected group). The attenuation in the model with both measures was much less in HIV-monoinfected participants (from 21% to 17% higher sCD14 levels).
In analysis restricted to HIV-infected participants (Table 2), after controlling for HIV-specific variables in addition to demographic characteristics, lifestyle factors, and body composition, coinfected participants had 13% higher sCD14 levels than HIV-monoinfected participants, suggesting that the effect of each infection is additive. This association was not attenuated by adjusting for I-FABP level (which yielded 13% higher sCD14 levels in both models) but was substantially attenuated by adjustment for liver stiffness (from 13% to 7% higher sCD14 levels) and no longer statistically significant (P = .101).
In additional analyses, restricted to 535 individuals for whom complete data were available, the associations of HIV and HCV with plasma sCD14 concentrations in models adjusted for I-FABP level and liver stiffness were similar to those where the full information maximum likelihood model was used to account for missing data (Supplementary Table 1).
Percentage of the Effect on sCD14 Level That Is Mediated by the I-FABP Level and Liver Stiffness During Infection With HIV and/or HCV
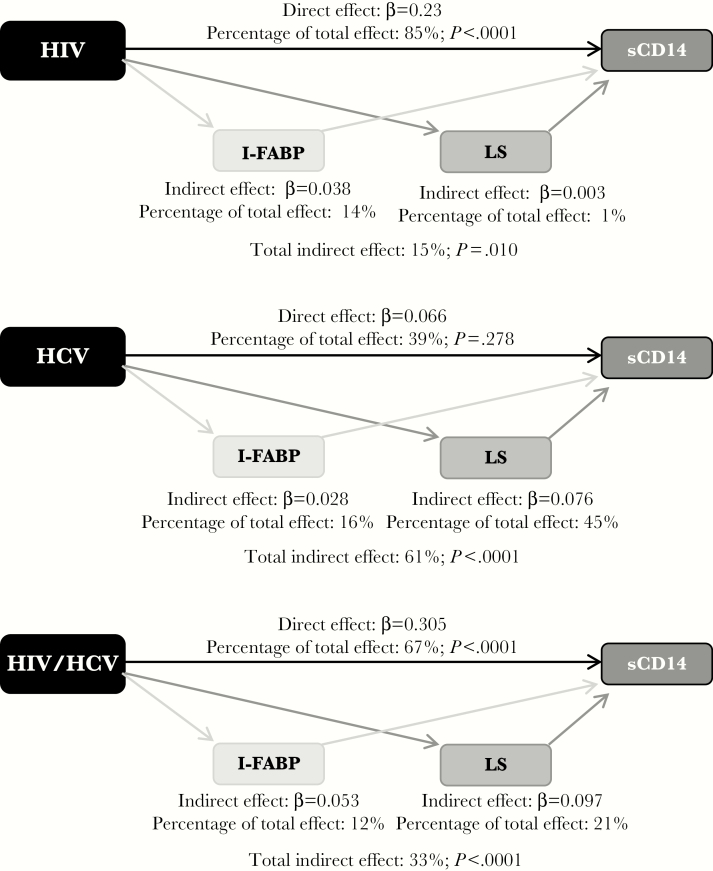
Using path analysis, we estimated the percentage of the effect on the sCD14 level that might be mediated by gut translocation (determined on the basis of I-FABP levels) and liver stiffness (measured by TE). Figure 1 shows the fully adjusted associations of HIV and HCV infection with relative differences in sCD14 levels, compared with the uninfected group; the percentage of the total effect attributable to direct (ie, infection status) and indirect (ie, I-FABP level and liver stiffness) effects of infection status is depicted. In coinfected participants, the I-FABP level accounted for 12% of the higher sCD14 levels, liver stiffness accounted for 21%, and coinfection accounted for 67%. By contrast, in HIV-monoinfected participants, the I-FABP level accounted for 14% of the higher sCD14 levels, whereas liver stiffness accounted for only 1%, while in HCV-monoinfected participants, the I-FABP level accounted for 16% of the higher sCD14 levels, whereas liver stiffness accounted for 45%.
Figure 1.
Path analysis showing multivariable adjusted direct and indirect effects of human immunodeficiency virus (HIV) and hepatitis C virus (HCV) infection status on soluble CD14 (sCD14) level. Standardized β coefficients for each path are shown, as well as percentages of the total effect attributable to direct (infection status) and indirect (intestinal fatty acid binding protein [I-FABP] level and liver stiffness [LS]) effects of infection status. Models are adjusted for HIV status, HCV status, age, sex, race/ethnicity, smoking status, alcohol use, and waist circumference.
We also examined whether the sCD14 level and liver stiffness mediated the association with I-FABP in individuals with HIV and/or HCV infection, compared with uninfected individuals. In coinfected participants, the sCD14 level accounted for 15% of the higher I-FABP level, and coinfection accounted for the remaining 85% (β coefficients, 0.16 for indirect effect [P = .006] and 0.89 for direct effect [P < .0001]). In HIV-monoinfected and HCV-monoinfected individuals, the sCD14 level accounted for 11% of the higher I-FABP level, and monoinfection accounted for 15% (β coefficients, 0.097 for indirect effect [P = .007] and 0.76 for direct effect [P < .0001] in the HIV-monoinfected group and 0.048 for indirect effect [P = .042] and 0.89 for direct effect [P = .063] in the HCV-monoinfected group). By contrast, there was not a mediation effect of liver stiffness on the I-FABP level for any infection group.
DISCUSSION
In our cohort of women and men with coinfection, HIV monoinfection, HCV monoinfection, or no infection, we found that HIV and HCV were independently associated with higher levels of sCD14 and that coinfection appeared to have an additive effect on levels of sCD14. We also provide evidence of an association of intestinal epithelial damage, as measured by I-FABP level, with immune activation in all three infection groups, although the association was most pronounced and significant in the HIV-infected group, regardless of HCV status.
Liver fibrosis was an important mediator of higher sCD14 levels in the HCV-infected group, regardless of HIV status, but had little effect on the association of HIV monoinfection with sCD14 levels. Importantly, we also found that monocyte activation but not liver fibrosis was an important mediator of the association of HIV and HCV infections with gut epithelial damage. Our findings suggest that microbial translocation contributes to immune activation in HIV infection, whereas liver fibrosis plays a stronger role in immune activation with HCV infection. Compared with the association of liver stiffness with sCD14 level in HCV infection, the association of I-FABP level with sCD14 level in HIV infection was small, which corroborates the findings of HIV studies that interventions to control microbial translocation did not lead to a decrease in markers of immune activation [22, 23]. Nevertheless, coinfected persons may be at greatest risk for progressive HIV and HCV disease, because of the independent effects of microbial translocation and liver fibrosis on immune activation.
Our study demonstrates that HIV and HCV infection have independent and additive effects on monocyte activation. Most studies that have examined monocyte activation during HIV infection and its adverse clinical sequelae have not included an HIV-uninfected comparison group [1, 6, 24]. Similarly, studies that examined the association of monocyte activation with liver disease progression in coinfected persons did not include an HCV-monoinfected comparison group, so the independent effects of HCV infection could not be examined [13], or they included persons with advanced liver disease [25].
While we demonstrated that I-FABP level was associated with monocyte activation in HIV-infected persons, a key finding in our analysis was the substantial independent effect of liver fibrosis on monocyte activation in HCV-infected persons. These data suggest that hepatocyte macrophage function could be sufficiently impaired in HCV-mediated fibrosis and, thus, that normal detoxification of endotoxins via lipoprotein scavenging does not occur. Our findings support the hypothesis that when liver function is impaired, the products of microbial translocation bypass hepatic filtration and activate circulating monocytes in the peripheral blood that in turn release sCD14. While 45% of the higher level of sCD14 in HCV-monoinfected persons was attributable to liver stiffness, only 1% was attributable to liver stiffness in HIV-monoinfected persons. This striking difference suggests a small role of liver fibrosis on monocyte activation in HIV infection, which could be explained by the fact that there are other mechanisms of immune activation in the setting of HIV infection and that HIV infection does not typically cause liver fibrosis in most people; in our study sample, we also noted that few HIV-monoinfected persons had clinically significant liver fibrosis. Interestingly, although liver fibrosis plays a substantial role in monocyte activation in HCV-infected persons, the association between coinfection and sCD14 levels remained significant even after adjusting for both I-FABP level and liver stiffness. Our findings could indicate that treating HCV infection, especially at early stages of fibrosis, will not only eradicate the virus but also mitigate the progression of liver fibrosis and inflammation-related complications.
A novel and unexpected finding of our analysis was that sCD14 had a significant mediation effect between coinfection and I-FABP. This suggests that increased monocyte activation or the resulting increased inflammation may be an important determinant of gut epithelial damage. Our finding is consistent with data that have demonstrated that proinflammatory cytokines are key modulators of gut barrier function [26, 27] and supports the hypothesis that both HIV and HCV infection drive reciprocal interactions between immune activation and gut epithelial damage: sustained monocyte activation induces a cycle whereby gut microbial translocation leads to cytotoxic and inflammatory responses, which in turn increase damage to the intestinal gut barrier and stimulate further translocation [28]. Our finding is contrary to the established notion that microbial translocation leads to immune activation and warrants further study.
Our study has some important limitations. First, we were unable to examine causal relationships because of the cross-sectional design of the study. While mediation analysis helps to clarify the nature of the relationship between the independent and dependent variables, further prospective research is necessary to validate our results. Second, since many potentially important factors, including specific comorbid data and adipose tissue parameters, were not measured in this analysis, we are unable to rule out confounding by these factors. Third, we used I-FABP level as a surrogate of microbial translocation instead of blood concentrations of bacterial lipopolysaccharide. However, Lipopolysaccharide is technically difficult to measure, and results are often inconsistent, even under research conditions [29]. For this reason, we chose to use I-FABP level, which provides an accurate, albeit indirect assessment of microbial translocation. We also used imaging to estimate liver fibrosis. Performance of the clinical gold standard, liver biopsy, would not be practical in this large population. In addition, TE data were not available for inclusion in the analysis in 11% of participants, but findings were similar when imputed analysis and complete case multivariable analysis were performed. Finally, while we pooled data from 2 cohorts, the VAHH was designed to allow for pooled analysis of its data with WIHS data; multivariable regression analysis was also used to control for differences in characteristics between the 2 cohorts.
In summary, HIV and HCV infection are independently associated with higher sCD14 levels. Gut epithelial damage is associated with immune activation in the setting of HIV, whereas liver fibrosis plays a greater role in determining immune activation in the setting of HCV. Because immune activation has been associated with HIV and HCV disease progression, our findings highlight the importance of treating both HIV and HCV infection to curb immune activation and its clinical sequelae in patients with either or both infections.
STUDY SITES AND INVESTIGATORS
WIHS I–WIHS V sites (principal investigator[s]; National Institutes of Health award number) are as follows: University of Alabama–Birmingham School of Medicine WIHS (Michael Saag, Mirjam-Colette Kempf, and Deborah Konkle-Parker; U01-AI-103401); Atlanta WIHS (Ighovwerha Ofotokun and Gina Wingood; U01-AI-103408); Bronx WIHS (Kathryn Anastos; U01-AI-035004); Brooklyn WIHS (Howard Minkoff and Deborah Gustafson; U01-AI-031834); Chicago WIHS (Mardge Cohen and Audrey French; U01-AI-034993); Metropolitan Washington WIHS (Seble Kassaye; U01-AI-034994); Miami WIHS (Margaret Fischl and Lisa Metsch; U01-AI-103397); University of North Carolina–Chapel Hill WIHS (Adaora Adimora; U01-AI-103390); Connie Wofsy Women’s HIV Study, Northern California (Ruth Greenblatt, Bradley Aouizerat, and Phyllis Tien; U01-AI-034989); WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub; U01-AI-042590); and Southern California WIHS (Joel Milam; U01-HD-032632).
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health.
Financial support. This work was supported by the University of California–San Francisco Liver Center (grant P30 DK026743), the National Institute of Allergy and Infectious Diseases (NIAID; grant K24 AI 108516 [to P. C. T.] and grant R01 AI 087176 [to P. C. T.], which was administered by the Northern California Institute for Research and Education and with resources of the Veterans Affairs Medical Center, San Francisco, CA), the University of California –San Francisco Gladstone Institute of Virology and Immunology Center for AIDS Research (grant P30-AI027763 to J. C. P.), and the American College of Gastroenterology (Junior Faculty Development Award to J. C. P.).
The Women’s Interagency HIV Study (WIHS) is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional cofunding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute on Mental Health. Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research, the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Deafness and other Communication Disorders, and the National Institutes of Health (NIH) Office of Research on Women’s Health. WIHS data collection is also supported by the NIH (Clinical and Translational Science awards UL1-TR000004 [to the University of California–San Francisco] and UL1-TR000454 [to the Atlanta WIHS]).
Potential conflicts of interest. A. L. F. reports receiving grants (during the conduct of the study) from the National Institutes of Health (NIH). M. P. reports receiving grants (during the conduct of the study) from the NIH and personal fees (for activities outside the submitted work) from Abbott, Gilead, Genentech, Johnson & Johnson, Merck, and Roche. G. D. H. reports receiving grants and personal fees (for activities outside the submitted work) from Gilead Sciences, Janssen, and Viiv; grants (for activities outside the submitted work) from Bristol-Myers Squibb; and personal fees (for activities outside the submitted work) from CSL. All other authors report no potential conflicts.
Presented in part: 24th Conference on Retroviruses and Opportunistic Infections, Seattle, Washington, 13–16 February 2017. Poster 526.
References
- 1. Sandler NG, Wand H, Roque A et al. ; INSIGHT SMART Study Group Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 2011; 203:780–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aly AM, Adel A, El-Gendy AO, Essam TM, Aziz RK. Gut microbiome alterations in patients with stage 4 hepatitis C. Gut Pathog 2016; 8:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sandler NG, Koh C, Roque A et al. Host response to translocated microbial products predicts outcomes of patients with HBV or HCV infection. Gastroenterology 2011; 141:1220–30, 1230.e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brenchley JM, Price DA, Schacker TW et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006; 12:1365–71. [DOI] [PubMed] [Google Scholar]
- 5. Brenchley JM, Schacker TW, Ruff LE et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med 2004; 200:749–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hunt PW, Sinclair E, Rodriguez B et al. Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis 2014; 210:1228–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jiang W, Lederman MM, Hunt P et al. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis 2009; 199:1177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Andreu M, Sola R, Sitges-Serra A et al. Risk factors for spontaneous bacterial peritonitis in cirrhotic patients with ascites. Gastroenterology 1993; 104:1133–8. [DOI] [PubMed] [Google Scholar]
- 9. Reiberger T, Ferlitsch A, Payer BA et al. ; Vienna Hepatic Hemodynamic Lab Non-selective betablocker therapy decreases intestinal permeability and serum levels of LBP and IL-6 in patients with cirrhosis. J Hepatol 2013; 58:911–21. [DOI] [PubMed] [Google Scholar]
- 10. Cirera I, Bauer TM, Navasa M et al. Bacterial translocation of enteric organisms in patients with cirrhosis. J Hepatol 2001; 34:32–7. [DOI] [PubMed] [Google Scholar]
- 11. Pascual S, Such J, Esteban A et al. Intestinal permeability is increased in patients with advanced cirrhosis. Hepatogastroenterology 2003; 50:1482–6. [PubMed] [Google Scholar]
- 12. Steele AK, Lee EJ, Vestal B et al. Contribution of intestinal barrier damage, microbial translocation and HIV-1 infection status to an inflammaging signature. PLoS One 2014; 9:e97171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. French AL, Evans CT, Agniel DM et al. Microbial translocation and liver disease progression in women coinfected with HIV and hepatitis C virus. J Infect Dis 2013; 208:679–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bacon MC, von Wyl V, Alden C et al. The Women’s Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol 2005; 12:1013–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barkan SE, Melnick SL, Preston-Martin S et al. The Women’s Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology 1998; 9:117–25. [PubMed] [Google Scholar]
- 16. Swanson S, Ma Y, Scherzer R et al. Association of HIV, hepatitis C virus, and liver fibrosis severity with the enhanced liver fibrosis score. J Infect Dis 2016; 213:1079–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bailony MR, Scherzer R, Huhn G, Plankey MW, Peters MG, Tien PC. Association of HIV infection, hepatitis C virus infection, and metabolic factors with liver stiffness measured by transient elastography. J Infect Dis 2013; 208:1776–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Price JC, Ma Y, Scherzer R et al. Human immunodeficiency virus-infected and uninfected adults with non-genotype 3 hepatitis C virus have less hepatic steatosis than adults with neither infection. Hepatology 2017; 65:853–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Singh S, Muir AJ, Dieterich DT, Falck-Ytter YT. American gastroenterological association institute technical review on the role of elastography in chronic liver diseases. Gastroenterology 2017; 152:1544–77. [DOI] [PubMed] [Google Scholar]
- 20. Wright S. The method of path coefficients. Ann Math Statist 1934; 5. [Google Scholar]
- 21. Allison P. Handling missing data by maximum likelihood. SAS GLobal Forum2012, 312. [Google Scholar]
- 22. Sandler NG, Zhang X, Bosch RJ et al. ; AIDS Clinical Trials Group A5296 Team Sevelamer does not decrease lipopolysaccharide or soluble CD14 levels but decreases soluble tissue factor, low-density lipoprotein (LDL) cholesterol, and oxidized LDL cholesterol levels in individuals with untreated HIV infection. J Infect Dis 2014; 210:1549–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tenorio AR, Chan ES, Bosch RJ et al. ; A5286 Team Rifaximin has a marginal impact on microbial translocation, T-cell activation and inflammation in HIV-positive immune non-responders to antiretroviral therapy - ACTG A5286. J Infect Dis 2015; 211:780–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wallet MA, Rodriguez CA, Yin L et al. Microbial translocation induces persistent macrophage activation unrelated to HIV-1 levels or T-cell activation following therapy. AIDS 2010; 24:1281–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. de Oca Arjona MM, Marquez M, Soto MJ et al. Bacterial translocation in HIV-infected patients with HCV cirrhosis: implication in hemodynamic alterations and mortality. J Acquir Immune Defic Syndr (1999) 2011; 56:420–7. [DOI] [PubMed] [Google Scholar]
- 26. Harris HW, Grunfeld C, Feingold KR et al. Chylomicrons alter the fate of endotoxin, decreasing tumor necrosis factor release and preventing death. J Clin Invest 1993; 91:1028–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Read TE, Harris HW, Grunfeld C et al. Chylomicrons enhance endotoxin excretion in bile. Infect Immun 1993; 61:3496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bruewer M, Luegering A, Kucharzik T et al. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol 2003; 171:6164–72. [DOI] [PubMed] [Google Scholar]
- 29. Balagopal A, Gama L, Franco V et al. ; NWCS 319 and ACTG 5175 study team Detection of microbial translocation in HIV and SIV infection using the Limulus amebocyte lysate assay is masked by serum and plasma. PLoS One 2012; 7:e41258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.