Abstract
Background
In TEMPO 3:4, the vasopressin V2 receptor antagonist tolvaptan slowed total kidney volume (TKV) growth and estimated glomerular filtration rate (eGFR) decline relative to placebo.
Methods
TEMPO 4:4 was designed to provide an additional 2 years of data on the long-term safety and efficacy of tolvaptan in subjects completing TEMPO 3:4. The objective was to assess the disease-modifying effects of tolvaptan on TKV and eGFR end-points including change from baseline over the combined duration of TEMPO 3:4 and TEMPO 4:4, and non-inferiority of slopes during TEMPO 4:4.
Results
Of the 1445 subjects randomized to TEMPO 3:4, 871 (60.3%) enrolled in TEMPO 4:4. Percent changes in TKV from TEMPO 3:4 baseline to TEMPO 4:4 Month 24 were 29.9% and 31.6% (prior tolvaptan versus prior placebo, P = 0.38). Adjusting for baseline covariates improved the TKV treatment difference at Month 24 in TEMPO 4:4 from −1.70% to − 4.15% between the groups (P = 0.04). Slopes of TKV growth during TEMPO 4:4 were higher in early- versus delayed-treatment groups (6.16% versus 4.96% per year, P = 0.05). Analysis of secondary eGFR endpoints demonstrated a persistent effect on eGFR (3.15 mL/min/1.73 m2, P < 0.001), and non-inferiority in eGFR slopes. The safety profile on exposure to tolvaptan in TEMPO 4:4 was similar to that in TEMPO 3:4.
Conclusions
The results of TEMPO 4:4 support a sustained disease-modifying effect of tolvaptan on eGFR. The lack of a sustained treatment difference on TKV may be accounted for by limitations of the trial design, including loss of randomization and baseline imbalances ensuing TEMPO 3:4. The safety profile was similar to that observed in TEMPO 3:4.
Keywords: autosomal dominant polycystic kidney disease, chronic kidney disease, polycystin kidney disease, vasopressin, vasopressin v2 receptor antagonist
INTRODUCTION
Autosomal dominant polycystic kidney disease (ADPKD) is the fourth most common cause of end-stage renal disease. Mutations to polycystin-1 or polycystin-2 disrupt intracellular calcium homeostasis leading to upregulated adenosine 3′,5′-cyclic monophosphate (cAMP) signaling, increased chloride-driven fluid secretion, cell proliferation and cystogenesis [1, 2]. Cyst proliferation and expansion destroy the renal parenchyma and cause kidney failure [3]. Vasopressin V2 receptor activation promotes the generation of cAMP and cystogenesis. Genetic elimination of circulating vasopressin or pharmacological V2 receptor blockade inhibits disease progression in animal models orthologous to human ADPKD, ARPKD (Autosomal Recessive Polycystic Kidney Disease) and nephronophthisis [4–6].
Tolvaptan, an oral selective vasopressin V2 receptor antagonist, lowers cAMP within the epithelial cells of collecting ducts and distal nephrons, the major sites of cyst development in ADPKD [7]. In the pivotal TEMPO 3:4 (Tolvaptan Efficacy and safety in Management of Polycystic kidney disease and its Outcomes) Clinical Trial, tolvaptan slowed total kidney volume (TKV) growth by 49.2% relative to placebo (2.8% versus 5.5% per year, P < 0.001). Most of the treatment effect on TKV, observed during the first year, was thought to be due to inhibition of fluid secretion into cysts. Smaller incremental benefits observed throughout the 3 years were attributed to inhibition of epithelial cell proliferation [8]. Other benefits of treatment included lower rates of estimated glomerular filtration rate (eGFR) decline by 26% (0.98 mL/min/1.737 m2 per year) and of adjudicated events of renal function decline (equivalent to 30% reduction in eGFR) and kidney pain (requiring medical intervention or absence from work).
The open-label extension trial TEMPO 4:4 was designed to provide an additional 2 years of data on long-term safety and efficacy of tolvaptan in subjects who completed TEMPO 3:4. Its objective was to assess whether tolvaptan has disease-modifying effects on TKV growth and eGFR decline, measured by change from baseline over the combined duration of TEMPO 3:4 and TEMPO 4:4, and non-inferiority of TKV and eGFR slopes in early- versus delayed-treated subjects (prior tolvaptan and prior placebo in TEMPO 3:4) during TEMPO 4:4.
MATERIALS AND METHODS
Trial design and oversight
TEMPO 4:4 was an open-label, extension trial. Participation was elective for TEMPO 3:4 (clinical trial identifier: NCT00428948, 2006-002768-24) sites and their subjects. In Japan, a separate long-term follow-up extension trial was offered to most subjects (NCT01280721).
Eligible subjects had to enroll within 6 months of successful completion of TEMPO 3:4 and have an eGFR by Modification of Diet in Renal Disease (MDRD ≥30 mL/min/1.73 m2 within 45 days prior to the baseline visit. Detailed inclusion and exclusion criteria are presented in Supplementary data, Table S1. Oral tolvaptan was administered in daily split-dose regimens of 45/15 mg, 60/30 mg or 90/30 mg upon waking and approximately 9 h later. Subjects were titrated to the highest dose tolerated in TEMPO 3:4 and were able to titrate up or down per investigator discretion to maximize tolerability and urine osmolality suppression. Adherence to treatment was self-reported and monitored by tablet count. Use of diuretics and drugs inhibiting the cytochrome P-450 enzyme CYP3A4 were discouraged.
TEMPO 4:4 (clinical trial identifier: CT01214421, 2010-018401-10) was in compliance with International Conference on Harmonization Good Clinical Practice guidelines. The informed consent form, protocol and amendments were approved by institutional review boards or independent ethics committees of all trial sites. A steering committee of investigators and sponsor representatives oversaw the trial design and conduct with assistance of the Independent Data Monitoring Committee and Hepatic Adjudication Committee.
Trial assessments
Evaluations were performed in-clinic, as previously described in the TEMPO 3:4 Trial [8], at baseline and on Months 1, 3, 6 and every 6 months thereafter for 24 months. Interim laboratory tests [alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, total bilirubin and serum creatinine] were measured centrally at 3-month intervals between the scheduled 6-month visits. In TEMPO 3:4, the IDMS (isotope dilution mass spectrometry)-traceable Roche enzymatic method was used to measure creatinine. Initially, assays in TEMPO 4:4 used the rate blank method, which was corrected (2–3 months into the trial) through an amendment to the protocol requiring both methods moving forward. Only serum creatinine values obtained by the IDMS-traceable Roche enzymatic method were used in eGFR analyses. For subjects who did not complete the trial, final MRI scans were obtained within 2 weeks of withdrawal if not acquired during the previous 6 months.
Outcome measures
The primary endpoint was the change in TKV from TEMPO 3:4 baseline to TEMPO 4:4 Month 24 in early- versus delayed-treated subjects. Key secondary endpoints, hierarchal and tested sequentially, were changes in eGFR [Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI)] from TEMPO 3:4 baseline to TEMPO 4:4 Month 24 and TKV and eGFR slopes during TEMPO 4:4 in early- and delayed-treated subjects. Additional analyses included changes from baseline in TKV and eGFR by prespecified subgroups (e.g. gender). Safety endpoints assessed at all clinic visits comprised reported treatment-emergent adverse events (TEAEs) and results of clinical laboratory tests, vital signs and electrocardiograms.
Statistical analysis
No formal sample size calculation was performed. It was assumed that up to 900 subjects (i.e. 300 and 600 subjects from the placebo and tolvaptan groups, respectively) would enroll from TEMPO 3:4.
The primary efficacy endpoint was tested by Mixed-Effect Model Repeated Measures (MMRM) with factors of treatment group (the early-treatment group and the delayed-treatment group), TEMPO 4:4 visit, treatment group visit interaction, region, TEMPO 3:4 baseline hypertension, estimated creatinine clearance and renal volume status, covariate baseline (TEMPO 3:4) and baseline visit interaction with unknown variance covariance structure for the repeated visits was applied. Comparison between early- and delayed-treatment groups at Month 24 in TEMPO 4:4 was based on least squares (LS) means at Month 24 of the MMRM to test the superiority of early tolvaptan treatment. The primary analysis was based on the efficacy sample, with alpha level 0.05.
Key secondary efficacy endpoints were tested in hierarchical order using an alpha level of 0.05. Analysis of the first key secondary endpoint was identical to the analysis of the primary endpoint, except the model used a heterogeneous Toeplitz variance covariance matrix. Other key secondary objectives were tested by a model of random coefficient regression, with fixed effects of intercept, time, treatment group (early versus delayed), treatment group time interaction and random effects of intercept and time with unknown variance covariance structure, to the data collected in TEMPO 4:4, from baseline to Month 24. In the model, time was a continuous variable, which was equal to the time spent from baseline to collection of the data.
The 95% confidence interval (CI), based on a contrast between the early- and delayed-treatment group and obtained from the treatment group time interaction, was used to test the non-inferiority null hypotheses. For TKV, log transformed data were used, and the non-inferiority null hypothesis was rejected if the upper limit of the 95% CI was less than two-thirds of the treatment effect in slowing TKV (log-transformed) growth of the TEMPO 3:4 Trial. The non-inferiority null hypothesis was rejected if the upper limit of the 95% CI was less than two-thirds of the treatment effect in slowing eGFR decline in TEMPO 3:4.
Post hoc covariate adjustment analysis was derived from an MMRM analysis of the percent change from baseline in TKV employing the model of the primary analysis with the addition of copeptin and baseline covariates of age, gender, gender visit interaction, eGFR, eGFR visit interaction and urine albumin/creatinine ratio (ACR), using an unstructured variance covariance matrix to model the repeated measures.
RESULTS
Subjects
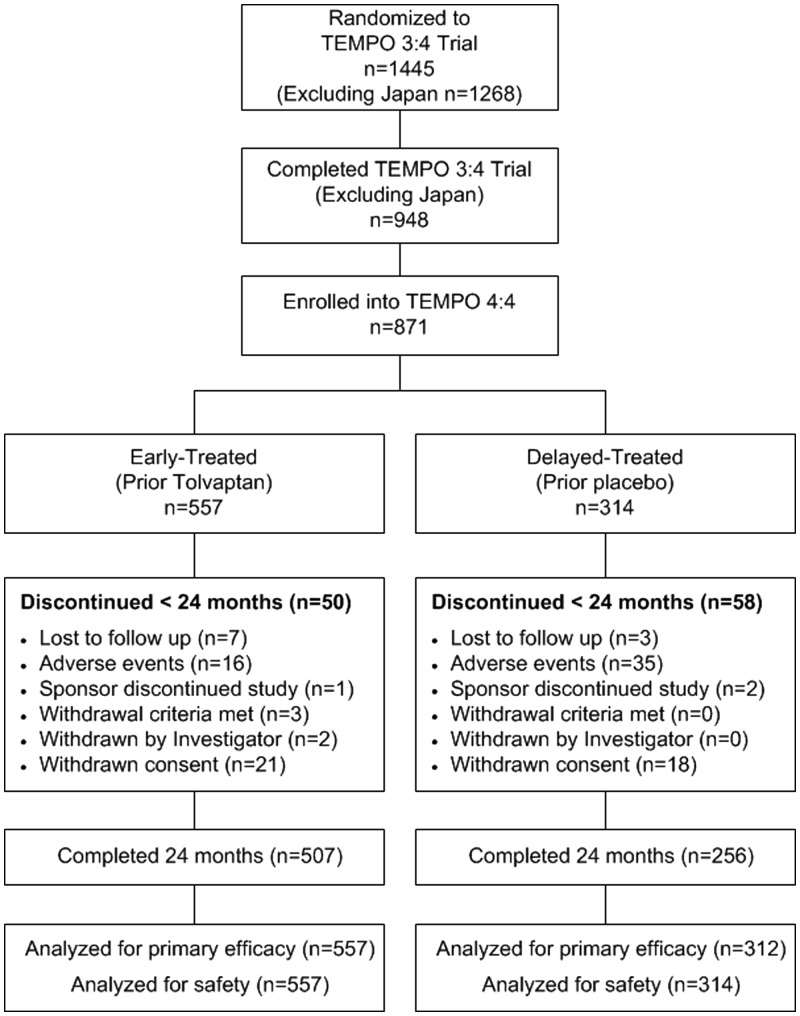
Of 1445 subjects (n = 961 tolvaptan, n = 484 placebo) randomized in TEMPO 3:4, 948 (excluding Japan) completed the trial and 871 (91.9% of eligible, 60.3% overall) of them elected to enroll in TEMPO 4:4 (n = 557, early-treatment group, 58.0% of those on prior tolvaptan; n = 314, delayed-treatment group, 64.9% of those on prior placebo) (Figure 1).
FIGURE 1.
Subject disposition in TEMPO 4:4.
The interval between completion of TEMPO 3:4 and initiation of TEMPO 4:4 varied between 13 and 829 days (mean = 81 days, median = 37 days) but was not different between early- and delayed-treated subjects (Table 1). Subject demographics and baseline characteristics of early- and delayed-treatment groups revealed baseline differences or growing imbalances from TEMPO 3:4 baseline to TEMPO 4:4 (Table 1) in gender, copeptin, ACR, TKV and eGFR.
Table 1.
Demographics characteristics at TEMPO 3:4 and TEMPO 4:4 baseline
| Characteristics | TEMPO 3:4 |
TEMPO 4:4 |
||||
|---|---|---|---|---|---|---|
| Tolvaptan (n = 557) | Placebo (n = 314) | P-value | Early-treateda (n = 557) | Delayed-treateda (n = 314) | P-value | |
| Male, n (%) | 303 (54.4) | 159 (50.3) | 0.25 | |||
| Age, years [mean (SD)] | 38.9 (6.9) | 39.2 (7.3) | 0.30 | 42.2 (6.9) | 42.5 (7.2) | 0.32 |
| Race, n (%) | 0.55 | |||||
| Caucasian | 535 (96.1) | 302 (96.2) | ||||
| Black or African American | 9 (1.6) | 2 (0.6) | ||||
| Asian | 3 (0.5) | 2 (0.6) | ||||
| Other | 10 (1.8) | 8 (2.5) | ||||
| Height, cm [mean (SD)] | 174.6 (10.4) | 173.9 (9.8) | 0.32 | 174.8 (10.3) | 174.1 (9.7) | 0.35 |
| Weight, kg [mean (SD)] | 80.5 (17.7) | 79.2 (17.2) | 0.30 | 82.6 (18.4) | 81.0 (17.3) | 0.23 |
| Age at diagnosis, years [mean (SD)] | 27.1 (9.3) | 27.2 (9.6) | 0.76 | |||
| TKV, mL [median (IQR)] | 1498 (977) | 1468 (751) | 0.60 | 1706 (1199) | 1835 (1162) | 0.05 |
| TKV, % change from TEMPO 3:4 BL | 15.8 (17.0) | 23.8 (16.9) | <0.0001 | |||
| eGFR, mL/min/1.73 m2 [mean (SD)] | 82.2 (20.6) | 83.5 (22.6) | 0.59 | 72.3 (24.5) | 70.4 (25.0) | 0.38 |
| eGFR, change from TEMPO 3:4 BL [mean (SD)] | −8.1 (−10.3) | −10.6 (−13.71) | 0.003 | |||
| Blood pressure, mmHg (SD) | ||||||
| Diastolic | 82.6 (9.1) | 82.7 (9.0) | 0.92 | 80.8 (8.6) | 81.1 (8.8) | 0.41 |
| Systolic | 128.6 (13.2) | 128.8 (13.2) | 0.86 | 126.6 (12.4) | 127.0 (12.1) | 0.68 |
| ACE/ARB use, % (n) | 72.0 (401) | 71.0 (223) | 0.76 | 81.1 (452) | 78.7 (247) | 0.38 |
| ACR, mg/mmol (SD) | 6.41 (11.75) | 6.65 (16.31) | 0.85 | 6.07 (12.46) | 7.69 (19.27) | 0.04 |
| Copeptina, pmol/L (SD) | 8.7 (10.0) | 10.9 (25.5) | 0.70 | |||
| Days between TEMPO 3:4 and 4:4, days [mean, median (range)] | 81, 39 (13–566) | 80, 36 (13–829) | 0.84 | |||
Data are given as means with standard deviation (SD), or as median with interquartile range (IQR) when appropriate. BL, baseline.
The early-treatment group received tolvaptan in TEMPO 3:4 and the late-treatment group received placebo.
In TEMPO 4:4, n = 50 (9.0%) early-treated subjects and n = 58 (18.5%) delayed-treated subjects withdrew from the trial prior to the Month 24 visit. The most common reasons for withdrawal were adverse event [n = 16 (2.9%) early-treated versus n = 35 (11.1%) delayed-treated] and consent withdrawal [n = 21 (3.8%) versus n = 18 (5.7%)]. All subjects were analyzed for safety and all but two subjects in the delayed-treated group were evaluated for efficacy.
Change from baseline in TKV
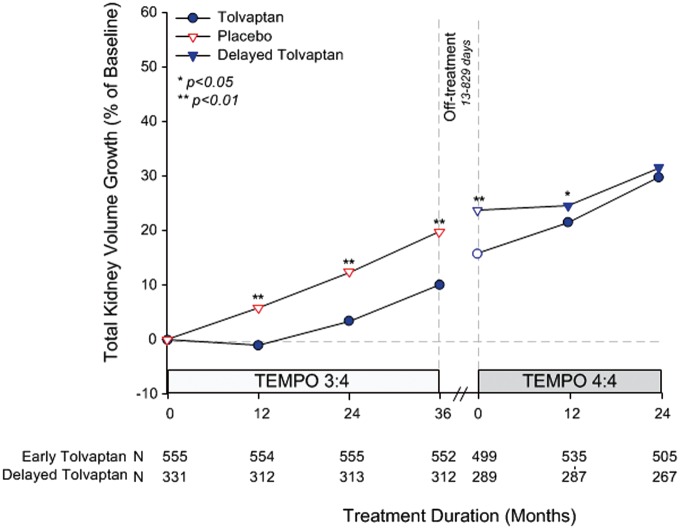
The primary efficacy endpoint was the maintenance of difference in percent change in TKV from TEMPO 3:4 baseline to TEMPO 4:4 Month 24 in early- versus delayed-treatment groups. TKV increased by 29.9% in early- versus 31.6% in delayed-treated subjects (P = 0.38) (Figure 2). The largest effect of tolvaptan on TKV growth during TEMPO 3:4 in early-treated subjects occurred during the first year of treatment and, while still statistically significant, it became smaller in subsequent years. Similarly, the largest effect of tolvaptan on TKV growth during TEMPO 4:4 in the delayed-treated subjects occurred in the first year and became smaller in the second year (Figure 2). Deceleration of TKV growth after reintroduction of tolvaptan at the initiation of TEMPO 4:4 in the early-treated subjects was correlated with the time delay between the two studies (R =−0.19, P < 0.001) (Supplementary data, Figure S1) and observed mainly in the quartile with the longest off-treatment duration (treatment effect from TEMPO 4:4 baseline to M12 comparing Q4 to Q1 −2.97% per year, P = 0.06) (Supplementary data, Figure S2). Since the primary endpoint of this trial was not reached, prespecified analyses of the secondary endpoints proceed at the risk of Type I (false positive) error. Thus, relying upon the results of these analyses, as well as well as additional posthoc analyses described below, must be considered exploratory, not confirmatory.
FIGURE 2.
Percentage change in TKV from TEMPO 3:4 baseline to TEMPO 4:4 Month 24 visit. Open circles and triangles represent off-treatment time points. Break in treatment from TEMPO 3:4 to TEMPO 4:4 ranged from 13 to 829 days from the Month 36 visit in TEMPO 3:4.
Change from baseline in eGFR
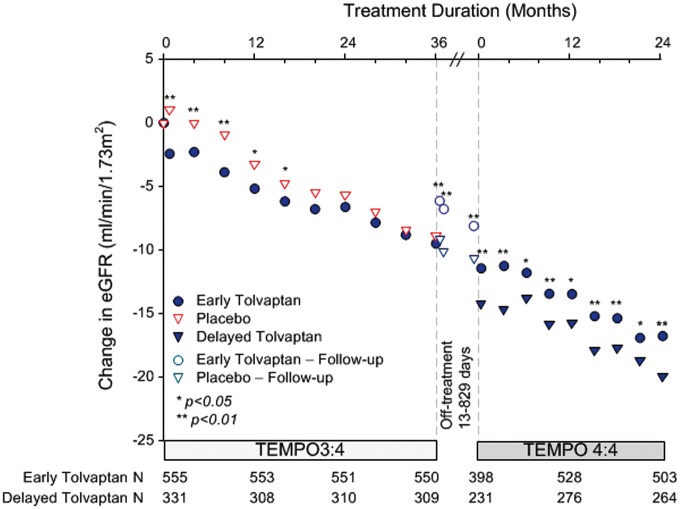
The key secondary endpoint was the change in eGFR from TEMPO 3:4 baseline to TEMPO 4:4 Month 24 in early- versus delayed-treatment groups. The nominally significant difference between early- and delayed-treatment groups was maintained at each time point, demonstrating that the effect of tolvaptan on slowing renal function decline in TEMPO 3:4 was maintained for an additional 2 years in TEMPO 4:4 (3.15 mL/min/1.73 m2, P < 0.001), (Figure 3). Contrary to the changes in TKV, changes in eGFR during the first year (or during the first month) of TEMPO 4:4 in early-treated subjects were not correlated with the time delays between the two studies (Supplementary data, Figure S3).
FIGURE 3.
Change in eGFR from the TEMPO 3:4 baseline to the Month 24 visit of TEMPO 4:4. The inter-trial period is plotted as a 5-month interval, which approximates the 80th percentile of the treatment holiday (range 13–829 days). Open circles and triangles represent off-treatment time points.
Slope of TKV in TEMPO 4:4
TKV slopes in TEMPO 4:4 were higher in early- compared with delayed-treated subjects (6.16 versus 4.96% per year; treatment difference 1.011, 95% CI, 1.00, 1.02, P = 0.05) (Table 2). After initiation of tolvaptan in TEMPO 4:4, a substantial effect on TKV growth during the first year of treatment occurred only in the delayed-treated subjects (Figure 2), while it was blunted or absent in the early-treatment group (Supplementary data, Figure S2). Since the prespecified Statistical Analysis Plan for non-inferiority required that a TKV slope difference between treatment groups should have an upper limit of the 95% CI not greater than two-third of the effect size seen in TEMPO 3:4 (0.0076 in log-10 scale) and the observed upper limit was 1.02 (0.0099 in log-10 scale), non-inferiority could not be established.
Table 2.
Slope of TKV and eGFR from baseline to Month 24 in TEMPO 4:4
| Treatment Group | n | Slope (% per year) | Treatment difference | 95% CI | P-value | NI margin |
|---|---|---|---|---|---|---|
| TKV (mL) | ||||||
| Early-treateda | 509 | 6.16 | 1.01 | 1.00, 1.02 | 0.046 | 0.65 |
| Delayed-treateda | 268 | 4.96 | ||||
| eGFR, mL/min/1.73 m2b | ||||||
| Early-treateda | 548 | −3.26 | −0.11 | −0.75, 0.52 | 0.73 | 0.65 |
| Delayed-treateda | 304 | −3.14 | ||||
NI, non-inferiority.
The early-treatment group received tolvaptan in TEMPO 3:4 and the late-treatment group received placebo.
eGFR by CKD-EPI equation
Slope of eGFR in TEMPO 4:4
eGFR slopes in TEMPO 4:4 were similar in early- and delayed-treated subjects (−3.26 versus −3.14 mL/min/1.73 m2 per year; treatment difference −0.11, 95% CI, −0.75, 0.52, P = 0.73) (Table 2). This secondary endpoint met the protocol predefined non-inferiority criterion, since the upper limit of the 95% CI (0.520) is less two-thirds of the eGFR slope difference in TEMPO 3:4 (0.98, 95% CI 0.60, 1.36). In addition, the upper limit of the 95% CI (0.52) is also smaller than the lower limit of the TEMPO 3:4 95% CI (0.60), showing that the non-inferiority criterion based on the FDA (Food and Drug Administration) M1 margin was also met [9].
Impact of non-randomization and covariate imbalance
Randomization and stratification covariates such as gender were well balanced at baseline in TEMPO 3:4 (ratio of males/females, 1.06 versus 1.08 in tolvaptan versus placebo groups) [8]. At baseline in TEMPO 4:4, however, there was a gender imbalance between early and delayed treatment groups (ratio of males/females, 1.19 versus 1.02), which further increased at Month 24 (ratio of males/females, 1.22 versus 1.02) due to higher rates of withdrawal of females compared with males in both trials.
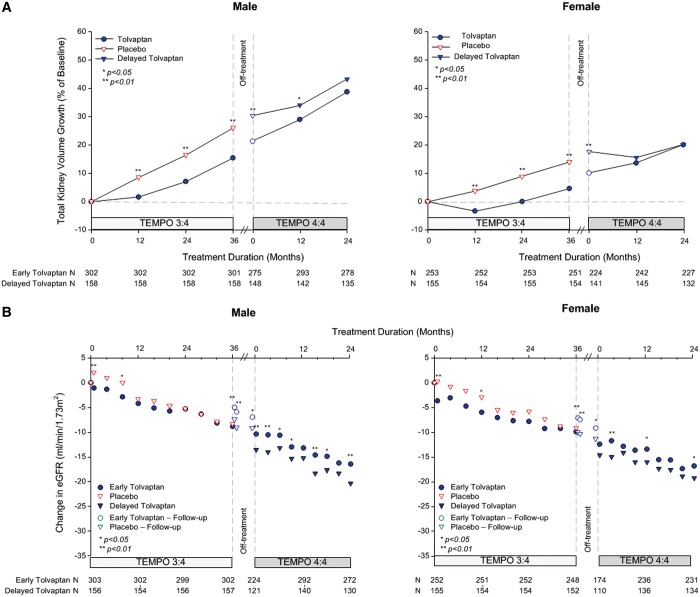
In the placebo arm of TEMPO 3:4, TKV growth in males progressed at nearly twice the rate as female subjects (LS mean change from baseline to M36; 26.0% versus 14.0%, respectively). In addition, based on TEMPO 3:4, treated by tolvaptan, male subjects had TKV annual growth rate of 4.4% versus female subjects of 1.4%. Thus, the drift from balanced randomization towards an increasing proportion of males in the early-treatment group would be confounded with the primary endpoint of TKV growth and expected to narrow the treatment difference in the overall analysis. Analysis by gender reveals the narrowing to be more significant for females (decreasing to −0.06%, P = 0.98) compared with males (decreasing to −4.48%, P = 0.104), potentially due to a larger acute response in delayed-treated female subjects in Year 1, (Figure 4A). Thus, the temporal drift in this covariate may have contributed to a lack of continued separation for TKV during TEMPO 4:4.
FIGURE 4.
Change from baseline in TKV and eGFR by gender. (A) Percentage change in TKV from TEMPO 3:4 baseline to Month 24 in TEMPO 4:4 for males (left panel) and females (right panel). (B) Change in eGFR from TEMPO 3:4 baseline to Month 24 in TEMPO 4:4 for males (left panel) and females (right panel). Open circles and triangles represent off-treatment time points.
In contrast, the change in renal function in the placebo arm of TEMPO 3:4 was less influenced by gender (LS mean change from baseline to FU (follow-up) visit 2; −9.35 versus −10.62 mL/min/1.73 m2, male versus female) and, treated by tolvaptan, male and female subjects in TEMPO 3:4 had a similar annual rate of eGFR decline (2.80 versus 2.64 mL/min/1.73 m2). Male and female subjects in TEMPO 3:4 experienced a similar tolvaptan treatment difference on eGFR over 3 years (3.45 versus 3.18 mL/min/1.73 m2, P = 0.001 versus P = 0.005, respectively) and both genders maintained the treatment difference for an additional 2 years in TEMPO 4:4 (3.94 versus 2.49 mL/min/1.73 m2, P = 0.001 versus 0.04, respectively) (Figure 4B).
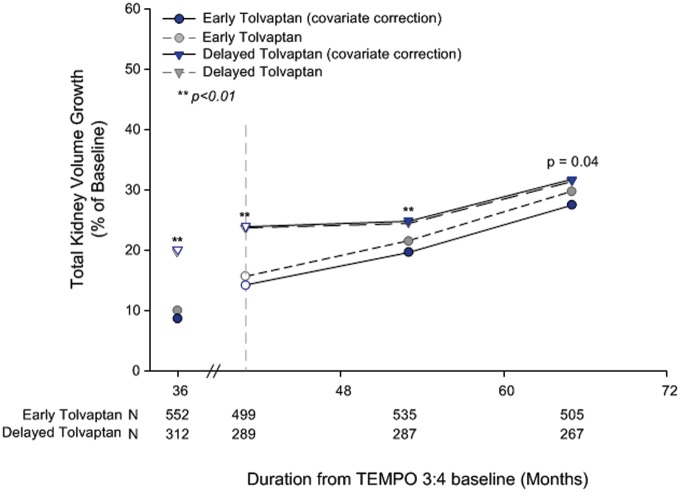
Because of the treatment effect of tolvaptan in TEMPO 3:4, there were additional imbalances in TKV, eGFR and urine ACR at the baseline of TEMPO 4:4. Adjusting for these imbalances, other important covariates (age, height, hypertensive status and region) and baseline characteristics that in posthoc analyses predicted the response to tolvaptan treatment (urine ACR and serum copeptin) [10, 11], improved the TKV treatment difference at Month 24 in TEMPO 4:4 from −1.70% to −4.15% between the groups and the P-value from P = 0.38 to P = 0.04 (Figure 5 and Supplementary data, Figure S4).
FIGURE 5.
Percentage change from baseline in TKV when adjusted for covariates. Open circles and triangles represent off-treatment time points. Blue and gray symbols are slightly offset for ease of interpretation. The following variables were included in an unstructured variance covariance matrix with fixed factors as in the primary analysis with the addition of copeptin and baseline covariates of age, gender, gender visit interaction, eGFR, eGFR visit interaction and urine ACR.
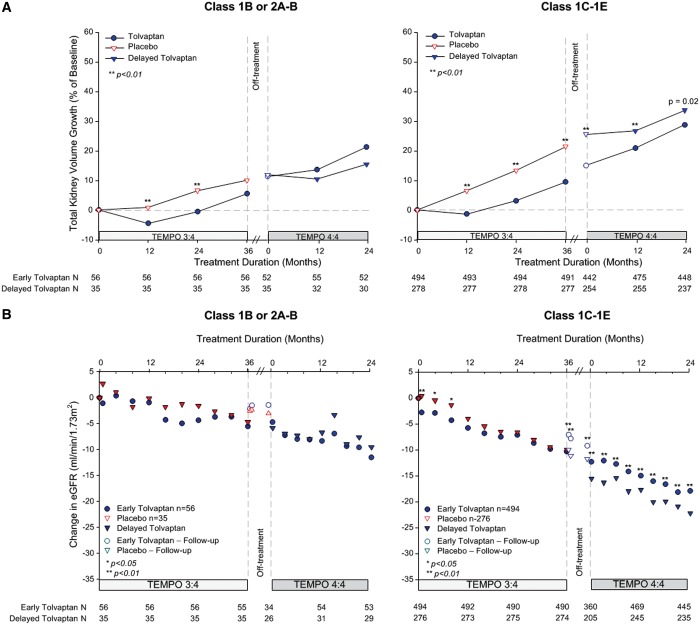
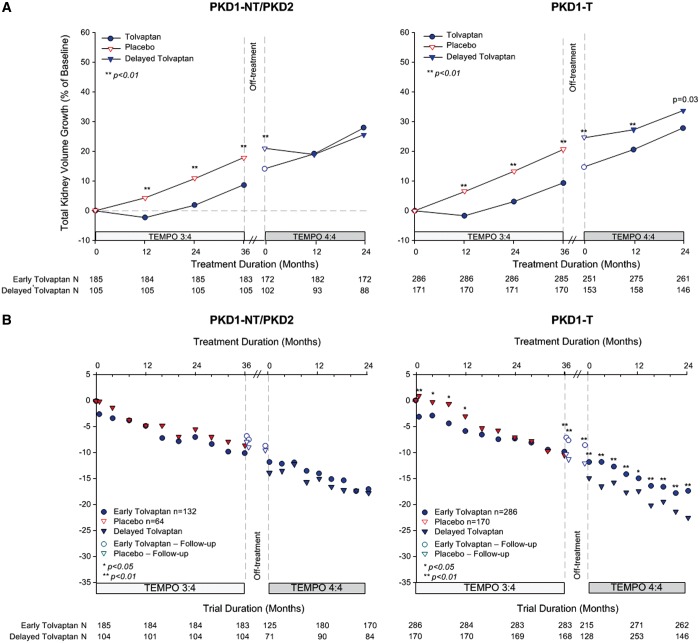
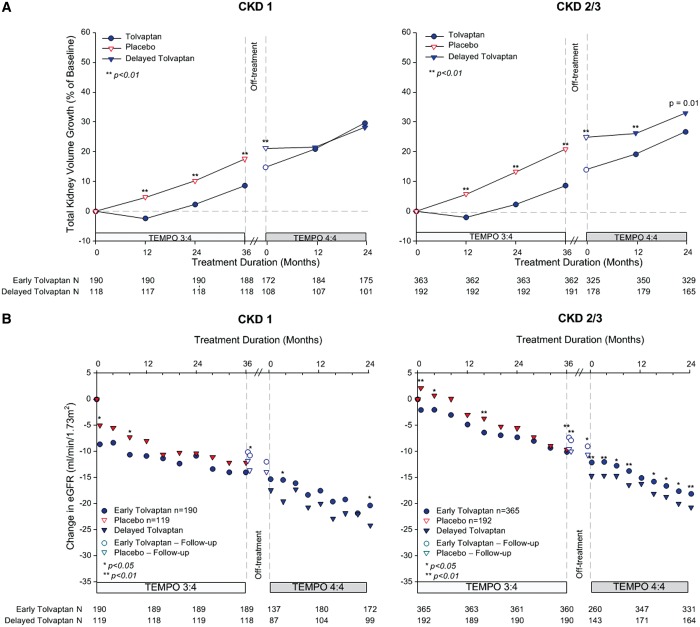
In previous posthoc analyses of TEMPO 3:4, we found that the beneficial effect of tolvaptan on TKV and eGFR was greater and/or easier to demonstrate in the subjects with more severe (as determined either by imaging [12] or genotype [13]) or more advanced (as determined by CKD stage at baseline) ADPKD [14]. We also noted that the treatment effect of tolvaptan on TKV during Year 1 was similar in subjects with CKD 1, 2 and 3, whereas it was greater during years 2 and 3 in CKD 2 and CKD 3 compared with CKD 1 subjects, where it was very small [14]. Based on these observations, we performed posthoc analyses to determine whether the treatment effects of tolvaptan on TKV and eGFR at the end of TEMPO 3:4 were maintained during TEMPO 4:4 in the subjects with more severe (i.e. image class 1C–E or with truncating PKD1 mutations) or more advanced (CKD 2–3) disease. The treatment effects of tolvaptan on TKV and on eGFR were maintained and statistically significant in groups with more severe (Figures 6 and 7) or more advanced disease (Figure 8). In imaging class 1B or 2A–B (because of the entry criteria enrichment no class 1A subjects were enrolled in TEMPO 3:4) subjects the tolvaptan treatment effects were not significant either at the end of TEMPO 3:4 or at the end of TEMPO 4:4. A significant difference between tolvaptan- and placebo-treated CKD 1 subjects and those with non truncating PKD 1 or PKD 2 mutations was observed at the end of TEMPO 3:4, but it was not sustained at the end of TEMPO 4:4. While the treatment effect on eGFR was present at the end of TEMPO 3:4 and TEMPO 4:4 in CKD 1 subjects, it was more easily demonstrable in the CKD 2 and CKD 3 subjects.
FIGURE 6.
Change from baseline in TKV and eGFR by imaging classification. (A) Percentage change in TKV from TEMPO 3:4 baseline to Month 24 in TEMPO 4:4 for subjects in class 2A–B and 1B (left panel) and class 1C, D, E (right panel). (B) Change in eGFR from TEMPO 3:4 baseline to Month 24 in TEMPO 4:4 for subjects in class 2A–B and 1B (left panel) and class 1C, D, E (right panel). Open circles and triangles represent off-treatment time points.
FIGURE 7.
Change from baseline in TKV and eGFR by genotype. (A) Percentage change in TKV from TEMPO 3:4 baseline to Month 24 in TEMPO 4:4 for subjects with PKD2 (left panel), PKD1 non-truncating (NT, middle panel) and PKD1 truncating (T, right panel) genotypes. (B) Change in eGFR from TEMPO 3:4 baseline to Month 24 in TEMPO 4:4 for subjects PKD2 (left panel), PKD1 non-truncating (NT, middle panel) and PKD1 truncating (T, right panel) genotypes. Open circles and triangles represent off-treatment time points.
FIGURE 8.
Change from baseline in TKV and eGFR by CKD stage. (A) Percent change in TKV from TEMPO 3:4 baseline to Month 24 in TEMPO 4:4 for subjects in CKD 1 (left panel) and CKD 2/3 (right panel). (B) Change in eGFR from TEMPO 3:4 baseline to Month 24 in TEMPO 4:4 for subjects in CKD 1 (left panel) and CKD 2/3 (right panel). Open circles and triangles represent off-treatment time points.
Adverse events
Overall, 92.3% and 96.5% of early- and delayed-treated subjects, respectively, experienced at least one TEAE over 24 months in TEMPO 4:4 (Table 3). Aquaretic adverse events were more frequently reported in the delayed-treatment group who had not been previously exposed to tolvaptan.
Table 3.
Adverse and treatment-emergent adverse events in TEMPO 4:4a
| Variable | Early-treatmentb(n = 557) | Delayed-treatmentb(n = 314) |
|---|---|---|
| TEAEs (%) | 92.6 | 96.5 |
| Serious TEAEs (%) | 16.0 | 17.5 |
| Subjects discontinued due to AEs (%) | 5.4 | 15.0 |
| TEAEs occurring in >10% of subjects in either rollover group, n (%) | ||
| Hypertension | 160 (28.7) | 83 (26.4) |
| Renal pain | 98 (17.6) | 60 (19.1) |
| Nasopharyngitis | 62 (11.1) | 38 (12.1) |
| Thirst | 260 (46.7) | 158 (50.3) |
| Polyuria | 229 (41.1) | 173 (55.1) |
| Polydipsia | 61 (11.0) | 51 (16.2) |
| Nocturia | 142 (25.5) | 107 (34.1) |
| Fatigue | 38 (6.8) | 44 (14.0) |
| Dry mouth | 45 (8.1) | 44 (14.0) |
| Dizziness | 18 (3.2) | 32 (10.2) |
| Headache | 58 (10.4) | 47 (15.0) |
| Deaths, n (%)c | 4 | 0 |
| ALT or AST >3× ULN, n (%) | 14 (2.5) | 12 (3.8) |
TEAEs and deaths reported within 24 months of first IMP of the open-label extension trial including those that discontinued drug.
The early-treatment group received tolvaptan in TEMPO 3:4 and delayed-treatment group received placebo.
Four deaths occurred within the first 24 months of the open-label extension trial; all deaths occurred after discontinuation of tolvaptan. Causes of death were cardiac arrest, gunshot wound, intracranial aneurysm and subarachnoid hemorrhage.
Transaminase elevations >3× upper limit of normal (ULN) during TEMPO 4:4 occurred in 12 of 314 (3.8%) delayed- compared with 14 of 557 (2.5%) early-treated subjects (Table 3). These rates are similar to those observed in the non-Japanese population from TEMPO 3:4, where 35 of 840 (4.2%) tolvaptan-treated subjects and 4 of 425 (1.6%) placebo-treated subjects had similar elevations. One delayed-treated subject in TEMPO 4:4 met the criterion for Hy’s Law (ALT >3× ULN, BT >2× ULN, alkaline phosphatase <2× ULN and no alternative explanation) and recovered completely after discontinuation of tolvaptan [15].
In all, four deaths occurred within the first 24 months of the open-label extension trial; all deaths occurred after discontinuation of tolvaptan, (Table 3). Causes of death were cardiac arrest, gunshot wound, intracranial aneurysm and subarachnoid hemorrhage.
DISCUSSION
In the pivotal TEMPO 3:4 clinical trial, tolvaptan slowed the rates of TKV growth by 49% and eGFR decline by 26%. TEMPO 4:4 was designed to provide an additional 2 years of data on the long-term safety and efficacy of tolvaptan in subjects who completed TEMPO 3:4. Its objective was to ascertain whether tolvaptan has disease-modifying effects on TKV growth and eGFR decline [16].
The primary endpoint, that is, preservation of the TKV benefit between tolvaptan-treated and placebo-treated subjects obtained at the end of TEMPO 3:4 after both groups received treatment with tolvaptan for an additional 2 years in TEMPO 4:4, was not achieved. Several factors may account for this result, including: (i) staggered and delayed roll-over from TEMPO 3:4 to TEMPO 4:4; (ii) large effect on TKV growth during the first year of exposure to tolvaptan and smaller effects in subsequent years; (iii) blunted or absent acute treatment effect on TKV upon reintroduction of tolvaptan in early-treated subjects; (iv) imbalance of relevant covariates in TEMPO 4:4 due to uneven withdrawal and re-enrollment; and (v) different age and disease stage in tolvaptan- and delayed-treated subjects upon first tolvaptan exposure, along with TKV growth acceleration with disease progression. The first four factors were not foreseeable when the trial was designed.
The TEMPO 4:4 Trial design was finalized January 2010 prior to completion of the pivotal TEMPO 3:4 Trial. Since the trials were not fully integrated, investigators and patients could elect to participate and opt to delay reentry for up to 6 months. Delays in ethics committee and administrative approvals led to further delays in treatment resumption at some sites for many subjects. Therefore, baseline visits for TEMPO 4:4 were potentially impacted by variable waning of TEMPO 3:4 effects. Half of the subjects were rolled over into the new trial within 37 days, while those remaining had longer off-treatment periods (up to 829 days). This obscured the ability to accurately model off-treatment and re-treatment effects on TKV. It also complicated the factor of ‘time’ and its impact on the stage of disease progression in our analyses.
Approximately 65% of the tolvaptan-induced reduction in the rate of TKV relative to placebo over the 3 years of TEMPO 3:4 (9.56% versus 18.75%, i.e. 9.19%) occurred during the first year of treatment (−1.16% versus 5.05%, i.e. 6.27%). This was consistent with prior reports of 3.1% and 3.7% reductions in TKV, 1 and 3 weeks after starting treatment, respectively, likely due to acute effects on fluid secretion [17, 18]. Smaller proportions of the treatment effect, accounting for reductions in TKV growth of 1.5–2% per year during the 3 years of the trial, were likely due to effects on proliferation of the cystic epithelium [19]. This means that the chronic benefit of tolvaptan treatment may amount to half of the total 49% reduction seen after TEMPO 3:4. Following discontinuation of tolvaptan at the end of TEMPO 3:4, we expected that the antisecretory effect would rapidly dissipate, leaving only one-half (5%) of the overall treatment effect on TKV (10%). The expectation was that early- and delayed-treated subjects would experience comparable acute effects on TKV upon initiation of tolvaptan treatment in TEMPO 4:4 and the 5% difference between the groups would be maintained during the open-label extension trial. The observed response of TKV, however, did not match the predicted response. During TEMPO 4:4, an acute effect of tolvaptan on TKV growth at Month 12 was clearly observed in delayed-treated subjects, but was absent or, in the subjects with a long off-treatment period, blunted upon reintroduction of tolvaptan in the early-treated subjects. This different response accounts in part for the reduction of the TKV difference between the groups and fully for the superiority of the TKV slope in delayed-treated subjects during TEMPO 4:4.
The first exposure to tolvaptan occurred at a more advanced stage of the disease in the delayed-treatment group compared with the early-treatment group. During TEMPO 3:4, the apparent rate of TKV growth in the placebo-treated subjects increased annually from 5.05%, to 6.44% and 7.36% in years 1 through 3, respectively. The gradual increase in the rate of TKV growth as the disease progresses hindered direct comparisons between the rates of TKV growth in early- and delayed-treated subjects during TEMPO 3:4 and TEMPO 4:4. In the absence of a placebo control group in the open-label extension, it is difficult to compare longer term tolvaptan treatment (5 years) to an untreated population. Extrapolation of the placebo-treated group’s trajectory beyond 3 years suggests that the TKV growth after 5 years would be approximately 40% (Figure 2). The observed rate of TKV growth in both early- and delayed- treatment groups was approximately 30% over 5 years, supporting an approximate 25% reduction of TKV growth rate by tolvaptan, beyond its acute effect. These estimates suggest that both early- and delayed-treated subjects benefited from treatment with tolvaptan.
Measuring the TKV effect is further confounded by the apparent imbalance in certain subject baseline characteristics in TEMPO 4:4. Gender is an important predictor of TKV growth, with men generally exhibiting faster TKV growth rates over time. While the proportion of men and women was nearly equivalent on randomization in TEMPO 3:4, it became progressively imbalanced during study conduct and upon enrollment in the extension trial such that nearly 20% more men completed the TEMPO 4:4 Trial in the early-treatment group versus the delayed-treatment group. The reasons for this growing imbalance are unclear but include a differential withdrawal rate during the pivotal trial and selective re-enrollment into TEMPO 4:4. Additional imbalances in TKV, eGFR and urine ACR at the TEMPO 4:4 baseline were due to the treatment effect of tolvaptan during TEMPO 3:4. Controlling for these and other covariates previously shown to predict the response to tolvaptan (urine ACR and serum copeptin) [10, 11] uncovered a significant effect of early intervention on TKV (−4.15%) comparable to the effect of tolvaptan at the end of TEMPO 3:4 after discounting the acute antisecretory effect (−5%), as discussed above.
TEMPO 4:4 demonstrated that the eGFR benefit, achieved at the end of TEMPO 3:4 in the early-treated subjects, was maintained for an additional 2 years in TEMPO 4:4. Moreover, eGFR slopes of early-treated subjects during TEMPO 4:4 were comparable and statistically non-inferior to those of the delayed-treated subjects. The more frequent estimation of GFR by serum creatinine, more acute onset and offset of hemodynamic effects, and lesser impact of covariates such as gender make the GFR endpoint more compatible with the design of this open-label extension trial. These results, therefore, support a disease-modifying effect of tolvaptan in slowing ADPKD progression, that is, renal function decline.
While it might be tempting to conclude from the divergent TKV and eGFR outcomes of TEMPO 4:4 that the effects of tolvaptan on TKV and eGFR are unrelated, it is clear from the results of the longest, ongoing longitudinal natural history study in ADPKD, the Consortium for Radiologic Studies of Polycystic Kidney Disease (CRISP), that changes in TKV and eGFR are not concurrent and that the increase in TKV precedes and significantly predicts the change in eGFR [20, 21]. Nevertheless, TEMPO 3:4 and TEMPO 4:4 also suggest that tolvaptan may have effects on eGFR that are independent of changes in TKV. TEMPO 3:4 showed that tolvaptan has an acute effect on eGFR (replicated in the delayed-treated subjects in TEMPO 4:4), which is rapidly reversible when the drug is discontinued even if this occurs years later [8]. On the other hand, after prolonged treatment, the acute reduction in cyst volume thought to be due to an antisecretory effect of tolvaptan appears to take months to reverse once the drug is discontinued. This suggests a persistent, structural and not just functional effect of tolvaptan on the secretory epithelium. Therefore, we cannot exclude the possibility that tolvaptan is renoprotective through a renal hemodynamic effect [22] or potentially other mechanisms in addition to its antisecretory and antiproliferative effects on cystic epithelium.
In previous posthoc analyses of TEMPO 3:4, we found that the treatment effect of tolvaptan on TKV and eGFR was greater and/or easier to demonstrate in the subjects with more severe or more advanced disease. The posthoc analyses of TEMPO 4:4 in the current study show that the subjects with image class 1C–E, with truncating PKD1 mutations or with CKD 2–3 maintained the treatment effect of tolvaptan not only on eGFR but also on TKV for an additional 2 years in TEMPO 4:4. In smaller numbers of subjects with image classification 1A–B or with non-truncating PKD1 or PKD2 mutations, tolvaptan had no demonstrable significant effect on TKV at the end of either TEMPO 3:4 or TEMPO 4:4. Interestingly, in CKD 1 subjects, tolvaptan had a significant effect on TKV at the end of TEMPO 3:4 but it was not maintained in TEMPO 4:4. This may be due to smaller incremental effect of tolvaptan on TKV, relative to the initial antisecretory effects during years 2 and 3 of TEMPO 3:4 in CKD 1, compared with that seen in CKD 2 and 3 subjects. In these subjects, TKV growth is faster and cyst expansion may be more dependent on proliferation than secretion.
In general, the safety profile for tolvaptan in TEMPO 4:4 was similar to that of the tolvaptan arm of TEMPO 3:4. Most adverse events were related to the aquaretic effect of tolvaptan. The incidence of hepatic events in the delayed-treated subjects was similar to that observed in the tolvaptan-treated subjects in TEMPO 3:4. One subject in the delayed-treatment group met the criterion for Hy’s Law and has been previously reported [15].
Despite significant strengths, including the large number of subjects treated with tolvaptan for at least 5 years and the comparison of early and delayed interventions, TEMPO 4:4 has several obvious limitations, including a design that did not take into account the changing treatment effect of tolvaptan over time, the changing rate of TKV growth over time, loss of randomization from TEMPO 3:4 due to unequal distribution of dropouts and optional rollover to open-label extension with highly variable intervals between the two studies.
In summary, TEMPO 4:4 did not demonstrate a sustained expected beneficial difference in TKV achieved during TEMPO 3:4 (primary endpoint of the trial) and non-inferiority in the slope of TKV growth during TEMPO 4:4 in early- compared with delayed-treated subjects. On the other hand, the trial showed a sustained beneficial effect on eGFR and non-inferiority in the slopes of eGFR, supporting a disease-modifying effect. While the TKV endpoint did not meet its prespecified statistical threshold for proof of disease modification, this may be accounted for by unforeseen limitations when the trial was designed, including loss of randomization and baseline imbalances ensuing TEMPO 3:4.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org. The investigators in the TEMPO 4:4 trial are listed in Supplementary Table S2.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the patients involved in the TEMPO 3:4 Trial for their participation and contribution; the trial sub-investigators, radiologists, study coordinators and nurses; the trial managers, trial monitors, data managers, programmers and statisticians; the staff at the University of Wisconsin Statistical Data Analysis Center; and Dr K. Bae for assistance in the development of the imaging protocol. The authors want to acknowledge in memoriam the contributions of Dr Vincent H. Gattone 2nd for his insights into the connection between vasopressin and polycystic kidney disease, and of Mr Akihiko Otsuka and Dr Taro Iwamoto for their dedication to finding a treatment for ADPKD. The authors also thank David Norris, PhD (Ecosse Medical Communications, Falmouth, MA, USA) for editorial assistance provided during the preparation of this report. The study was funded by Otsuka Pharmaceutical Development and Commercialization, Inc.
CONFLICT OF INTEREST STATEMENT
R.T.G., R.D.P., A.B.C., O.D. and V.E.T are members of the TEMPO 3:4 and 4:4 steering committee. J.B.D, F.S.C, A.D. and J.O are employees of Otsuka Pharmaceutical Development and Commercialization Inc. (Princeton, NJ, USA). V.E.T., O.D., A.B.C., R.T.G. and R.D.P. have received research funding from Otsuka Pharmaceutical Development & Commercialization, Inc. (Princeton, NJ, USA); A.B.C. have received consultancy fees from Otsuka Pharmaceutical Development & Commercialization, Inc.
REFERENCES
- 1. Ong AC, Devuyst O, Knebelmann B. et al. Autosomal dominant polycystic kidney disease: the changing face of clinical management. Lancet 2015; 385: 1993–2002 [DOI] [PubMed] [Google Scholar]
- 2. Chapman AB, Devuyst O, Eckardt KU. et al. Autosomal dominant polycystic kidney disease (ADPKD): executive summary from a kidney disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 2015; 88: 17–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grantham JJ, Mulamalla S, Swenson-Fields KI.. Why kidneys fail in autosomal dominant polycystic kidney disease. Nat Rev Nephrol 2011; 7: 556–566 [DOI] [PubMed] [Google Scholar]
- 4. Gattone VH, Wang X, Harris PC. et al. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med 2003; 9: 1323–1326 [DOI] [PubMed] [Google Scholar]
- 5. Torres VE, Wang X, Qian Q. et al. Effective treatment of an orthologous model of autosomal dominant polycystic kidney disease. Nat Med 2004; 10: 363–364 [DOI] [PubMed] [Google Scholar]
- 6. Wang X, Wu Y, Ward CJ. et al. Vasopressin directly regulates cyst growth in polycystic kidney disease. J Am Soc Nephrol 2008; 19: 102–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Torres VE, Harris PC.. Strategies targeting cAMP signaling in the treatment of polycystic kidney disease. J Am Soc Nephrol 2014; 25: 18–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Torres VE, Chapman AB, Devuyst O. et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 2012; 367: 2407–2418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Non-Inferiority Clinical Trials to Establish Effectiveness: Guidance for Industry, Food and Drug Administration, Division of Drug Information, Office of Communications, Center for Drug Evaluation and Research http://www.fda.gov/drugs/guidancecomplianceregulatorinformation/guidances/default.htm
- 10. Gansevoort RT, Meijer E, Chapman AB. et al. Albuminuria and tolvaptan in autosomal-dominant polycystic kidney disease: results of the TEMPO 3:4 Trial. Nephrol Dial Transplant 2016; 31: 1887–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gansevoort R, van Gastel M, Chapman A. et al. Copeptin, a surrogate for Vasopressin, predicts disease progression and Tolvaptan treatment efficacy in ADPKD. Results of the TEMPO 3:4 trial. J Am Soc Nephrol 2016; 27: 34A [Google Scholar]
- 12. Irazabal MV, Blais JD, Perrone RP. et al. Prognostic enrichment design in clinical trials for ADPKD: The TEMPO 3:4 clinical trial. Kidney Int Rep 2016; 1: 213–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cornec-Le Gall E. Can we further enrich ADPKD clinical trials for rapidly progressive patients? Application of the PROPKD score in the TEMPO trial. J Am Soc Nephrol 2016; 27: 766A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Torres VE, Higashihara E, Devuyst O. et al. Effect of tolvaptan in autosomal dominant polycystic kidney disease by CKD stage: results from the TEMPO 3:4 Trial. Clin J Am Soc Nephrol 2016; 11: 803–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Watkins PB, Lewis JH, Kaplowitz N. et al. Clinical pattern of tolvaptan-associated liver injury in subjects with autosomal dominant polycystic kidney disease: analysis of clinical trials database. Drug Saf 2015; 38: 1103–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bhattaram VA, Siddiqui O, Kapcala LP. et al. Endpoints and analyses to discern disease-modifying drug effects in early Parkinson's disease. Am Assoc Pharm Sci J 2009; 11: 456–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Irazabal MV, Torres VE, Hogan MC. et al. Short-term effects of tolvaptan on renal function and volume in patients with autosomal dominant polycystic kidney disease. Kidney Int 2011; 80: 295–301 [DOI] [PubMed] [Google Scholar]
- 18. Boertien WE, Meijer E, de Jong PE, et al. Short-term effects of tolvaptan in individuals with autosomal dominant polycystic kidney disease at various levels of kidney function. Am J Kidney Dis 2015; 65: 833–841 [DOI] [PubMed] [Google Scholar]
- 19. Reif GA, Yamaguchi T, Nivens E. et al. Tolvaptan inhibits ERK-dependent cell proliferation, Cl(-) secretion, and in vitro cyst growth of human ADPKD cells stimulated by vasopressin. Am J Physiol Renal Physiol 2011; 301: F1005–F1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grantham JJ, Torres VE, Chapman AB. et al. Volume progression in polycystic kidney disease. N Engl J Med 2006; 354: 2122–2130 [DOI] [PubMed] [Google Scholar]
- 21. Chapman AB, Bost JE, Torres VE. et al. Kidney volume and functional outcomes in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 2012; 7: 479–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bankir L, Bouby N, Ritz E.. Vasopressin: a novel target for the prevention and retardation of kidney disease? Nat Rev Nephrol 2013; 9: 223–239 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.