Abstract
Zika virus (ZIKV) is an emerging viral pathogen that continues to spread throughout different regions of the world. Herein we report a case that provides further evidence that ZIKV transmission can occur through breastfeeding by providing a detailed clinical, genomic, and virological case-based description.
Keywords: Zika virus, Venezuela, asymptomatic, breast milk, breastfeeding transmission
Zika virus (ZIKV) continues to spread throughout tropical and subtropical regions of the world [1]. Transmission of ZIKV can occur through mosquito vectors, sexual contact, blood transfusion, or accidental laboratory exposure [2]. There is evidence that postnatal transmission between mother and child can occur during breastfeeding, delivery, or close contact between the mother and her newborn [1–7]. We present additional evidence supporting transmission of ZIKV through breastfeeding.
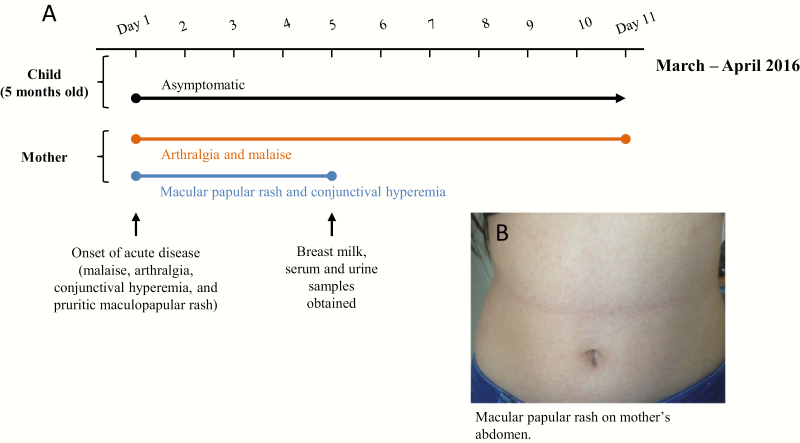
In March 2016, a 32-year-old female patient from Barquisimeto, Venezuela presented with a 1-day history of malaise, arthralgia, conjunctival hyperemia, and pruritic maculopapular rash (Figure 1). At that time, she was exclusively breastfeeding her 5-month-old child, who was asymptomatic. Breast milk, plasma, and urine were collected from the mother 4 days later, as were plasma and urine from the child, who was asymptomatic. Specimens were collected, analyzed, and cryopreserved for shipment and virus isolation as detailed in the Supplementary Data. For virus detection and isolation, Vero E6 and LLC-MK2 cells were inoculated with aliquots of the mother’s milk (whole, lipid, and aqueous fractions) and urine and child’s plasma and urine specimens. Cytopathic effects (CPE) characteristic of ZIKV infection [8] (perinuclear vacuoles prior to cell death: Supplementary Figure 1) were observed 9 and 12 days postinoculation of the mother’s and child’s specimens, respectively. Virus-induced CPE were initially most plentiful and detected first in cells inoculated with the lipid fraction of breast milk. The cultures were screened for chikungunya virus (CHIKV), dengue viruses (DENVs) 1, 2, 3, and 4, and ZIKV genomic RNA by reverse-transcription polymerase chain reaction (RT-PCR). All cultures were negative for CHIKV and DENV1–4.
Figure 1.
Symptoms of Zika virus infection in mother and child. A, Timeline of symptom onset and progression for mother–child pair. B, Maculopapular rash on mother’s abdomen. Photograph was taken with permission for publication in March 2016.
The presence of ZIKV genomic RNA (vRNA) was confirmed in all cultures by RT-PCR. The mother’s urine was positive for ZIKV vRNA by real-time RT-PCR (cycle threshold [Ct], 26.73; level of detection, Ct 36.8). The child’s plasma and urine were positive for ZIKV by real-time RT-PCR, with Ct 35.57 and 35.36. Serologic analysis was performed using the Zika Virus ViraStripe IgG/IgM Test Kit (Viramed Planegg, Germany) revealing that the mother had immunoglobulin M (IgM) and borderline immunoglobulin G (IgG) antibodies against ZIKV, and no detectable antibodies against CHIKV. She also exhibited IgG against DENV, but no IgM against DENV. The mother was negative for parvovirus, cytomegalovirus, Epstein-Barr virus, varicella zoster virus, and herpes simplex virus types 1 and 2. ZIKV genomes were sequenced by extracting vRNA from virions in the spent media of LLC-MK2 cells 14 days postinoculation with mother’s milk (lipid-enriched fraction) and child’s urine, and sequenced as described previously [8] and in the Supplementary Data. In the mother, arthralgias and malaise lasted 10 days, with the rash and conjunctival hyperemia resolving 4 days after the onset of symptoms. The child remained asymptomatic throughout the observation period.
Full-genome sequencing of ZIKV isolated from breast milk and the child’s urine (GenBank accession numbers KX702400 and KX893855) revealed 99% identity, with only 2 synonymous nucleotide substitutions at third codon positions between the 2 strains [9]. Both genomes were different from the genomic sequences of other ZIKV strains in the laboratory. Moreover, sequencing of the NS5 gene of the other isolates indicated that identical virus was in all specimens from both mother and child. Mock-infected cells did not develop CPE, and their spent media was RT-PCR negative for ZIKV vRNA. A phylogenetic analysis was performed using all currently available ZIKV full-genome sequences downloaded from the National Center for Biotechnology Information (NCBI; http://www.ncbi.nlm.nih.gov/). Results showed that the 2 strains cluster with high bootstrap support (99%) within a larger clade of Colombian sequences (Supplementary Figure 2). Interestingly, the mother had no history of traveling to Colombia. However, Barquisimeto is on a major trade route to Colombia where a large number of Venezuelans regularly travel due to food and medicine shortages in Venezuela.
The mother and child lived in an air-conditioned, screened house, where the risk of mosquito transmission would be minimized. They had no recent travel history, and the child spent most of the time within the house. While the source of the child’s infection cannot be determined, the virological and phylogenetic results of this study strongly suggest the occurrence of postnatal transmission from mother to child during breastfeeding.
The full-genome sequences of ZIKV isolates from breast milk and the child’s urine were deposited under NCBI GenBank accession numbers KX702400 and KX893855, respectively, and reported in [9].
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. All authors contributed substantially to the completion of this study and preparation of this manuscript.
Financial support. Components of the work were supported by grants from the National Science Foundation (award number 1515734) and the National Institutes of Health (grant number R01 AI126357-01S1).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Zika virus, microcephaly, and Guillain Barré syndrome. Situation report Available at: http://apps.who.int/iris/bitstream/ 10665/251648/1/zikasitrep24Nov16-eng.pdf?ua=1. Accessed 25 September 2017.
- 2. Centers for Disease Control and Prevention. Zika virus transmission and risks Available at: https://www.cdc.gov/zika/transmission/. Accessed 25 September 2017.
- 3. Besnard M, Lastère S, Teissier A, et al. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill 2014; 19: 13. [PubMed] [Google Scholar]
- 4. Cavalcanti MG, Cabral-Castro MJ, Gonçalves JL, Santana LS, Pimenta ES, Peralta JM. Zika virus shedding in human milk during lactation: an unlikely source of infection?Int J Infect Dis 2017; 57:70–2. [DOI] [PubMed] [Google Scholar]
- 5. Colt S, Garcia-Casal MN, Peña-Rosas JP, et al. Transmission of Zika virus through breast milk and other breastfeeding-related bodily-fluids: a systematic review. PLoS Negl Trop Dis 2017; 11:e0005528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dupont-Rouzeyrol M, Biron A, O’Connor O, Huguon E, Descloux E. Infectious Zika viral particles in breastmilk. Lancet 2016; 387:1051. [DOI] [PubMed] [Google Scholar]
- 7. Sotelo JR, Sotelo AB, Sotelo FJB, et al. Persistence of Zika virus in breast milk after infection in late stage of pregnancy. Emerg Infect Dis 2017; 23:856–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lednicky J, Beau De Rochars VM, El Badry M, et al. Zika virus outbreak in Haiti in 2014: molecular and clinical data. PLoS Negl Trop Dis 2016; 10:e0004687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Blohm GM, Lednicky JA, Márquez M, et al. Complete genome sequences of identical Zika viruses in a nursing mother and her infant. Genome Announc 2017; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.