Abstract
We investigated individual differences in longitudinal trajectories of brain aging in cognitively normal healthy adults from the Seattle Longitudinal Study covering 8 years of longitudinal change (across 5 occasions) in cortical thickness in 249 midlife and older adults (52–95 years old). We aimed to understand true brain change; examine the influence of salient risk factors that modify an individual’s rate of cortical thinning; and compare cross-sectional age-related differences in cortical thickness to longitudinal within-person cortical thinning. We used Multivariate Multilevel Modeling to simultaneously model dependencies among 5 lobar composites (Frontal, Parietal, Temporal, Occipital, and Cingulate [CING]) and account for the longitudinal nature of the data. Results indicate (1) all 5 lobar composites significantly atrophied across 8 years, showing nonlinear longitudinal rate of cortical thinning decelerated over time, (2) longitudinal thinning was significantly altered by hypertension and Apolipoprotein-E ε4 (APOEε4), varying by location: Frontal and CING thinned more rapidly in APOEε4 carriers. Notably, thinning of parietal and occipital cortex showed synergistic effect of combined risk factors, where individuals who were both APOEε4 carriers and hypertensive had significantly greater 8-year thinning than those with either risk factor alone or neither risk factor, (3) longitudinal thinning was 3 times greater than cross-sectional estimates of age-related differences in thickness in parietal and occipital cortices.
Keywords: aging, APOE, cortical thinning, hypertension, longitudinal modeling
Aging is associated with adverse cognitive outcomes believed to be associated with changes in the macrostructure of the brain (Raz and Rodrigue 2006; MacDonald et al. 2009; Rodrigue and Kennedy 2011). Cross-sectional and longitudinal MRI studies of aging of brain structural properties, such as cortical and subcortical volume (Jernigan et al. 1991; Pfefferbaum et al. 1994; Good et al. 2001; Liu et al. 2003; Scahill et al. 2003; Raz et al. 2005; Carmichael et al. 2012), white matter quality and connectivity (Salat et al. 2005; Kennedy and Raz 2009; Sullivan et al. 2010; Lebel et al. 2012; Raz et al. 2012), have shown detrimental effects across the lifespan that are modified by individual difference variables, such as genetic and health risk factors (e.g., Swan et al. 1998; Kennedy and Raz 2009; Salat et al. 2012). More recently, it has been suggested that cortical thinning in individuals at risk for dementia may be even more sensitive to early preclinical pathological changes than measures of cortical volume (Burggren et al. 2008), and that cortical thinning is the primary contributor to reductions in cortical volume with aging and thus, may be a more specific index of structural brain changes with advancing age (Thambisetty et al. 2010; Storsve et al. 2014).
Primarily, inferences about effects of aging on brain structure, including cortical thinning, are drawn from cross-sectional research (Salat et al. 2004; Fjell et al. 2009; Gautam et al. 2011, 2013; Leritz et al. 2011). Although cross-sectional designs allow for the comparison of a wide age range (e.g., 20 s vs. 80 s), they do not allow measurement of true within-person change over time (cf. Hofer et al. 2006). Further, it is suggested that cross-sectional studies have underestimated actual changes in individual brain size as we age (Kennedy and Raz 2015). Thus, only longitudinal follow-up of individuals over time can assess the accurate rate of brain aging. Accordingly, there is a growing body of longitudinal studies of structural brain aging (Raz et al. 2005, 2010; Thambisetty et al. 2010; Pillai et al. 2012; Doré et al. 2013; Pfefferbaum et al. 2013; Fjell et al. 2014; Jiang et al. 2014; Storsve et al. 2014; Walhovd et al. 2014; Pacheco et al. 2015). To better understand the effects of age and developmental change on the brain, longitudinal studies need to follow individuals on multiple occasions and for several years (to take advantage of advanced multi-sampling data analysis techniques), examine multiple brain regions (for investigation of regional patterns), include potential modifying predictor variables, sample widely from the lifespan as preclinical changes often occur in midlife, and accommodate nonlinear time trajectories. Therefore, there remain two major issues to address in the need to document within cognitively normal adults the regional variability in thinning across the cortex: Long-term longitudinal designs and advanced statistical procedures need to be applied to understand actual change and variability of change, and to examine the salient factors that modify an individual’s rate of cortical thinning. The current study sought to incorporate these strengths.
The majority of longitudinal studies of brain aging have underutilized the richness of the data by using suboptimal analysis methods such as using simple repeated-measures t-tests or other univariate linear models rather than mixed models or latent growth curve modeling (but for example, see Raz et al. 2005, 2010, 2012 for exceptions). Multivariate multilevel modeling approaches (MMLM; MacCallum et al. 1997) are sophisticated data analysis techniques that are more appropriate to capture and model the richness of multivariate longitudinal data when more than two measurement occasions have been collected. These models are able to take into account not only the dependence of individual data points due to repeated measurements but also due to the similarity in cortical thickness among brain regions within individuals. Moreover, multilevel or mixed-effects models make full use of all available data, do not require that all participants have the same number of follow-up time points, nor do they require that all participants be measured at the same time intervals (cf. Raudenbush 2001).
Two salient vascular risk factors have been often studied because they convey increased risk for Alzheimer’s disease and alter cognition and brain structure in normal aging: Apolipoprotein-E ε4 (APOEε4) genotype and diagnosis of hypertension (HT). APOEε4 is the strongest genetic risk factor for sporadic AD (Corder et al. 1993), with 60% of AD individuals carrying at least one ε4 allele. These ε4 carriers display structural alterations in APOE protein, which impacts lipid and beta-amyloid binding, reducing its function in neuronal integrity maintenance (Frieden and Garai 2012). Individuals who are ε4 positive accumulate greater amyloid, and at an earlier age of onset (Fleisher et al. 2013), likely because the APOEε4 isoform reduces amyloid clearance and increases Aβ aggregation (Verghese et al. 2011). Without adequate clearance, beta-amyloid can form oligomers and plaque which may be neurotoxic to neurons (Liu et al. 2013; Scheltens et al. 2016; but see Hardy 2009 for a different viewpoint). This neurotoxicity brings with it damaged neuronal repair, neuronal inflammation, weakened blood barrier integrity, upregulated tau phosphorylation and neurofibrillary tangle formation (Mahley and Huang 2012). Thus, APOE has been associated with increased risk for AD, modified cognitive and brain aging, and exacerbated cerebrovascular dysfunction. Carrying a diagnosis of HT in midlife has also been associated with increased later life dementia risk (Kivipelto et al. 2001). HT diagnosis has been associated with greater 5-year shrinkage of brain volumes in otherwise healthy adults (Raz et al. 2005) and this shrinkage was associated with poorer fluid intelligence (Raz et al. 2008). Vuorinen et al. (2013) found that HT in midlife was associated with differences in cortical thickness 28 years later. Although this was not a longitudinal study, it suggests that carrying a diagnosis of HT over many years may lead to thinning of the cortex. Finally, Donix et al. (2010) found that APOEε4 carriers had greater thinning over 2 years in the medial temporal lobe structures.
Thus, in the present study, we sought to examine the regional pattern of cortical thinning of association cortices in a large sample of cognitively normal adults with a wide age range including midlife through older age (52–95 years). In the Seattle Longitudinal Study (SLS), we have collected 5 measurement occasions of data spanning 8 years and here we use MMLM to optimally model within-person change and variability of change. We further investigate individual difference factors that likely contribute substantially to variance in rate of cortical thinning; APOEε4 status and diagnosis of HT. Beyond these primary aims, we also sought to compare cross-sectional age-related differences in cortical thickness with longitudinal within-person change in thickness.
Materials and Methods
Seattle Longitudinal Study
Data were obtained from community-living, nondemented participants in the SLS, a cohort-sequential longitudinal study of the relationship between aging, health, cognition, and lifestyle (Schaie 2013). SLS members at recruitment represented a stratified-by-age and gender random sample of the membership of the Group Health Cooperative of Puget Sound, a large health maintenance organization in western Washington State. Cognitive and behavioral assessments have been conducted every 7 years starting in 1956 on a mixed age cohort (age 22–88) with follow-up and recruitment of new samples every 7 years (1956 through 1998). This study has been approved by the Group Health Cooperative of Puget Sound Internal Review Board. All participants provided written informed consent.
Participants
The current analyses included 249 individuals from the SLS who had MRI scans between 2006 and 2014. The interval between the MRI scans was 2 years and some participants had up to 5 scans (n = 69 participants had 5 scans, 49 had 4, 50 had 3, 55 had 2, and 26 participants had 1 scan; Interval 1 between waves 1 and 2 was on average 23 months with SD = 1.9, interval 2 mean was 24 months and SD = 3.6, interval 3 mean was 24 months and SD = 3.9, and interval 4 was 17 months and SD = 2.5). The mean age at the first MRI scan was 66.3 years (SD = 8.0 years); mean education was 16 years; sex distribution: 45% male. Racial composition included 241 Caucasian, 3 African American, 4 Asian, and 1 Hispanic participant. The racial composition of the MRI sample is similar to that of the total SLS population and to the health maintenance organization at the time of recruitment. Note that the MRI sample is a longitudinal sample who have been participating in the SLS for at least 14 years prior to the first MRI scan. Participants were assessed using an expanded CERAD neuropsychological battery (Morris et al. 1989, 1993; Schaie 2013) within 3 years of baseline scan and assessed at 2–3 year intervals thereafter. Mini Mental State Examination (MMSE) and Wechsler Adult Intelligence Scale (WAIS) Vocabulary scores at time of first scan are reported in Table 1. Neuropsychological test performance was reviewed by 2 neuropsychologists, taking into consideration age norms and participant education and occupational status. No participants met criteria for dementia or mild cognitive impairment (MCI) at baseline scan. Since the baseline scan, 12 participants have been assessed as demented and 2 participants as having MCI. Participants assessed as demented or MCI after the first scan were included in the analyses. Further, 13 participants are now deceased.
Table 1.
Descriptive statistics and sample characteristics
| Variable | Total sample | APOEa | HT | ||
|---|---|---|---|---|---|
| E4 | Noncarrier | Yes | No | ||
| N (%) | 249 | 71 (32%) | 150 (68%) | 119 (48%) | 129 (52%) |
| M/F (%F) | 112/137 (55%) | 30/41 (33%) | 65/85 (67%) | 57/62 (45%) | 55/75 (55%) |
| Age first scan | |||||
| M (SD) | 66 (8.0) | 65 (7.2) | 67 (8.5) | 68 (8.7) | 65 (7.1) |
| Range | 52–89 | 53–87 | 52–89 | 53–89 | 52–81 |
| Education (SD) | 16.2 (2.5) | 16.5 (2.6) | 16.1 (2.4) | 16 (2.5) | 16.4 (2.4) |
| MMSE (SD) | 29 (1.1) | 29.4 (0.8) | 29.2 (1.2) | 29.1(1.3) | 29.3 (1.0) |
| WAIS Voc (SD) | 60.8 (7.3) | 61.8 (5.8) | 60.3 (7.8) | 59.8 (7.4) | 61.5 (7.1) |
aAPOE allele was available on N = 221 subjects.
Chi square of APOE × HT = 6.05, P < 0.01. Fewer ɛ4 carriers were hypertensive than expected. APOEɛ4 carriers versus noncarriers differed significant in age, but not in education or cognitive scores. Hypertensive versus nonhypertensive differed significantly (P < 0.01) in age, but not in education or cognitive scores.
Participants were selected from the larger SLS sample with cognitive assessments in middle age. Selection criteria were: (1) had undergone 2 or more cognitive assessments in midlife and/or old age, (2) participated in the 2005 SLS longitudinal data wave, (3) were cognitively normal based on cognitive assessment and consensus review, and (4) were willing and capable of undergoing multiple MRI scans. Of the 249 participants, 161 entered the study in 2006, 46 entered in 2008, and 42 entered the study in 2010. Regarding attrition, of the participants that entered the study in 2006, 91% returned for the second occasion after that, 80% returned to the third, 75% to the fourth, and 85% to the fifth occasion. Of those who entered in 2008, 91% returned for the second occasion after that, 83% returned to the third, and 74% returned to the fourth measurement occasion. The attrition was larger for the group that had their first scan in 2010. Of those participants, 81% returned for their second measurement occasion and after that 53% returned for their third occasion. Dropouts from the first recruitment did not differ from the study sample in age, education, or neuropsychological test scores.
Risk Factor Predictor Variables
Apolipoprotein-E
APOE genotyping was performed at Northwest Lipid Research Laboratory, Seattle using restriction isotyping (Hixson and Vernier 1990) on 221 participants. Individuals were categorized as APOEε4 allele carriers (homozygotes and heterozygotes) versus noncarriers for analysis. Of these, 32% possessed at least one ε4 allele (Table 1). Given the unknown effect of an APOE ε2 allele in the ε2ε4 carriers (5 participants), we ran sensitivity analyses excluding these participants. Given that the results without the ε2ε4 carriers were almost identical, we included the 5 individuals in the final sample.
Hypertension
HT status was determined based on medical records and confirmed on self-report provided at baseline. According to these self-reports, 119 participants (48%) were hypertensive and 129 participants (52%) were normotensive. One person did not provide information on her HT status and thus was not included in the final model.
Hence, the final model with both predictors, APOE and HT, was based on 220 participants. Participants with HT or an APOEε4 allele did not differ in education or neuropsychological test scores from normotensives or non ε4-carriers.
MRI Acquisition and Processing
T1-weighted high-resolution structural imaging employed a magnetization-prepared rapid gradient echo imaging sequence on a Philips 3.0 T Achieva scanner using the following parameters: repetition time = 7 milliseconds (ms), echo time = 3.20 ms; flip angle = 8 degrees; matrix = 256 × 256; NEX = 2; FOV = 256 × 256; and 0.859 millimeter thick sagittal slices. Cortical reconstruction was performed with FreeSurfer v5.1.0 (Dale et al. 1999; Fischl et al. 1999; Fischl and Dale 2000). After automatic analysis, the gray/white matter surfaces were visually inspected, and when deemed necessary, 2 trained raters made control point and white matter edits to improve pial and white matter boundary-finding. Analyses were then reiterated to ensure that tissue classification was as accurate as possible. The mapping to standard spherical coordinates allowed for automated anatomical parcellation of the cortical surface into 34 gyral parcels per hemisphere (Desikan et al. 2006). The time points for the present analyses were obtained from Freesurfer’s cross-sectional pipeline. Following Reuter et al. (2012) recommendation we did not use the data points from Freesurfer’s longitudinal pipeline due to the potential risks of “underestimating change and accuracy… when measuring longitudinal change over longer periods of time” (Reuter et al. 2012, p. 1404).
Creation of Lobar Composites
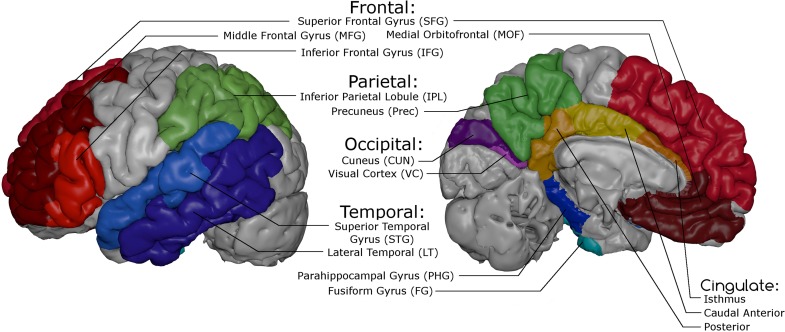
For data reduction purposes, we aggregated cortical parcels across biological regions of interest to create lobar composites as follows. Cortical thickness values for each of the 68 parcels for each subject were first obtained at all time points. Given our interest in association and paralimbic cortices, likely to be most susceptible to age-related cortical thinning, we included a subset of 46 parcels that were used to define 13 regions and 5 composites (cf. Table 2 and Fig. 1), plus visual cortex (VC) as a control region. To allow broader levels of analysis and to minimize comparisons required, parcels were averaged to form relevant regions (e.g., pars triangularis, parsopercularis, and pars orbitalis combined to form inferior frontal gyrus [IFG]) which were combined to also form appropriate lobar composites: Frontal, Parietal, Temporal, Occipital, and CING. To account for different sizes of the Desikan parcels when averaging, we computed average weighted means for each region and each composite. Due to difficulties in obtaining adequate parcellation of the entorhinal cortex that were not resolved after careful manual edits of individual segmentations we decided to remove this region from our analyses.
Table 2.
Component construction of lobar composites, from aggregated regions, from relevant association cortex Parcels
| Composite | Region | Desikan parcel |
|---|---|---|
| Frontal | SFG | SFG |
| MFG | Rostral MFG | |
| Caudal MFG | ||
| IFG | Parsopercularis | |
| Pars Triangularis | ||
| Pars Orbitalis | ||
| mF | Rostral Anterior CING | |
| Medial Orbital Frontal | ||
| Parietal | IPL | Supramarginal Gyrus |
| Inferior Parietal Gyrus | ||
| PREC | PREC Cortex | |
| Temporal | PHC | PHC Gyrus |
| FG | FG | |
| STG | STG | |
| Transverse Temporal Gyrus | ||
| LT | Middle Temporal Gyrus | |
| Inferior Temporal Gyrus | ||
| Banks of STG | ||
| Occipital | VC | Pericalcarine Sulcusa |
| CUN | CUN | |
| CING | CING | Posterior CING Gyrus |
| Caudal Anterior CING Gyrus | ||
| Isthmus CING Gyrus |
SFG, Superior Frontal Gyrus; MFG, Middle Frontal Gyrus; mF, Medial Frontal; IPL, Inferior Parietal Lobule; PREC, Precuneus; PHC, Parahippocampal; FG, Fusiform Gyrus; STG, Superior Temporal Gyrus; LT, Lateral Temporal; CUN, Cuneus; CING, Cingulate.
aWe included early VC (pericalcarine sulcus and CUN) as nonheteromodal association control regions.
Figure 1.
Representation of the 5 lobar composites (Frontal, Parietal, Occipital, Temporal, and CING) and the 13 subordinate regions of interest.
Statistical Modeling Approach: MMLM
The longitudinal data used here have several levels of dependency that need to be included in the statistical models. For one, the measurements of the composites, regions and parcels are nested in two hemispheres within individuals who have been measured repeatedly over time. Cortical thickness measures of different regions taken from the same individual share the same history of growth, atrophy and exogenous and endogenous influences making data points dependent as they cannot be considered random draws. In addition, repeated-measures over time will result in thickness measures that are more similar within individual than across individuals thus adding another layer of data dependency. Currently, multilevel or mixed-effects models are best suited to account for these dependencies and to obtain unbiased parameter estimates and standard errors of cortical thickness and atrophy rates.
To account for the multivariate nature of the data resulting from multiple regions (Carmichael et al. 2012) or composites measured at the same time across both hemispheres, and to jointly examine the longitudinal cortical thinning among these composites and regions, we applied MMLM (MacCallum et al. 1997). In addition to estimating average effects of cortical thickness and cortical atrophy (fixed effects), these models also estimate individual differences expressed as variances of the fixed effects (random effects). The multivariate approach allows us to model simultaneously the interplay (covariances between cortical thickness and annual atrophy rate) among the 5 composites and the effect of the predictor variables on atrophy.
In contrast to ANOVA, the MMLM approach takes advantage of different coding techniques to directly test comparisons of interest, omitting the need for post hoc testing. Further, multilevel techniques shrink group-level variances toward the mean, reducing the number of statistically significant comparisons, and with it counterbalancing the risk of Type I errors arising from multiple comparisons (cf. Gelman et al. 2012).
In the Supplementary Material, we provide a detailed formal description of the statistical model and the approach to derive the best model for these data. While the current analyses focus on cortical thickness changes across composites (cf. Table 2), we provide additional analyses of change within each of the 5 composites across its regions in the Supplementary Material. Briefly, we employed a series of nested joint MMLM models to analyze cortical thickness of the 5 broad lobar composites: Frontal, Temporal, Parietal, Occipital, and CING. This process consisted of 4 modeling steps that incrementally modified model complexity, starting with the “Intercept Model” that only includes intercept terms of cortical thickness. Next, we expanded that model to the “Baseline Model” which includes age-at-study-entry and time-in-study effects. In a third step, we constrained (co)variance parameters to be equal across composites to reduce overall model complexity while maintaining model fit (“Constrained Baseline Model”). The final, “Full Model”, expanded that latter model to include explanatory variables such as APOEε4 and HT, to explain differences in between-person cortical thickness and within-person thinning rates. All estimates reported in Table 3, as well as Figs 2 and 3 are based on the “Full Model”. Model fitting steps and resulting parameters (from Intercept to Constrained Baseline Model) are reported as Supplementary Materials.
Table 3.
Full model fixed effects estimates from joint MMLM of cortical thinning of the 5 lobar composites
| Lobar composite | Intercept | Time | Age | APOE | HT | Time2 | Time × Age | Time × APOE | Time × HT | APOE × HT | Age × Time2 | Time × APOE × HT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frontal | 2.3766 | −0.0031 | −0.0038 | −0.0159 | −0.0087 | 0.0007 | 0.0003 | −0.0056 | −0.0001 | 0.0106 | −0.0001 | −0.0005 |
| ∆% | −0.13 | −0.16 | −0.67 | −0.37 | 0.03 | 0.01 | −0.24 | 0.00 | 0.45 | 0.00 | −0.02 | |
| Parietal | 2.2419 | −0.0155 | −0.0058 | −0.0096 | −0.0107 | 0.002 | −0.0001 | −0.0016 | −0.0006 | 0.0337 | 0.0000 | −0.0066 |
| ∆% | −0.69 | −0.26 | −0.43 | −0.48 | 0.09 | 0.00 | −0.07 | −0.03 | 1.5 | 0.00 | −0.29 | |
| Temporal | 2.4876 | −0.0074 | −0.0074 | −0.0117 | −0.0189 | 0.0012 | 0.0001 | −0.001 | −0.0011 | 0.0509 | −0.0001 | −0.0037 |
| ∆% | −0.30 | −0.30 | −0.47 | −0.76 | 0.05 | 0.00 | −0.04 | −0.04 | 2.05 | 0.00 | −0.15 | |
| Occipital | 1.6417 | −0.0119 | −0.0034 | 0.0026 | −0.0043 | 0.0011 | −0.0002 | −0.0004 | 0.0003 | 0.0058 | 0.0000 | −0.0068 |
| ∆% | −0.72 | −0.21 | 0.16 | −0.26 | 0.07 | −0.01 | 0.02 | 0.02 | 0.35 | 0.00 | −0.41 | |
| CING | 2.3764 | 0.0019 | −0.0050 | 0.0169 | 0.0134 | 0.0006 | 0.0004 | −0.0057 | −0.001 | 0.0128 | −0.0001 | −0.0011 |
| ∆% | 0.08 | −0.21 | 0.71 | 0.56 | 0.03 | 0.02 | −0.24 | −0.04 | 0.54 | 0.00 | −0.05 |
Note: Bolded estimates are statistically significant at P < 0.05. N = 220. Composites are dependent variables modeled simultaneously from a MMLM (see Analysis Plan). APOE and HT are dummy coded with the reference group being the noncarriers of the ε4 allele and the normotensives (e.g., the interaction term Time × APOE represents the deviation of APOE-ε4 carriers in the atrophy rate from noncarriers). ∆% represents average annual percent change with respect to initial cortical thickness for a 65-year-old person. ∆% for dummy coded variables represents the change in APOE-ε4 carriers or hypertensives with respect to noncarriers or normals. Age is centered at 65 years of age and represents age at Wave 1. Time captures the atrophy rate of the cortex on an annual basis and Time2 represents quadratic rate of change.
Figure 2.
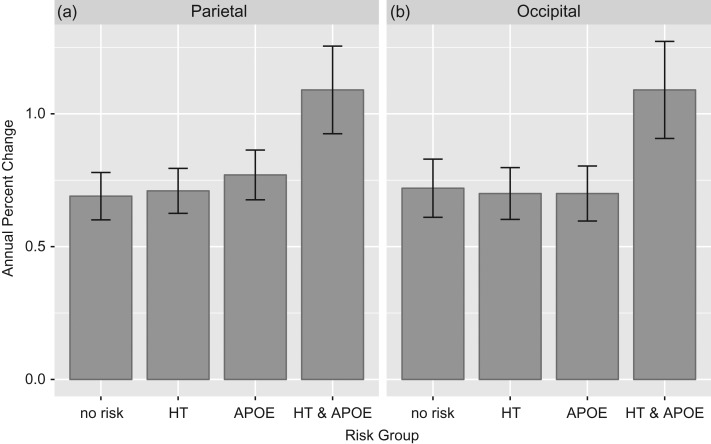
Cortical thinning in parietal and occipital cortex over 8 years depend on APOE and HT status. Breakdown of significant Time × APOE × HT interaction. Bars show annual initial percent change in cortical thickness illustrated in 4 risk groups: participants with “No Risk” factors (i.e., APOE-, normotensive); participants with one risk factor, either HT or APOEε4+ (APOE); and participants with both risk factors (APOEε4+, hypertensive). Annual percent change for Occipital (Panel A) and Parietal (Panel B).
Figure 3.
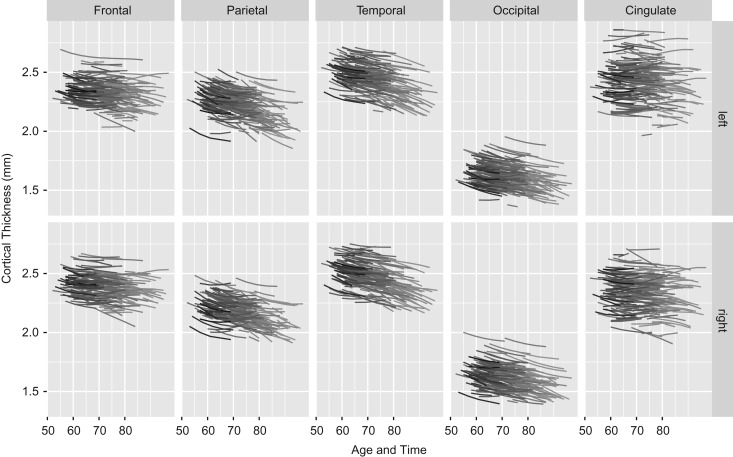
Spaghetti plots for each lobar composite hemisphere rate of cortical thinning Loss. Each line represents an individual across the occasions of study. Predicted cortical thickness based on the full model with all predictors across 5 composites and both hemispheres. LH, left hemisphere; RH, right hemisphere. N = 220 individuals.
All models were estimated using the statistical software package R with the LME command from the NLME library (Pinheiro and Bates 2000; R Core Team 2017). The code for the final model is provided in the Supplementary Material (Code section). The significance of random effects was obtained using likelihood ratio tests.
Results
MMLM of Cortical Thinning in Lobar Composites and Risk Modifiers
The Full Model is the major test of our hypotheses and results are presented in Table 3. The significant effects in Table 3 are noted in bold font and are explained in turn here. For each of the 5 lobar composites, we examined Age, Time, Time2, APOEε4, and HT effects and the interactions among these effects.
Age indicates the significance of cross-sectional age differences in cortical thickness and refers to age at baseline. Significant cross-sectional age differences were found for all 5 composites indicating thinner cortex with older age. Time indicates the significance of linear slope (rate of thinning) across the longitudinal occasions for a given composite at study entry—also referred to as instantaneous rate of change. Linear change over time was found in Parietal, Temporal, and Occipital composites; however, a significant quadratic function would supersede these effects of linear change. Time2 term was included to investigate curvilinear (accelerated or decelerated) thinning over time. We found significant Time2 effects for all 5 composites indicating that the rate of atrophy significantly reduced as time passed; in other words, participants’ cortex tended to thin out at a slower pace as the study occasions progressed. Interestingly, for the Frontal, Temporal, and CING cortex there was additionally a significant Age × Time2 interaction, indicating that this deceleration of rate of thinning was stronger in older participants.
Risk Modifiers
Interestingly, neither the main effects of APOE nor HT were significant for any composite, indicating that at study entry cortical thickness did not differ between carriers and noncarriers or hypertensives and normotensives. However, there was a significant Time × APOE interaction for Frontal and CING composites, indicating that thinning rate depended on APOE status, with ε4 carriers having larger atrophy rates, amounting to a 0.24% initial thinning per year compared with noncarriers. HT did not affect the rate of thinning in any lobar composite, neither on initial thickness nor on the atrophy rate. Rather, a significant Time × APOE × HT interaction was found in the Parietal and Occipital composites and indicated that participants who were both hypertensive and APOEε4 carriers showed significantly faster thinning over time than their nonrisk counterparts (Fig. 2). As such, atrophy rates for those participants evidenced an additional loss of cortical thickness of 0.3% to 0.4% per year, over and above the overall atrophy rate of approximately 0.7% per year (Table 3).
Notably, APOE genotype explained 20% of the variance between participants in initial slope (change) in longitudinal thinning associated with the Frontal, CING, and Temporal composites, 16% in the Parietal and 10% in the Occipital composite, indicating that APOE is a major explanatory factor in individual differences in cortical thinning. APOE genotype also explained 11% of between-hemisphere differences in the Temporal composite. As a comparison, participants’ age explained 4% of slope variance in the Frontal and 10% of the slope variance in the CING composite while it explained less than 1% in all other regions.
After accounting for individual differences due to APOE and HT, large individual differences persist (cf. Supplementary Table 4), as there is a myriad of factors that influence brain aging. Both cortical thickness at study entry and thinning rates differed among individuals. These individual differences in cortical thinning are illustrated in Fig. 3. All lines represent predicted individual cortical thickness trajectories from the full model for each of the 5 lobar composites for both hemispheres. Each line represents individual estimates across the 8-year duration of the study.
Cross-Sectional Versus Longitudinal Estimates of Aging of Cortical Thickness
Longitudinal data offer the possibility to compare change due to the passage of time within individuals (longitudinal) and the differences in time lived across individuals, operationalized as age-differences (cross-sectional). Both of these sets of estimates are reported in Table 3. For both Age and Time, the unit of change is one year. The differences/changes reported in the table reflect the slope of Time and Age at study entry for a, on average, 65-year old. At later points in the study or for different ages, these slopes and their ratios will differ due to the quadratic term in the model which makes a comparison of these 2 effects difficult as they are contingent on centering choices. However, given that cross-sectional studies essentially correspond to the first measurement occasion in longitudinal studies the comparison of atrophy rates at the first measurement occasion with cross-sectional age effects are still informative. In the Parietal, Temporal, and Occipital composites, the significant main effect of longitudinal thinning (Time) indicated an annual −0.7%Δ for Parietal and Occipital and −0.3%Δ for Temporal composites. The longitudinal thinning rate in the Parietal and Occipital composites was almost three times larger than the effect of cross-sectional age differences in cortical thickness. This suggests that for these brain regions, cross-sectional estimates are underestimated compared with true within-person change over time. For the Temporal composite, however, longitudinal thinning and cross-sectional age differences were identical. In the Frontal and CING composites only APOEε4 carriers’ cortices thinned initially by approximately −0.25% per year compared with noncarriers. Again, in both of these composites longitudinal thinning was similar or slightly larger compared with cross-sectional thickness differences but, overall, atrophy rates decelerated over the course of the study.
Discussion
This study aimed to contribute to our understanding of aging of brain structure in 2 important ways: By utilizing long-term multiple occasion longitudinal designs with appropriate sophisticated statistical procedures needed to understand true brain change, and by examining the potential influence of salient risk factors that modify an individual’s rate of brain structural loss. To this end, we used MMLM to measure 8-year longitudinal change (across 5 occasions) in cortical thickness in cognitively healthy midlife and older adults in the SLS, as well as the modifying effects of APOEε4 and HT. As a secondary aim, we compared cross-sectional age-related differences in cortical thickness with true within-person cortical thinning over time, as it is believed that for some cortical composites, cross-sectional aging studies routinely underestimate an individual’s rate of actual shrinkage over time. This design and modeling approach allowed us to detect three major results: (1) in all 5 areas examined (frontal, parietal, temporal, occipital, and CING) there was significant loss of cortical thickness across 8 years and this thinning rate decelerated over time in midlife through older age when change is assessed via quadratic rather than only linear trajectories, (2) thinning of parietal and occipital cortex was dependent on combined risk factors, where individuals who were both APOEε4 carriers and hypertensive had significantly greater 8-year thinning than those with either risk factor alone or neither risk factor, and (3) that for some brain regions (parietal, occipital) longitudinal loss of thickness was 3 times greater than cross-sectional estimates of age-related thickness differences. To our knowledge, this is the first report of long-term longitudinal change in cortical thickness extending over both midlife and old age and reporting synergistic modifying effects of APOE genotype and HT status.
Risk Modifiers of Within-Person Brain Aging
Humans age at different rates and this is apparent across all organs and systems. Underlying this variability are individual difference factors that include one’s genetic, health, and lifestyle/environmental factors. Some individuals maintain sharp cognitive prowess as they age, whereas others less so, and still others develop neurodegenerative conditions such as Alzheimer’s disease or other dementias. Two of the major risk factors that have been linked to cognitive and brain aging and pathology are the presence of APOEε4 allele and diagnosis of HT. Elevated blood pressure and HT have been associated with poorer brain health, including greater white matter hyperintensity lesions (Raz et al. 2007, 2012), decreased white matter connectivity (Kennedy and Raz 2009; Salat et al. 2012), smaller regional brain volumes (Raz et al. 2005, 2007), and most recently thinner cortical regions (Leritz et al. 2011; Vuorinen et al. 2013). HT putatively exerts its negative effects on the brain because it brings about cerebral dysautoregulation and alters perfusion of the cortex. We find effects of HT on thinning of posterior cortices (parietal and occipital) in the Time × APOE × HT interaction, in line with studies of loss of regional posterior volumes (Raz et al. 2007) and of decreased white matter integrity (Raz et al. 2007; Kennedy and Raz 2009). Given the perfusion of the posterior cortices is limited to the posterior cerebral arteries territory, as compared with the better perfused anterior territories of the brain, posterior brain regions would be more susceptible to negative effects of HT (Sorond et al. 2005). These vascular alterations are associated with an increased risk for Alzheimer’s disease and other dementias (Kivipelto et al. 2001). Indeed, it has been proposed that HT, and other vascular risk factors, may increase risk for dementia possibly because of an association with decreased cortical thickness (Alosco et al. 2014; Villeneuve et al. 2014; Villeneuve and Jagust 2015).
APOEε4 positivity, in addition to a genetic risk for AD, also increases risk for cerebrovascular dysfunction as its mechanisms are, in part, through clearance and transport of cholesterol and beta-amyloid protein. APOEε4 carriers have been found to have greater hippocampal shrinkage (Moffat et al. 2000), smaller regional brain volumes and thickness (Liu et al. 2010), and poorer white matter integrity (Persson et al. 2006). APOEε4 carriers also show greater reduced metabolism in temporal cortex across 2 years (Reiman et al. 2001) and greater beta-amyloid deposition in temporal cortex with increased APOEε4 dose (Reiman et al. 2009). Notably, in the current study, APOE’s effects on rate of thinning over time exceeded those of the effects of age, explaining 20% of the variance in change in Frontal, Temporal, and CING cortices.
Of particular importance is the impact of multiple risk factors. Meta-analyses have identified at least 10 major risk factors suggesting that brain atrophy is likely due to multiple risk factors in any individual (Anstey et al. 2013; Deckers et al. 2014; Exalto et al. 2014). Interestingly, Rodrigue et al. (2013) found in a cross-sectional study that among cognitively healthy older adults, individuals with both HT and APOEε4 risk factors had significant elevation in beta-amyloid deposition. Quite similarly, we report here accelerated rate of thinning across 8 years in ε4 carriers in frontal and CING cortex, but also a significantly greater rate of thinning in the parietal and occipital cortex in ε4 carriers who were also hypertensive. These synergistic effects on brain health markers may suggest that there is a threshold at which the brain can no longer resist damage. For example, having a genetic risk for AD may not have detrimental effects on the brain unless another exacerbating condition is present, such as HT.
Although the larger and longer longitudinal studies of cortical thinning have not systematically investigated the effects of APOE genotype or HT, a few smaller longitudinal investigations have done so (Thompson et al. 2011). Donix et al. (2010) examined 2-year longitudinal change in cortical thickness in cognitively normal older adults varying by APOEε4 status. Those with an APOEε4 allele exhibited greater cortical thinning in entorhinal cortex, and all medial temporal cortex regions compared with noncarriers. In relation to the moderating effect of HT on cortical thinning, Walhovd et al. (2014) examined effects of several cardiovascular-related factors (omega-3 fatty acids, vitamin D, physical exercise, cholesterol, systolic blood pressure, and body mass) and reported that better cardiovascular indices related to less cortical thinning in temporal and frontal regions. In a cross-sectional study, hypertensives exhibited lower perfusion in temporal and occipital cortices, along with thinner temporal, frontal, parietal cortex than nonhypertensives (Alosco et al. 2014). Overall, it seems fairly consistent that HT has an effect on posterior cortical thickness, and although the mechanism behind this is yet unknown, it may be due in part to altered perfusion of those territories in hypertensives. Indeed, we show that APOE by itself has an effect on thinning in frontal and CING regions; however, it required the synergistic effect of both APOE and HT risk factors to affect change in the most posterior regions (parietal and occipital). This finding mirrors and extends the previous findings of posterior regions being most affected longitudinally by HT in white matter hyperintensities and volume loss (Raz et al. 2007) to cortical thinning loss.
Comparison of Magnitude of Cross-Sectional Versus Longitudinal Findings Across Regions
There has been debate regarding the magnitude of cross-sectional age differences versus longitudinal annual rate of atrophy. This is a very important issue, both methodologically, and for the proper understanding of true effects of aging on a person’s brain. Some researchers have suggested that neural longitudinal effects may be larger than cross-sectional age difference estimates (Raz and Lindenberger 2010; Kennedy and Raz 2015; Pfefferbaum and Sullivan 2015). Similar to prior cross-sectional studies, we found age differences in all composites.
Although few in number thus far, longitudinal evidence suggests that there are regional differences in rate of cortical thinning and highlight the considerable individual variability in rates of thinning. Although some longitudinal studies report general agreement with cross-sectional studies regarding regions showing age effects (Resnick et al. 2003), other longitudinal studies report patterns of associations that differ from cross-sectional reports (Raz et al. 1997; Du et al. 2006; Pfefferbaum and Sullivan 2015). The primary problem with cross-sectional studies of aging, however is that it is impossible to know whether these ubiquitous detrimental effects of aging on the brain are actually due to the passage of time, or from differences across individuals in the sample. Longitudinal designs, in contrast, hold variation at the individual level constant, essentially utilizing each person as his or her own control subject. Therefore, although limited by shorter time windows, longitudinal designs are the only way to measure true (e.g., intraindividual) change in brain structure as a person ages. We found that although initial atrophy rates tended to be larger than cross-sectional differences (in some regions 3 times greater), atrophy rates also were nonlinear, decelerating over the course of the study reducing the rate of atrophy as time went by.
Individual Differences and Between- Versus Within-Person Variance
Importantly, in some brain regions, APOE genotype predicted from 13% to 20% of the variance among participants (compared with HT, which explained less than 1% of between-person variance). This is an impressive amount of variance to be explained in atrophy in normally aging individuals. Despite this, even after including person-level predictors, the amount of individual heterogeneity in cortical thickness and atrophy rates remains substantial, given that there are multitude of variables that affect how people age. In contrast, within-person variability was much smaller, with the main source residing at the measurement (i.e., FreeSurfer) level, comprising both fluctuations due to longitudinal change over 5 time points (thinning and stability and thickening) and to measurement error. This remaining unexplained variance in brain aging leaves opportunities for the investigation in future studies of additional risk factors.
Study-Specific Considerations
Any study’s findings should be interpreted with regard to the study design and sample characteristics. As noted previously, there is naturally a much wider age range (35 years) available for examining cross-sectional age differences, compared with an 8-year interval for examining longitudinal within-subject thinning effects. This may have implications for directly comparing magnitude of age differences and age-related change. The prevalence of significant longitudinal effects and individual differences in atrophy rates in all 5 composites indicates that age-related thinning can be reliably assessed across 5 measurement occasions spanning 8 years (cf. Rast and Hofer 2014) and corroborates findings from studies with just 2 years (e.g., Fjell et al. 2014).
Additionally, sample characteristics must be considered in interpreting study findings. The sample represents cognitively healthy adults of above average educational level within the midlife to old age range. No participants were diagnosed as MCI or demented at baseline. The restriction of the age range to midlife and old age has both advantages and limitations. Lack of a younger adult group restricts comparisons to other longitudinal studies that do include a younger age group. On the other hand, restricting the age range studied allows for an increase in power and the number of participants studied in midlife, which is the critical period for examining preclinical levels of brain and cognitive decline. Likewise, the inclusion of older adults in the high 80’s has extended these findings to an undersampled population of older ages. Related, given the lengthy latent prodromal phase of AD, it is unknown what portion of the sample transitions to dementia over time. Future time points of the SLS will reveal which individuals, if any, are on a trajectory toward dementia and it will be important to relate AD onset to brain aging and preclinical information obtained in the early phases of the study. This extension in age range, however, may have also increased age-differential selectivity in the sense that the older participants have fared particularly well in terms of brain atrophy. This might be a contributing factor to smaller cross-sectional age differences compared with atrophy rates at study entry.
Summary
In conclusion, we used sophisticated MMLM to demonstrate with longitudinal data spanning 8 years and 5 time points that regional cortical thickness is lost in heteromodal association cortex in middle-aged and older adults ranging in age from 51 to 86 years. This loss of cortex is decelerated with time, and negatively modified by at least 2 markers of vascular risk, APOEε4 genotype and presence of HT. Parietal and Occipital cortex appear to be particularly vulnerable to the synergistic combination of both APOEε4 allele and hypertensive vascular risk factors. Because HT is amenable to treatment, especially in midlife, it may be a viable strategy for Alzheimer’s disease prevention (Villeneuve and Jagust 2015).
Supplementary Material
Notes
Conflict of Interest: None declared.
Supplementary Material
Funding
The National Institutes of Health (grant number R01AG050720 as well as additional support from P01AG043362 to P.R.), (grant number AG-024102 to S.L.W./K.W.S.), (grant number R00 AG-036818-05 to K.M.K.), (grant number R00 AG-036848-05 to K.M.R.), (grant number AG-042228 to D.G.M.), and (grant number RC4 NS-073008 to T.G.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Alosco ML, Gunstad J, Xu X, Clark US, Labbe DR, Riskin-Jones HH, Terrero G, Schwarz NF, Walsh EG, Poppas A, et al. 2014. The impact of hypertension on cerebral perfusion and cortical thickness in older adults. J Am Soc Hypertens. 8:561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstey KJ, Cherbuin N, Herath PM. 2013. Development of a new method for assessing global risk of Alzheimer’s disease for use in population health approaches to prevention. Prev Sci. 14:411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burggren AC, Zeineh MM, Ekstrom AD, Braskie MN, Thompson PM, Small GW, Bookheimer SY. 2008. Reduced cortical thickness in hippocampal subregions among cognitively normal apolipoprotein E e4 carriers. Neuroimage. 41:1177–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael O, Mungas D, Beckett L, Harvey D, Tomaszewski Farias S, Reed B, Olichney J, Miller J, Decarli C. 2012. MRI predictors of cognitive change in a diverse and carefully characterized elderly population. Neurobiol Aging. 33:83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder EH, Saunders M, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses D, Haines JL, Pericak-Vance M. 1993. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 261:921–923. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. 1999. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage. 9:179–194. [DOI] [PubMed] [Google Scholar]
- Deckers K, van Boxtel MPJ, Schiepers OJG, de Vugt M, Sanchez JLM, Anstey KJ, Brayne C, Dartigues JF, et al. 2014. Target risk factors for dementia prevention: a systematic review and Delphi consensus study on the evidence from observational studies. Int J Geriatr Psychiatry. 4245:234–246. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, et al. 2006. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 31:968–980. [DOI] [PubMed] [Google Scholar]
- Donix M, Burggren AC, Suthana NA, Siddarth P, Ekstrom AD, Krupa AK, Jones M, Rao A, Martin-Harris L, Ercoli LM, et al. 2010. Longitudinal changes in medial temporal cortical thickness in normal subjects with the APOE-4 polymorphism. Neuroimage. 53:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doré V, Villemagne VL, Bourgeat P, Fripp J, Acosta O, Chetélat G, Zhou L, Martins R, Ellis KA, Masters CL, et al. 2013. Cross-sectional and longitudinal analysis of the relationship between Aβ deposition, cortical thickness, and memory in cognitively unimpaired individuals and in Alzheimer disease. JAMA Neurol. 70:903–911. [DOI] [PubMed] [Google Scholar]
- Du AT, Schuff N, Chao LL, Kornak J, Jagust WJ, Kramer JH, Reed BR, Miller BL, Norman D, Chui HC, et al. 2006. Age effects on atrophy rates of entorhinal cortex and hippocampus. Neurobiol Aging. 27:733–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exalto LG, Quesenberry CP, Barnes D, Kivipelto M, Biessels GJ, Whitmer RA. 2014. Midlife risk score for the prediction of dementia four decades later. Alzheimers Dement. 10:562–570. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. 2000. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 97:11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. 1999. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 9:195–207. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve DN, Fischl B, et al. 2009. High consistency of regional cortical thinning in aging across multiple samples. Cereb Cortex. 19:2001–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Grydeland H, Amlien I, Espeseth T, Reinvang I, Raz N, Dale AM, Walhovd KB, for the Alzheimer Disease Neuroimaging Initiative . 2014. Accelerating cortical thinning: unique to dementia or universal in aging? Cereb Cortex. 24:919–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisher AS, Chen K, Liu X, Ayutyanont N, Roontiva A, Thiyyagura P, Protas H, Joshi AD, Sabbagh M, Sadowsky CH, et al. 2013. Apolipoprotein E ε4 and age effects on florbetapir positron emission tomography in healthy aging and Alzheimer disease. Neurobiol Aging. 34:1–12. [DOI] [PubMed] [Google Scholar]
- Frieden C, Garai K. 2012. Structural differences between apoE3 and apoE4 may be useful in developing therapeutic agents for Alzheimer’s disease. Proc Natl Acad Sci USA. 109:8913–8918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam P, Cherbuin N, Sachdev PS, Wen W, Anstey KJ. 2011. Relationships between cognitive function and frontal grey matter volumes and thickness in middle aged and early old-aged adults: the PATH Through Life Study. Neuroimage. 55:845–855. [DOI] [PubMed] [Google Scholar]
- Gautam P, Cherbuin N, Sachdev PS, Wen W, Anstey KJ. 2013. Sex differences in cortical thickness in middle aged and early old-aged adults: Personality and Total Health Through Life study. Neuroradiology. 55:697–707. [DOI] [PubMed] [Google Scholar]
- Gelman A, Hill J, Yajima M. 2012. Why we (usually) don’t have to worry about multiple comparisons. J Res Educ Eff. 5:189–211. [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. 2001. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 14:21–36. [DOI] [PubMed] [Google Scholar]
- Hardy J. 2009. The amyloid hypothesis for Alzheimer’s disease: a critical reappraisal. J Neurochem. 110:1129–1134. [DOI] [PubMed] [Google Scholar]
- Hixson JE, Vernier DT. 1990. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 31:545–548. [PubMed] [Google Scholar]
- Hofer SM, Flaherty BP, Hoffman L. 2006. Cross-sectional analysis of time-dependent data: problems of mean-induced association in age-heterogeneous samples and an alternative method based on sequential narrow age-cohorts. Multivar Behav Res. 41:165–187. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Berhow MT, Sowell ER, Foster DS, Hesselink JR. 1991. Cerebral structure on MRI, part I: localization of age-related changes. Biol Psychiatry. 29:55–67. [DOI] [PubMed] [Google Scholar]
- Jiang J, Sachdev P, Lipnicki DM, Zhang H, Liu T, Zhu W, Suo C, Zhuang L, Crawford J, Reppermund S, et al. 2014. A longitudinal study of brain atrophy over two years in community-dwelling older individuals. Neuroimage. 86:203–211. [DOI] [PubMed] [Google Scholar]
- Kennedy KM, Raz N. 2009. Pattern of normal age-related regional differences in white matter microstructure is modified by vascular risk. Brain Res. 1297:41–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KM, Raz N. 2015. Normal aging of the brain In: Toga AW, editor. Brain Mapping: An Encyclopedic Reference. London: Academic Press - Elsevier; p. 603–617. [Google Scholar]
- Kivipelto M, Helkala EL, Laakso MP, Hänninen T, Hallikainen M, Alhainen K, Soininen H, Tuomilehto J, Nissinen A. 2001. Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. BMJ. 322:1447–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. 2012. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage. 60:340–352. [DOI] [PubMed] [Google Scholar]
- Leritz EC, Salat DH, Williams VJ, Schnyer DM, Rudolph JL, Lipsitz L, Fischl B, McGlinchey RE, Milberg WP. 2011. Thickness of the human cerebral cortex is associated with metrics of cerebrovascular health in a normative sample of community dwelling older adults. Neuroimage. 54:2659–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C-C, Kanekiyo T, Xu H, Bu G. 2013. Apolipoprotein E and Alzheimer disease: risk, mechanisms, and therapy. Nat Rev Neurol. 9:106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RS, Lemieux L, Bell G, Sisodiya S, Shorvon S, Sander JWA, Duncan J. 2003. A longitudinal study of brain morphometrics using quantitative magnetic resonance imaging and difference image analysis. Neuroimage. 20:22–33. [DOI] [PubMed] [Google Scholar]
- Liu Y, Paajanen T, Westman E, Wahlund LO, Simmons A, Tunnard C, Sobow T, Proitsi P, Powell J, Mecocci P, et al. , AddNeuroMed Consortium . 2010. Effect of APOE ε4 allele on cortical thicknesses and volumes: the AddNeuroMed study. J Alzheimers Dis. 21:947–966. [DOI] [PubMed] [Google Scholar]
- MacCallum RC, Kim C, Malarkey WB, Kiecolt-Glaser JK. 1997. Studying multivariate change using multilevel models and latent curve models. Multivar Behav Res. 32:215–253. [DOI] [PubMed] [Google Scholar]
- MacDonald SW, Li SC, Bäckman L. 2009. Neural underpinnings of within-person variability in cognitive functioning. Psychol Aging. 24:792–808. [DOI] [PubMed] [Google Scholar]
- Mahley RW, Huang Y. 2012. Apolipoprotein e sets the stage: response to injury triggers neuropathology. Neuron. 76:871–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat SD, Szekely CA, Zonderman AB, Kabani NJ, Resnick SM. 2000. Longitudinal change in hippocampal volume as a function of apolipoprotein E genotype. Neurology. 55:134–136. [DOI] [PubMed] [Google Scholar]
- Morris JC, Edland S, Clark C, Galasko D, Koss E, Mohs R, van Belle G, Fillenbaum G, Heyman A. 1993. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD): Part IV. Rates of cognitive change in the longitudinal assessment of probable Alzheimer’s disease. Neurology. 43:2457–2457. [DOI] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C. 1989. The consortium to establish a registry for Alzheimer’s disease (CERAD): I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 39:1159–1165. [DOI] [PubMed] [Google Scholar]
- Pacheco J, Goh JO, Kraut MA, Ferrucci L, Resnick SM. 2015. Greater cortical thinning in normal older adults predicts later cognitive impairment. Neurobiol Aging. 36:903–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Lind J, Larsson A, Ingvar M, Cruts M, Van Broeckhoven C, Adolfsson R, Nilsson LG, Nyberg L. 2006. Altered brain white matter integrity in healthy carriers of the APOE epsilon4 allele: a risk for AD? Neurology. 66:1029–1033. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. 1994. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 51:874–887. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rohlfing T, Rosenbloom MJ, Chu W, Colrain IM, Sullivan EV. 2013. Variation in longitudinal trajectories of regional brain volumes of healthy men and women (ages 10 to 85 years) measured with atlas-based parcellation of MRI. Neuroimage. 65:176–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV. 2015. Cross-sectional versus longitudinal estimates of age-related changes in the adult brain: overlaps and discrepancies. Neurobiol Aging. 36:2563–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai JA, McEvoy LK, Hagler DJ, Holland D, Dale AM, Salmon DP, Galasko D, Fennema-Notestine C. 2012. Higher education is not associated with greater cortical thickness in brain areas related to literacy or intelligence in normal aging or mild cognitive impairment. J Clin Exp Neuropsychol. 34:925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro JC, Bates DM. 2000. Mixed-Effects Models in S and S-PLUS. New York: Springer. [Google Scholar]
- R Core Team 2017. R: A Language and Environment for Statistical Computing.
- Rast P, Hofer SM. 2014. Longitudinal design considerations to optimize power to detect variances and covariances among rates of change: simulation results based on actual longitudinal studies. Psychol Methods. 19:133–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush SW. 2001. Comparing personal trajectories and drawing causal inferences from longitudinal data. Annu Rev Psychol. 52:501–525. [DOI] [PubMed] [Google Scholar]
- Raz N, Ghisletta P, Rodrigue KM, Kennedy KM, Lindenberger U. 2010. Trajectories of brain aging in middle-aged and older adults: Regional and individual differences. Neuroimage. 51:501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Loken WJ, Thornton AE, Acker JD. 1997. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb Cortex. 7:268–282. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U. 2010. News of cognitive cure for age-related brain shrinkage is premature: a comment on Burgmans et al. (2009). Neuropsychology. 24:255–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Ghisletta P, Rodrigue KM, Kennedy KM, Acker JD. 2008. Neuroanatomical correlates of fluid intelligence in healthy adults and persons with vascular risk factors. Cereb Cortex. 18:718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. 2005. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 15:1676–1689. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM. 2006. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev. 30:730–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Acker JD. 2007. Vascular health and longitudinal changes in brain and cognition in middle-aged and older adults. Neuropsychology. 21:149–157. [DOI] [PubMed] [Google Scholar]
- Raz N, Yang YQ, Rodrigue KM, Kennedy KM, Lindenberger U, Ghisletta P. 2012. White matter deterioration in 15 months: latent growth curve models in healthy adults. Neurobiol Aging. 33:429.e1–e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Caselli RJ, Chen K, Alexander GE, Bandy D, Frost J. 2001. Declining brain activity in cognitively normal apolipoprotein E ε4 heterozygotes: a foundation for using positron emission tomography to efficiently test treatments to prevent Alzheimer’s disease. Proc Natl Acad Sci USA. 98:3334–3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Liu X, Bandy D, Yu M, Lee W, Ayutyanont N, Keppler J, Reeder SA, Langbaum JBS, et al. 2009. Fibrillar amyloid-β burden in cognitively normal people at 3 levels of genetic risk for Alzheimer’s disease. Proc Natl Acad Sci USA. 106:6820–6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. 2003. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci. 23:3295–3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Schmansky N, Rosas H, Fischl B. 2012. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 61:1402–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigue KM, Kennedy KM. 2011. The cognitive consequences of structural changes to the aging brain In: Schaie KW, Willis SL, editors. Handbook of the Psychology of Aging. 7th ed New York: Elsevier; p. 73–92. Ch 5. [Google Scholar]
- Rodrigue KM, Rieck JR, Kennedy KM, Devous MD, Diaz-Arrastia R, Park DC. 2013. Risk factors for amyloid deposition in healthy aging: interactive effects of vascular and genetic risk. JAMA Neurol. 70:600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RSR, Busa E, Morris JC, Dale AM, Fischl B. 2004. Thinning of the cerebral cortex in aging. Cereb Cortex. 14:721–730. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Greve DN, van der Kouwe AJ, Hevelone ND, Zaleta AK, Rosen BR, Fischl B, Corkin S, Rosas HD, et al. 2005. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging. 26:1215–1227. [DOI] [PubMed] [Google Scholar]
- Salat DH, Williams VJ, Leritz EC, Schnyer DM, Rudolph JL, Lipsitz LA, McGlinchey RE, Milberg WP. 2012. Inter-individual variation in blood pressure is associated with regional white matter integrity in generally healthy older adults. Neuroimage. 59:181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scahill RI, Frost C, Jenkins R, Whitwell JL, Rossor MN, Fox NC. 2003. A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Arch Neurol. 60:989–994. [DOI] [PubMed] [Google Scholar]
- Schaie KW. 2013. Developmental Influences on Adult Intelligence: The Seattle Longitudinal Study. 2nd ed New York: Oxford University Press. [Google Scholar]
- Scheltens P, Blennow K, Breteler MMB, de Strooper B, Frisoni GB, Salloway S, Van der Flier WM. 2016. Alzheimer’s disease. Lancet. 388:505–517. [DOI] [PubMed] [Google Scholar]
- Sorond FA, Khavari R, Serrador JM, Lipsitz LA. 2005. Regional cerebral autoregulation during orthostatic stress: age-related differences. J Gerontol A Biol Sci Med Sci. 60:1484–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storsve AB, Fjell AM, Tamnes CK, Westlye LT, Overbye K, Aasland HW, Walhovd KB. 2014. Differential longitudinal changes in cortical thickness, surface area and volume across the adult life span: regions of accelerating and decelerating change. J Neurosci. 34:8488–8498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Rohlfing T, Pfefferbaum A. 2010. Quantitative fiber tracking of lateral and interhemispheric white matter systems in normal aging: relations to timed performance. Neurobiol Aging. 31:464–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan GE, DeCarli C, Miller BL, Reed T, Wolf PA, Jack LM, Carmelli D. 1998. Association of midlife blood pressure to late-life cognitive decline and brain morphology. Neurology. 51:986–993. [DOI] [PubMed] [Google Scholar]
- Thambisetty M, Wan J, Carass A, An Y, Prince JL, Resnick SM. 2010. Longitudinal changes in cortical thickness associated with normal aging. Neuroimage. 52:1215–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson WK, Hallmayer J, O’Hara R. 2011. Design considerations for characterizing psychiatric trajectories across the lifespan: application to effects of APOEε4 on cerebral cortical thickness in Alzheimer’s disease. Am J Psychiatry. 168:894–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese PB, Castellano JM, Holtzman DM. 2011. Apolipoprotein E in Alzheimer’s disease and other neurological disorders. Lancet Neurol. 10:241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve S, Reed BR, Madison CM, Wirth M, Marchant NL, Kriger S, Mack WJ, Sanossian N, DeCarli C, Chui HC, et al. 2014. Vascular risk and Abeta interact to reduce cortical thickness in AD vulnerable brain regions. Neurology. 83:40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve S, Jagust WJ. 2015. Imaging vascular disease and amyloid in the aging brain: implications for treatment. J Prev Alzheimers Dis. 2:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuorinen M, Kåreholt I, Julkunen V, Spulber G, Niskanen E, Paajanen T, Soininen H, Kivipelto M, Solomon A. 2013. Changes in vascular factors 28 years from midlife and late-life cortical thickness. Neurobiol Aging. 34:100–109. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Espeseth T. 2014. Cognitive decline and brain pathology in aging--need for a dimensional, lifespan and systems vulnerability view. Scand J Psychol. 55:244–254. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.