Abstract
Tumors with a neuronal component comprise a small percentage of central nervous system (CNS) neoplasms overall, but the presence of neuronal differentiation has important diagnostic, prognostic, and therapeutic implications. Insulinoma-associated protein 1 (INSM1) is a transcription factor with strong nuclear immunostaining in neuroendocrine cells and neoplasms of neuroendocrine origin; however, its expression in the CNS in normal brain and in neoplastic cells has not been fully explored. Here, we present immunostaining results from a large number of archival CNS tissue specimens, including 416 tumors. Nuclear immunostaining for INSM1 was frequently seen in medulloblastomas (87%, n = 94). Diffuse nuclear INSM1 immunostaining was detected in all central neurocytomas and pituitary adenomas. Patchy to rare staining with INSM1 was also seen in other high-grade embryonal tumors and high-grade gliomas. In normal brain tissue, specific nuclear INSM1 immunohistochemical staining was only seen in early neuronal development. Notably, nuclear INSM1 staining was not seen in adult normal brain, including areas of gliosis. These findings indicate that nuclear INSM1 immunostaining may serve as a strong nuclear marker in the brain for neoplasms of neuroendocrine or immature neuronal differentiation, when used in concert with other immunostains.
Keywords: Biomarker, Immunohistochemistry, INSM1, Medulloblastoma, Neurodevelopment, Neuroendocrine
INTRODUCTION
Clinical diagnosis of central nervous system (CNS) neoplasms depends on a combination of morphologic changes and immunohistochemical stains to differentiate between tumor types and diagnose disease. Commonly used immunohistochemical markers of neuronal differentiation, such as synaptophysin and chromogranin, have diffuse and cytoplasmic staining that includes background brain, making it particularly difficult to identify infiltrating tumor cells. Although the nuclear marker NeuN, an antibody targeted against the human RNA binding protein RBFOX3, can be used to recognize mature neuronal differentiation, immature neuronal cells frequently show negative or dim staining for this protein. As such, additional nuclear markers that recognize immature neuronal differentiation could be a valuable diagnostic tool.
As a transcription factor, insulinoma-associated protein 1 (INSM1) is localized to the nucleus and is a marker of mature neuroendocrine and intermediate neuronal differentiation (1, 2). This DNA-binding protein is essential for development, as mice with homozygous deletion of this gene do not survive embryogenesis (3). Recent studies have shown that a new monoclonal anti-INSM1 antibody (clone A8, Santa Cruz Biotechnology, Dallas, TX) is a highly sensitive marker of neuroendocrine differentiation in many different anatomic sites, including the thoracic cavity, gastrointestinal system, and genitourinary tract (4–6). However, immunostaining with clone A8 has not been extensively tested in the CNS, although there is evidence that this may be a histologic marker of medulloblastoma and expressed in some oligodendrogliomas (7, 8). Although INSM1 is involved in neuronal development and shows loss of gene expression in the adult brain (2), the overall utility of antibodies against this protein in diagnosing brain neoplasms is not well understood. The clone A8 monoclonal antibody targeting INSM1 has recently been approved for clinical use in our College of American Pathology (CAP)-accredited laboratory for the staining of neuroendocrine tumors, so we decided to test this antibody in a combination of preexisting human tumor microarrays (9–13), archival tissue, and autopsy specimens.
Our studies indicate that INSM1 is indeed a sensitive marker of immature neuronal neoplasms, mature CNS neuroendocrine neoplasms (i.e. pituitary adenoma), and some glial tumors with immature neuronal differentiation. Most significantly, we have found that nuclear INSM1 immunostaining is not significantly identified in areas of adult neurogenesis or in inflammatory mimics of neoplasia. While increased practical application of this immunohistochemical stain will refine its utility, we anticipate that INSM1 immunohistochemistry will be a powerful addition to immunohistochemical panels in the evaluation of CNS neoplasms.
MATERIALS AND METHODS
Tissue Acquisition
This study was performed in accordance with the Johns Hopkins Institutional Review Board guidelines. Tissues used in this study included previously constructed microarrays (9–13), archival paraffin-embedded CNS tissues, and archival autopsy brain tissue. Tissue microarrays (TMAs) were previously constructed from specimens obtained between 1984 and 2014. Glioblastoma and low-grade glioma specimens were previously evaluated for IDH1 R132H status; however, no additional molecular diagnostic information was available for these specimens. Whole archival unstained slides were stained for evaluation of a subset of glioblastoma specimens, central neurocytoma, pituitary adenoma, atypical rhabdoid/teratoid tumors, and normal tissue. Normal adult and fetal brains for anatomical study were obtained from the autopsy archives of Johns Hopkins Hospital.
Immunohistochemistry
Immunostaining was performed using monoclonal antibodies for INSM1 (Santa Cruz Biotechnology; clone A8; 1:200 dilution) and NeuN (Millipore Sigma, Temecula, CA, clone MAB377, 1:4000 dilution). Immunohistochemical staining was performed in a CLIA certified laboratory as reported previously (4). Expression of both INSM1 and NeuN were graded from rare staining to diffuse, with patchy staining representing the staining of subsets of adjacent cells (Supplementary Data Fig. S1).
Data Analysis
All statistical comparative analysis of INSM1 and NeuN staining was performed using TMA data in order to control for specimen size. Comparison of INSM1 staining and NeuN staining in embryonal tumors was performed in GraphPad Prism using the Fisher exact test.
The data shown in Figure 3 was evaluated using previously published microarray analysis data (Affymetrix array U133) (14). The raw data were downloaded from the publically available Gene Expression Omnibus (GEO) of the National Center of Biotechnology Information (NCBI). Molecular subtype analysis of the medulloblastoma and CNS embryonal tumor samples was not available. The raw data were then normalized and evaluated for differential gene expression using the limma (21) package in the R statistical analysis environment. Genes with statistically significant differential expression were then subset into only those in the “transcription factor” Gene Ontology (GO: 0003700) pathway for heat map visualization.
FIGURE 3.
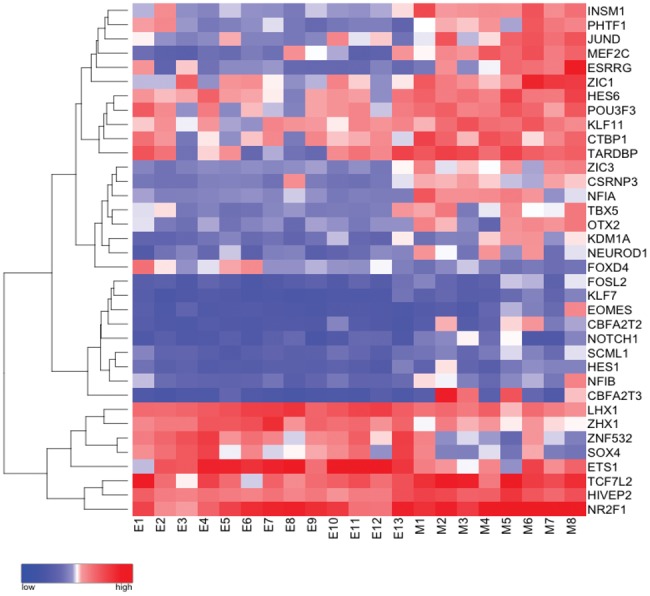
Differentially expressed transcription factors in medulloblastoma as compared with central nervous system embryonal tumors, not otherwise specified. Heat map with unsupervised clustering demonstrating differential expression of genes in the gene ontology pathway “transcription factor activity, sequence specific” (GO: 0003700) between medulloblastoma (M) and other CNS embryonal tumors (E). Red = relative upregulation, Blue = relative downregulation.
RESULTS
INSM1 Is Frequently Expressed in CNS Neoplasms
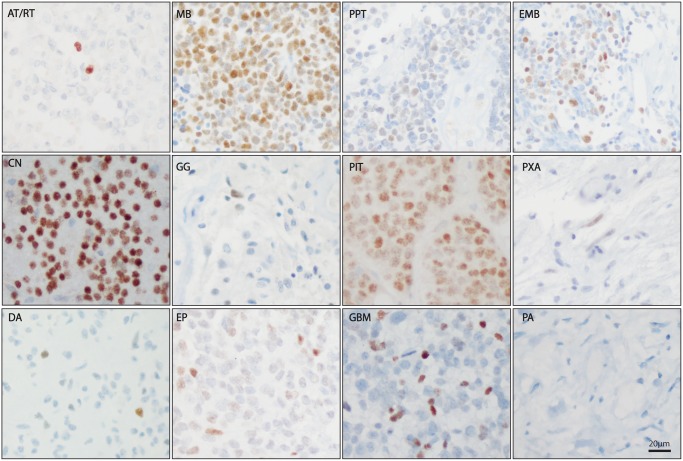
INSM1 immunostaining of archival paraffin-embedded tissue (Supplementary Data Table S1), including previously constructed tumor TMAs, showed a universally strong level of staining in all pituitary adenomas (100%, n = 10) and central neurocytomas (100%, n = 11) evaluated (Fig. 1; Table 1). INSM1 positivity was also observed in a very large proportion of medulloblastomas (87%, n = 94), and a moderate number of other embryonal tumors, including pineal parenchymal tumors (43%, n = 28). Additionally, CNS embryonal neoplasms showed a moderate amount of staining (43%, n = 14). Only very rare INSM1 immunostaining was seen in the high-grade polyphenotypic embryonal tumor, atypical teratoid/rhabdoid tumor (11%, n = 9) and in low-grade tumors with ganglion cell differentiation (i.e. ganglioglioma, 7%, n = 13).
FIGURE 1.
INSM1 immunostaining in central nervous system neoplasms. Representative INSM1 immunostaining in atypical rhabdoid/teratoid tumor (AT/RT), medulloblastoma (MB), pineal parenchymal tumors (PPT), CNS embryonal tumor, not otherwise specified (EMB), central neurocytoma (CN), ganglioglioma (GG), pituitary adenoma (PIT), pleomorphic xanthoastrocytoma (PXA), diffuse astrocytoma (DA), ependymoma (EP), glioblastoma (GBM), pilocytic astrocytoma (PA). Scale bar = 20 µm.
TABLE 1.
INSM1 Immunostaining in CNS Neoplasms
| Diagnosis | Total (%) | Diffuse (%) | Patchy (%) | Rare (%) |
|---|---|---|---|---|
| Medulloblastoma (n = 94) | 82 (87%) | 22 (23%) | 43 (46%) | 17 (18%) |
| Atypical teratoid/rhabdoid tumor (n = 9) | 1 (11%) | 0 (0%) | 0 (0%) | 1 (11%) |
| Pineal parenchymal tumor (n = 28) | 12 (43%) | 1 (4%) | 6 (21%) | 4 (14%) |
| CNS embryonal, other (n = 14) | 6 (43%) | 0 (0%) | 2 (14%) | 4 (29%) |
| Central neurocytoma (n = 11) | 11 (100%) | 7 (64%) | 4 (36%) | 0 (0%) |
| Pituitary adenoma (n = 10) | 10 (100%) | 7 (70%) | 3 (30%) | 0 (0%) |
| Ganglioglioma (n = 14) | 1 (7%) | 0 (0%) | 0 (0%) | 1 (7%) |
| Ependymoma (n = 62) | 3 (5%) | 0 (0%) | 0 (0%) | 3 (5%) |
| Pleomorphic xanthoastrocytoma (n = 8) | 1 (13%) | 0 (0%) | 0 (0%) | 1 (13%) |
| Pilocytic astrocytoma (n = 43) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Diffuse astrocytoma (n = 29) | 6 (21%) | 0 (0%) | 0 (0%) | 6 (21%) |
| Glioblastoma (n = 94) | 41 (44%) | 1 (1%) | 10 (11%) | 30 (32%) |
| Total (n = 416) | 174 (42%) | 38 (9%) | 68 (16%) | 67 (16%) |
More surprisingly, a large number of glial neoplasms showed at least focal nuclear INSM1 staining. Of the glial neoplasms evaluated, the most abundant immunostaining was seen in infiltrating gliomas, including glioblastoma (43%, n = 96) and diffuse astrocytoma (21%, n = 29). There was no significant association between INSM1 immunostaining and the presence of an IDH1 R132H mutation or a primitive neuroectodermal component in the TMAs on prior diagnostic evaluation (data not shown). Of note, INSM1 immunostaining in ependymomas was very low (5%, n = 68) and the only ependymomas expressing detectable amounts of nuclear INSM1 protein were diagnosed as atypical (grade III) due to increased mitotic activity. One of the INSM1-positive ependymomas was noted to have focal synaptophysin staining in the original pathology report. INSM1 immunostaining was not present in the grade I astrocytoma, pilocytic astrocytoma (0%, n = 43), and was only rarely present in the well-circumscribed grade II astrocytoma, pleomorphic xanthoastrocytoma (13%, n = 8).
Nuclear Immunostaining for INSM1 Is More Sensitive for Medulloblastoma Than NeuN
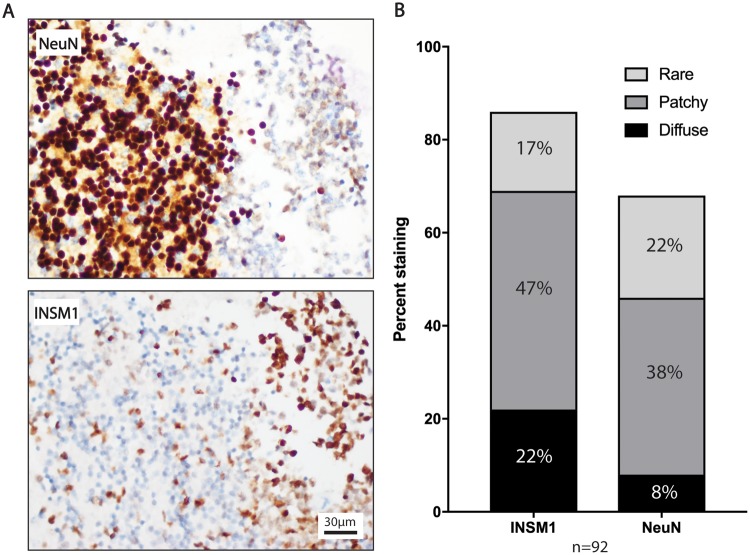
In order to compare INSM1 immunostaining in these tumors with another nuclear marker of neuronal differentiation, the same TMAs were immunostained for NeuN. Both INSM1 and NeuN demonstrated a mosaic pattern of staining, with interspersed positive and negative cells, regardless of the overall percentage of positive cells. In this comparison, INSM1 immunostaining was more sensitive than NeuN for medulloblastoma (87% vs 70%), and had a trend of increased sensitivity in other embryonal tumors (Fig. 2; Table 2). In contrast, NeuN was more frequently positive than INSM1 in the differentiated ganglion cells of ganglioglioma, staining 54% versus 8% tumors. Both immunostains were positive in all of the central neurocytomas evaluated.
FIGURE 2.
INSM1 shows stronger expression in medulloblastoma than NeuN and does not stain the granular cell layer of the cerebellum. (A) Tissue microarray (TMA) specimen with medulloblastoma infiltrating the internal granular cell layer of the cerebellum. Levels of the same specimen are stained with NeuN and INSM1. Microscopic images presented are taken at 40×. (B) Bar graph shows density of INSM1 and NeuN staining in medulloblastoma as a percentage of all TMA specimens with sufficient tissue (>1 core) stained by both antibodies.
TABLE 2.
Comparison of INSM1 Immunostaining to NeuN Immunostaining in Embryonal and Neuronally Differentiated Tumors
| Diagnosis | INSM1+ (%) | NeuN+ (%) |
|---|---|---|
| MB (n = 92)** | 80 (87%) | 64 (70%) |
| AT/RT (n = 8) | 0 (0%) | 2 (25%) |
| PPT (n = 18) | 4 (22%) | 5 (28%) |
| EMB (n = 11) | 4 (36%) | 1 (9%) |
| CN (n = 11) | 11 (100%) | 11 (100%) |
| GG (n = 13) | 1 (8%) | 7 (54%) |
| Total (n = 143) | 173 (41%) | 38 (9%) |
p < 0.01 for medulloblastoma, Fisher exact test.
Both INSM1 and NeuN had a mosaic pattern of staining, therefore these stains were graded from rare staining to diffuse, with patchy staining representing the staining of subsets of adjacent cells (Supplementary Data Fig. S1). These results showed that in addition to having higher sensitivity for medulloblastoma, INSM1 was more likely to have diffuse or patchy staining of medulloblastoma as compared with NeuN (Fig. 2). This finding is clinically significant, as NeuN also prominently stains normal neurons that may be infiltrated by tumor. Figure 2 illustrates this point, showing INSM1-positive medulloblastoma cells infiltrating the INSM1 negative granular cell layer in the cerebellum. In comparison, NeuN darkly stained granular cells and only faintly stained medulloblastoma cells.
Both medulloblastoma and CNS embryonal tumors demonstrated INSM1 immunostaining. However, the frequency and extent of INSM1 immunostaining in medulloblastoma seemed to be increased over other CNS embryonal tumors. In order to determine if this difference in apparent expression reflected different transcriptional regulation changes, raw data from previously published RNA expression microarrays of medullolastoma and other CNS embryonal tumors was evaluated (14). Differentially expressed genes in the transcription factor gene ontology group (GO: 0003700) were selected among all genes with significantly altered expression. Many transcription factors known to be involved in medulloblastoma pathogenesis, including OTX2, were upregulated in the medulloblastoma group (Fig. 3). As expected based on immunohistochemical analysis, INSM1 expression was upregulated in medulloblastoma as compared with other embryonal tumors.
INSM1 Expression Is Enriched in Medulloblastoma, Regardless of Subtype, and Is Expressed in Partially Differentiated Medulloblastoma Nodules
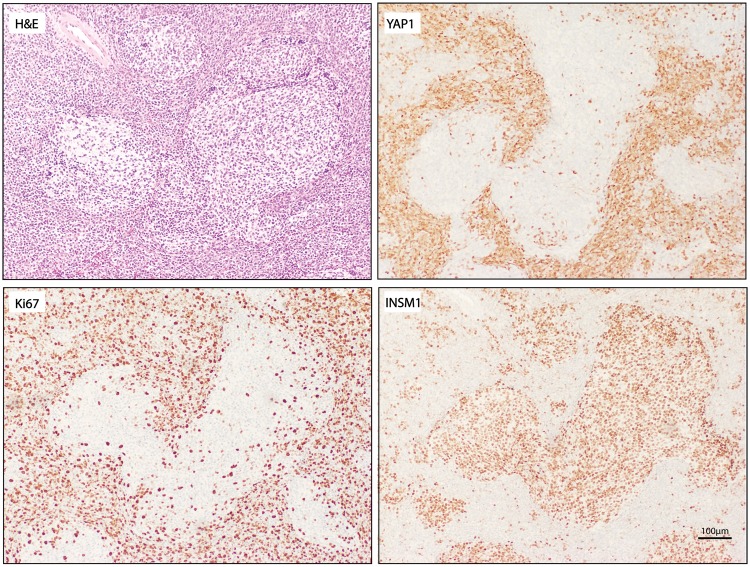
Previous reports have suggested that INSM1 expression is activated by the sonic hedgehog (SHH) pathway in the cerebellum (15). These findings would suggest that INSM1 expression is specifically upregulated in the SHH-activated subtype of medulloblastoma. To test the correlation of subtype and INSM1 protein abundance, the percentage of each tumor type that stained with INSM1 was calculated and no significant differences were found (Supplementary Data Table S2). Interestingly, the nodular-desmoplastic histological subtype that is highly associated with SHH activation showed a unique pattern of INSM1 staining. In these tumors, INSM1 was positive in the mitotically inert nodules that often also have positive staining with synaptophysin (16) (Fig. 4); however, INSM1 immunostaining was absent in the Yes activated protein (YAP1)-positive areas, which demonstrate increased SHH pathway activation.
FIGURE 4.
INSM1 expression and immunostaining in medulloblastoma subtypes. (A) Hematoxylin and eosin (H&E) staining, and INSM1, YAP1, and Ki67 immunostaining in nodular desmoplastic medulloblastoma. Scale bar = 100 µm.
INSM1 Immunostaining in the Developing and Adult Brain
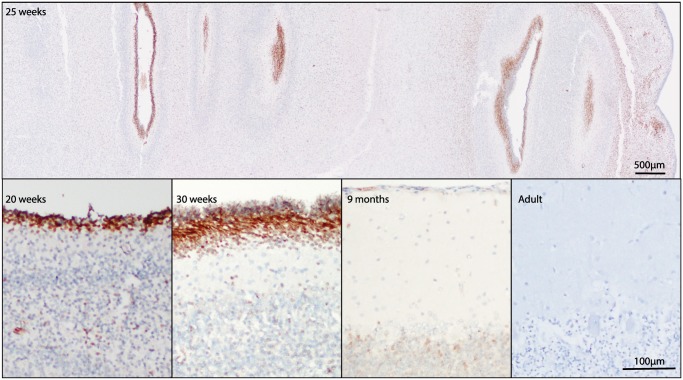
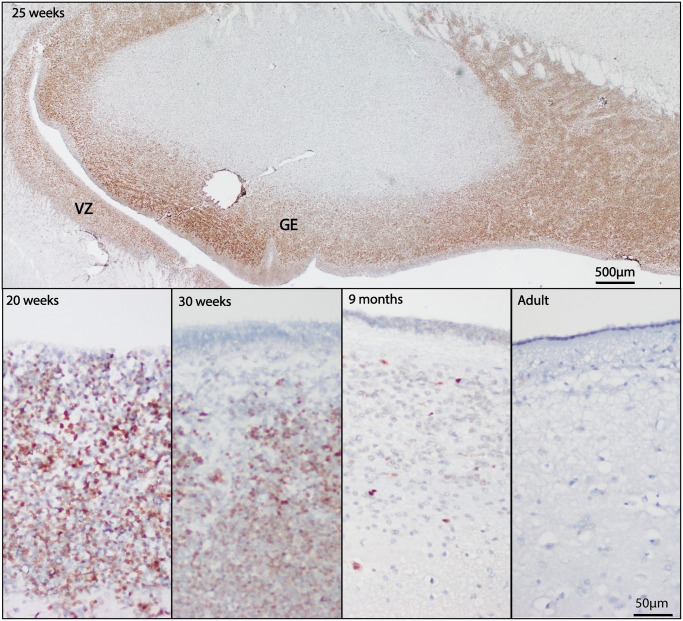
Numerous studies of INSM1 mRNA expression have shown that INSM1 is transiently expressed in the developing brain; however, protein expression, as assessed by immunohistochemistry, has not been assessed in normal fetal and adult human brain. Autopsy brain specimens from the second and third trimester of development and in adulthood were immunostained for INSM1. At 20 weeks gestation, fetal brain tissue showed dark immunostaining for INSM1 in developing neurons of the external granular cell layer of the cerebellum (Fig. 5) and in the subventricular zones of the lateral ventricles (Fig. 6). This staining was particularly strong at 25 weeks and was seen throughout the ventricular zone and in the ganglionic eminence. In the cerebellum at 25 weeks, INSM1 remained limited to the external granular cell layer and in scattered cells in the roof of the fourth ventricle. By 30 weeks gestation, INSM1-positive cells in the external granular cell layer showed nuclear elongation and faintly positive cells were seen in the molecular layer and external portion of the internal granular cell layer. In postnatal month 9, faint nuclear staining was still present in the internal granular cell layer; however, this disappeared by adulthood. In the subventricular zone, INSM1 expression started to recede by gestational week 30, with only rare positive cells detected at postnatal month 9, and none detected in adulthood.
FIGURE 5.
INSM1 immunostaining in developing cerebellum. INSM1 expression in the cerebellum at 20, 25, and 30 weeks and 9 months postnatally. No significant INSM1 immunostaining is present in the adult cerebellum. Scale bars = 500 µm, 100 µm.
FIGURE 6.
INSM1 immunostaining in developing subventricular zone. INSM1 expression in the cerebellum at 20, 25, and 30 weeks and 9 months postnatally. No significant INSM1 immunostaining is present in the adult cerebellum. GE = ganglionic eminence, VZ = ventricular zone. Scale bars = 500 µm, 50 µm.
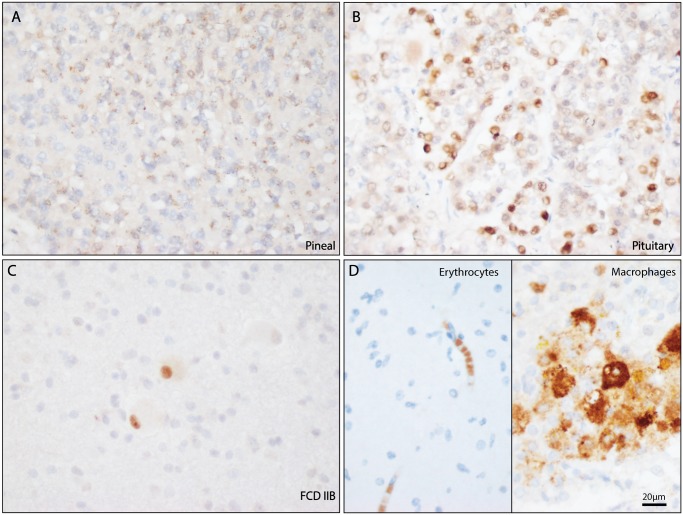
INSM1 is known to be expressed in mature neuroendocrine cells of the gastrointestinal tract, therefore the areas of the brain with endocrine function were evaluated for INSM1 expression using normal adult pituitary and pineal gland specimens. In normal adult autopsy brain specimens, INSM1 had perinuclear granular staining in the pineal gland (Fig. 7A, n = 2). INSM1 also had variable nuclear staining of normal cells in the pituitary gland (Fig. 7B, n = 2). No significant nuclear staining was seen in the remaining adult normal brain. Areas of reactive gliosis also lacked nuclear INSM1 immunostaining. Nonspecific cytoplasmic staining for INSM1 was abundant in foamy macrophages and in erythrocytes in the slides evaluated for this study.
Figure 7.
INSM1 immunostaining in nonneoplastic brain. (A) The normal adult pineal gland shows punctate cytoplasmic staining with A8. (B) The normal pituitary gland shows variable amounts of nuclear staining. (C) Specific nuclear staining seen in balloon cells a case of focal cortical dysplasia. (D) Nonspecific cytoplasmic INSM1 immunostaining is frequently seen in erythrocytes and macrophages. Scale bar = 20 µm.
DISCUSSION
These findings represent the most extensive analysis of immunohistochemical detection of INSM1 in the CNS to date. The results of this study are clinically applicable in that INSM1 was very specific for neoplastic and dysplastic processes and showed higher sensitivity for immature neuronal neoplasms than NeuN. This study also illustrated spatial heterogeneity in the expression of INSM1 protein expression in both neuronal- and glial-derived tumor types.
Regarding clinical usage of the A8 monoclonal antibody, these findings show that INSM1 immunostaining is not exclusively expressed in neuroendocrine-derived tumors and tissue. INSM1 is also expressed during early stages on neuronal development; therefore, this antibody cannot differentiate between primary and metastatic primitive neuroectodermal tumors in the brain. More significantly, the immunostaining of normal and reactive brain tissue showed that this antibody may have a p53-like role in differentiating neoplasms from normal brain parenchyma. Unlike p53 immunostaining, the presence of even rare, faint nuclear staining by this antibody was strongly indicative of the presence of a neoplastic cell. This is particularly relevant in the tumors of primitive neuroectodermal origin, for which p53 mutation is rare and INSM1 expression is frequent.
Among the primary neuroectodermal types of CNS tumors, variable expression amongst subtypes was seen at both transcriptional and protein expression levels. Analysis of publically available microarray data showed high levels of INSM1 transcript in medulloblastoma as compared with extra-cerebellar embryonal tumors (14). These findings have several implications as indicators of the cellular origins of these neoplasms. Given the strong expression of INSM1 in medulloblastoma, regardless of subtype, and in the external granular cell layer in the cerebellum, these findings support the hypothesis that most medulloblastomas are derived from the immature neuronal precursors of the external granular cell layer, resulting in their localization to the pediatric cerebellum. Many of the other transcription factors that showed increased expression in medulloblastoma have previously been linked to medulloblastoma development, including NFIB, NFIA (17), HES1 (18), and OTX2 (19). Subdivision of the nonmedulloblastoma CNS-embryonal tumors by INSM1 expression would also be valuable in future studies, as diagnosis by 2017 criteria was not available for this historic data set.
It has been previously shown that forced expression of recombinant SHH in granular cell culture progenitors leads to INSM1 upregulation (15). The SHH pathway activation also drives development of SHH medulloblastoma. One might hypothesize, therefore, that INSM1 immunostaining would be particularly strong in this subtype. In our findings, strong INSM1 immunostaining was present in all subtypes of medulloblastoma examined and there was no correlation of expression frequency and subtype. Interestingly, INSM1 followed an opposite pattern of immunostaining to SHH medulloblastoma markers in nodular desmoplastic medulloblastoma (20). Unlike YAP1 and GAB1, which are expressed mainly in the mitotically active desmoplastic regions, INSM1 expression was predominantly present in the mitotically inert nodules with synaptophysin. These nodules, therefore, may represent postmitotic differentiation of medulloblastoma cells through INSM1 expression.
Unlike neuroendocrine cells, where INSM1 expression appears to be a critical for both differentiation and maintenance, the role for INSM1 in CNS development appears to be much more transient. Specifically, it is necessary for the terminal division of neurons in the developing olfactory bulb (3). This makes INSM1 expression in brain neoplasms more associated with a particular stage of differentiation rather that limited to a particular tumor type, such as neuroendocrine neoplasms in other organs. For this reason, INSM1 expression in brain tumors must be evaluated by clinical setting and in concert with other markers of differentiation. This is particularly important when considering the possibility of tumors metastatic to the brain. INSM1 remains an ectopically expressed protein in neoplastic cells of the brain in a manner that could increase our ability to differentiate neoplastic processes from reactive processes. As such, this protein could also leave these neoplasms vulnerable to molecular targeting with minimal collateral damage if administered locally—away from normally expressing cells, such as those in the pituitary and pineal glands.
One caveat to note in this study is that, because frequency of staining with this marker is often rare or focal, true frequencies of INSM1 immunostaining may increase as a greater number of full slides are stained, rather than the majority small TMA sections that were evaluated in this study. For example, among the glioblastoma cases that were stained, the percent of INSM1-positive tumors greatly increased in larger specimens (88%, n = 17) as compared that found in the much smaller TMA samples (34%, n = 77). This phenomenon may also explain the lack of statistical correlation between the presence of a primitive neuroectodermal component in glioblastoma and the presence of INSM1 staining. Additionally, this effect was seen in a larger atypical rhabdoid/teratoid tumor specimen, where only a small cluster of sparsely distributed cells showed INSM1-positive immunostaining. The INSM1 A8 antibody used in this study, as many do, may also have increased sensitivity in new, rather than archived biopsy tissue. Together, it is expected that a higher percentage of INSM1-positive CNS neoplasms may ultimately be identified than the percentages that this study predicts.
Supplementary Material
ACKNOWLEDGMENT
The authors gratefully acknowledge Dr. Rajni Sharma for technical assistance.
REFERENCES
- 1. Breslin MB, Zhu M, Notkins AL et al. , Neuroendocrine differentiation factor, IA-1, is a transcriptional repressor and contains a specific DNA-binding domain: Identification of consensus IA-1 binding sequence. Nucleic Acids Res 2002;30:1038–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Duggan A, Madathany T, de Castro SC et al. , Transient expression of the conserved zinc finger gene INSM1 in progenitors and nascent neurons throughout embryonic and adult neurogenesis. J Comp Neurol 2008;507:1497–520 [DOI] [PubMed] [Google Scholar]
- 3. Rosenbaum JN, Duggan A, Garcia-Anoveros J.. Insm1 promotes the transition of olfactory progenitors from apical and proliferative to basal, terminally dividing and neuronogenic. Neural Dev 2011;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rooper LM, Sharma R, Li QK et al. , INSM1 demonstrates superior performance to the individual and combined use of synaptophysin, chromogranin and CD56 for diagnosing neuroendocrine tumors of the thoracic cavity. Am J Surg Pathol 2017;41:1561–9 [DOI] [PubMed] [Google Scholar]
- 5. Kuji S, Watanabe R, Sato Y et al. , A new marker, insulinoma-associated protein 1 (INSM1), for high-grade neuroendocrine carcinoma of the uterine cervix: Analysis of 37 cases. Gynecol Oncol 2017;144:384–90 [DOI] [PubMed] [Google Scholar]
- 6. Tanigawa M, Nakayama M, Taira T et al. , Insulinoma-associated protein 1 (INSM1) is a useful marker for pancreatic neuroendocrine tumor. Med Mol Morphol 2017[Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7. Rosenbaum JN, Guo Z, Baus RM et al. , INSM1: A novel immunohistochemical and molecular marker for neuroendocrine and neuroepithelial neoplasms. Am J Clin Pathol 2015;144:579–91 [DOI] [PubMed] [Google Scholar]
- 8. Bielle F, Ducray F, Mokhtari K et al. , Tumor cells with neuronal intermediate progenitor features define a subgroup of 1p/19q co-deleted anaplastic gliomas. Brain Pathol 2017;27:567–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bar EE, Lin A, Tihan T et al. , Frequent gains at chromosome 7q34 involving BRAF in pilocytic astrocytoma. J Neuropathol Exp Neurol 2008;67:878–87 [DOI] [PubMed] [Google Scholar]
- 10. Orr BA, Bai H, Odia Y et al. , Yes-associated protein 1 is widely expressed in human brain tumors and promotes glioblastoma growth. J Neuropathol Exp Neurol 2011;70:568–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ho C-Y, Bar E, Giannini C et al. , MicroRNA profiling in pediatric pilocytic astrocytoma reveals biologically relevant targets, including PBX3, NFIB, and METAP2. Neuro-Oncology 2013;15:69–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ames HM, Yuan M, Vizcaíno MA et al. , MicroRNA profiling of low-grade glial and glioneuronal tumors shows an independent role for cluster 14q32.31 member miR-487b. Mod Pathol 2017;30:204–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kahlert UD, Suwala AK, Raabe EH et al. , ZEB1 promotes invasion in human fetal neural stem cells and hypoxic glioma neurospheres. Brain Pathol 2015;25:724–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rogers HA, Ward JH, Miller S et al. , The role of the WNT/beta-catenin pathway in central nervous system primitive neuroectodermal tumours (CNS PNETs). Br J Cancer 2013;108:2130–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. De Smaele E, Fragomeli C, Ferretti E et al. , An integrated approach identifies Nhlh1 and Insm1 as Sonic Hedgehog-regulated genes in developing cerebellum and medulloblastoma. Neoplasia 2008;10:89–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eberhart CG, Kaufman WE, Tihan T et al. , Apoptosis, neuronal maturation, and neurotrophin expression within medulloblastoma nodules. J Neuropathol Exp Neurol 2001;60:462–9 [DOI] [PubMed] [Google Scholar]
- 17. Łastowska M, Al-Afghani H, Al-Balool HH et al. , Identification of a neuronal transcription factor network involved in medulloblastoma development. Acta Neuropathol Commun 2013;1:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fiaschetti G, Abela L, Nonoguchi N et al. , Epigenetic silencing of miRNA-9 is associated with HES1 oncogenic activity and poor prognosis of medulloblastoma. Br J Cancer 2014;110:636–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Di C, Liao S, Adamson DC et al. , Identification of OTX2 as a medulloblastoma oncogene whose product can be targeted by all-trans retinoic acid. Cancer Res 2005;65:919–24 [PubMed] [Google Scholar]
- 20. Ellison DW, Dalton J, Kocak M et al. , Medulloblastoma: Clinicopathological correlates of SHH, WNT, and non-SHH/WNT molecular subgroups. Acta Neuropathol 2011;121:381–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ritchie ME, Phipson B, Wu D et al. , limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 2015;43:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.