Abstract
Objectives
Several epidemiological studies suggest declining trends in dementia over the last three decades with both decreasing age-specific prevalence and incidence. There is limited data on whether this delayed clinical onset is accompanied by a shorter postdiagnosis survival.
Methods
A total of 5,205 participants from the Framingham Original and Offspring cohorts were studied. Four epochs were considered from 1977–1984 to 2004–2008. Gender and education adjusted 5-year mortality risks were estimated using delayed entry Cox models with the earliest epoch as reference category. Stratified analyses by sex, education, and age were undertaken. A nested case control study of 317 dementia cases and 317 controls matched on age, gender and epoch was initiated.
Results
In the whole sample, 5-year mortality risk has decreased with time, it was 33% lower in the last epoch compared to the earliest. In the 317 persons who developed dementia, age at onset increased (1.5 years/epoch), and years alive with dementia decreased (1 year/epoch) over time. We observed however, a decreased adjusted relative mortality risk (by 18%) in persons with dementia in 1986–1991 compared to 1977–1983 and no significant change from then to the latest epoch. The nested case control study suggested in matched controls that 5-year mortality relative risk had increased by 60% in the last epoch compared to Epoch 1.
Discussion
In the FHS, in the last 30 years, disease duration in persons with dementia has decreased. However, age-adjusted mortality risk has slightly decreased after 1977–1983. Consequences of such trends on dementia prevalence should be investigated.
Keywords: Alzheimer’s disease, Dementia, morbidity, prevention, secular trends, survival
Background
The 20th century was marked by a continuous increase in average life expectancy (LE) which is ranked as one of society’s greatest achievements. Based on the Global Burden of Disease 2015 Study (GBD 2015 Mortality and Causes of Death Collaborators) that provides a comprehensive assessment of all-cause and cause-specific mortality for 249 causes in 195 countries and territories, LE at birth rose from 61·7 years in 1980 to 71·8 years in 2015. In high-income countries, in 2015, LE at birth was 78 and 84 years, respectively in men and women. Despite the current debates on life-span limits (Brown, Albers, & Ritchie, 2017; Dong, Milholland, & Vijg, 2016; Lenart & Vaupel, 2017; Rozing, Kirkwood, & Westendorp, 2017), there is a consensus that the rapid ageing of the population in developed countries will go on accelerate in the coming years. In a recent study in United Kingdom and Wales (Guzman-Castillo et al., 2017), projections suggest an increase by 19% of persons aged 65 years and above between 2015 and 2025, and as a consequence, an increase, over the same period, by 25% of persons living with disability. The quality of added years of life accompanying improved life expectancy is a concern both at an individual level and at the societal level.
Until 1980, the predominant view on the consequences of gains in LE was that it would inevitably lead to an increased number of years lived with chronic diseases, i.e., people would tend to live longer and sicker (Gruenberg, 1977) lives. In opposition to this approach, Fries et al. (Fries, 1980) proposed the concept of “compression of morbidity” according to which the same changes that lead to decreased mortality will also be linked with a lower incidence of chronic diseases and delayed age at onset of chronic diseases therefore shortening the number of years spent with morbidity. A third hypothesis entitled “dynamic equilibrium” was developed by Manton (Manton, 1982) in which he elaborates that decreases in mortality result from reductions in the rate of chronic disease progression. As declines in the rate of disease progression delay the onset of more serious disease stages, the dynamic equilibrium scenario implies that mortality reductions will be associated with a redistribution of disease and disability from more to less severe states. Under this scenario, the proportion of the life span with serious illness or disability stabilizes or decreases, whereas the proportion with moderate disability or less severe illness increases.
Among the health conditions occurring in older age, dementia is frequent, the estimated prevalence being above 5% after 65 years old in developed countries. Despite the absence of prevention or effective treatment, there is a convergence of evidence suggesting that the prevalence of dementia has decreased in the last 20 years in high-income countries (Dodge et al., 2012; Grasset et al., 2016; Matthews et al., 2013; Matthews et al., 2016; Rocca et al., 2011; Satizabal et al., 2016; Schrijvers et al., 2012; Wu et al., 2017) and reports suggesting a decrease in dementia incidence are yet more scarce (Dodge et al., 2012; Grasset et al., 2016; Matthews et al., 2013; Matthews et al., 2016; Rocca et al., 2011; Satizabal et al., 2016; Schrijvers et al., 2012; Wu et al., 2017). These unexpected declining trends are not yet fully understood but two contributing conditions have been suggested to explain them: improved education and social conditions and primary/secondary prevention of cardiovascular diseases and stroke through better control of their risk factors which are also factors associated with brain health.(Jones & Greene, 2016) However, studies that attempted to, failed to explain dementia trends by these changes in education or cardiovascular diseases. Changes in dementia prevalence could also be the result of decrease in disease duration, i.e., decrease over time of survival after dementia diagnosis. The associations between these declining trends and future mortality have not been investigated yet.
In this paper, we use data from the Framingham Heart Study to assess the trends in survival over a 30-year period among persons with and without dementia. We also prospectively compared trends in survival in two age-matched samples.
Methods
The Framingham Study is a population-based, longitudinal cohort study initiated in 1948 to prospectively investigate the risk factors associated with cardiovascular disease. The Original Cohort enrolled 5,209 residents of Framingham, Massachusetts, and they have been under continuous surveillance through biennial examinations, including a detailed medical history, physical examinations and laboratory measures. In1971, 5,214 children of the Original Cohort and their spouses were enrolled in the study. These participants, referred to as the Offspring Cohort, have undergone similar examinations to the Original Cohort once every 4 years. Detailed descriptions of the study design have been previously published. All participants provided written informed consent at each examination. The Framingham Study protocols and participant consent forms were approved by the Institutional Review Board of Boston University School of Medicine.
Dementia Surveillance
The methods for dementia surveillance have been detailed in prior publications. Cognitive status has been monitored in the Original Cohort since 1975 with a comprehensive neuropsychological battery administered between 1975 and 1977, followed by neurological assessment of participants with lower cognitive scores (bottom 10%). Since 1981, this cohort has been assessed at each examination with a Mini-Mental State Examination (MMSE), where participants were flagged for further cognitive screening if they scored below predefined education- and prior performance-based cutoffs. The Offspring cohort has undergone similar monitoring with serial MMSEs since 1991. Participants identified as having possible cognitive impairment based on these screening assessments were invited to undergo additional annual neurological and neuropsychological examinations. Additional examination was also pursued whenever subjective cognitive decline was self-, or family-reported, or upon referral by a treating physician, Framingham ancillary study investigators, or through review of outside medical records (Farmer et al., 1987). A dementia review panel including a neurologist and a neuropsychologist has reviewed each case of possible cognitive decline and dementia. For cases detected before 2001, reviews have been repeated applying current diagnostic criteria. The panel determined whether or not a person had dementia according to DSM-IV criteria, and date of diagnosis using data from prospectively ascertained serial neurological and neuropsychological assessments, telephone interviews with caregivers, medical records, neuroimaging, and when available, autopsy data.
Mortality Surveillance
After participants died an investigator interviewed the surviving next of kin and the participant’s physician in addition to reviewing all hospital, clinic and nursing home records. If a participant might have experienced cognitive decline, especially since their last examination, this person’s records were referred for further assessment by the panel, which could include a retrospective clinical dementia rating based on telephone interviews with family members.
Education
The Framingham Heart Study (FHS) collects extensive information at each examination cycle, including educational achievement. For the purpose of this analyses, education will be studied in either four categories (No high school degree, High school degree, Some years of college, College degree) or two categories (No high school degree vs at least high school degree).
Statistical Analysis
Our aim was to explore how dementia trends in FHS has influenced trends in mortality after diagnosis. Therefore, our analytical strategy conformed to that of our earlier dementia trends publication (Satizabal et al., 2016). Four nonoverlapping 5-year epochs anchored at the second, fourth, sixth, and eighth examination cycles for the Offspring Cohort, and the closest corresponding four examinations for the Original Cohort had been considered. The epochs had been defined based on the dates of the baseline examinations so as to optimize person-years of surveillance data available. The mean baseline years were 1978, 1989, 1996, and 2006 for the corresponding first, second, third, and fourth epochs. Participants aged 60 years or older who were free of dementia at the start of each of the four epochs were included in the analyses, and could contribute to more than one epoch. For the dementia trends publication (Satizabal et al., 2016), the occurrence of dementia cases over 5 years of follow-up had been determined in the four epochs and Cox proportional hazard models adjusting for age and sex had been estimated for comparisons across epochs using the earliest epoch as the reference category.
For the current analyses, we compared mortality in the four epochs using delayed entry (i.e., age as time scale) Cox proportional hazards models, adjusted for sex and education, with the earliest epoch as reference category. Because of overlapping epochs, we did not investigate mortality trends after 5 years of follow-up.
In a first step, we looked at 5-year mortality trends in the full sample across epochs. Follow-up was from baseline up to 5 years; participants who did not die were censored at the last date when they were known to be alive, or up to a maximum of 5 years. Analyses were performed on the whole sample and stratified by age, sex, and education.
In a second step, we studied the vital status of the subset of participants who developed dementia during the original 5 years follow-up in each epoch. Follow-up for these analyses ranged from the diagnosis of dementia over 5 years. Participants who did not die were censored at the age when they were known to be alive, or up to a maximum of 5 years after they were diagnosed as having dementia. Analyses undertaken were run in the full sample of persons with incident dementia as well as by age (<80 vs 80 and above), gender and by education level (less than high school level, vs high school level and above).
In a third step, in order to more directly compare 5-year mortality trends across epochs between participants with and without dementia, we used a population-based nested case–control study design. Cases were dementia cases that had occurred in the 5-year follow-up of each epoch. For each case, at the date of dementia diagnosis, a dementia-free control was identified, matched on age, epoch, cohort (Original vs Offspring), and sex. We compared the 5-years hazards ratio of death in cases and controls using Cox proportional hazard models with follow-up to a maximum of 5 years after dementia diagnosis/matching date, with the earliest epoch as reference category.
We implemented robust sandwich estimators to account for the inclusion of repeated individuals in more than one epoch, except in analyses performed on dementia cases only.
Analyses were also undertaken restricting dementia cases to those of Alzheimer’s type (71% of dementia cases) but as results were unchanged, we did not report them in the manuscript.
All statistical analyses were performed with the software SAS, version 9.4 (SAS Institute, Cary, NC).
Results
Table 1 shows the baseline characteristics of the sample and the distribution of incident events (dementia, death) by epoch. The sample comprised 5,205 unique individuals distributed over four epochs. Each epoch included over 2,000 individuals and the participants’ ages ranged between 60 and 101 years at baseline, with a tendency to have older participants (p for trend <.001) and slightly lower proportions of women (p for trend <.001) in more recent epochs. In total, 371 cases of dementia and 1,026 deaths occurred during the 5 years after baseline. Forty-three percent of dementia cases survived more than 5 years after diagnosis.
Table 1.
Sample description at baseline and occurrence of events (deaths, dementia, death after dementia)
| Time-periods | ||||
|---|---|---|---|---|
| Epoch 1 (1977–1983) | Epoch 2 (1986–1991) | Epoch 3 (1992–1998) | Epoch 4 (2004–2008) | |
| N | 2,457 | 2,135 | 2,333 | 2,090 |
| Mean Agea (SD), years (range) | 69 (7) (60–89) | 72 (7) (60–96) | 72 (8) (60–101) | 72 (9) (60–101) |
| Womena, % | 59 | 57 | 57 | 56 |
| Educational levela, % | ||||
| No high school degree | 36 | 24 | 15 | 5 |
| High school degree | 32 | 37 | 37 | 32 |
| Some years of college | 19 | 21 | 24 | 29 |
| College degree | 13 | 17 | 24 | 34 |
| Number of deaths over 5 years (N) | 276 | 231 | 262 | 257 |
| Number with dementia over 5 years (N) | 98 | 93 | 103 | 77 |
| Survival after dementia diagnosis | ||||
| Above 5 years, n (%) | 47 (47.9) | 47 (50.5) | 45 (43.7) | 22 (28.9)a |
| Above 10 years, n (%) | 19 (19.4) | 5 (5.4) | 8 (7.8) | NA (NA) |
| Mean age (SDb) at dementia diagnosis in years | 80.00 (6.8) | 82.41 (7.0) | 83.58 (6.5) | 86.38 (7.7) |
| Mean survival in years (SE)c after dementia diagnosis | 5.3 (0.3) | 5.1 (0.3) | 5.2 (0.3)d | 4.2 (0.4)e |
| Mean survival in years (SE)c after baseline in persons who developed dementia during the 5-year epoch only | 8.5 (0.3) | 7.6 (0.3) | 8.0 (0.3) | 6.3 (0.4) |
Note: aN = 76 (one participant had not yet full 5-year follow-up).
bSD = Standard deviation; SE = Standard error.
cAdjusted for age at diagnosis, sex, and education.
d n = 102 (1 participant is still alive).
e n = 66 (10 have not yet had full 10-year follow-up).
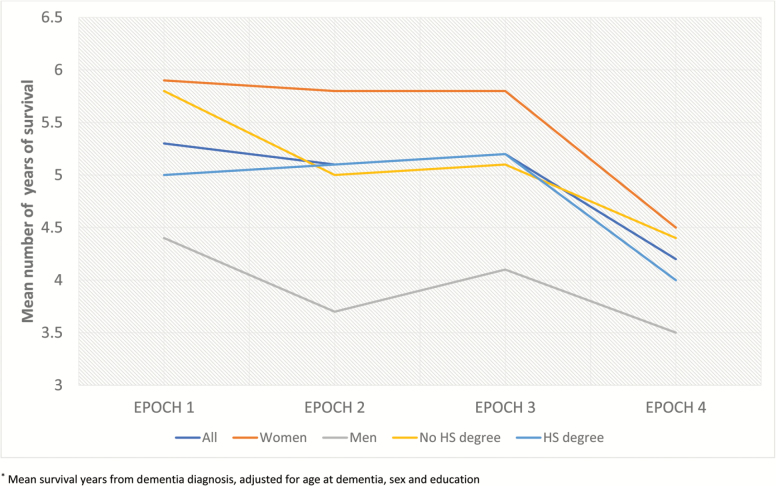
Table 2 describes age at dementia and age at deaths of participants that were diagnosed with dementia within each epoch. Mean age at dementia has increased by around 2 years per epoch whereas age at death has increased by around 1 year per epoch. These trends were consistent in stratified analyses by sex, education. Figure 1 illustrates that postdementia mean survival years (adjusted for age at dementia, sex and education) has decreased over time globally as well as in men and women and in both education level groups. Postdiagnosis survival was almost 6 years in epoch 1 whereas it was 3 years in epoch 4. This suggests that the absolute number of years with dementia has decreased with time
Table 2.
Temporal trends in age at dementia, age at death in persons who became demented, Stratified by Sex, Educational Level
| Time – periods | ||||
|---|---|---|---|---|
| Epoch 1 (1977–1983) | Epoch 2 (1986–1991) | Epoch 3 (1992–1998) | Epoch 4 (2004–2008) | |
| Mean age at dementia (SD) | 80.00 (6.8) | 82.41 (7.0) | 83.58 (6.5) | 86.38 (7.7) |
| Mean age at death (SD) | 85.92 (6.8) | 87.51 (6.9) | 88.42 (6.3) | 89.40 (7.1) |
| By Sex | ||||
| Women | ||||
| Mean age at dementia (SD) | 80.68 (6.6) | 82.77 (6.7) | 84.36 (6.2) | 87.10 (8.7) |
| Mean age at death (SD) | 87.07 (6.6) | 88.66 (6.1) | 89.75 (5.7) | 90.51 (7.8) |
| Men | ||||
| Mean age at dementia (SD) | 78.36 (7.1) | 81.86 (7.4) | 82.46 (6.8) | 85.27 (5.8) |
| Mean age at death (SD) | 83.19 (6.6) | 85.68 (6.2) | 86.52 (6.6) | 87.70 (5.5) |
| By Education | ||||
| No high school degree | ||||
| Mean age at dementia (SD) | 78.94 (7.8) | 82.84 (6.5) | 85.39 (6.0) | 92.03 (7.3) |
| Mean age at death (SD) | 85.61 (7.5) | 87.91 (6.1) | 89.80 (5.7) | 94.08 (7.6) |
| High school degree | ||||
| Mean age at dementia (SD) | 80.51 (6.1) | 82.22 (7.4) | 82. 68 (6.6) | 85.08 (7.3) |
| Mean age at death (SD) | 86.01 (6.2) | 87.35 (6.5) | 87.77 (6.5) | 88.35 (6.7) |
Figure 1.
Trends in survival in persons who developed dementia*. *Mean survival years from dementia diagnosis, adjusted for age at dementia, sex, and education.
In the whole sample, the 5-year sex and education adjusted hazard ratio (HR) of death declined by 33% between epoch 1 and 2 and remained stable from epoch 2 to epoch 4 (Table 3). In stratified analyses by sex, education level and age, we observed the same pattern except in participants with lower education level (no high school degree) where a linear trend toward declining 5-year mortality risk across epochs was found.
Table 3.
Temporal trends in mortality risk, Stratified by Sex, Educational Level and age
| Cases/N | 5-year hazard ratio (95% CI)a | ||||
|---|---|---|---|---|---|
| Epoch 1 (1977–1983) | Epoch 2 (1986–1991) | Epoch 3 (1992–1998) | Epoch 4 (2004–2008) | ||
| Overall mortality | 983/8,779 | Ref | 0.67 (0.59–0.84) | 0.62 (0.51–0.74) | 0.70 (0.55–0.81) |
| By Sex | |||||
| Women | 468/5,049 | Ref | 0.66 (0.51–0.86) | 0.58 (0.45–0.76) | 0.66 (0.50–0.87) |
| Men | 515/3,730 | Ref | 0.74 (0.58–0.94) | 0.65 (0.50–0.83) | 0.69 (0.53–0.89) |
| By Education level | |||||
| No HSb degree | 299/1,831 | Ref | 0.85 (0.64–1.13) | 0.77 (0.56–1.07) | 0.56 (0.34–0.89) |
| HS degree | 684/6,948 | Ref | 0.63 (0.50–0.79) | 0.56 (0.45–0.70) | 0.65 (0.52–0.80) |
| By age at baseline | |||||
| <80 years old | 586/7,438 | Ref | 0.69 (0.55–0.86) | 0.68 (0.55–0.86) | 0.67 (0.53–0.86) |
| ≥80 years old | 397/1,341 | Ref | 0.73 (0.53–1.00) | 0.53 (0.38–0.73) | 0.65 (0.48–0.88) |
Note: aComputed from delayed entry Cox models adjusted for sex, and education.
bHS = High school.
In participants who developed dementia during an epoch (Table 4), the 5-year sex and education adjusted hazard ratio (HR) of death declined during the epochs 2 and 3 compared to epoch one (by 18% and 32% respectively) but it rose in epoch 4 compared to epoch 3 to a level comparable to epoch 2 (Table 3). Stratified analyses by sex or education showed some variations that are consistent with chance. None of the interaction by sex or education level was significant.
Table 4.
Temporal trends in mortality in persons who became demented, Stratified by Sex, Educational Level
| Cases/N | 5-year hazard ratio (95% CI)a | ||||
|---|---|---|---|---|---|
| Epoch 1 (1977–1983) | Epoch 2 (1986–1991) | Epoch 3 (1992–1998) | Epoch 4 (2004–2008) | ||
| Overall mortality | 199/356 | Ref | 0.82 (0.55–1.21) | 0.70 (0.47–1.03) | 0.88 (0.55–1.34) |
| By Sex | |||||
| Women | 109/226 | Ref | 0.60 (0.36–1.04) | 0.69 (0.41–1.16) | 0.81 (0.46–1.44) |
| Men | 90/130 | Ref | 1.13 (0.62–2.07) | 0.74 (0.40–1.37) | 0.95 (0.50–1.81) |
| By Education level | |||||
| No HSb degree | 67/128 | Ref | 0.85 (0,45-1.60) | 0.53 (0.26–1.09) | 0.78 (0.31–1.96) |
| HS degree | 132/228 | Ref | 0.76 (0.46–1.26) | 0.74 (0.47–1.19) | 0.92 (0.57–1.49) |
| By age at baseline | |||||
| <80 years old | 44/121 | Ref | 0.95 (0.46–1.98) | 0.62 (0.29–1.33) | 1.04 (0.42–2.57) |
| ≥80 years old | 155/235 | Ref | 0.74 (0.46–1.18) | 0.68 (0.43–1.08) | 0.85 (0.52–1.38) |
Note: aComputed from delayed entry Cox models adjusted for sex and education.
bHS = High school.
We further explored the relative mortality risks across epochs and compared trends in 5-year mortality risk between persons with and without dementia in a nested case control study (Table 5) (Matched on age, sex, epoch, and cohort) show differential trends. In persons without dementia, 5-year mortality risk did not change significantly in epochs 2 and 3 compared to epoch 1 but increased significantly, by 69%, in epoch 4 compared to epoch 1. In persons with dementia, mortality risk has decreased between epoch 1 and 2 and almost remained unchanged between epoch 2 and 4. However, the interaction between dementia status (case/control) and epoch on mortality risk was not statistically significant (p = .41).
Table 5.
Temporal trends in 5-year mortality risk, Nested-case control study
| 5-year cumulative hazard ratio (95% CI)a | ||||
|---|---|---|---|---|
| Epoch 1 (1977–1983) | Epoch 2 (1986–1991) | Epoch 3 (1992–1998) | Epoch 4 (2004–2008) | |
| Dementia cases during an epoch | ||||
| Deaths/N | 47/93 | 45/89 | 55/99 | 52/75 |
| Mortality Risk | Ref | 0.82 (0.55–1.21) | 0.70 (0.47–1.03) | 0.88 (0.57–1.34) |
| Matched Controls | ||||
| Deaths/N | 39/97 | 34/91 | 45/101 | 31/76 |
| Mortality Risk | Ref | 0.75 (0.44–1.27) | 1.07 (0.64–1.78) | 1.69 (1.00–2.84) |
Note: aComputed from Cox models with the earliest epoch as reference category. Controls were matched to dementia cases on age (at the date of dementia diagnosis), epoch, cohort (Original vs. Offspring), and sex.
Discussion
Recent evidence suggests that dementia incidence and prevalence have decreased in high income countries in the last 30 years but no data so far has explored whether survival of demented individuals has been modified over the same period. This analysis of the Framingham Heart Study data investigated trends in survival after dementia diagnosis over 30 years of monitoring of brain health of FHS participants. The results are in favor of compression of dementia morbidity: age at dementia onset increased on average by around 1.5 years per epoch whereas years alive with dementia decreased on average by 1 year per epoch over time. However, in multivariable analyses (delayed entry cox models taking into account “left truncation” selection bias) when we estimated the 5-year relative of death in persons with dementia there was a decrease by 18% of 5-year mortality risk between 1986–1991 compared to 1977–1983 and it remained stable from 1986–1991 to 2004–2008.
During the same period of observation, in the whole FHS population, the age-adjusted 5-year mortality risks have decreased by more than 30% between 1977–1984 and 2004–2008, reflecting the increased life-expectancy seen in all developed nations over the last 30 years.
On average, the age at onset of dementia has increased by 2 years every decade whereas the survival after dementia diagnosis has fallen from 6 years on average in 1977–1984 to 3 years on average in 2004–2008. This could be interpreted as compression of morbidity but the accurate multivariable modeling of mortality risk using age as a time scale do no suggest a compression a morbidity across time but rather an increase in age-adjusted survival in epochs 2 to 4 compared to the first one.
One prior study using Health and Retirement Study data investigated trends in cognitive impairment between 1993 and 2004 (Langa et al., 2008). The authors reported a decrease in prevalence of cognitive impairment after 70 years of age between the two periods and an increased risk of death after cognitive impairment in 2004 compared to 1993.
There are so far only putative explanations of declining dementia trends (Wu et al., 2017; Wu, Matthews, & Brayne, 2014). They could be the consequences of this last century improvements in education achievement, medical care, lifestyle changes and primary and secondary prevention of cardiovascular and cerebrovascular conditions that would have been beneficial for preserving cognitive health longer.
Our data suggests that age at dementia onset has increased more rapidly in participants without a high school degree (more than 13 years increase in age at onset between epoch 1 and epoch 4) than in participants with at least a high school degree (around 5 years increase in age at onset between epoch 1 and epoch 4). However, multivariable survival analyses suggest that did not suggest any significant interaction with education level.
This study has some limitations. The population for which inferences are being made is a European American population of diverse European origins that includes persons who had arrived in the United States prior to 1950 and their children, grand-children and spouses of these children and grand-children. Generalizability to other European American persons is reasonable but these results cannot be generalized to the more diverse current population residing in the United States (Leaverton et al., 1987; Vasan et al., 2005).
This investigation of older participants of the FHS suggests evidence of absolute compression of dementia morbidity over 30 years. Population is ageing, therefore the absolute number of dementia cases will continue to grow (Hebert, Beckett, Scherr, & Evans, 2001; Jacqmin-Gadda et al., 2013). The impact of a decrease in mortality risk in persons with dementia on the disease prevalence will need to be investigated when sufficient data with long-term follow-up is available.
Funding
Framingham Heart Study (FHS). This work was supported by the National Heart, Lung, and Blood Institute’s Framingham Heart Study (contracts N01-HC-25195 and HHSN268201500001I). This study was also supported by grants from the National Institute on Aging (AG054076, P30 AG012846, U01-AG049505, and AG008122 [S. Seshadri]). S. Seshadri and A. Beiser were also supported by additional grants from the National Institute on Aging (R01AG049607, AG033193, AG033040, AG052409) and the National Institute of Neurological Disorders and Stroke (R01-NS017950).
Author Contributions
All authors planned the study and contributed to designing the statistical analyses, A Beiser ran the statistical analyses. C Dufouil wrote the paper. All authors reviewed and revised the manuscript.
Conflict of Interest
None reported.
References
- Brown N. J. L. Albers C. J. & Ritchie S. J (2017). Contesting the evidence for limited human lifespan. Nature, 546, E6–E7. doi:10.1038/nature22784 [DOI] [PubMed] [Google Scholar]
- Dodge H. H. Buracchio T. J. Fisher G. G. Kiyohara Y. Meguro K. Tanizaki Y. & Kaye J. A (2012). Trends in the prevalence of dementia in Japan. International Journal of Alzheimer’s Disease, 2012, 956354. doi:10.1155/2012/956354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X. Milholland B. & Vijg J (2016). Evidence for a limit to human lifespan. Nature, 538, 257–259. doi:10.1038/nature19793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer M. E. White L. R. Kittner S. J. Kaplan E. Moes E. McNamara P. … Feinleib M (1987). Neuropsychological test performance in Framingham: A descriptive study. Psychological Reports, 60, 1023–1040. doi:10.2466/pr0.1987.60.3c.1023 [DOI] [PubMed] [Google Scholar]
- Fries J. F. (1980). Aging, natural death, and the compression of morbidity. The New England Journal of Medicine, 303, 130–135. doi:10.1056/NEJM198007173030304 [DOI] [PubMed] [Google Scholar]
- GBD 2015 Mortality and Causes of Death Collaborators.(2016). Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet, 388, 1459–1544. doi:10.1016/S0140-6736(16)31012-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasset L. Brayne C. Joly P. Jacqmin-Gadda H. Peres K. Foubert-Samier A. … Helmer C (2016). Trends in dementia incidence: Evolution over a 10-year period in France. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 12, 272–280. doi:10.1016/j.jalz.2015.11.001 [DOI] [PubMed] [Google Scholar]
- Gruenberg E. M. (1977). The failures of success. The Milbank Memorial Fund Quarterly. Health and Society, 55, 3–24. doi:10.1111/j.1468-0009.2005.00400.x [PubMed] [Google Scholar]
- Guzman-Castillo M., Ahmadi-Abhari S., Bandosz P., Capewell S., Steptoe A., Singh-Manoux A., O’Flaherty M (2017). Forecasted trends in disability and life expectancy in England and Wales up to 2025: A modelling study. Lancet Public Health, 2, e307–e313. doi:10.1016/S2468-2667(17)30091–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert L. E. Beckett L. A. Scherr P. A. & Evans D. A (2001). Annual incidence of Alzheimer disease in the United States projected to the years 2000 through 2050. Alzheimer Disease and Associated Disorders, 15, 169–173. [DOI] [PubMed] [Google Scholar]
- Jacqmin-Gadda H. Alperovitch A. Montlahuc C. Commenges D. Leffondre K. Dufouil C.… Joly P (2013). 20-Year prevalence projections for dementia and impact of preventive policy about risk factors. European Journal of Epidemiology, 28, 493–502. doi:10.1007/s10654-013-9818-7 [DOI] [PubMed] [Google Scholar]
- Jones D. S., & Greene J. A (2016). Is dementia in decline? Historical trends and future trajectories. The New England Journal of Medicine, 374, 507–509. doi:10.1056/NEJMp1514434 [DOI] [PubMed] [Google Scholar]
- Langa K. M. Larson E. B. Karlawish J. H. Cutler D. M. Kabeto M. U. Kim S. Y. & Rosen A. B (2008). Trends in the prevalence and mortality of cognitive impairment in the United States: Is there evidence of a compression of cognitive morbidity?Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 4, 134–144. doi:10.1016/j.jalz.2008.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaverton P. E. Sorlie P. D. Kleinman J. C. Dannenberg A. L. Ingster-Moore L. Kannel W. B. & Cornoni-Huntley J. C (1987). Representativeness of the Framingham risk model for coronary heart disease mortality: A comparison with a national cohort study. Journal of Chronic Diseases, 40, 775–784. doi:10.1016/0021-9681(87)90129-9 [DOI] [PubMed] [Google Scholar]
- Lenart A. & Vaupel J. W (2017). Questionable evidence for a limit to human lifespan. Nature, 546, E13–E14. doi:10.1038/nature22790 [DOI] [PubMed] [Google Scholar]
- Manton K. G. (1982). Changing concepts of morbidity and mortality in the elderly population. The Milbank Memorial Fund Quarterly. Health and Society, 60, 183–244. [PubMed] [Google Scholar]
- Matthews F. E. Arthur A. Barnes L. E. Bond J. Jagger C. Robinson L. … Brayne C; Medical Research Council Cognitive Function and Ageing Collaboration (2013). A two-decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: results of the Cognitive Function and Ageing Study I and II. Lancet (London, England), 382, 1405–1412. doi:10.1016/S0140-6736(13)61570-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews F. E. Stephan B. C. Robinson L. Jagger C. Barnes L. E. Arthur A.… Brayne C; Cognitive Function and Ageing Studies (CFAS) Collaboration (2016). A two decade dementia incidence comparison from the Cognitive Function and Ageing Studies I and II. Nature Communications, 7, 11398. doi:10.1038/ncomms11398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca W. A. Petersen R. C. Knopman D. S. Hebert L. E. Evans D. A. Hall K. S. … White L. R (2011). Trends in the incidence and prevalence of Alzheimer’s disease, dementia, and cognitive impairment in the United States. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 7, 80–93. doi:10.1016/j.jalz.2010.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozing M. P. Kirkwood T. B. L. & Westendorp R. G. J (2017). Is there evidence for a limit to human lifespan?Nature, 546, E11–E12. doi:10.1038/nature22788 [DOI] [PubMed] [Google Scholar]
- Satizabal C. L. Beiser A. S. Chouraki V. Chêne G. Dufouil C. & Seshadri S (2016). Incidence of dementia over three decades in the Framingham Heart Study. The New England Journal of Medicine, 374, 523–532. doi:10.1056/NEJMoa1504327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrijvers E. M. Verhaaren B. F. Koudstaal P. J. Hofman A. Ikram M. A. & Breteler M. M (2012). Is dementia incidence declining? Trends in dementia incidence since 1990 in the Rotterdam Study. Neurology, 78, 1456–1463. doi:10.1212/WNL.0b013e3182553be6 [DOI] [PubMed] [Google Scholar]
- Vasan R. S. Sullivan L. M. Wilson P. W. Sempos C. T. Sundström J. Kannel W. B. … D’Agostino R. B (2005). Relative importance of borderline and elevated levels of coronary heart disease risk factors. Annals of Internal Medicine, 142, 393–402. doi:10.7326/0003-4819-142-6-200503150-00005 [DOI] [PubMed] [Google Scholar]
- Wu Y. T. Beiser A. S. Breteler M. M. B. Fratiglioni L. Helmer C. Hendrie H. C. … Brayne C (2017). The changing prevalence and incidence of dementia over time - current evidence. Nature Reviews. Neurology, 13, 327–339. doi:10.1038/nrneurol.2017.63 [DOI] [PubMed] [Google Scholar]
- Wu Y. T. Matthews F. E. & Brayne C (2014). Dementia: Time trends and policy responses. Maturitas, 79, 191–195. doi:10.1016/j.maturitas.2014.06.020 [DOI] [PubMed] [Google Scholar]