Abstract
Understanding how trees mediate the effects of chronic anthropogenic disturbance is fundamental to developing forest sustainable management strategies. The role that intraspecific functional diversity plays in such process is poorly understood. Several tree species are repeatedly defoliated at large scale by cattle breeders in Africa to feed livestock. In addition, these tree species are also debarked for medicinal purposes. These human-induced disturbances lead to biomass loss and subsequent decline in the tree growth. The main objective of this work is to investigate how functional traits mediate tree response to chronic anthropogenic disturbance. We used a unique data set of functional traits and growth rate of 503 individual tree of Afzelia africana. We collected data on leaf mass per area (LMA), wood density (WD) and growth rate, and recorded history of human disturbances (debarking, pruning) on individual tree from 12 populations of A. africana distributed in two ecological zones in Benin (West Africa). We tested the effect of disturbances on absolute growth rate across ontogenetic stages, assessed the role of intraspecific trait variability on growth and tested the role of tree functional strategy on the tree growth response to debarking and pruning. We found that debarking did not affect stem growth, suggesting a fast compensatory regrowth of bark wounded. Moreover, tree response to debarking was independent of the functional strategy. By contrast, we found that pruning reduced tree absolute growth; however, trees with low WD were more strongly affected by pruning than trees with high WD. Our results emphasize the importance for plant functioning of the interplay between the availability of leaves for resource acquisition and a resilience strategy by mobilizing stored resources in stem wood to be reinvested for growth under severe disturbances.
Keywords: Debarking, growth performance, pruning, resilience strategy, wood density
This study demonstrates that functional traits can mediate the response of a tropical tree to chronic anthropogenic disturbance. Pruning by indigenous Fulani people, a cultural practice in West African savanna, can reduce tree growth. From a conservation perspective, tree location also affected their functional traits indicating that even classical conservation measures can affect tree ecology. Trees that are in protected areas where indigenous Fulani people do not have access tend to have heavier wood density (WD). We showed that these trees with higher WD are most resilient to branch pruning.
Introduction
Functional traits can aid in our understanding of the variation in species response to environment (McGill et al. 2006). Wood density (WD) and leaf mass per area (LMA) are key plant traits that define resource allocation strategies for essential functions (acquisition, storage), and particularly reflecting the trade-offs between construction cost of new plant structures (leaf or stem wood) and survival under different conditions of the environment (Poorter et al. 2008, 2010; Iida et al. 2014). First, Poorter et al. (2008) examined the relationship between functional and demographic rate for 240 tree species in Neotropical forests, and found that growth and mortality rate decreased with increasing WD, suggesting a trade-off between stem construction cost and resistance to damage. Second, with high LMA, leaves exhibit high thickness and toughness that reflect greater resource allocation into defence than into growth (Wright and Cannon 2001). This leads to more robust leaves with long lifespan (Sterck et al. 2006) and therefore less damaged from herbivory (Wright and Westoby 2002).
Most studies linking functional traits to growth rates have focused on the interspecific trait variability as a convenient proxy of the ecological strategies which vary largely among coexisting species (Westoby et al. 2002). However, our understanding of intraspecific functional trait variation and how this mediates plant demography response to environmental variation and disturbance is limited (Poorter et al. 2008; Martínez-Vilalta et al. 2010; Wright et al. 2010; Albert et al. 2011; Iida et al. 2014). Furthermore, several studies independently demonstrated ontogenetic variation in plant growth/survival and functional traits (Poorter and Bongers 2006; Hérault et al. 2011; Iida et al. 2014). For example, growth, survival and reproduction vary positively with individual size or age (Ellner and Rees 2006; Zuidema et al. 2010; Gaoue 2016). In addition, functional traits such as WD, seed mass and adult stature are good predictors of plant growth and/or mortality (Poorter et al. 2008). However, the role of ontogeny in the relationship between functional traits and demography is rarely examined with most studies focusing on a single life stage, such as sapling or adult (Poorter et al. 2008). Recent studies have highlighted the importance of considering ontogenetic shifts in the relationship between functional traits and disturbance (e.g. Herault et al. 2010).
In tropical regions, many tree species are debarked or pruned for subsistence, medicinal and economic purposes (Ouédraogo-Koné et al. 2006; Gaoue and Ticktin 2007; Nacoulma et al. 2017). These types of damage alter tree ecophysiological functioning (Gaoue et al. 2011) and can ultimately reduce the demographic performance of harvested plants (Rijkers et al. 2006; Gaoue et al. 2011, 2013). Stem bark wound is a direct biomass removal of an important plant tissue that induces shift in internal resource allocation patterns and often leads to a decrease in tree growth (Bazzaz et al. 1987), even if compensatory growth may sometimes occur when bark removal is not recurrent (Gaoue et al. 2011). However, the wounded organ can recover through several internal mechanisms (Ryan and Yoder 1997) depending on the debarking intensity and the intrinsic recover ability of the species (Gaoue and Ticktin 2007; Delvaux et al. 2010).
Pruning trees creates significant branch and leaf biomass loss, with long-lasting impacts on the whole-plant photosynthesis performance (Anten and Ackerly 2001; Anten et al. 2003; Gaoue et al. 2011). Consequently, pruning may result into a shortage of the amount of resources allocated for growth. From an evolutionary perspective, many tree species have evolved to cope with repeated disturbances (Gutschick and BassiriRad 2003) and exhibit various recovery processes that are ultimately shaped by the tree functional strategy. Previous studies have shown that the effects of both debarking and pruning are size-dependent (Sinsin et al. 2004; Gaoue and Ticktin 2007; Mensah et al. 2014; Amahowe et al. 2017), because resource availability and quality are linked to the tree life stage. Failure to account for this size-dependent growth, both in tree performance and in assessing the effect of pruning and debarking, may result into suboptimal management decisions.
In this study, we used a unique data set of 503 individual trees from 12 populations of Afzelia africana, a tropical tree distributed from the Sudano-Guinean to the Sudanian zones in Benin, to test the effects of pruning and debarking on the absolute growth rate while accounting for the ontogenetic tree growth trajectory. We assessed the effects of intraspecific variability in the leaf and wood economic spectrum on individual tree growth performance and tested the effects of the individual tree functional strategy on tree growth response to debarking and pruning.
Methods
Study area
This study was conducted in the Republic of Benin (6°–12°50′ N and 1°–3°40′ E), West Africa. The country covers 114763 km2 and comprises three main ecological zones including Guineo-Congolean (6°25–7°30 N), Sudano-Guinean (7°30–9°30 N) and Sudanian (9°30–12° N) ecological zones. The study was conducted in the latter two zones, where Afzelia populations were mostly distributed. The physical characteristics of the two zones were reported in Gaoue and Ticktin (2008). Rainfall ranges from 800 to 1300 mm, while temperature varies from 24 to 31 °C. The vegetation is dominated by savannas, woodlands, dry dense forests and gallery forests.
Study species
Afzelia africana is a multi-use tree species belonging to Fabaceae family (Angiosperm Phylogeny Group: APG III). Its crown can spread up to 35 m in height, with a diameter at breast height (DBH) measuring up to 1 m (Arbonnier 2000; Akoègninou et al. 2006; Assogbadjo et al. 2010). Flowering occurs at the end of dry season (April–May), fruiting in the middle of rainy season and fruits mature in December/January onward (Sacande 2007; Orwa et al. 2009). Seeds are dispersed by wind and animals (Orwa et al. 2009). In Sudanian and Sudano-Guinean zones of Benin, A. africana is distributed in savannas, woodlands, dry dense forests, gallery forests (Mensah et al. 2014), edge of dry forest and mountainous zones (Akoègninou et al. 2006), while it is found in dense semi-deciduous forest (Bonou et al. 2009) in Southern Benin. The species is defoliated by indigenous Fulani people to feed cattle (Ouédraogo-Koné et al. 2006; Nacoulma et al. 2017), and debarked for medicinal purposes (Kone et al. 2004; Delvaux et al. 2009; Tshisikhawe et al. 2012). In addition to debarking, the species trunk bark is frequently wounded and shows large deep scars. Pruning and debarking intensity are size-dependent (Amahowe et al. 2017). The species is sensitive to fire at early age and this is worsened by insect attack (Stark 1986; Delvaux et al. 2009).
Sampling strategy
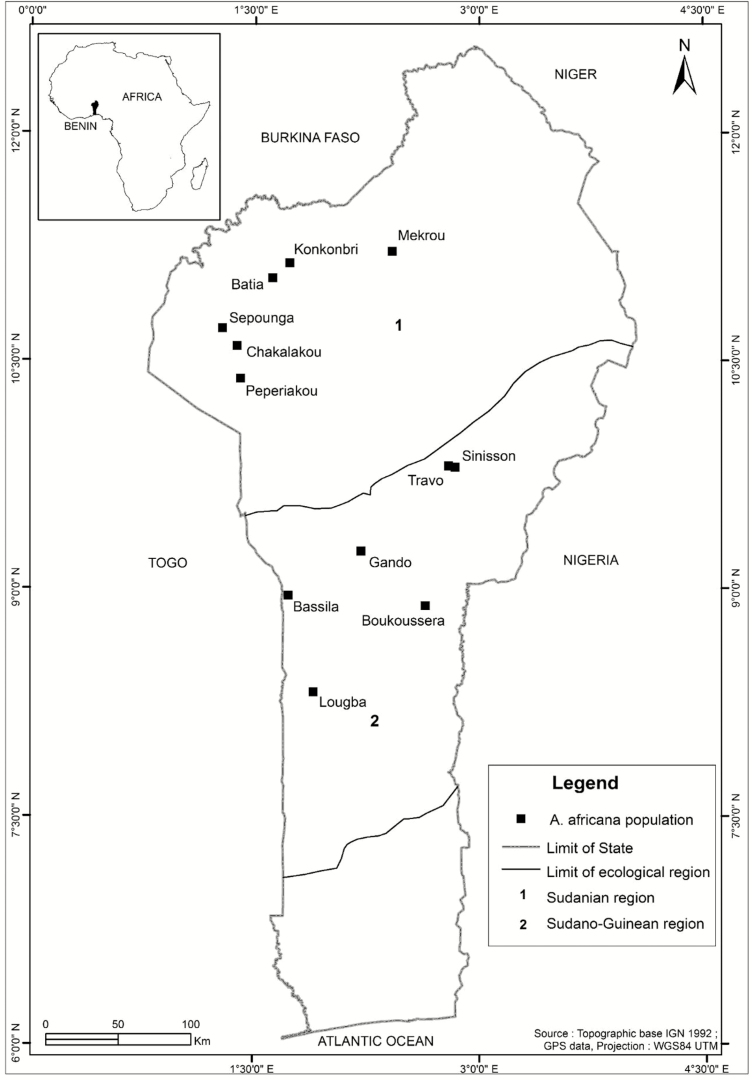
We randomly sampled 12 populations of A. africana out of 52 populations surveyed in Benin during an exploratory study in December 2014. These populations were equally distributed across two main ecological zones: Sudano-Guinean and Sudanian zones. In each zone populations were separated by a minimum of 10 km from each other (Fig. 1). Within each population, 1 ha plot was used to record the DBH for all A. africana individuals with DBH ≥ 2 cm. This represents a total number of 503 trees sampled and measured in Sudano-Guinean and in Sudanian zones. Debarking and pruning intensity were assessed on each sampled tree. For each tree, we recorded if it was pruned or not. Pruning intensity was estimated at the tree level as the proportion of branches pruned related to the total number of branches. We also calculated the percentage of area covered by the wound on the trunk up to the first main ramification to assess the debarking intensity as in previous studies (Cunningham 2001; Gaoue and Ticktin 2007).
Figure 1.
Study area and location of the 12 sampled populations of Afzelia africana studied. Number 1 indicates the drier Sudanian zone and ‘2’ indicates the wetter Sudano-Guinean ecological zone.
Functional trait measurements
To measure WD, we collected wood samples from all the 503 individual of A. africana trees using the increment borer that offered cylindrical-shaped wood cores (Chave 2005), after the growth period (December 2014 to December 2015), at the end of rainy season. Then, we measured the length (L) and the cross-section diameter (D) with a calliper, and subsequently calculated the volume (V). Cores were oven-dried up to 100 °C for 5 days then weighed using an electronic balance. Wood density was estimated as a ratio of the wood fresh volume by wood dry mass (Cornelissen et al. 2003; Chave 2005; Matilo et al. 2013). Wood density is a key trait defining the wood economic spectrum (Chave 2005) often seen as orthogonal to the leaf economic spectrum (Baraloto et al. 2010).
Leaf mass per area is a key plant trait of the leaf economic spectrum related to biomass investment (Cornelissen et al. 2003) and is a good predictor of growth (Grime et al. 1997; Wright et al. 2004). To measure LMA, we collected for each tree, three visibly intact and sunlight-exposed leaves discarding any leaf that suffered herbivory (Cornelissen et al. 2003). These leaves were scanned on flatbed scanner and each image was processed to calculate leaf area using ImageJ (https://imagej.nih.gov/ij/). Leaves were oven-dried at 60 °C for 72 h, before measuring their dry weight and calculate the LMA.
Modelling framework
We modelled the ontogenetic absolute growth rate of A. africana following a model developed by Hérault et al. (2011) to fit a sigmoid curve to tree growth trajectory (Model 0). We then tested for the effects of disturbance (individual tree pruning and debarking, Model 1), intraspecific functional trait variation (Model 2) on tree absolute growth rate and finally the effect of functional strategy on tree absolute growth response to disturbance (Model 3). The model without any effect of disturbance or functional strategy (Model 0) is as follows:
Model 0
| (0) |
where represents diameter at breast height of individual tree i and is its absolute growth rate, i.e. the difference in for each individual tree i measured between two consecutive years, 2014 and 2015. Parameters Gmax and Dopt, respectively, refer to the maximum value of and the DBH value at which this maximum value is reached, and σ2 is the variance. The effects of covariates (debarking, pruning and functional traits) were tested on Gmax. We did not test for the effects of covariates on Dopt and σ because of (i) convergence problems when introducing similar covariates in different part of a model and (ii) no clear biologically relevant hypothesis to test for.
Model 1—This model tests the effect of disturbance (debarking, pruning) on tree absolute growth rate includes Model 0 (see equation 0 above) with
| (1) |
where is the value of Gmax where there is no disturbance (Debark and Prun equal to 0), Debark and Prun are values of, respectively, the debarking and pruning variables (from 0 to 100 %) and and the model parameters.
Model 2—This model tests for the role of intraspecific trait variability on tree absolute growth rate includes Model 0 (see equation 0) with
| (2) |
where is the value of Gmax for an average (i.e. mean values of WD and LMA) tree, WD and LMA are values of, respectively, the WD and LMA variables and and the model parameters.
Model 3—This model tests for the importance of the individual functional strategy on tree absolute growth response to disturbance. To avoid over-parameterization, all interaction terms between disturbance (Debark and Prun) and traits (WD and LMA) were included sequentially in the model using a forward variable selection procedure. The final model includes Model 0 with
| (3) |
where is the value of Gmax for an average (i.e. mean values of WD and LMA) tree with no disturbance (Debark and Prun equal to 0) and the model parameter that was retained by the selection procedure and reflected the interaction effect between disturbance and the individual functional strategy (WD). All models were written and inferred in a Bayesian framework using Monte Carlo Markov Chain (MCMC) algorithms under the R environment (R Development Core Team 2016).
Results
The growth curve of A. africana showed a clear hump-shape (Fig. 2). The overall size-dependent model (Model 0) yielded a maximum growth rate of 0.36 cm per year with a diameter at maximum growth of 8.76 cm (Fig. 3, Table 1). We found no significant effect of debarking (Table 1) on maximum growth rate relative to the Model 0 without disturbance (Fig. 3). However, pruning exerted significantly negative effects on absolute growth rate (Table 1, Fig. 3). Pruned trees at optimal DBH, grew at an average of 0.24 cm per year less than non-pruned trees (Table 1,
Figure 2.
Size-dependent growth rate of Afzelia africana. The highest growth rates are obtained at intermediate DBH justifying the use of a hump-shaped growth trajectory to model the effect of stress on tree performance. White rectangles span from the first to the third quartile. A segment inside the rectangle shows the median and black lines above and below the box show the locations of the minimum and maximum. Black dots refer to outliers.
Figure 3.
Effect of stress disturbance (debarking and pruning) on the absolute growth rate of Afzelia africana (Model 1). Model predictions (lines) are represented for not-pruned (yellow), pruned (red) and debarked (orange) growth trajectories with envelops showing the prediction credibility intervals.
Table 1.
Posterior values for the three growth models parameterized in a Bayesian framework. Model 0: the model without any effect of disturbance or functional strategy; Model 1: testing the effect of disturbance (debarking, pruning) on growth performance; Model 2: role for intraspecific trait variability on individual performance; Model 3: importance of the individual functional strategy on the growth response to disturbance. Gmax and Dopt are, respectively, to the maximum value of and the DBH value at which this maximum value is reached. is the value of Gmax where there is no disturbance (Debark and Prun equal to 0), and and the model parameters. is the value of Gmax for an average (i.e. mean values of WD and LMA) tree, and and the model parameters. is the value of Gmax for an average (i.e. mean values of WD and LMA) tree with no disturbance (Debark and Prun equal to 0) and the model parameter.
| Parameter | Value at maximum likelihood | 95 % credibility intervals | |
|---|---|---|---|
| Model 0 | Gmax | 0.36 | [0.29; 0.43] |
| Dopt | 8.76 | [6.77; 11.54] | |
| σ | 0.51 | [0.48; 0.55] | |
| Model 1 | 0.44 | [0.35; 0.52] | |
| 0.12 | [−0.44; 0.69] | ||
| −0.24 | [−0.44; −0.03] | ||
| Model 2 | 0.34 | [0.24; 0.48] | |
| −0.07 | [−0.16; 0.06] | ||
| −0.02 | [−0.13; 0.09] | ||
| Model 3 | 0.41 | [0.27; 0.57] | |
| −0.18 | [−0.37; 0.02] | ||
| −0.10 | [−0.22; 0.03] | ||
| 0.12 | [−0.09; 0.32] |
Across populations, the mean LMA was 58.17 ± 7.62 g m−2 and mean WD was 0.56 ± 0.005 g cm−3. When we included the effect of intraspecific functional traits variability in our model (Model 2), we found no significant effect of LMA and WD on growth rate (Table 1, Figs. 4 and 5). However, there was a significant interactive effect of WD and pruning on absolute growth rate (Table 1). Pruning significantly reduced the growth of A. africana trees by up to 0.35 cm per year, and this negative effect was highest for intermediate aged trees, i.e. around 10 cm with lower WD (Fig. 6). The growth reduction caused by pruning was greater for trees with lower WD than the opposite (Fig. 6). Notably, trees with very dense wood (above 0.70 g cm−3), independent of their size, experienced less effect of pruning (close to 0) than trees with low WD. However, this advantage of high WD trees became negligible for very large trees (Fig. 6) which may be due to the low growth rates of these old trees (Fig. 2).
Figure 4.
Weak role of intraspecific WD variability in observed growth of Afzelia africana (Model 2). Data are binned into low (WD values < quantile 0.33), high (WD values > quantile 0.66) and medium (in between) categories.
Figure 5.
Weak role of intraspecific LMA variability in observed growth of Afzelia africana (Model 2). Data are binned into low (LMA values < quantile 0.33), high (LMA values > quantile 0.66) and medium (in between) categories.
Figure 6.
Model predictions showing how the individual WD value mediates the tree response to pruning (Model 3). The cost of being pruned is calculated using the difference in predicted growth between a pruned and a not-pruned tree taking into account both WD and the ontogenetic stage.
Based on our results, we further tested the hypothesis that trees sampled in populations with low pruning pressure and trees sampled outside forest reserves, where pruning intensity is high, exhibit different population level WD values. We demonstrated that on average, WD was 0.06 g cm−3 lower in trees found within forest reserves (Student’s t-test, P < 0.001).
Discussion
This study investigated the effect of intraspecific functional trait variation on the growth rate of a tropical tree and how it responds to chronic anthropogenic disturbance. We found a hump-shaped diameter growth curve which reaches a maximum growth of 0.4–0.6 cm per year (Table 1). This maximum growth rate was reached for trees with DBH of 10 cm. Growth rates for trees larger than this threshold were slow. Not surprisingly, trees tend to first grow proportionally to their size given that the largest individuals have more access to light, enhancing photosynthesis (Colbert et al. 2002; Motallebi and Kangur 2016). However, when individual trees reach the maturity, they invest more in reproduction, maintenance and defence rather than growth (Coley et al. 1985; Ryan and Yoder 1997; Poorter et al. 2008). For A. africana, the maximum growth rate was reached at smaller diameter values than reported for other tropical trees (Bragg 2002; Karyati et al. 2017). For example, in the Amazonian forest, while many canopy species have diameter at maximum growth of 30–50 cm, understory species show lower values (Hérault et al. 2011). In South-East Asia, widespread species such as Endospermum diadenum, Macaranga gigantean or Cratoxylum spp. expressed their highest growth rates between 15 and 22 cm (Karyati et al. 2017). Consistent with the lower than expected diameter of maximum growth rate in A. africana, we also found that the maximum growth was far lower than many other tropical species (Karyati et al. 2017). Altogether, this slower growth of our study species may be explained by the harsh growing environment in which this species occurs where it is restricted to poor and rocky soils (Sinsin et al. 2004; Orwa et al. 2009).
Effects of debarking and pruning on growth rates
We tested for the effect of human disturbance on tree absolute growth rate and found that our study species responds differently to several types of disturbance. For example, trunk debarking had no significant effect on tree growth. However, pruning had negative effect on absolute growth rates (Fig. 2), indicating a significant difference in the compensatory tree response to biomass loss depending on plant organs that are targeted. Consistent with this differential response of plant’s organs to disturbance, Ticktin (2004) showed that bark and root removal tend to have more effect on plant demography than leaf harvest. However, in this study, we found an opposite trend. The tolerance of A. africana to debarking could be driven by disturbance-induced increase in photosynthetic rates, readjustment and reallocation of resource to compensatory growth (Caldwell et al. 1981; Hamerlynck et al. 2016). Similar results have been found in Khaya senegalensis, where the tree can quickly recover from partial debarking (Gaoue and Ticktin 2007) while the 100 % ringbarked trees will die (Delvaux et al. 2009) suggesting that the intensity of bark removed affects plant responses to debarking. Afzelia africana is one of the tropical species that exhibited the highest ecological resilience to debarking under stressful arid and semi-arid climate in Benin (Delvaux et al. 2010), and this may explain its wide distribution across disturbance and climate gradient in Benin (Orwa et al. 2009; Mensah et al. 2014). The lack of clear and significant effect of debarking on tree growth may suggest that current bark harvesting intensities might be sustainable (N’Dri 2012; Amahowe et al. 2017). This contrasts with results from previous studies which suggest that the growth rates of many other species in West Africa, i.e. Prunus africanus, Grevillea spp. and Mondia whytei, are negatively impacted by debarking (Vuyiya et al. 2014). Debarking often breaks some xylem vessel elements that would consequently affect water and nutrient transportation from soil interface to stem and leaves (Maherali and DeLucia 2000; Schreiber et al. 2015). This would subsequently exert a negative effect on stomatal conductance and carbon gain for photosynthesis (Panek 1996; Aasamaa et al. 2001) and resources to be allocated for growth. Further investigations are needed to understand why this is apparently not the case for A. africana.
Contrary to bark removal, pruning reduced diameter growth rate (Fig. 3). Similarly, several studies reported chronic defoliation-driven reduction in diameter growth (Sydnor and McCartney 1996), which would affect carbon sequestration and storage. Pruning A. africana is an important activity for cattle breeders because of their high leaf nitrogen content (Orwa et al. 2009). As a result, outside of protected areas most individual trees are severely pruned (Ouédraogo-Koné et al. 2006; Amahowe et al. 2017). Defoliation obviously reduces photosynthesis, hence affecting carbon storage, which can lead to reduced stem growth. Moreover, leaf removal and associated nitrogen loss can affect tree stoichiometry (Beadle 1966; Gaoue et al. 2011), and the high cost of post-harvest leaf replacement can shift biomass allocation patterns (Chapin 1980; Dianda et al. 2009; Zhou et al. 2014). This can ultimately reduce fruit production (Gaoue and Ticktin 2008; Nacoulma et al. 2017) and tree population dynamics (Gaoue et al. 2013). Environmental conditions including nutrient availability can influence such species response to defoliation (Lovelock et al. 1999).
A weak role for intraspecific trait variability in tree performance
Although several studies have investigated the link between functional traits and plant demography (see Poorter et al. 2008, 2010), most studies failed to account for the intraspecific variability in these traits (Burton et al. 2017). We explored how intraspecific variation in functional traits predicts growth rate in A. africana. Particularly, LMA reflects a trade-off between cheap construction cost and leaf longevity with the fastest growing individuals having the lowest LMA values (Kunstler et al. 2016). However, consistent with other studies in tropical regions (Poorter et al. 2008; Wright et al. 2010), we did not find any significant effect of LMA on tree growth (Table 1, Fig. 4). This lack of relationship between LMA and the absolute growth rate could be due to ontogenetic shifts in biomass investment. However, in a prior study, we found no change in LMA across ontogeny (Amahowe et al. 2016) indicating that the biomass investment in leaves per unit of area for A. africana is similar across DBH classes. Contrary to LMA, we did find a slight but not significant effect of WD on growth rates (Table 1). Wood density is an indicator of a trade-off in stems between growth performance and strength in stem (Kunstler et al. 2016). Indeed, high WD trees are often associated with a slow potential growth rate, while low WD trees are usually associated with rapid growth rate (Herault et al. 2010). Individuals with light wood grow faster simply because they produce more volume per unit of biomass (King et al. 2006; Poorter et al. 2008). There is a body of evidence that individuals with low WD have larger vessels diameter and hydraulic conductance favouring the sapwood efficiency (Baraloto et al. 2010; Poorter et al. 2010; Hoeber et al. 2014) and thus increasing the carbon gain that would be allocated to growth (Santiago et al. 2004). However, the global weak relationship between intraspecific functional traits and growth rate in A. africana trees may be due to the additional effect of disturbance.
Individual functional strategy mediates the tree response to disturbance
We demonstrated that WD strongly mediates the tree growth response to pruning (Table 1, Fig. 6). Low WD trees were strongly more affected by pruning, especially at intermediate ages and individuals with high WD tolerated pruning more than those with low WD. Furthermore, we predict that trees with WD > 0.73 g cm−3 will incur no pruning-related reduction in growth rate (Fig. 6). This result shows strong evidence of a functional trade-off between WD and response to pruning. Low WD trees tend to grow faster but experience high growth costs when pruned while trees with high WD grow at a slower rate but experience low costs after pruning. High WD trees should thus be selected in high-pruning-pressure environment and vice versa. We further tested this hypothesis splitting our data between trees sampled in forest reserves with low pruning pressure and trees sampled outside forest reserves where pruning intensity is high. Wood density was lower in trees found within forest reserves, and this provides a compelling example of an individual-level adaptation to disturbance.
Internal physiological mechanisms are activated in trees in order to survive and recover from severe disturbance (Vargas 2012). Individual trees with high WD have important resource reserves, i.e. carbohydrates, that could be used not only to compensate for biomass loss (Würth et al. 2005), but also to continuously allocate resources to growth in stressful conditions (McDowell et al. 2011). Numerous studies provide support for the postulate that stored resources including carbohydrate and nitrogen allow defoliated trees to maintain their growth (Gleason and Ares 2004; Myers and Kitajima 2007; Genet et al. 2009). The importance of carbohydrate storage may depend on the tree life history and the local environment. Carbohydrate contents are often lower in dry forest species than in moist ones, where carbohydrate concentration declines with light requirement (Poorter and Kitajima 2007). Given that A. africana is a shade-tolerant species in the early stages (Orwa et al. 2009; Biaou et al. 2011), we expect that carbohydrate storage would be particularly important for this species. Our results emphasize the importance for plant functioning on the interplay between photosynthesis activity and stored resource mobilization (Richardson et al. 2013).
The negative effect of pruning on the absolute growth rate suggests that A. africana requires increasing attention from forest managers to limit over-exploitation. The unique resilience observed with individuals of high WD provides insights for the phenotype of tree to be selected as good candidates for sustainable pruning. For instance, due to the great socio-economic impact of this activity (Sinsin et al. 2004; Ouédraogo-Koné et al. 2006), it is unrealistic to prevent Fulani harvesters from pruning the tree; however, forest management programmes can encourage harvesters to target individual trees with high WD which are known to be the larger individuals. Preferably harvesting larger trees can limit the overall negative demographic cost of pruning because our results showed that these individuals are more resilient to harvest.
In addition, for another fodder tree species in our study region, K. senegalensis, the Fulani adopt a traditional sustainable management practice, which consists of leaving the top branches (known locally as ‘Sopoodu’) during pruning (Gaoue and Ticktin 2009). This Sopoodu practice can also be encouraged for A. africana trees to ensure the viability of this species.
Conclusions
This study investigates the effect of intraspecific functional trait variation on the growth rate of a tropical tree species and how it responds to anthropogenic disturbance. The compensatory response of A. africana to debarking is good news for its sustainable management. However, ring barking should be avoided to allow the recovery of the damage. Environmental education sessions should be implemented to enhance traditional healers and local communities’ awareness on the sustainable debarking method on woody species. The negative impact of pruning on the absolute growth rate of A. africana is highlighted in this work, thus emphasizing the importance of leaving significant part of foliage and branches on tree to allow photosynthesis and subsequently improve tree growth. Furthermore, our research illustrates a strong evidence of a functional trade-off between WD and response to pruning. The cost of being pruned for low dense trees is higher than the cost for trees of high WD, suggesting a resilience strategy of A. africana by mobilizing stored resources in stem wood to be reinvested for growth under severe disturbances.
Sources of Funding
This work was financially supported by a grant from the International Foundation for Science (Grant N° D/5623-1) and IDEA Wild to I.O.A. O.G.G. was supported by a start-up grant from the University of Tennessee, Knoxville.
Contributions by the Authors
I.O.A., O.G.G. and B.H. conceived the idea and designed the research project. I.O.A. and A.K.N. collected the data. B.H., I.O.A., C.P. and I.C.Z. performed the statistical modelling. I.O.A. drafted the initial manuscript with contribution from O.G.G. and B.H. All the authors contributed critically to the discussion and edited the manuscript before submission.
Conflict of Interest
None declared.
Acknowledgements
We are grateful to the « Unité mixte de Recherche EcoFoG de Kourou » and Labex-Ceba for offering I.O.A. the opportunity to attend the Thematic School on “Functional Ecology of Tropical rainforests in the context of Climate Changes » at Kourou, French Guiana. We thank A. C. Christian, D.-Z. Vincent, B. Sévérin, A. Dieudonné, A. Atchade, S. Boni and A. Nassirou for field assistance. We are grateful to the editors and two anonymous reviewers for comments which improved an early version of this manuscript.
Literature Cited
- Aasamaa K, Sõber A, Rahi M. 2001. Leaf anatomical characteristics associated with shoot hydraulic conductance, stomatal conductance and stomatal sensitivity to changes of leaf water status in temperate deciduous trees. Functional Plant Biology 28:765–774. [Google Scholar]
- Akoègninou A, van der Burg W, van der Maesen L. 2006. Flore analytique du Bénin. The Netherlands: Backhuys Publishers. [Google Scholar]
- Albert CH, Grassein F, Schurr FM, Vieilledent G, Violle C. 2011. When and how should intraspecific variability be considered in trait-based plant ecology?Perspectives in Plant Ecology, Evolution and Systematics 13:217–225. [Google Scholar]
- Amahowe OI, Biaou SSH, Natta AK, Balagueman RO. 2016. Functional traits variation of Afzelia africana Sm & Pers across ontogenetic and light gradients: implication for plant adaptive strategy in Benin (West Africa). Sciences de la vie, de la Terre et Agronomie 4:51–58. [Google Scholar]
- Amahowe OI, Biaou SSH, Natta AK, Balagueman RO. 2017. Multiple disturbance patterns and population structure of a tropical tree species, Afzelia africana (Leguminosae–Caesalpinioideae), in two contrasting bioclimatic zones of the Republic of Benin. Southern Forests: A Journal of Forest Science 80:95–103. [Google Scholar]
- Anten NPR, Ackerly DD. 2001. Canopy-level photosynthetic compensation after defoliation in a tropical understorey palm. Functional Ecology 15:252–262. [Google Scholar]
- Anten NPR, Martínez-Ramos M, Ackerly DD. 2003. Defoliation and growth in an understory palm: quantifying the contributions of compensatory responses. Ecology 84:2905–2918. [Google Scholar]
- Arbonnier M. 2000. Arbres, arbustes et lianes des zones sèches d’Afrique de l’Ouest. Paris, France: CIRAD. [Google Scholar]
- Assogbadjo A, Glèlè Kakai R, Sinsin B. 2010. Afzelia africana Caesalpiniaceae. In: Sinsin B, Kampmann D, eds. Biodiversity atlas of West Africa. Burkina Faso: BIOTA Publisher. [Google Scholar]
- Baraloto C, Timothy Paine CE, Poorter L, Beauchene J, Bonal D, Domenach AM, Hérault B, Patiño S, Roggy JC, Chave J. 2010. Decoupled leaf and stem economics in rain forest trees. Ecology Letters 13:1338–1347. [DOI] [PubMed] [Google Scholar]
- Bazzaz FA, Chiariello NR, Coley PD, Pitelka LF. 1987. Allocating resources to reproduction and defense. BioScience 37:58–67. [Google Scholar]
- Beadle NCW. 1966. Soil phosphate and its role in molding segments of the Australian flora and vegetation, with special reference to xeromorphy and sclerophylly. Ecology 47:992–1007. [Google Scholar]
- Biaou SSH, Holmgren M, Sterck FJ, Mohren GMJ. 2011. Stress-driven changes in the strength of facilitation on tree seedling establishment in West African woodlands. Biotropica 43:23–30. [Google Scholar]
- Bonou W, Glèlè Kakaï R, Assogbadjo AE, Fonton HN, Sinsin B. 2009. Characterisation of Afzelia africana Sm. habitat in the Lama forest reserve of Benin. Forest Ecology and Management 258:1084–1092. [Google Scholar]
- Bragg D. 2002. Empirically derived optimal growth equations for hardwoods and softwoods in Arkansas (GTRS 48, ed.). Asheville, NC: US Department of Agriculture, Forest Service, Southern Research Station. [Google Scholar]
- Burton JI, Perakis SS, McKenzie SC, Lawrence CE, Puettmann KJ. 2017. Intraspecific variability and reaction norms of forest understorey plant species traits. Functional Ecology 31:1881–1893. [Google Scholar]
- Caldwell MM, Richards JH, Johnson DA, Nowak RS, Dzurec RS. 1981. Coping with herbivory: photosynthetic capacity and resource allocation in two semiarid Agropyron bunchgrasses. Oecologia 50:14–24. [DOI] [PubMed] [Google Scholar]
- Chapin FS. 1980. The mineral nutrition of wild plants. Annual Review of Ecology and Systematics 11:233–260. [Google Scholar]
- Chave J. 2005. Measuring wood density for tropical forest trees. Measuring wood density for tropical forest trees-a field manual for the CTFS sites, 7. http://chave.ups-tlse.fr/projects/wood-density-protocol.pdf. [Google Scholar]
- Colbert KC, Larsen DR, Lootens JR. 2002. Height-diameter equations for thirteen Midwestern Bottomland hardwood species. Northern Journal of Applied Forestry 19:171–176. [Google Scholar]
- Coley PD, Bryant JP, Chapin FS. 1985. Resource availability and plant antiherbivore defense. Science 230:895–899. [DOI] [PubMed] [Google Scholar]
- Cornelissen JHC, Lavorel S, Garnier E, Diaz S, Buchmann N, Gurvich DE, Reich PB, Ter Steege H, Morgan HD, Van Der Heijden MGA, Pausas JG. 2003. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Australian Journal of Botany 51:335–380. [Google Scholar]
- Cunningham A. 2001. Applied ethnobotany: people, wild plant use and conservation UK: Earthscan. [Google Scholar]
- Delvaux C, Sinsin B, Darchambeau F, Van Damme P. 2009. Recovery from bark harvesting of 12 medicinal tree species in Benin, West Africa. Journal of Applied Ecology 46:703–712. [Google Scholar]
- Delvaux C, Sinsin B, Van Damme P. 2010. Impact of season, stem diameter and intensity of debarking on survival and bark re-growth pattern of medicinal tree species, Benin, West Africa. Biological Conservation 143:2664–2671. [Google Scholar]
- Dianda M, Bayala J, Dicop T, Ouedraogo SJ. 2009. Improving growth of shea butter tree (Vitellaria paradoxa CFGaertn.) seedlings using mineral N, P and arbuscular mycorrhizal (AM) fungi. Biotechnologie Agronomie Societe et Environnement 13:93–102. [Google Scholar]
- Ellner SP, Rees M. 2006. Integral projection models for species with complex demography. The American Naturalist 167:410–428. [DOI] [PubMed] [Google Scholar]
- Gaoue OG. 2016. Transient dynamics reveal the importance of early life survival to the response of a tropical tree to harvest. Journal of Applied Ecology 53:112–119. [Google Scholar]
- Gaoue OG, Horvitz CC, Ticktin T, Steiner UK, Tuljapurkar S. 2013. Defoliation and bark harvesting affect life-history traits of a tropical tree. Journal of Ecology 101:1563–1571. [Google Scholar]
- Gaoue OG, Sack L, Ticktin T. 2011. Human impacts on leaf economics in heterogeneous landscapes: the effect of harvesting non-timber forest products from African mahogany across habitats and climates. Journal of Applied Ecology 48:844–852. [Google Scholar]
- Gaoue OG, Ticktin T. 2007. Patterns of harvesting foliage and bark from the multipurpose tree Khaya senegalensis in Benin: variation across ecological regions and its impacts on population structure. Biological Conservation 137:424–436. [Google Scholar]
- Gaoue OG, Ticktin T. 2008. Impacts of bark and foliage harvest on Khaya senegalensis (Meliaceae) reproductive performance in Benin. Journal of Applied Ecology 45:34–40. [Google Scholar]
- Gaoue OG, Ticktin T. 2009. Fulani knowledge of the ecological impacts of Khaya senegalensis (Meliaceae) foliage harvest in Benin and its implications for sustainable harvest. Economic Botany 63:256–270. [Google Scholar]
- Genet H, Bréda N, Dufrêne E. 2009. Age-related variation in carbon allocation at tree and stand scales in beech (Fagus sylvatica L.) and sessile oak (Quercus petraea (Matt.) Liebl.) using a chronosequence approach. Tree Physiology 30:177–192. [DOI] [PubMed] [Google Scholar]
- Gleason SM, Ares A. 2004. Photosynthesis, carbohydrate storage and survival of a native and an introduced tree species in relation to light and defoliation. Tree Physiology 24:1087–1097. [DOI] [PubMed] [Google Scholar]
- Grime JP, Thompson K, Hunt R, Hodgson JG, Cornelissen JHC, Rorison IH, Hendry GAF, Ashenden TW, Askew AP, Band SR, Booth RE. 1997. Integrated screening validates primary axes of specialisation in plants. Oikos 79:259–281. [Google Scholar]
- Gutschick VP, BassiriRad H. 2003. Extreme events as shaping physiology, ecology, and evolution of plants: toward a unified definition and evaluation of their consequences. New Phytologist 160:21–42. [DOI] [PubMed] [Google Scholar]
- Hamerlynck EP, Smith BS, Sheley RL, Svejcar TJ. 2016. Compensatory photosynthesis, water-use efficiency, and biomass allocation of defoliated exotic and native bunchgrass seedlings. Rangeland Ecology and Management 69:206–214. [Google Scholar]
- Hérault B, Bachelot B, Poorter L, Rossi V, Bongers F, Chave J, Paine CET, Wagner F, Baraloto C. 2011. Functional traits shape ontogenetic growth trajectories of rain forest tree species. Journal of Ecology 99:1431–1440. [Google Scholar]
- Herault B, Ouallet J, Blanc L, Wagner F, Baraloto C. 2010. Growth responses of neotropical trees to logging gaps. Journal of Applied Ecology 47:821–831. [Google Scholar]
- Hoeber S, Leuschner C, Köhler L, Arias-Aguilar D, Schuldt B. 2014. The importance of hydraulic conductivity and wood density to growth performance in eight tree species from a tropical semi-dry climate. Forest Ecology and Management 330:126–136. [Google Scholar]
- Iida Y, Kohyama TS, Swenson NG, Su SH, Chen CT, Chiang JM, Sun I. 2014. Linking functional traits and demographic rates in a subtropical tree community: the importance of size dependency. Journal of Ecology 102:641–650. [Google Scholar]
- Karyati K, Ipor I, Jusoh I, Wasli M. 2017. The diameter increment of selected tree species in a secondary tropical forest in Sarawak, Malaysia. Biodiversitas Journal of Biological Diversity 18:304–311. [Google Scholar]
- King D, Davies S, Tan S, Nur M. 2006. The role of wood density and stem support costs in the growth and mortality of tropical trees. Journal of Ecology 94:670–680. [Google Scholar]
- Kone WM, Atindehou KK, Terreaux C, Hostettmann K, Traore D, Dosso M. 2004. Traditional medicine in north Cote-d’Ivoire: screening of 50 medicinal plants for antibacterial activity. Journal of Ethnopharmacology 93:43–49. [DOI] [PubMed] [Google Scholar]
- Kunstler G, Falster D, Coomes DA, Hui F, Kooyman RM, Laughlin DC, Poorter L, Vanderwel M, Vieilledent G, Wright SJ, Aiba M. 2016. Plant functional traits have globally consistent effects on competition. Nature 529:204–207. [DOI] [PubMed] [Google Scholar]
- Lovelock CE, Posada J, Winter K. 1999. Effects of elevated CO2 and defoliation on compensatory growth and photosynthesis of seedlings in a tropical tree, Copaifera aromatica. Biotropica 31:279–287. [Google Scholar]
- Maherali H, DeLucia EH. 2000. Xylem conductivity and vulnerability to cavitation of ponderosa pine growing in contrasting climates. Tree Physiology 20:859–867. [DOI] [PubMed] [Google Scholar]
- Martínez-Vilalta J, Mencuccini M, Vayreda J, Retana J. 2010. Interspecific variation in functional traits, not climatic differences among species ranges, determines demographic rates across 44 temperate and Mediterranean tree species. Journal of Ecology 98:1462–1475. [Google Scholar]
- Matilo A, Iida Y, Kohyama T. 2013. Tree species composition and stand structure of woody savanna in Dahomey Gap. Tropics 22:39–57. [Google Scholar]
- McDowell NG, Beerling DJ, Breshears DD, Fisher RA, Raffa KF, Stitt M. 2011. The interdependence of mechanisms underlying climate-driven vegetation mortality. Trends in Ecology and Evolution 26:523–532. [DOI] [PubMed] [Google Scholar]
- McGill BJ, Enquist BJ, Weiher E, Westoby M. 2006. Rebuilding community ecology from functional traits. Trends in Ecology and Evolution 21:178–185. [DOI] [PubMed] [Google Scholar]
- Mensah S, Houehanou TD, Sogbohossou EA, Assogbadjo AE, Glèlè Kakaï R. 2014. Effect of human disturbance and climatic variability on the population structure of Afzelia africana Sm. ex pers. (Fabaceae-Caesalpinioideae) at country broad-scale (Bénin, West Africa). South African Journal of Botany 95:165–173. [Google Scholar]
- Motallebi A, Kangur A. 2016. Are allometric relationships between tree height and diameter dependent on environmental conditions and management?Trees - Structure and Function 30:1429–1443. [Google Scholar]
- Myers JA, Kitajima K. 2007. Carbohydrate storage enhances seedling shade and stress tolerance in a neotropical forest. Journal of Ecology 95:383–395. [Google Scholar]
- Nacoulma BMI, Lykke AM, Traoré S, Sinsin B, Thiombiano A. 2017. Impact of bark and foliage harvesting on fruit production of the multipurpose tree Afzelia africana in Burkina Faso (West Africa). Agroforestry Systems 91:565–576. [Google Scholar]
- N’Dri AB. 2012. Short term effects of fire intensity and fire regime on vegetation dynamic in a tropical humid savanna (Lamto, central Côte d’Ivoire). Natural Science 4:1056–1064. [Google Scholar]
- Orwa C, Mutua A, Kindt R, Jamnadass R, Simons A. 2009. Agroforestree database: a tree species reference and selection guide version 4.0. Nairobi, Kenya: World Agroforestry Centre ICRAF. [Google Scholar]
- Ouédraogo-Koné S, Kaboré-Zoungrana CY, Ledin I. 2006. Behaviour of goats, sheep and cattle on natural pasture in the sub-humid zone of West Africa. Livestock Science 105:244–252. [Google Scholar]
- Panek JA. 1996. Correlations between stable carbon-isotope abundance and hydraulic conductivity in Douglas-fir across a climate gradient in Oregon, USA. Tree Physiology 16:747–755. [DOI] [PubMed] [Google Scholar]
- Poorter L, Bongers F. 2006. Leaf traits are good predictors of plant performance across 53 raun forest species. Ecology 87:1733–1743. [DOI] [PubMed] [Google Scholar]
- Poorter L, Kitajima K. 2007. Carbohydrate storage and light requirements of tropical moist and dry forest tree species. Ecology 88:1000–1011. [DOI] [PubMed] [Google Scholar]
- Poorter L, McDonald I, Alarcón A, Fichtler E, Licona JC, Peña‐Claros M, Sterck F, Villegas Z, Sass‐Klaassen U. 2010. The importance of wood traits and hydraulic conductance for the performance and life history strategies of 42 rainforest tree species. New Phytologist 185:481–492. [DOI] [PubMed] [Google Scholar]
- Poorter L, Wright SJ, Paz H, Ackerly DD, Condit R, Ibarra-Manríquez G, Harms KE, Licona JC, Martinez-Ramos M, Mazer SJ, Muller-Landau HC. 2008. Are functional traits good precictors of demographic rates? Evidence from five neotropical forests. Ecology 89:1908–1920. [DOI] [PubMed] [Google Scholar]
- R Development Core Team 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Richardson AD, Carbone MS, Keenan TF, Czimczik CI, Hollinger DY, Murakami P, Schaberg PG, Xu X. 2013. Seasonal dynamics and age of stemwood nonstructural carbohydrates in temperate forest trees. New Phytologist 197:850–861. [DOI] [PubMed] [Google Scholar]
- Rijkers T, Ogbazghi W, Wessel M, Bongers F. 2006. The effect of tapping for frankincense on sexual reproduction in Boswellia papyrifera. Journal of Applied Ecology 43:1188–1195. [Google Scholar]
- Ryan MG, Yoder BJ. 1997. Hydraulic limits to tree height and tree growth. BioScience 47:235–242. [Google Scholar]
- Sacande M. 2007. Afzelia africana. Seed Leaflet 118:24–26. [Google Scholar]
- Santiago LS, Goldstein G, Meinzer FC, Fisher JB, Machado K, Woodruff D, Jones T. 2004. Leaf photosynthetic traits scale with hydraulic conductivity and wood density in Panamanian forest canopy trees. Oecologia 140:543–550. [DOI] [PubMed] [Google Scholar]
- Schreiber SG, Hacke UG, Hamann A. 2015. Variation of xylem vessel diameters across a climate gradient: insight from a reciprocal transplant experiment with a widespread boreal tree. Functional Ecology 29:1392–1401. [Google Scholar]
- Sinsin B, Matig OE, Assogbadjo AE, Gaoue OG, Sinadouwirou T. 2004. Dendrometric characteristics as indicators of pressure of Afzelia africana Sm. dynamic changes in trees found in different climatic zones of Benin. Biodiversity and Conservation 13:1555–1570. [Google Scholar]
- Stark MA. 1986. Relationship between fire and basal scarring on Afzelia africana in Benoue National Park, Cameroon. African Journal of Ecology 24:263–271. [Google Scholar]
- Sterck FJ, Poorter L, Schieving F. 2006. Leaf traits determine the growth‐survival trade‐off across rain forest tree species. The American Naturalist 167:758–765. [DOI] [PubMed] [Google Scholar]
- Sydnor TD, McCartney RB. 1996. The influence of defoliation on flowering dogwood. Journal of Arboriculture 22:218–221. [Google Scholar]
- Ticktin T. 2004. The ecological implications of harvesting non-timber forest products. Journal of Applied Ecology 41:11–21. [Google Scholar]
- Tshisikhawe MP, van Rooyeni MW, Bhat RB. 2012. An evaluation of the extent and threat of bark harvesting of medicinal plant species in the Venda Region, Limpopo Province, South Africa. Phyton-International Journal of Experimental Botany 81:89–100. [Google Scholar]
- Vargas R. 2012. How a hurricane disturbance influences extreme CO2 fluxes and variance in a tropical forest. Environmental Research Letters 7:035704. [Google Scholar]
- Vuyiya E, Konje M, Tsingalia H, et al. 2014. The impacts of human activities on tree species richness and diversity in Kakamega Forest, Western Kenya. International Journal of Biodiversity and Conservation 6:428–435. [Google Scholar]
- Westoby M, Falster DS, Moles AT, Vesk PA, Wright IJ. 2002. Plant ecological strategies: some leading dimensions of variation between species. Annual Review of Ecology and Systematics 33:125–159. [Google Scholar]
- Wright IJ, Cannon K. 2001. Relationships between leaf lifespan and structural defences in a low-nutrient, sclerophyll flora. Functional Ecology 15:351–359. [Google Scholar]
- Wright SJ, Kitajima K, Kraft NJB, Reich PB, Wright IJ, Bunker DE, Condit R, Dalling JW, Davies SJ, Diaz S, Engelbrecht BM. 2010. Functional traits and the growth-mortality trade-off in tropical trees. Ecology 91:3664–3674. [DOI] [PubMed] [Google Scholar]
- Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JH, Diemer M, Flexas J. 2004. The worldwide leaf economics spectrum. Nature 428:821–827. [DOI] [PubMed] [Google Scholar]
- Wright IJ, Westoby M. 2002. Leaves at low versus high rainfall: coordination of structure, lifespan and physiology. New Phytologist 155:403–416. [DOI] [PubMed] [Google Scholar]
- Würth MKR, Peláez-Riedl S, Wright SJ, Körner C. 2005. Non-structural carbohydrate pools in a tropical forest. Oecologia 143:11–24. [DOI] [PubMed] [Google Scholar]
- Zhou X, Zhang Y, Niklas KJ. 2014. Sensitivity of growth and biomass allocation patterns to increasing nitrogen: a comparison between ephemerals and annuals in the Gurbantunggut Desert, north-western China. Annals of Botany 113:501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuidema PA, Jongejans E, Chien PD, During HJ, Schieving F. 2010. Integral projection models for trees: a new parameterization method and a validation of model output. Journal of Ecology 98:345–355. [Google Scholar]