Abstract
Recent success with B cell depletion therapies has revitalized efforts to understand the pathogenic role of B cells in Multiple Sclerosis (MS). Using the adoptive transfer system of experimental autoimmune encephalomyelitis (EAE), a murine model of MS, we have previously shown that mice in which B cells are the only MHCII-expressing antigen presenting cell (APC) are susceptible to EAE. However, a reproducible delay in the day of onset of disease driven by exclusive B cell antigen presentation suggests that B cells require optimal conditions to function as APCs in EAE. In this study, we utilize an in vivo genetic system to conditionally and temporally regulate expression of MHCII to test the hypothesis that B cell APCs mediate attenuated and delayed neuroinflammatory T cell responses during EAE. Remarkably, induction of MHCII on B cells following the transfer of encephalitogenic CD4 T cells induced a rapid and robust form of EAE, while no change in the time to disease onset occurred for recipient mice in which MHCII is induced on a normal complement of APC subsets. Changes in CD4 T cell activation over time did not account for more rapid onset of EAE symptoms in this new B cell-mediated EAE model. Our system represents a novel model to study how the timing of pathogenic cognate interactions between lymphocytes facilitates the development of autoimmune attacks within the CNS.
Introduction
Multiple sclerosis (MS) is a debilitating autoimmune disease of the central nervous system (CNS) with an unknown etiology despite being the subject of intense study for over a century [1]. MS is characterized by the chronologically and spatially distinct formation of lesions (“plaques”) comprised of cellular and humoral inflammation, demyelination, and axonal damage. Experimental autoimmune encephalomyelitis (EAE) is the main animal model for MS used to investigate the cellular mechanisms of disease as well as to develop new MS treatments [2, 3]. Early experiments with EAE identified the CD4 T cell as both necessary and sufficient for disease and prompted further investigation into the characteristics of MHCII+ antigen presenting cells (APCs) responsible for the regulation of CD4 T cell behavior during neuro-inflammation [4].
In MS, B cell depletion therapies (BCDTs) have recently been shown to be highly effective at amelioration of disease [5, 6]. BCDT reduces relapses and decreases inflammatory lesions [5, 7] but does not affect cerebral spinal fluid (CSF) levels of immunoglobulin nor deplete the long-lived antibody-secreting plasma cells from within the CSF or other tissues [8, 9]. Various approaches with BCDT in EAE also demonstrate that B cells can have an enormous influence on cognate encephalitogenic T cell pathogenicity and highlight the importance of antibody-independent B cell functions for the pathogenesis of CNS autoimmunity [10–12]. B cells are not highly phagocytic yet are very efficient at presenting antigens acquired via receptor-mediated endocytosis [13–15]. Although the target antigens for MS are unknown, recombinant B cell receptors (BCRs) derived from CSF-localized B cell clones exhibit specificity for myriad CNS components [16–19]. Through the process of linked recognition, a non-auto-reactive B cell could still present self-peptide antigens associated with internalized immune complexes to activate auto-reactive CD4 T cells [20]. Understanding how B cell-mediated antigen presentation influences neuro-inflammation and tolerance in MS could lead to potent and more specific immunomodulatory therapies.
Our previous work demonstrated that B cells are capable of serving as the only APC during passive EAE [15]. However, transgenic mice with elevated B cell specificity for MOG (IgHMOG mice) crossed to mice expressing MHCII exclusively by CD19+ B cells (CD19-BMHCII mice)–referred to as CD19-BMHCIIxIgHMOG mice because CD19Cre drives MHCII expression on B cells–develop passive EAE with a statistically significant and reproducible delay in onset compared to WT mice [15]. Thus, B cells are capable of propagating auto-antigen-specific CNS demyelination on their own but may be limited in their efficiency as APCs. To explore the kinetics of B cell cognate interactions during EAE, BMHCIIxIgHMOG mouse were bred to a Tamoxifen (Tam)-inducible CD20Tam-Cre murine reagent generated by Shlomchik and colleagues [21]. By carefully controlling of the timing of cognate interactions between B cells and CD4 T cells during EAE, we have observed a rapid induction of disease, demonstrating that B cells can efficiently drive auto-reactive CD4 T cell responses targeting the CNS.
Materials & methods
Mice
WT C57BL/6 (B6) mice, MOG-specific TCR-transgenic (2D2) mice, and UBCTam-Cre mice were obtained from The Jackson Laboratory (Bar Harbor, ME). CD19-BMHCIIxIgHMOG mice, in which B cells expressing a transgene driving a MOG-specific BCR exclusively express MHCII, were generated as previously reported [15]. CD20Tam-Cre mice [21] were bred to IAßbstopflox/floxxIgHMOG mice [15] to generate CD20-BMHCIIxIgHMOG and UBCTam-Cre mice were bred to IAßbstopflox/flox mice to obtain UBCMHCII mice [21, 22]. Littermates from IAßbstopflox/floxxIgHMOG crossed to CD20Tam-CrexIAßbstopflox/flox breedings were used for comparison during experiments. 2D2 mice were crossed with CD19-BMHCIIxIgHMOG mice, which lack a complete endogenous CD4 T cell compartment. Three-week-old 2D2xCD19-BMHCIIxIgHMOG progeny received a thymic transplant from WT animals to support 2D2 thymocyte development as previously described, and were harvested after 8–12 weeks [23]. Animals were housed in a specific pathogen-free barrier facility at the Washington University School of Medicine (WUSM). All breeding and experimental protocols were performed in accordance with protocols reviewed and approved by the WUSM Animal Studies Committee/Institutional Animal Care and Use Committee (IACUC). Death was not considered an experimental outcome for any of the experiments reported herein. Nevertheless, humane endpoints were considered for all experiments, and according to IACUC protocols all experiments were designed to minimize suffering and distress of mice.
EAE
Active EAE with rhMOG (Children’s Hospital of Pennsylvania) was induced by s.c. immunization of 150 μg rhMOG protein (residues 1–125 of human MOG protein [23]) emulsified in CFA (Sigma, St. Louis, MO). Mice received 200ng Pertussis toxin (Enzo, Farmingdale, NY) i.p. at the time of immunization and 48h later. Passive EAE was induced by adoptive transfer of 5x106 MOG-specific Thy1.1+ or CD45.1+ encephalitogenic CD4 T cells, as previously reported [23]. Transfer experiments in which encephalitogenic donor T cells were ‘rested’ in vivo in an MHCII-deficient host were performed by initially transferring 5x106 encephalitogenic CD4 T cells into MHCII-deficient IAßbstopflox/floxxIgHMOG mice. Three weeks later, CD4 T cells were isolated from those mice and immediately to transferred into CD19-BMHCIIxIgHMOG or WT mice. In other experiments, MHCII expression was induced in UBCMHCII and CD20-BMHCIIxIgHMOG mice by oral gavage with 5mg Tam in 50μL corn oil. Tam was either administered three days prior to donor T cell transfer or at various time points after donor T cell transfer. MHCII expression was verified by flow cytometric analysis of blood collected from the cheek. Mice were observed daily and clinical scores were determined by a five point scoring system, as follows: 0 = no disease; 1 = limp tail; 2 = mild hind limb paresis; 3 = severe hind limb paresis; 4 = complete hind limb plegia or quadriplegia; 5 = moribund or dead.
Flow cytometry
Spleens were harvested from Avertin-anesthetized mice and single cell suspensions were treated with ACK erythrocyte lysis buffer. Mice were perfused with 25mL of ice-cold PBS and CNS tissues were isolated. Mononuclear cells were purified from homogenized brains and spinal cords by centrifugation for 30 min in 30% Percoll (GE Healthcare) solution as previously reported [22]. Cells were incubated with the anti-Fc receptor antibody 2.4G2 prior to the addition of staining antibodies. Intracellular cytokine (BD Cytofix/Cytoperm Plus, BD Biosciences, San Diego CA) and FoxP3 staining (Mouse Regulatory T cell Staining Kit #1, eBioscience San Diego, CA) of donor CD4 T cells was performed in accordance with kit protocols. The following anti-mouse antibodies were purchased from BD Biosciences (San Jose, CA): anti-CD69 FITC clone H1.2F3, anti-CD45 FITC, anti-GR-1 PE clone RB6-8C5, anti-CD3e PE-CF594 clone 145.2C11, anti-B220 PE-CF594 clone RA3-6B2, anti-CD44 PerCP/Cy5.5 clone IM7, anti-CD19 APC clone 1D3. The following antibodies were purchased from eBioscience (San Diego, CA): anti-CD45.1 FITC clone A20, anti-Foxp3 PE clone FJK-16s, rat anti-IgG2a isotype PE clone eBR2a, anti-CD25 APC clone PC61.5, anti-IFNγ APC clone XMG1.2. The following antibodies were purchased from BioLegend (San Diego, CA): anti-IL-17 FITC clone TC11-18H10.1, anti-CD4 clone GK1.5 in FITC and BV 421, anti-CD19 PE clone 1D3, anti-GM-CSF PE clone MP1-22E9, anti-CD49d clone R1-2 in PE and PE/Cy7, anti-Thy1.1 PerCP clone OX-7, anti-CD11b clone M1.7 in PerCP/Cy5.5 and PE/Cy7, anti-GR-1 PE/Cy7 clone RB6-8C5, anti-MHCII clone M5/114.15.2 in AF700 and Pacific Blue, anti-CD44 AF700 clone IM7, anti-CD8α APC/Cy7 clone 53.6.7, anti-CD45 AF700 clone 30-F11 in AF700, Pacific Blue, and BV 510. Cells were acquired on a Gallios flow cytometer (Beckman Coulter) and analyzed with FlowJo software version 8.5.2 (TreeStar, Ashland, OR) with doublets excluded.
Histology and immunofluorescence
Mice were sacrificed and perfused with 25mL ice cold PBS followed by 20mL 4% paraformaldehyde (Sigma-Aldrich). Spinal cord tissue was removed from the vertebrae and then fixed in 4% paraformaldehyde for more than 12 hours, followed by dehydration in 30% sucrose for 48 hours. The tissue was embedded in optimal cutting temperature media (TissueTek, Torrence, CA) and cut at 8–10μm thick sections using a Leica CM1900 cryostat (Germany). The WUSM Developmental Biology Histology & Microscopy Core stained sections with Luxol Fast Blue to detect myelin. Slides were examined by light microscopy using a Nikon 90i motorized upright digital microscope with camera and Metamorph software (Molecular Devices). Sections were also stained to quantify myelinated white matter using goat anti-MOG (Invitrogen) with isotype control goat IgG2a (abcam) and secondary antibody donkey anti-goat Alexa-555 (Invitrogen). Slides were mounted with Fluoroshield Mounting Medium with DAPI (abcam). Slides were examined by immunofluorescent microscopy using a Nikon 90i motorized upright digital microscope with CoolSNAP ES2 camera (Photometrics). Inflammation score was recorded by blinded observer using the following scale system: 0 = normal, no inflammation; 1 = mild inflammation with a few small regions of increased cellularity; 2 = moderate inflammation with one large lesion or several smaller inflammatory foci; 3 = severe inflammation with dense parenchymal infiltration and many large lesions; 4 = massive inflammation in which most of the white matter has dense cellularity. Demyelination was quantified by blinded calculation of lesion area in thoracic spinal cord sections using Metamorph software (Molecular Devices, Inc).
Statistical analysis
All data generated are reported as mean ± the standard error of the mean (SEM). Unpaired t-tests were used for comparison of cellular infiltrates. Time-to-EAE-onset incidence curves were compared by Log-rank (Mantel-Cox) tests. Group effects were compared via analysis of variance (ANOVA) with Tukey’s test or Kruskal-Wallis test with Dunn’s test for multiple comparisons when assumptions were not violated. Mann-Whitney tests with two-tailed P values were performed for flow cytometry assays quantifying lymphocytes in the CNS. All statistical analyses were completed using PRISM 7 software (GraphPad).
Results
B cell antigen presentation is insufficient to initiate spontaneous autoimmune CNS demyelination
Increasing the frequency of MOG-specific B cells in 2D2 mice, in which T cell receptors are highly specific for MOG, routinely leads to spontaneous inflammatory demyelination within the spinal cord and optic nerve, indicating that naïve MOG-specific B and T cells collaborate to induce neuro-inflammation [24, 25]. Additionally, transgenic SJL/J mice with MOG-specific T cells require an intact B cell compartment for susceptibility to spontaneous EAE [26]. To test the hypothesis that antigen presentation by B cells can independently trigger spontaneous EAE, we crossed CD19-BMHCIIxIgHMOG with 2D2 mice and salvaged 2D2 CD4 T cell development in progeny by thymus transplant. While both 2D2 and 2D2xIgHMOG mice with WT MHCII expression spontaneously developed optic neuritis, only 2D2xIgHMOG mice developed spontaneous EAE, confirming previously published data (Table 1). However, 2D2xCD19-BMHCIIxIgHMOG mice were completely resistant to both optic nerve inflammation and spontaneous EAE (Table 1) despite a substantial reconstitution of the CD4 T cell compartment with MOG-specific T cells as reported [23]. These results suggested that antigen-specific B cells alone were not sufficient to elicit CD4 T cell-dependent spontaneous inflammatory demyelination within the CNS, even when a high frequency of cognate, auto-reactive T cells were present. When these data are considered in conjunction with our previous observation that EAE is delayed in CD19-BMHCIIxIgHMOG mice [15], it suggests that B cells are intrinsically less efficient than other APCs in their ability to initiate and direct CD4 T cell auto-reactivity to MOG.
Table 1. B cells are not sufficient APCs to support spontaneous EAE or optic neuritis.
| Genotype | Optic Neuritis | Thymus Transplant | Spontaneous EAE |
|---|---|---|---|
| 2D2 | 5/11 | No | 0/11 |
| 2D2xIgHMOG | 3/3 | No | 15/19 |
| 2D2xCD19-BMHCIIxIgHMOG | 0/19 | Yes | 0/21 |
| WT hMOG* | 3/5 | No | 5/5 |
* Active EAE (not spontaneous)
Tamoxifen-inducible systems for induction of MHCII expression
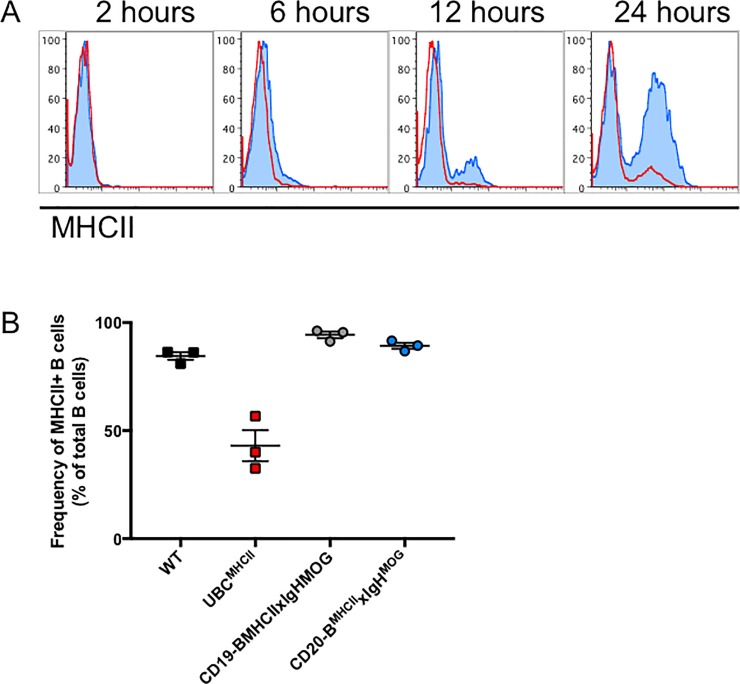
By breeding the IAßbstopflox/floxxIgHMOG mouse to Tam-inducible CD20Tam-Cre mice, we aimed to temporally regulate MHCII expression exclusively by B cells. To assess the efficiency of recombination at the IA-ß locus following Tam administration, peripheral blood was collected from CD20-BMHCIIxIgHMOG and UBCMHCII mice prior to oral gavage with Tam and at various time points thereafter. MHCII expression was undetectable prior to Tam treatment for both CD20-BMHCIIxIgHMOG and UBCMHCII mice similar to the two hour timepoint (Fig 1A). 52.3 ± 4.7% (Mean ± SEM) of B cells from CD20-BMHCIIxIgHMOG mice expressed MHCII 24 hours after Tam administration (Fig 1A). After 72 hours, the mean frequency of MHCII+ B cells in CD20-BMHCIIxIgHMOG spleens (89.3 ± 1.4%) was similar to that of WT (84.5 ± 1.8%) and CD19-BMHCIIxIgHMOG mice (94.3 ± 1.5%) (Fig 1B). Following Tam administration, fewer B cells in UBCMHCII mice expressed MHCII compared to WT B cells, yet MHCII was induced on other APC subsets in UBCMHCII mice (Fig 1B).
Fig 1. Temporal regulation of MHCII expression in vivo.
(A) MHCII expression on peripheral blood B cells collected from UBCMHCII (red line) and CD20-BMHCIIxIgHMOG (blue shaded) at several time points after Tam administration. Data are representative of two experiments, (n = 2 mice per time point from each genotype). (B) 72 hours post Tam treatment, spleens were harvested from naïve mice 72 hours after oral gavage with Tam for UBCMHCII (red squares) and CD20-BMHCIIxIgHMOG (blue circles) or corn oil vehicle for WT (black squares) and CD19-BMHCIIxIgHMOG mice (grey circles). The frequency of MHCII+ B cells as a percentage of total B cells is presented as mean ± SEM from n = 3 mice per genotype.
B cell-mediated EAE is delayed in CD20-BMHCIIxIgHMOG mice
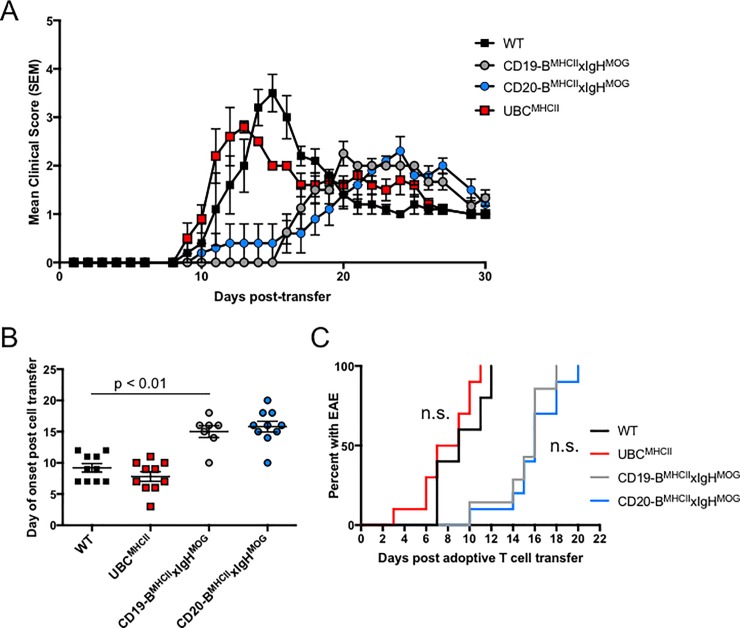
Based on the high efficiency of MHCII induction by Tam administration, we hypothesized that expression of MHCII by B cells in CD20-BMHCIIxIgHMOG mice would manifest EAE similar to CD19-BMHCIIxIgHMOG mice. Thus, we treated CD20-BMHCIIxIgHMOG mice and UBCMHCII mice with Tam three days prior to the adoptive transfer of encephalitogenic T cells. In WT mice, adoptive transfer of encephalitogenic CD4 T cells typically resulted in clinical and pathological signs within 10 days (Fig 2A). The time to onset and disease course for WT and UBCMHCII mice treated with Tam prior to the adoptive transfer of CD4 T cells were not significantly different (Fig 2B and 2C). As previously reported, CD19-BMHCIIxIgHMOG mice exhibited a statistically significant delay in the day of onset of EAE compared to WT mice, with EAE signs beginning on average at 15.0 ± 1.0 days following transfer of donor T cells [15]. CD20-BMHCIIxIgHMOG mice treated with Tam before T cell transfer exhibited similar disease kinetics, with an average day of EAE onset of 15.8 ± 0.9 days (Fig 2B and 2C). Hence, in our Tam-inducible MHCII system, B cell-restricted MHCII expression requires an extended period of time to facilitate disease mediated by encephalitogenic CD4 T cells compared to a full complement of MHCII+ APC subsets. These results indicate that the conditional and temporal regulation of MHCII in vivo is suitable for investigating the timing of B cell involvement during EAE.
Fig 2. Tam-inducible MHCII expression models recapitulate WT and B-cell mediated adoptive transfer EAE models.
(A) Mean ± SEM EAE scores recorded for CD20-BMHCIIxIgHMOG (blue circles), and UBCMHCII (red squares) mice treated with Tam by oral gavage and WT (black squares), CD19-BMHCIIxIgHMOG (grey circles), treated with corn oil vehicle by oral gavage once, three days prior to receiving 5x106 encephalitogenic CD4 T cells. Data is representative of three independent experiments with n = 3–5 mice per genotype. (B) Day of EAE onset post cell transfer for WT (black squares), UBCMHCII (red squares), CD19-BMHCIIxIgHMOG (grey circles) treated with corn oil 72 hours prior to CD4 T cell transfer and CD20-BMHCIIxIgHMOG (blue circles) recipient mice treated with Tam 72 hours prior to CD4 T cell transfer. Graph shows mean day of onset ± SEM from two pooled, independent experiments with n = 2–4 mice per genotype. Unpaired t test performed to compare day of onset between WT and CD19-BMHCIIxIgHMOG mice. (C) Mice were treated with Tam 72 hours prior to CD4 T cell transfer. Time to EAE onset for WT (black) and UBCMHCII (red) mice is not significantly different by log-rank test (p = 0.107). Time to EAE onset for CD19-BMHCIIxIgHMOG (grey), and CD20-BMHCIIxIgHMOG (blue) mice is not significantly different by log-rank test (p = 0.481). Incidence curves generated from two pooled, independent experiments with n = 2–4 mice per genotype.
B cells are capable of inducing accelerated onset EAE
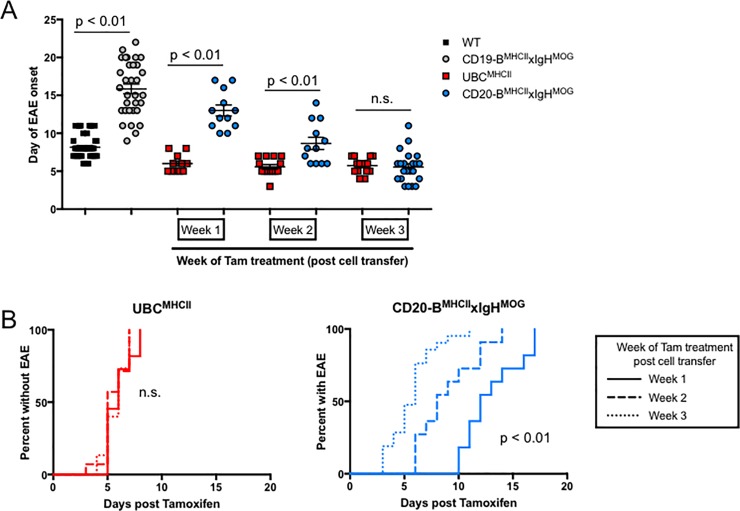
Antigen acquisition and presentation by B cells is implicated as a critical precursor to CD4 T cell activation and demyelination in B cell-dependent models of EAE [27–29]. It is possible that B cells require more time to collect and process antigen because they are generally sequestered in peripheral lymphoid tissues where CNS antigens are less accessible. We reasoned that B cell-mediated EAE would be delayed regardless of when MHCII expression was induced relative to CD4 T cell transfer. To test this, encephalitogenic CD4 T cells were transferred into CD20-BMHCIIxIgHMOG mice and UBCMHCII mice prior to the induction of MHCII expression. No animals developed EAE prior to Tam treatment. Regardless of the amount of time between CD4 T cell transfer and Tam-induced MHCII expression, the time to EAE onset for UBCMHCII mice after Tam administration was not significantly different; the average day of EAE onset for UBCMHCII mice treated with Tam one week after T cell transfer was 6.0 ± 0.4 days post Tam, whereas the average for mice treated with Tam after two or three weeks post T cell transfer was 5.6 ± 0.3 days and 5.7 ± 0.3 days post Tam, respectively (Fig 3A and 3B). Surprisingly, we observed a rapid onset of EAE signs for CD20-BMHCIIxIgHMOG mice that was dependent on the length of time between T cell transfer and MHCII induction (Fig 3A and 3B). The mean day of onset for CD20-BMHCIIxIgHMOG mice was significantly delayed compared to UBCMHCII mice when Tam was administered at either week one or week two after T cell transfer (Fig 3A). However, when mice received Tam three weeks after CD4 T cell transfer, the mean time to EAE onset for CD20-BMHCIIxIgHMOG and UBCMHCII mice was not significantly different (Fig 3B). These results demonstrate that B cell-mediated EAE has the capacity to develop more quickly than even the conventional WT passive EAE model. Thus, our Tam-inducible MHCII expression system provides a means to assess the kinetic differences in neuro-inflammation coordinated by antigen-specific B cells.
Fig 3. Tam treatment after T cell transfer results in accelerated B cell mediated EAE.
(A) Mean ± SEM day of EAE onset for WT (black squares) and CD19-BMHCIIxIgHMOG (grey circles) post T cell transfer, and for UBCMHCII (red squares) and CD20-BMHCIIxIgHMOG (blue circles) treated with Tam at either one week, two weeks, or three weeks post T cell transfer. Data is pooled from 12 different experiments with n = 1–5 mice per genotype and time point evaluated, p values calculated by unpaired t tests. (B) Time to EAE onset is not significantly different for UBCMHCII mice (left graph) treated with Tam at either Week 1 (solid line), Week 2 (dashed line), or Week 3 (dotted line) post CD4 T cell transfer. Time to EAE onset is significantly delayed for CD20-BMHCIIxIgHMOG mice (right graph) treated with Tam at either Week 1 (solid line), compared to Week 2 (dashed line), or Week 3 (dotted line) post CD4 T cell transfer. Incidence curves generated from is pooled from 12 different experiments with n = 1–5 mice per genotype and time point, with significance evaluated by log-rank test.
Accelerated EAE is not due to immune cell trafficking to the CNS prior to Tam administration
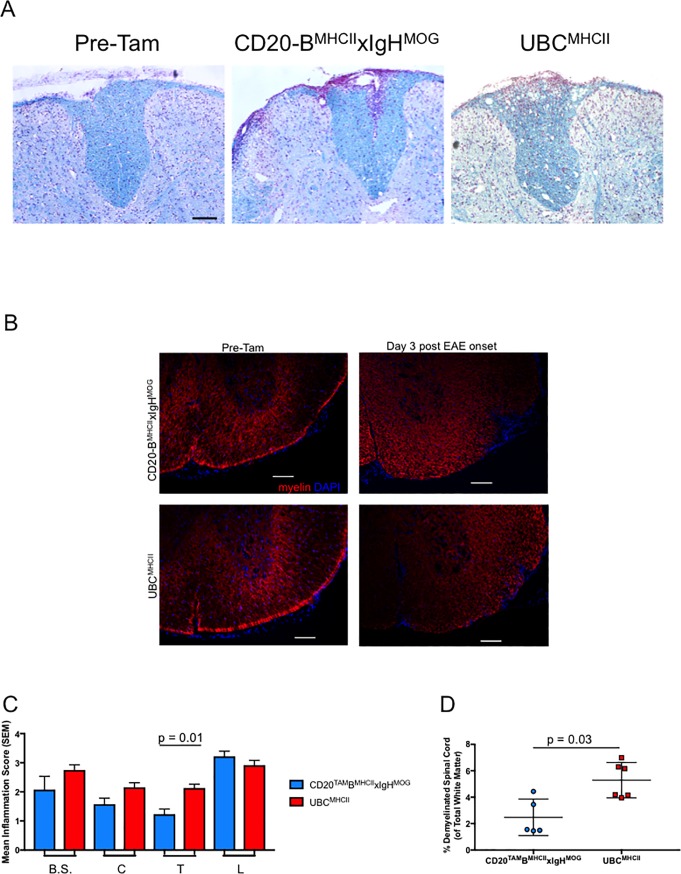
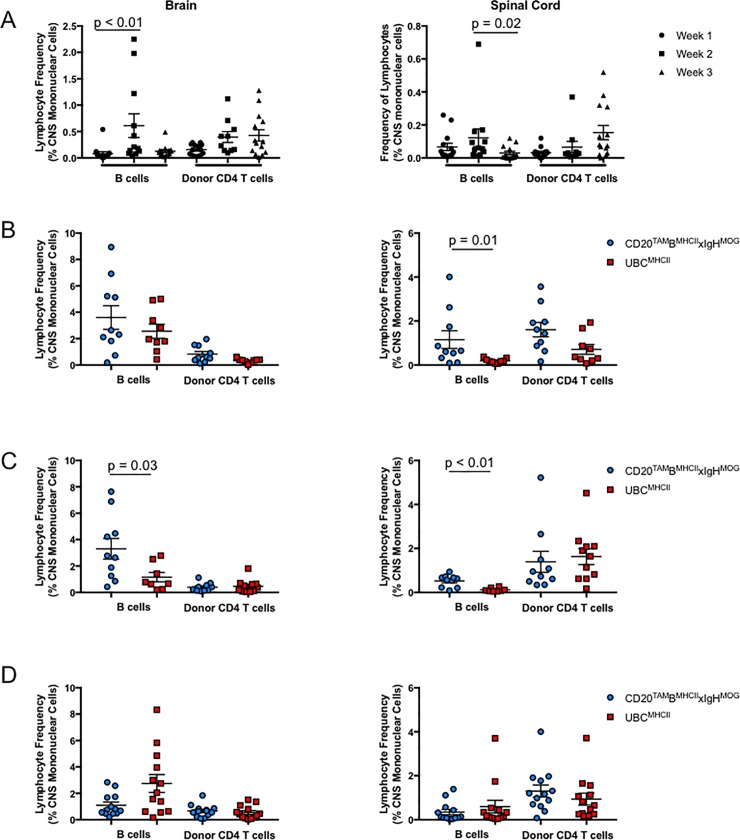
A low frequency of CNS MHCII+ B cells can support neuro-inflammation in CD19-BMHCIIxIgHMOG mice with EAE [15]. Localization to the CNS compartment prior to Tam administration would give a small number of B cells ready access to both MOG antigens and cognate T cells primed to respond rapidly upon MHCII expression by the B cells. Hence, antigen-specific lymphocytes entering the CNS over time prior to the induction of MHCII expression could explain the increasingly rapid onset of EAE signs observed in CD20-BMHCIIxIgHMOG mice. To assess this possibility, the spinal cords of CD20-BMHCIIxIgHMOG mice and UBCMHCII mice were examined for inflammation and demyelination before and after treatment with Tam three weeks post-T cell transfer. Histological examination of these tissues did not reveal any signs of inflammation or demyelination prior to Tam treatment (Fig 4A). Both CD20-BMHCIIxIgHMOG mice and UBCMHCII mice that developed EAE after receiving Tam on week three post-T cell transfer had similar inflammatory foci (Fig 4A and 4B). Semi-quantitative assessment of inflammation of the spinal cord at the level of the brainstem and cervical, thoracic, and lumbar regions of the spinal cord revealed similar degrees of inflammation (Fig 4C). However, we observed a significant difference in the extent of inflammation between the genotypes at the thoracic level (Fig 4B). The difference in area of demyelinated white matter within the thoracic spinal cords of CD20-BMHCIIxIgHMOG and UBCMHCII mice with EAE was significantly different, though this could be due to a higher mean EAE score for the UBCMHCII mice (3.33 ± 0.4 vs. 2.4 ± 0.3) (Fig 4D). These observations were verified by flow cytometric analyses of the composition of infiltrating mononuclear cells in brain and spinal cord tissues examined prior to Tam treatment at various time points post T cell transfer (Fig 5 and S1 Fig). UBCMHCII, CD20-BMHCIIxIgHMOG, and MHCII deficient Cre-IAßbstopflox/floxxIgHMOG littermate control mice were harvested one, two, or three weeks post T cell transfer but prior to Tam treatment and brains and spinal cords were analyzed by flow cytometry to detect infiltrating B cells and donor CD4 T cells. Prior to Tam treatment, these recipient mice are all MHCII-deficient and analysis of donor T cell cytokine production after incubation in different genotypes does not indicate that the genotype of the recipient influences encephalitogenicity over time (Fig 6 and S2 Fig). While the mean frequency of B cells in the brain at week two post cell transfer was significantly different compared to week one post cell transfer (p < 0.01), there was no difference at week three and no overall increase in the frequency of lymphocytes in the CNS over time prior to Tam treatment (Fig 5A). Altogether, very few infiltrating lymphocytes were detected in the brain and spinal cord before MHCII expression was induced, and the cellular composition of CNS tissues from experimental mice was comparable to the number of B cells and endogenous CD4 T cells detected in the CNS of naïve WT mice (S1 Fig). Taken together this suggests that the rapid onset of disease is not a result of anticipatory migration of CD4 T or B cells into the CNS. Flow cytometry of CNS tissues harvested from CD20-BMHCIIxIgHMOG mice three days post-EAE onset showed that the frequency of B cells in the brains and spinal cords tended to be more variable when Tam is administered soon after T cell transfer (Fig 5B–5D). Additionally, although there is a higher frequency of B cells in the CNS of CD20-BMHCIIxIgHMOG mice with EAE when treated with Tam two weeks after T cell transfer (Fig 5C), the difference is not apparent when treated with Tam three weeks after T cell transfer (Fig 5D). These results suggest that the rapid onset of EAE signs observed in CD20-BMHCIIxIgHMOG mice treated with Tam weeks after T cell transfer is not the result of enhanced localization of B cells or T cells in the CNS compartment.
Fig 4. Inflammation and demyelination is not evident in spinal cords of encephalitic CD4 T cell recipients prior to Tam administration.
(A) Representative spinal cord sections from recipients of encephalitogenic CD4 T cells (n = 5, CD20-BMHCIIxIgHMOG mice; n = 6, UBCMHCII mice) were stained with Luxol Fast Blue, scale bar = 100um; all images generated from 10x magnification. (A) Spinal cords from CD20-BMHCIIxIgHMOG mice harvested three weeks after T cell transfer and before Tam administration (left). CD20-BMHCIIxIgHMOG mice treated with Tam three weeks after T cell transfer (middle) and harvested three days post EAE onset. UBCMHCII mice treated with Tam three weeks after T cell transfer (right) and harvested three days post EAE onset. (B) Representative spinal cord sections from recipients of encephalitogenic CD4 T cells (at least mice 5 per genotype) were stained with antibodies to detect MOG, scale bar = 100um. Spinal cords from CD20-BMHCIIxIgHMOG mice (top left) and UBCMHCII mice (bottom left) harvested three weeks after T cell transfer and before Tam administration. CD20-BMHCIIxIgHMOG (top right) and UBCMHCII (bottom right) mice treated with Tam three weeks after T cell transfer and harvested three days post EAE onset. (C) Regions from rostral to caudal sections of spinal cords from CD20-BMHCIIxIgHMOG (blue) and UBCMHCII mice (red) were harvested and scored for inflammation. Graph shows mean +/- SEM inflammation scores and Kruskal-Wallis test with Dunn’s correction for multiple comparisons was applied. B.S. = brainstem; C = cervical; T = thoracic; L = lumbar. (D) Mean (SD) percent area of demyelinated white matter was quantified for thoracic spinal cord sections from CD20-BMHCIIxIgHMOG (blue) and UBCMHCII (red) mice. Significance determined by Mann-Whitney test with two-tailed p value.
Fig 5.
Flow cytometric analysis of lymphocytes from brains (left) and spinal cords (right). (A) Mean ± SEM frequency of B cells and donor CD4 T cells as a percent of total mononuclear cells in the CNS of mice at week 1 (circles), week 2 (squares), or week 3 (triangles) post CD4 T cell transfer. Data is pooled from CD20-BMHCIIxIgHMOG mice, UBCMHCII mice, and IAßbstopflox/floxxIgHMOG (Cre-) littermate controls from 9 different experiments with n = 3–5 mice at each time point prior to Tam treatment. Significance determined by Kruskal-Wallis test and Dunn’s correction for multiple comparisons. (B-D) Mean ± SEM frequency of B cells and donor CD4 T cells as a percent of total mononuclear cells in the brains (left) and spinal cords (right) harvested UBCMHCII (red squares) and CD20-BMHCIIxIgHMOG (blue circles) mice approximately three days post EAE onset when mice were treated with Tam at (B) week 1, (C) week 2, or (D) week 3 post encephalitogenic CD4 T cell transfer. Data is pooled from 9 different experiments with n = 1–5 mice per genotype at each time point evaluated. Significance determined by Mann-Whitney test with two-tailed p value.
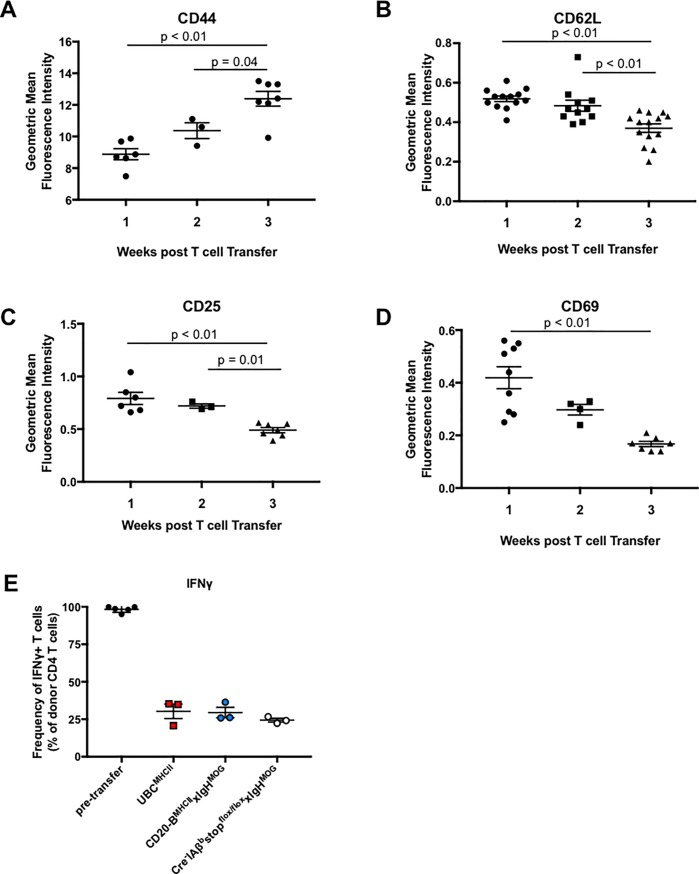
Fig 6.
(A-D) Mean ± SEM of the geometric mean fluorescence intensity of activation marker expression on donor CD4 T cells harvested from spleens of recipient mice at week 1 (circles), week 2 (squares), or week 3 (triangles) post CD4 T cell transfer. Data is pooled from CD20-BMHCIIxIgHMOG mice, UBCMHCII mice, and IAßbstopflox/floxxIgHMOG (Cre-) littermate control recipients from five independent experiments with n = 2–4 mice per time point evaluated. Significance tested by one way ANOVA with Tukey’s test for multiple comparisons. Geometric mean fluorescence intensity of (A) CD44, (B) CD62L, (C) CD25, and (D) CD69 expression on donor CD4 T cells. (E) Frequency of IFNγ-expressing donor CD4 T cells pre-transfer and following in vivo incubation for three weeks in MHCII-deficient hosts. Graph is representative of n = 5 independent experiments with at least two mice from each genotype. Donor T cells were harvested from the spleen. Significance tested by Kruskal-Wallis test and Dunn’s correction for multiple comparisons.
Changes in donor T cells over time do not account for rapid onset of B cell-mediated EAE
It is possible that donor encephalitogenic CD4 T cells become more pathogenic over time prior to MHCII induction in CD20-BMHCIIxIgHMOG mice. To rule out the possible effects of increasing donor CD4 T cell activation, peripheral CD4 T cell markers were assayed by flow cytometry at various time points post-transfer and prior to Tam administration. The mean fluorescence intensity (MFI) of CD44, a marker of effector-memory CD4 T cells, increased over time while CD69, a marker of T cell activation, decreased over time (Fig 6A and 6D). The MFI of two other markers, CD62L, which is expressed by naïve T cells homing to secondary lymphoid tissues, and CD25, expressed by activated T cells and regulatory T cells, also decreased over time in MHCII deficient recipients prior to Tam administration (Fig 6B and 6C). Hence, deviations in activation status were observed in donor CD4 T cells after incubation in MHCII-deficient hosts. However, a mixture of changes, inconsistent with a selection of a singular population of activated encephalitogenic CD4 T cells, was observed after prolonged incubation in the absence of cognate interactions.
Subsequently, we reasoned that if the pre-Tam time period induces changes in donor T cells to enhance their encephalitogenic potential, we would then be able to induce rapid-onset EAE in WT or CD19-BMHCIIxIgHMOG mice by re-transferring the T cells into new naïve hosts. If the observed changes in donor T cells are responsible for the increasingly rapid EAE onset observed in CD20-BMHCIIxIgHMOG mice, donor T cells collected after an initial incubation in an MHCII-deficient host would induce rapid-onset EAE after a subsequent passive transfer into naïve recipients. Therefore, MHCII deficient Cre-IAßbstopflox/floxxIgHMOG mice received encephalitogenic CD4 T cells, which were harvested from spleens and lymph nodes after three weeks of in vivo incubation. These freshly isolated T cells were MACS-purified by CD4 positive selection and immediately transferred into naïve WT or CD19-BMHCIIxIgHMOG recipients. The average day of onset was 18.2 for CD19-BMHCIIxIgHMOG receiving encephalitogenic CD4 T cells rested for three weeks in a MHCII-deficient environment, similar to the day of onset described previously for CD19-BMHCIIxIgHMOG mice receiving freshly in vitro generated donor CD4 T cell lines (Table 2). Likewise, the day of onset for WT and UBCMHCII mice was similar to the day of onset for WT mice whether donor CD4 T cells were rested in vivo for three weeks or transferred immediately. Flow cytometry to detect intracellular cytokines revealed the frequency of IFNγ-producing donor T cells harvested three weeks after transfer into an MHCII-deficient recipient is drastically reduced compared to the frequency of cytokine-producing donor CD4 T cells prior to transfer. This same reduction in IFNγ production was observed in CD4 T cells harvested from CD20-BMHCIIxIgHMOG and UBCMHCII mice prior to Tam administration (Fig 6E and S2 Fig). Taken together, the data indicate that prolonged incubation in a MHCII-deficient environment prior to Tam treatment does not lead to enhanced encephalitogenicity of donor CD4 T cells. Indeed, it is apparent that B cells are sufficient to provide the necessary APC functions to rapidly re-stimulate rested effector memory T cells to initiate EAE symptoms.
Table 2. Accelerated EAE is not induced by CD4 T cells harvested from MHCII-deficient hosts.
| Recipient Genotype | Donor CD4 T cells | Incidence of EAE | Mean Day of onset (± SEM) |
|---|---|---|---|
| WT | in vivo incubated* | 8/9 | 6.2 ± 0.6 |
| CD19-BMHCIIxIgHMOG | in vivo incubated | 9/13 | 16.7 ± 2.3 |
* ≥ 3 weeks
Discussion
The contributions of antigen specificity, antigen presentation, and cytokine production by individual APC populations on secondary T cell activation are complex and poorly understood. In MS and EAE, variation in T cell activation and effector lineage may be due to subtle differences in the abilities of APCs to acquire and present certain antigens at different times. For example, B6 mice are susceptible to MOG35-55 peptide-induced active EAE even if MHCII expression is restricted to DCs or if B cells are genetically ablated [23, 28, 30, 31]. However, resistance to MOG protein-induced active EAE can be seen in B cell deficient mice, mice with DC-specific MHCII expression, or mice in which B cells are MHCII deficient [28, 32, 33]. Taken together, it is apparent that B cell antigen presentation is critical for supporting CNS auto-reactivity to whole protein antigens.
Whether B cells facilitate initial CD4 T cell activation toward protein antigens in EAE has not been directly examined to date. In restricting MHCII expression to B cells, we found that the transgenic combination of B and T cell receptor specificities for MOG no longer evoked spontaneous inflammatory demyelination within the spinal cord (Table 1). While it is possible that 2D2xCD19-BMHCIIxIgHMOG mice do not develop spontaneous EAE due to the incomplete reconstitution of CD4 T cells by thymic grafting, our method has been utilized previously with success [23] and wild-type levels of CD4 T cells can be observed in secondary lymphoid tissue 9 weeks post-graft [23]. Other constraints such as a limited humoral MOG response in 2D2xCD19-BMHCIIxIgHMOG mice are unlikely to limit inflammatory demyelinating disease, as we believe that soluble MOG-specific antibody is not essential for EAE development. This is based on our observation that passive transfer of MOG-specific antibodies is not sufficient for disease development in CD19-BMHCII mice [15] and the observation by the Zamvil group that mice in which MOG-specific antibody is tethered to B cells (and therefore incapable of being secreted) are susceptible to EAE [33]. While we did not collect serum from our cohort of 2D2xIgHMOG mice, either with or without MHCII expression restricted to B cells, we have detected MOG-specific IgG in CD19-BMHCIIxIgHMOG mice, suggesting that antibody production in 2D2xCD19-BMHCIIxIgHMOG mice would not be absent or deficient. The lack of spontaneous EAE or optic neuritis in 2D2xCD19-BMHCIIxIgHMOG mice is in agreement with the previously reported limitations for B cells to prime CD4 T cells [22, 34]. On the other hand, B cells can indeed prime naïve T cells as long as valid B cell antigens are delivered to them [35]. Hence, in the EAE model, limitations in B cell APC function may be rooted in restricted access to relevant auto-antigens sequestered in the CNS. Hence, the responsibility for CD4 T cell activation and initiation of demyelination in spontaneous EAE most likely falls on DCs, which have been found to be capable of independently initiating spontaneous optic neuritis [23]. Whether the physical location of DCs in and around the CNS [36, 37] or the intrinsic ability for DCs to prime CD4 T cells [38] bestows this capacity for disease initiation is not apparent. Clearly, these features and others are not mutually exclusive.
Given the limitations for B cell antigen presentation in initiating disease, along with the restrictions in eliciting full recall production of cytokines by MOG-specific CD4 T cells in secondary responses [22], we sought to assess the limitations of B cells in supporting cognate CD4 T cell-mediated CNS autoimmunity after priming during EAE. Using a Tam-inducible MHCII expression system, we now show that antigen presentation by B cells is sufficient to induce EAE signs just as rapidly as EAE induced by a full complement of MHCII+ APCs. This result is contrary to our expectations given the reproducible delay in disease onset when B cells function as the sole APC during EAE [15]. Our findings raise questions regarding what drives accelerated disease onset in CD20-BMHCIIxIgHMOG mice. One critical component of neuro-inflammation worthy of consideration is lymphocyte trafficking. For example, the reduced variability in B cell frequency detected in the CNS of CD20-BMHCIIxIgHMOG mice with EAE induced by Tam administration after T cell transfer (Fig 5B–5D) could be explained by chronological changes in the concentration of chemotactic factors driving lymphocytes to the CNS. This theory could also explain the increasingly rapid onset of EAE symptoms observed for CD20-BMHCIIxIgHMOG mice treated with Tam after CD4 T cell transfer, as immune cells may traffic to, or organize within, the CNS more quickly if chemokines promoting B cell accumulation were up-regulated.
Our data support a model in which non-B cell APCs prime CD4 T cells toward myelin antigens, enabling subsequent cognate interactions with B cell APCs to support auto-reactive B cell proliferation and differentiation. Clinical studies have demonstrated that memory B cells and plasmablasts are the most common B cell subtype in the CSF of patients with MS [39]. This expansion of antigen-specific B cells during CNS autoimmunity could amplify cognate interactions between dysregulated B and CD4 T cells, which in turn could independently drive neuro-inflammation and relapses at later stages of MS. The high frequency of antigen-specific B cells in our EAE system may imitate the prevalence or expansion of B cells in MS patients [40–42]. During an MS remission, previously primed encephalitogenic T cells rest in the periphery (reflected in our EAE model as the period of time prior to Tam treatment). Of note, peripheral B cells from relapsing-remitting MS patients exhibit exaggerated pro-inflammatory cytokine responses that can directly promote T cell activation [43–45]. Increased MHCII expression promotes lymphocyte trafficking to the myelin-containing CNS compartment to initiate plaque formation (mimicked by what we observed soon after Tam treatment). It is possible that BCDTs eliminate pathogenic B cell antigen presentation, reducing germinal center reactions that may be occurring in the periphery and CNS. Studying the interdependency and amplification of pro-inflammatory interactions between dysregulated lymphocytes could lead to more targeted therapeutic interventions that specifically ablate the pathogenic effects of B cells in situ.
The reproducibly rapid onset of EAE that we have demonstrated shows that the time required for B cell trafficking from the periphery to the CNS is not a major contribution to the observed delay in disease onset for CD19-BMHCIIxIgr mice. However, our findings raise questions about where antigen presentation needs to occur in the context of B cell-driven CNS autoimmunity. While germinal center reactions mainly occur in the spleen and lymph nodes, various reports describe formation of functional ectopic lymphoid follicles in the CNS of MS patients and in mice with EAE [46–48]. Exclusion of B cells from the CNS results in reduced severity of EAE induced by rhMOG yet exacerbates EAE induced by MOG35-55 peptide, indicating that access to the CNS is required for EAE mediated by B cell antigen presentation yet can also be important for regulatory B cells to ameliorate disease [49–51]. In contrast, Tesfagiorgis et al. found that antigen-nonspecific B cells are recruited to the CNS compartment while myelin-specific B cells remain in the peripheral draining lymph nodes in a model of active EAE that requires B cells [52]. In our model, the relative infrequency of B cells observed in the CNS of mice that develop EAE may reflect the ease with which MOG-specific B cells capture their soluble protein antigens to present to T cells within the CNS compartment. The formation of ectopic lymphoid follicles in the CNS could create an environment in which very few B cells have ready access to antigen and ample interactions with many cognate encephalitogenic CD4 T cells.
Overall, the results described herein reveal that myelin-specific B cells are not able to support spontaneous EAE, although they have the potential to rapidly induce EAE with similar kinetics as WT APCs. Our new inducible MHCII expression EAE system enables precise control of the timing of cognate interactions between B cells and CD4 T cells, revealing that B cells do not harbor an intrinsic deficit in the promotion of auto-reactive CD4 T cell responses targeting the CNS.
Supporting information
Flow cytometric analysis was used to determine mean +/- SEM absolute number of B cells (A) and CD4 T cells (B) isolated from spinal cords (SC). CD20-BMHCIIxIgHMOG mice, UBCMHCII mice, and Cre-IAßbstopflox/floxxIgHMOG littermate controls harvested at week 1 (filled circles), week 2 (squares), or week 3 (triangles) post CD4 T cell transfer in comparison to lymphocytes in the spinal cords of naïve WT mice (open circles). Graphs show absolute number of donor CD4 T cells for mice harvested prior to Tam treatment and number of endogenous CD4 T cells from WT mice. Data is pooled from CD20-BMHCIIxIgHMOG mice, UBCMHCII mice, and Cre- Cre-IAßbstopflox/floxxIgHMOG littermate controls from 9 different experiments with n = 3–5 mice at each time point prior to Tam treatment. Significance determined by Kruskal-Wallis test with Dunn’s correction for multiple comparisons, with alpha = 0.05.
(TIFF)
Intracellular cytokine expression of IFNγ, IL-17, and GM-CSF by CD4 T cells prior to adoptive transfer is represented by black circles. After ‘resting’ in MHCII-deficient mice for 3 weeks, CD4 T cells were harvested from spleens of UBCMHCII (red squares), CD20-BMHCIIxIgHMOG (blue circles) or CD20Tam-Cre-IAßbstopflox/floxxIgHMOG (white circles) littermates and tested for intracellular cytokine expression. Kruskal-Wallis nonparametric test with Dunn’s correction for multiple comparisons did not identify significant differences in the percentage of T cells expressing various cytokines after incubation in MHCII-deficient mice with different genotypes (p>0.05).
(TIFF)
Acknowledgments
We would like to thank Dr. Amber Salter for assistance with statistical analysis. We would also like to thank Nicholas Jarjour and Sindhu Manivasagam for technical assistance and Drs. Anne H. Cross and Brian T. Edelson for thoughtful feedback and editing of the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was funded by the National Institutes of Health grants and 1R01NS083678 and 1F31NS096824 (NINDS) and R01-AI043603 (NIAID).
References
- 1.Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372(9648):1502–17. Epub 2008/10/31. doi: 10.1016/S0140-6736(08)61620-7 . [DOI] [PubMed] [Google Scholar]
- 2.Steinman L. Optic neuritis, a new variant of experimental encephalomyelitis, a durable model for all seasons, now in its seventieth year. J Exp Med. 2003;197(9):1065–71. doi: 10.1084/jem.20030320 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rangachari M, Kuchroo VK. Using EAE to better understand principles of immune function and autoimmune pathology. J Autoimmun. 2013;45:31–9. doi: 10.1016/j.jaut.2013.06.008 ; PubMed Central PMCID: PMC3963137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goverman J. Autoimmune T cell responses in the central nervous system. Nat Rev Immunol. 2009;9(6):393–407. Epub 2009/05/16. doi: 10.1038/nri2550 ; PubMed Central PMCID: PMC2813731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358(7):676–88. doi: 10.1056/NEJMoa0706383 . [DOI] [PubMed] [Google Scholar]
- 6.Hauser SL, Bar-Or A, Comi G, Giovannoni G, Hartung HP, Hemmer B, et al. Ocrelizumab versus Interferon Beta-1a in Relapsing Multiple Sclerosis. N Engl J Med. 2017;376(3):221–34. doi: 10.1056/NEJMoa1601277 . [DOI] [PubMed] [Google Scholar]
- 7.Bar-Or A, Calabresi PA, Arnold D, Markowitz C, Shafer S, Kasper LH, et al. Rituximab in relapsing-remitting multiple sclerosis: a 72-week, open-label, phase I trial. Ann Neurol. 2008;63(3):395–400. doi: 10.1002/ana.21363 . [DOI] [PubMed] [Google Scholar]
- 8.Petereit HF, Moeller-Hartmann W, Reske D, Rubbert A. Rituximab in a patient with multiple sclerosis—effect on B cells, plasma cells and intrathecal IgG synthesis. Acta Neurol Scand. 2008;117(6):399–403. doi: 10.1111/j.1600-0404.2007.00958.x . [DOI] [PubMed] [Google Scholar]
- 9.Cross AH, Stark JL, Lauber J, Ramsbottom MJ, Lyons JA. Rituximab reduces B cells and T cells in cerebrospinal fluid of multiple sclerosis patients. J Neuroimmunol. 2006;180(1–2):63–70. doi: 10.1016/j.jneuroim.2006.06.029 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li R, Rezk A, Healy LM, Muirhead G, Prat A, Gommerman JL, et al. Cytokine-Defined B Cell Responses as Therapeutic Targets in Multiple Sclerosis. Front Immunol. 2015;6:626 doi: 10.3389/fimmu.2015.00626 ; PubMed Central PMCID: PMC4705194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li R, Rezk A, Miyazaki Y, Hilgenberg E, Touil H, Shen P, et al. Proinflammatory GM-CSF-producing B cells in multiple sclerosis and B cell depletion therapy. Sci Transl Med. 2015;7(310):310ra166. doi: 10.1126/scitranslmed.aab4176 . [DOI] [PubMed] [Google Scholar]
- 12.Yoshizaki A, Miyagaki T, DiLillo DJ, Matsushita T, Horikawa M, Kountikov EI, et al. Regulatory B cells control T-cell autoimmunity through IL-21-dependent cognate interactions. Nature. 2012;491(7423):264–8. doi: 10.1038/nature11501 ; PubMed Central PMCID: PMC3493692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casten LA, Pierce SK. Receptor-mediated B cell antigen processing. Increased antigenicity of a globular protein covalently coupled to antibodies specific for B cell surface structures. J Immunol. 1988;140(2):404–10. . [PubMed] [Google Scholar]
- 14.Lanzavecchia A. Receptor-mediated antigen uptake and its effect on antigen presentation to class II-restricted T lymphocytes. Annu Rev Immunol. 1990;8:773–93. doi: 10.1146/annurev.iy.08.040190.004013 . [DOI] [PubMed] [Google Scholar]
- 15.Parker Harp CR, Archambault AS, Sim J, Ferris ST, Mikesell RJ, Koni PA, et al. B cell antigen presentation is sufficient to drive neuroinflammation in an animal model of multiple sclerosis. J Immunol. 2015;194(11):5077–84. doi: 10.4049/jimmunol.1402236 ; PubMed Central PMCID: PMC4433779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brennan KM, Galban-Horcajo F, Rinaldi S, O'Leary CP, Goodyear CS, Kalna G, et al. Lipid arrays identify myelin-derived lipids and lipid complexes as prominent targets for oligoclonal band antibodies in multiple sclerosis. J Neuroimmunol. 2011;238(1–2):87–95. doi: 10.1016/j.jneuroim.2011.08.002 ; PubMed Central PMCID: PMC3400538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ilyas AA, Chen ZW, Cook SD. Antibodies to sulfatide in cerebrospinal fluid of patients with multiple sclerosis. J Neuroimmunol. 2003;139(1–2):76–80. . [DOI] [PubMed] [Google Scholar]
- 18.Kanter JL, Narayana S, Ho PP, Catz I, Warren KG, Sobel RA, et al. Lipid microarrays identify key mediators of autoimmune brain inflammation. Nat Med. 2006;12(1):138–43. doi: 10.1038/nm1344 . [DOI] [PubMed] [Google Scholar]
- 19.Lambracht-Washington D, O'Connor KC, Cameron EM, Jowdry A, Ward ES, Frohman E, et al. Antigen specificity of clonally expanded and receptor edited cerebrospinal fluid B cells from patients with relapsing remitting MS. J Neuroimmunol. 2007;186(1–2):164–76. doi: 10.1016/j.jneuroim.2007.03.002 ; PubMed Central PMCID: PMC2709235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985;314(6011):537–9. . [DOI] [PubMed] [Google Scholar]
- 21.Khalil AM, Cambier JC, Shlomchik MJ. B cell receptor signal transduction in the GC is short-circuited by high phosphatase activity. Science. 2012;336(6085):1178–81. Epub 2012/05/05. doi: 10.1126/science.1213368 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Archambault AS, Carrero JA, Barnett LG, McGee NG, Sim J, Wright JO, et al. Cutting edge: Conditional MHC class II expression reveals a limited role for B cell antigen presentation in primary and secondary CD4 T cell responses. J Immunol. 2013;191(2):545–50. doi: 10.4049/jimmunol.1201598 ; PubMed Central PMCID: PMC3711531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu GF, Shindler KS, Allenspach EJ, Stephen TL, Thomas HL, Mikesell RJ, et al. Limited sufficiency of antigen presentation by dendritic cells in models of central nervous system autoimmunity. J Autoimmun. 2011;36(1):56–64. Epub 2010/11/26. doi: 10.1016/j.jaut.2010.10.006 ; PubMed Central PMCID: PMC3053076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bettelli E, Baeten D, Jager A, Sobel RA, Kuchroo VK. Myelin oligodendrocyte glycoprotein-specific T and B cells cooperate to induce a Devic-like disease in mice. J Clin Invest. 2006;116(9):2393–402. Epub 2006/09/07. doi: 10.1172/JCI28334 ; PubMed Central PMCID: PMC1555670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krishnamoorthy G, Lassmann H, Wekerle H, Holz A. Spontaneous opticospinal encephalomyelitis in a double-transgenic mouse model of autoimmune T cell/B cell cooperation. J Clin Invest. 2006;116(9):2385–92. Epub 2006/09/07. doi: 10.1172/JCI28330 ; PubMed Central PMCID: PMC1555668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pollinger B, Krishnamoorthy G, Berer K, Lassmann H, Bosl MR, Dunn R, et al. Spontaneous relapsing-remitting EAE in the SJL/J mouse: MOG-reactive transgenic T cells recruit endogenous MOG-specific B cells. J Exp Med. 2009;206(6):1303–16. Epub 2009/06/03. doi: 10.1084/jem.20090299 ; PubMed Central PMCID: PMC2715069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pierson ER, Stromnes IM, Goverman JM. B cells promote induction of experimental autoimmune encephalomyelitis by facilitating reactivation of T cells in the central nervous system. J Immunol. 2014;192(3):929–39. doi: 10.4049/jimmunol.1302171 ; PubMed Central PMCID: PMC3934009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lyons JA, San M, Happ MP, Cross AH. B cells are critical to induction of experimental allergic encephalomyelitis by protein but not by a short encephalitogenic peptide. Eur J Immunol. 1999;29(11):3432–9. doi: 10.1002/(SICI)1521-4141(199911)29:11<3432::AID-IMMU3432>3.0.CO;2-2 . [DOI] [PubMed] [Google Scholar]
- 29.Oliver AR, Lyon GM, Ruddle NH. Rat and human myelin oligodendrocyte glycoproteins induce experimental autoimmune encephalomyelitis by different mechanisms in C57BL/6 mice. J Immunol. 2003;171(1):462–8. . [DOI] [PubMed] [Google Scholar]
- 30.Hjelmstrom P, Juedes AE, Fjell J, Ruddle NH. B-cell-deficient mice develop experimental allergic encephalomyelitis with demyelination after myelin oligodendrocyte glycoprotein sensitization. J Immunol. 1998;161(9):4480–3. . [PubMed] [Google Scholar]
- 31.Wolf SD, Dittel BN, Hardardottir F, Janeway CA Jr., Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. J Exp Med. 1996;184(6):2271–8. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Albouz-Abo S, Wilson JC, Bernard CC, von Itzstein M. A conformational study of the human and rat encephalitogenic myelin oligodendrocyte glycoprotein peptides 35–55. Eur J Biochem. 1997;246(1):59–70. . [DOI] [PubMed] [Google Scholar]
- 33.Molnarfi N, Schulze-Topphoff U, Weber MS, Patarroyo JC, Prod'homme T, Varrin-Doyer M, et al. MHC class II-dependent B cell APC function is required for induction of CNS autoimmunity independent of myelin-specific antibodies. J Exp Med. 2013;210(13):2921–37. doi: 10.1084/jem.20130699 ; PubMed Central PMCID: PMC3865476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barnett LG, Simkins HM, Barnett BE, Korn LL, Johnson AL, Wherry EJ, et al. B cell antigen presentation in the initiation of follicular helper T cell and germinal center differentiation. J Immunol. 2014;192(8):3607–17. doi: 10.4049/jimmunol.1301284 ; PubMed Central PMCID: PMC4380085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shlomchik MJ, Weisel F. Germinal center selection and the development of memory B and plasma cells. Immunol Rev. 2012;247(1):52–63. doi: 10.1111/j.1600-065X.2012.01124.x . [DOI] [PubMed] [Google Scholar]
- 36.Bulloch K, Miller MM, Gal-Toth J, Milner TA, Gottfried-Blackmore A, Waters EM, et al. CD11c/EYFP transgene illuminates a discrete network of dendritic cells within the embryonic, neonatal, adult, and injured mouse brain. J Comp Neurol. 2008;508(5):687–710. doi: 10.1002/cne.21668 . [DOI] [PubMed] [Google Scholar]
- 37.Matyszak MK, Perry VH. Dendritic cells in inflammatory responses in the CNS. Adv Exp Med Biol. 1997;417:295–9. . [DOI] [PubMed] [Google Scholar]
- 38.Steinman RM. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol. 2012;30:1–22. doi: 10.1146/annurev-immunol-100311-102839 . [DOI] [PubMed] [Google Scholar]
- 39.Cepok S, Rosche B, Grummel V, Vogel F, Zhou D, Sayn J, et al. Short-lived plasma blasts are the main B cell effector subset during the course of multiple sclerosis. Brain. 2005;128(Pt 7):1667–76. doi: 10.1093/brain/awh486 . [DOI] [PubMed] [Google Scholar]
- 40.Palanichamy A, Apeltsin L, Kuo TC, Sirota M, Wang S, Pitts SJ, et al. Immunoglobulin class-switched B cells form an active immune axis between CNS and periphery in multiple sclerosis. Sci Transl Med. 2014;6(248):248ra106. doi: 10.1126/scitranslmed.3008930 ; PubMed Central PMCID: PMC4176763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stern JN, Yaari G, Vander Heiden JA, Church G, Donahue WF, Hintzen RQ, et al. B cells populating the multiple sclerosis brain mature in the draining cervical lymph nodes. Sci Transl Med. 2014;6(248):248ra107. doi: 10.1126/scitranslmed.3008879 ; PubMed Central PMCID: PMC4388137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.von Budingen HC, Kuo TC, Sirota M, van Belle CJ, Apeltsin L, Glanville J, et al. B cell exchange across the blood-brain barrier in multiple sclerosis. J Clin Invest. 2012;122(12):4533–43. Epub 2012/11/20. doi: 63842 [pii] doi: 10.1172/JCI63842 ; PubMed Central PMCID: PMC3533544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lisak RP, Nedelkoska L, Benjamins JA, Schalk D, Bealmear B, Touil H, et al. B cells from patients with multiple sclerosis induce cell death via apoptosis in neurons in vitro. J Neuroimmunol. 2017;309:88–99. doi: 10.1016/j.jneuroim.2017.05.004 . [DOI] [PubMed] [Google Scholar]
- 44.Bar-Or A, Fawaz L, Fan B, Darlington PJ, Rieger A, Ghorayeb C, et al. Abnormal B-cell cytokine responses a trigger of T-cell-mediated disease in MS? Ann Neurol. 2010;67(4):452–61. Epub 2010/05/04. doi: 10.1002/ana.21939 . [DOI] [PubMed] [Google Scholar]
- 45.Harp CT, Lovett-Racke AE, Racke MK, Frohman EM, Monson NL. Impact of myelin-specific antigen presenting B cells on T cell activation in multiple sclerosis. Clin Immunol. 2008;128(3):382–91. Epub 2008/07/05. doi: S1521-6616(08)00653-0 [pii] doi: 10.1016/j.clim.2008.05.002 . [DOI] [PubMed] [Google Scholar]
- 46.Kuerten S, Schickel A, Kerkloh C, Recks MS, Addicks K, Ruddle NH, et al. Tertiary lymphoid organ development coincides with determinant spreading of the myelin-specific T cell response. Acta Neuropathol. 2012;124(6):861–73. doi: 10.1007/s00401-012-1023-3 . [DOI] [PubMed] [Google Scholar]
- 47.Peters A, Pitcher LA, Sullivan JM, Mitsdoerffer M, Acton SE, Franz B, et al. Th17 cells induce ectopic lymphoid follicles in central nervous system tissue inflammation. Immunity. 2011;35(6):986–96. Epub 2011/12/20. doi: 10.1016/j.immuni.2011.10.015 ; PubMed Central PMCID: PMC3422678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Magliozzi R, Columba-Cabezas S, Serafini B, Aloisi F. Intracerebral expression of CXCL13 and BAFF is accompanied by formation of lymphoid follicle-like structures in the meninges of mice with relapsing experimental autoimmune encephalomyelitis. J Neuroimmunol. 2004;148(1–2):11–23. Epub 2004/02/21. doi: 10.1016/j.jneuroim.2003.10.056 . [DOI] [PubMed] [Google Scholar]
- 49.Lehmann-Horn K, Sagan SA, Bernard CC, Sobel RA, Zamvil SS. B-cell very late antigen-4 deficiency reduces leukocyte recruitment and susceptibility to central nervous system autoimmunity. Ann Neurol. 2015;77(5):902–8. doi: 10.1002/ana.24387 ; PubMed Central PMCID: PMC4405474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lehmann-Horn K, Sagan SA, Winger RC, Spencer CM, Bernard CC, Sobel RA, et al. CNS accumulation of regulatory B cells is VLA-4-dependent. Neurol Neuroimmunol Neuroinflamm. 2016;3(2):e212 doi: 10.1212/NXI.0000000000000212 ; PubMed Central PMCID: PMC4794810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Glatigny S, Wagner CA, Bettelli E. Cutting Edge: Integrin alpha4 Is Required for Regulatory B Cell Control of Experimental Autoimmune Encephalomyelitis. J Immunol. 2016;196(9):3542–6. doi: 10.4049/jimmunol.1502614 ; PubMed Central PMCID: PMC4868662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tesfagiorgis Y, Zhu SL, Jain R, Kerfoot SM. Activated B Cells Participating in the Anti-Myelin Response Are Excluded from the Inflamed Central Nervous System in a Model of Autoimmunity that Allows for B Cell Recognition of Autoantigen. J Immunol. 2017;199(2):449–57. doi: 10.4049/jimmunol.1602042 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flow cytometric analysis was used to determine mean +/- SEM absolute number of B cells (A) and CD4 T cells (B) isolated from spinal cords (SC). CD20-BMHCIIxIgHMOG mice, UBCMHCII mice, and Cre-IAßbstopflox/floxxIgHMOG littermate controls harvested at week 1 (filled circles), week 2 (squares), or week 3 (triangles) post CD4 T cell transfer in comparison to lymphocytes in the spinal cords of naïve WT mice (open circles). Graphs show absolute number of donor CD4 T cells for mice harvested prior to Tam treatment and number of endogenous CD4 T cells from WT mice. Data is pooled from CD20-BMHCIIxIgHMOG mice, UBCMHCII mice, and Cre- Cre-IAßbstopflox/floxxIgHMOG littermate controls from 9 different experiments with n = 3–5 mice at each time point prior to Tam treatment. Significance determined by Kruskal-Wallis test with Dunn’s correction for multiple comparisons, with alpha = 0.05.
(TIFF)
Intracellular cytokine expression of IFNγ, IL-17, and GM-CSF by CD4 T cells prior to adoptive transfer is represented by black circles. After ‘resting’ in MHCII-deficient mice for 3 weeks, CD4 T cells were harvested from spleens of UBCMHCII (red squares), CD20-BMHCIIxIgHMOG (blue circles) or CD20Tam-Cre-IAßbstopflox/floxxIgHMOG (white circles) littermates and tested for intracellular cytokine expression. Kruskal-Wallis nonparametric test with Dunn’s correction for multiple comparisons did not identify significant differences in the percentage of T cells expressing various cytokines after incubation in MHCII-deficient mice with different genotypes (p>0.05).
(TIFF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.