Abstract
Objectives
The traditional approach to stable blunt thoracic aortic injury (BTAI) endorsed by the Society for Vascular Surgery is early (<24 hours) thoracic endovascular aortic repair (TEVAR). Recently, some studies have shown improved mortality in stable BTAI patients repaired in a delayed manner (≥24 hours). However, the indications for use of delayed TEVAR for BTAI are not well characterized, and its overall impact on patient survival remains poorly understood. We sought to determine if delayed TEVAR is associated with a decrease in mortality compared to early TEVAR in this population.
Methods
We conducted a retrospective cohort study of adult patients with BTAI (ICD-9 diagnosis code 901.0) who underwent TEVAR (ICD-9 procedure code 39.73) from 2009-2013 using the National Sample Program dataset. Missing physiologic data was imputed using chained multiple imputation. Patients were parsed into groups based on the timing of TEVAR (early, <24 hours versus delayed ≥24 hours). Chi-square, Mann-Whitney, and Fisher’s exact tests were used to compare baseline characteristics and outcomes of interest between groups. Multivariable logistic regression for mortality was performed that included all variables significant at P ≤ .2 in univariate analyses.
Results
A total of 2,045 adult patients with BTAI were identified, of whom 534 (26%) underwent TEVAR. Patients with missing data on TEVAR timing were excluded (n=27), leaving a total of 507 patients for analysis (75% male, 69% Caucasian, median age 40 (IQR27-56), median ISS 34 (IQR26-41)). Of these, 378 patients underwent early TEVAR and 129 underwent delayed TEVAR. The two groups were similar with regards to age, sex, race, ISS, and presenting physiology. Mortality was 11.9% in the early TEVAR group versus 5.4% in the delayed group, with the early group displaying a higher odds of death (OR 2.36 (95%CI 1.03-5.36), P = .042). After adjusting for age, ISS, and admission physiology, the association between early TEVAR and mortality was preserved (adjusted OR 2.39 (95%CI 1.01-5.67), P = .047).
Conclusions
Consistent with current Society for Vascular Surgery recommendations, more BTAI patients underwent early TEVAR than delayed TEVAR during the study period. However, delayed TEVAR was associated with significantly reduced mortality in this population. Together, these findings support a need for critical appraisal and clarification of existing practice guidelines in management of BTAI. Future studies should seek to understand this survival disparity and determine optimal patient selection for early versus delayed TEVAR.
Background
Blunt thoracic aortic injury (BTAI) is a rare but serious event with exceedingly high mortality (75-90%).1, 2 While no randomized controlled trials exist, survival and major operative morbidity have improved significantly as thoracic endovascular aortic repair (TEVAR) for BTAI has largely supplanted open aortic repair in most major trauma centers.3–6 Based on this evidence, in 2011 the Society for Vascular Surgery put forth guidelines endorsing early TEVAR as the preferred operative approach in BTAI patients, with delayed TEVAR (defined as repair after the first 24 hours of care) reserved for patients with prohibitive operative risk due to major associated injuries or severe comorbidities.3
In recent years, however, a number of studies have suggested a mortality benefit in stable BTAI patients repaired in a delayed manner.5, 7–10 The latest multicenter trial published by the American Association for the Surgery of Trauma in 2008 showed an increase in the average time from admission to aortic repair from 15 to 55 hours compared to their original trial in 1997, and patients undergoing delayed repair had 65% lower mortality.2, 4 Using this same population, Demetriades et al. showed a strong survival advantage with delayed repair in patients with and without severe associated injuries.5 Other single-institution series also support improved survival and safety with delayed repair, specifically in patients with BTAI and traumatic brain injury (TBI), again with the conceptual underpinning for these findings being that delayed TEVAR allows for stabilization and focused treatment of these associated injuries.9, 10
Despite these findings, the indications for use of delayed TEVAR for BTAI are not well characterized and its overall impact on patient survival remains poorly understood. To address these problems, we examined rates of early versus delayed TEVAR and associated mortality in BTAI patients across a five-year period in a national sample of trauma patients. Our primary hypothesis was that delayed TEVAR would be associated with lower mortality than early TEVAR. We secondarily hypothesized that rates of early TEVAR would be higher than delayed TEVAR over the study period.
Methods
This study was reviewed and approved by the University of Pennsylvania Institutional Review Board. Need for consent of the patient was waived because of the retrospective nature of the study design.
Population
We conducted a retrospective cohort study of adult patients using the National Trauma Data Bank (NTDB) National Sample Program (NSP) from 2009-2013.11 The NSP is based on a nationally representative sample of the NTDB and contains data from 100 Level I and Level II trauma centers in the United States. Inclusion criteria for our study were age ≥18 year and BTAI as indicated by ICD-9 diagnosis code 901.0. Exclusion criteria for this study were age <18 years of age and injury mechanism other than blunt trauma. Patients with BTAI who underwent TEVAR were identified by ICD-9 procedure code 39.73. Patients with missing data on TEVAR timing (n=27) were excluded. Missing physiologic data was imputed using chained multiple imputation.12 To describe the patients in the cohort, we first examine demographics, injury severity, presenting physiology, injury patterns, and procedures by repair type (none, open aortic repair, or TEVAR) across the study period. To characterize injury patterns of patients in the cohort and procedures they underwent, we used ICD-9 diagnosis codes define the following: rib fractures (807.[0–1][0–9]), splenic injury (865.[0–1][0–9]), hepatic injury 864.[0–1][0–9]), and pelvic fractures (808.[0–9][0–9]). We used a head abbreviated injury score (AIS) of ≥ 3 to define traumatic brain injury (TBI). To describe the procedures that the patients in the cohort underwent, we used ICD-9 procedure codes to define the following: craniotomy (1, 1.2[4–5]), thoracostomy tube placement (34.0[1,9]), and laparotomy (54.1[1,2,9]; 41.5; 45.[0–9][0–9]), and long bone fixation (79.3[1,2,5,6]).
Exposure and Outcomes
The primary exposure of this study was the timing of TEVAR. Patients were grouped by the timing of TEVAR, with early TEVAR defined as repair < 24 hours after injury, and delayed TEVAR defined as ≥24 hours after injury, consistent with current nomenclature. The primary outcome measure was in-hospital mortality. As secondary outcome measures, we examined rates of common complications recorded in the NSP dataset (defined here as pre- and post-operative complications occurring in ≥2% of the overall cohort), ventilator days, intensive care unit (ICU) and hospital lengths of stay. We also examined rates of secondary aortic interventions (ICD-9 procedure codes 39.7[3–9]) and rates of subclavian revascularization (ICD-9 procedure code 39.22) between the early and delayed TEVAR groups. We additionally examined trends in incidence of early and delayed TEVAR across the study period. Finally, as an exploratory analysis we examined the frequency of early TEVAR by trauma center for each of the centers contributing patients to the study.
Statistical analysis
We examined the association between TEVAR timing and in-hospital mortality using multivariable logistic regression models. Variables assessed for inclusion into multivariable mortality models included age, race, sex, motor component of the Glasgow Coma Scale (GCS) score on arrival, Injury Severity Score (ISS), admission systolic blood pressure, and admission heart rate. Variables associated with mortality significant at P ≤ .2 in univariate analyses were included in multivariable logistic regression models using forced entry. Model discrimination and calibration for the final model were assessed using ROC curves and Hosmer-Lemeshow test, respectively. Differences in rates of complications between early versus delayed TEVAR groups were assessed using Chi-square or Fisher’s exact tests as appropriate, while differences in ventilator days, ICU and hospital lengths of stay between groups were assessed using Kruskal-Wallis test. To evaluate temporal trends in incidence of early versus delayed repair in the TEVAR cohort over the study period, we used a variation of the Cochran-Armitage nonparametric tests of trend. Significance was defined as a P value <.05. All analyses were conducted using Stata (version 14.1, 2015; StataCorp, College Station, TX).
Results
In total, 2,045 adult patients presenting with BTAI were identified over the five-year study period. Of these, 534 (26%) underwent TEVAR. Among the remaining 1,511 BTAI patients who did not undergo TEVAR, 101 underwent open aortic repair (OAR), and 1410 underwent conservative management on their initial inpatient stay (Figure 1). Twenty-seven patients with missing data on timing of TEVAR were excluded, leaving a total of 507 patients for the primary analysis.
Figure I.
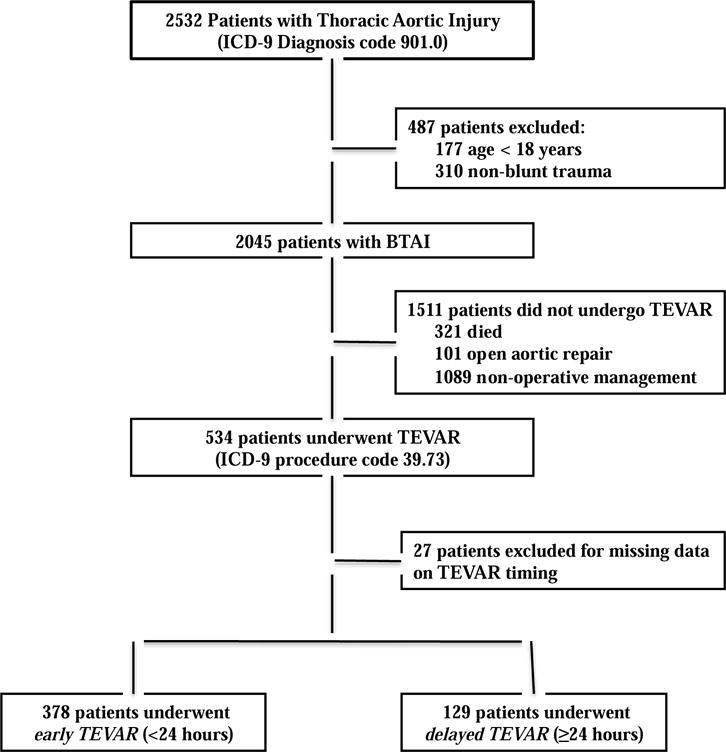
Study design flowchart.
Patient characteristics
The characteristics of patients in the study by type of aortic repair can be seen in Table I. There were significant differences between patients who had no repair versus open aortic repair (OAR) versus TEVAR. Patients who had no repair tended to be older than patients undergoing OAR or TEVAR (median ages 47 (IQR 31-61.0%) vs. 43 (IQR 28-59) vs. 40.5 (27-56) respectively; P<.001) and also had higher rates of specific injuries, most notably TBI (38.7% vs. 31.7% in the OAR group vs. 26.0% in the TEVAR group; P<.001). Conversely, rates of long bone fixation were lowest in the group that received no repair compared to the OAR group and the TEVAR group (11.9% vs. 26.7% vs. 32.8%, respectively; P<.001). Mortality was 20.9% for the no intervention group, 25.7% for the OAR group, and 10.1% for the TEVAR group (P<.001).
Table I.
Characteristics of patients in the cohort by aortic repair type. Data for nonparametric continuous variables expressed as median (Interquartile Range); categorical values expressed as n (%). P values are for Kruskal-Wallis test for nonparametric continuous variables and Chi square test for categorical variables.
| None (n =1410) |
Repair Type Open (n =101) |
TEVAR (n = 534) |
P | |
|---|---|---|---|---|
| Age | 47 (IQR 31-61.0) | 43 (IQR 28-59) | 40.5 (27-56) | <0.001 |
| Race | ||||
| Caucasian | 947 (67.2%) | 69 (68.3%) | 373 (69.9%) | 0.46 |
| African American | 234 (16.6%) | 12 (11.9%) | 76 (14.2%) | |
| Asian | 31 (2.2%) | 1 (1.0%) | 14 (2.6%) | |
| Other | 198 (14.0%) | 19 (18.8%) | 71 (13.3%) | |
| Male | 1009 (71.6%) | 76 (75.2%) | 400 (74.9%) | 0.57 |
| Motor GCS<6 | 783 (55.5%) | 38 (37.6%) | 182 (34.1%) | <0.001 |
| SBP < 90mmHg | 521 (37.0%) | 21 (20.8%) | 72 (13.5%) | <0.001 |
| Pulse>120 BPM | 296 (21.0%) | 27 (26.7%) | 111 (20.8%) | 0.38 |
| ISS | 35 (IQR 29-48) | 38 (IQR 29-48) | 34 (IQR 26-41) | <0.001 |
| Traumatic Brain Injury | 545 (38.7%) | 32 (31.7%) | 139 (26.0%) | <0.001 |
| Craniotomy | 10 (0.7%) | 0 (0.0%) | 5 (0.9%) | 0.59 |
| Rib Fractures | 1079 (76.5%) | 75 (74.3%) | 379 (71.0%) | 0.04 |
| Thoracostomy Tube | 530 (37.6%) | 64 (63.4%) | 216 (40.4%) | <0.001 |
| Splenic Injury | 359 (25.5%) | 28 (27.7%) | 138 (25.8%) | 0.88 |
| Hepatic Injury | 434 (30.8%) | 22 (21.8%) | 163 (30.5%) | 0.16 |
| Laparotomy | 240 (17.0%) | 30 (29.7%) | 100 (18.7%) | 0.01 |
| Pelvic Fractures | 465 (33.0%) | 35 (34.7%) | 198 (37.1%) | 0.23 |
| Long Bone Fixation | 168 (11.9%) | 27 (26.7%) | 175 (32.8%) | <0.001 |
| Mortality | 295 (20.9%) | 26 (25.7%) | 54 (10.1%) | <0.001 |
| ICU days | 3 (IQR 0-10) | 12 (IQR 4-21) | 9 (IQR 4-18) | <0.001 |
| Ventilator days | 1 (IQR 0-7) | 9 (IQR 2-16) | 9 (IQR 3-17) | <0.001 |
| Length of Stay, days | 3 (IQR 1-15) | 16 (IQR 3-27) | 17 (IQR 8-27) | 0.001 |
| Any Complication | 920 (65.3%) | 73 (72.3%) | 387 (72.5%) | 0.01 |
Abbreviations: GCS = Glasgow Coma Scale; SBP = Systolic Blood Pressure; BPM = Beats Per Minute.
Demographic characteristics, injury severity, physiology, injury patterns, and procedural interventions of BTAI patients undergoing early versus delayed TEVAR are shown in Table II. Rates of missingness for physiologic data were quite low in the overall cohort and in the TEVAR group, ranging from 3.6-4%. Missingness was non-differential between the early and delayed groups. Overall, the TEVAR cohort was 75% male and 69% Caucasian with a median age of 40 years (IQR 27-56) and a median ISS of 34 (IQR 26-41). Of these, 378 (74.6%) underwent early repair (<24 hours) and 129 (25.4%) underwent delayed repair (≥24 hours). The median time to repair in the early TEVAR group was 5 (IQR 4-11) hours, compared to 55 (IQR 36-102) hours in the delayed group. The two groups were similar with regards to age, sex, race, ISS, and presenting physiology. Rates of specific injuries and procedures were very similar between the two groups with the exception of the rate of TBI, which was higher in the delayed group (38.8% vs. 22.5%, P<.001).
Table II.
Characteristics of patients by early vs. delayed TEVAR. Data for nonparametric continuous variables expressed as median (Interquartile Range); categorical values expressed as n (%). P values are for Kruskal-Wallis test for nonparametric continuous variables and Chi square test for categorical variables.
| N = 507 | n=378 | n=129 | P | |
|---|---|---|---|---|
| Age | 40 (IQR 27–56) | 40 (IQR 27–56) | 42 (IQR26–58) | 0.14 |
| Race | ||||
| Caucasian | 351 (69.2%) | 261 (69.0%) | 90 (69.8%) | 0.66 |
| African American | 75 (14.8%) | 56 (14.8%) | 19 (14.7%) | |
| Asian | 13 (2.6%) | 8 (2.1%) | 5 (3.9%) | |
| Other | 68 (13.4%) | 53 (14.0%) | 15 (11.6%) | |
| Male | 381 (75.1%) | 285 (75.4%) | 96 (74.4%) | 0.82 |
| Motor GCS<6 | 174 (34.3%) | 123 (32.5%) | 51 (39.5%) | 0.15 |
| SBP < 90mmHg | 68 (13.4%) | 49 (13.0%) | 19 (14.7%) | 0.61 |
| Pulse>120 BPM | 107 (21.1%) | 82 (21.7%) | 25 (19.4%) | 0.58 |
| ISS | 34 (IQR 26-41) | 34 (IQR 26-41) | 33 (IQR 26-41) | 0.63 |
| Traumatic Brain Injury | 135 (26.6%) | 85 (22.5%) | 50 (38.8%) | <0.001 |
| Craniotomy | 5 (1.0%) | 2 (0.5%) | 3 (2.3%) | 0.08 |
| Rib Fractures | 384 (75.7%) | 264 (69.8%) | 95 (73.6%) | 0.41 |
| Thoracostomy Tube | 206 (40.6%) | 160 (42.3%) | 46 (35.7%) | 0.18 |
| Splenic Injury | 131 (25.8%) | 97 (25.7%) | 34 (26.4%) | 0.88 |
| Hepatic Injury | 151 (29.8%) | 114 (30.2%) | 37 (28.7%) | 0.75 |
| Laparotomy | 97 (19.1%) | 69 (18.3%) | 28 (21.7%) | 0.39 |
| Pelvic Fractures | 184 (36.3%) | 135 (35.7%) | 49 (38.0%) | 0.64 |
| Long Bone Fixation | 163 (32.1%) | 117 (31.0%) | 46 (35.7%) | 0.32 |
| Mortality | 52 (10.3%) | 45 (11.9%) | 7 (5.4%) | 0.04 |
| Length of stay, days | 17 (IQR 8-27) | 15 (IQR 8-26) | 20 (IQR 11-32) | <0.001 |
Abbreviations: GCS = Glasgow Coma Scale; SBP = Systolic Blood Pressure; BPM = Beats Per Minute.
In-hospital mortality
In patients undergoing TEVAR, 52/507 patients died for an overall mortality of 10.3%. Observed mortality was 11.9% in the early TEVAR group versus 5.4% in the delayed group, for an unadjusted odds ratio of 2.36 (95%CI 1.03-5.36), P=.042) for the association between early TEVAR and mortality. Variables associated with mortality with P < .2 in univariate logistic regression analysis included age, ISS, GCS motor, and admission SBP <90mmHg and TBI. Although TBI was associated with mortality, because of concerns for co-linearity with ISS and model parsimony, this variable was not included in our final multivariable model. After controlling for age, ISS, GCS motor and admission SBP <90mmHg in multivariable logistic regression analysis, the association between early TEVAR and mortality was preserved (adjusted OR 2.39 (95%CI 1.01-5.67, P=.047)) (Table III). The discrimination of this model was good (AUC 0.79, 95% CI (0.72-0.85)) as was the calibration (Hosmer-Lemeshow Chi-square=3.64, P=.96).
Table III.
Univariable (A) and multivariable (B) logistic regression analyses of potential risk factors for mortality. Factors significant at p ≤ 0.2 in univariate analyses (age, ISS, and admission GCS motor score) were included in the final multivariable regression model.
| A Univariate Logistic Regression | |||
|---|---|---|---|
| Variable | OR | 95% CI | P |
| Early TEVAR | 2.36 | (1.03 – 5.36) | 0.042 |
| Age, per year Race | 1.04 | (1.03 – 1.06) | <0.001 |
| Caucasian | ref | ||
| African American | 0.63 | (0.24 – 1.65) | 0.343 |
| Asian | 2.63 | (0.69 – 9.98) | 0.157 |
| Other | 1.17 | (0.52 – 2.63) | 0.711 |
| Female sex | 1.26 | (0.66 – 2.38) | 0.482 |
| ISS, per point | 1.04 | (1.02 – 1.07) | 0.002 |
| GCS motor | 0.85 | (0.75 – 0.97) | 0.017 |
| SBP < 90mmHg | 2.14 | (1.06 – 4.32) | 0.034 |
| HR> 120 BPM | 1.14 | (0.57 – 2.25) | 0.713 |
| B Multivariate Logistic Regression | |||
| Variable | OR | 95% CI | P |
|
| |||
| Early TEVAR | 2.39 | (1.01 – 5.67) | 0.047 |
| Age, per year | 1.05 | (1.03 – 1.07) | <0.001 |
| ISS, per point | 1.04 | (1.01 – 1.07) | 0.005 |
| GCS motor | 0.89 | (0.77 – 1.04) | 0.151 |
| SBP < 90mmHg | 1.95 | (0.87 – 4.37) | 0.104 |
Abbreviations: ISS = Injury Severity Score; GCS = Glasgow Coma Scale; SBP = Systolic Blood Pressure; HR = Heart Rate; OR = Odds Ration; CI = Confidence interval.
Complications and Resource Utilization
Overall, the incidence of complications was 50% in patients undergoing TEVAR. The rate tended to be higher in the delayed versus early TEVAR group, but this did not reach statistical significance (47% vs. 57%, P=.07). In examining rates of specific complications, we found that rates of bleeding, decubitus ulcer, superficial surgical site infection, and pneumonia were significantly higher in the delayed group (Table IV). Patients undergoing early TEVAR had fewer hospital days (median 15 (IQR 8-26) vs. 20 (IQR 11-32) P<.001), ICU days (8 (IQR 4-17) vs. 13 (IQR 6-23), P<.001), and ventilator days (median 8 (IQR 3-16) vs. 12 (IQR 5-21), P=.001) compared to the delayed TEVAR group. The overall rate of secondary aortic interventions was low in patients undergoing TEVAR (10/507, 2%) but was significantly lower in those undergoing early repair (4/374 (1.1%) vs. 6/129 (4.7%), P=.02). The rate of subclavian revascularization in patients undergoing TEVAR was 1.8% and did not differ significantly between early and delayed groups.
Table IV.
Incidence of complications in patients undergoing early versus delayed TEVAR, stratified by number of complications per patient. Data is expressed as n (%). Chi square test or Fisher’s exact test (for comparisons with cell counts <5) was used to compare incidence of complications between groups.
| Early TEVAR n=378 |
Delayed TEVAR n=129 |
P | |
|---|---|---|---|
|
| |||
| AKI | 26 (6.9%) | 10 (7.8%) | 0.74 |
| ARDS | 53 (14.0%) | 23 (17.8%) | 0.30 |
| Bleeding | 3 (0.8%) | 6 (4.7%) | 0.01* |
| Cardiac Arrest | 25 (6.6%) | 4 (3.1%) | 0.14 |
| CVA | 13 (3.4%) | 7 (5.4%) | 0.32 |
| Decubitus Ulcer | 21 (5.6%) | 14 (10.9%) | 0.04* |
| DVT | 33 (8.7%) | 9 (7.0%) | 0.53 |
| Graft failure | 0 (0.0%) | 2 (1.6%) | 0.06 |
| Organ Space SSI | 11 (2.9%) | 1 (0.8) | 0.31 |
| Superficial SSI | 6 (1.6%) | 6 (4.7%) | 0.05* |
| Severe Sepsis | 11 (2.9%) | 1 (0.8) | 0.31 |
| Unplanned Intubation | 13 (3.4%) | 4 (3.1%) | 1.00 |
| Pneumonia | 74 (19.8%) | 40 (31.0%) | 0.01* |
| Pulmonary Embolism | 15 (4.0%) | 7 (5.4%) | 0.48 |
| UTI | 16 (4.2%) | 11 (8.5%) | 0.06 |
| Any Complication | 179 (47.4%) | 73 (56.6%) | 0.07 |
| Total Complications | 0 (IQR 0-2) | 1 (IQR 0-2) | 0.07 |
Abbreviations: AKI = Acute Kidney Injury; ARDS = Adult Respiratory Distress Syndrome; CVA = Cerebrovascular Accident; DVT = Deep Venous Thrombosis; SSI = Surgical Site Infection; UTI = Urinary Tract Infection.
Rates of early and delayed TEVAR over time and by center
The incidence of early repair among BTAI patients undergoing TEVAR was 77.3% in 2009 and 71.1% in 2013. However, there was no significant trend across the study period (P=.31; Figure 2). The 509 TEVARS in our dataset were performed at 71 trauma centers. Center volume ranged between 1-31 TEVARs per center, while rates of early TEVAR ranged between 0 and 100% (Figure 3).
Figure II.
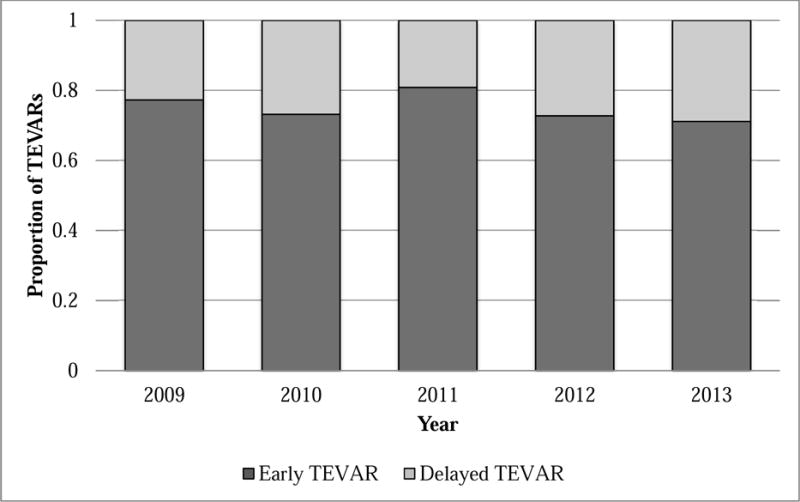
Incidence of early TEVAR for BTAI by year over the study period. A variation of the Cochran-Armitage non-parametric tests of trend was used to evaluate temporal trends in incidence of early TEVAR (P=.37).
Figure III.
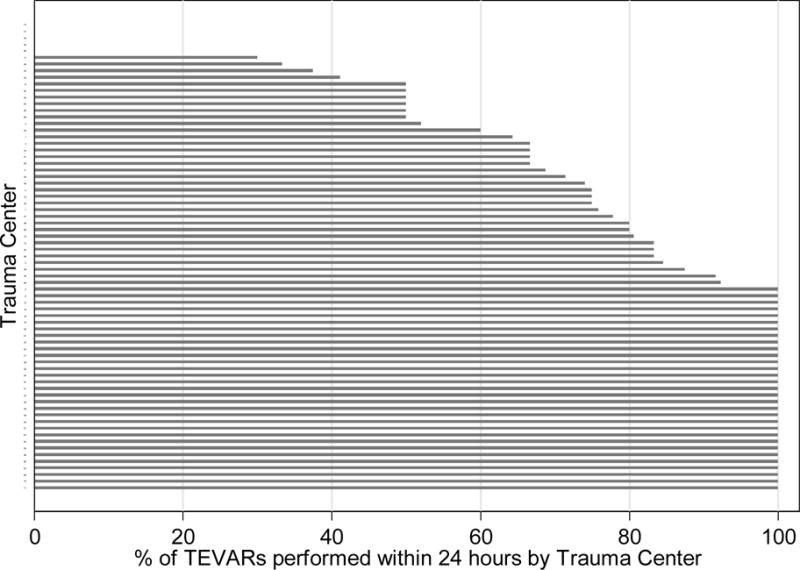
Percentage of early vs. delayed TEVAR at the 71 trauma centers in the cohort. Percentage of early TEVARs ranged from 0 to 100%.
Discussion
In this study, we examined rates of early versus delayed TEVAR and associated mortality in BTAI patients across a five-year period in a national sample of trauma patients. We found that the majority of BTAI patients undergoing TEVAR received an early repair and that the rate of early repair was stable throughout the study period. Further, we showed that BTAI patients undergoing early repair had lower rates of bleeding, decubitus ulcer, superficial surgical site infection, and pneumonia as well as fewer hospital, ICU, and ventilator days. However, these patients still had a significantly higher odds of mortality compared to those undergoing delayed repair. These findings suggest that current practice guidelines be reviewed to reflect consideration of delayed TEVAR in a select subset of patients.
The current Society for Vascular Surgery guidelines endorse early TEVAR as the preferred operative approach in stable BTAI patients, with delayed TEVAR reserved for patients with major associated injuries or severe comorbidities.3 In keeping with these recommendations, our study showed that the majority of BTAI patients undergoing TEVAR between the years of 2009 to 2013 did so within 24 hours of admission. Our findings are consistent with data from other studies placing the percentage of patients with BTAI who undergo TEVAR between 4 – 65%.10, 13–18 We also demonstrate a continued role for conservative management in a subset of patients with BTAI, as over half of adult patients in our cohort were managed non-operatively.
Despite the prevalence of early TEVAR, several recent studies have shown that delayed TEVAR may be associated with improved survival in BTAI patients, even those without severe polytrauma. As evidenced by our findings, these studies have yet to inspire a national change to our standard of care for BTAI. There are several possible explanations for this. First, these studies do not characterize contemporary practice patterns with regards to timing of TEVAR across various trauma centers, which limits our ability to identify major obstacles to effecting widespread change in temporal management of BTAI. Further, some of the earlier studies lack sufficient power to generate meaningful recommendations. Our project addresses these challenges by providing an improved understanding of how TEVAR for BTAI is approached nationwide and how time of repair impacts mortality. Unlike other studies to date, we show that most BTAI patients undergoing TEVAR in this country still receive an early repair. We also show significantly reduced mortality with delayed TEVAR in this population. This important finding strengthens work presented in existing literature and calls into question our traditional understanding that BTAI requires early TEVAR. Our findings may suggest some flexibility in the timing of TEVAR for BTAI, which may help to minimize conflicts in medical management and enable aortic repair under optimized circumstances.
Due to the design of our study, we are unable to definitively say what accounts for the difference in timing between the early and delayed TEVAR groups. Given the center-level variability in rates of early TEVAR, it is likely that center and provider factors play a role, but it is also probable that patient factors contribute. While we did not see many differences in patient demographics, admission physiology, or injury severity between the early and delayed TEVAR groups, we did note that rates of TBI were higher in the delayed group. Since TEVAR is often performed under systemic anticoagulation, which is generally contraindicated in TBI, it is plausible that this could account for some of the delay between the groups, but definitive answers to this question will require prospective study.
Few studies have examined non-fatal outcomes in patients undergoing early versus delayed TEVAR in BTAI. The largest existing study in BTAI patients undergoing TEVAR (n=115) showed similar complication rates between early and delayed repair groups; however delayed repair was associated with significantly longer ICU and hospital lengths of stay.5 With our cohort, we reaffirmed that BTAI patients undergoing delayed TEVAR have longer ICU and hospital stays, but we also showed that delayed TEVAR patients have increased ventilator days and higher rates of bleeding, decubitus ulcer, superficial surgical site infection, and pneumonia. Thus, our study provides novel insight into the course of BTAI patients undergoing early versus delayed TEVAR. While patients undergoing delayed TEVAR have improved mortality, they also experience higher rates of other potentially detrimental outcomes, suggesting a trade-off function between death and complication rates with respect to the timing of TEVAR.
Strengths of our study include scope of information and sample size. Our study was designed to examine epidemiologic trends in timing of TEVAR for BTAI and associated outcomes. Use of the NSP dataset allowed us to examine nationwide rates of early and delayed TEVAR for BTAI over time and compare mortality rates across these two groups. Given the breadth of this nationally representative cohort, we believe that our findings are relevant for the management of most BTAI patients seen by level I and II trauma centers across the United States. Importantly, BTAI is a very rare injury, and many existing datasets do not have a sufficient sample size to generate meaningful findings. The large NSP dataset, however, has untapped potential for studies on vascular trauma. This dataset afforded us with the largest BTAI population studied to date and enabled us to demonstrate a significant decrease in mortality with delayed TEVAR.
We acknowledge several limitations to this work. Although use of the NSP allows for a much larger sample size than has been achieved with previous single and multicenter studies, this dataset only records information pertaining to the initial hospital stay after injury and does not include data beyond discharge. We are therefore unable to describe the long-term outcomes including mortality repair beyond the initial hospital admission. Other variables which may have been informative to explore between the early and delayed groups (such as transfusion requirements) are also not collected by the NSP. Although we are able to describe differences in rates of complications captured by the NSP between the early and delayed TEVAR groups, there are complications specific to TEVAR that are not captured by the NSP. As such, we are not able to describe rates of access site complications, paraplegia, paraparesis, or left subclavian artery coverage between the two groups. However, because the exposure of interest is the timing of the TEVAR and not technique itself, we have no a priori reason to suspect differential rates of these occurrences between the two groups. As is common in large trauma databases, data on admission physiology were missing at rates of up to 4%. However, we were able to partially mitigate the issue of bias introduced by case-wise deletion by imputing missing physiologic data using chained multiple imputation.
Second, we do not have information on the grades of aortic injury of the patients in our study. This data is unfortunately not collected by the NSP, and as a result we were unable to control for injury grade in evaluating rates of early versus delayed repair and associated mortality. This is an important limitation of this study, as aortic injury grade has been shown to predict overall mortality and aortic-related mortality in BTAI.17, 19, 20 It is therefore possible that our findings reflect confounding by indication, or the phenomenon in which higher-grade injuries are associated with both our primary outcome of mortality and the exposure of early repair.16 While our data do not permit us to rule this out, prior studies have demonstrated improved survival with delayed repair even in BTAI patients with high-grade aortic injuries.5, 21 One study even suggests that a subset of patients with grade III aortic injury can be safely managed non-operatively.22 In addition, adjusting for ISS in our multivariable model may have at least partially mitigated this issue given that higher velocity injuries are associated with more multisystem trauma.23 Finally, we are not able to distinguish cause-specific mortality in the NSP cohort, specifically mortality attributable to BTAI versus other causes. As a result, BTAI patients who suffer fatal aortic rupture prior to TEVAR are not captured in our cohort and thus are not accounted for in our mortality analysis comparing early versus delayed repair. Despite this potential limitation, numerous studies including the most recent AAST multicenter study on BTAI demonstrate that the risk of in-hospital aortic rupture while awaiting aortic repair is actually exceedingly low with appropriate blood pressure control.14, 24, 25
In summary, our study shows that most BTAI patients undergoing TEVAR from 2009 to 2013 received an early repair. However, delayed TEVAR was associated with reduced mortality in this population, even after adjusting for the degree of concomitant injuries. These findings support a need for critical reappraisal and clarification of existing practice guidelines in management of BTAI. A recent study has already proposed a new classification system and treatment strategy for BTAI, recommending that only SVS grade 4 injury (free aortic rupture) requires emergency TEVAR while grade 3 injury (external contour abnormality or intimal tear >10mm) can be managed with semi-elective TEVAR and grade 1 and 2 injury (external contour abnormality or intimal tear <10mm) managed nonoperatively.16 Future studies should seek to understand the decrease in mortality seen with delayed TEVAR and determine optimal patient selection for early versus delayed repair. Additionally, further examination of patient and institutional factors associated with early versus delayed TEVAR in BTAI will help improve our understanding of current practice patterns and identify potential barriers to optimal care, knowledge that is currently lacking and that promises to yield novel insights into how to best improve operative management of these injuries.
Conclusions
Consistent with current Society for Vascular Surgery recommendations, more BTAI patients underwent early TEVAR than delayed TEVAR during the study period. Patients undergoing delayed TEVAR had higher rates of specific complications and increased resource utilization, but lower rates of risk-adjusted mortality. Existing practice guidelines in management of BTAI should be re-appraised and clarified. Future studies should seek to understand the decrease in mortality seen with delayed TEVAR and determine optimal patient selection for early versus delayed TEVAR.
Acknowledgments
This project was supported by Award Number K08 131995 from the National Heart, Lung, and Blood Institute (NHLBI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This study was presented in the Poster Competition at the 2017 Vascular Annual Meeting of the Society for Vascular Surgery, San Diego, California, May 31-June 3, 2017.
Author Contributions
Conception and design: CM, DH
Analysis and interpretation: CM, RD, YH, WY, GW, DH
Data collection: Not applicable
Writing the article: CM
Critical revision of the article: CM, RD, YH, WY, GW, DH
Final approval of the article: CM, RD, YH, WY, GW, DH
Statistical analysis: CM, YH, WY, DH
Obtained funding: Not applicable
Overall responsibility: DH
Disclosure:
Conflicts of interest: The authors report no proprietary or commercial interest in any product or concept discussed in this paper.
References
- 1.Yamane BH, Tefera G, Hoch JR, Turnipseed WD, Acher CW. Blunt thoracic aortic injury: Open or stent graft repair? Surgery. 2008;144(4):575–82. doi: 10.1016/j.surg.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 2.Fabian TC, Richardson JD, Croce MA, Smith JS, Jr, Rodman G, Jr, Kearney PA, et al. Prospective study of blunt aortic injury: Multicenter trial of the American Association for the Surgery of Trauma. Journal of Trauma - Injury, Infection and Critical Care. 1997;42(3):374–82. doi: 10.1097/00005373-199703000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Lee WA, Matsumura JS, Mitchell RS, Farber MA, Greenberg RK, Azizzadeh A, et al. Endovascular repair of traumatic thoracic aortic injury: Clinical practice guidelines of the Society for Vascular Surgery. J Vasc Surg. 2011;53(1):187–92. doi: 10.1016/j.jvs.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 4.Demetriades D, Velmahos GC, Scalea TM, Jurkovich GJ, Karmy-Jones R, Teixeira PG, et al. Diagnosis and treatment of blunt thoracic aortic injuries: Changing perspectives. Journal of Trauma - Injury, Infection and Critical Care. 2008;64(6):1415–9. doi: 10.1097/TA.0b013e3181715e32. [DOI] [PubMed] [Google Scholar]
- 5.Demetriades D, Velmahos GC, Scalea TM, Jurkovich GJ, Karmy-Jones R, Teixeira PG, et al. Blunt traumatic thoracic aortic injuries: Early or delayed repair - Results of an American Association for the Surgery of Trauma prospective study. Journal of Trauma - Injury, Infection and Critical Care. 2009;66(4):967–73. doi: 10.1097/TA.0b013e31817dc483. [DOI] [PubMed] [Google Scholar]
- 6.Pang D, Hildebrand D, Bachoo P. Thoracic endovascular repair (TEVAR) versus open surgery for blunt traumatic thoracic aortic injury. The Cochrane database of systematic reviews. 2015;9:CD006642. doi: 10.1002/14651858.CD006642.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Langanay T, Verhoye J-P, Corbineau H, Agnino A, Derieux T, Menestret P, et al. Surgical treatment of acute traumatic rupture of the thoracic aorta a timing reappraisal? Eur J Cardiothorac Surg. 2002;21(2):282–7. doi: 10.1016/s1010-7940(01)01133-2. [DOI] [PubMed] [Google Scholar]
- 8.Duwayri Y, Abbas J, Cerilli G, Chan E, Nazzal M. Outcome after thoracic aortic injury: experience in a level-1 trauma center. Ann Vasc Surg. 2008;22(3):309–13. doi: 10.1016/j.avsg.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Di Eusanio M, Folesani G, Berretta P, Petridis FD, Pantaleo A, Russo V, et al. Delayed management of blunt traumatic aortic injury: Open surgical versus endovascular repair. Ann Thorac Surg. 2013;95(5):1591–7. doi: 10.1016/j.athoracsur.2013.02.033. [DOI] [PubMed] [Google Scholar]
- 10.Estrera AL, Miller CC, III, Guajardo-Salinas G, Coogan S, Charlton-Ouw K, Safi HJ, et al. Update on blunt thoracic aortic injury: Fifteen-year single-institution experience. J Thorac Cardiovasc Surg. 2013;145(3 SUPPL):S154–S8. doi: 10.1016/j.jtcvs.2012.11.074. [DOI] [PubMed] [Google Scholar]
- 11.National Trauma Data Bank Version NSP 2009–2013. Committee on Trauma American College of Surgeons; Chicago, IL: 2016. [Google Scholar]
- 12.Royston P, White IR. Multiple imputation by chained equations (MICE): implementation in Stata. Journal of Statistical Software. 2011;45(4):1–20. [Google Scholar]
- 13.Arthurs ZM, Starnes BW, Sohn VY, Singh N, Martin MJ, Andersen CA. Functional and survival outcomes in traumatic blunt thoracic aortic injuries: An analysis of the National Trauma Databank. J Vasc Surg. 2009;49(4):988–94. doi: 10.1016/j.jvs.2008.11.052. [DOI] [PubMed] [Google Scholar]
- 14.Demetriades D, Velmahos GC, Scalea TM, Jurkovich GJ, Karmy-Jones R, Teixeira PG, et al. Operative repair or endovascular stent graft in blunt traumatic thoracic aortic injuries: Results of an American Association for the Surgery of Trauma multicenter study. Journal of Trauma - Injury, Infection and Critical Care. 2008;64(3):561–70. doi: 10.1097/TA.0b013e3181641bb3. [DOI] [PubMed] [Google Scholar]
- 15.Starnes BW, Lundgren RS, Gunn M, Quade S, Hatsukami TS, Tran NT, et al. A new classification scheme for treating blunt aortic injury. J Vasc Surg. 2012;55(1):47–54. doi: 10.1016/j.jvs.2011.07.073. [DOI] [PubMed] [Google Scholar]
- 16.Heneghan RE, Aarabi S, Quiroga E, Gunn ML, Singh N, Starnes BW. Call for a new classification system and treatment strategy in blunt aortic injury. J Vasc Surg. 2016;64(1):171–6. doi: 10.1016/j.jvs.2016.02.047. [DOI] [PubMed] [Google Scholar]
- 17.DuBose JJ, Leake SS, Brenner M, Pasley J, O’Callaghan T, Luo-Owen X, et al. Contemporary management and outcomes of blunt thoracic aortic injury: a multicenter retrospective study. journal of trauma and acute care surgery. 2015;78(2):360–9. doi: 10.1097/TA.0000000000000521. [DOI] [PubMed] [Google Scholar]
- 18.Shackford SR, Dunne CE, Karmy-Jones R, Long W, III, Teso D, Schreiber MA, et al. The Evolution of Care Improves Outcome in Blunt Thoracic Aortic Injury: A Western Trauma Association Multicenter Study. Journal of Trauma and Acute Care Surgery. 2017 doi: 10.1097/TA.0000000000001555. [DOI] [PubMed] [Google Scholar]
- 19.Fortuna GR, Perlick A, DuBose JJ, Leake SS, Charlton-Ouw KM, Miller CC, et al. Injury grade is a predictor of aortic-related death among patients with blunt thoracic aortic injury. J Vasc Surg. 2016;63(5):1225–31. doi: 10.1016/j.jvs.2015.11.046. [DOI] [PubMed] [Google Scholar]
- 20.Gombert A, Barbati ME, Storck M, Kotelis D, Keschenau P, Pape H-C, et al. Treatment of blunt thoracic aortic injury in Germany—Assessment of the TraumaRegister DGU®. PLoS One. 2017;12(3):e0171837. doi: 10.1371/journal.pone.0171837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smeds MR, Wright MP, Eidt JF, Moursi MM, Escobar GA, Spencer HJ, et al. Delayed management of Grade III blunt aortic injury: Series from a Level I trauma center. Journal of Trauma and Acute Care Surgery. 2016;80(6):947–51. doi: 10.1097/TA.0000000000001027. [DOI] [PubMed] [Google Scholar]
- 22.Gandhi SS, Blas JV, Lee S, Eidt JF, Carsten CG. Nonoperative management of grade III blunt thoracic aortic injuries. J Vasc Surg. 2016;64(6):1580–6. doi: 10.1016/j.jvs.2016.05.070. [DOI] [PubMed] [Google Scholar]
- 23.Staff T, Eken T, Wik L, Røislien J, Søvik S. Physiologic, demographic and mechanistic factors predicting New Injury Severity Score (NISS) in motor vehicle accident victims. Injury. 2014;45(1):9–15. doi: 10.1016/j.injury.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Fabian TC, Davis KA, Gavant ML, Croce MA, Melton SM, Patton JH, Jr, et al. Prospective study of blunt aortic injury: helical CT is diagnostic and antihypertensive therapy reduces rupture. Ann Surg. 1998;227(5):666. doi: 10.1097/00000658-199805000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holmes JH, Bloch RD, Hall RA, Carter YM, Karmy-Jones RC. Natural history of traumatic rupture of the thoracic aorta managed nonoperatively: a longitudinal analysis. The Annals of thoracic surgery. 2002;73(4):1149–54. doi: 10.1016/s0003-4975(01)03585-8. [DOI] [PubMed] [Google Scholar]


