Abstract
Objective
The number of people living with HIV (PLWH) over 50 years old in sub-Saharan Africa is predicted to triple in the coming decades, to 6-10 million. Yet, there is a paucity of data on the determinants of health and quality of life for older PLWH in the region.
Methods
A review was undertaken to describe the impact of HIV infection on aging for PLWH in sub-Saharan Africa.
Results
We (a) summarize the pathophysiology and epidemiology of aging with HIV in resource-rich settings, and (b) describe how these relationships might differ in sub-Saharan Africa, (c) propose a conceptual framework to describe determinants of quality of life for older PLWH, and (d) suggest priority research areas needed to ensure long-term gains in quality of life for PLWH in the region.
Conclusions
Differences in traditional, lifestyle, and envirnomental risk factors, as well as unique features of HIV epidemiology and care delivery appear to substantially alter the contribution of HIV to aging in sub-Saharan Africa. Meanwhile, unique preferences and conceptualizations of quality of life will require novel measurement and intervention tools. An expanded research and public health infrastructure is needed to ensure that gains made in HIV prevention and treamtent are translated into long-term benefits in this region.
Keywords: HIV/AIDS, sub-Saharan Africa, aging, gerontology, inflammation, noncommunicable diseases
The Aging of People Living With HIV (PLWH) in Sub-Saharan Africa
HIV is a CD4+ T-cell tropic virus that, if untreated, results in an accelerated acquired immunodeficiency and death for the vast majority of those infected. However, the advent of combination of antiretroviral therapy (ART), which inhibits HIV viral replication and enables repopulation of target immune cells, can convert the disease from a routinely fatal infection to a treatable, chronic disease (Hammer et al., 1997; Palella et al., 1998).
At the turn of the 21st century, ART access was largely restricted to research-rich settings. However, over the past 15 years, massive global investments have been made into HIV care programs in sub-Saharan Africa (Bendavid, Holmes, Bhattacharya, & Miller, 2012; El-Sadr et al., 2012), where more than 25 million people are infected and the adult prevalence of HIV reaches more than 20% in multiple countries. (UNAIDS Global AIDS Epidemic Update) These investments, which have enabled more than 12 million people in the region gain access to ART, have resulted in significant reductions in AIDS-related mortality (Bor, Herbst, Newell, & Barnighausen, 2013; Mills et al., 2011; Nsanzimana et al., 2015; Reniers et al., 2017). Some of the best evidence for this benefit comes from South Africa, which is home to more than 7 million PLWH and the world’s largest HIV epidemic. A demographic and health survey following nearly 100,000 individuals in the KwaZulu-Natal Province has demonstrated that, in the 8 years after implementation of large-scale ART distribution, the general population life expectancy increased from 49 to 61 years, and these gains were fully accounted for by increases in life span among PLWH (Bor et al., 2013). Similar improvements in life expectancy have been reported in Uganda and Rwanda among PLWH (Mills et al., 2011; Nsanzimana et al., 2015); and life expectancies in the region for PLWH who access ART are now approaching those of uninfected individuals (Johnson et al., 2013; Mills et al., 2011).
Consequently, the number of PLWH in sub-Saharan Africa above 50 years old is predicted to triple in the coming decades; to up to 10 million by 2040 (Hontelez et al., 2012). Despite these gains, and multiple calls for research and public health programs aimed at enhancing quality of life for older aged PLWH (Green, 2016; High et al., 2012; Mills, Barnighausen, & Negin, 2012; Negin, Barnighausen, Lundgren, & Mills, 2012), there is a paucity of data on the determinants of health and quality of life for older PLWH in the region. This review will summarize what is known about the pathophysiology and epidemiology of aging with HIV in developed settings, how these relationships might differ in sub-Saharan Africa, and discuss priority research areas to ensure that the benefits realized by providing ART to PLWH in sub-Saharan Africa are translated into long-term gains in health and quality of life.
Pathophysiology of Aging With HIV in Developed Settings
Heightened Risk of Noncommunicable Disease With HIV in Developed Settings
In resource-rich settings, the discovery that combination ART improves survival (Hammer et al., 1997; Mocroft et al., 1998; Palella et al., 1998) was followed by the realization that PLWH were at higher risk of non-AIDS-related comorbidities, including cardiovascular disease (D:A:D Study Group et al., 2008; Friis-Moller et al., 2007; Obel et al., 2007; Triant, Lee, Hadigan, & Grinspoon, 2007), cancer (Engels et al., 2006; Smith et al., 2014), and geriatric syndromes such as frailty and decreased physical functioning (Desquilbet et al., 2007; Greene et al., 2015; Greene, Justice, Lampiris, & Valcour, 2013; Gustafson et al., 2016; Pathai, Gilbert, et al., 2013; Piggott et al., 2013). Traditional risk factors only partially account for the increased risk of many of these comorbidities among PLWH (Justice & Braithwaite, 2012; Rodriguez-Penney et al., 2013). ART regimen toxicities were once thought to also be the major cause (Friis-Moller et al., 2007; Sabin et al., 2016); however, this hypothesis has been convincingly rejected, because ART-mediated virologic suppression has been subsequently demonstrated to significantly decrease the incidence of most chronic comorbidities and mortality among PLWH (El-Sadr et al., 2006; The INSIGHT START Study Group et al., 2015).
Chronic Inflammation and Noncommunicable Disease Risk With HIV in Developed Settings
Instead, immune activation caused by direct HIV infection of end-organ tissues (Dube et al., 2008; Eugenin et al., 2008) and coinfections (Hunt et al., 2008; Sylwester et al., 2005; Wilson et al., 2014) is thought to precipitate chronic activation of both innate and adaptive immunologic pathways (Boasso & Shearer, 2008; Esser et al., 2001; Hardy, Graham, Shearer, & Herbeuval, 2007; Martin et al., 2013). This phenomenon persists despite suppressive ART (French, King, Tschampa, da Silva, & Landay, 2009; Hearps et al., 2012; Hunt et al., 2003). Moreover, HIV infection promotes the so-called senescence-associated secretory phenotype (Coppe, Desprez, Krtolica, & Campisi, 2010), characterized by proliferation of CD8+CD57+CD28− T-lymphocytes, which have been implicated in increased cytokine production (Macaulay, Akbar, & Henson, 2013), decreased naïve T-cell precursor populations, diminished humoral immunity, and attenuated vaccine responses (Xie & McElhaney, 2007). For example, virologically suppressed PLWH have higher relative populations of CD8+CD57+CD28− T-lymphocytes as HIV-uninfected individuals of the same age and similar levels to those decades older (Desai & Landay, 2010; Serrano-Villar et al., 2014).
The resulting inflammatory state as well as markers of immune senescence have been correlated with frailty, neurocognitive dysfunction, functional impairment, and mortality in older aged PLWH (Brothers & Rockwood, 2014; Deeks, 2011; Erlandson et al., 2013; Hsu et al., 2016; Knudsen et al., 2016; S. A. Lee et al., 2014; Lyons et al., 2011; Piggott et al., 2015; Tien et al., 2010). The association of HIV infection with the frailty phenotype has been consistently reported across HIV cohorts. In the Women’s Interagency HIV Study, women with low CD4 counts had 2 to 3 times the odds of frailty (as measured by the Fried Frailty Phenotype Criteria; Fried et al., 2001) compared with HIV-uninfected women independent of age (Gustafson et al., 2016). The Multicenter AIDS Cohort Study (MACS) also reported increased odds of the frailty phenotype, as measured by an adapted Fried scale that removed the grip strength criterion and substituted self-reported measures for gait speed, among men living with HIV with greater duration of HIV infection (adjusted odds ratio [AOR] 3.4 for HIV duration <4 years, AOR > 12 for HIV duration >4 years; Desquilbet et al., 2007). Intriguingly, in the Veterans Aging Cohort Study (>95% male), which used a similar adapted version of the Fried criteria to the MACS investigators, frailty also appeared to be more common in PLWH with active viremia than HIV-uninfected comparators but less common among PLWH with a suppressed viral load (Akgun et al., 2014). Physical functioning, measured by both self-report (Oursler et al., 2011) and using the Short Physical Functioning Battery (Greene et al., 2014), has also been consistently shown to be impaired in PLWH versus age-matched HIV-uninfected counterparts. Importantly, decreased physical function and a frailty phenotype appear to be both of greater severity and develop at younger ages among PLWH in developed settings (Desquilbet et al., 2007; Gustafson et al., 2016; Oursler et al., 2011), leading some to consider HIV a cause of both accelerated and accentuated aging (Pathai, Bajillan, Landay, & High, 2014). For example, in the MACS cohort, the prevalence of a frailty phenotype was similar in recently infected PLWH less than 55 years old as in HIV-uninfected men greater than 65 years (Desquilbet et al., 2007).
Chronic Comorbidities and Inflammation With HIV in Sub-Saharan Africa
Traditional Chronic Disease Risk Factors Vary in Sub-Saharan Africa
Yet, extrapolation of these data to the sub-Saharan African region, where more than two thirds of PLWH reside, should be done cautiously. Differences in genetics, the social-environmental milieu, timing of ART initiation, commonly used ART regimens, and prevalence of coinfections in sub-Saharan Africa will almost certainly alter the risk profile and morbidity phenotypes among PLWH. For example, rates of smoking and alcohol use, diet and physical activity patterns (Amare et al., 2012; Guthold et al., 2011; Steyn, Nel, Parker, Ayah, & Mbithe, 2011), the epidemiology of HIV coinfections (Appay et al., 2011; Borkow & Bentwich, 2004; Cannon, Schmid, & Hyde, 2010; de Mast et al., 2015; Elliott, Summers, & Weinstock, 2007; Hochman & Kim, 2009; Hsue et al., 2006; Turner et al., 2003), nadir CD4 counts (M. J. Siedner, Ng, et al., 2015), and recommended first- and second-line regimens (World Health Organization, 2016) are markedly different in sub-Saharan Africa than in the United States and Europe.
Such differences have been particularly apparent in studies measuring prevalence of chronic comorbidities and their traditional risk factors among PLWH in sub-Saharan Africa. In the Ugandan Non-Communicable Diseases and Aging Cohort Study (Clinicaltrials.gov registration number: NCT 02445079), a population-based cohort study in rural Uganda which includes both PLWH on suppressive ART and age and gender-matched HIV-negative comparators (Siedner et al., 2016, #443), more than 70% of PLWH meet World Health Organization (WHO) criteria for high physical activity, less than 10% are current smokers, and less than 5% have a fasting low-density lipoprotein greater than 130 mg/dL (Feinstein et al., 2017). In stark contrast to cohort studies from the United States, PLWH were less likely to be active smokers, and had better blood pressure and diabetes control. Studies from elsewhere in the African region have also generally demonstrated a lower prevalence of obesity and hypertension among PLWH compared with HIV-uninfected comparators (Gaziano et al., 2017; Kwarisiima et al., 2016; Malaza, Mossong, Barnighausen, & Newell, 2012).
Although reductions in hypertension prevalence might be partially attenuated after ART initiation (Okello et al., 2016; Okello et al., 2015; Peck et al., 2014), a growing body of literature argues that the HIV care infrastructure in sub-Saharan Africa might offer secondary benefits through primary care delivery and improved chronic disease risk factor management (Gupta & Bukhman, 2015; Manne-Goehler et al., 2017). Yet, whereas many of the traditional cardiovascular disease risk factors appear to be decreased in HIV infection in sub-Saharan Africa, arterial stiffness (Siedner et al., 2016, stroke (Benjamin et al., 2015), and cancer (Dryden-Peterson et al., 2015) preliminarily appear to be associated with HIV infection in the region. Taken together, these results highlight the need to collect data on regional risk factors for chronic comorbidities among older PLWH, which are likely to alter the disease profile of the population (Figure 1; Alwan et al., 2010; Riha et al., 2014).
Figure 1.
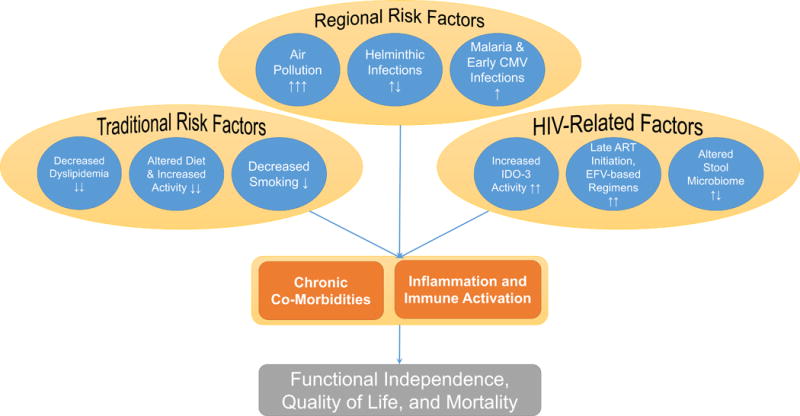
Altered risk profile for cardiopulmonary disease risk among PLWH in rural sub-Saharan Africa.
Note. PLWH = people living with HIV.
Chronic Inflammation With HIV in Sub-Saharan Africa
There is likewise compelling evidence for altered inflammatory pathways among PLWH in rural Africa (Anthony, Rutitzky, Urban, Stadecker, & Gause, 2007; Banerjee, Mondal, Das, & Ray, 2012; de Mast et al., 2015; Dutta, Ray, & Banerjee, 2012; Geffken et al., 2001), compared with the United States and Europe. For example, the Ugandan AIDS Rural Cohort Study has demonstrated novel elevations in indoleamine 2,3-dioxygenase (IDO) activity among PLWH in Uganda, resulting in increased tryptophan metabolism and an increased kynurenine:tryptophan ratio (K:T). This alteration in tryptophan metabolism, which has since been additionally reported elsewhere in the region, including urban areas of South Africa (Bipath, Levay, & Viljoen, 2015), has been associated with an increased risk of atherosclerosis, depression, AIDS-related cancer, and all-cause mortality (Byakwaga et al., 2015; S. Lee et al., 2017; Martinez et al., 2014; M. J. Siedner, Kim et al., 2015).
Multiple groups in the region have also demonstrated key differences in inflammatory marker profiles in sub-Saharan Africa compared with U.S.-based cohorts. Three such markers—sCD14, a biomarker of innate immune activation; IL-6, a generalized marker of systemic inflammation; and sCD163, a specific marker of macrophage activation—have been systematically demonstrated to be elevated among PLWH compared with HIV-uninfected comparators in the United States and Europe, and independently associated with cardiovascular events, the frailty phenotype, and mortality, despite suppressive ART (Duprez et al., 2012; Hsu et al., 2016; Knudsen et al., 2016; Kuller et al., 2008; S. A. Lee et al., 2014; Leng, Chaves, Koenig, & Walston, 2002; Lien et al., 1998; Longenecker et al., 2014; Mendez-Lagares et al., 2013; Sandler et al., 2011; Wada et al., 2015). In Uganda, however, although we have found significantly increased levels of sCD14 in PLWH versus community-based, HIV-uninfected comparators (p < .001), we found no differences by serostatus in IL-6 or sCD163 (p > .40 for both, unpublished data). Other cohort studies from South Africa and Uganda comparing HIV-uninfected individuals with PLWH on and off ART have also failed to detect significant differences in IL-6 by HIV serostatus (Fourie, Schutte, Smith, Kruger, & van Rooyen, 2015; Olwenyi et al., 2016).
Although markers of systemic inflammation and immune activation might be muted in sub-Saharan Africa, the single study that has assessed molecular aging among PLWH in sub-Saharan Africa did demonstrate evidence of accelerated aging. Shorter telomeres and greater CD2NKA leukocyte expression, which is inversely related to cellular replicative capacity, were found in PLWH compared with age- and sex-matched HIV-uninfected comparators, and low CD4 counts predicted accelerated biological aging (Pathai, Lawn, et al., 2013).
Nontraditional Chronic Disease Risk Factors in Sub-Saharan Africa
While the mechanisms that drive these putatively unique inflammatory pathways require further study, early evidence points to genetic, environmental, and coinfection exposures. For example, a polymorphism has been identified that might predispose populations in Uganda to increased IDO activity, resulting in increased K:T ratio (S. A. Lee et al., 2016). Biomass cooking fuel exposure, which is a another distinctively regional risk factor (Brook et al., 2010; Salvi & Barnes, 2009), is independently associated with carotid atherosclerosis (Painschab et al., 2013), cardiovascular disease events (Brook et al., 2010; M. S. Lee et al., 2012), and chronic obstructive pulmonary disease (Salvi & Barnes, 2009), and shares pathways of systemic inflammation, immune activation, oxidative stress, and pulmonary epithelial barrier dysfunction with HIV infection (Ghio & Devlin, 2001; Hajat et al., 2015; Hu et al., 2013; Li, Gilmour, Donaldson, & MacNee, 1996; Park et al., 2011; Rylance et al., 2015; Sussan et al., 2014). The potential impact of air pollution on health of PLWH is further supported by the fact that nonsmoking exposures account for more than 50% of cardiopulmonary disease (COPD)-associated mortality in males and more than 80% of COPD-associated mortality in females in low- and middle-income countries (Eisner et al., 2010).
Highly prevalent tropical coinfections might similarly differentiate interactions between HIV, inflammation, and chronic disease risk in resource-poor settings. For example, malaria immunity and susceptibility are reduced in HIV infection (Finney et al., 2013; Hochman & Kim, 2009), and malaria infection triggers HIV viral replication (Hoffman et al., 1999). Subclinical malaria has been associated with elevated markers of immune activation in adults (de Mast et al., 2015), and malaria infection has recently been shown to induce a chronic proinflammatory response among HIV-infected apes (Trott, Richardson, Hudgens, & Abel, 2013). Soil-transmitted helminth infections are also highly endemic in much of sub-Saharan Africa, (Kabatereine et al., 2005; Karagiannis-Voules et al., 2015), induce complex effects on gut and systemic immunity, and unlike HIV, cytomegalovirus (CMV) and malaria, activate Th2 immune responses (Anthony et al., 2007; Salgame, Yap, & Gause, 2013). Whereas prior studies have investigated relationships between CD4 count trajectories and helminthic infection and HIV treatment (Lankowski et al., 2014; Walson et al., 2012), less is known about how these intestinal infections alter gut immunity, microbial translocation, and chronic immune activation among PLWH in the region.
Contributions of HIV to Geriatric Syndromes in Sub-Saharan Africa
Whereas chronic morbidity prevalence estimates in sub-Saharan Africa abound, much less has been described on multifactorial geriatric syndromes, functioning, and disability among older aged PLWH in the region. Such syndromes are more predictive of self-reported health, healthcare utilization, and mortality than classical medical syndromes in developed settings (Koroukian et al., 2016; Wang, Shamliyan, Talley, Ramakrishnan, & Kane, 2013). Consequently, defining their epidemiology might illustrate more global determinants of well-being for older PLWH in the region. To date, data have been contrasting about relationships between HIV and geriatric syndromes in sub-Saharan Africa. A cross-sectional study from South Africa including clinic-based PLWH in care and community-based controls found more than twice the odds of frailty among PLWH, defined using an adapted version of the Fried Frailty Scale (Pathai, Gilbert, et al., 2013). This finding is in keeping with similar estimates about HIV and a frailty phenotype from the United States as described above. In contrast, baseline data from the large community-based, Health and Aging in Africa Longitudinal Study in South Africa (HAALSI) have demonstrated a similar occurrence of age-adjusted, self-reported disability and functioning between relatively old age PLWH (greater than 40 years) and HIV-uninfected comparators, and faster gait speeds among PLWH (Payne, Gomez-Olive, Kahn, & Berkman, 2017). Similarly, a study comparing functioning, disability, and chronic comorbidities between older PLWH (>50 years) and age-matched HIV-affected individuals (family member with HIV or loss of family member to HIV) in Uganda found generally similar rates of disability and comorbidity between groups (Scholten et al., 2011). Given these contrasting findings, a clear priority for the region is to better classify both the locally relevant syndromes which comprise health and functioning for older PLWH in the region, and expand data collection to comprehensively describe their prevalence and determinants.
The epidemiology of cognitive deficits with HIV infection in sub-Saharan Africa, as well as contributions from inflammation and local risk factors, also remains poorly defined. In the pre-ART era in the United States and Europe, HIV was associated with frank dementia at early age (Sacktor et al., 1996). This syndrome has become rare after widespread ART availability, replaced with more subtle HIV-associated neurocognitive deficits (McArthur, Steiner, Sacktor, & Nath, 2010; McCutchan et al., 2007). Chronic immune activation of central nervous system macrophages is hypothesized to mediate this condition (Saylor et al., 2016). In sub-Saharan Africa, advanced HIV is most strongly associated with neurocognitive deficits, and ART similarly mitigates the prevalence and severity of these deficits (Habib et al., 2013). However, there are few data on the pathophysiologic processes responsible. Moreover, whether the heightened risk of central nervous system infections (e.g., cryp-tococcal meningitis, childhood cerebral malaria, tuberculous meningitis) as well as nutritional and metabolic factors that are considerably more common in sub-Saharan Africa affect these relationships remains an important, but an unexplored, area of study.
Quality of Life for Older PLWH in Sub-Saharan Africa
Importantly, significant work also remains in bridging the gap between medical conceptualizations of health and more global elements of well-being among PLWH in sub-Saharan Africa. To date, the lion’s share of research on quality of life among PLWH in the sub-Saharan African region has focused on the impact of ART on health-related quality of life. Not surprisingly, these studies have identified strong and rapid gains in quality of life after ART initiation (Louwagie et al., 2007; Pitt, Myer, & Wood, 2009; Wouters, Meulemans, Van Rensburg, Heunis, & Mortelmans, 2007). Most of these studies have relied on structured scales with a specific focus on health-related quality of life, such as EuroQOL (EuroQol, 1990), which have been validated in sub-Saharan African populations for this purpose (Jelsma & Ferguson, 2004; Jelsma, Mkoka, Amosun, & Nieuwveldt, 2004), including among PLWH (Jelsma, Maclean, Hughes, Tinise, & Darder, 2005). In addition to expected gains in quality of life after ART initiation, these data help shed light on other health system factors that might contribute to the quality of life for PLWH in sub-Saharan Africa. For example, accessing additional HIV/AIDS-related services, being visited by community health workers, and having HIV treatment supporters were associated with increasing health and/or emotional quality of life, and speak to the ability of the formal healthcare and public health institutions to affect well-being for PLWH in the region (Booysen Fle, Van Rensburg, Bachmann, Louwagie, & Fairall, 2007).
A limitation of many of the studies that describe quality of life among PLWH is the narrow focus on physical and health-related quality of life at the exclusion of more global elements of well-being, particularly in aging populations (Higgs, Hyde, Wiggins, & Blane, 2003). Qualitative work in the United States on psychosocial determinants of healthy aging and coping mechanisms for older aged PLWH has often identified coping domains related to self-reliance, drug use, and accessing support from networks of other PLWH (DeGrezia & Scrandis, 2015; Emlet, Tozay, & Raveis, 2011; Psaros et al., 2015).
In contrast, definitions and expectations of health and aging in sub-Saharan Africa tend to focus more on obligations to family and social networks (Mudege & Ezeh, 2009). Indeed, available formative data suggest that quality of life in older aged groups is defined as a capacity to meet expectations related to family roles and responsibilities (Ogunmefun, Gilbert, & Schatz, 2011; Schatz, 2007; Schatz & Gilbert, 2014; Ssengonzi, 2009). Moreover, the pervasiveness of the HIV epidemic and its high mortality in young adults early in the epidemic have frequently altered these roles, and in many cases increased obligations of (and burdens on) older aged family members (Kautz, Bendavid, Bhattacharya, & Miller, 2010; Madhavan & Schatz, 2007; Mudege & Ezeh, 2009; Muga & Onyango-Ouma, 2009). Specifically, the classic role of the younger generation family member as the caregiver and income generator for both young and elderly adults has often been reversed (Schatz, 2007; Schatz & Gilbert, 2014), and may be of particular relevance for female-headed households (Schatz, Madhavan, & Williams, 2011). Critical areas of future reserach include studies to better estimate how PLWH alters the risk of chronic diseases among older-individuals, and how the regional public health system will help relieve any additional burden on familial caregivers Bendavid, Ford, & Mills, 2012; de-Graft Aikins et al., 2010; Kautz et al., 2010).
Thus, developing a conceptual framework to better describe contributors and barriers to quality of life for older aged PLWH in sub-Saharan Africa will be a critical addition to the field, and necessary to design future studies and interventions to improve health in these populations. Recently, preliminary work has begun to accomplish this goal by establishing the consistency and validity of more global quality of life measures in PLWH in Eastern Africa. The Functional Assessment of HIV Infection Score intends to more broadly consider domains of physical, emotional, functional/global, social and cognitive well-being (Cella, McCain, Peterman, Mo, & Wolen, 1996; O’Brien et al., 2010). It has recently been evaluated and adapted in Kenya with reasonable internal consistency and preliminary evidence of external validity (Nyongesa et al., 2017). Of interest in this study was the relatively low factor loadings and low correlation observed between social and cognitive functioning subscores and external functional measures, compared with the physical and emotional functioning subscores, suggesting that global conceptualizations of well-being for aging PLWH are likely to differ in ways that will require novel frameworks, data collection tools, and interventions that focus on unique domains of aging.
A Conceptual Framework for Aging With HIV in Sub-Saharan Africa
Proposed here is a conceptual framework that incorporates the relevant data from the field as summarized above to describe determinants of health-related quality of life for older aged PLWH in rural sub-Saharan Africa (Figure 2). The framework incorporates a syndromic approach to gerontology (Fried et al., 2001; Mitnitsky, Mogilner, & Rockwood, 2001) to include domains of physical, cognitive, and social functioning (Rubtsova et al., 2017; Thurn & Gustafson, 2017). It includes key determinants of these domains derived from prior work on HIV and aging in the United States, which have demonstrated independent effects of HIV-related inflammation on domains of physical and neurocognitive functioning (Erlandson et al., 2013; Lyons et al., 2011; Scully et al., 2016). It also adds locally relevant elements described in qualitative studies among older aged PLWH in Kenya and South Africa, which demonstrate the importance of familial deaths, and their impact on caregiving and household responsibility structures (Madhavan & Schatz, 2007; Mudege & Ezeh, 2009; Muga & Onyango-Ouma, 2009), as well as the importance of HIV stigma, which disrupts access to social support mechanisms in this population (Takada et al., 2014; Tsai et al., 2013). The framework intends to serve as a launching point for future work both to validate and to alter these conceptualizations, and further priority public health and research domains for older PLWH in the sub-Saharan African region.
Figure 2.
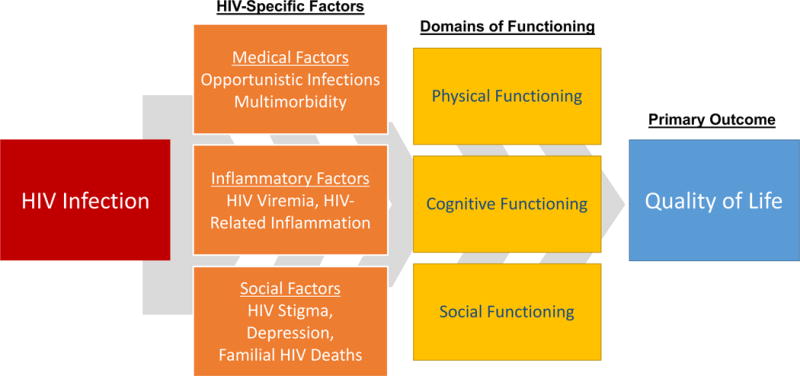
Proposed conceptual framework to describe health-related quality of life for older aged PLWH in Uganda.
Note. PLWH = people living with HIV.
Knowledge Gaps and Future Research Priorities
Notwithstanding over two decades of research on the HIV epidemic in sub-Saharan Africa that have revealed the devastation of the early epidemic, identified priority interventions to improve health, and documented extraordinary gains with implementation of ART programs, there remains a paucity of data on the determinants of well-being among older PLWH in sub-Saharan Africa. Critical to this process will be formative work that considers relevant domains to geriatric health, such as independence, functioning, and social well-being more broadly, and accounts for locally adapted features of quality of life. Such information will be required to ensure that data collection platforms and public health responses are targeted to locally established preferences for health and quality of life, and not transplanted conceptions of these domains that might be relevant to other regions.
Additional quantitative data are needed to specify biomedical, psychosocial, and environmental determinants of health and quality of life, and their relative contributions to both, for the many millions of people who have survived the AIDS epidemic into middle and late life. In the biomedical realm, there are vanishingly little data on priority causes of morbidity in sub-Saha-ran Africa among PLWH above the age of 45, the upper age limit of most demographic and health surveys, which have historically focused on child and maternal health. Because few countries have death registries in the region, most estimates on causes of death are derived from verbal autopsy, or modeling studies that predict disease burden based on general population prevalent morbidities assessed by the WHO Sage and 10/66 Dementia surveys (Kowal et al., 2010; Prince et al., 2007; Prince et al., 2015). However, few of these studies are powered to make conclusions by HIV serostatus, and they tend to focus on a handful of countries in sub-Saharan Africa—most notably South Africa—which is one of only six middle-income countries in the region.
Multicenter, longitudinal cohorts of PLWH and/or demographic health surveys that enroll sufficient PLWH and HIV-uninfected comparators could serve such a purpose. They must include both traditional and regionally relevant risk factor data, as well as incident morbidity, and mortality data to better discern health priorities for older PLWH. For example, the most compelling evidence that HIV infection is associated with non-AIDS conditions, including geriatric syndromes such as frailty and decreased physical functioning, has come from longitudinal studies in the United States and Europe, such as the MACS, the Women’s Interagency Health Study, and the Veterans’ Aging Cohort Study, which study both HIV-infected and uninfected populations (Currier et al., 2003; Freiberg et al., 2013; Hadigan et al., 2001; Kaplan et al., 2008; Kingsley et al., 2008; Klein et al., 2015; Seaberg et al., 2010). Plausible estimation of associations between HIV infection and chronic disease risk requires a well-matched comparator group, ideally enrolled from the community to minimize selection bias.
Finally, interventional studies, targeted at the individual, health center, and community level, will be needed to translate observational findings into improvements in health for older PLWH in sub-Saharan Africa. While many traditional interventions that focus on known determinants of health and quality of life (e.g., smoking cessation, exercise) may play a role, it is also highly likely that entirely new interventions will be necessary to improve health in the local context. This is due to both the unique risk factors and health determinants in the region (e.g., cooking fuel exposure, loss of familial caregivers in the HIV epidemic) and the profoundly different structure and capacity of healthcare delivery in the sub-Saharan African region.
An Actionable Public Health Response
The infrastructure supported by HIV care programs has built an ideal platform through which to evaluate and deliver sustainable interventions to address non-HIV-related health needs (Gupta & Bukhman, 2015). Currently, this HIV care system supports millions of individuals across the region to receive routine medical care through multiple annual visits with clinicians, pharmacists, counselors, and other ancillary staff with a primary goal of sustaining health. Thus, a foundation is in place to deliver interventions if priority targets and cost-effective approaches can be identified. Innumerable potential solutions could be envisioned that meet these criteria. As one example, if familial HIV-related deaths and their deleterious effects on social support and caregiver provision meaningfully undermine quality of life for older PLWH in the region, clinic-based solutions that target caregiver support have been found to be highly effective in such settings (Uebel, Nash, & Avalos, 2007).
Conclusion
To sustain the benefits of global investments in HIV care in sub-Saharan Africa, there is a need for increased research on determinants of health and quality of life for older aged PLWH in sub-Saharan Africa. To date, most studies of aging with HIV in the region have been cross-sectional, have focused on single morbidity domains (e.g., hypertension or obesity), and lack local insight about preferences for quality of life. Available evidence suggests that the determinants of quality of life among older aged PLWH in rural sub-Saharan Africa differ meaningfully from those in the United States and Europe; and that elucidating these determinants and their relative contributions is essential to developing effective interventions to optimize health. Priority areas for future research on aging among PLWH include (a) developing locally and regionally validated conceptual frameworks to describe determinants of health and quality of life; (b) examining the characteristics and epidemiology of aging-related syndromes in this population; (c) assessing the contributions of genetic, environmental, sociodemographic, and HIV-specific (e.g., inflammatory, ART related) determinants; and (d) leveraging these data to develop and evaluate interventions that can meaningfully improve health and well-being for this population. The well-established HIV care infrastructure in the region affords a ready foundation on which to design such interventions. Failing to accomplish these goals risks squandering the extraordinarily successful investments made to extend the life of PLWH in the region.
Acknowledgments
Funding
The author disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: M.J.S. receives research support from the National Institutes of Health and the Harvard Center for AIDS Research (K23 MH099916, R21 HL124712, P30 AI060354, R24 AG044325).
Footnotes
Declaration of Conflicting Interests
The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Akgun KM, Tate JP, Crothers K, Crystal S, Leaf DA, Womack J, Oursler KK. An adapted frailty-related phenotype and the VACS index as predictors of hospitalization and mortality in HIV-infected and uninfected individuals. Journal of Acquired Immune Deficiency Syndromes. 2014;67:397–404. doi: 10.1097/QAI.0000000000000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alwan A, Maclean DR, Riley LM, d’Espaignet ET, Mathers CD, Stevens GA, Bettcher D. Monitoring and surveillance of chronic non-communicable diseases: Progress and capacity in high-burden countries. The Lancet. 2010;376:1861–1868. doi: 10.1016/S0140-6736(10)61853-3. [DOI] [PubMed] [Google Scholar]
- Amare B, Moges B, Moges F, Fantahun B, Admassu M, Mulu A, Kassu A. Nutritional status and dietary intake of urban residents in Gondar, Northwest Ethiopia. BMC Public Health. 2012;12 doi: 10.1186/1471-2458-12-752. Article 752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony RM, Rutitzky LI, Urban JF, Stadecker MJ, Jr, Gause WC. Protective immune mechanisms in helminth infection. Nature Reviews Immunology. 2007;7:975–987. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appay V, Fastenackels S, Katlama C, Ait-Mohand H, Schneider L, Guihot A, Sauce D. Old age and anti-cytomegalovirus immunity are associated with altered T-cell reconstitution in HIV-1-infected patients. AIDS. 2011;25:1813–1822. doi: 10.1097/QAD.0b013e32834640e6. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Mondal NK, Das D, Ray MR. Neutrophilic inflammatory response and oxidative stress in premenopausal women chronically exposed to indoor air pollution from biomass burning. Inflammation. 2012;35:671–683. doi: 10.1007/s10753-011-9360-2. [DOI] [PubMed] [Google Scholar]
- Bendavid E, Ford N, Mills EJ. HIV and Africa’s elderly: The problems and possibilities. AIDS. 2012;26(Suppl. 1):S85–S91. doi: 10.1097/QAD.0b013e3283558513. [DOI] [PubMed] [Google Scholar]
- Bendavid E, Holmes CB, Bhattacharya J, Miller G. HIV development assistance and adult mortality in Africa. Journal of the American Medical Association. 2012;307:2060–2067. doi: 10.1001/jama.2012.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin LA, Corbett EL, Connor MD, Mzinganjira H, Kampondeni S, Choko A, Solomon T. HIV, antiretroviral treatment, hypertension, and stroke in Malawian adults: A case-control study. Neurology. 2015 doi: 10.1212/WNL.0000000000002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bipath P, Levay PF, Viljoen M. The kynurenine pathway activities in a sub-Saharan HIV/AIDS population. BMC Infectious Diseases. 2015;15 doi: 10.1186/s12879-015-1087-5. Article 346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boasso A, Shearer GM. Chronic innate immune activation as a cause of HIV-1 immunopathogenesis. Clinical Immunology. 2008;126:235–242. doi: 10.1016/j.clim.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booysen Fle R, Van Rensburg HC, Bachmann M, Louwagie GM, Fairall LR. The Heart in HAART: Quality of life of patients enrolled in the public sector antiretroviral treatment programme in the Free State Province of South Africa. Social Indicators Research. 2007;81:283–329. [Google Scholar]
- Bor J, Herbst AJ, Newell ML, Barnighausen T. Increases in adult life expectancy in rural South Africa: Valuing the scale-up of HIV treatment. Science. 2013;339:961–965. doi: 10.1126/science.1230413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkow G, Bentwich Z. Chronic immune activation associated with chronic helminthic and human immunodeficiency virus infections: Role of hyporesponsiveness and anergy. Clinical Microbiology Reviews. 2004;17:1012–1030. doi: 10.1128/CMR.17.4.1012-1030.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA, Brook JR, Bhatnagar A, Diez-Roux AV, Kaufman JD. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Brothers TD, Rockwood K. Biologic aging, frailty, and age-related disease in chronic HIV infection. Current Opinion in HIV and AIDS. 2014;9:412–418. doi: 10.1097/COH.0000000000000070. [DOI] [PubMed] [Google Scholar]
- Byakwaga H, Hunt PW, Laker-Oketta M, Glidden DV, Huang Y, Bwana BM, Martin JN. The kynurenine pathway of tryptophan catabolism and AIDS-associated Kaposi Sarcoma in Africa. Journal of Acquired Immune Deficiency Syndromes. 2015;70:296–303. doi: 10.1097/QAI.0000000000000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus sero-prevalence and demographic characteristics associated with infection. Reviews in Medical Virology. 2010;20:202–213. doi: 10.1002/rmv.655. [DOI] [PubMed] [Google Scholar]
- Cella DF, McCain NL, Peterman AH, Mo F, Wolen D. Development and validation of the Functional Assessment of Human Immunodeficiency Virus Infection (FAHI) quality of life instrument. Quality of Life Research. 1996;5:450–463. doi: 10.1007/BF00449920. [DOI] [PubMed] [Google Scholar]
- Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annual Review of Pathology. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier JS, Taylor A, Boyd F, Dezii CM, Kawabata H, Burtcel B, Hodder S. Coronary heart disease in HIV-infected individuals. Journal of Acquired Immune Deficiency Syndromes. 2003;33:506–512. doi: 10.1097/00126334-200308010-00012. [DOI] [PubMed] [Google Scholar]
- D:A:D Study Group. Sabin CA, Worm SW, Weber R, Reiss P, El-Sadr W, Lundgren JD. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D:A:D study: A multi-cohort collaboration. The Lancet. 2008;371:1417–1426. doi: 10.1016/S0140-6736(08)60423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annual Review of Medicine. 2011;62:141–155. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de-Graft Aikins A, Unwin N, Agyemang C, Allotey P, Campbell C, Arhinful D. Tackling Africa’s chronic disease burden: From the local to the global. Global Health. 2010;6 doi: 10.1186/1744-8603-6-5. Article 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGrezia MG, Scrandis D. Successful coping in urban, community-dwelling older adults with HIV. Journal of the Association of Nurses in AIDS Care. 2015;26:151–163. doi: 10.1016/j.jana.2014.11.008. [DOI] [PubMed] [Google Scholar]
- de Mast Q, Brouwers J, Syafruddin D, Bousema T, Baidjoe AY, de Groot PG, Fijnheer R. Is asymptomatic malaria really asymptomatic? Hematological, vascular and inflammatory effects of asymptomatic malaria para-sitemia. Journal of Infection. 2015;71:587–596. doi: 10.1016/j.jinf.2015.08.005. [DOI] [PubMed] [Google Scholar]
- Desai S, Landay A. Early immune senescence in HIV disease. Current HIV/AIDS Reports. 2010;7:4–10. doi: 10.1007/s11904-009-0038-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desquilbet L, Jacobson LP, Fried LP, Phair JP, Jamieson BD, Holloway M, Multicenter ACS. HIV-1 infection is associated with an earlier occurrence of a phenotype related to frailty. Journal of Gerontology, Series A: Biological Sciences & Medical Sciences. 2007;62:1279–1286. doi: 10.1093/gerona/62.11.1279. [DOI] [PubMed] [Google Scholar]
- Dryden-Peterson S, Medhin H, Kebabonye-Pusoentsi M, Seage GR, Suneja G, III, Kayembe MK, Lockman S. Cancer incidence following expansion of HIV treatment in Botswana. PLoS ONE. 2015;10(8):e0135602. doi: 10.1371/journal.pone.0135602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube MP, Lipshultz SE, Fichtenbaum CJ, Greenberg R, Schecter AD, Fisher SD, Working Group 3 Effects of HIV infection and anti-retroviral therapy on the heart and vasculature. Circulation. 2008;118:e36–e40. doi: 10.1161/CIRCULATIONAHA.107.189625. [DOI] [PubMed] [Google Scholar]
- Duprez DA, Neuhaus J, Kuller LH, Tracy R, Belloso W, De Wit S, INSIGHT SMART Study Group Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS ONE. 2012;7(9):e44454. doi: 10.1371/journal.pone.0044454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta A, Ray MR, Banerjee A. Systemic inflammatory changes and increased oxidative stress in rural Indian women cooking with biomass fuels. Toxicology and Applied Pharmacology. 2012;261:255–262. doi: 10.1016/j.taap.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Eisner MD, Anthonisen N, Coultas D, Kuenzli N, Perez-Padilla R, Postma D, Environmental and Occupational Health Assembly An official American Thoracic Society public policy statement: Novel risk factors and the global burden of chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 2010;182:693–718. doi: 10.1164/rccm.200811-1757ST. [DOI] [PubMed] [Google Scholar]
- Elliott DE, Summers RW, Weinstock JV. Helminths as governors of immune-mediated inflammation. International Journal for Parasitology. 2007;37:457–464. doi: 10.1016/j.ijpara.2006.12.009. [DOI] [PubMed] [Google Scholar]
- El-Sadr WM, Holmes CB, Mugyenyi P, Thirumurthy H, Ellerbrock T, Ferris R, Whiteside A. Scale-up of HIV treatment through PEPFAR: A historic public health achievement. Journal of Acquired Immune Deficiency Syndromes. 2012;60(Suppl. 3):S96–S104. doi: 10.1097/QAI.0b013e31825eb27b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sadr WM, Lundgren JD, Neaton JD, Gordin F, Abrams D, Arduino RC, Rappoport C. CD4+ count-guided interruption of antiretroviral treatment. New England Journal of Medicine. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- Emlet CA, Tozay S, Raveis VH. “I’m not going to die from the AIDS”: Resilience in aging with HIV disease. Gerontologist. 2011;51:101–111. doi: 10.1093/geront/gnq060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels EA, Pfeiffer RM, Goedert JJ, Virgo P, McNeel TS, Scoppa SM, HIV/AIDS Cancer Match Study Trends in cancer risk among people with AIDS in the United States 1980-2002. AIDS. 2006;20:1645–1654. doi: 10.1097/01.aids.0000238411.75324.59. [DOI] [PubMed] [Google Scholar]
- Erlandson KM, Allshouse AA, Jankowski CM, Lee EJ, Rufner KM, Palmer BE, Campbell TB. Association of functional impairment with inflammation and immune activation in HIV type 1-infected adults receiving effective antiretroviral therapy. The Journal of Infectious Diseases. 2013;208:249–259. doi: 10.1093/infdis/jit147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser MT, Bess JW, Suryanarayana K, Jr, Chertova E, Marti D, Carrington M, Lifson JD. Partial activation and induction of apoptosis in CD4(+) and CD8(+) T lymphocytes by conformationally authentic noninfectious human immunodeficiency virus type 1. Journal of Virology. 2001;75:1152–1164. doi: 10.1128/JVI.75.3.1152-1164.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin EA, Morgello S, Klotman ME, Mosoian A, Lento PA, Berman JW, Schecter AD. Human immunodeficiency virus (HIV) infects human arterial smooth muscle cells in vivo and in vitro: Implications for the pathogenesis of HIV-mediated vascular disease. American Journal of Pathology. 2008;172:1100–1111. doi: 10.2353/ajpath.2008.070457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EuroQol G. EuroQol—A new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- Feinstein MJ, Kim JH, Bibangambah P, Sentongo R, Martin JN, Tsai AC, Siedner MJ. Ideal cardiovascular health and carotid atherosclerosis in a mixed cohort of HIV-infected and uninfected Ugandans. AIDS Research and Human Retroviruses. 2017;33:49–56. doi: 10.1089/AID.2016.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney CA, Ayi K, Wasmuth JD, Sheth PM, Kaul R, Loutfy MR, Serghides L. HIV infection deregulates innate immunity to malaria despite combination antiretroviral therapy. AIDS. 2013;27:325–335. doi: 10.1097/QAD.0b013e32835b3dfa. [DOI] [PubMed] [Google Scholar]
- Fourie CM, Schutte AE, Smith W, Kruger A, van Rooyen JM. Endothelial activation and cardiometabolic profiles of treated and never-treated HIV infected Africans. Atherosclerosis. 2015;240:154–160. doi: 10.1016/j.atherosclerosis.2015.03.015. [DOI] [PubMed] [Google Scholar]
- Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, Justice AC. HIV infection and the risk of acute myocardial infarction. JAMA Internal Medicine. 2013;173:614–622. doi: 10.1001/jamain-ternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French MA, King MS, Tschampa JM, da Silva BA, Landay AL. Serum immune activation markers are persistently increased in patients with HIV infection after 6 years of antiretroviral therapy despite suppression of viral replication and reconstitution of CD4+ T cells. The Journal of Infectious Diseases. 2009;200:1212–1215. doi: 10.1086/605890. [DOI] [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Cardiovascular Health Study Collaborative Research Group Frailty in older adults: Evidence for a phenotype. Journals of Gerontology, Series A: Biological Sciences & Medical Sciences. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- Friis-Moller N, Reiss P, Sabin CA, Weber R, Monforte A, El-Sadr W, Lundgren JD. Class of antiretroviral drugs and the risk of myocardial infarction. New England Journal of Medicine. 2007;356:1723–1735. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- Gaziano TA, Abrahams-Gessel S, Gomez-Olive FX, Wade A, Crowther NJ, Alam S, Tollman S. Cardiometabolic risk in a population of older adults with multiple co-morbidities in rural South Africa: The HAALSI (Health and Aging in Africa: Longitudinal studies of INDEPTH communities) study. BMC Public Health. 2017;17(1) doi: 10.1186/s12889-017-4117-y. Article 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geffken DF, Cushman M, Burke GL, Polak JF, Sakkinen PA, Tracy RP. Association between physical activity and markers of inflammation in a healthy elderly population. American Journal of Epidemiology. 2001;153:242–250. doi: 10.1093/aje/153.3.242. [DOI] [PubMed] [Google Scholar]
- Ghio AJ, Devlin RB. Inflammatory lung injury after bronchial instillation of air pollution particles. American Journal of Respiratory and Critical Care Medicine. 2001;164:704–708. doi: 10.1164/ajrccm.164.4.2011089. [DOI] [PubMed] [Google Scholar]
- Green A. NIH project focuses on integration of HIV and NCD care. The Lancet. 2016;388:1869. doi: 10.1016/S0140-6736(16)31846-3. [DOI] [PubMed] [Google Scholar]
- Greene M, Covinsky KE, Astemborski J, Piggott DA, Brown T, Leng S, Kirk GD. The relationship of physical performance with HIV disease and mortality. AIDS. 2014;28:2711–2719. doi: 10.1097/QAD.0000000000000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene M, Covinsky KE, Valcour V, Miao Y, Madamba J, Lampiris H, Deeks SG. Geriatric syndromes in older HIV-infected adults. Journal of Acquired Immune Deficiency Syndromes. 2015;69:161–167. doi: 10.1097/QAI.0000000000000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene M, Justice AC, Lampiris HW, Valcour V. Management of human immunodeficiency virus infection in advanced age. Journal of the American Medical Association. 2013;309:1397–1405. doi: 10.1001/jama.2013.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Bukhman G. Leveraging the lessons learned from HIV/AIDS for coordinated chronic care delivery in resource-poor settings. Healthcare. 2015;3:215–220. doi: 10.1016/j.hjdsi.2015.09.006. [DOI] [PubMed] [Google Scholar]
- Gustafson DR, Shi Q, Thurn M, Holman S, Minkoff H, Cohen M, Hoover D. Frailty and constellations of factors in aging HIV-infected and uninfected women—The Women’s Interagency HIV Study. The Journal of Frailty & Aging. 2016;5:43–48. doi: 10.14283/jfa.2016.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthold R, Louazani SA, Riley LM, Cowan MJ, Bovet P, Damasceno A, Armstrong TP. Physical activity in 22 African countries: Results from the World Health Organization STEPwise approach to chronic disease risk factor surveillance. American Journal of Preventive Medicine. 2011;41:52–60. doi: 10.1016/j.amepre.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Habib AG, Yakasai AM, Owolabi LF, Ibrahim A, Habib ZG, Gudaji M, Nashabaru I. Neurocognitive impairment in HIV-1-infected adults in sub-Saharan Africa: A systematic review and meta-analysis. International Journal of Infectious Diseases: IJID: Official Publication of the International Society for Infectious Diseases. 2013;17:e820–e831. doi: 10.1016/j.ijid.2013.06.011. [DOI] [PubMed] [Google Scholar]
- Hadigan C, Meigs JB, Corcoran C, Rietschel P, Piecuch S, Basgoz N, Grinspoon S. Metabolic abnormalities and cardiovascular disease risk factors in adults with human immunodeficiency virus infection and lipodystro-phy. Clinical Infectious Diseases. 2001;32:130–139. doi: 10.1086/317541. [DOI] [PubMed] [Google Scholar]
- Hajat A, Allison M, Diez-Roux AV, Jenny NS, Jorgensen NW, Szpiro AA, Kaufman JD. Long-term exposure to air pollution and markers of inflammation, coagulation, and endothelial activation: A repeat-measures analysis in the Multi-Ethnic Study of Atherosclerosis (MESA) Epidemiology. 2015;26:310–320. doi: 10.1097/EDE.0000000000000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer SM, Squires KE, Hughes MD, Grimes JM, Demeter LM, Currier JS, Fischl MA. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. New England Journal of Medicine. 1997;337:725–733. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- Hardy AW, Graham DR, Shearer GM, Herbeuval JP. HIV turns plasmacytoid dendritic cells (pDC) into TRAIL-expressing killer pDC and down-regulates HIV coreceptors by Toll-like receptor 7-induced IFN-alpha. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:17453–17458. doi: 10.1073/pnas.0707244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearps AC, Maisa A, Cheng WJ, Angelovich TA, Lichtfuss GF, Palmer CS, Crowe SM. HIV infection induces age-related changes to mono-cytes and innate immune activation in young men that persist despite combination antiretroviral therapy. AIDS. 2012;26:843–853. doi: 10.1097/QAD.0b013e328351f756. [DOI] [PubMed] [Google Scholar]
- Higgs P, Hyde M, Wiggins RD, Blane D. Researching quality of life in early old age: The importance of the sociological dimension. Social Policy & Administration. 2003;37:239–252. [Google Scholar]
- High KP, Brennan-Ing M, Clifford DB, Cohen MH, Currier J, Deeks SG, OAR Working Group on HIV Aging HIV and aging: State of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. Journal of Acquired Immune Deficiency Syndromes. 2012;60(Suppl. 1):S1–S18. doi: 10.1097/QAI.0b013e31825a3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochman S, Kim K. The impact of HIV and malaria coinfection: What is known and suggested venues for further study. Interdisciplinary Perspectives on Infectious Diseases. 2009;2009 doi: 10.1155/2009/617954. Article 617954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman IF, Jere CS, Taylor TE, Munthali P, Dyer JR, Wirima JJ, Molyneux ME. The effect of Plasmodium falciparum malaria on HIV-1 RNA blood plasma concentration. AIDS. 1999;13:487–494. doi: 10.1097/00002030-199903110-00007. [DOI] [PubMed] [Google Scholar]
- Hontelez JA, de Vlas SJ, Baltussen R, Newell ML, Bakker R, Tanser F, Barnighausen T. The impact of antiretroviral treatment on the age composition of the HIV epidemic in sub-Saharan Africa. AIDS. 2012;26(Suppl. 1):S19–30. doi: 10.1097/QAD.0b013e3283558526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu DC, Ma YF, Hur S, Li D, Rupert A, Scherzer R, Hsue PY. Plasma IL-6 levels are independently associated with atherosclerosis and mortality in HIV-infected individuals on suppressive antiretroviral therapy. AIDS. 2016;30:2065–2074. doi: 10.1097/QAD.0000000000001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsue PY, Hunt PW, Sinclair E, Bredt B, Franklin A, Killian M, Deeks SG. Increased carotid intima-media thickness in HIV patients is associated with increased cytomegalovirus-specific T-cell responses. AIDS. 2006;20:2275–2283. doi: 10.1097/QAD.0b013e3280108704. [DOI] [PubMed] [Google Scholar]
- Hu G, Zhou Y, Hong W, Tian J, Hu J, Peng G, Ran P. Development and systematic oxidative stress of a rat model of chronic bronchitis and emphysema induced by biomass smoke. Experimental Lung Research. 2013;39:229–240. doi: 10.3109/01902148.2013.797521. [DOI] [PubMed] [Google Scholar]
- Hunt PW, Brenchley J, Sinclair E, McCune JM, Roland M, Page-Shafer K, Deeks SG. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. The Journal of Infectious Diseases. 2008;197:126–133. doi: 10.1086/524143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt PW, Martin JN, Sinclair E, Bredt B, Hagos E, Lampiris H, Deeks SG. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. The Journal of Infectious Diseases. 2003;187:1534–1543. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- The INSIGHT START Study Group. Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, Neaton JD. Initiation of antiretroviral therapy in early asymptomatic HIV infection. New England Journal of Medicine. 2015;373:795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelsma J, Ferguson G. The determinants of self-reported health-related quality of life in a culturally and socially diverse South African community. Bulletin of the World Health Organization. 2004;82:206–212. [PMC free article] [PubMed] [Google Scholar]
- Jelsma J, Maclean E, Hughes J, Tinise X, Darder M. An investigation into the health-related quality of life of individuals living with HIV who are receiving HAART. AIDS Care. 2005;17:579–588. doi: 10.1080/09540120412331319714. [DOI] [PubMed] [Google Scholar]
- Jelsma J, Mkoka S, Amosun L, Nieuwveldt J. The reliability and validity of the Xhosa version of the EQ-5D. Disability and Rehabilitation. 2004;26:103–108. doi: 10.1080/09638280310001629705. [DOI] [PubMed] [Google Scholar]
- Johnson LF, Mossong J, Dorrington RE, Schomaker M, Hoffmann CJ, Keiser O, International Epidemiologic Databases to Evaluate AIDS Southern Africa Collaboration Life expectancies of South African adults starting antiretroviral treatment: Collaborative analysis of cohort studies. PLoS Medicine. 2013;10(4):e1001418. doi: 10.1371/journal.pmed.1001418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice AC, Braithwaite RS. Lessons learned from the first wave of aging with HIV. AIDS. 2012;26(Suppl. 1):S11–S18. doi: 10.1097/QAD.0b013e3283558500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabatereine NB, Tukahebwa EM, Kazibwe F, Twa-Twa JM, Barenzi JF, Zaramba S, Brooker S. Soil-transmitted helminthiasis in Uganda: Epidemiology and cost of control. Tropical Medicine & International Health. 2005;10:1187–1189. doi: 10.1111/j.1365-3156.2005.01509.x. [DOI] [PubMed] [Google Scholar]
- Kaplan RC, Kingsley LA, Gange SJ, Benning L, Jacobson LP, Lazar J, Hodis HN. Low CD4+ T cell count as a major atherosclerosis risk factor in HIV-infected women and men. AIDS. 2008;22(13):1615–1624. doi: 10.1097/QAD.0b013e328300581d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiannis-Voules DA, Biedermann P, Ekpo UF, Garba A, Langer E, Mathieu E, Vounatsou P. Spatial and temporal distribution of soil-transmitted helminth infection in sub-Saharan Africa: A systematic review and geostatistical meta-analysis. The Lancet Infectious Diseases. 2015;15:74–84. doi: 10.1016/S1473-3099(14)71004-7. [DOI] [PubMed] [Google Scholar]
- Kautz T, Bendavid E, Bhattacharya J, Miller G. AIDS and declining support for dependent elderly people in Africa: Retrospective analysis using demographic and health surveys. British Medical Journal. 2010;340:c2841. doi: 10.1136/bmj.c2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsley LA, Cuervo-Rojas J, Munoz A, Palella FJ, Post W, Witt MD, Kuller L. Subclinical coronary atherosclerosis, HIV infection and antiretroviral therapy: Multicenter AIDS Cohort Study. AIDS. 2008;22:1589–1599. doi: 10.1097/QAD.0b013e328306a6c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DB, Leyden WA, Xu L, Chao CR, Horberg MA, Towner WJ, Silverberg MJ. Declining relative risk for myocardial infarction among HIV-positive compared with HIV-negative individuals with access to care. Clinical Infectious Diseases. 2015;60:1278–1280. doi: 10.1093/cid/civ014. [DOI] [PubMed] [Google Scholar]
- Knudsen TB, Ertner G, Petersen J, Moller HJ, Moestrup SK, Eugen-Olsen J, Benfield T. Plasma soluble CD163 level independently predicts all-cause mortality in HIV-1-infected individuals. The Journal of Infectious Diseases. 2016;214:1198–1204. doi: 10.1093/infdis/jiw263. [DOI] [PubMed] [Google Scholar]
- Koroukian SM, Schiltz N, Warner DF, Sun J, Bakaki PM, Smyth KA, Given CW. Combinations of chronic conditions, functional limitations, and geriatric syndromes that predict health outcomes. Journal of General Internal Medicine. 2016;31:630–637. doi: 10.1007/s11606-016-3590-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowal P, Kahn K, Ng N, Naidoo N, Abdullah S, Bawah A, Tollman SM. Ageing and adult health status in eight lower-income countries: The INDEPTH WHO-SAGE collaboration. Global Health Action. 2010;3 doi: 10.3402/gha.v3i0.5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, Neaton JD. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Medicine. 2008;5(10):e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwarisiima D, Balzer L, Heller D, Kotwani P, Chamie G, Clark T, Kamya MR. Population-based assessment of hypertension epidemiology and risk factors among HIV-positive and general populations in rural Uganda. PLoS ONE. 2016;11(5):e0156309. doi: 10.1371/journal.pone.0156309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankowski AJ, Tsai AC, Kanyesigye M, Bwana M, Haberer JE, Wenger M, Siedner MJ. Empiric deworming and CD4 count recovery in HIV-infected Ugandans initiating antiretroviral therapy. PLoS Neglected Tropical Diseases. 2014;8:e3036. doi: 10.1371/journal.pntd.0003036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MS, Hang JQ, Zhang FY, Dai HL, Su L, Christiani DC. In-home solid fuel use and cardiovascular disease: A cross-sectional analysis of the Shanghai Putuo study. Environmental Health. 2012;11:18. doi: 10.1186/1476-069X-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Byakwaga H, Boum Y, Burdo TH, Williams KC, Lederman MM, Hunt PW. Immunologic pathways that predict mortality in HIV-infected Ugandans initiating ART. The Journal of Infectious Diseases. 2017;215(8):1270–1274. doi: 10.1093/infdis/jix113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SA, Mefford JA, Huang Y, Witte JS, Martin JN, Haas DW, Kroetz DL. Host genetic predictors of the kynurenine pathway of tryp-tophan catabolism among treated HIV-infected Ugandans. AIDS. 2016;30:1807–1815. doi: 10.1097/QAD.0000000000001124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SA, Sinclair E, Jain V, Huang Y, Epling L, Van Natta M, Hunt PW. Low proportions of CD28- CD8+ T cells expressing CD57 can be reversed by early ART initiation and predict mortality in treated HIV infection. The Journal of Infectious Diseases. 2014;210:374–382. doi: 10.1093/infdis/jiu109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng S, Chaves P, Koenig K, Walston J. Serum interleukin-6 and hemoglobin as physiological correlates in the geriatric syndrome of frailty: A pilot study. Journal of the American Geriatrics Society. 2002;50:1268–1271. doi: 10.1046/j.1532-5415.2002.50315.x. [DOI] [PubMed] [Google Scholar]
- Li XY, Gilmour PS, Donaldson K, MacNee W. Free radical activity and pro-inflammatory effects of particulate air pollution (PM10) in vivo and in vitro. Thorax. 1996;51:1216–1222. doi: 10.1136/thx.51.12.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien E, Aukrust P, Sundan A, Muller F, Froland SS, Espevik T. Elevated levels of serum-soluble CD14 in human immunodeficiency virus type 1 (HIV-1) infection: Correlation to disease progression and clinical events. Blood. 1998;92:2084–2092. [PubMed] [Google Scholar]
- Longenecker CT, Jiang Y, Orringer CE, Gilkeson RC, Debanne S, Funderburg NT, McComsey GA. Soluble CD14 is independently associated with coronary calcification and extent of subclinical vascular disease in treated HIV infection. AIDS. 2014;28:969–977. doi: 10.1097/QAD.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louwagie GM, Bachmann MO, Meyer K, Booysen Fle R, Fairall LR, Heunis C. Highly active antiretroviral treatment and health related quality of life in South African adults with human immunodeficiency virus infection: A cross-sectional analytical study. BMC Public Health. 2007;7:244. doi: 10.1186/1471-2458-7-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons JL, Uno H, Ancuta P, Kamat A, Moore DJ, Singer EJ, Gabuzda D. Plasma sCD14 is a biomarker associated with impaired neurocog-nitive test performance in attention and learning domains in HIV infection. Journal of Acquired Immune Deficiency Syndromes. 2011;57:371–379. doi: 10.1097/QAI.0b013e3182237e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaulay R, Akbar AN, Henson SM. The role of the T cell in age-related inflammation. Age. 2013;35:563–572. doi: 10.1007/s11357-012-9381-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhavan S, Schatz EJ. Coping with change: Household structure and composition in rural South Africa, 1992-2003. Scandinavian Journal of Public Health. 2007;69:85–93. doi: 10.1080/14034950701355627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaza A, Mossong J, Barnighausen T, Newell ML. Hypertension and obesity in adults living in a high HIV prevalence rural area in South Africa. PLoS ONE. 2012;7(10):e47761. doi: 10.1371/journal.pone.0047761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manne-Goehler J, Montana L, Gomez-Olive FX, Rohr J, Harling G, Wagner GR, Gaziano JM. The ART advantage: Healthcare utilization for diabetes and hypertension in rural South Africa. Journal of Acquired Immune Deficiency Syndromes. 2017;75(5):561–567. doi: 10.1097/QAI.0000000000001445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GE, Gouillou M, Hearps AC, Angelovich TA, Cheng AC, Lynch F, Crowe SM. Age-associated changes in monocyte and innate immune activation markers occur more rapidly in HIV infected women. PLoS ONE. 2013;8(1):e55279. doi: 10.1371/journal.pone.0055279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez P, Tsai AC, Muzoora C, Kembabazi A, Weiser SD, Huang Y, Hunt PW. Reversal of the Kynurenine pathway of tryptophan catabolism may improve depression in ART-treated HIV-infected Ugandans. Journal of Acquired Immune Deficiency Syndromes. 2014;65:456–462. doi: 10.1097/QAI.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur JC, Steiner J, Sacktor N, Nath A. Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap. Annals of Neurology. 2010;67:699–714. doi: 10.1002/ana.22053. [DOI] [PubMed] [Google Scholar]
- McCutchan JA, Wu JW, Robertson K, Koletar SL, Ellis RJ, Cohn S, Williams PL. HIV suppression by HAART preserves cognitive function in advanced, immune-reconstituted AIDS patients. AIDS. 2007;21:1109–1117. doi: 10.1097/QAD.0b013e3280ef6acd. [DOI] [PubMed] [Google Scholar]
- Mendez-Lagares G, Romero-Sanchez MC, Ruiz-Mateos E, Genebat M, Ferrando-Martinez S, Munoz-Fernandez MA, Leal M. Long-term suppressive combined antiretroviral treatment does not normalize the serum level of soluble CD14. The Journal of Infectious Diseases. 2013;207:1221–1225. doi: 10.1093/infdis/jit025. [DOI] [PubMed] [Google Scholar]
- Mills EJ, Bakanda C, Birungi J, Chan K, Ford N, Cooper CL, Hogg RS. Life expectancy of persons receiving combination antiretroviral therapy in low-income countries: A cohort analysis from Uganda. Annals of Internal Medicine. 2011;155:209–216. doi: 10.7326/0003-4819-155-4-201108160-00358. [DOI] [PubMed] [Google Scholar]
- Mills EJ, Barnighausen T, Negin J. HIV and aging—Preparing for the challenges ahead. New England Journal of Medicine. 2012;366:1270–1273. doi: 10.1056/NEJMp1113643. [DOI] [PubMed] [Google Scholar]
- Mitnitsky AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. The Scientific World Journal. 2001;1:323–336. doi: 10.1100/tsw.2001.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocroft A, Vella S, Benfield TL, Chiesi A, Miller V, Gargalianos P, Lundgren JD. Changing patterns of mortality across Europe in patients infected with HIV-1. EuroSIDA Study Group. The Lancet. 1998;352:1725–1730. doi: 10.1016/s0140-6736(98)03201-2. [DOI] [PubMed] [Google Scholar]
- Mudege NN, Ezeh AC. Gender, aging, poverty and health: Survival strategies of older men and women in Nairobi slums. Journal of Aging Studies. 2009;23:245–257. doi: 10.1016/j.jaging.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muga GO, Onyango-Ouma W. Changing household composition and food security among the elderly caretakers in rural Western Kenya. Journal of Cross-Cultural Gerontology. 2009;24:259–272. doi: 10.1007/s10823-008-9090-6. [DOI] [PubMed] [Google Scholar]
- Negin J, Barnighausen T, Lundgren JD, Mills EJ. Aging with HIV in Africa: The challenges of living longer. AIDS. 2012;26(Suppl. 1):S1–S5. doi: 10.1097/QAD.0b013e3283560f54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nsanzimana S, Remera E, Kanters S, Chan K, Forrest JI, Ford N, Mills EJ. Life expectancy among HIV-positive patients in Rwanda: A retrospective observational cohort study. The Lancet Global Health. 2015;3:e169–e177. doi: 10.1016/S2214-109X(14)70364-X. [DOI] [PubMed] [Google Scholar]
- Nyongesa MK, Sigilai A, Hassan AS, Thoya J, Odhiambo R, Van de Vijver FJ, Abubakar A. A mixed methods approach to adapting and evaluating the functional assessment of HIV infection (FAHI), Swahili version, for use with low literacy populations. PLoS ONE. 2017;12(4):e0175021. doi: 10.1371/journal.pone.0175021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obel N, Thomsen HF, Kronborg G, Larsen CS, Hildebrandt PR, Sorensen HT, Gerstoft J. Ischemic heart disease in HIV-infected and HIV-uninfected individuals: A population-based cohort study. Clinical Infectious Diseases. 2007;44:1625–1631. doi: 10.1086/518285. [DOI] [PubMed] [Google Scholar]
- O’Brien KK, Bayoumi AM, Strike C, Young NL, King K, Davis AM. How do existing HIV-specific instruments measure up? Evaluating the ability of instruments to describe disability experienced by adults living with HIV. Health and Quality of Life Outcomes. 2010;8:88. doi: 10.1186/1477-7525-8-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogunmefun C, Gilbert L, Schatz E. Older female caregivers and HIV/AIDS-related secondary stigma in rural South Africa. Journal of Cross-Cultural Gerontology. 2011;26:85–102. doi: 10.1007/s10823-010-9129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okello S, Asiimwe SB, Kanyesigye M, Muyindike WR, Boum Y, Mwebesa BB, II, Siedner MJ. D-dimer levels and traditional risk factors are associated with incident hypertension among HIV-infected individuals initiating antiretroviral therapy in Uganda. Journal of Acquired Immune Deficiency Syndromes. 2016 doi: 10.1097/QAI.0000000000001074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okello S, Kanyesigye M, Muyindike WR, Annex BH, Hunt PW, Haneuse S, Siedner MJ. Incidence and predictors of hypertension in adults with HIV-initiating antiretroviral therapy in South-Western Uganda. Journal of Hypertension. 2015;33:2039–2045. doi: 10.1097/HJH.0000000000000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olwenyi OA, Naluyima P, Cham F, Quinn TC, Serwadda D, Sewankambo NK, Eller MA. Brief report: Differential associations of interleukin 6 and intestinal fatty acid-binding protein with progressive untreated HIV-1 infection in Rakai, Uganda. Journal of Acquired Immune Deficiency Syndromes. 2016;72:15–20. doi: 10.1097/QAI.0000000000000915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oursler KK, Goulet JL, Crystal S, Justice AC, Crothers K, Butt AA, Sorkin JD. Association of age and comorbidity with physical function in HIV-infected and uninfected patients: Results from the Veterans Aging Cohort Study. AIDS Patient Care and STDs. 2011;25:13–20. doi: 10.1089/apc.2010.0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painschab MS, Davila-Roman VG, Gilman RH, Vasquez-Villar AD, Pollard SL, Wise RA, CRONICAS Cohort Study Group Chronic exposure to biomass fuel is associated with increased carotid artery intima-media thickness and a higher prevalence of atherosclerotic plaque. Heart. 2013;99:984–991. doi: 10.1136/heartjnl-2012-303440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palella FJ, Delaney KM, Jr, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Holmberg SD. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. New England Journal of Medicine. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- Park EJ, Roh J, Kim Y, Park K, Kim DS, Yu SD. PM 2.5 collected in a residential area induced Th1-type inflammatory responses with oxidative stress in mice. Environmental Research. 2011;111:348–355. doi: 10.1016/j.envres.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Pathai S, Bajillan H, Landay AL, High KP. Is HIV a model of accelerated or accentuated aging? Journal of Gerontology, Series A: Biological Sciences & Medical Sciences. 2014;69:833–842. doi: 10.1093/gerona/glt168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathai S, Gilbert C, Weiss HA, Cook C, Wood R, Bekker LG, Lawn SD. Frailty in HIV-infected adults in South Africa. Journal of Acquired Immune Deficiency Syndromes. 2013;62:43–51. doi: 10.1097/QAI.0b013e318273b631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathai S, Lawn SD, Gilbert CE, McGuinness D, McGlynn L, Weiss HA, Shiels PG. Accelerated biological ageing in HIV-infected individuals in South Africa: A case-control study. AIDS. 2013;27:2375–2384. doi: 10.1097/QAD.0b013e328363bf7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne CF, Gomez-Olive FX, Kahn K, Berkman L. Physical function in an aging population in rural South Africa: Findings from HAALSI and cross-national comparisons with HRS sister studies. Journals of Gerontology, Series B: Psychological Sciences & Social Sciences. 2017 doi: 10.1093/geronb/gbx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck RN, Shedafa R, Kalluvya S, Downs JA, Todd J, Suthanthiran M, Kataraihya JB. Hypertension, kidney disease, HIV and anti-retroviral therapy among Tanzanian adults: A cross-sectional study. BMC Medicine. 2014;12:125. doi: 10.1186/s12916-014-0125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggott DA, Muzaale AD, Mehta SH, Brown TT, Patel KV, Leng SX, Kirk GD. Frailty, HIV infection, and mortality in an aging cohort of injection drug users. PLoS ONE. 2013;8(1):e54910. doi: 10.1371/journal.pone.0054910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggott DA, Varadhan R, Mehta SH, Brown TT, Li H, Walston JD, Kirk GD. Frailty, inflammation, and mortality among persons aging with HIV infection and injection drug use. Journal of Gerontology, Series A: Biological Sciences & Medical Sciences. 2015;70:1542–1547. doi: 10.1093/gerona/glv107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt J, Myer L, Wood R. Quality of life and the impact of drug toxicities in a South African community-based antiretroviral programme. Journal of the International AIDS Society. 2009;12:5. doi: 10.1186/1758-2652-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince M, Ferri CP, Acosta D, Albanese E, Arizaga R, Dewey M, Uwakwe R. The protocols for the 10/66 dementia research group population-based research programme. BMC Public Health. 2007;7:165. doi: 10.1186/1471-2458-7-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince MJ, Wu F, Guo Y, Gutierrez Robledo LM, O’Donnell M, Sullivan R, Yusuf S. The burden of disease in older people and implications for health policy and practice. The Lancet. 2015;385:549–562. doi: 10.1016/S0140-6736(14)61347-7. [DOI] [PubMed] [Google Scholar]
- Psaros C, Barinas J, Robbins GK, Bedoya CA, Park ER, Safren SA. Reflections on living with HIV over time: Exploring the perspective of HIV-infected women over 50. Aging & Mental Health. 2015;19:121–128. doi: 10.1080/13607863.2014.917608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reniers G, Blom S, Calvert C, Martin-Onraet A, Herbst AJ, Eaton JW, Hosegood V. Trends in the burden of HIV mortality after roll-out of anti-retroviral therapy in KwaZulu-Natal, South Africa: An observational community cohort study. The Lancet HIV. 2017;4:e113–e121. doi: 10.1016/S2352-3018(16)30225-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riha J, Karabarinde A, Ssenyomo G, Allender S, Asiki G, Kamali A, Seeley J. Urbanicity and lifestyle risk factors for cardiometabolic diseases in rural Uganda: A cross-sectional study. PLoS Medicine. 2014;11(7):e1001683. doi: 10.1371/journal.pmed.1001683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Penney AT, Iudicello JE, Riggs PK, Doyle K, Ellis RJ, Letendre SL, The HIV Neurobehavioral Research Program (HNRP) Group Co-morbidities in persons infected with HIV: Increased burden with older age and negative effects on health-related quality of life. AIDS Patient Care and STDs. 2013;27:5–16. doi: 10.1089/apc.2012.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubtsova AA, Kempf MC, Taylor TN, Konkle-Parker D, Wingood GM, Holstad MM. Healthy aging in older women living with HIV infection: A systematic review of psychosocial factors. Current HIV/AIDS Reports. 2017;14:17–30. doi: 10.1007/s11904-017-0347-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rylance J, Fullerton DG, Scriven J, Aljurayyan AN, Mzinza D, Barrett S, Gordon SB. Household air pollution causes dose-dependent inflammation and altered phagocytosis in human macrophages. American Journal of Respiratory Cell and Molecular Biology. 2015;52:584–593. doi: 10.1165/rcmb.2014-0188OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabin CA, Reiss P, Ryom L, Phillips AN, Weber R, Law M, D:A:D Study Group Is there continued evidence for an association between aba-cavir usage and myocardial infarction risk in individuals with HIV? A cohort collaboration. BMC Medicine. 2016;14:61. doi: 10.1186/s12916-016-0588-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor NC, Bacellar H, Hoover DR, Nance-Sproson TE, Selnes OA, Miller EN, McArthur JC. Psychomotor slowing in HIV infection: A predictor of dementia, AIDS and death. Journal of NeuroVirology. 1996;2:404–410. doi: 10.3109/13550289609146906. [DOI] [PubMed] [Google Scholar]
- Salgame P, Yap GS, Gause WC. Effect of helminth-induced immunity on infections with microbial pathogens. Nature Immunology. 2013;14:1118–1126. doi: 10.1038/ni.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in nonsmokers. The Lancet. 2009;374:733–743. doi: 10.1016/S0140-6736(09)61303-9. [DOI] [PubMed] [Google Scholar]
- Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, INSIGHT SMART Study Group Plasma levels of soluble CD14 independently predict mortality in HIV infection. The Journal of Infectious Diseases. 2011;203:780–790. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, McArthur JC. HIV-associated neurocognitive disorder—Pathogenesis and prospects for treatment. Nature Reviews Neurology. 2016;12:234–248. doi: 10.1038/nrneurol.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz EJ. “Taking care of my own blood”: Older women’s relationships to their households in rural South Africa. Scandinavian Journal of Public Health. 2007;35(Suppl. 69):147–154. doi: 10.1080/14034950701355676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz EJ, Gilbert L. “My legs affect me a lot…. I can no longer walk to the forest to fetch firewood”: Challenges related to health and the performance of daily tasks for older women in a high HIV context. Health Care for Women International. 2014;35:771–788. doi: 10.1080/07399332.2014.900064. [DOI] [PubMed] [Google Scholar]
- Schatz EJ, Madhavan S, Williams J. Female-headed households contending with AIDS-related hardship in rural South Africa. Health Place. 2011;17:598–605. doi: 10.1016/j.healthplace.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholten F, Mugisha J, Seeley J, Kinyanda E, Nakubukwa S, Kowal P, Grosskurth H. Health and functional status among older people with HIV/AIDS in Uganda. BMC Public Health. 2011;11:886. doi: 10.1186/1471-2458-11-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scully E, Lockhart A, Huang L, Robles Y, Becerril C, Romero-Tejeda M, Lin NH. Elevated levels of microbial translocation markers and CCL2 among older HIV-1-infected men. The Journal of Infectious Diseases. 2016;213:771–775. doi: 10.1093/infdis/jiv501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaberg EC, Benning L, Sharrett AR, Lazar JM, Hodis HN, Mack WJ, Kaplan RC. Association between human immunodeficiency virus infection and stiffness of the common carotid artery. Stroke: A Journal of Cerebral Circulation. 2010;41:2163–2170. doi: 10.1161/STROKEAHA.110.583856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Villar S, Sainz T, Lee SA, Hunt PW, Sinclair E, Shacklett BL, Deeks SG. HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS Pathogens. 2014;10(5):e1004078. doi: 10.1371/journal.ppat.1004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siedner MJ, Kim JH, Haberer JE, Martin JN, Boum Y, II, Tsai AC, Bangsberg DR. HIV infection and vascular stiffness among older adults taking antiretroviral therapy in rural Uganda; 8th International AIDS Society Conference on HIV Pathogenesis; Vancouver, British Columbia, Canada. 2015. Jul 19-22, [Google Scholar]
- Siedner MJ, Ng CK, Bassett IV, Katz IT, Bangsberg DR, Tsai AC. Trends in CD4 count at presentation to care and treatment initiation in sub-Saharan Africa, 2002-2013: A meta-analysis. Clinical Infectious Diseases. 2015;60:1120–1127. doi: 10.1093/cid/ciu1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siedner MJ, Kim JH, Nakku RS, Hemphill L, Triant VA, Haberer JE, Bangsberg DR. HIV infection and arterial stiffness among older-adults taking antiretroviral therapy in rural Uganda. AIDS. 2016;30(4):667–670. doi: 10.1097/QAD.0000000000000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CJ, Ryom L, Weber R, Morlat P, Pradier C, Reiss P, D:A:D Study Group Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): A multicohort collaboration. The Lancet. 2014;384:241–248. doi: 10.1016/S0140-6736(14)60604-8. [DOI] [PubMed] [Google Scholar]
- Ssengonzi R. The impact of HIV/AIDS on the living arrangements and well-being of elderly caregivers in rural Uganda. AIDS Care. 2009;21:309–314. doi: 10.1080/09540120802183461. [DOI] [PubMed] [Google Scholar]
- Steyn NP, Nel JH, Parker WA, Ayah R, Mbithe D. Dietary, social, and environmental determinants of obesity in Kenyan women. Scandinavian Journal of Public Health. 2011;39:88–97. doi: 10.1177/1403494810384426. [DOI] [PubMed] [Google Scholar]
- Sussan TE, Ingole V, Kim JH, McCormick S, Negherbon J, Fallica J, Biswal S. Source of biomass cooking fuel determines pulmonary response to household air pollution. American Journal of Respiratory Cell and Molecular Biology. 2014;50:538–548. doi: 10.1165/rcmb.2013-0201OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, Picker LJ. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. The Journal of Experimental Medicine. 2005;202:673–685. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada S, Weiser SD, Kumbakumba E, Muzoora C, Martin JN, Hunt PW, Tsai AC. The dynamic relationship between social support and HIV-related stigma in rural Uganda. Annals of Behavioral Medicine. 2014;48:26–37. doi: 10.1007/s12160-013-9576-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurn M, Gustafson DR. Faces of frailty in aging with HIV infection. Current HIV/AIDS Reports. 2017;14:31–37. doi: 10.1007/s11904-017-0348-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien PC, Choi AI, Zolopa AR, Benson C, Tracy R, Scherzer R, Grunfeld C. Inflammation and mortality in HIV-infected adults: Analysis of the FRAM study cohort. Journal of Acquired Immune Deficiency Syndromes. 2010;55:316–322. doi: 10.1097/QAI.0b013e3181e66216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. The Journal of Clinical Endocrinology & Metabolism. 2007;92:2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott KA, Richardson A, Hudgens MA, Abel K. Immune activation and regulation in simian immunodeficiency virus-Plasmodium fragile-coinfected rhesus macaques. Journal of Virology. 2013;87:9523–9537. doi: 10.1128/JVI.00861-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai AC, Bangsberg DR, Kegeles SM, Katz IT, Haberer JE, Muzoora C, Weiser SD. Internalized stigma, social distance, and disclosure of HIV seropositivity in rural Uganda. Annals of Behavioral Medicine. 2013;46:285–294. doi: 10.1007/s12160-013-9514-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JD, Faulkner H, Kamgno J, Cormont F, Van Snick J, Else KJ, Bradley JE. Th2 cytokines are associated with reduced worm burdens in a human intestinal helminth infection. The Journal of Infectious Diseases. 2003;188:1768–1775. doi: 10.1086/379370. [DOI] [PubMed] [Google Scholar]
- Uebel KE, Nash J, Avalos A. Caring for the caregivers: Models of HIV/AIDS care and treatment provision for health care workers in Southern Africa. The Journal of Infectious Diseases. 2007;196(Suppl. 3):S500–S504. doi: 10.1086/521113. [DOI] [PubMed] [Google Scholar]
- UNAIDS Global AIDS Epidemic Update. Geneva: UN Joint Programme on HIV/AIDS. Available at: http://www.unaids.org/sites/default/files/media_asset/global-AIDS-update-2016_en.pdf (accessed 15 June 2017)
- Wada NI, Jacobson LP, Margolick JB, Breen EC, Macatangay B, Penugonda S, Bream JH. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS. 2015;29:463–471. doi: 10.1097/QAD.0000000000000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walson J, Singa B, Sangare L, Naulikha J, Piper B, Richardson B, John-Stewart G. Empiric deworming to delay HIV disease progression in adults with HIV who are ineligible for initiation of antiretroviral treatment (the HEAT study): A multi-site, randomised trial. The Lancet Infectious Diseases. 2012;12:925–932. doi: 10.1016/S1473-3099(12)70207-4. [DOI] [PubMed] [Google Scholar]
- Wang SY, Shamliyan TA, Talley KM, Ramakrishnan R, Kane RL. Not just specific diseases: Systematic review of the association of geriatric syndromes with hospitalization or nursing home admission. Archives of Gerontology and Geriatrics. 2013;57:16–26. doi: 10.1016/j.archger.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Wilson EM, Singh A, Hullsiek KH, Gibson D, Henry WK, Lichtenstein K, Study to Understand the Natural History of HIV/AIDS in the Era of Effective Therapy (SUN Study) Investigators Monocyte-activation phenotypes are associated with biomarkers of inflammation and coagulation in chronic HIV infection. The Journal of Infectious Diseases. 2014;210:1396–1406. doi: 10.1093/infdis/jiu275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach. 2nd. Geneva: World Health Organization; 2016. [PubMed] [Google Scholar]
- Wouters E, Meulemans H, Van Rensburg HC, Heunis JC, Mortelmans D. Short-term physical and emotional health outcomes of public sector ART in the Free State province of South Africa. Quality of Life Research. 2007;16:1461–1471. doi: 10.1007/s11136-007-9260-y. [DOI] [PubMed] [Google Scholar]
- Xie D, McElhaney JE. Lower GrB+ CD62Lhigh CD8 TCM effector lymphocyte response to influenza virus in older adults is associated with increased CD28null CD8 T lymphocytes. Mechanisms of Ageing and Development. 2007;128:392–400. doi: 10.1016/j.mad.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


