Abstract
Recent preclinical studies have suggested an antifibrotic role for tricyclic antidepressants. Using the Electronically Retrieved Cohort of HCV Infected Veterans, we aimed to evaluate the impact of TCA use on fibrosis progression and development of hepatocellular carcinoma (HCC) among HCV-infected persons. Subjects were categorized according to use of tricyclic antidepressants, selective serotonin reuptake inhibitors, or no antidepressants. tricyclic antidepressants or selective serotonin uptake inhibitors use was defined according to cumulative defined daily dose, and categories were mutually exclusive. Subjects with HIV coinfection, HbsAg positivity, cirrhosis, or HCC at baseline were excluded. Outcomes were liver fibrosis progression measured by APRI scores and incident HCC. We utilized Cox proportional hazards regression to determine predictors of cirrhosis, defined as APRI > 2, and iHCC. Among 128,201 eligible HCV+ persons, 4% received tricyclic antidepressants, 43% received selective serotonin uptake inhibitors, and 53% received no antidepressants. Fewer tricyclic antidepressants users had drug abuse (34% and 43%) and alcohol abuse (32% vs 42%) compared to selective serotonin uptake inhibitor users. After adjusting for age, baseline APRI score, diabetes, hypertension, alcohol use, drug abuse, and HCV RNA levels, tricyclic antidepressants use was associated with decreased risk of cirrhosis (hazard ratio [HR] = 0.77, 95% CI = 0.60, 0.99) and delayed time to development of cirrhosis, but not with decreased iHCC. In conclusion among a large cohort of HCV-positive Veterans, tricyclic antidepressants use was associated with decreased fibrosis progression and lower risk of developing cirrhosis. These data provide supportive evidence for the beneficial effects of tricyclic antidepressants on progression of liver fibrosis in patients with chronic HCV infection.
Keywords: HCV, tricyclic antidepressant, fibrosis, cirrhosis, ERCHIVES
End stage liver disease affects approximately 600,000 Americans and accounts for 30,000 deaths per year [1, 2]. Regression of liver fibrosis can occur with cessation of chronic liver injury, including eradication of hepatitis C virus (HCV) [3–6], but pharmacological therapies able to treat this lethal endpoint of chronic liver disease have yet to be developed [7].
Recent evidence has suggested that tricyclic antidepressant (TCA) use may reduce fibrogenesis in hepatic stellate cells [8, 9], the primary cell type responsible for liver fibrosis [10, 11]. Moreover, treatment with amitriptyline, a TCA, has been shown to reduce the development of fibrosis in vivo [9, 12]. Specifically, mice treated with carbon tetrachloride (CCl4) to induce fibrosis experienced less fibrosis when treated concomitantly with amitriptyline [9]. In addition, mice pretreated with CCl4 for 5 weeks prior to treatment with amitriptyline experienced fibrosis regression [9]. Similarly, in a high fat diet model of nonalcoholic steatohepatitis (NASH), mice receiving amitriptyline experienced less fibrosis [12]. Recent data have also suggested a possible antifibrotic role of TCAs in chronic renal disease [13] and cystic fibrosis [14].
TCAs have been used in clinical practice for over forty years for several indications, including depression and pain [15]. There have been no studies to determine the relationship between TCA use and fibrosis progression among patients with chronic liver disease. Thus, we performed this study in a national cohort of HCV-infected persons to delineate the impact of TCAs upon fibrosis progression, development of liver cirrhosis, and incidence of HCC.
Methods
Study Population
The study population consisted of persons with HCV infection in the Electronically Retrieved Cohort of HCV Infected Veterans (ERCHIVES). ERCHIVES is a national cohort of HCV infected and uninfected Veterans [16–18]. Briefly, we identified all HCV infected Veterans between 2001 and 2015 through the VHA’s (Veterans Health Administration) Corporate Data Warehouse (CDW) based on a positive HCV antibody test. Corporate Data Warehouse is a repository that consists of data from VHA administrative and clinical systems. Demographic, laboratory, pharmacy, clinical encounters, health factors, and vital signs data were retrieved from the CDW using established algorithms to create a comprehensive database. Subjects with human immunodeficiency virus (HIV) coinfection and those with positive hepatitis B surface antigen (HBsAg) were excluded. Furthermore, we excluded subjects with missing laboratory data at baseline to calculate Aspartate Aminotransferase Platelet Ratio Index (APRI) scores as well as those for whom we could not determine an APRI score at least once after use of a TCA or a selective serotonin reuptake inhibitor (SSRI). Subjects with cirrhosis at baseline, defined as APRI > 2, were also excluded from the analysis.
Definitions
Baseline was defined as the date of first positive HCV antibody test. Laboratory data were collected at annual intervals and the APRI score was determined as follows:
Cirrhosis was defined as an APRI score >2 based on prior studies [19]. An average of two values closest to the start of each year (± 6 months) was used to calculate APRI.
Subjects were defined as having diabetes if any of the following criteria were met: (1) glucose ≥200 mg/dL on two separate occasions; (2) International Classification of Diseases, Ninth Revision (ICD-9) codes (two or more outpatient or one or more inpatient) plus treatment with insulin or an oral hypoglycemic for 30 days or more; (3) ICD-9 codes (two outpatient or one inpatient) plus glucose ≥126 mg/dL on two separate occasions; and (4) glucose ≥200 mg/dL on one occasion plus treatment with insulin or an oral hypoglycemic for 30 days or more [20, 21]. History of alcohol dependence or abuse, history of drug dependence or abuse, and hypertension were determined according to ICD-9 codes (at least two outpatient or one inpatient) [22]. Diagnosis of HCC was identified according to the presence of one inpatient or two outpatient ICD-9 diagnoses [20, 23]. Anemia was defined as hemoglobin < 12 g/dL for women and <13 g/dL for men [16]. Lipid profiles included total cholesterol (TC), low-density lipoprotein (LDL), high-density lipoprotein (HDL), and triglyceride (TG).
Exposures to Antidepressant Medications
TCA prescriptions included amoxapine, amitriptyline, desipramine, doxepin, protriptyline, nortriptyline, imipramine, and trimipramine. Selective serotonin reuptake inhibitors (SSRIs) included escitalopram, fluoxetine, citalopram, fluvoxamine, sertraline, paroxetine, and duloxetine. We collected the numbers of days prescribed, the dates of prescriptions ordered, number of pills per prescription, and number of refills prescribed. As defined by the World Health Organization, the defined daily dose (DDD) is the average maintenance dose per day of a drug consumed by an adult [24]. The TCA DDD was then calculated for each subject using the following formula: DDD = (total amount of drug prescribed on a daily basis to a patient)/(amount of drug in a DDD) [24].
The cumulative defined daily dose (cDDD) is defined as the sum of DDDs dispensed, and was recalculated each year during the study period. TCA use was defined as TCA ≥28 cDDD and no SSRI use during the study period. SSRI use was defined as SSRI ≥28 cDDD and no TCA use. Subjects who received both TCA ≥28 cDDD and SSRI ≥28 cDDD were excluded from the study. Subjects were identified to use no antidepressants if any of the following criteria were met: (1) no receipt of SSRIs or TCAs; (2) SSRI receipt <28 cDDD; and (3) TCA receipt <28 cDDD. Long-term users of TCAs were defined as TCA ≥ 180 cDDD.
Outcomes
Our primary outcome measure was development of cirrhosis as defined by APRI > 2, and time to cirrhosis was determined to be the date of first APRI > 2. Our secondary outcome measure was incident hepatocellular carcinoma (iHCC) based on ICD-9 codes as defined above, and time to HCC was defined as date of first ICD-9 code of HCC. Time at risk was defined from first positive HCV antibody date to the first diagnosis of cirrhosis, iHCC, last observation date in ERCHIVES, or death.
Statistical Analysis
The study population was divided into three groups according to receipt of antidepressants: those who received only TCAs, those who received only SSRIs, and those who received no antidepressants. To analyze baseline characteristics between the groups, we utilized the chi- squared test for categorical and the t test for continuous variables. We plotted mean APRI score for each group to compare fibrosis progression over the study period. We generated Kaplan-Meier’s curves to compare time to cirrhosis and iHCC by receipt group. We utilized Cox’s proportional hazards model to determine predictors of cirrhosis. A P value < 0.05 was considered significant where comparisons were made. We used SAS version 9.4 (SAS Institute Inc., Cary, NC) for statistical analyses.
Regulatory Approval
The institutional review board at VA Pittsburgh Healthcare System approved the study. We obtained appropriate permissions from the data sources that provided ERCHIVES data.
Results
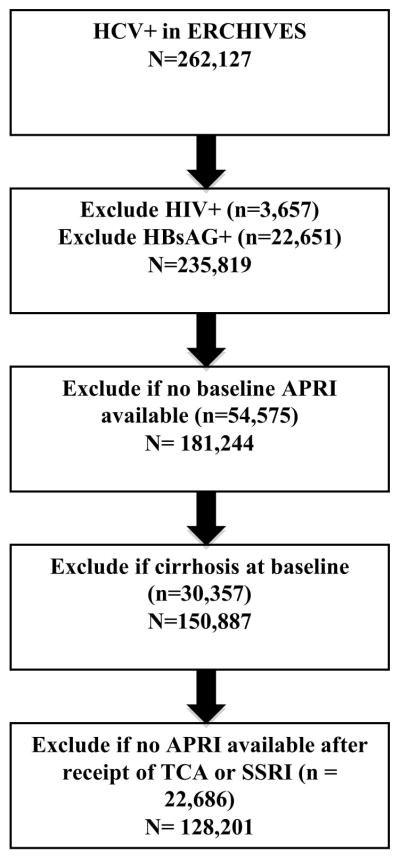
We identified 262,127 persons with a positive HCV serology without a subsequent negative serology in the ERCHIVES database. Subjects with HIV coinfection (n = 3,657), positive HBsAg (n = 22,651), missing baseline labs to calculate APRI score (n = 54,575), cirrhosis at baseline (n = 30,357), and missing labs to calculate APRI score at least one time after receipt of TCA or SSRI (n = 22,686) were excluded. Following these exclusions, our dataset consisted of 128,201 subjects (Figure 1).
Figure 1.
Study flow diagram.
Among the 128,201 remaining subjects, 5,190 (4%) received TCAs; 54,948 (43%) received SSRIs; and 68,063 (53%) received no antidepressants. Comparison of subjects receiving TCAs, SSRIs, and subjects receiving no antidepressants is displayed in Table 1. Among TCA users, the median age (interquartile range; IQR) was 52 (48, 57) and 97% were male. The majority of subjects with available genotype information had genotype 1 infection, and we observed similar HCV RNA levels at baseline among the three groups. HCV RNA results were available for 89,863 persons at baseline, of whom 19,141 (21.3%) had undetectable HCV RNA. Of 4313 patients with a documented HCV RNA any time after EOT+12 weeks, 1594 (37%) achieved a sustained virologic response.
Table 1.
Baseline* Characteristics
| Characteristic | Receipt of TCAs† (N = 5,190) | Receipt of SSRIs† (N = 54,948) | No Antidepressant Use† (N = 68,063) | P value |
|---|---|---|---|---|
|
| ||||
| Age in years, median (IQR) | 52.0 (48.0, 57.0) | 52.0 (47.3, 56.0) | 55.0 (50.0, 60.0) | <0.01 |
|
| ||||
| Race, % | ||||
|
| ||||
| White | 54.9 | 57.4 | 52.5 | <0.01 |
|
| ||||
| Black | 32.3 | 28.6 | 31.7 | |
|
| ||||
| Hispanic | 3.6 | 4.4 | 3.3 | |
|
| ||||
| Other | 9.2 | 9.6 | 12.4 | |
|
| ||||
| Sex, % male | 97.0 | 96.0 | 97.5 | <0.01 |
|
| ||||
| Baseline median HCV RNA (IQR), log10 | 4.7 (1.6, 6.5) 1683 missing |
5.0 (1.6, 6.5) 17624 missing |
5.0 (1.6, 6.6) 19038 missing |
0.70 |
|
| ||||
| HCV genotype, % | ||||
|
| ||||
| 1 | 27.7 | 31.0 | 29.0 | |
|
| ||||
| 2 | 3.1 | 3.9 | 3.6 | |
|
| ||||
| 3 | 2.2 | 2.4 | 1.9 | |
|
| ||||
| 4 | 0.3 | 0.3 | 0.2 | <0.01 |
|
| ||||
| Mix | 0.2 | 0.2 | 0.1 | |
|
| ||||
| Missing | 66.6 | 62.3 | 65.1 | |
|
| ||||
| BMI, median (IQR) | 27.2 (23.9, 30.9) | 27.0 (23.9, 30.7) | 26.9 (23.7, 30.5) | <0.01 |
|
| ||||
| Diabetes, % | 25.1 | 15.6 | 17.0 | <0.01 |
|
| ||||
| Hypertension, % | 51.7 | 44.5 | 47.4 | <0.01 |
|
| ||||
| Alcohol abuse or dependence, % | 32.1 | 41.9 | 25.6 | <0.01 |
|
| ||||
| Drug abuse or dependence, % | 34.0 | 42.6 | 25.9 | <0.01 |
|
| ||||
| Baseline APRI score, median (IQR) | 0.39 (0.25,0.65) | 0.41(0.26,0.68) | 0.40(0.26,0.67) | <0.01 |
|
| ||||
| Median ALT (IQR), IU/mL | 41 (27, 66) | 43 (27, 70) | 40 (26, 66) | <0.01 |
|
| ||||
| Median AST (IQR), IU/mL | 35 (25, 53) | 36 (26, 55) | 35 (25, 54) | <0.01 |
|
| ||||
| Completed a course of HCV treatment, % | 3.2 | 4.6 | 3.6 | <0.01 |
|
| ||||
| Baseline lipid levels median, (IQR) | ||||
| Triglycerides | 122 (85,184) Missing: 746 |
117(81,174) Missing: 8940 |
109 (76,161) Missing: 9195 |
<0.01 |
| LDL | 101 (78, 126) Missing: 1065 |
103 (81, 127) Missing: 11577 |
101 (79, 152) Missing: 11811 |
<0.01 |
| HDL | 42 (34, 54) Missing: 879 |
43 (35, 54) Missing: 10568 |
43 (35, 54) Missing: 10382 |
<0.01 |
| Total cholesterol | 173 (149, 202) Missing: 628 |
176 (151, 203) Missing: 7315 |
173 (148, 200) Missing: 7841 |
<0.01 |
|
| ||||
| Anemia, % | 13.2 | 9.9 | 13.2 | <0.01 |
|
| ||||
| Follow-up variables, % | ||||
|
| ||||
| Developed incident cirrhosis (APRI > 2) | 20.8 | 24.3 | 17.3 | <0.01 |
|
| ||||
| Developed HCC | 2.9 | 2.6 | 2.5 | 0.12 |
Values obtained at baseline or closest value within 12 months of baseline.
TCA use was defined as TCA ≥28 cDDD per year and no SSRI use, and SSRI use was defined as ≥28 cDDD per year and no TCA use. Subjects were considered to use no antidepressants if they met any of the following criteria: (1) no receipt of SSRIs or TCAs; (2) SSRI receipt <28 cDDD; and (3) TCA receipt <28 cDDD.
Abbreviations: BMI, body mass index; ALT, alanine aminotransferase; AST, aspartate aminotransferase; PEG, pegylated interferon; LDL, low- density lipoprotein; HDL, high-density lipoprotein; HCC, hepatocellular carcinoma.
TCA users were more likely to have diabetes, compared with SSRI users and no antidepressant users (25%, 16%, and 17% respectively) and hypertension (52%, 45%, and 47%, respectively). TCA users had a higher median body mass index compared with SSRI users and no antidepressant users (27.2, 27.0, and 26.9 respectively). Compared to SSRI users, less TCA users had alcohol abuse (32%, 42% and 26%) and drug abuse (34%, 43% and 26%) (P <0.01 for all comparisons).
TCA users had decreased APRI scores at baseline compared to SSRI users and those receiving no antidepressants (0.39, 0.41, and 0.40, respectively) (P < 0.01). In trivariate analysis, those who received TCAs were less likely to develop cirrhosis compared to SSRI users but more likely than those receiving no antidepressants (21%, 24%, and 17.3%, respectively) (P < 0.01). The proportion of persons developing iHCC was higher among those who received TCAs compared with those who receiving SSRIs and those who received no antidepressants in the unadjusted analysis, but the difference was not statistically significant (2.9%, 2.6%, and 2.5%, respectively) (P = 0.12).
In multivariate Cox regression analysis adjusted for baseline APRI score, receipt of TCAs (cDDD≥ 180) was significantly associated with a reduced risk of developing cirrhosis (hazard ratio [HR] = 0.77, 95% confidence interval [CI] = 0.60, 0.99; Table 2). Older age, white race, baseline APRI score, diabetes mellitus, hypertension, higher baseline HCV RNA, alcohol use or dependence, and drug use or dependence were associated with a higher risk of cirrhosis. Infection with HCV genotype 1 (vs 2–6) was associated with a lower risk of cirrhosis. SSRI use was not associated with a reduction in risk of cirrhosis (HR = 1.03, 95% CI = 0.98, 1.08). In a subset analysis, TCA use defined as cDDD ≥ 28 was not associated with a reduced risk of cirrhosis development (HR 0.93, 95% CI = 0.83, 1.05) (Supplemental Table 1).
Table 2.
Predictors of Cirrhosis, multivariable Cox regression mode adjusted for baseline APRI score.
| Characteristic | HR | 95% Cl | P Value |
|---|---|---|---|
| TCA use (cDDD ≥180 days) † | 0.77 | 0.60, 0.99 | 0.04 |
| SSRI use (cDDD ≥180 days) † | 1.03 | 0.98, 1.08 | 0.21 |
| Sex (male) | 1.12 | 0.97, 1.31 | 0.13 |
| Age, per 10-year increase | 1.13 | 1.09, 1.17 | <0.01 |
| Race (white vs. non-white) | 1.45 | 1.38, 1.52 | <0.01 |
| Baseline APRI score (continuous) | 4.95 | 4.73, 5.19 | <0.01 |
| Diabetes | 1.21 | 1.14, 1.28 | <0.01 |
| Hypertension | 1.08 | 1.03, 1.13 | <0.01 |
| BMI, per unit increase | 1.00 | 0.99, 1.00 | 0.64 |
| HCV genotype (1 vs. 2–6) | 0.94 | 0.89, 0.99 | 0.02 |
| HCV RNA, per log10 increase | 1.03 | 1.02, 1.04 | <0.01 |
| Alcohol use or dependence | 1.31 | 1.24, 1.39 | <0.01 |
| Drug abuse or dependence | 1.07 | 1.01, 1.13 | 0.01 |
TCA, tricyclic antidepressants; SSRI, selective serotonin reuptake inhibitors; cDDD, cumulative defined daily dose; BMI, body mass index.
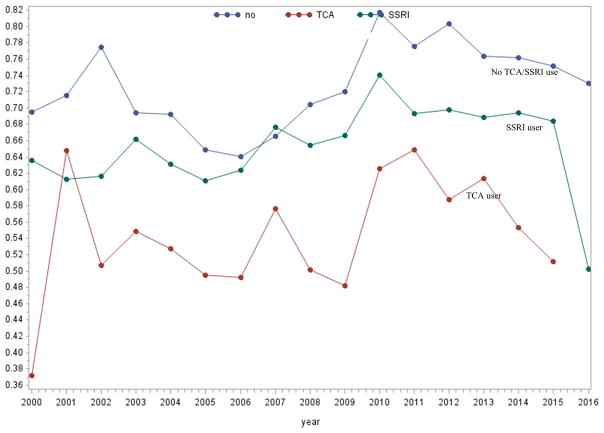
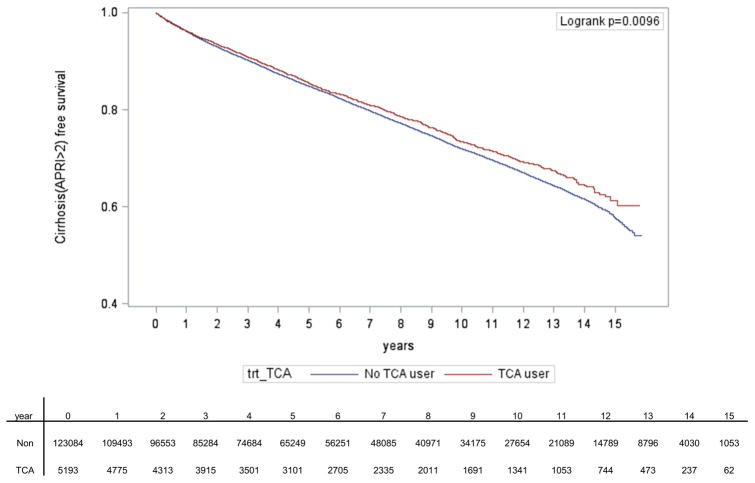
Mean APRI score are shown for the three comparison groups during the study period (Figure 2). Throughout the majority of the study period, subjects who received TCAs had lower mean APRI scores compared to those receiving SSRIs or no antidepressants (Figure 2). Over a median time of follow-up of 5.4 years (IQR 2.4, 9.6), TCA use was significantly associated with delayed time to cirrhosis development (p = 0.01) (Figure 3).
Figure 2. Mean APRI score over time according to exposure group.
*TCA use was defined as TCA ≥28 cDDD per year and no SSRI use, and SSI use was defined as ≥28 cDDD per year and no TCA use.
Figure 3. Kaplan-Meier curves showing time to first diagnosis of cirrhosis.
TCA use was defined as TCA ≥28 cDDD per year and no SSRI use, and SSRI use was defined as ≥28 cDDD per year and no TCA use.
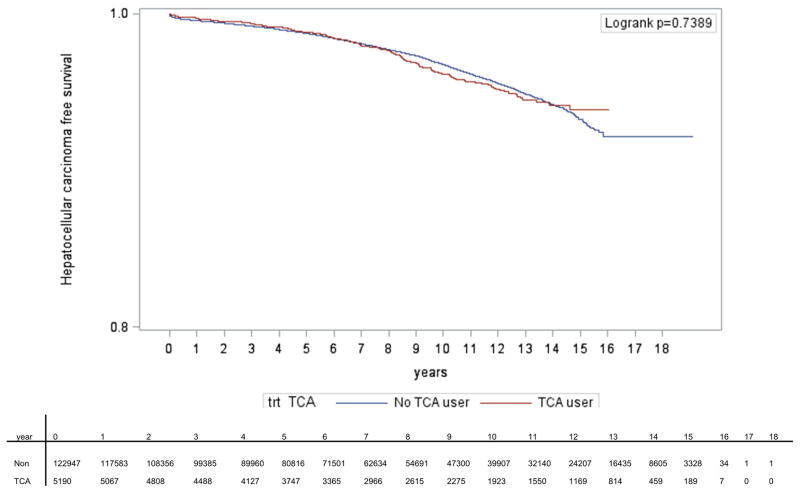
Treatment with TCAs was not associated with a reduced risk of iHCC in multivariate analysis (HR = 0.40, 95% CI = 0.06, 2.84; Table 3). Older age, baseline APRI score, diabetes mellitus, hypertension, body mass index (BMI), higher baseline HCV RNA, and alcohol use or dependence were associated with a higher risk of iHCC. Of note, SSRI use was associated with a lower risk of iHCC (HR = 0.81, 95% CI = 0.69, 0.95). Over a median time of follow-up of 7.2 years (IQR 3.8, 11.2), TCA use was not associated with delayed time to HCC development compared to SSRI use and use of no antidepressants (Figure 4).
Table 3.
Predictors of HCC, multivariable Cox regression mode adjusted for baseline APRI score.
| Characteristic | HR | 95% Cl | P Value |
|---|---|---|---|
| TCA use (cDDD ≥180 days) † | 0.40 | 0.06, 2.84 | 0.36 |
| SSRI use (cDDD ≥180 days) † | 0.81 | 0.69, 0.95 | <0.01 |
| Sex (male) | 1.88 | 0.97, 3.62 | 0.06 |
| Age, per 10-year increase | 2.39 | 2.18, 2.63 | <0.01 |
| Race (white vs. non-white) | 1.08 | 0.95, 1.23 | 0.24 |
| Baseline APRI score (continuous) | 4.71 | 4.13, 5.38 | <0.01 |
| Diabetes | 1.38 | 1.19, 1.62 | <0.01 |
| Hypertension | 1.28 | 1.12, 1.46 | <0.01 |
| BMI, per unit increase | 0.99 | 0.97, 1.00 | 0.04 |
| HCV genotype (1 vs. 2–6) | 0.95 | 0.81, 1.12 | 0.56 |
| HCV RNA, per log10 increase | 1.04 | 1.01. 1.06 | <0.01 |
| Alcohol use or dependence | 1.34 | 1.15, 1.57 | <0.01 |
| Drug abuse or dependence | 0.89 | 0.76, 1.05 | 0.17 |
TCA, tricyclic antidepressants; SSRI, selective serotonin reuptake inhibitors; cDDD, cumulative defined daily dose; BMI, body mass index.
Figure 4. Kaplan-Meier curves showing time to first diagnosis of hepatocellular carcinoma.
TCA use was defined as TCA ≥28 cDDD per year and no SSRI use, and SSRI use was defined as ≥28 cDDD per year and no TCA use.
Discussion
Among a large, national cohort of HCV-positive Veterans, we found that the use of tricyclic antidepressants was significantly associated with decreased fibrosis progression. This study is the first to analyze and demonstrate the effect of TCAs on clinical outcomes in a large, unselected group of patients with chronic HCV infection. These critical outcomes included fibrosis progression to cirrhosis, and the antifibrotic benefits of TCAs remained significant even after adjusting for the potentially confounding effects of baseline level of fibrosis, HCV RNA level, hypertension, diabetes, drug abuse, and alcohol use or dependence.
Previous studies have reported the effects on liver fibrosis of other drug classes not typically associated with treatment of liver disease. Statins have been found to have varying effects dependent on the liver disease type, slowing fibrosis progression in patients with chronic HCV [23] but having no effect on progression of nonalcoholic fatty liver disease [25]. A recent study found that use of aspirin was associated with decreased APRI scores in patients with suspected chronic liver disease [26]. The relevance of antidepressant use to liver disease progression, however, has been unexplored until now.
Our study describes a potentially novel application of tricyclic antidepressants, a class of FDA- approved drugs with a well-documented side effect profile [27]. Preclinical studies have defined an antifibrotic role of TCAs in liver disease. Treatment with amitriptyline led to decreased fibrosis development and increased fibrosis regression in a CCl4-mouse model of hepatic fibrosis. Fucho et al. recently highlighted the antifibrotic role of amitriptyline in a model of NASH [12]. TCAs have been shown to inactivate hepatic stellate cells and decrease collagen expression [8, 9], which further suggests that TCAs may exert their effects independent of the etiology of liver injury. It must be noted that the TCA doses used in vitro and in in vivo models are significantly higher than that used for the treatment of depression in patients. For example, our work has shown that nortriptyline dosed at 7000 ng/ml leads to significant reduction in expression of type 1 collagen and alpha-1 smooth muscle actin in human hepatic stellate cells [28]. In the CCl4-mouse experiment described above, mice received 180 mcg/ml of amitriptyline, a TCA that is structurally similar to nortriptyline [9]. However, the serum level seen in patients treated with nortriptyline for depression ranges between 50 to 140 ng/ml [29].
Given this preclinical data, we focused our analyses on TCA users receiving a cDDD of 180 days or greater. Interestingly, our subset analyses of TCA users receiving a cDDD of 28 days or more did not reveal a difference in cirrhosis compared to SSRI users and subjects receiving no antidepressants, suggesting that longer exposure to TCAs may be necessary to observe an antifibrotic effect (Supplemental Table 1). Furthermore, when we plotted mean APRI score over time for each group, we observed that TCA users had lower mean scores throughout the study period, including at baseline. TCA users may have initiated the medication prior to the study period, which may account for the lower mean APRI scores seen at baseline and at the end of the follow-up period. However, when we controlled for baseline APRI score in our multivariate analyses, TCA use remained significantly associated with a reduction in cirrhosis development.
TCA use was not associated with a reduction in iHCC in multivariate analyses, but there were few events observed in the study, which may limit interpretation of these findings. Of note, a nationwide population-based study by Chen et al, which included 49,998 cases with HCC and 244,236 randomly selected controls, found that use of TCAs and SSRIs was associated with a reduced risk of HCC [30], suggesting that additional studies are needed to elucidate the relationship between use of TCAs and SSRIs and risk of HCC.
TCAs have a myriad of targets including 5-hydroxytryptamine, muscarinic acetylcholine, adrenergic, and histamine H1 and H2 receptors and the serotonin and norepinephrine transporters [15, 31]. Recent evidence has suggested that TCAs exert their antifibrotic effect in hepatic stellate cells through inhibition of the sphingomyelinase pathway [8, 9], and our work highlights the specific role of acid ceramidase [28]. Moreover, in the study by Fucho et al, treatment with TCAs ameliorated the hyperglycemia, body weight gain, and hepatomegaly induced among mice receiving a high fat diet (HFD) [12]. TCA treatment also reduced HFD-induced hepatic steatosis, suggesting that TCAs may reduce NASH through regulating endoplasmic reticulum (ER) stress, lysosomal membrane permeabilization, and autophagy [12]. Given treatment with TCAs has been associated with increased weight gain and insulin resistance among patients with depression [32, 33], additional studies are warranted to elucidate the complex relationship between depression, weight gain, insulin resistance, receipt of antidepressants including TCAs, and development of NASH fibrosis.
Although this study was performed on a large, well-characterized HCV cohort, several limitations must be recognized. Selection bias and unaccounted-for confounders are possible, though we did control for many relevant variables including baseline APRI score, diabetes, HCV viral load at baseline, and alcohol use. We utilized APRI scores rather than liver biopsy to estimate the progression of liver fibrosis. However, the use of the APRI index as a marker of liver fibrosis is well substantiated and is associated with an area under the receiver operating characteristic curve of 0.97 for identifying patients with cirrhosis [34]. The use of ICD-9 codes to identify HCC may also be interpreted as a study limitation, but this has been previously validated in the VA healthcare setting [35]. For the treatment of depression, TCAs have been widely replaced by SSRIs and this was reflected in the smaller percentage of subjects receiving TCAs during the study period. However, despite the smaller numbers, we did observe an association with TCA use and reduction in cirrhosis. Additionally, differences between the effects of distinct TCAs were not specifically assessed, nor were the effects of duration of TCA use on fibrosis progression. We also did not measure the impact of TCA use on achievement of SVR. These factors may be the focus of future studies. Moreover, additional studies are warranted to determine the impact of TCA use on fibrosis progression in other etiologies of chronic liver disease, including NASH and alcoholic liver disease.
Conclusions
Our results show a significant reduction in risk of cirrhosis development among TCA users in a well-characterized cohort of veterans with chronic HCV infection. These results suggest a potential role for TCAs in the prevention of fibrosis progression in patients with chronic HCV infection. Additional studies to elucidate the specific basis for the effects of TCAs on liver fibrosis are warranted.
Supplementary Material
Acknowledgments
We gratefully acknowledge the resources provided by the VA Pittsburgh Healthcare System. We are grateful for the use of the central data repositories maintained by the VA Information Resource Center, which included the Pharmacy Benefits Management Database, National Patient Care Database, and Decisions Support System Database. The views expressed in this article are those of the authors and do not represent those of the Department of Veterans Affairs.
Funding Sources: This current research project was not funded by any organization. ERCHIVES was created through a Career Development Award from National Institutes of Health/National Institute on Drug Abuse (Butt: K23 DA01675-01A1), and maintained through grants and cooperative agreements from Merck, Valenant, AbbVie, and Gilead.
List of abbreviations
- TCA
tricyclic antidepressant
- HCC
hepatocellular carcinoma
- ERCHIVES
Electronically Retrieved Cohort of HCV Infected Veterans
- SSRI
selective serotonin reuptake inhibitor
- HCV
hepatitis C virus
- CCl4
carbon tetrachloride
- NASH
nonalcoholic steatohepatitis
- VHA
Veterans Health Administration
- CDW
Corporate Data Warehouse
- HIV
human immunodeficiency virus
- HBsAg
hepatitis B surface antigen
- APRI
Aspartate Aminotransferase Platelet Ratio Index
- ICD-9
International Classification of Diseases, Ninth Revision
- TC
total cholesterol
- LDL
low- density lipoprotein
- HDL
high-density lipoprotein
- TG
triglyceride
- DDD
defined daily dose
- cDDD
cumulative defined daily dose
- IQR
interquartile range
- iHCC
incident hepatocellular carcinoma
- BMI
body mass index
Footnotes
Authorship Statement: Dr. Butt had full access to data at all times and is responsible for the integrity of this work.
Disclosures: AAB has disclosed investigator initiated grant support (to the institution) from Merck and Gilead Sciences. Other authors have reported no disclosures.
References
- 1.Dufour M. Chronic liver disease and cirrhosis. In: Everhart JE, editor. Digestive diseases in the United States: epidemiology and impact US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Vol. 1994 Washington, DC: US Government Printing Office; 1994. pp. 613–646. NIH Publication No 94-1447. [Google Scholar]
- 2.Kochanek KD, Xu J, Murphy SL, Minino AM, Kung HC. Deaths: final data for 2009. Natl Vital Stat Rep. 2011 Dec 29;60(3):1–116. [PubMed] [Google Scholar]
- 3.Poynard T, McHutchison J, Manns M, et al. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology. 2002 May;122(5):1303–13. doi: 10.1053/gast.2002.33023. [DOI] [PubMed] [Google Scholar]
- 4.George SL, Bacon BR, Brunt EM, Mihindukulasuriya KL, Hoffmann J, Di Bisceglie AM. Clinical, virologic, histologic, and biochemical outcomes after successful HCV therapy: a 5-year follow-up of 150 patients. Hepatology. 2009 Mar;49(3):729–38. doi: 10.1002/hep.22694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mallet V, Gilgenkrantz H, Serpaggi J, et al. Brief communication: the relationship of regression of cirrhosis to outcome in chronic hepatitis C. Ann Intern Med. 2008 Sep 16;149(6):399–403. doi: 10.7326/0003-4819-149-6-200809160-00006. [DOI] [PubMed] [Google Scholar]
- 6.Saez-Royuela F, Linares P, Cervera LA, et al. Evaluation of advanced fibrosis measured by transient elastography after hepatitis C virus protease inhibitor-based triple therapy. Eur J Gastroenterol Hepatol. 2016 Mar;28(3):305–12. doi: 10.1097/MEG.0000000000000533. [DOI] [PubMed] [Google Scholar]
- 7.Schuppan D, Kim YO. Evolving therapies for liver fibrosis. J Clin Invest. 2013 May 1;123(5):1887–901. doi: 10.1172/JCI66028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moles A, Tarrats N, Morales A, et al. Acidic sphingomyelinase controls hepatic stellate cell activation and in vivo liver fibrogenesis. Am J Pathol. 2010 Sep;177(3):1214–24. doi: 10.2353/ajpath.2010.091257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quillin RC, 3rd, Wilson GC, Nojima H, et al. Inhibition of acidic sphingomyelinase reduces established hepatic fibrosis in mice. Hepatol Res. 2015 Mar;45(3):305–14. doi: 10.1111/hepr.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedman SL, Roll FJ, Boyles J, Bissell DM. Hepatic lipocytes: the principal collagen- producing cells of normal rat liver. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8681–5. doi: 10.1073/pnas.82.24.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mederacke I, Hsu CC, Troeger JS, et al. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun. 2013;4:2823. doi: 10.1038/ncomms3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fucho R, Martinez L, Baulies A, et al. ASMase regulates autophagy and lysosomal membrane permeabilization and its inhibition prevents early stage non-alcoholic steatohepatitis. J Hepatol. 2014 Nov;61(5):1126–34. doi: 10.1016/j.jhep.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Achar E, Maciel TT, Collares CF, Teixeira VP, Schor N. Amitriptyline attenuates interstitial inflammation and ameliorates the progression of renal fibrosis. Kidney Int. 2009 Mar;75(6):596–604. doi: 10.1038/ki.2008.578. [DOI] [PubMed] [Google Scholar]
- 14.Teichgraber V, Ulrich M, Endlich N, et al. Ceramide accumulation mediates inflammation, cell death and infection susceptibility in cystic fibrosis. Nat Med. 2008 Apr;14(4):382–91. doi: 10.1038/nm1748. [DOI] [PubMed] [Google Scholar]
- 15.Gillman PK. Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br J Pharmacol. 2007 Jul;151(6):737–48. doi: 10.1038/sj.bjp.0707253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butt AA, Yan P, Lo Re V, 3rd, et al. Liver fibrosis progression in hepatitis C virus infection after seroconversion. JAMA Intern Med. 2015 Feb;175(2):178–85. doi: 10.1001/jamainternmed.2014.6502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butt AA, Xiaoqiang W, Budoff M, Leaf D, Kuller LH, Justice AC. Hepatitis C virus infection and the risk of coronary disease. Clin Infect Dis. 2009 Jul 15;49(2):225–32. doi: 10.1086/599371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butt AA, Wang X, Moore CG. Effect of hepatitis C virus and its treatment on survival. Hepatology. 2009 Aug;50(2):387–92. doi: 10.1002/hep.23000. [DOI] [PubMed] [Google Scholar]
- 19.Chou R, Wasson N. Blood tests to diagnose fibrosis or cirrhosis in patients with chronic hepatitis C virus infection: a systematic review. Ann Intern Med. 2013 Jun 4;158(11):807–20. doi: 10.7326/0003-4819-158-11-201306040-00005. [DOI] [PubMed] [Google Scholar]
- 20.Simon TG, Bonilla H, Yan P, Chung RT, Butt AA. Atorvastatin and fluvastatin are associated with dose-dependent reductions in cirrhosis and hepatocellular carcinoma, among patients with hepatitis C virus: Results from ERCHIVES. Hepatology. 2016 Jul;64(1):47–57. doi: 10.1002/hep.28506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butt AA, Khan UA, Shaikh OS, et al. Rates of HCV treatment eligibility among HCV- monoinfected and HCV/HIV-coinfected patients in tertiary care referral centers. HIV Clin Trials. 2009 Jan-Feb;10(1):25–32. doi: 10.1310/hct1001-025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGinnis KA, Fine MJ, Sharma RK, et al. Understanding racial disparities in HIV using data from the veterans aging cohort 3-site study and VA administrative data. Am J Public Health. 2003 Oct;93(10):1728–33. doi: 10.2105/ajph.93.10.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butt AA, Yan P, Bonilla H, et al. Effect of addition of statins to antiviral therapy in hepatitis C virus-infected persons: Results from ERCHIVES. Hepatology. 2015 Apr 6; doi: 10.1002/hep.27835. [DOI] [PubMed] [Google Scholar]
- 24.Organization WH; WHOCCfDS, editor. Methodology. Oslo: 2003. ATC Index with Defined Daily Dose. [Google Scholar]
- 25.Oni ET, Sinha P, Karim A, et al. Statin use is not associated with presence of and severity of nonalcoholic fatty liver disease. Arch Med Res. 2014 Jan;45(1):52–7. doi: 10.1016/j.arcmed.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Jiang ZG, Feldbrugge L, Tapper EB, et al. Aspirin use is associated with lower indices of liver fibrosis among adults in the United States. Aliment Pharmacol Ther. 2016 Mar;43(6):734–43. doi: 10.1111/apt.13515. [DOI] [PubMed] [Google Scholar]
- 27.Trindade E, Menon D, Topfer LA, Coloma C. Adverse effects associated with selective serotonin reuptake inhibitors and tricyclic antidepressants: a meta-analysis. CMAJ. 1998 Nov 17;159(10):1245–52. [PMC free article] [PubMed] [Google Scholar]
- 28.Chen JYNB, Zhou C, Pondick JV, Ghoshal S, York S, Motola D, Coant N, Yi JK, Mao C, Tanabe KK, Bronova I, Berdyshev EV, Fuchs BC, Hannun Y, Chung RT, Mullen AC. Tricyclic Antidepressants Promote Ceramide Accumulation to Regulate Collagen Production in Human Hepatic Stellate Cells. Sci Rep. 2017;7(44867) doi: 10.1038/srep44867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asberg MC, Borje, Folke Sjoqvist, Dick Tuck. Relationship between Plasma Level and Therapeutic Effect of Nortriptyline. British Medical Journal. 1971;3:331–4. doi: 10.1136/bmj.3.5770.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen VC, Lin CF, Hsieh YH, et al. Hepatocellular carcinoma and antidepressants: a nationwide population-based study. Oncotarget. 2017 May 02;8(18):30464–70. doi: 10.18632/oncotarget.12826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rudorfer MV, Potter WZ. Metabolism of tricyclic antidepressants. Cell Mol Neurobiol. 1999 Jun;19(3):373–409. doi: 10.1023/A:1006949816036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kivimaki M, Hamer M, Batty GD, et al. Antidepressant medication use, weight gain, and risk of type 2 diabetes: a population-based study. Diabetes Care. 2010 Dec;33(12):2611–6. doi: 10.2337/dc10-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andersohn F, Schade R, Suissa S, Garbe E. Long-term use of antidepressants for depressive disorders and the risk of diabetes mellitus. Am J Psychiatry. 2009 May;166(5):591–8. doi: 10.1176/appi.ajp.2008.08071065. [DOI] [PubMed] [Google Scholar]
- 34.Jain P, Tripathi BK, Gupta B, Bhandari B, Jalan D. Evaluation of Aspartate Aminotransferase-to-Platelet Ratio Index as a Non-Invasive Marker for Liver Cirrhosis. J Clin Diagn Res. 2015 Nov;9(11):OC22–4. doi: 10.7860/JCDR/2015/13944.6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldberg DS, Lewis JD, Halpern SD, Weiner MG, Lo Re V., 3rd Validation of a coding algorithm to identify patients with hepatocellular carcinoma in an administrative database. Pharmacoepidemiol Drug Saf. 2013 Jan;22(1):103–7. doi: 10.1002/pds.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.