Abstract
Objective
Currently, there are no accepted nonsurgical therapies which improves the delivery of blood-derived nutrients to patients with critical limb ischemia (CLI). Here, we describe the ongoing phase I/II CHAMP trial, which will provide crucial evidence regarding the safety profile of mesenchymal stromal cells (MSC) and explore their therapeutic mechanisms of in the setting of CLI requiring below-knee amputation (BKA).
Methods
In the CHAMP and the parallel marrowCHAMP trials (hereafter grouped together as CHAMP), a total of 32 extremities with rest pain or tissue loss requiring BKA will be enrolled to receive intramuscular (IM) injections of allogeneic MSCs (CHAMP, n=16) or autogenous concentrated bone marrow aspirate (cBMA, marrowCHAMP, n=16) along the distribution of the BKA myocutaneous flap and proximal tibialis anterior.
After treatment, subjects are randomized to BKA at four timepoints post-injection (days 3, 7, 14, 21). At the time of amputation, skeletal muscle is collected at 2 cm increments from the tibialis injection site and used to determine proangiogenic cytokine description, MSC retention, quantification of proangiogenic hematopoietic progenitor cells (PHCs), and histological description. Clinical limb perfusion pre- and post-treatment will be quantified using TcPO2, TBI, ABI, and indocyanine angiography (ICA). Additional clinical endpoints include all-cause mortality, need for amputation revision, and gangrene incidence during the 6-month post-treatment follow-up.
Results
Enrollment is underway with ten patients treated per protocol thus far. We anticipate full conclusion of follow-up within the next 24 months.
Conclusion
CHAMP will be pivotal in characterizing the safety, efficacy, and most importantly, the therapeutic mechanism of allogeneic MSCs and autogenous cBMA in ischemic skeletal muscle.
Background
CLI refers to a pathologic state in which blood flow to the leg is insufficient to deliver the resting metabolic needs of skeletal muscle. Unfortunately, those who are not candidates for surgical revascularization, the gold standard therapy, are at a higher risk of amputation secondary to refractory pain or persistent infection.1 Cell-based therapies represent a potential treatment for patients without a suitable revascularization option, but the mechanism of action in which this occurs has yet to be clearly defined. IM injection of cBMA, containing CD34+ PHCs and MSCs, have shown promise in small case series and large randomized controlled trials alike.2-6
However, the source of cBMA must be autogenous given a robust host rejection response and therefore limited by the comorbidities of the treated patient. MSCs, as an isolated treatment product, potentially represent a more efficacious therapy as they do not constitutively express major histocompatibility complex (MHC) II and largely escape host immune recognition allowing the use of healthy allogeneic donors for diseased hosts.7
Methods
Synopsis
CHAMP (Clinical and Histological Analysis of Mesenchymal Stromal Cells in AmPutations) is a single-center, phase I/II, open-label clinical trial that will enroll 32 total CLI patients requiring semi-elective BKA within a 30-days for rest pain or dry gangrene. The Indiana University Institutional Review Board (IRB #1510579216A015) reviewed and approved the trial protocol which was designed in concordance with the latest iteration of the Declaration of Helsinki.8 After informed consent is obtained, participants receive IM injections of autogenous cBMA or allogeneic MSCs along the distal thigh, gastrocnemius, and the proximal aspect of the tibialis anterior before randomization to BKA and tissue harvest at four timepoints within 21 days.
cBMA/MSC Preparation
The protocol for harvest, concentration, and injection of cBMA has been described by our group in detail elsewhere.9 In short, a large bore needle is inserted into the superior iliac crests under local anesthesia and approximately 330 mL of bone marrow is aspirated. The MarrowStim PAD Kit (Zimmer Biomet, Warsaw, IN) is used to create 33 mL of cBMA from this dilute suspension via centrifugation. Small aliquots (∼1 mL) of the treatment product is injected into the muscle beds of the BKA myocutaneous flap and proximal tibialis anterior at set points. Subjects enrolled into the MSC cohort are typed for HLA-A2 (Human Leukocyte Antigen A2) prior to selection of appropriately mismatched MSCs to allow for ease of identification of between host and donor at the time of tissue harvest via fluorescent in situ hybridization (FISH). HLA-A2− subjects will receive HLA-A2+ donor cells, and HLA-A2+ patients will receive gender mismatched donor cells.
All MSCs in the treatment product are isolated from young, healthy donors at the Center for Advanced Cellular Therapeutics (University of Louisville). Mononuclear cells from the aspirate are cultured in 175 cm2 flasks. Adherent cells are isolated, proliferated, and collected at passage two to ensure homogeneity of treatment product. MSC phenotype is confirmed via Fluorescence Activated Cell Sorting (FACS) observation of CD73, CD90, and CD105 coexpression.10
Inclusion/Exclusion, Randomization, and Treatment
All patients with CLI between the ages of 40 and 80, without clinical evidence of infection, requiring BKA within 30 days are eligible for inclusion into the cBMA or MSC cohorts (Table I). Screening protocols, treatment timing, and follow-up schedule are detailed in Table II. Patency of lower extremity arteries are not routinely performed as part of the CHAMP screening protocol.
Table 1. Inclusion/exclusion criteria for CHAMP.
| Inclusion | Exclusion |
|---|---|
| Age between 40 and 80 years | Evidence of infection
|
| Requires BKA, as determined by an independent vascular surgeon, for rest pain, non-suppurative gangrene, or tissue loss | Ulceration/gangrene due to venous insufficiency or lymphedema |
| Resting ABI ≤ 0.55 or TBI ≤ 0.40 | Patent superficial femoral artery as determined by Duplex ultrasound, angiography (including CT), or MRI within 6 months prior to enrollment |
| TcPO2 at the calf ≤ 40mmHg | Patients who are pregnant, planning to become pregnant in the next 12 months, or lactating |
| Tissue loss distal to the malleoli | Hospitalization for congestive heart failure exacerbation within the last 1 month prior to enrollment |
| BKA can be safely performed up to 30-days after screening | Acute coronary syndrome in the last 1 month prior to enrollment |
| Females of childbearing potential must be willing to use one form of birth control for the duration of the study. Female participants must undergo a blood or urine pregnancy test at screening | HIV positive, active HBV or HCV |
| History of cancer within the last 5 years, except basal cell skin carcinoma | |
| Any bleeding diathesis defined as an INR ≥ 2.0 (off anticoagulation therapy) or history of platelet countless than 70,000 or hemophilia | |
| Any condition requiring immunosuppressant medications | |
| Presence of any clinical condition that in the opinion of the PI or the sponsor makes the patient not suitable to participate in the trial |
Table 2. Follow-up protocol for CHAMP.
The following tests are performed at baseline: (1) baseline blood tests (CBC, CMP); (2) infectious disease panel; (3) medical history and physical examination; (4) medication history; (5) pregnancy test; (6) 12-lead EKG. Additional noninvasive clinical testing of the ischemic limb includes ankle-brachial index (ABI), toe-brachial index (TBI), transcutaneous oximetry (TcPO2), and SPY-Elite angiography with IV indocyanine (ICA).
| Evaluation | Baseline | MSC Injection | BKA (Day varies) | Day 3 | Day 14 | Day 45 | Week 12 | Week 18 | Week 24 |
|---|---|---|---|---|---|---|---|---|---|
| Informed Consent | X | ||||||||
| Medical History | X | ||||||||
| Physical Exam | X | X | X | X | X | X | X | X | X |
| Vital Signs | X | X | X | X | X | X | X | X | X |
| Medication List | X | X | X | X | X | X | X | X | |
| Serum Pregnancy Test | X | ||||||||
| Adverse Event Evaluation | X | X | X | X | X | X | X | X | X |
| Infectious Disease Labs | X | ||||||||
| General Labs | X | X | X | X | X | X | |||
| 12-Lead EKG | X | X | X | X | X | X | |||
| Lower Extremity Arterial Duplex | X | ||||||||
| Randomization: Time to BKA | X |
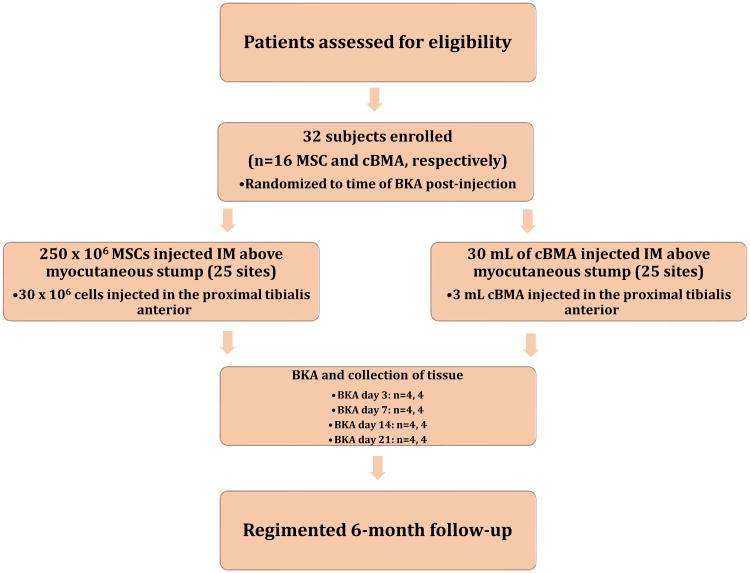
Computer-assisted, nonstratified randomization is performed to assign patients to BKA and tissue harvest at days 3, 7, 14, or 21 post-injection (Figure 1). Cells are administered into 25 different sites within the gastrocnemius and distal thigh (Figure 2) muscle beds that comprises the BKA myocutaneous stump. An additional injection point at a fixed point from the tibial tuberosity is performed in the most proximal aspect of the tibialis anterior for downstream mechanistic analysis. Tissue from the untouched soleus of the deep posterior compartment serve as an internal control.
Figure 1. Trial design of CHAMP.
After screening, patients are enrolled into either the cBMA or MSC arm of CHAMP. Subjects are randomized to one of four time points within 21 days of cell therapy administration. At that time, BKA is performed and tissue is harvested. All patients are followed for six months post-amputation.
Figure 2. Simplified treatment plan of the CHAMP trial.
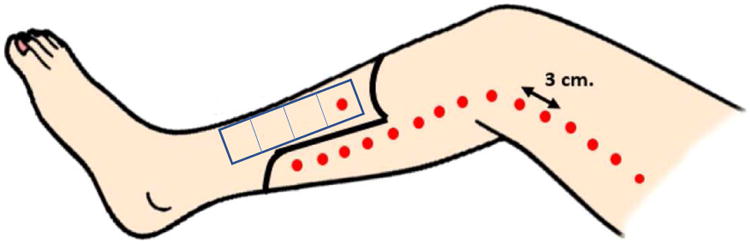
A total of 280 million allogeneic MSCs (33 mL cBMA) are injected into the muscle beds of patients undergoing BKA (dots). 250 million MSCs (30 mL cBMA) are injected proximal to the myocutaneous flap along the gastrocnemius and distal thigh muscle beds. One additional site in the proximal tibialis anterior is injected with 30 million MSCs (3 mL cBMA). Tissue harvest sites of the tibialis anterior at time of BKA are denoted (rectangle) along the length of the muscle bed in 2 cm increments. Untouched soleus muscle will serve as an internal tissue control and harvested concurrently.
Tissue Harvest
Before BKA on the assigned day, the patient is subjected to a clinical limb perfusion evaluation with ABI, TBI, TcPO2, and ICA for comparison to baseline studies. Intraoperatively, at least four muscle samples from the tibialis anterior in 2 cm increments distal to the cellular injection point are collected for downstream applications. Amputation proceeds in study patients in the standard fashion for our institution consisting of the anterior rotation of a posterior compartment myocutaneous flap.
Endpoints and Follow-Up
Through a review of adverse events via clinic visits over six months post-procedure, we plan to test the hypothesis that autogenous cBMA and allogeneic MSCs do not result in significant cardiovascular, respiratory, or infectious complications (Table II). Peripheral blood is also collected from the patients at days 3, 14, 45, 90, 135, 180 and compared to baseline tests to determine changes in peripheral cytokine signaling, miRNA expression, and proangiogenic/inflammatory mononuclear phenotypes. Tissue collected at time of amputation will be subject to multiplex arrays, immunohistochemistry, FACS, and FISH. In this way, we can characterize histological appearance of muscle, local proangiogenic cytokine expression, inflammatory cell infiltrates, donor MSC retention, and the clinically important measure of capillary density at various time points post-treatment. The plan for statistical analysis of data points isolated are detailed in the appendix (Statistical Supplement).
Additional clinical investigations will be made into the clinical efficacy of MSCs and cBMA in promoting freedom from gangrene, above-knee amputation (AKA) conversion, and death after BKA. Treatment groups will be compared to patients who qualified for CHAMP but declined participation.
Current Status
Recruitment for CHAMP is ongoing and the first ten patients have been treated per protocol. Anticipated conclusion of recruitment, treatment, and follow-up will be within 24 months.
Discussion
When amputation is required, below-knee creation of a myocutaneous stump is preferred as knee preservation decreases energy expenditure for prosthesis-assisted ambulation.11,12 However, contemporary proximal conversion rates secondary to stump ischemia are reported at 20% creating massive morbidity for the patient and increasing the overall healthcare burden.12-14 Therefore, the effort to reduce BKA failure represents an important area of research for all patients with CLI.
CHAMP will be decisive in characterizing the biological activity of allogeneic MSCs in human tissue. The idea for this study matured secondary to completion of our previous open label phase I trial investigating the utility of IM injections of cBMA containing MSCs into 30 limbs with nonrevascularizable CLI. We found these patients exhibited a 1- and 5-year amputation free survival rates of 86% and 74%, respectively, which is comparable to the CLI population that receives surgical revascularization.6 Additionally, we noted improvements in ABI, TcPO2, and rest pain. Results from that preliminary study allowed us to initiate a multicenter randomized controlled phase III study (MOBILE) which demonstrated a statistically significant ability of cBMA to prevent major amputation in nonrevascularizable CLI patients with the caveat that they are not diabetic and do not have Rutherford class five disease. The number needed to treat to prevent one amputation was merely six patients.3
Although these studies reported promising results, several glaring limitations has prevented the advancement and adoption of cell-based therapy as a widespread treatment modality. First, isolation of autologous cells requires a harvest procedure under anesthesia placing CLI patients, often of advanced age and severe cardiovascular comorbidities, at risk for additional complications. Secondly, autologous stem cells from patients with cardiovascular disease have shown limited neovascularization ability in experimental stroke and heart disease models as compared to healthy controls.15-17
MSCs, on the other hand, can be sourced from healthy allogeneic donors and injected into diseased limbs because of reduced constitutive MHC-II expression bypassing the limitations of cell-based therapies.7 This readily available, unique subset of cells has demonstrated the ability to induce angiogenesis, decrease muscle fiber apoptosis, and stimulate reepithelialization of wound beds.18-20 Our preliminary unpublished in vitro experiments indicated MSCs secrete increased quantities of the proangiogenic cytokines VEGF, HGF, and Ang-1 in response to hypoxic conditions. However, the current overall understanding of the bioactivity of this subset of stem cells arise entirely from in vitro or animal studies, which are limited by poor generalizability and genomic discordance in pathologic conditions.18,21,22
The lack of clarity regarding MSC bioactivity in ischemic human tissue continues to be a problem despite multiple clinical trials.23-25 Initially, MSCs were thought to engraft, differentiate, and replace damaged tissue. However, subsequent work has established low levels of engraftment (<0.1%) of donor cells.21 Current opinion reflects a mechanism of crosstalk between MSCs and injured cells to limit tissue destruction and enhance repair. Specifically, this phenomenon may occur secondary to (1) secretion of bioactive proteins that act in a paracrine or autocrine fashion, (2) upregulation of genes that inhibit excessive inflammatory and immune reactions, (3) and transfer of vesicular components that contain mitochondria and miRNA (exosomes).22
The MSC dosage selected in CHAMP was based on mouse models (by body surface area) and previous clinical trials, notably POSEIDON, in which MSCs were directly injected into the myocardium.23 Therefore, it is entirely possible we may not observe efficacy secondary to inadequate dosing. Additionally, the primary goal of this phase I/II trial is to assess safety and therefore not powered for clinical efficacy endpoints.
To describe the bioactivity of allogeneic MSCs in ischemic muscle, we plan to define donor MSC retention over time, host immune responses, recruitment of PHCs, changes in capillary density, and variation of skeletal muscle fiber morphology by histology. Based on previous animal models, we anticipate the donor population will be significantly depleted by day seven as MSCs begin to upregulate the expression MHC-II antigen.22 A peak infiltration of PHCs in the more perfused regions of the anterior tibialis should occur concurrently to peak cytokine concentration around this time. Capillary formation should continue to expand secondary to PHC recruitment until the last timepoint at 21 days.
CHAMP is designed to deliver critical evidence regarding the safety profile of IM MSCs in CLI. Tissues harvested from the participants receiving both cBMA and MSCs will provide data which may elucidate MSC therapeutic mechanism. Comparison to concurrent nonCHAMP risk factor matched BKA patients at the same institution will provide controls to assess improvements in wound complications. As the next iteration after CHAMP conclusion, we hope to proceed with a larger, randomized controlled trial with escalating MSC dosages powered to provide outcomes data for real world use.
Conclusion
Because CHAMP aims to harvest human tissue post-IM MSC and cBMA injection at time points relevant to tissue reparation and cell survival, it will have the unique ability to provide a vital description of the in vivo biological activity of MSCs and cBMA in ischemic limbs undergoing BKA.
Supplementary Material
Acknowledgments
The work presented in this manuscript was made possible through generous funding from the National Institutes of Health (1UM1HL113457 and R01HL128827).
Footnotes
Clinical Trial Registration: https://clinicaltrials.gov/ct2/show/NCT02685098, NCT02685098
Disclosures: The authors of this manuscript have no relevant disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Norgren L, Hiatt W, Dormandy J, Nehler M, Harris K, Fowkes F, et al. Inter-society consensus for the management of peripheral arterial disease. J Vasc Surg. 2007 doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 2.Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002 doi: 10.1016/S0140-6736(02)09670-8. 0140-6736 (Print) [DOI] [PubMed] [Google Scholar]
- 3.Wang SK, Green LA, Motaganahalli RL, Wilson MG, Fajardo A, Murphy MP. Rationale and Design of the MOBILE Trial Investigating Autologous Bone Marrow Cell Therapy for Critical Limb Ischemia (In Press) Journal of vascular surgery. 2017 doi: 10.1016/j.jvs.2017.01.054. [DOI] [PubMed] [Google Scholar]
- 4.Giles KA, Rzucidlo EM, Goodney PP, Walsh DB, Powell RJ. Bone marrow aspirate injection for treatment of critical limb ischemia with comparison to patients undergoing high-risk bypass grafts. Journal of vascular surgery. 2015;61(1):134–137. doi: 10.1016/j.jvs.2014.06.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang TW, Jester A, Motaganahalli RL, Wilson MG, G'Sell P, Akingba GA, et al. Autologous bone marrow mononuclear cell therapy for critical limb ischemia is effective and durable. Journal of vascular surgery. 2016;63(6):1541–1545. doi: 10.1016/j.jvs.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 6.Murphy M, Lawson J, Rapp B, Dalsing M, Klein J, Wilson M, et al. Autologous bone marrow mononuclear cell therapy is safe and promotes amputation-free survival in patients with critical limb ischemia. J Vasc Surg. 2011 doi: 10.1016/j.jvs.2011.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryan JM, Barry FP, Murphy JM, Mahon BP. Mesenchymal stem cells avoid allogeneic rejection. Journal of inflammation (London, England) 2005;2:8. doi: 10.1186/1476-9255-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. Jama. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 9.Wang SK, Green LA, Motaganahalli RL, Wilson MG, Fajardo A, Murphy MP. Rationale and design of the MarrowStim PAD Kit for the Treatment of Critical Limb Ischemia in Subjects with Severe Peripheral Arterial Disease (MOBILE) trial investigating autologous bone marrow cell therapy for critical limb ischemia. Journal of vascular surgery. 2017;65(6):1850–1857.e1852. doi: 10.1016/j.jvs.2017.01.054. [DOI] [PubMed] [Google Scholar]
- 10.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 11.Dillingham TR, Pezzin LE, Shore AD. Reamputation, mortality, and health care costs among persons with dysvascular lower-limb amputations. Archives of physical medicine and rehabilitation. 2005;86(3):480–486. doi: 10.1016/j.apmr.2004.06.072. [DOI] [PubMed] [Google Scholar]
- 12.Stone PA, Flaherty SK, Hayes JD, AbuRahma AF. Lower extremity amputation: a contemporary series. The West Virginia medical journal. 2007;103(5):14–18. [PubMed] [Google Scholar]
- 13.Lim TS, Finlayson A, Thorpe JM, Sieunarine K, Mwipatayi BP, Brady A, et al. Outcomes of a contemporary amputation series. ANZ journal of surgery. 2006;76(5):300–305. doi: 10.1111/j.1445-2197.2006.03715.x. [DOI] [PubMed] [Google Scholar]
- 14.Nehler MR, Coll JR, Hiatt WR, Regensteiner JG, Schnickel GT, Klenke WA, et al. Functional outcome in a contemporary series of major lower extremity amputations. Journal of vascular surgery. 2003;38(1):7–14. doi: 10.1016/s0741-5214(03)00092-2. [DOI] [PubMed] [Google Scholar]
- 15.Heeschen C, Lehmann R, Honold J, Assmus B, Aicher A, Walter DH, et al. Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation. 2004;109(13):1615–1622. doi: 10.1161/01.CIR.0000124476.32871.E3. [DOI] [PubMed] [Google Scholar]
- 16.Sugihara S, Yamamoto Y, Matsuura T, Narazaki G, Yamasaki A, Igawa G, et al. Age-related BM-MNC dysfunction hampers neovascularization. Mechanisms of ageing and development. 2007;128(9):511–516. doi: 10.1016/j.mad.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Zhuo Y, Li SH, Chen MS, Wu J, Kinkaid HY, Fazel S, et al. Aging impairs the angiogenic response to ischemic injury and the activity of implanted cells: combined consequences for cell therapy in older recipients. The Journal of thoracic and cardiovascular surgery. 2010;139(5):1286–1294. 1294.e1281–1282. doi: 10.1016/j.jtcvs.2009.08.052. [DOI] [PubMed] [Google Scholar]
- 18.Maxson S, Lopez EA, Yoo D, Danilkovitch-Miagkova A, Leroux MA. Concise review: role of mesenchymal stem cells in wound repair. Stem cells translational medicine. 2012;1(2):142–149. doi: 10.5966/sctm.2011-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy MP, Wang H, Patel AN, Kambhampati S, Angle N, Chan K, et al. Allogeneic endometrial regenerative cells: an “Off the shelf solution” for critical limb ischemia? Journal of translational medicine. 2008;6:45. doi: 10.1186/1479-5876-6-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem cells (Dayton, Ohio) 2007;25(10):2648–2659. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 21.Kotton DN, Fabian AJ, Mulligan RC. Failure of bone marrow to reconstitute lung epithelium. American journal of respiratory cell and molecular biology. 2005;33(4):328–334. doi: 10.1165/rcmb.2005-0175RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prockop DJ. Repair of tissues by adult stem/progenitor cells (MSCs): controversies, myths, and changing paradigms. Molecular therapy : the journal of the American Society of Gene Therapy. 2009;17(6):939–946. doi: 10.1038/mt.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, et al. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. Jama. 2012;308(22):2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371(9624):1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 25.Weiss DJ, Casaburi R, Flannery R, LeRoux-Williams M, Tashkin DP. A placebo-controlled, randomized trial of mesenchymal stem cells in COPD. Chest. 2013;143(6):1590–1598. doi: 10.1378/chest.12-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.