Abstract
Background
Acute kidney injury (AKI) is a common serious complication following cardiac surgery. Doppler-determined renal resistive index (RRI) is a promising early AKI biomarker in this population. However, the relationship between aortic valve pathology (insufficiency and/or stenosis) and RRI is unknown. This study aimed to investigate RRI variability related to aortic valve pathology.
Methods
In a retrospective review of cardiac surgery patients, RRI and aortic valve pathology were assessed prior to cardiopulmonary bypass using transesophageal echocardiography. Aortic valve status was categorized into four subgroups: normal (insufficiency and stenosis, none/trace/mild), insufficiency (insufficiency, moderate/severe; stenosis, none/trace/mild), combined insufficiency/stenosis (insufficiency and stenosis, moderate/severe), or stenosis (insufficiency, none/trace/mild; stenosis, moderate/severe). RRI, and time-matched hemodynamic and Doppler measurements were compared among subgroups.
Results
Of 175 patients, 60 had aortic valve pathology (16 insufficiency, 18 insufficiency/stenosis, 26 stenosis). Compared to the normal subgroup, patients with aortic insufficiency had lower diastolic blood pressure and trough renal Doppler velocities, and higher RRI (0.77 vs 0.69; p<0.001); patients with combined insufficiency/stenosis also had higher RRI (0.72 vs 0.69, p=0.042).
Conclusions
Patients with aortic insufficiency and combined insufficiency/stenosis had higher median RRI values compared to normal patients. For these individuals, diastolic flow differences related to AI may explain why their pre-surgery RRI values often exceeded postoperative thresholds typically associated with AKI. Strategies to account for the potentially confounding effects of aortic insufficiency on renal flow patterns, independent of renal injury, may add to the value of RRI as an early AKI biomarker.
Classifications: Kidney, renal function failure, dialysis, Echocardiography, Cardiac, Surgery, complications, Ultrasound
Graphical Abstract
Acute kidney injury (AKI) is a common and serious complication of cardiac surgery associated with significant morbidity and mortality.[1] Recognition of AKI is often delayed up to 48 hours postoperatively due to reliance on serum creatinine for diagnosis.[2] A search for earlier AKI biomarkers is, therefore, underway.[2] In this regard, there is considerable interest in the Doppler-determined renal resistive index (RRI; Figure 1) using transabdominal ultrasound or transesophageal echocardiography (TEE).[3–8] As an index of renal arterial pulsatility, RRI elevation that exceeds a critical value (e.g., 0.74[3]) may reflect increased renal intracapsular pressure due to AKI.[9, 10] During cardiac surgery, TEE-determined elevation of RRI after separation from cardiopulmonary bypass (CPB) correlates with subsequent AKI diagnosis.[4, 7] Similarly, transabdominal-determined RRI predicts AKI when measured in critically ill patients[5] and soon after various surgical procedures.[3, 6, 8] However, the potential for hemodynamic effects of heart valve abnormalities such as aortic insufficiency (AI) or stenosis (AS) to confound the value of RRI as a biomarker, independent of AKI, is poorly understood.
Figure 1.
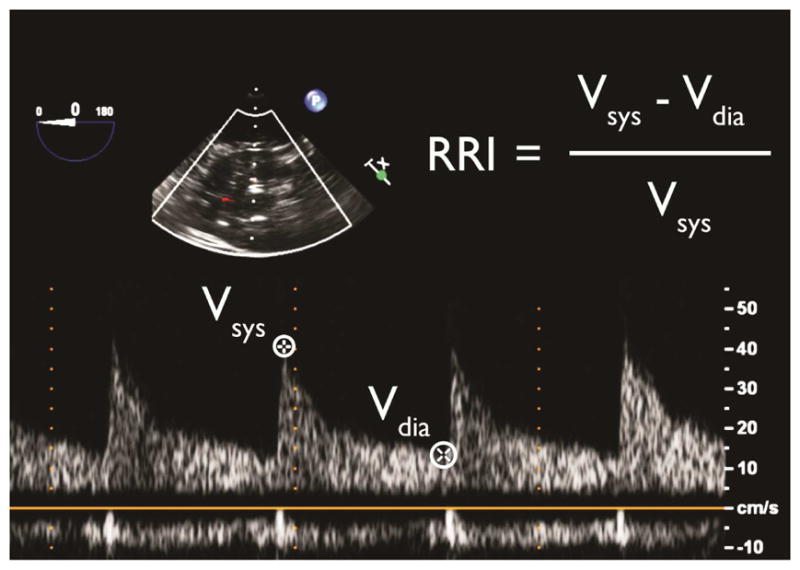
Calculation of renal resistive index (RRI) using transesophageal echocardiography. Vdia-trough diastolic velocity, Vsys-peak systolic velocity.
Serious aortic valve pathology, which may affect renal artery blood flow, is a common indication for cardiac surgery.[11, 12] Consequently, better understanding of the relationship among the various types of aortic valve pathology and RRI may add to its value as an AKI biomarker. Therefore, we hypothesized that patients with and without aortic valve pathology have different intraoperative RRI values prior to surgery-related renal insult (i.e., pre-CPB).
Patients and Methods
Study Population
After Institutional Review Board approval, a retrospective review was conducted using prospectively collected data from the Duke Anesthesiology TEE Database and Electronic Medical Record (Epic Systems, Verona, WI). Since RRI determination was introduced as an optional requirement during the study period, a subset of cardiac surgery patients was available for analysis.
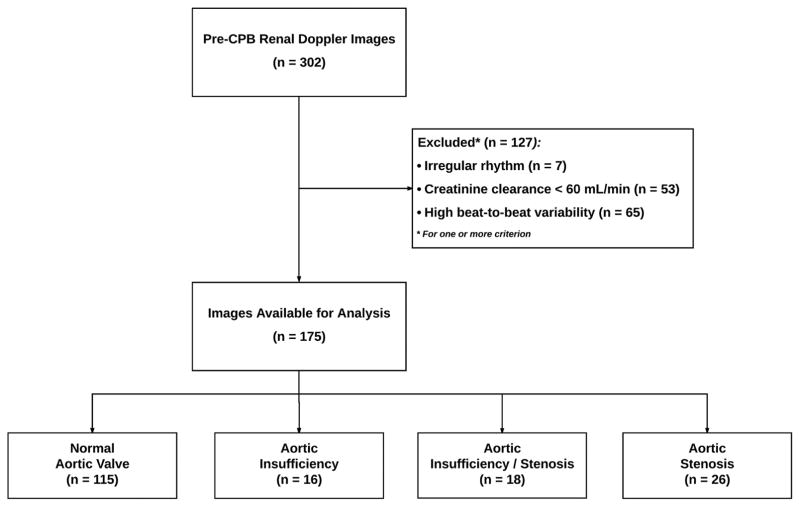
Subjects included patients ≥18 years old who underwent cardiac surgery requiring CPB between 7/1/2013 and 7/10/2014 at Duke University Medical Center (Durham, NC) for whom pre-CPB TEE aortic valve evaluation and renal blood flow measurements were documented. Exclusion criteria included: missing pre- or post-operative creatinine; chronic kidney disease or recent AKI (preoperative estimated creatinine clearance <60 mL/min [Cockroft-Gault]); non-sinus rhythm during RRI acquisition; and renal Doppler images deemed poor quality by blinded reviewers.
Intraoperative Ultrasound Imaging and Post-Hoc Assessment
A comprehensive examination using a multiplane TEE probe (Philips X7-2t; Philips iE33, Andover, MA) is routine prior to, and after, CPB at the reference institution. The examination is performed by an adult cardiothoracic anesthesiology fellow with an attending anesthesiologist certified in advanced peri-operative TEE by the National Board of Echocardiography (Advanced PTEeXAM). Pre-CPB TEE examinations were selected a priori for two primary reasons: to (1) maximize the range of uncorrected aortic valve abnormalities, and (2) minimize the effects on RRI of any procedural factors (e.g., surgery-related renal insult).
Assessment of Aortic Valve Status
Severity of AI and AS (none/trace/mild/moderate/severe) was reported in the TEE Database using standardized criteria.[13, 14] For analysis, subjects were grouped by aortic valve status: normal (AI and AS, none/trace/mild), insufficiency (AI, moderate/severe; AS, none/trace/mild), combined insufficiency/stenosis (AI and AS, moderate/severe), or stenosis (AI, none/trace/mild; AS, moderate/severe).
Assessment of Pre-CPB Renal Resistive Index
Left renal artery Doppler velocity images were obtained using a transgastric approach.[15] Seven TEE-certified cardiothoracic anesthesiologists (blinded to aortic valve status) re-reviewed de-identified renal images to determine RRI values. Doppler images were distributed among reviewers, for whom intra- and inter-rater reliability for RRI determination was previously confirmed.[4] Renal arterial peak systolic (Vsys) and trough diastolic (Vdia) velocities were measured for all suitable cardiac cycles (1–3); RRI was calculated for each cycle (Figure 1), and averaged for each subject.
Additional Measurements
Hemodynamic Parameters
Intra-arterial (radial or femoral) blood pressure parameters (systolic [SBP], diastolic [DBP], and mean arterial pressure) both at baseline (pre-induction) and time-matched to RRI determination were obtained from the medical record. Values that were likely erroneous (related to instrument error or line flushes), were excluded if they fell outside pre-defined ranges: SBP 40–250, DBP 15–200, mean arterial pressure 30–250, and pulse pressure (PP) >5 mmHg. Baseline measures were the median of the first 5 intraoperative values. Time-matched measures were the mean of values within 1 minute of RRI acquisition.
Renal Function Parameters
Serum creatinine was measured using the Jaffe technique (UniCel DxC-800, Beckman Coulter, Brea, CA). Per institutional protocol, creatinine was measured preoperatively and at least daily postoperatively. If multiple assessments occurred on one day, the earliest was utilized. Preoperative creatinine was defined as the value closest to, but not on, the day of surgery within 5 days preoperatively. Due to lack of accurate urine output data for all patients, AKI was defined using the Kidney Disease: Improving Global Outcomes (KDIGO) criteria based on creatinine.[2]
Statistical Analysis
Statistics are presented as median (Q1–Q3) or frequency (%). Patient and procedural characteristics were compared between aortic valve subgroups using the Kruskal-Wallis or Chi-Squared test.
In the primary analysis, pre-CPB RRI was compared among subgroups utilizing the Kruskal-Wallis test. Due to small subgroup sizes, post-hoc pairwise comparisons were performed using two-sample t-tests with permutation and Benjamini-Hochberg adjustment to control false-discovery rate at 0.05.
Secondary analyses comparing time-matched hemodynamic and Doppler parameters among subgroups were performed using the same methodology as the primary analysis. The relationship between RRI and aortic valve status was further examined with multiple linear regression adjusted for age, sex, DBP, PP, left ventricular ejection fraction, and postoperative AKI status.
Significance was defined as p<0.05 (R version 3.3.3; R Foundation for Statistical Computing, Vienna, Austria).
Results
Of 175 patients meeting inclusion criteria (Figure 2), 60 had documented aortic valve pathology (16 AI, 18 AI/AS, 26 AS). Patient and procedural characteristics were similar to those observed in other populations (Table 1).[16] Baseline differences among aortic valve subgroups included age, left ventricular ejection fraction, and DBP. Procedural factors (related to events following RRI measurement) also varied, including surgery type, and duration of aortic cross-clamp application, but rate and severity of AKI were similar among subgroups (Table 2).
Figure 2.
STROBE study population flow-chart. Abbreviations: CPB-cardiopulmonary bypass.
Table 1.
Patient and Procedural Characteristics
| Aortic Valve Status | |||||
|---|---|---|---|---|---|
|
|
|||||
| Normal (n=115) | Insufficiency (n=16) | Insufficiency & Stenosis (n=18) | Stenosis (n=26) | p-value | |
| Demographics and Comorbidities | |||||
| Age (years) | 58 (48–66) | 64 (49–69) | 67 (61–76) | 68 (65–76) | <0.0001 |
| Female | 38 (33%) | 6 (38%) | 6 (33%) | 11 (42%) | 0.83 |
| Body Mass Index | 30 (28–34) | 31 (28–32) | 28 (26–35) | 30 (26–34) | 0.76 |
| Diabetes | 34 (30%) | 5 (31%) | 3 (17%) | 5 (20%) | 0.53 |
| Hypertension | 53 (46%) | 12 (75%) | 9 (50%) | 13 (52%) | 0.20 |
| Proteinuria | 8 (7%) | 1 (6%) | 1 (6%) | 4 (15%) | 0.50 |
| Stroke | 5 (4%) | 0 (0%) | 3 (17%) | 1 (4%) | 0.12 |
| Peripheral Vascular Disease | 3 (3%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.66 |
| Left Ventricular Ejection Fraction | 0.005 | ||||
| <45% | 25 (22%) | 2 (12%) | 1 (6%) | 3 (12%) | |
| >55% | 49 (43%) | 12 (75%) | 15 (83%) | 18 (72%) | |
| 45–55% | 41 (36%) | 2 (12%) | 2 (11%) | 4 (16%) | |
|
| |||||
| Procedural Characteristics | |||||
| Procedure | <0.0001 | ||||
| Aortic | 3 (3%) | 0 | 0 | 0 | |
| CABG | 59 (51%) | 2 (12%) | 0 | 0 | |
| Combined | 6 (5%) | 11 (69%) | 7 (39%) | 9 (35%) | |
| Other | 19 (17%) | 0 | 0 | 0 | |
| Valve | 28 (24%) | 3 (19%) | 11 (61%) | 17 (65%) | |
| CPB Time (minutes) | 142 (105–194) | 168 (141–196) | 138 (122–213) | 155 (121–172) | 0.19 |
| Cross Clamp Time (minutes) | 73 (32–102) | 117 (105–138) | 103 (82–141) | 94 (88–117) | <0.0001 |
|
| |||||
| Baseline Hemodynamics | |||||
| Systolic Blood Pressure (mmHg) | 142 (127–166) | 155 (143–169) | 132 (117–160) | 144 (132–166) | 0.35 |
| Diastolic Blood Pressure (mmHg) | 64 (57–72) | 51 (46–60) | 60 (47–70) | 66 (54–71) | 0.027 |
| Pulse Pressure (mmHg) | 77 (64–94) | 92 (81–104) | 76 (64–90) | 81 (68–96) | 0.094 |
| Mean Arterial Pressure (mmHg) | 90 (80–100) | 88 (75–97) | 89 (73–105) | 90 (84–106) | 0.75 |
CABG-coronary artery bypass grafting, CPB-cardiopulmonary bypass. Values represent median (Q1–Q3) or frequency (%).
Table 2.
Renal Characteristics
| Aortic Valve Status | |||||
|---|---|---|---|---|---|
|
|
|||||
| Normal (n=115) | Insufficiency (n=16) | Insufficiency & Stenosis (n=18) | Stenosis (n=26) | p-value | |
| Baseline Serum Creatinine (mg/dL) | 0.9 (0.8–1.1) | 0.9 (0.8–1.1) | 0.9 (0.8–1.0) | 0.9 (0.7–1.1) | 0.59 |
| Baseline Creatinine Clearance (mL/min) | 98 (82–125) | 91 (71–124) | 86 (74–95) | 86 (80–100) | 0.030 |
| Maximum Postoperative Creatinine (mg/dL) | 1.2 (1.0–1.4) | 1.6 (1.1–1.9) | 1.1 (1.0–1.4) | 1.1 (1.0–1.4) | 0.045 |
| Maximum Creatinine Increase (%) | 20.0 (8.3–50.0) | 47.3 (20.4–78.1) | 23.6 (11.5–39.6) | 23.6 (0.0–48.2) | 0.14 |
| KDIGO AKI | 56 (49%) | 12 (75%) | 9 (50%) | 14 (54%) | 0.27 |
| KDIGO Stage | 0.25 | ||||
| 0 | 59 (51%) | 4 (25%) | 9 (50%) | 12 (46%) | |
| 1 | 46 (40%) | 8 (50%) | 9 (50%) | 11 (42%) | |
| 2 | 6 (5%) | 1 (6%) | 0 (0%) | 1 (4%) | |
| 3 | 4 (3%) | 3 (19%) | 0 (0%) | 2 (8%) | |
KDIGO-Kidney Disease: Improving Global Outcomes, AKI-Acute Kidney Injury. Values represent median (Q1–Q3) or frequency (%).
Renal Resistive Index
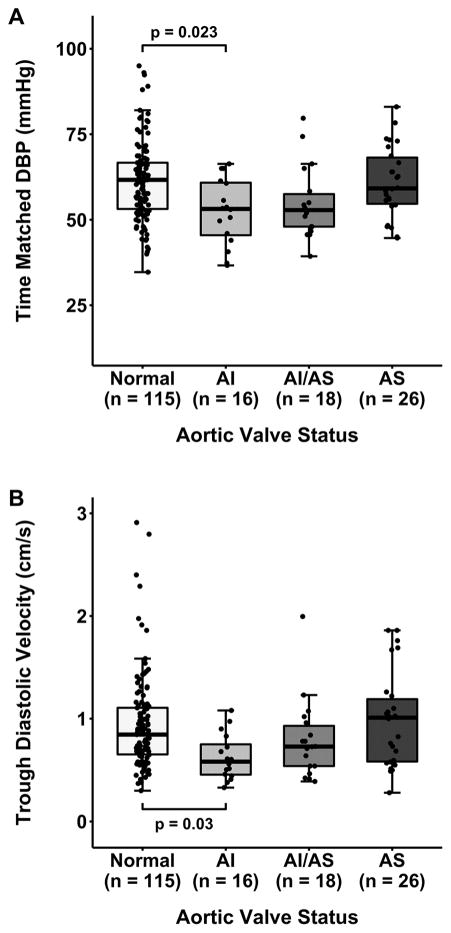
Aortic valve pathology was strongly associated with variability in RRI (p=0.0003; Table 3). In post-hoc analysis when compared to the normal subgroup median RRI was elevated in the AI (0.77 vs 0.69; adjusted p<0.001) and AI/AS (0.72 vs 0.69; adjusted p=0.042) but not the AS subgroup (adjusted p=0.38; Figure 3).
Table 3.
Doppler and Hemodynamic Parameters Prior to Initiation of Cardiopulmonary Bypass
| Aortic Valve Status | |||||
|---|---|---|---|---|---|
|
|
|||||
| Normal (n=115) | Insufficiency (n=16) | Insufficiency & Stenosis (n=18) | Stenosis (n=26) | p-value | |
| Doppler Parameters | |||||
| Renal Resistive Index | 0.69 (0.64–0.73) | 0.77 (0.71–0.82) | 0.72 (0.66–0.79) | 0.67 (0.62–0.76) | 0.0003 |
| Peak Systolic Velocity (cm/s) | 2.81 (2.16–3.50) | 2.71 (2.03–3.28) | 2.54 (2.03–3.56) | 3.15 (2.15–4.12) | 0.87 |
| Trough Diastolic Velocity (cm/s) | 0.84 (0.65–1.11) | 0.58 (0.46–0.75) | 0.73 (0.54–0.93) | 1.01 (0.58–1.19) | 0.006 |
|
| |||||
| Time-Matched Hemodynamic Parameters | |||||
| Heart Rate (bpm) | 69 (60–81) | 72 (67–83) | 71 (68–82) | 70 (61–82) | 0.57 |
| Systolic Blood Pressure (mmHg) | 120 (105–132) | 113 (107–123) | 113 (97–139) | 120 (103–135) | 0.80 |
| Diastolic Blood Pressure (mmHg) | 62 (53–67) | 53 (46–61) | 53 (48–58) | 59 (55–68) | 0.007 |
| Pulse Pressure (mmHg) | 58 (49–68) | 60 (50–74) | 50 (47–85) | 56 (49–66) | 0.92 |
| Mean Arterial Pressure (mmHg) | 80 (71–88) | 73 (67–81) | 79 (67–88) | 81 (71–93) | 0.24 |
Bpm-beats per minute. Values represent median (Q1–Q3) or frequency (%).
Figure 3.
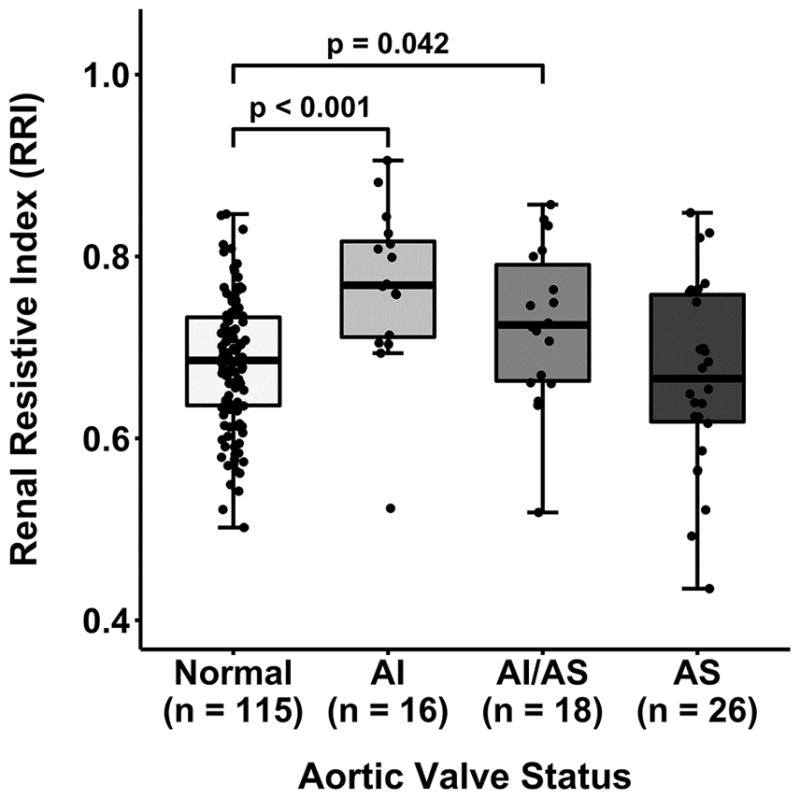
Renal resistive index determined prior to cardiopulmonary bypass stratified by aortic valve status. P-values represent corrected post-hoc comparisons. Additional differences: AI vs. AS (p=0.013). AI-aortic insufficiency, AI/AS-combined aortic insufficiency/stenosis, AS-aortic stenosis.
Time-Matched Hemodynamic and Doppler Parameters
To better understand our primary observation regarding the association of RRI with aortic valve pathology, Doppler components of RRI were compared among subgroups (Table 3). Vsys was similar (p=0.87), while Vdia varied significantly among subgroups (p=0.006). In post-hoc analysis, median Vdia was lower with AI compared to both the normal (0.58 vs 0.84; adjusted p=0.03; Figure 4B) and AS subgroups (0.58 vs 1.01; adjusted p=0.03). Similarly, there was no difference in time-matched SBP among subgroups (p=0.80) while a difference existed in time-matched DBP (p=0.007). In post-hoc analysis, median time-matched DBP was lower in the AI subgroup compared to both the normal (53 vs 62; adjusted p=0.023; Figure 4A) and AS subgroups (53 vs 59; adjusted p=0.035). In a multiple linear regression model adjusted for age, sex, time-matched DBP and PP, left ventricular ejection fraction, and postoperative AKI status, only isolated AI remained associated with elevation in RRI (0.06, 95% CI [0.02, 0.10]).
Figure 4.
Diastolic blood pressure (A) and trough diastolic velocity (B) at time of RRI determination, stratified by aortic valve status. P-values represent corrected post-hoc comparisons. Additional differences: diastolic blood pressure (p=0.035), trough diastolic velocity (p=0.03) in AI vs. AS. AI-aortic insufficiency, AI/AS-combined aortic insufficiency/stenosis, AS-aortic stenosis, DBP-diastolic blood pressure.
Comment
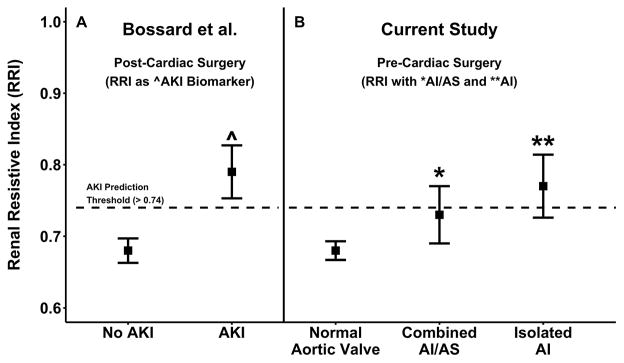
In this retrospective review of cardiac surgery patients, we confirmed variation in RRI associated with aortic valve pathology that preceded any potential renal insult from the surgical procedure (i.e., pre-CPB). Specifically, we found that RRI values in the subgroups with AI and combined AI/AS were elevated compared to normal patients. Importantly, these elevations with AI (0.08 units) and AI/AS (0.03 units) caused pre-surgery RRI values to considerably overlap with post-procedure thresholds previously associated with AKI (Figure 5). Notably, AKI rates in the AI and AI/AS subgroups were not different from the normal subgroup (Table 2). Hemodynamic and Doppler analyses expose the components of RRI estimation contributing to this observation. Time-matched DBP and Vdia were lower in patients with AI but not with other aortic valve pathologies. These findings suggest that the value of, and thresholds for, RRI as an early AKI biomarker may be further improved with appropriate consideration of aortic valve pathology.
Figure 5.
Renal resistive index (mean±95% CI) for cardiac surgery patients in two studies. Values from (A) Bossard et al., [3] stratified by AKI status and determined postoperatively by transabdominal ultrasound and (B) the current study, stratified by aortic valve status, determined prior to CPB. Notably, pre-intervention values in the AI and AI/AS subgroups from the current study considerably overlap post-intervention values in the AKI subgroup. The horizontal hatched line reflects the RRI threshold (>0.74) for AKI prediction developed by Bossard et al. AI-aortic insufficiency, AI/AS-combined aortic insufficiency/stenosis, AKI-acute kidney injury.
Although no previous reports have explored the relationship between aortic valve pathology and RRI, one study involving similar hemodynamic perturbations is informative to compare with the current investigation. Sinning and colleagues noted elevated RRI in patients with moderate/severe paravalvular leaks following transcatheter aortic valve replacement (TAVR)[17]. RRI was 0.17 units higher in patients with moderate/severe leaks relative to others. While our findings are consistent with those of Sinning et al., and similar hemodynamic abnormalities (lower DBP and Vdia) likely underpin elevated RRI in both settings, notable differences between the studies deserve mention. The greater magnitude of RRI elevation with paravalvular leak vs. isolated AI (0.17 vs. 0.08) may be related to larger regurgitant volumes with the former condition. However, the post-procedure timing of RRI measurement by Sinning et al. complicates comparison of the two studies. AKI is a recognized complication of TAVR[18] and is inextricably inserted into post-procedure consideration of the effects of paravalvular leak on RRI estimation. This is particularly relevant since AKI risk may differ between patients with and without paravalvular leak. Post-TAVR paravalvular leaks occur more often with calcified valves[19], and additional contrast dye may be required to image a leak, both presumably raising the risk for AKI. Furthermore, higher AKI risk may also be accompanied by the consequences of acute paravalvular leak on perfusion in the setting of renal swelling, whose implications for the evolution of a renal injury are unknown. Such potential confounding effects are not equivalent between studies and it is unlikely they are fully accounted for by adjustment for comorbidities and AKI status. Nonetheless, the most pertinent observation from both studies is that regurgitant aortic lesions are associated with elevated RRI values that overlap importantly with the range associated with AKI (Figure 5). In the current study, elevated RRI values appear to be hemodynamic “artifacts” of aortic insufficiency, rather than related to any renal insult, and likely confound the value of RRI as an early AKI biomarker.
Our analysis is limited by its retrospective nature. However, the variables utilized involve data electronically transferred to the medical record as part of regular clinical care. Furthermore, blinded re-analysis of prospectively collected TEE images by certified experts should eliminate potential bias in interpreting RRI in relation to existing valve pathology. The small subgroup sizes limit statistical power for comparisons. However, we observed significant associations using empirical p-values obtained using permutation and adjusted for multiple comparisons. Naturally, patients with aortic valve pathology were more likely to be undergoing aortic valvular procedures, associated with varying duration of aortic cross-clamp time. Additionally, as expected, patients with aortic valve pathology were older, and those with AI had lower baseline DBP (Table 1). In order to address the inhomogeneity of such variables, including some that are confounded with RRI (e.g., age, PP), [20] we included an adjusted analysis.
Hemodynamic factors that both affect RRI and are associated with AI could potentially confound our study findings. Variation in the use of radial vs. femoral arterial catheters was not assessed, and wider PP with the latter could vary among groups. However, we have no reason to suspect there was systematic variation in the arterial catheter site across subgroups. Hypertension is associated with RRI variation in some studies, and a reported history of hypertension in our sample was (non-significantly) higher in the AI than other subgroups (Table 1).[21–23] However, the relationship between RRI and hypertension is complex; although RRI is not elevated in patients with primary essential hypertension relative to unaffected patients, RRI does differentiate treatment-resistant from treatment-responsive hypertension.[21–23] Since these distinguishing factors are not included in the hypertension documentation available for the current study, we conducted subanalyses to further assess the potential for hypertension to have introduced bias into our primary finding. As previously reported in general populations, patients with (n=53) and without (n=62) a diagnosis of hypertension (from our normal aortic valve subgroup) had very similar RRI values (0.70 vs. 0.68, p=0.33)[21]. Furthermore, a primary re-analysis limited to patients with a history of hypertension (AI, n=12 vs normal valve, n=53) continues to demonstrate a significant difference in RRI (0.78 vs 0.70, p=0.001). Lastly, preoperative proteinuria was not significantly different among subgroups (Table 1). While these analyses do not completely eliminate concerns, we believe they provide reassurance that hypertension is not exhibiting a major confounding effect. Nonetheless, the current study represents the only assessment of aortic valve pathology and its relationship with RRI, including observations that may enhance the value of RRI as an AKI biomarker.
The implications of predictable baseline variation in RRI that could confound its value as a marker of renal insult are worthy of speculation. First, since these observations are predictable, RRI may be amenable to adjustment as a strategy to enhance its value as an early AKI biomarker. No published AKI biomarker studies have integrated adjustment for aortic valve status into their RRI estimation [3, 7, 8], yet even without adjustments RRI is associated with AKI in these cohorts, while such valve lesions likely exist among them. Accounting for aortic valve status may, in fact further enhance the value of RRI as an AKI biomarker by addressing previously unexplained variability. Further investigations are essential to validate refinements accounting for aortic insufficiency in RRI estimation for AKI prediction. For example, data from the current study suggests that adjusting RRI downward by 0.08 for subjects with moderate/severe AI and by 0.03 for subjects with AI/AS may account for these valvular pathologies. Unfortunately, validation of an approach to improve RRI as an AKI biomarker through “adjustment” is not possible using this cohort since the post-CPB valve status of this subset typically involves valve replacement. Until such validation is complete findings from the current and other studies suggest that RRI should be used with caution as an AKI biomarker in the context of aortic regurgitant lesions. In this regard, our AI subgroup findings align with the abovementioned TAVR report [17], but also analyses in ex-vivo perfusion systems and human subjects showing associations between systemic hemodynamics and RRI similar to the current investigation.[20, 24] Finally, as an AKI biomarker, RRI may benefit from a search for other factors that confound its value and are amenable to adjustment. Such information may be useful at the bedside or within decision support tools to make an already valuable biomarker potentially even more useful. This is particularly relevant for cardiac surgery patients given the routine use of intraoperative TEE, and that other early AKI biomarker candidates (e.g., neutrophil gelatinase-associated lipocalin) are more expensive and less immediately available, yielding information with several hours delay following renal insult relative to intraoperative RRI [25].
In summary, in cardiac surgery patients, pre-procedure intraoperative RRI values determined by TEE were elevated in patients with isolated AI and combined AI/AS compared to those with normal valvular function, likely related to effects on diastolic renal blood flow. Regarding RRI as an AKI biomarker, these observations suggest that regurgitant aortic lesions create a hemodynamic “artifact” unrelated to renal insult that may confound its value. Given the common occurrence of aortic valve pathology in patients undergoing cardiac surgery and the need for reliable early AKI biomarkers, additional research is needed to better understand this issue. Furthermore, if the role of aortic pathology and other potential confounders can be clarified, such improvements may add value to RRI as an early AKI biomarker.
Abbreviations
- AI
Aortic insufficiency
- AKI
Acute kidney injury
- AS
Aortic stenosis
- CPB
Cardiopulmonary bypass
- DBP
Diastolic blood pressure
- KDIGO
Kidney Disease: Improving Global Outcomes
- PP
Pulse pressure
- RRI
Renal resistive index
- SBP
Systolic blood pressure
- TAVR
Transcatheter aortic valve replacement
- TEE
Transesophageal echocardiography
- Vsys
Peak systolic velocity
- Vdia
Trough diastolic velocity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stafford-Smith M, Patel UD, Phillips-Bute BG, Shaw AD, Swaminathan M. Acute kidney injury and chronic kidney disease after cardiac surgery. Advances in chronic kidney disease. 2008;15(3):257–277. doi: 10.1053/j.ackd.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Khwaja A. Kdigo clinical practice guidelines for acute kidney injury. Nephron Clinical practice. 2012;120(4):c179–184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 3.Bossard G, Bourgoin P, Corbeau JJ, Huntzinger J, Beydon L. Early detection of postoperative acute kidney injury by doppler renal resistive index in cardiac surgery with cardiopulmonary bypass. British journal of anaesthesia. 2011;107(6):891–898. doi: 10.1093/bja/aer289. [DOI] [PubMed] [Google Scholar]
- 4.Cherry A, Hauck J, Andrew B, et al. Renal resistive index and acute kidney injury prediction using transesophageal echocardiography in cardiac surgery. Anesthesiology. 2015:A2034. [Google Scholar]
- 5.Darmon M, Schortgen F, Vargas F, et al. Diagnostic accuracy of doppler renal resistive index for reversibility of acute kidney injury in critically ill patients. Intensive care medicine. 2011;37(1):68–76. doi: 10.1007/s00134-010-2050-y. [DOI] [PubMed] [Google Scholar]
- 6.Marty P, Szatjnic S, Ferre F, et al. Doppler renal resistive index for early detection of acute kidney injury after major orthopaedic surgery: A prospective observational study. Eur J Anaesthesiol. 2015;32(1):37–43. doi: 10.1097/EJA.0000000000000120. [DOI] [PubMed] [Google Scholar]
- 7.Regolisti G, Maggiore U, Cademartiri C, et al. Renal resistive index by transesophageal and transparietal echo-doppler imaging for the prediction of acute kidney injury in patients undergoing major heart surgery. Journal of nephrology. 2016 doi: 10.1007/s40620-016-0289-2. [DOI] [PubMed] [Google Scholar]
- 8.Wu HB, Qin H, Ma WG, et al. Can renal resistive index predict acute kidney injury after acute type a aortic dissection repair? The Annals of thoracic surgery. 2017 doi: 10.1016/j.athoracsur.2017.03.057. [DOI] [PubMed] [Google Scholar]
- 9.Cruces P, Salas C, Lillo P, Salomon T, Lillo F, Hurtado DE. The renal compartment: A hydraulic view. Intensive Care Med Exp. 2014;2(1):26. doi: 10.1186/s40635-014-0026-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy ME, Tublin ME. Understanding the doppler ri: Impact of renal arterial distensibility on the ri in a hydronephrotic ex vivo rabbit kidney model. J Ultrasound Med. 2000;19(5):303–314. [PubMed] [Google Scholar]
- 11.Yang PL, Wong DT. Resistive index may not accurately reflect renal flow resistance in the presence of significant aortic insufficiency. Anesth Analg. 2009;109(6):2033. doi: 10.1213/ane.0b013e3181bc77a5. author reply 2033–2034. [DOI] [PubMed] [Google Scholar]
- 12.Grover FL, Vemulapalli S, Carroll JD, et al. 2016 annual report of the society of thoracic surgeons/american college of cardiology transcatheter valve therapy registry. The Annals of thoracic surgery. 2017;103(3):1021–1035. doi: 10.1016/j.athoracsur.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Zoghbi WA, Enriquez-Sarano M, Foster E, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and doppler echocardiography. J Am Soc Echocardiogr. 2003;16(7):777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 14.Baumgartner H, Hung J, Bermejo J, et al. Echocardiographic assessment of valve stenosis: Eae/ase recommendations for clinical practice. J Am Soc Echocardiogr. 2009;22(1):1–23. doi: 10.1016/j.echo.2008.11.029. quiz 101–102. [DOI] [PubMed] [Google Scholar]
- 15.Bandyopadhyay S, Kumar Das R, Paul A, Sundar Bhunia K, Roy D. A transesophageal echocardiography technique to locate the kidney and monitor renal perfusion. Anesth Analg. 2013;116(3):549–554. doi: 10.1213/ANE.0b013e31827ab3b1. [DOI] [PubMed] [Google Scholar]
- 16.Brown JR, Hisey WM, Marshall EJ, et al. Acute kidney injury severity and long-term readmission and mortality after cardiac surgery. The Annals of thoracic surgery. 2016;102(5):1482–1489. doi: 10.1016/j.athoracsur.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sinning JM, Adenauer V, Scheer AC, et al. Doppler-based renal resistance index for the detection of acute kidney injury and the non-invasive evaluation of paravalvular aortic regurgitation after transcatheter aortic valve implantation. EuroIntervention. 2014;9(11):1309–1316. doi: 10.4244/EIJV9I11A221. [DOI] [PubMed] [Google Scholar]
- 18.Aalaei-Andabili SH, Pourafshar N, Bavry AA, et al. Acute kidney injury after transcatheter aortic valve replacement. J Card Surg. 2016;31(7):416–422. doi: 10.1111/jocs.12768. [DOI] [PubMed] [Google Scholar]
- 19.Fonseca P, Figueiredo B, Almeida C, et al. Aortic valve calcium volume predicts paravalvular regurgitation and the need for balloon post-dilatation after transcatheter aortic valve implantation. Journal of interventional cardiology. 2016;29(1):117–123. doi: 10.1111/joic.12267. [DOI] [PubMed] [Google Scholar]
- 20.Kuznetsova T, Cauwenberghs N, Knez J, et al. Doppler indexes of left ventricular systolic and diastolic flow and central pulse pressure in relation to renal resistive index. Am J Hypertens. 2015;28(4):535–545. doi: 10.1093/ajh/hpu185. [DOI] [PubMed] [Google Scholar]
- 21.Bruno RM, Daghini E, Landini L, et al. Dynamic evaluation of renal resistive index in normoalbuminuric patients with newly diagnosed hypertension or type 2 diabetes. Diabetologia. 2011;54(9):2430–2439. doi: 10.1007/s00125-011-2148-y. [DOI] [PubMed] [Google Scholar]
- 22.Prejbisz A, Warchol-Celinska E, Florczak E, et al. Renal resistive index in patients with true resistant hypertension: Results from the resist-pol study. Kardiol Pol. 2016;74(2):142–150. doi: 10.5603/KP.a2015.0114. [DOI] [PubMed] [Google Scholar]
- 23.Kintis K, Tsioufis C, Kasiakogias A, et al. Noninvasive assessment of haemodynamics in resistant hypertension: The role of the renal resistive index. J Hypertens. 2017;35(3):578–584. doi: 10.1097/HJH.0000000000001206. [DOI] [PubMed] [Google Scholar]
- 24.Tublin ME, Tessler FN, Murphy ME. Correlation between renal vascular resistance, pulse pressure, and the resistive index in isolated perfused rabbit kidneys. Radiology. 1999;213(1):258–264. doi: 10.1148/radiology.213.1.r99oc19258. [DOI] [PubMed] [Google Scholar]
- 25.Ho J, Tangri N, Komenda P, et al. Urinary, plasma, and serum biomarkers’ utility for predicting acute kidney injury associated with cardiac surgery in adults: A meta-analysis. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2015;66(6):993–1005. doi: 10.1053/j.ajkd.2015.06.018. [DOI] [PubMed] [Google Scholar]