Abstract
Age-dependent impairments in the central control of compensatory responses to body fluid challenges have received scant experimental attention, especially in females. In the present study, we found that water drinking in response to β-adrenergic activation with isoproterenol (30 μg/kg, s.c.) was reduced by more than half in aged (25 mo) vs. young (5 mo) ovariectomized female Brown Norway rats. To determine whether this age-related decrease in water intake was accompanied by changes in central nervous system areas associated with fluid balance, we assessed astrocyte density and neuronal activation in the SFO, OVLT, SON, AP and NTS of these rats using immunohistochemical labeling for GFAP and c-fos, respectively. GFAP labeling intensity was increased in the SFO, AP, and NTS of aged females independent of treatment, and was increased in the OVLT of isoproterenol-treated rats independent of age. Fos immunolabeling in response to isoproterenol was reduced in both the SFO and the OVLT of aged females compared to young females, but was increased in the SON of female rats of both ages. Finally, fos labeling in the AP and caudal NTS of aged rats was elevated after vehicle control treatment and did not increase in response to isoproterenol as it did in young females. Thus, age-related declines in water drinking are accompanied by site-specific, age-related changes in astrocyte density and neuronal activation. We suggest that astrocyte density may alter the detection and/or processing of signals related to isoproterenol treatment, and thereby alter neuronal activation in areas associated with fluid balance.
Keywords: subfornical organ, organum vasculosum of the lamina terminalis, area postrema, nucleus of the solitary tract, supraoptic nucleus
1. Introduction
The elderly are at major risk for dehydration [1], because of both increased fluid losses [2] and decreased sensation of thirst [3–5]. Indeed, diminished thirst is perhaps the major factor contributing to problems of fluid balance in the elderly [6]. As a result, the elderly are more vulnerable than are the young to the health consequences of dehydration such as confusion, hypotension, and renal dysfunction [1–2]. Accordingly, investigations of water ingestion are critical for understanding declines in body fluid regulation that accompany aging. Notably, the experimental literature contains only a few studies using aging rats to investigate the capacity of behavioral systems, such as thirst, to maintain adequate hydration. Furthermore, most current studies on age-related declines in water intake and body fluid regulation, including our own, have used male rats. In these efforts, we and others have advanced understanding of how the behavioral, renal, and cardiovascular underpinnings of body fluid homeostasis change with age in males [7–13]. However, despite increasing appreciation of the importance of sex differences for body fluid balance in young adult rats (e.g., [14–15]; for review, see [16]), with few exceptions (e.g., [17–18]), aged females have been virtually excluded from studies of body fluid homeostasis.
Isoproterenol (ISOP) is a mixed β-adrenergic agonist that has been extensively employed to investigate thirst mechanisms in rats, including aged rats of both sexes [11, 17–18]. ISOP activates the renin-angiotensin system (RAS) both directly by stimulating β1-adrenergic receptors located on the juxtaglomerular apparatus of the kidney and indirectly from hypotension caused by stimulating vascular β2-adrenergic receptors [11]. Water drinking in response to ISOP results from high levels of circulating angiotensin II (ANG II) which acts at receptors in the forebrain circumventricular organ, subfornical organ (SFO; [19–20]) to promote thirst. We and others have shown that estradiol (E2) reduces ISOP-induced water intake, neural activation, and ANG II receptors in the SFO of young female rats [21–27], findings that are consistent with an extensive literature focused on central areas involved in body fluid homeostasis [19–20, 28–31]. In those studies, circumventricular organs in addition to the SFO (i.e., the organum vasculosum of the lamina terminalis (OVLT) and the area postrema (AP)) have been implicated in detecting hormonal signals including ANG II [28–33], while the Nucleus of the Solitary Tract (NTS) receives baroreceptor input related to the hypotension produced by ISOP [30–31]). Moreover, other central areas play a role in compensatory responses to ISOP, with the hypothalamic paraventricular and supraoptic nuclei (PVN, SON) releasing the vasoactive hormones, vasopressin and oxytocin [30–31], and the NTS modifying autonomic function, in part via activation of norepinephrine-containing neurons [34].
Clearly, a wealth of information concerning central controls of water intake has resulted from previous studies. Nonetheless, two oversights are noteworthy. First, nearly all of these studies have been conducted in males, especially male rats (e.g., [19–20]. Much less is known about these central areas in females and, with very few exceptions [17–18], virtually nothing is known about the central controls in aged females. Second, this literature has focused primarily on neurons, neurotransmitters, and neural pathways; however, it has become increasingly apparent that non-neuronal cells such as astrocytes play more than a ‘supporting role’. One of the primary functions of astrocytes is their contribution to the blood-brain-barrier via interactions between astrocyte end feet and vascular epithelia [35–37]. In the context of circumventricular organs, which detect circulating hormones by virtue of an incomplete blood-brain-barrier, changes in astrocytes might therefore be expected to alter detection of circulating signals associated with ISOP. In addition, astrocytes may more directly influence neural activity by buffering extracellular potassium concentration, or by the uptake and synthesis of glutamate and GABA [37–38]. In short, astrocytes have the potential to affect ISOP-induced water intake through altering activation in central areas by contributing to neural membrane potential or to levels of neurotransmitters, or by contributing to the blood-brain-barrier.
At present, it is not known whether aging or ovarian hormones such as estrogen may alter astrocytes in circumventricular organs. However, a possible mechanism for age-related changes in astrocytes or astrocyte function that, in turn, might alter neuronal activity is suggested by the neuroprotective effects of the α subtype of estrogen receptors in astrocytes in the central nervous system of mice [39]. Accordingly, the present work examined water drinking, astrocyte density, and neural activation following ISOP treatment in young and aged female rats. In this preliminary assessment of age-related changes in astrocytes associated with ISOP-induced water intake, we focused on SFO, OVLT, and AP (circumventricular organs involved in the detection of ANG II), and the NTS (the primary terminal site of baroreceptor afferents). We also sought to investigate potential functional consequences of any differences in astrocyte density by examining neuronal activation in those areas, in oxytocinergic neurons in the SON, and in norepinephrine-containing neurons in the NTS. To better discriminate between effects due to aging and those due to depletion of ovarian hormones, all experiments were conducted using ovariectomized (OVX) young and aged female rats.
2. Methods
2.1 Animals
Young (5 mo) and aged (25 mo) female Brown Norway rats were obtained from Charles River, Raleigh, NC. They were housed singly in hanging, wire-mesh cages with ad libitum access to standard rodent diet (Teklad) and tap water provided from 100-ml graduated cylinders with attached stainless-steel spouts unless otherwise stated. Room temperature was maintained at 23°C with a 12:12 light dark cycle. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committees of the University of Iowa and of the Oklahoma State University Center for Health Sciences.
2.2 Surgical Procedures
Under isofluorane anesthesia, bilateral ovariectomy was performed by making a single 2- to 3-cm dorsal midline incision in the skin and underlying muscles. The ovaries were isolated, tied-off with sterile suture and removed, and the incisions were closed. Ovariectomized (OVX) rats were allowed to recover for 8–10 days before testing. Subsets of these OVX rats (aged: n = 9, young: n = 8) were tested in the following studies; the numbers included in each experimental condition are given in the Results sections pertaining to the individual studies.
2.3 Drugs
The mixed β1,β2-adrenergic receptor agonist isoproterenol (ISOP; Isuprel, Hospira, Lake Forest, IL) was diluted 3:1 with isotonic saline. In all experiments, ISOP was injected s.c. (30 μg/kg body weight).
2.4 Experiment 1: Water Intake
On the morning of testing, drinking tubes were removed from cages, rats were weighed and then injected with ISOP. A single graduated burette filled with water was placed on the cage and intakes were recorded every 30 min for 90 min.
2.5 Experiments 2 and 3: Immunohistochemistry
One week after the water intake study, rats were randomly subdivided into groups receiving either ISOP or vehicle (VEH; 0.15 M NaCl; 750 μl/kg, s.c.) and then returned to their home cages with no water or food available. Ninety minutes after injections, rats were deeply anesthetized with sodium pentobarbital (Nembutal) and then perfused with 0.15 M NaCl followed by 4% paraformaldehyde. Brains were removed and placed in 30% sucrose until cut into 40-μm serial coronal sections using a cryostat (Leica). Forebrain and hindbrain blocks each were cut in a 1:3 series and stored in a cryoprotectant solution [40] at −20° C until processed for immunolabeling.
2.5.1 Experiment 2: Glial Fibrillary Acidic Protein (GFAP) immunolabeling
One series each of free-floating forebrain and hindbrain sections was used to label GFAP, a marker of glial cells such as astrocytes after ISOP. Tissue was rinsed with 0.05 M Tris-NaCl, treated with 0.5% H2O2 for 30 min, and rinsed again with 0.05 M Tris-NaCl on a rocker at room temperature (RT). Tissue was soaked for 60 min at RT in 10% normal goat serum (NGS) mixed in 0.5% Triton-X in 0.05 M Tris-NaCl, then incubated for ~48 hr on a rocker at 4°C in the primary antibody (Millipore, mouse anti-GFAP; clone GA5, diluted 1:6000 in 2% NGS in 0.05 M Tris-NaCl with 0.5% Triton-X). Sections were rinsed, incubated in Cy3-conjugated goat anti-mouse IgG (Jackson Immunoresearch; diluted 1:200 in 2% NGS) for 4–6 hrs, and then rinsed multiple times. Sections were ordered, mounted on gelatinized slides, and dried overnight. Tissue was dehydrated in a series of EtOHs, defatted in xylenes, and then coverslipped using Cytoseal 60 (Fisher Scientific).
Quantification
The SFO, organum vasculosum of the lamina terminalis (OVLT), area postrema (AP), and the nucleus of the solitary tract (NTS) at the middle of the rostral-caudal extent (mNTS) were identified under brightfield microscopy using a Nikon Eclipse 80i microscope with rhodamine and FITC filter sets based on neuroanatomical landmarks and coordinates [41]. These areas were captured using NIS-Elements AR software 3.2 (Nikon) with exposure times held constant. SFO, OVLT, AP each were traced; a square of uniform size was placed on the mNTS ventral to the lateral extent of the AP and dorsal to the dorsal motor nucleus of the vagus. GFAP immunolabeling in these areas was quantified as intensity of labeling using NIS-Elements. All quantification was conducted by personnel blind to the experimental condition. GFAP intensity in each area from each animal was averaged over 2–3 sections that were matched among groups. Group means for each area were calculated from the averaged counts.
2.5.2 Experiment 3: fos immunolabeling
A second series of free-floating forebrain and hindbrain sections were processed for fos immunoreactivity using the avidin-biotin-peroxidase technique as described in our previous publications [23–24, 42–43]. Briefly, tissue sections were rinsed in 0.05 M Tris-NaCl, incubated in 0.5% H2O2 for 30 min, rinsed again, and then incubated in 10% NGS in 0.05 M Tris-NaCl with 0.5% Tween for 60 min. Tissue sections were incubated overnight in the primary antibody (Santa Cruz; rabbit anti-c-fos, diluted 1:30,000 in 2% NGS) on a rocker at 4° C, then rinsed with 2% NGS and incubated in the secondary antibody (biotinylated goat anti-rabbit IgG; Vector, diluted 1:300 in 2% NGS) for 2 hr on a rocker at RT. Following additional rinses with 0.05 M Tris-NaCl, labeling was amplified using Vectastain ABC kit (Vector) before incubating for 10–15 min with a nickel-intensified diaminobenzidine solution (Vector) to visualize fos immunoreactivity. The reaction was stopped with multiple rinses of 0.05 M Tris-NaCl. After completion of fos immunolabeling, forebrain sections were further processed to label oxytocin (OT). Sections were incubated in a mouse anti-OT monoclonal primary antibody (Chemicon; diluted 1:20,000 in 2% NGS in 0.05 M Tris-NaCl with 0.5% Triton-X) for ~48 hr, and then incubated in in a Cy2-conjugated goat anti-mouse secondary antibody (Jackson Immunoresearch; diluted 1:300 in 2% NGS in 0.05 M Tris-NaCl with 0.5% Triton-X) for 4–6 hrs before multiple rinses. Hindbrain sections were further processed to label dopamine-β-hydroxylase (DBH), a marker of norepinephrine-containing neurons, using a mouse anti-DBH monoclonal primary antibody (Chemicon; diluted 1:1,000 in 2% NGS in 0.05 M Tris-NaCl with 0.5% Triton-X) for ~48 hr. These sections then were incubated for 4–6 hrs in a Cy3-conjugated goat anti-mouse secondary antibody (Jackson Immunoresearch; diluted 1:200), and then rinsed multiple times.
Finally, sections were ordered, mounted on gelatinized slides, and dried overnight. Tissue was dehydrated in a series of EtOHs, defatted in xylenes, and then coverslipped using Cytoseal 60 (Fisher Scientific).
Quantification
The SFO, OVLT, SON, AP, mNTS, and caudal NTS (cNTS) were identified as described for GFAP immunolabeling. Brightfield images were captured, and fos-immunoreactive (fos-ir) neurons (i.e., those with dark brown-stained nuclei) were counted manually in 2–3 sections from each area for each animal, matched between animals. Group means for each area were calculated from the averaged counts.
OT-immunoreactive (OT-ir) neurons in the SON and DBH-immunoreactive (DBH-ir) neurons in the cNTS were visualized under fluorescence microscopy, and images were captured with exposure times held constant. Brightfield and fluorescent images were merged to allow manual counting of fos-ir nuclei in OT-ir neurons in the SON or fos-ir nuclei in DBH-ir neurons in the NTS.
2.6 Statistics
For experiment 1, cumulative water intake was expressed as ml/100 g body weight. All data are shown as group means ± SEM.
Body weight on the day of water intake testing was analyzed with an independent, 2-tailed t-test; the magnitude of the difference between groups was assessed using effect size, calculated as eta2 (η2). GFAP intensity and numbers of fos-ir neurons in AP, cNTS, mNTS, OVLT, SFO, and SON were analyzed using separate 2-way analyses of variance (ANOVAs) with age (young, aged) and treatment (VEH, ISOP) as factors. Numbers of fos- and OT-ir neurons in SON, and numbers of fos- and DBH-ir neurons in cNTS also were analyzed using separate 2-way ANOVA with age and treatment as factors. Cumulative water intake after ISOP treatment was analyzed using 2-way repeated measures (rm) ANOVA with age (young, aged) and time (30, 60, 90 min) as factors, repeated for time. For all ANOVAs, significant (p<0.05) main effects or interactions were further evaluated using Fishers LSD tests. Specific planned comparisons were made using Bonferroni corrections. The magnitude of the difference was assessed using effect size, calculated as partial eta2 (pη2).
3. Results
3.1 Body weight and water intake
On the day of water intake testing, aged females weighed significantly more than did young females (242.8 + 6.9 g vs. 208.9 + 5.4 g, respectively; t = −3.79, p<0.01; η2= 0.49).
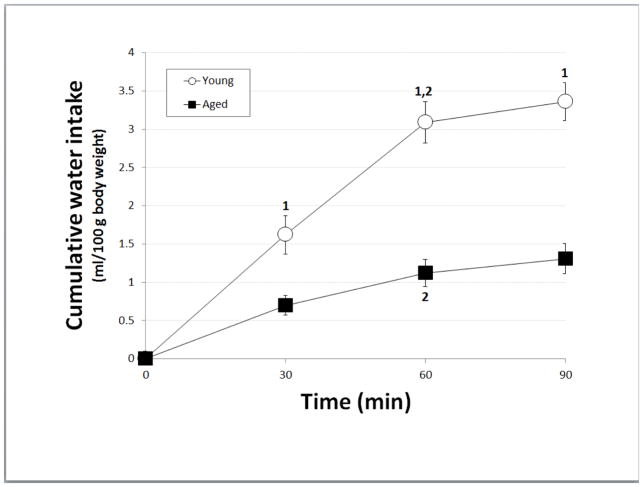
Figure 1 shows water intake stimulated by ISOP treatment. There were main effects of both age [F(1,15) = 35.59, p<0.001; pη2 = 0.70] and time [F(2,30) = 63.07, p<0.001; pη2 = 0.81]. There also was a significant interaction between age and time [F(2,30) = 16.09. p<0.001; pη2 = 0.52]. Pairwise comparisons of the interaction showed that water intake by females of both ages (aged: n = 9, young: n = 8) was significantly greater at 60 min than at 30 min (ps<0.01-0.001), with no further increase thereafter in either age group. However, water intake by young females was significantly greater than that by aged females at each time point (ps<0.01-0.001).
Figure 1. Water intake after isoproterenol.
Cumulative water intake by young (open circles) and aged (black squares) female Brown Norway rats after treatment with isoproterenol. Measurements were taken at 30-min intervals for 90 min after s.c. isoproterenol injection; intakes were normalized to body weight. 1 = significantly greater than aged females at corresponding time point (ps<0.01-0.001); 2 = significantly greater than preceding time point within age group (ps<0.01-0.001)
3.2 GFAP immunolabeling
Figures 2–4 show that ISOP treatment had age-dependent effects on GFAP labeling intensity that depended on the specific area.
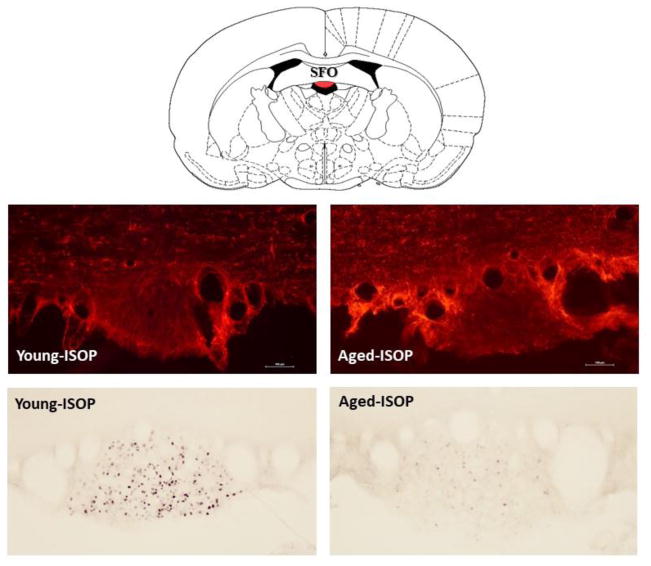
Figure 2. GFAP or fos immunolabeling in the SFO.
Top: line drawing (adapted from [41]) of the subfornical organ (SFO; red shading). Middle: representative digital photomicrographs of immunolabeling for glial fibrillary acidic protein (GFAP; red labeling) in the SFO of young (left panel) or aged (right panel) female Brown Norway rats that were given s.c. injections of isoproterenol (ISOP). Bottom: representative digital photomicrographs of immunolabeling for the fos protein (brown/black nuclear staining) in the SFO of young (left panel) or aged (right panel) female Brown Norway rats that were given s.c. injections of ISOP.
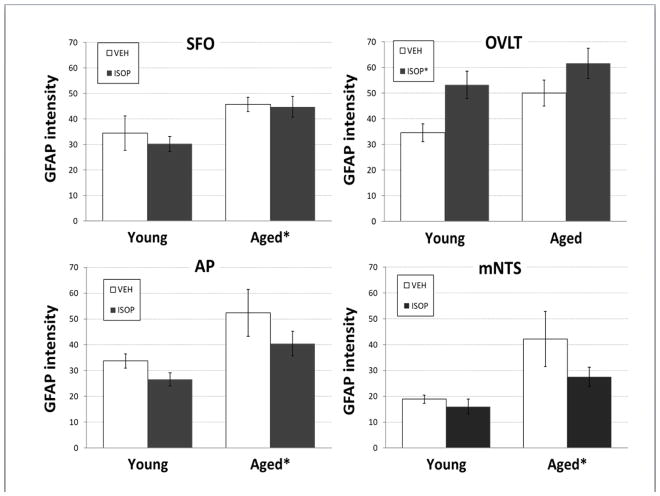
Figure 4. GFAP intensity.
Mean intensity of glial fibrillary acid protein (GFAP) immunolabeling in the subfornical organ (SFO; top left), organum vasculosum of the lamina terminalis (OVLT; top right), Area postrema (AP; bottom left), and middle level of the nucleus of the solitary tract (mNTS; bottom right) of young or aged female Brown Norway rats. Rats were given s.c. injections of the 0.15M NaCl vehicle (VEH; open bars) or isoproterenol (ISOP; black bars).
* = significant main effect of age or drug treatment.
Forebrain
Two-way ANOVA on GFAP intensity in SFO (Figure 2, 4; ISOP-aged: n = 4, ISOP-young: n = 3, VEH-aged: n= 4, VEH-young: n = 2) revealed a significant main effect of age [F(1,9) = 10.32, p<0.01; pη2 = 0.53], with greater GFAP intensity in the SFO of aged females, overall. There was no effect of treatment and no interaction between age and treatment. In contrast, two-way ANOVA on GFAP intensity in OVLT (Figure 4; ISOP-aged: n = 5, ISOP-young: n = 4, VEH-aged: n= 4, VEH-young: n = 3) revealed a significant main effect of treatment [F(1,12) = 7.51, p<0.01; pη2 = 0.38], with greater GFAP intensity in the OVLT after ISOP treatment, overall. There was no effect of age and no interaction between age and treatment on GFAP intensity in the OVLT.
Hindbrain
Two-way ANOVA on GFAP intensity in both AP and mNTS (Figure 3, 4; ISOP-aged: n = 5, ISOP-young: n = 5, VEH-aged: n= 4, VEH-young: n = 3) revealed significant main effects of age ([F(1,13) = 8.67, p<0.05; pη2 = 0.40]; [F(1,13) = 8.81, p<0.05; pη2 = 0.40], respectively). In both areas, GFAP intensity was greater in aged females, with no effects of treatment and no interactions between age and treatment.
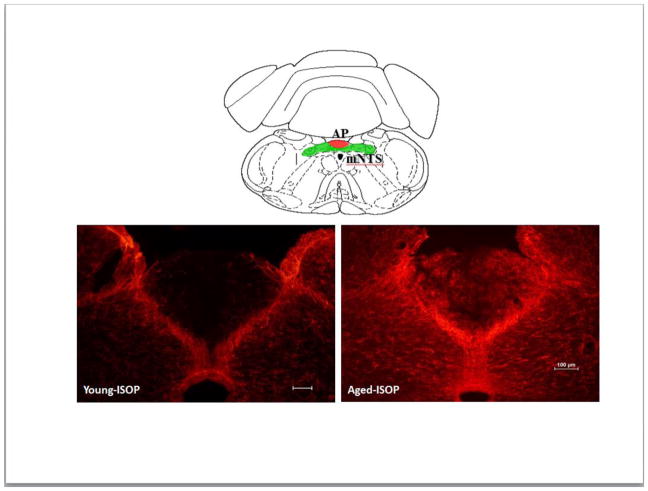
Figure 3. GFAP immunolabeling in the AP and mNTS.
Top: line drawing (adapted from [41]) of the area postrema (AP; red shading) and the middle level of the nucleus of the solitary tract (mNTS; green shading). Bottom: representative digital photomicrographs of immunolabeling for glial fibrillary acidic protein (GFAP; red labeling) in the AP and mNTS of young (left panel) or aged (right panel) female Brown Norway rats that were given s.c. injections of isoproterenol (ISOP).
3.3 fos immunolabeling
Figures 2, 5, 6, 7 show that ISOP treatment had age-dependent effects on fos immunolabeling that were site-specific.
Figure 5. fos immunolabeling.
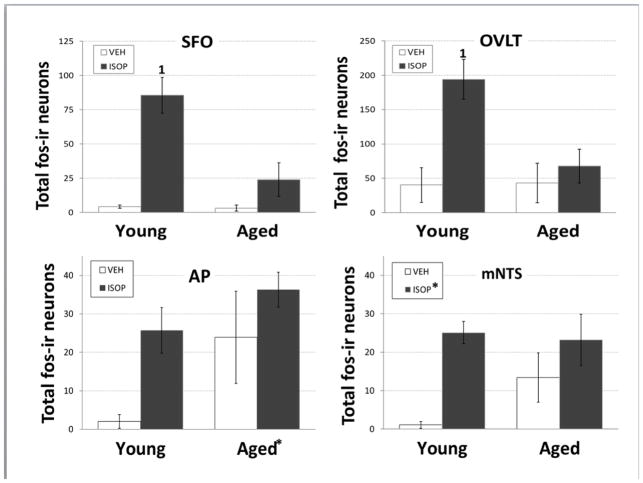
Mean number of fos-immunoreactive (fos-ir) neurons in the subfornical organ (SFO; top left), organum vasculosum of the lamina terminalis (OVLT; top right), Area postrema (AP; bottom left), and middle level of the nucleus of the solitary tract (mNTS; bottom right) of young or aged female Brown Norway rats. Rats were given s.c. injections of the 0.15M NaCl vehicle (VEH; open bars) or isoproterenol (ISOP; black bars).
* = significant main effect of age or drug treatment. 1 = significantly greater than all other conditions (ps<0.01-0.001).
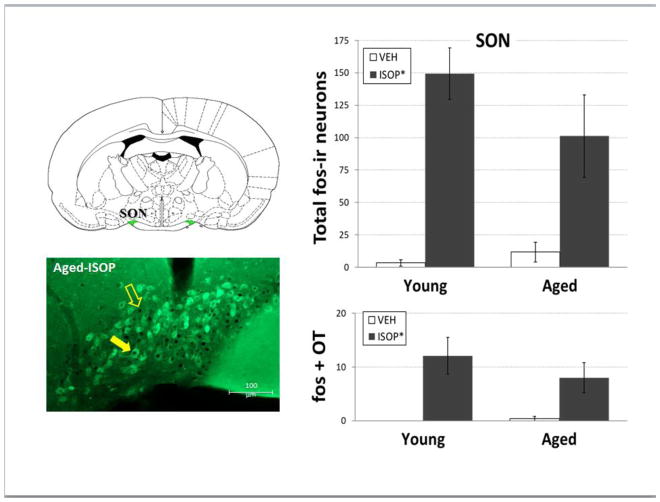
Figure 6. fos and OT immunolabeling in the SON.
Left panels: Line drawing (top; adapted from [41]) of the hypothalamic supraoptic nucleus (SON; green shading). Representative merged digital photomicrograph (bottom) of double immunolabeling for fos (brown/black nuclear staining) and oxytocin (OT; green cytoplasmic staining) in the SON of and female Brown Norway rat that was given s.c. injection of isoproterenol (ISOP). Right panels: mean total number of fos-immunoreactive (fos-ir) neurons (top) and mean number of fos and OT double labeled neurons (bottom) in young and aged female Brown Norway rats that were given s.c. injections of the 0.15M NaCl vehicle (VEH; open bars) or ISOP (black bars).
*= significant main effect of drug; open arrow = fos-ir nucleus; filled arrow = fos and OT double labeled neuron
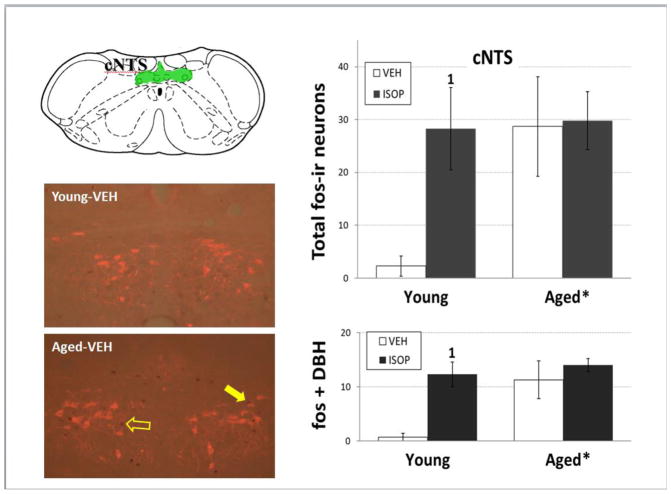
Figure 7. fos and DBH immunolabeling in the cNTS.
Left panels: Line drawing (top; adapted from [41]) of the caudal level of the nucleus of the solitary tract (cNTS; green shading). Representative merged digital photomicrographs of double immunolabeling for fos (brown/black nuclear staining) and dopamine-β-hydroxylase (DBH; red cytoplasmic labeling) in the cNTS of young (middle) and aged (bottom) female Brown Norway rats that were given s.c. injections of the 0.15M NaCl vehicle (VEH). Right panels: mean total number of fos-immunoreactive (fos-ir) neurons (top) and mean number of fos and DBH double labeled neurons (bottom) in young and aged female Brown Norway rats that were given s.c. injections of VEH (open bars) or isoproterenol (ISOP; black bars).
*= significant main effect of age; 1 = significantly greater than VEH-treated rats within age group (ps<0.05); open arrow = fos-ir nucleus; filled arrow = fos and DBH double labeled neuron
Forebrain
Two-way ANOVA on numbers of fos-ir neurons in SFO (Figure 2, 5; ISOP-aged: n = 4, ISOP-young: n = 5, VEH-aged: n= 3, VEH-young: n = 3) revealed significant main effects of both age [F(1,11) = 7.27, p<0.05 pη2 = 0.40] and treatment [F(1,11) = 19.28, p<0.01; pη2 = 0.64], as well as an interaction between age and treatment [F(1,11) = 6.76, p<0.05; pη2 = 0.38]. Pairwise comparisons of the interaction showed that the number of fos-ir neurons in young animals treated with ISOP was significantly greater than those in all other conditions (ps<0.01-0.001). Two-way ANOVA on numbers of fos-positive neurons in the OVLT (Figure 5; ISOP-aged: n = 4, ISOP-young: n = 5, VEH-aged: n= 3, VEH-young: n = 3) also revealed a significant main effect of treatment [F(1,11) = 9.50, p<0.05; pη2 = 0.46] and an interaction between age and treatment [F(1,11) = 5.01, p<0.05; pη2 = 0.31]. Pairwise comparisons of the interaction showed that here, too, the number of fos-ir neurons in young animals treated with ISOP was significantly greater than those in all other conditions (ps<0.01). In contrast, two-way ANOVA on numbers of fos-ir neurons in SON (Figure 6) revealed a significant main effect of treatment [F(1,11) = 26.77, p<0.001; pη2 = 0.71], with greater numbers after ISOP treatment, independent of age. There was no effect of age and no interaction between age and treatment. Similarly, two-way ANOVA on numbers of OT-ir neurons in the SON that also were fos-ir revealed a significant main effect of treatment [F(1,11) = 11.41, p<0.01; pη2 = 0.51], but no effect of age and no interaction.
Hindbrain
Two-way ANOVA on numbers of fos-ir neurons in AP (Figure 5; ISOP-aged: n = 4, ISOP-young: n = 4, VEH-aged: n= 3, VEH-young: n = 3) revealed a main effect of age [F(1,10) = 7.25, p<0.05; pη2 = 0.42], with greater numbers in aged females, overall. There was no effect of treatment and no interaction between age and treatment. In contrast, two-way ANOVA on numbers of fos-ir neurons in mNTS (Figure 5; ISOP-aged: n = 4, ISOP-young: n = 4, VEH-aged: n= 3, VEH-young: n = 3) revealed a main effect of treatment [F(1,10) = 5.49, p<0.05; pη2 = 0.35], with greater numbers after ISOP treatment, independent of age. There was no effect of age and no interaction between age and treatment on numbers of fos-ir neurons in mNTS. Finally, two-way ANOVA on numbers of fos-ir neurons in cNTS (Figure 7) revealed a main effect of age [F(1,10) = 5.62, p<0.05; pη2 = 0.36], with greater numbers in aged females, overall. There was no effect of treatment, and no interaction between age and treatment. Planned comparisons of the numbers of fos-ir neurons in cNTS showed significantly greater numbers after ISOP compared to those after VEH in young females (p<0.05), but no differences between VEH and ISOP in aged females. Two-way ANOVA on numbers of DBH-ir neurons that also were fos-ir revealed main effects of both age [F(1,10) = 10.90, p<0.01; pη2 = 0.52]and treatment [F(1,10) = 11.46, p<0.01; pη2 = 0.53], with no interaction between age and treatment. Here, too planned comparisons showed significantly greater numbers of DBH-ir neurons that also were fos-ir after ISOP compared to those after VEH in young females (p<0.05), but not in aged females.
4. Discussion
Several studies have shown that E2 reduces ISOP-stimulated water intake by young female rats [21–22, 24–25]; nonetheless, despite the elimination of ovarian hormones by OVX in both young and aged female rats in the present investigation, we found that water intake was reduced in aged OVX rats compared to their young OVX counterparts (see also [17–18]). However, the pattern of drinking was similar in both groups, indicating that group differences in water consumed were not attributable to longer latencies to begin drinking by aged females, or to shorter durations of drinking. Rather, the similar patterns of ISOP-induced water intake suggest that the central nervous system (CNS) may detect and/or process signals that underlie thirst differently in aged females. Here, it is important to note that although aged male rats also drink less water in response to ISOP, renin release is blunted [11] and, therefore, likely is insufficient to stimulate water intake. Consistent with this idea, when ANG II was given by intravenous infusion, water intake by aged male rats was comparable to that by young males [8, 18]. We cannot rule out the possibility that aging also interferes with the RAS in females, as plasma renin activity was not assessed in the previous study [18] or in the present. However, when aged female rats were given ANG II intravenously, they drank less water than did younger females [18]. These findings suggest that, unlike aged male rats which appear to retain the capacity to respond to ANG II with water intake, the capacity to respond to ANG II is blunted in aged female rats. Certainly, numerous central areas and mechanisms that mediate water intake stimulated by ANG II may be involved in these alterations, but the possibility of age-related changes in astrocytes is a novel and exciting prospect.
Until recently, it was thought that the primary role of astrocytes was their contribution to the blood-brain-barrier [35–37]. However, astrocytes are increasingly receiving attention for additional functions that may more directly influence neural activity, including buffering extracellular potassium concentration, and regulating and synthesizing specific neurotransmitters [37–38]. Clearly, then, changes in astrocytes in aged females could affect ISOP-induced water intake by altering central detection or processing of signals associated with stimulated water intake (and see [37]). Therefore, these experiments focused on astrocytes in central areas involved in body fluid homeostasis in aged female rats, with two main goals. First, we examined GFAP immunolabeling as a marker of astrocyte density in the SFO, OVLT, and AP, all of which are circumventricular organs known to have receptors for ANG II [29–33], and in the NTS, which receives baroreceptor input [30–31]. Second, we examined changes in neuronal activation in these areas, as well as in other CNS areas associated with body fluid balance, as a preliminary assessment of the functional consequences of changes in astrocyte density.
The SFO is the circumventricular organ that is most implicated in ANG II-induced drinking [19–20], and it showed greater GFAP intensity in aged female rats. These findings suggest that the blood-brain-barrier is enhanced in the SFO of aged females, which may decrease the binding of circulating ANG II to receptors in the SFO, thereby reducing ISOP-induced water intake. Interestingly, GFAP intensity also was elevated in the hindbrain circumventricular organ, the AP, as well as in the mNTS of aged females. The AP, which has receptors for both ANG II and E2 [33], has been implicated in blood pressure control, and baroreceptor afferents terminate in the NTS [30–31]. These observations raise the possibility of parallel age-related changes in astrocyte density in the SFO and AP/NTS, with functional differences (e.g., water intake vs. blood pressure control) attributable to the specific site in which differences in density occurred. Alternatively, age-related differences in blood pressure control (see e.g., [8, 11, 44–45]) may contribute to the blunted water intake observed in aged females. Additional studies will be necessary to distinguish between these possibilities.
At present, however, our findings of increased GFAP intensity in these areas suggest age-related differences in the detection of and/or central responses to signals associated with ISOP treatment. We used fos immunolabeling as a marker of neuronal activation to explore this idea and found that the number of fos-ir neurons in the SFO after ISOP treatment was less in aged females compared to their young counterparts. This difference may be attributable to the reduced ability of ANG II to bind to, and activate, receptors in the SFO as a result of the enhanced blood-brain-barrier suggested by greater GFAP labeling [35–37]. Alternatively, the increased astrocyte density in the SFO of aged females may directly influence neuronal activity via, e.g., decreased glutamate or increased GABA release, or changes in excitability [37–38], any of which could account for decreased fos-ir in the SFO of aged females. Whatever the specific mechanism by which astrocytes in the SFO affect neuronal activity, the decreased fos-ir in the SFO of aged females is consistent with their reduced water intake, as has been reported in young female rats [22, 24]. The relationship between fos-ir and GFAP intensity in the other areas is less easily interpreted.
In the OVLT, GFAP intensity was not affected by age, but was increased by ISOP treatment. Interestingly, then, fos labeling in the OVLT followed the same pattern as that observed in the SFO, with ISOP treatment increasing the number of fos-ir neurons in young female rats but not in aged females. It should be noted, however, that the increase in GFAP labeling in the OVLT after ISOP treatment was more pronounced in young females. Thus, astrocytes in the OVLT may play a role in neuronal activation in responses to ISOP treatment, perhaps via changes in neurotransmitter levels or neural excitability and consequently contribute to greater ISOP-induced water intake by young female rats. Given that the OVLT has been implicated in mediating responses to sodium loss (e.g., [28, 38]), an alternative explanation is that these alterations are involved with stimulated salt intake, rather than with water intake. Consistent with this idea, ANG II is typically elevated in conditions of sodium need, as well as after ISOP [22, 31], and the compensatory salt intake is blunted in both aged male [9–10, 12] and aged female [18] rats.
As was observed in the SFO, GFAP intensity was increased in the hindbrain AP and subadjacent mNTS of aged female rats. However, neuronal activity in these areas was unlike neuronal activity in the SFO. Specifically, fos-ir was elevated after VEH treatment in aged females compared to their young counterparts, and did not increase after ISOP treatment. Thus, while there was a similar lack of activation in AP, mNTS, SFO, and OVLT in response to ISOP treatment in aged females, the lack of activation in AP and mNTS may have been attributable to elevated baseline activity which precluded detecting further increases after ISOP treatment. Similar trends for fos labeling were observed in the cNTS, both for the total number of fos-ir neurons and for the number of fos and DBH double-labeled neurons. Given that these areas are important for autonomic regulation [30–31, 34], differences in baseline neuronal activity may be related to the control of blood pressure, as supported by observations that aging alters baroreflex function and blood pressure control in both aged male [8, 11] and female [44–45] rats. This idea suggests that increased astrocyte density in the AP and mNTS of aged females may promote increased neural activation via changes in neurotransmitter release, or alterations in membrane potential [37–38] and, in the cNTS, the effects include norepinephrine-containing neurons.
Despite age-related differences in neuronal activation by ISOP in these other CNS areas, fos-ir in the SON was comparable between young and aged females. These findings may indicate that, despite attenuated activation in other areas, the SON received sufficient input to activate both OT and vasopressin neurosecretory neurons. The contributions of these hormones to maintaining body fluid balance may be particularly important for aged females with impaired water drinking responses. Alternatively, what appears to be comparable activation in young and aged females may, in fact, indicate insufficient activation in the aged group, presuming the hypotension produced by ISOP treatment is exaggerated in aged females as it is in aged males [8, 11]. The elevated basal blood pressure reported in aged female rats [44–45] may mitigate that possibility; however, it will be necessary to directly assess ISOP-induced blood pressure changes, as well as the effects on circulating OT, vasopressin, and renin to fully understand how changes in the various compensatory responses to hypotension produced by ISOP treatment—or indeed, by other manipulations—interact with and influence each other in aged females.
Taken together, our findings suggest that changes in astrocyte density in CNS areas of aged female rats may be associated with the detection of and responses to signals related to ISOP treatment. At present, the clearest evidence for a relationship between astrocytes and neuronal activation related to water intake exists for the SFO, an area classically thought to be critical for ANG II-stimulated drinking [19–20]. In the SFO, astrocytes show an age-dependent increase, and neuronal activity stimulated by ISOP shows an aged-related decrease that is reflective of the reduced water intake by aged female rats, as also has been reported in young females [21–22, 24]. In contrast, GFAP intensity and fos-ir in other CNS areas involved in body fluid balance show different patterns of changes that may be related to blood pressure control and/or salt intake, as has been shown in young female rats [23]. As a first approach toward understanding how astrocytes may contribute to central pathways involved in body fluid homeostasis in aged female rats, the present findings provide intriguing possibilities for further studies to advance our understanding of age effects on fluid balance in females, an area currently underemphasized and largely overlooked.
Highlights.
Isoproterenol-induced water drinking was blunted in aged female Brown Norway rats
Astrocyte density in most CNS areas involved in body fluid balance was increased in aged females
Isoproterenol-induced neuronal activation in these areas was differentially affected in aged females
Thus, aging may alter the detection of or responses to signals related to isoproterenol
Acknowledgments
These studies were supported by Oklahoma Center for the Advancement of Science and Technology (OCAST) Health Research Program 12-196 (KSC), OK INBRE program 8P20GM103447 (ZS, BC), AG25465 (RLT) and HL098207, MH80241 and HL14388 (AKJ).
Portions of these data were presented at the annual meetings of the Society for Neuroscience (Washington, DC, 2017), and the Society for the Study of Ingestive Behaviors (Denver, CO, 2015; Montreal, Quebec, 2017).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brocklehurst JC. Textbook of Geriatric Medicine and Gerontology. Churchill Livingstone; New York: 1978. [Google Scholar]
- 2.Weinberg AD, Minaker KL Council on Scientific Affairs. Dehydration: evaluation and management in older adults. JAMA. 1995;274:1552–1556. doi: 10.1001/jama.274.19.1552. [DOI] [PubMed] [Google Scholar]
- 3.Phillips PA, Bretherton M, Johnston CI, Gray L. Reduced osmotic thirst in healthy elderly men. Am J Physiol Regul Integr Comp Physiol. 1991;261:R166–R171. doi: 10.1152/ajpregu.1991.261.1.R166. [DOI] [PubMed] [Google Scholar]
- 4.Phillips PA, Rolls BJ, Ledingham JGG, Forsling ML, Morton JJ, Crowe MJ, Wollner L. Reduced thirst after water deprivation in healthy elderly men. N Engl J Med. 1984;311:753–759. doi: 10.1056/NEJM198409203111202. [DOI] [PubMed] [Google Scholar]
- 5.Silver AJ. Aging and risks for dehydration. Cleve Clin J Med. 1990;57:341–344. doi: 10.3949/ccjm.57.4.341. [DOI] [PubMed] [Google Scholar]
- 6.Kenney WL, Chiu P. Influence of age on thirst and fluid intake. Med Sci Sports Exerc. 2001;33:1524–1532. doi: 10.1097/00005768-200109000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Thunhorst RL, Beltz TG, Johnson AK. Hypotension- and osmotically induced thirst in old Brown Norway rats. Am J Physiol Regul Integr Comp Physiol. 2009;297:R149–R157. doi: 10.1152/ajpregu.00118.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thunhorst RL, Beltz TG, Johnson AK. Drinking and arterial blood pressure responses to ANG II in young and old rats. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1135–R1141. doi: 10.1152/ajpregu.00360.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thunhorst RL, Beltz TG, Johnson AK. Effects of aging on mineralocorticoid-induced salt appetite in rats. Am J Physiol Regul Integr Comp Physiol. 2013;305:R1498–R1505. doi: 10.1152/ajpregu.00349.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thunhorst RL, Beltz T, Johnson AK. Age-related declines in thirst and salt appetite responses in male Fischer 344×Brown Norway rats. Physiol Behav. 2014;135:180–188. doi: 10.1016/j.physbeh.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thunhorst RL, Grobe CL, Beltz TG, Johnson AK. Effects of β-adrenergic receptor agonists on drinking and arterial blood pressure in young and old rats. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1001–R1008. doi: 10.1152/ajpregu.00737.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thunhorst RL, Johnson AK. Thirst and salt appetite responses in young and old Brown Norway rats. Am J Physiol Regul Integr Comp Physiol. 2003;284:R317–R327. doi: 10.1152/ajpregu.00368.2002. [DOI] [PubMed] [Google Scholar]
- 13.Whyte DG, Thunhorst RL, Johnson AK. Reduced thirst in old, thermally dehydrated rats. Physiol Behav. 2004;81:569–576. doi: 10.1016/j.physbeh.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 14.Jonklass J, Buggy J. Angiotensin-estrogen interaction in female brain reduces drinking and pressor responses. Am J Physiol. 1984;247:R167–R172. doi: 10.1152/ajpregu.1984.247.1.R167. [DOI] [PubMed] [Google Scholar]
- 15.Santollo J, Torregrossa A-M, Daniels D. Sex differences in the drinking response to angiotensin II (AngII): Effect of body weight. Horm Behav. 2017;93:128–136. doi: 10.1016/j.yhbeh.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santollo J, Daniels D. Control of fluid intake by estrogens in the female rat: role of the hypothalamus. Front Systems Neurosci. 2015;9:25. doi: 10.3389/fnsys.2015.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rowland NE, Morien A, Garcea M, Fregly MJ. Aging and fluid homeostasis in rats. Am J Physiol. 1997;273:R1441–R1450. doi: 10.1152/ajpregu.1997.273.4.R1441. [DOI] [PubMed] [Google Scholar]
- 18.Begg DP, Sinclair AJ, Weisinger RS. Reductions in water and sodium intake by aged male and female rats. Nutr Res. 2012;32:865–872. doi: 10.1016/j.nutres.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 19.Fitts DA. Angiotensin II receptors in SFO but not in OVLT mediate isoproterenol-induced thirst. Am J Physiol. 1994;267:R7–15. doi: 10.1152/ajpregu.1994.267.1.R7. [DOI] [PubMed] [Google Scholar]
- 20.Krause EG, Melhorn SJ, Davis JF, Scott KA, Ma LY, de Kloet AD, Benoit SC, Woods SC, Sakai RR. Angiotensin type 1 receptors in the subfornical organ mediate the drinking and hypothalamic-pituitary-adrenal response to systemic isoproterenol. Endocrinology. 2008;149:6416–6424. doi: 10.1210/en.2008-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones AB, Curtis KS. Differential effects of estradiol on drinking by ovariectomized rats in response to hypertonic NaCl or isoproterenol: Implications for hyper- vs. hypo-osmotic stimuli for water intake. Physiol Behav. 2009;98:421–426. doi: 10.1016/j.physbeh.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krause EG, Curtis KS, Davis LM, Stowe JR, Contreras RJ. Estrogen influences stimulated water intake by ovariectomized female rats. Physiol Behav. 2003;79:267–274. doi: 10.1016/s0031-9384(03)00095-7. [DOI] [PubMed] [Google Scholar]
- 23.Krause EG, Curtis KS, Markle JP, Contreras RJ. Oestrogen affects the cardiovascular and central responses to isoproterenol of female rats. J Physiol. 2007;582:435–447. doi: 10.1113/jphysiol.2007.131151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krause EG, Curtis KS, Stincic TL, Markle JP, Contreras RJ. Oestrogen and weight loss decrease isoproterenol-induced Fos immunoreactivity and angiotensin type 1 mRNA in the subfornical organ of female rats. J Physiol. 2006;573:251–262. doi: 10.1113/jphysiol.2006.106740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Findlay AL, Fitzsimons JT, Kucharczyk J. Dependence of spontaneous and angiotensin-induced drinking in the rat upon the oestrous cycle and ovarian hormones. J Endocrinol. 1979;82:215–225. doi: 10.1677/joe.0.0820215. [DOI] [PubMed] [Google Scholar]
- 26.Kisley LR, Sakai RR, Fluharty SJ. Estrogen decreases hypothalamic angiotensin II AT1 receptor binding and mRNA in the female rat. Brain Res. 1999;844:34–42. doi: 10.1016/s0006-8993(99)01815-6. [DOI] [PubMed] [Google Scholar]
- 27.Rosas-Arellano MP, Sloano-Flores LP, Ciriello J. Co-localization of estrogen and angiotensin receptors within subfornical organ neurons. Brain Res. 1999;837:254–262. doi: 10.1016/s0006-8993(99)01672-8. [DOI] [PubMed] [Google Scholar]
- 28.Fitzsimons JT. Angiotensin, thirst and sodium appetite. Phys Rev. 1998;78:583–686. doi: 10.1152/physrev.1998.78.3.583. [DOI] [PubMed] [Google Scholar]
- 29.Gizowski C, Bourque CW. The neural basis of homeostatic and anticipatory thirst. Nat Rev Nephrology online advance publication. 2017:1–15. doi: 10.1038/nrneph.2017.149. [DOI] [PubMed] [Google Scholar]
- 30.Dampney RAL. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev. 1994;74:323–364. doi: 10.1152/physrev.1994.74.2.323. [DOI] [PubMed] [Google Scholar]
- 31.Johnson AK, Thunhorst RL. The neuroendocrinology of thirst and salt appetite: visceral sensory signals and mechanisms of central integration. Front Neuroendocrinol. 1997;18:292–353. doi: 10.1006/frne.1997.0153. [DOI] [PubMed] [Google Scholar]
- 32.Wright JW, Harding JW. Brain renin-angiotensin—A new look at an old system. Prog Neurobiol. 2011;95:49–67. doi: 10.1016/j.pneurobio.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Pamidimukkala J, Hay M. 17β-Estradiol inhibits angiotensin II activation of area postrema neurons. Am J Physiol Heart Circ Physiol. 2003;285:H1515–H1520. doi: 10.1152/ajpheart.00174.2003. [DOI] [PubMed] [Google Scholar]
- 34.Rinaman L. Hindbrain noradrenergic A2 neurons: diverse role in autonomic, endocrine, cognitive, and behavioral functions. Am J Physiol Regul Integr Comp Physiol. 2011;300:R222–R235. doi: 10.1152/ajpregu.00556.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abbot NJ, Ronnback L, Hansson E. Astrocyte endothelial interactions at the blood brain barrier. Nature Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 36.Alvarez JI, Katayama T, Prat A. Glial influence on the blood brain barrier. Glia. 2013;61:1939–1958. doi: 10.1002/glia.22575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prager-Khoutorsky M, Bourque CW. Anatomical organization of the rat organum vasculosum laminae terminalis. Am J Physiol Regul Integr Comp Physiol. 2015;309:R324–R337. doi: 10.1152/ajpregu.00134.2015. [DOI] [PubMed] [Google Scholar]
- 38.Kimelberg HK, Nedergaard Functions of astrocytes and their potential as therapeutic targets. Neurotherapeutics. 2010;7:338–353. doi: 10.1016/j.nurt.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spence RD, Hamby ME, Umeda E, Itoh H, Du S, Wisdom AJ, Cao Y, Bondar G, Lam J, Ao Y, Sandoval F, Suriany S, Sofroniew MV, Voskuh RR. Neuroprotection mediated through estrogen receptor-α in astrocytes. PNAS. 2011;108:8867–8872. doi: 10.1073/pnas.1103833108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watson RE, Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 1986;7:155–159. doi: 10.1016/0196-9781(86)90076-8. [DOI] [PubMed] [Google Scholar]
- 41.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. Academic Press; San Diego: 1998. [DOI] [PubMed] [Google Scholar]
- 42.Curtis KS. Estradiol and osmolality: behavioral responses and central pathways. Physiol Behav. 2015;152:422–430. doi: 10.1016/j.physbeh.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jones AB, Bass EE, Fan L, Curtis KS. Estradiol selectively reduces central neural activation induced by hypertonic NaCl infusion in ovariectomized rats. Physiol Behav. 2012;107:192–200. doi: 10.1016/j.physbeh.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clark JT, Chakraborty-Chatterjee M, Hamblin M, Wyss JM, Fentie IH. Estrogen depletion differentially affects blood pressure depending on age in Long-Evans rats. Endocrine. 2004;25:1731–86. doi: 10.1385/ENDO:25:2:173. [DOI] [PubMed] [Google Scholar]
- 45.Tezini GC, Becari C, Zanotto CZ, Salgado MC, de Passaglia RC, Souza HC. Ageing is the main determinant of haemodynamics and autonomic cardiac changes observed in post-menopausal female rats. Auton Neurosci. 2013;174:36–41. doi: 10.1016/j.autneu.2012.12.003. [DOI] [PubMed] [Google Scholar]