Abstract
Background
Frailty is an important predictor of outcomes after cardiac surgery, but utility is limited by difficult assessment and quantification. We hypothesize that sarcopenia defined as psoas muscle cross-sectional area is a useful predictor of surgical aortic valve replacement (SAVR) outcomes in moderate to high-risk patients.
Methods
Moderate to high-risk (predicted risk of mortality [PROM] >3%) patients who underwent SAVR with or without coronary bypass were extracted from an institutional database (2009–2016). Psoas index was calculated as the cross-sectional area of the psoas muscle at the L4 vertebral level normalized to body surface area. Patients were stratified by sarcopenia status, defined as <25th gender-specific percentile. Multivariable regression analysis identified risk-adjusted associations with psoas index using STS predicted risk scores.
Results
Of the 240 patients included, the median PROM was 6%, median age 80 years, and 40% were female. Patients with (33.3%) and without (66.7%) sarcopenia had equivalent baseline risk (median PROM 5.7% vs 6.0%, p=0.29). Patients with sarcopenia had higher 1-year mortality (31.9% vs 16.9% p=0.03). Psoas index significantly predicted risk-adjusted 1-year mortality (OR 0.84, p=0.02), long-term mortality (HR 0.92, p=0.04), as well as risk-adjusted major morbidity, prolonged ventilation, length of stay, discharge to a facility and hospital cost. Finally, psoas index measurements were highly reproducible (Pearson correlation coefficient 0.944).
Conclusions
Psoas index is an easily obtained and reproducible measure of frailty that predicts risk-adjusted resource utilization, morbidity, and long-term mortality. Psoas index may improve procedural selection and risk-adjustment in high-risk patients with aortic valve disease.
Classifications: Aortic valve replacement, frailty, sarcopenia, resource utilization
The trend towards more complex and higher risk patients undergoing cardiac surgery has been underway for decades.[1] Patients undergoing surgical aortic valve replacement (SAVR) today have greater comorbid disease than in prior years.[2] Additionally, the population of the United States is aging, leading to an increase in the number of older patients undergoing cardiac surgery.[3] The inclusion of standard risk factors in current prediction models, such as the Society of Thoracic Surgeons and EUROScore, has resulted in highly accurate in risk prediction algorithms that are now the gold standard.[4] However, use of the so called “eyeball test” to determine patient appropriateness for surgery persists, with its importance highlighted by its inclusion as a selection criterion in the Placement of Aortic Transcatheter Valves (PARTNER) trials.[5]
Preoperative surgeon judgment of a patient attempts to assess frailty, the missing ingredient in current risk models. By definition, frailty is the diminished reserve across multiple organ systems resulting in a patient incapable of adapting to stressors.[5–7] Whether frailty is a phenotype or a series of accumulated deficits is a topic of debate, although the former tends to be more useful for cardiovascular purposes.[8] Comprehensive frailty assessments evaluate all aspects of this phenotype (weight loss, exhaustion, low energy, weakness, slowness) but are cumbersome and time consuming.[5, 9–11] While not the sole etiology of frailty, sarcopenia is a physical manifestation of frailty with significant overlap across the phenotypic model.[12] This can be observed, measured and objectively inserted into risk prediction models.
Psoas muscle size is a relatively new technique for quantifying sarcopenia and is recognized as a useful measure of frailty across multiple surgical specialties.[13–17] It is simple to obtain, highly reproducible, and validated. Sarcopenia has been shown to correlate with body fat percentage, lean muscle mass, grip strength, short physical performance battery scores and VO2 max.[18–20] Psoas muscle size is predictive of mortality, major complications, and resource utilization in areas as diverse as emergency general surgery, abdominal aortic aneurysms, colorectal surgery and pancreatic surgery.[13–17]
The purpose of this study was to evaluate the utility of psoas muscle cross-sectional area as a quantitative measure of frailty in moderate to high-risk patients undergoing SAVR. Psoas muscle area is an easily quantified measure of sarcopenia that is typically available in higher risk SAVR patients due to procedural planning for potential transcatheter aortic valve replacement (TAVR). We hypothesized that patients with decreased psoas muscle cross-sectional area would have increased risk-adjusted morbidity, mortality and resource utilization following surgical aortic valve replacement.
Patients and Methods
Patient Data
This study was approved by the University of Virginia Institutional Review Board, #19762. All patients who underwent aortic valve replacement between January of 2009 and December of 2016 were extracted from an institutional Society of Thoracic Surgeons (STS) database. Inclusion criteria included first time aortic valve replacement for severe aortic stenosis, a STS predicted risk of mortality (PROM) >3% and a preoperative abdominal computed tomography (CT) scan available for review. Patients with endocarditis were excluded. Medical records were reviewed for 1,384 patients and of these, 240 met criteria for inclusion as demonstrated in the consort diagram (Supplemental Figure 1). The STS database contained clinical information that was paired with cost and long-term mortality information. The long-term mortality information was obtained from three separate sources including clinical records, the Virginia Department of Health and the Social Security Death Master File.
The cost data was abstracted from the Clinical Data Repository and is derived from finance department records. Each patient has charges identified by Current Procedural Terminology code and converted to costs based on monthly updates that include direct and indirect component costs. The sum cost was then adjusted for inflation to 2016 dollars using the market basket for the inpatient prospective payment system at the Centers for Medicare and Medicaid Services.
Preoperative CT scans were used to calculate the cross-sectional area of the psoas muscle at the level of the L4 vertebra using multiplanar reconstruction to account for rotation and kyphosis (Supplemental Figure 2). Three measurements were taken of the left and right psoas with the average used for analysis. This methodology has been described previously, and validated as a measure to estimate total body sarcopenia and cardiorespiratory fitness.[19–21] The mean psoas cross-sectional area was divided by the body surface area to obtain the psoas index. Sarcopenia was defined as a psoas index below the 25th gender specific percentile, based on definitions used in the original description of the frailty phenotype and prior analyses.[8, 14] Outcome measures evaluated included operative, one-year, and long-term mortality, in hospital complications, intensive care unit (ICU) or postoperative length of stay, and hospital cost.
Statistical Analyses
Categorical variables are presented as count (percent) while continuous variables are presented as mean ± standard deviation (SD) or if skewed then median [interquartile range, Q1–Q3]. Normality was evaluated by Shapiro-Wilk Statistics. For univariate analysis, comparisons were made by Chi Square test, Independent T-test or Mann Whitney U test as appropriate. Multivariable logistic regression was used to evaluate psoas index as a predictive measure for categorical outcomes. Generalized linear models were used to evaluate hospital cost using a gamma distribution, length of stay using a negative binomial distribution, and albumin using a normal distribution.[22] Models were fit in linear form except for hospital cost which performed best as a logarithmic link making interpretation more difficult. Risk-adjustment was performed using STS risk scores relevant to the outcome of interest, and if no specific risk model was available for a given outcome adjustment was performed using predicted risk of morbidity or mortality. Long-term mortality was compared by Kaplan-Meier survival analysis for comparison of sarcopenia status and by Cox proportional hazard analysis for prediction of risk adjusted psoas index on long-term survival. Inter-observer agreement for psoas size measurements read by two independent physician readers was assessed by Pearson correlation. All statistical analyses were performed using SAS Version 9.4 (SAS Institute, Cary, NC) with p<0.05 defining statistical significance.
Results
Sarcopenia and Frailty
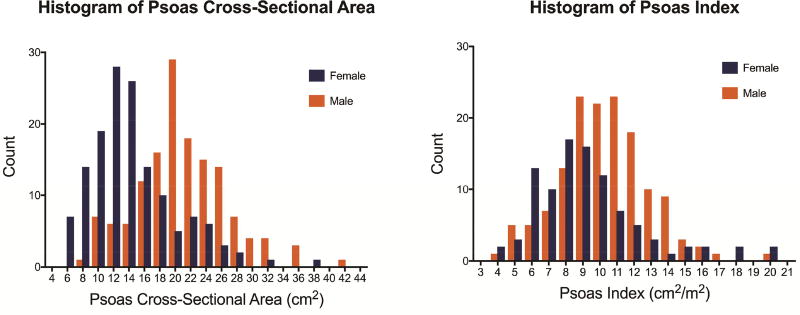
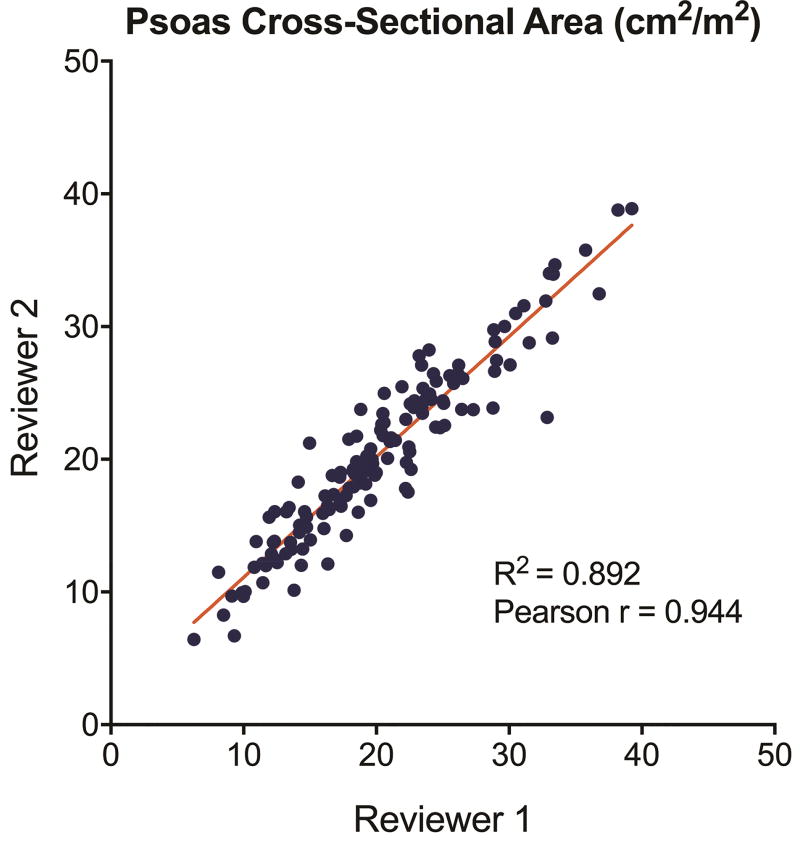
Patients included in the study were largely similar compared to those excluded for missing CT scans including rates of comorbidities and STS predicted risk of mortality, although there are some minor logical differences including being statistically older, more frequently elective, and having a higher rate of prior cardiac surgery (Supplemental Table 1). Of patients included in the study, the median psoas cross-sectional area was 18.9 cm2 (14.3–23.3) and was significantly lower in females (15.0 vs 20.5 cm2, p<0.0001). After adjusting for body surface area, the median psoas index was 9.6 cm2/m2 with both distributions by gender shown in Figure 1. The 25th percentile cutoff for sarcopenia was a psoas index of 6.96 cm2/m2 for women and 9.09 cm2/m2 for men. A subset of 145 (60%) patients had psoas measurements by both reviewers. This demonstrated high reproducibility with a Pearson correlation coefficient of 0.944 and by linear regression an R2 of 0.892 (Figure 2).
Figure 1.
Histogram of (A) psoas cross sectional area and (B) psoas index by sex.
Figure 2.
Plot of psoas cross-sectional area measurements for both reviewers with Pearson correlation coefficient and linear regression results.
There was no statistically significant correlation between psoas index and preoperative albumin level (parameter estimate = 0.012 [−0.006 – 0.031], p=0.195), nor with prolonged 5 meter walk time (OR 1.22 (0.94–1.59), p=0.138).
Baseline and Operative Characteristics
Baseline and operative characteristics for sarcopenic (33.3% [60]) and non-sarcopenic (66.7% [180]) patients are shown in Table 1. The only statistically significant baseline difference between patients with and without sarcopenia was the rate of smoking (6.7% vs 26.7%, p=0.001). There were no differences in rates of comorbid disease, valve disease, or prior cardiac surgery. There was no significant difference between groups in STS PROM (5.7% vs 6.0%, p=0.29). Similarly, there were no differences in rates of CABG, cross-clamp times or cardiopulmonary bypass times (Table 1).
Table 1.
Baseline and operative characteristics by sarcopenic status
| Baseline Characteristics | Sarcopenic (n = 60) |
Non-Sarcopenic (n = 180) |
p value |
|---|---|---|---|
| Psoas Index (cm2/m2) | 6.7 [5.7–7.9] | 10.6 [9.4–12.2] | <0.0001 |
| Age (years) | 81 [77–85] | 80 [75–85] | 0.165 |
| Body surface area (m2) | 1.96 ± 0.29 | 1.92 ±0.27 | 0.350 |
| Female | 24 (40.0%) | 73 (40.6%) | 0.940 |
| Smoker | 4 (6.7%) | 48 (26.7%) | 0.001 |
| Hypertension | 51 (85.0%) | 162 (90.0%) | 0.289 |
| Diabetes | 26 (43.3%) | 82 (45.6%) | 0.764 |
| Dialysis dependent renal failure | 2 (3.3%) | 1 (0.6%) | 0.094 |
| Prior stroke | 9 (15.0%) | 18 (10.0%) | 0.289 |
| Chronic lung disease, moderate/severe | 10 (16.7%) | 47 (26.3%) | 0.131 |
| Prior myocardial infarction | 20 (33.3%) | 65 (36.1%) | 0.697 |
| Heart failure within 2 weeks | 45 (75.0%) | 154 (85.6%) | 0.060 |
| Ejection fraction (%) | 57 [50–63] | 57 [43–63] | 0.160 |
| Aortic insufficiency (moderate/severe) | 4 (6.7%) | 19 (10.6%) | 0.376 |
| Mitral insufficiency (moderate/severe) | 15 (26.3%) | 40 (23.3%) | 0.639 |
| Predicted risk of mortality (%) | 5.7% [3.9–8.4%] | 6.0% [4.2–7.9%] | 0.285 |
| Operative Characteristics | |||
|
| |||
| Prior cardiac surgery | 15 (46.9%) | 45 (45.9%) | 0.925 |
| Urgent or Emergent status | 14 (23.3%) | 48 (26.7%) | 0.610 |
| Coronary artery bypass grafting | 17 (28.3%) | 67 (37.2%) | 0.211 |
| Cross clamp time (min) | 69 [61.5–85] | 73 [60–90] | 0.375 |
| Cardiopulmonary bypass time (min) | 105.5 [89.5–123] | 103.5 [87–124] | 0.363 |
Unadjusted Outcomes
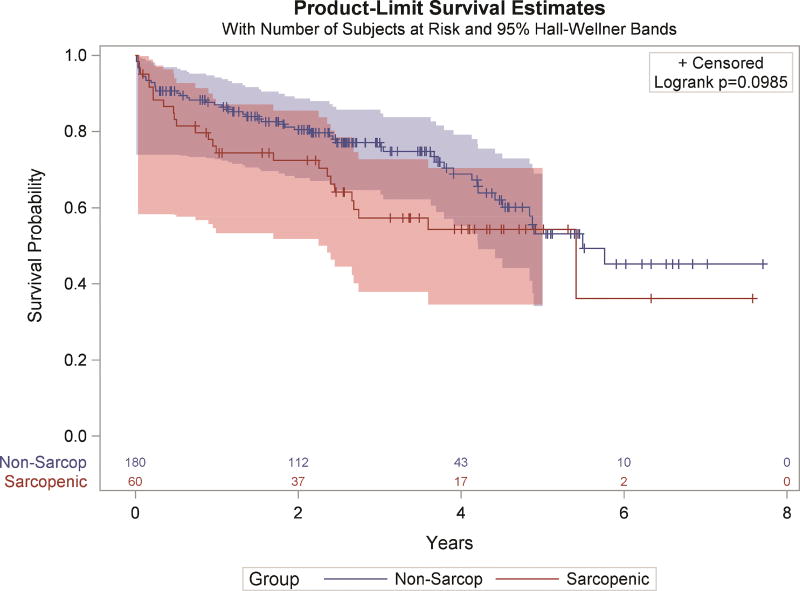
While there was no difference in operative mortality rate between groups, sarcopenic patients had a higher one-year mortality rate (31.9% vs 16.9%, p=0.03). There was a trend towards improved long-term survival in non-sarcopenic patients by Kaplan-Meier survival analysis (Figure 3). This trend becomes significant when utilizing the continuous psoas index instead of sarcopenia status (HR=0.916 [0.843–0.996], p=0.04). The median survival for sarcopenic patients was 5.41 years while for nonsarcopenic patients was 5.49 years (p=0.099). There were no significant differences in rates of major or minor complications (Table 2). Finally, there were no significant differences in measures of resource utilization by sarcopenia status including readmission and ICU length of stay. There were trends towards sarcopenic patients having a higher rate of discharge to a facility (63.2% vs 49.1%, p=0.07) and longer median postoperative length of stay (7.5 vs 7 days, p=0.06).
Figure 3.
Kaplan Meier Survival analysis by Sarcopenia status. Number at risk is displayed below the figure and 95% confidence intervals are displayed by the thin lines in the corresponding color.
Table 2.
Outcomes by sarcopenic status
| Sarcopenic (n = 60) |
Non-Sarcopenic (n = 180) |
p value | |
|---|---|---|---|
| STS operative mortality | 3 (5.0%) | 10 (5.6%) | 0.869 |
| One-year mortality (n=183) | 15 (31.9%) | 23 (16.9%) | 0.029 |
| STS major morbidity | 14 (23.3%) | 36 (20.0%) | 0.582 |
| Permanent stroke | 3 (5.0%) | 5 (2.8%) | 0.406 |
| Cardiac arrest | 2 (3.3%) | 3 (1.7%) | 0.434 |
| Prolonged ventilation | 11 (18.3%) | 19 (10.6%) | 0.115 |
| Renal failure requiring dialysis | 4 (6.7%) | 6 (3.3%) | 0.263 |
| Deep sternal wound infection | 0 (0%) | 1 (0.6%) | 0.563 |
| Atrial fibrillation | 20 (33.3%) | 42 (23.3% | 0.125 |
| Pneumonia | 5 (8.3%) | 7 (3.9%) | 0.171 |
| Transfusion, packed red blood cells | 34 (56.7%) | 101 (56.1%) | 0.940 |
| Reoperation for any reason | 7 (11.7%) | 15 (8.3%) | 0.438 |
| Readmission | 8 (13.6%) | 28 (16.2%) | 0.631 |
| Discharge to facility | 36 (63.2%) | 85 (49.1%) | 0.066 |
| Hospital cost (median) | $51905 | $51787 | 0.328 |
| Length of stay (days; median, IQR) | 7.5 (6–12) | 7 (5–9) | 0.056 |
| ICU stay (hrs; median, IQR) | 46.7 [30.0–139.5] | 87 [41.4–125.7] | 0.208 |
ICU = intensive care unit; STS = Society of Thoracic Surgeons
Risk Adjusted Outcomes
Risk-adjusted outcomes for logistic regression models using psoas index can be found in Table 3. As a continuous variable, psoas index is predictive of risk-adjusted one-year mortality (OR 0.84, p=0.02). Similarly, psoas index remains a significant predictor of long-term survival even after risk-adjustment (HR=0.917 (0.845–0.996), p=0.041). Psoas index is a significant independent predictor of STS major morbidity, as well as prolonged ventilation.
Table 3.
Risk-adjusted outcomes for psoas index
| Odds Ratio | 95% Confidence Limit | p-value | c-statistic | ||
|
| |||||
| STS operative mortality | 0.94 | 0.77 | 1.15 | 0.568 | 0.690 |
| One-year mortality (n=183) | 0.84 | 0.73 | 0.97 | 0.017 | 0.647 |
| STS major morbidity | 0.86 | 0.76 | 0.98 | 0.020 | 0.705 |
| Prolonged ventilation | 0.81 | 0.69 | 0.95 | 0.012 | 0.730 |
| Dialysis dependent renal failure | 0.84 | 0.65 | 1.10 | 0.200 | 0.770 |
| Permanent stroke | 0.89 | 0.67 | 1.17 | 0.401 | 0.728 |
| Atrial fibrillation | 0.90 | 0.81 | 1.00 | 0.058 | 0.574 |
| Readmission | 0.95 | 0.84 | 1.08 | 0.444 | 0.610 |
| Discharge to a facility | 0.88 | 0.80 | 0.96 | 0.005 | 0.612 |
|
| |||||
| Estimate | 95% Confidence Limit | p-value | χ2 | ||
|
| |||||
| Hospital cost ($; log form) | −0.03 | −0.05 | −0.01 | 0.001 | 69 |
| Postoperative length of stay (d) | −0.46 | −0.65 | −0.27 | <0.0001 | 407 |
| ICU length of stay (hr) | −2.41 | −5.40 | 0.57 | 0.113 | 477 |
ICU = intensive care unit; STS = Society of Thoracic Surgeons
Psoas index was also independently predictive of most measures of resource utilization including discharge to a facility (OR 0.88, p=0.005), postoperative length of stay (–0.46, p<0.0001) and hospital cost (−.03, p=0.001). There was also a trend towards psoas index significantly predicting risk-adjusted ICU length of stay, although it was not associated with rates of readmission.
Comment
Using data already obtained in most moderate to high-risk aortic valve cases, we demonstrate that psoas muscle size is a useful measure of sarcopenia and predicts risk-adjusted morbidity, mortality and resource utilization. Sarcopenic patients had higher mortality at 1-year with a trend towards higher long-term mortality. When utilized as a continuous variable, psoas index is predictive of risk-adjusted major morbidity, 1-year mortality and long-term mortality. In addition, psoas index is independently associated with the resource utilization metrics of discharge to a facility, postoperative length of stay and hospital cost.
It has been well established that frailty increases the risk of mortality after cardiac surgery. Using a variety of frailty measures, it has been found to increase the risk of short and mid-term mortality.[23–25] This finding holds true for sarcopenia assessed by psoas muscle size in the TAVR population at 6 months, and the TAVR or SAVR population at 2 years.[21, 26] This analysis did not demonstrate an association between psoas index and operative morality, but smaller psoas index was associated with increased odds of mortality at 1 year even after risk-adjustment. Moreover, this study is unique in finding that the mortality association persists long-term with psoas index predicting risk-adjusted long-term mortality. As quality metrics move past the 30-day threshold, psoas index could be a useful tool for accurate long-term risk prediction.
Frailty also appears to be an important driver of complications after cardiac surgery, although only a few studies have evaluated this question. One analysis demonstrated that slow gait speed in elderly cardiac surgery patients was an independent predictor of STS major morbidity or mortality.[27] The same group found that the addition of frailty and disability score improved the predictive abilities of multiple cardiac risk models including the STS risk model for morbidity and mortality.[28] Finally, a recent systematic review found a strong relationship between frailty and major adverse cardiac and cerebrovascular events following cardiac surgery.[29] In this analysis we also find that frailty as measured by psoas index is independently associated with increased risk of major morbidity as well as its component complication of prolonged ventilation. There was also a trend towards a risk-adjusted association with atrial fibrillation.
Another important aspect of complications after cardiac surgery is that they are an important driver of increased resource utilization.[30, 31] The most consistent finding is that frailty is associated with an increased length of stay.[23] This includes prior analyses using psoas muscle size to define sarcopenia after both cardiac surgery and TAVR.[18, 32] In this analysis, a 1 point increase in psoas index was independently associated with almost a half day less in the hospital. Complications and length of stay can increase hospital costs, and frailty has previously been associated with an additional $20,000.[33] This was corroborated in our analysis with a strong association between psoas index and hospital cost in a risk-adjusted model. Finally, with global billing efforts increasing discharges to a facility are going to be increasingly under scrutiny.[34] We found that increasing psoas index was strongly associated with a decreased risk of discharge to a facility (OR 0.88).
There are several limitations to this study including its single center, retrospective nature that inherently introduces some element of selection bias. The generalizability of the results is also limited by the prevalence of frailty in certain cardiac surgery populations and will be more useful in more frail cohorts. Psoas index, while a validated measure of sarcopenia that correlates with many different measures of frailty, is not a comprehensive frailty measure. This limited frailty assessment is going to be an inherent limitation of any simple, fast measurement. This is simply a tradeoff we believe is worth the downside if it enables inclusion in future risk models. Finally, risk adjustment was performed using the STS risk models. STS PROM was not designed to predict long-term mortality, although it has previously been validated to do so.[35]
Frailty and sarcopenia are known to be important predictors of morbidity, mortality and resource utilization in cardiac surgery. Here we demonstrate psoas index is a simple, reproducible measure of sarcopenia that is associated with short and long-term outcomes after SAVR. This makes it an ideal measure to incorporate into future risk models. As psoas size has demonstrated similar promise in the TAVR population, it would be useful in risk assessment across the spectrum of approaches currently available. In an era of emphasis on patient centered outcomes, autonomy and choice, the inclusion of frailty measures will be important to help patients and providers select the appropriate procedure. Additionally, with public reporting and value based payments the inclusion of a simple frailty metric such as psoas index could help improve longer-term risk prediction models.
Supplementary Material
Abbreviations
- CT
computed tomography
- ICU
intensive care unit
- PARTNER
Placement of AoRTic TraNscathetER Valve Trial
- PROM
predicted risk of mortality
- SAVR
surgical aortic valve replacement
- SD
standard deviation
- STS
Society of Thoracic Surgeons
- TAVR
transcatheter aortic valve replacement
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ferguson TB, Jr, Hammill BG, Peterson ED, DeLong ER, Grover FL Committee STSND. A decade of change--risk profiles and outcomes for isolated coronary artery bypass grafting procedures, 1990–1999: A report from the sts national database committee and the duke clinical research institute. Society of thoracic surgeons. Ann Thorac Surg. 2002;73:480–489. doi: 10.1016/s0003-4975(01)03339-2. discussion 489–490. [DOI] [PubMed] [Google Scholar]
- 2.Hawkins RB, Downs EA, Johnston LE, et al. Impact of transcatheter technology on surgical aortic valve replacement volume, outcomes, and cost. Ann Thorac Surg. 2017;103:1815–1823. doi: 10.1016/j.athoracsur.2017.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. [Accessed 2016 May 23];2016 Aging statistics. 2016 Available at http://www.aoa.acl.gov/aging_statistics/index.aspx.
- 4.O'Brien SM, Shahian DM, Filardo G, et al. The society of thoracic surgeons 2008 cardiac surgery risk models: Part 2--isolated valve surgery. Ann Thorac Surg. 2009;88:S23–42. doi: 10.1016/j.athoracsur.2009.05.056. [DOI] [PubMed] [Google Scholar]
- 5.Mack M. Frailty and aortic valve disease. J Thorac Cardiovasc Surg. 2013;145:S7–10. doi: 10.1016/j.jtcvs.2012.11.063. [DOI] [PubMed] [Google Scholar]
- 6.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Afilalo J, Karunananthan S, Eisenberg MJ, Alexander KP, Bergman H. Role of frailty in patients with cardiovascular disease. Am J Cardiol. 2009;103:1616–1621. doi: 10.1016/j.amjcard.2009.01.375. [DOI] [PubMed] [Google Scholar]
- 8.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 9.Shimura T, Yamamoto M, Kano S, et al. Impact of the clinical frailty scale on outcomes after transcatheter aortic valve replacement. Circulation. 2017;135:2013–2024. doi: 10.1161/CIRCULATIONAHA.116.025630. [DOI] [PubMed] [Google Scholar]
- 10.Stortecky S, Schoenenberger AW, Moser A, et al. Evaluation of multidimensional geriatric assessment as a predictor of mortality and cardiovascular events after transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2012;5:489–496. doi: 10.1016/j.jcin.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 11.Sundermann S, Dademasch A, Praetorius J, et al. Comprehensive assessment of frailty for elderly high-risk patients undergoing cardiac surgery. Eur J Cardiothorac Surg. 2011;39:33–37. doi: 10.1016/j.ejcts.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 12.Afilalo J. Conceptual models of frailty: The sarcopenia phenotype. Can J Cardiol. 2016;32:1051–1055. doi: 10.1016/j.cjca.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 13.Jones KI, Doleman B, Scott S, Lund JN, Williams JP. Simple psoas cross-sectional area measurement is a quick and easy method to assess sarcopenia and predicts major surgical complications. Colorectal Dis. 2015;17:O20–26. doi: 10.1111/codi.12805. [DOI] [PubMed] [Google Scholar]
- 14.Amini N, Spolverato G, Gupta R, et al. Impact total psoas volume on short- and long-term outcomes in patients undergoing curative resection for pancreatic adenocarcinoma: A new tool to assess sarcopenia. J Gastrointest Surg. 2015;19:1593–1602. doi: 10.1007/s11605-015-2835-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dirks RC, Edwards BL, Tong E, et al. Sarcopenia in emergency abdominal surgery. J Surg Res. 2017;207:13–21. doi: 10.1016/j.jss.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 16.Drudi LM, Phung K, Ades M, et al. Psoas muscle area predicts all-cause mortality after endovascular and open aortic aneurysm repair. Eur J Vasc Endovasc Surg. 2016;52:764–769. doi: 10.1016/j.ejvs.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Ikeno Y, Koide Y, Abe N, et al. Impact of sarcopenia on the outcomes of elective total arch replacement in the elderlydagger. Eur J Cardiothorac Surg. 2017;51:1135–1141. doi: 10.1093/ejcts/ezx050. [DOI] [PubMed] [Google Scholar]
- 18.Zuckerman J, Ades M, Mullie L, et al. Psoas muscle area and length of stay in older adults undergoing cardiac operations. Ann Thorac Surg. 2017;103:1498–1504. doi: 10.1016/j.athoracsur.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Shen W, Punyanitya M, Wang Z, et al. Total body skeletal muscle and adipose tissue volumes: Estimation from a single abdominal cross-sectional image. J Appl Physiol (1985) 2004;97:2333–2338. doi: 10.1152/japplphysiol.00744.2004. [DOI] [PubMed] [Google Scholar]
- 20.Fitzpatrick J, Chambers ES, Parkinson JR, Frost G, Bell JD, Thomas EL. Psoas major cross-sectional area: A potential marker of cardiorespiratory fitness. Int J Clin Exp Physiol. 2017;4:15–20. [Google Scholar]
- 21.Saji M, Lim DS, Ragosta M, et al. Usefulness of psoas muscle area to predict mortality in patients undergoing transcatheter aortic valve replacement. Am J Cardiol. 2016;118:251–257. doi: 10.1016/j.amjcard.2016.04.043. [DOI] [PubMed] [Google Scholar]
- 22.Osnabrugge RL, Speir AM, Head SJ, et al. Cost, quality, and value in coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2014;148:2729–2735. e2721. doi: 10.1016/j.jtcvs.2014.07.089. [DOI] [PubMed] [Google Scholar]
- 23.Lee DH, Buth KJ, Martin BJ, Yip AM, Hirsch GM. Frail patients are at increased risk for mortality and prolonged institutional care after cardiac surgery. Circulation. 2010;121:973–978. doi: 10.1161/CIRCULATIONAHA.108.841437. [DOI] [PubMed] [Google Scholar]
- 24.Afilalo J, Kim S, O'Brien S, et al. Gait speed and operative mortality in older adults following cardiac surgery. JAMA Cardiol. 2016;1:314–321. doi: 10.1001/jamacardio.2016.0316. [DOI] [PubMed] [Google Scholar]
- 25.Lytwyn J, Stammers AN, Kehler DS, et al. The impact of frailty on functional survival in patients 1 year after cardiac surgery. J Thorac Cardiovasc Surg. 2017 doi: 10.1016/j.jtcvs.2017.06.040. [DOI] [PubMed] [Google Scholar]
- 26.Paknikar R, Friedman J, Cron D, et al. Psoas muscle size as a frailty measure for open and transcatheter aortic valve replacement. J Thorac Cardiovasc Surg. 2016;151:745–751. doi: 10.1016/j.jtcvs.2015.11.022. [DOI] [PubMed] [Google Scholar]
- 27.Afilalo J, Eisenberg MJ, Morin JF, et al. Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J Am Coll Cardiol. 2010;56:1668–1676. doi: 10.1016/j.jacc.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 28.Afilalo J, Mottillo S, Eisenberg MJ, et al. Addition of frailty and disability to cardiac surgery risk scores identifies elderly patients at high risk of mortality or major morbidity. Circ Cardiovasc Qual Outcomes. 2012;5:222–228. doi: 10.1161/CIRCOUTCOMES.111.963157. [DOI] [PubMed] [Google Scholar]
- 29.Sepehri A, Beggs T, Hassan A, et al. The impact of frailty on outcomes after cardiac surgery: A systematic review. J Thorac Cardiovasc Surg. 2014;148:3110–3117. doi: 10.1016/j.jtcvs.2014.07.087. [DOI] [PubMed] [Google Scholar]
- 30.Osnabrugge RL, Speir AM, Head SJ, et al. Prediction of costs and length of stay in coronary artery bypass grafting. Ann Thorac Surg. 2014;98:1286–1293. doi: 10.1016/j.athoracsur.2014.05.073. [DOI] [PubMed] [Google Scholar]
- 31.Mehaffey JH, Hawkins RB, Byler M, et al. Cost of individual complications following coronary artery bypass grafting. J Thorac Cardiov Sur. 2017 doi: 10.1016/j.jtcvs.2017.08.144. Accepted. [DOI] [PubMed] [Google Scholar]
- 32.Garg L, Agrawal S, Pew T, et al. Psoas muscle area as a predictor of outcomes in transcatheter aortic valve implantation. Am J Cardiol. 2017;119:457–460. doi: 10.1016/j.amjcard.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 33.Goldfarb M, Bendayan M, Rudski LG, et al. Cost of cardiac surgery in frail compared with nonfrail older adults. Can J Cardiol. 2017;33:1020–1026. doi: 10.1016/j.cjca.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 34.Hawkins RB, Mehaffey JH, Yount KW, et al. Coronary artery bypass grafting bundled payment proposal will have significant financial impact on hospitals. J Thorac Cardiovasc Surg. 2017 doi: 10.1016/j.jtcvs.2017.07.035. [DOI] [PubMed] [Google Scholar]
- 35.Puskas JD, Kilgo PD, Thourani VH, et al. The society of thoracic surgeons 30-day predicted risk of mortality score also predicts long-term survival. Ann Thorac Surg. 2012;93:26–33. doi: 10.1016/j.athoracsur.2011.07.086. discussion 33-25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.