Abstract
Objective
To evaluate the feasibility of salpingectomy compared with standard bilateral tubal ligation at the time of cesarean delivery in women with undesired fertility.
Methods
We included women ≥ 35 weeks of gestation desiring permanent sterilization at the time of cesarean delivery. Patients were randomized after skin incision to bilateral salpingectomy or bilateral tubal ligation by a computer-generated scheme. If salpingectomy was unable to be completed on one or both sides, bilateral tubal ligation was attempted. Primary feasibility outcomes were total operative time and bilateral completion of randomized procedure. Secondary outcomes included clinically estimated blood loss (EBL) and surgical complications up to 6 weeks postpartum. We estimated that 80 patients(40 per group) would provide >80% power to identify a 10-minute difference in the primary outcome (time) with a standard deviation of 15 minutes and 2-sided α of 0.05. Analysis was by intent-to-treat.
Results
Of 221 women screened from June 2015 to April 2017, 115 (52%) consented to the study; 80 were randomized, 40 to salpingectomy and 40 to bilateral tubal ligation. Groups were similar at baseline. A total of 27 bilateral salpingectomies were successfully completed compared to 38 bilateral tubal ligations (68% vs. 95%, p = 0.002). Total operative time was on average 15 minutes longer for salpingectomies (75.4 ± 29.1 vs. 60.0 ± 23.3 min, p = 0.004). No adverse outcomes directly related to the sterilization procedure were noted in either group. While EBL of only the sterilization procedure (surgeon estimate) was greater for the salpingectomy group (median 10 [IQR 5–25] vs. 5 [IQR 5–10] cc, p<0.001), total EBL and safety outcomes were similar for both groups.
Conclusion
Adding 15 minutes to total operative times, salpingectomy can be successfully completed in approximately two thirds of women desiring permanent contraception with cesarean delivery.
Clinical Trial Registration
INTRODUCTION
Ovarian cancer remains the most lethal gynecologic malignancy, with greater than 22,000 cases diagnosed and over 14,000 deaths attributed to disease annually in the United States (1). Major contributors to this high mortality are the lack of effective screening strategies, diagnosis at advanced stage of presentation as well as the high risk of recurrence following seemingly effective primary therapy (2). As such, the recent focus of reducing the ovarian cancer burden has shifted toward primary prevention.
In the last 10 years, studies have suggested that the majority of ovarian cancers (up to 70%) may actually originate in the distal fallopian tube and metastasize to the ovary, creating the appearance of a primary ovarian malignancy (3–7). Given these new findings, both the American College of Obstetricians and Gynecologists and the Society of Gynecologic Oncology have recommended that total salpingectomy be considered for potential ovarian cancer risk reduction in benign gynecologic surgeries after completion of child-bearing (8,9). As implementation of this additional procedure into routine gynecologic practice has become more widespread, multiple studies have demonstrated its safety, lack of effect on hormonal function, and potential cost-effectiveness (8.9).
However, the data are limited – especially in terms of randomized trials – regarding the feasibility and surgical safety of salpingectomy compared to standard bilateral tubal ligation at the time of cesarean delivery. As such, our objective was to compare the technical feasibility and surgical outcomes between complete salpingectomy and standard postpartum tubal ligation at the time of cesarean delivery in women with undesired future fertility.
MATERIALS AND METHODS
Salpingectomy at Cesarean for Ovarian Cancer Reduction (SCORE) was a randomized clinical trial conducted at the University of Alabama at Birmingham (UAB). The trial was registered with clinicatrials.gov (NCT02374827) and approved by our institutional review board (#F140630003) with approval valid throughout the course of the trial. Written informed consent was obtained from all participants in the trial. Funding was provided in part by the Debra Kogan Lyda Memorial Ovarian Cancer Fund. An independent data and safety monitoring board oversaw the trial. The first author takes responsibility for the accuracy, completeness, and fidelity to the trial protocol.
All women receiving obstetric care at UAB desiring surgical sterilization at the time of their cesarean delivery at our academic, tertiary hospital were eligible to participate in this study. At our institution, consent for all surgical sterilization is obtained after 20 weeks’ gestation and consists of counseling the patient on the risks, benefits, and alternatives of surgical sterilization and signing of sterilization consent paperwork. Subsequently, desire for permanent sterilization is confirmed by signing surgical consent forms upon admission for delivery and re-confirmed (patient “yes” or “no”) upon the decision to proceed with cesarean delivery. Women underwent the consent procedure for this study in the outpatient clinics, on the inpatient antepartum service, or at admission for delivery. The majority of patients approached had planned elective cesarean deliveries or a history of cesarean with the decision for a trial of labor. Women without a prior history of cesarean could elect to participate in the trial if they desired surgical sterilization and they ultimately required cesarean delivery for obstetric or maternal indication.
In order to minimize the potential for patient regret with desire for future fertility as well as potential surgical complications we employed liberal exclusion criteria including inability to give informed consent, maternal age < 25 years, fetal death, prenatally diagnosed fetal anomalies, immune-compromising disease, chronic steroid/immunosuppressive therapy, anticoagulation therapy (prophylactic or therapeutic), previous tubal surgery, and known BRCA mutation carrier. For ethical considerations women were not approached for participation once in active labor, in the setting of non-reassuring fetal status, or if a decision was made for an urgent/emergent cesarean delivery. Patients were also excluded if they delivered < 35 weeks gestational age given the potential for participant regret of permanent sterilization with a preterm infant.
Patients were randomly assigned to receive either standard postpartum bilateral tubal ligation (partial salpingectomy per our institutional practice) or complete bilateral salpingectomy. A computer-generated randomization scheme with a block size of 100 was produced by our data center with only our lead biostatistician having access to the algorithm. Our standard practice for postpartum bilateral tubal ligation at the time of cesarean delivery is the modified Pomeroy technique (double ligation of midportion loop of tube with No.1 plain catgut suture and subsequent transection of tube) with the Parkland method only used in rare situations at the discretion of the attending physician when modified Pomeroy is deemed unfeasible or unsafe (10). A standard protocol was developed for the complete salpingectomy procedure. For complete salpingectomy, the fallopian tube was first grasped with Babcock clamp(s). Adhesiolysis was performed so that the fimbriae were free of the ovary and the ovarian vessels clearly identified away from the tube and fimbriae. A window was created in the avascular portion of the mesosalpinx by cautery or bluntly. A Kelly or Stille clamp was then placed laterally to medially across the mesosalpinx and through the avascular window. The mesosalpinx was then divided (leaving tissue on the clamp for a substantial pedicle) and suture ligated or free tied with 0-chromic or 2-0 chromic on an SH needle (second ligation placed by physician discretion). These steps were repeated until placement of final clamp at the proximal end of the fallopian tube (leaving <10mm of tube on the cornua), with these procedures repeated on the contralateral tube. If a salpingectomy was unable to completed on one or both sides, standard tubal ligation was attempted. The ability to perform salpingectomy or decision to not perform salpingectomy was solely at the discretion of the attending physician provider.
Upon patient transport to the operating room for cesarean delivery, research staff members retrieved the next sequentially numbered sealed opaque envelope containing the name of the allocated procedure. Prior to skin incision, the randomization was revealed to the case surgeons. At our institution, cesareans are typically performed by two residents (4th year resident with a first year or second year resident) under the direct supervision of a maternal fetal-medicine physician. Cesarean delivery commenced per routine practice. After closure of the hysterotomy (incision), the case surgeons notified the operating room “time keeper” at the beginning of the randomized tubal procedure for the “start time of the tubal procedure.” “End time of tubal procedure,” any complications, additional procedures, adhesiolysis, and physician-estimated blood loss during the tubal procedure only were reported to the research staff. All complications and adverse events were also noted in the operative summary in the patient chart. Completion of cesarean was conducted per routine practice with the patient taken to recovery. Start and end times of the complete operative procedure was maintained by anesthesia in the patient chart. After the completion of the procedure, a brief physician (satisfaction) survey was completed by the one primary surgeon performing the randomized tubal procedure (senior resident, fellow, or attending as appropriate). Patients were informed about their randomized procedure only on day of discharge.
The primary outcomes related to feasibility were total operative time (skin incision to skin closure) and bilateral completion rate of randomized procedure. Total operative time was specifically chosen as the primary outcome as it is more clinically relevant (as compared to operative time of sterilization only) for scheduling surgical cases. “Feasible” was defined as the procedure being successfully completed bilaterally in the majority of cases (>50%). Secondary outcomes included clinically estimated blood loss (total procedure and tubal procedure only), operative time of tubal procedure only, change in hematocrit (pre- and postoperatively), as well as an assessment of surgical complications up to 6 weeks postpartum. Surgical complications included intra- and postoperative complications, postpartum hemorrhage, need for blood transfusion (intra- or postoperative), pain assessment, length of maternal postoperative stay, admission to ICU, wound complications (infectious or non-infectious), need for reoperation, and readmissions (see Table 2 for all secondary outcomes). Other secondary outcomes included physician assessment and attitudes towards tubal procedure performed as well as patient attitudes regarding their concern about developing ovarian cancer at 1 week and 6 weeks postpartum.
Table 2.
Primary, secondary surgical and safety outcomes between salpingectomy and bilateral tubal ligation (BTL) groups [Data presented as n (%), mean± standard deviation, or median (interquartile range)]
| BTL (n=40) |
Salpingectomy (n=40) |
p- value |
|
|---|---|---|---|
|
| |||
| Bilateral completion of procedure | 38 (95) | 27 (68) | 0.002 |
|
| |||
| Total operative time (minutes) | 60.0 ± 23.3 | 75.4 ± 29.1 | 0.004 |
| 57.5 (45.5–67.5) | 71.0 (57.5–87.0) | ||
|
| |||
| Sterilization operative time (minutes) | 6.9 ± 5.0 | 18.5 ± 8.3 | < 0.001 |
| 5.0 (4.0–8.0) | 18.0 (14.0–22.0) | ||
|
| |||
| Total procedure EBL (cc) | 930 ± 221 | 1007 ± 426 | 0.56 |
| 800 (800–1000) | 807.5 (800–1000) | ||
|
| |||
| Sterilization procedure EBL | 6 ± 4 | 17 ± 14 | <0.001 |
| 5 (5–10) | 10 (5–25) | ||
|
| |||
| Intraoperative complications1 | 1 (3) | 0 (0) | 0.49 |
|
| |||
| Change in HCT (%) | 4.9 ± 3.4 | 7.2 ± 5.9 | 0.12 |
| 4.5 (2.9–7.2) | 6.0 (3.9–8.7) | ||
|
| |||
| Postpartum hemorrhage | 0 (0) | 3 (8) | 0.24 |
|
| |||
| Blood transfusion | 2 (5) | 3 (8) | >0.99 |
| Intraoperative | 1 (3) | 1 (3) | >0.99 |
| Postoperative | 1 (3) | 3 (8) | 0.62 |
|
| |||
| Called to evaluate possible postoperative complication | 1 (3) | 0 (0) | >0.99 |
|
| |||
| Average pain score | 8.9 ± 4.8 | 8.7 ± 4.8 | 0.91 |
| 8.1 (5.6–11.5) | 8.8 (6.4–11.7) | ||
|
| |||
| Postoperative complications2 | 2 (5) | 3 (8) | >0.99 |
|
| |||
| ICU admission3 | 0 (0) | 1 (3) | >0.99 |
|
| |||
| Maternal postoperative hospital stay | 3.9 ± 1.3 | 3.4 ± 0.6 | 0.018 |
| 4 (3–4) | 3 (3–4) | ||
|
| |||
| Presented to hospital within first week | 5 (13) | 5 (13) | >0.99 |
| Wound complaint | 5 (13) | 4 (10) | >0.99 |
| Abdominal Pain | 1 (3) | 1 (3) | >0.99 |
| Reported Fever | 1 (3) | 1 (3) | >0.99 |
|
| |||
| Readmission within first week4 | 1 (3) | 1 (3) | >0.99 |
| Non-infectious wound complication | 1 (3) | 0 (0) | >0.99 |
|
| |||
| Wound infection | 0 (0) | 1 (3) | >0.99 |
|
| |||
| Antibiotics | 0 (0) | 1 (3) | >0.99 |
| Wound debridement | 0 (0) | 1 (3) | >0.99 |
|
| |||
| Presented to hospital up to 6 weeks postpartum | 6 (15) | 7 (18) | 0.76 |
| 6 (15) | 5 (13) | 0.75 | |
| Wound complaint | 1 (3) | 2 (5) | >0.99 |
| Vaginal Bleeding | 4 (10) | 3 (8) | >0.99 |
| Abdominal Pain Fever | 2 (5) | 2 (5) | >0.99 |
| Fever | |||
|
| |||
| Readmission up to 6 week postpartum4 | 1 (3) | 3 (8) | 0.62 |
| Wound infection | 1 (3) | 2 (5) | >0.99 |
| Antibiotics | 1 (3) | 2 (5) | >0.99 |
| Wound debridement | 1 (3) | 2 (5) | >0.99 |
Complication was bleeding secondary to myomatous uterus
Complications: TL group - postoperative ileus and anemia of unknown source
SPG group: bleeding from hysterotomy (n=2), anemia of unknown source (n=1)
ICU admission secondary to hysterectomy for placenta accreta
All reoperations were for wound exploration/superficial infection and not noted by the data monitoring safety board to be related to sterilization procedure.
Trained and certified research staff members ascertained outcomes by reviewing the medical records from the delivery hospitalization, from visits to a postpartum clinic or emergency department, and from hospital admissions/readmissions. Patients were contacted by phone within 1 week of discharge and again at 6 weeks postpartum (by clinic visit or telephone) to ascertain maternal events as well as their concern about developing ovarian cancer in the future. Medical records (including those at other health care facilities) were required to verify study outcomes. All data were collected on case report forms and entered into the study’s REDCap database.
We estimated that 80 patients(40 per group) would provide >80% power to identify a 10-minute difference in the primary outcome (total operative time) from a baseline operative time of 50 minutes (per institutional data review) with a standard deviation of 15 minutes and a two-sided alpha of 0.05.
All analyses of the primary outcomes, secondary surgical outcomes, and provider attitudes were performed according to the intent-to-treat principle. To evaluate factors associated with successful completion of salpingectomy, we compared patient characteristics between patients with successfully completed bilateral salpingectomies and patients in whom bilateral salpingectomy was not completed (but surgical sterilization was performed, i.e. standard bilateral tubal ligation). In addition, we compared patient attitudes about their risk of ovarian cancer by the actual sterilization procedure performed. We used the chi-square or Fisher’s exact test for categorical variables and Student’s t-test or Wilcoxon rank-sum test for continuous variables. All analyses were performed using SAS version 9.4 (Cary, NC), and all outcomes were evaluated at a 0.05 level of significance.
RESULTS
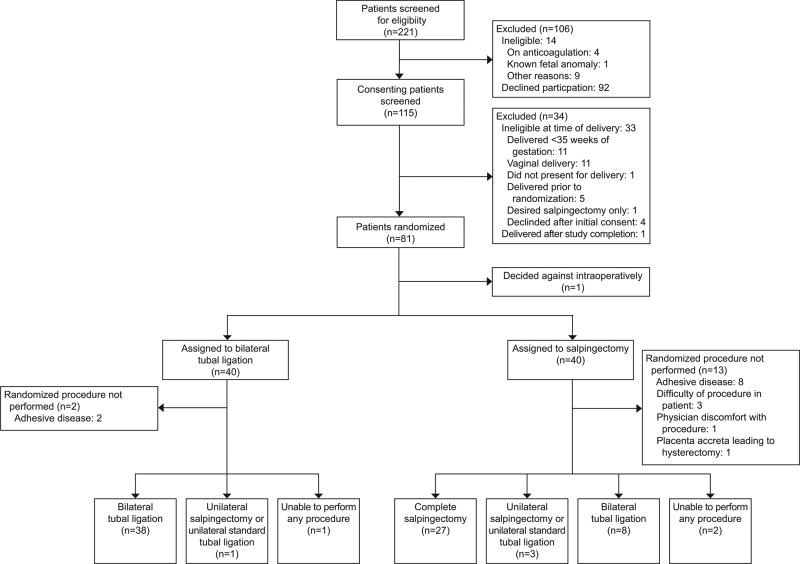
Of 221 women screened at the University of Alabama at Birmingham from June 2015 to April 2017, 115 (52%) consented to participate in the study. Eighty of these 115 patients were randomized: 40 to the salpingectomy group and 40 to the bilateral tubal ligation group (Figure 1). Baseline characteristics were similar between the two groups (Table 1).
Figure 1.
Enrollment and outcomes.
Table 1.
Characteristics of Patients at Baseline*
| BTL (N=40) |
Salpingectomy (N=40) |
|
|---|---|---|
|
| ||
| Maternal age (years) | 32.3 ± 3.9 | 33.0 ± 4.1 |
|
| ||
| Race | ||
| African American, Non-Hispanic | 18 (45) | 13 (33) |
| White, Non-Hispanic | 14 (35) | 14 (35) |
| White, Hispanic | 8 (20) | 13 (33) |
|
| ||
| Marital status | ||
| Married | 14 (35) | 14 (35) |
| Other | 26 (65) | 26 (65) |
|
| ||
| Education Levela | ||
| High school + | 17 (74) | 12 (57) |
| Less than high school | 6 (26) | 9 (43) |
|
| ||
| Payer status | ||
| Government Assisted/Medicaid | 33 (83) | 38 (95) |
| Private Insurance | 6 (15.0) | 1 (3) |
| No Insurance/Self-paid | 1 (3) | 1 (3) |
|
| ||
| Delivery BMI (kg/m2) | 39.4 ± 7.4 | 38.8 ± 10.0 |
|
| ||
| Multiparous | 40 (100) | 40 (100) |
|
| ||
| Chronic hypertension | 10 (25) | 10 (25) |
|
| ||
| Diabetes | 14 (35) | 9 (23) |
|
| ||
| Pregnancy-induced HTN | 11 (28) | 8 (20) |
|
| ||
| History of abdominal/pelvic surgery | 2 (5) | 3 (8) |
|
| ||
| Family history of cancer | 5 (13) | 7 (18) |
| Breast | 5 (13) | 5 (13) |
| Ovarian | 0 (0) | 2 (5) |
|
| ||
| Maternal tobacco use | 8 (20) | 14 (35) |
|
| ||
| Alcohol use | 2 (5) | 3 (8) |
|
| ||
| Drug use | 3 (8) | 4 (10) |
|
| ||
| Cesarean type | ||
| Primary | 7 (18) | 1 (3) |
| Repeat | 33 (83) | 39 (98) |
|
| ||
| Number of prior cesarean | 2.0 ± 1.0 | 2.0 ± 0.8 |
|
| ||
| Primary indication for cesarean | ||
| Repeat cesarean or prior surgery into uterine cavity (myomectomy) | 33 (83) | 38 (95) |
| Abnormal presentation | 3 (8) | 1 (3) |
| Otherc | 4 (10) | 1 (3) |
|
| ||
| Skin incision | ||
| Vertical | 3 (8) | 7 (18) |
| Pfannenstiel | 37 (93) | 33 (83) |
|
| ||
| Uterine incision | ||
| Low transverse | 35 (88) | 34 (85) |
| Other (classical, T, high transverse) | 5 (13) | 6 (15) |
Plus-minus values are means±SD.
Data not reported by patient and considered missing
Other includes low lying placenta, history of shoulder dystocia, labor arrest
Successful bilateral completion of assigned tubal procedure occurred in 27 women (68%) randomized to salpingectomy compared to 38 (95%) randomized to bilateral tubal ligation, (p = 0.002). Total operative time was on average 15 minutes longer for the salpingectomy group (75.4 ± 29.1 minutes vs. 60.0 ± 23.3 minutes, median 57.5 minutes (interquartile range IQR 45.5–67.5) vs. 71.0 minutes (57.5–87.0), p = 0.004). Successful bilateral completion rates (feasibility rate) were also significantly different between the salpingectomy and bilateral tubal ligation groups (68% [95% CI 53–82%] vs. 95% [95% CI 88–100%] respectively, p = 0.002). There were no adverse outcomes directly related to the sterilization procedure in either group. While there was an increase in operative time of the sterilization procedure alone and the associated sterilization procedure EBL in the salpingectomy group (median 5.0 minutes (IQR 4.0–8.0) vs. 18.0 minutes (IQR 14.0–22.0), p < 0.001; median 5 cc (IQR 5–10) vs. 10 cc (IQR 5–25), p < 0.001, respectively), overall EBL and change in hematocrit was not significantly different between the two groups (Table 2). There were no statistically significant differences in the rates of intraoperative complications, postoperative complications, blood transfusion, ICU admission, pain score, readmission, and reoperations between the salpingectomy and bilateral tubal ligation groups; however, there was a shorter maternal hospital stay associated with salpingectomy compared to the bilateral tubal ligation group (3.4 ± 0.6 days vs. 3.9 ± 1.3 days, p = 0.018).
To analyze factors associated with successful salpingectomy completion, we analyzed baseline patient characteristics from Table 1 between the 27 women in whom bilateral salpingectomy was completed and the 11 women in whom bilateral salpingectomy was not completed, but an alternative procedure (unilateral salpingectomy/unilateral standard tubal ligation or bilateral standard tubal ligation) was completed (Figure 1). There were no significant differences in the baseline characteristics between these groups – especially number of abdominal surgeries, prior cesarean deliveries, or skin incision type – with the exception of delivery BMI being higher in women in whom bilateral salpingectomies were not completed (46.2 ± 12.1 kg/m2 vs. 36.2 ± 7.9 kg/m2, p = 0.012). In addition, time from skin incision to tubal start (47.6 ± 23.2 minutes vs. 29.2 ± 14.7 minutes, p = 0.009) was also greater in the unsuccessful bilateral salpingectomy group.
Attitudes of the primary surgeon (4th year Obstetrics and Gynecology resident, Maternal-Fetal Medicine Fellow, or Maternal-Fetal Medicine Attending) performing the assigned tubal procedure are presented in Table 3. Providers were more satisfied with bilateral tubal ligation - responding that compared to bilateral tubal ligation, salpingectomy added difficulty to the case (71% vs. 8%, p <0.001) and took significantly more time than expected (57% vs. 8%, p<0.001). While all responders would perform bilateral tubal ligation again and as part of general practice, only 54% (n=19) would perform salpingectomy and only 35% (n=12) would perform salpingectomy at cesarean delivery as part of general practice (Table 3). When evaluating patient attitudes regarding their level of concern for developing ovarian cancer in the future, we compared the 27 women who successfully had a bilateral salpingectomy with the other 50 patients who underwent other methods of surgical sterilization (Figure 1)). While there was no significant difference at 1 week postpartum in patient attitudes (Scale of 0–10 with 0 = no concern) between those who received salpingectomy and those who received other surgical sterilization (1.2 ± 1.5 vs. 2.2 ± 3.2, p = 0.58), by the 6-week postpartum follow-up, patients receiving complete salpingectomies were significantly less concerned about the development of ovarian cancer (0.8± 1.7 vs. 2.8 ± 3.0, p = 0.005).
Table 3.
A comparison of physician satisfaction between standard bilateral tubal ligation (BTL) and complete salpingectomy at the time of cesarean delivery
| Tubal ligation (n=40) |
Salpingectomy (n=40) |
p-value | |
|---|---|---|---|
|
| |||
| Satisfaction with tubal segment exposure1 | 7.0 (6.0–7.0) | 6.5 (5.0–7.0) | 0.046 |
| Satisfied | 37 (95) | 29 (85) | 0.24 |
| Dissatisfied | 2 (5) | 5 (15) | 0.24 |
|
| |||
| Satisfaction with feasibility of procedure1 | 7.0 (6.0–7.0) | 5.0 (3.0–6.0) | <0.001 |
| Satisfied | 36 (92) | 21 (62) | 0.002 |
| Neutral | 1 (3) | 3 (9) | 0.33 |
| Dissatisfied | 2 (5) | 10 (29) | 0.005 |
|
| |||
| Satisfaction with safety of procedure1 | 7.0 (7.0–7.0) | 5.0 (3.0–6.0) | <0.001 |
| Satisfied | 38 (97) | 18 (53) | <0.001 |
| Neutral | 0 (0) | 7 (21) | 0.003 |
| Dissatisfied | 1 (3) | 9 (27) | 0.005 |
|
| |||
| Did procedure require adhesiolysis2 | 7 (18) | 14 (39) | 0.044 |
|
| |||
| Did procedure add difficulty to the case3 | 3 (8) | 25 (71) | <0.001 |
|
| |||
| Did procedure take more time than expected3 | 3 (8) | 20 (57) | <0.001 |
|
| |||
| Would you perform this procedure again3 | 39 (100) | 19 (54) | <0.001 |
|
| |||
| Would you perform this procedure as part of general practice1 | 39 (100) | 12 (35) | <0.001 |
Data presented as n (%) or median (interquartile range) on Likert scale (1–7, 7= very satisfied)
Missing 7 participants: BTL (n=39), Salpingectomy (n=34)
Missing 5 participants: BTL (n=39), Salpingectomy (n=36)
Missing 6 participants: BTL (n=39), Salpingectomy (n=35)
DISCUSSION
In this randomized trial, we have demonstrated that a complete salpingectomy can successfully be completed in approximately two-thirds of women desiring permanent contraception at the time of cesarean delivery, with higher patient BMI and time from skin incision to tubal procedure as factors associated with unsuccessful salpingectomy completion. While patients are more reassured about future ovarian cancer risk after complete salpingectomy compared to bilateral tubal ligation, salpingectomy per our protocol does add on average 15 minutes to operative times and obstetric providers are generally less satisfied performing salpingectomy compared to bilateral tubal ligation (as they report greater difficulty with the procedure). Even so, our trial supports that salpingectomy is a reasonable consideration as a surgical sterilization method during cesarean delivery as an ovarian cancer risk reducing strategy.
Complete or total salpingectomy for ovarian cancer risk reduction is not a new concept in general gynecologic practice – as studies estimate a 50% ovarian cancer risk reduction (up to 70%) associated with salpingectomy (11,12). In fact, multiple societies including Society of Gynecologic Oncology of Canada, the Society of Gynecologic Oncology (United States), and the American College of Obstetricians and Gynecologists have advocated for the adoption of this practice since 2011 (7). As such, the incorporation of salpingectomy into routine gynecologic surgery has increased dramatically over the last decade to the point that up to 75% of hysterectomies for benign disease are now accompanied by opportunistic risk-reducing bilateral salpingectomies (13, 14). Multiple studies evaluating the addition of bilateral salpingectomy to hysterectomy (abdominal, vaginal, laparoscopic) or as a form of surgical sterilization (not at cesarean) have failed to demonstrate increased surgical risks or short-term effects on ovarian function associated with this practice (7, 13–20). As a form of contraception, salpingectomy also offers a negligible risk of pregnancy and even lower risk of ectopic pregnancy compared to tubal ligation. Furthermore, studies have demonstrated that incorporation of salpingectomy into routine gynecologic surgery is a cost-effective strategy for ovarian-cancer risk reduction (11,12).
Two other studies have shown that salpingectomy is feasible at the time of cesarean delivery; however, both were small case series (21,22) In a review of the available literature, to date (using online published databases with search terms such as salpingectomy, cesarean, trial), only one randomized trial of salpingectomy compared to standard bilateral tubal ligation at cesarean delivery has been conducted. In a small pilot trial in Israel, Ganer et al. demonstrated no changes in ovarian reserve or significant differences in surgical outcomes or complications in 46 patients randomized to salpingectomy or bilateral tubal ligation at time of cesarean (23). While this study had an apparent completion rate of 100% (n=22 salpingectomies), operative times for cesareans with salpingectomies were on average 13 minutes longer, patients had lower BMI, and all salpingectomies were performed by the same two “expert” surgeons, perhaps limiting the generalizability to general practice, as there may be a significant learning curve to the salpingectomy procedure.
While our sample size was small (n=80), our study was adequately powered for both of our primary outcomes. We acknowledge that the sample size may be underpowered for thorough assessment of surgical complications and safety. Another limitation of our study is the lack of knowledge of the effect of salpingectomy as compared to standard bilateral tubal ligation on ovarian cancer risk. As ovarian cancer typically develops after the 5th decade of life, all data regarding the potential ovarian cancer risk-reduction of salpingectomy compared to tubal ligation is derived from epidemiologic population-based studies. Conclusive evidence regarding salpingectomy as an effective risk-reduction strategy is still decades away. Similarly, the cost-effectiveness of this strategy at the time of cesarean is also unknown. Finally, 106 women approached for inclusion into the study declined to participate. Limited clinical information is available about this group, which may affect generalizability of our findings.
In conclusion, our study supports that bilateral salpingectomy should be considered as a form of surgical sterilization at the time of cesarean delivery.
Acknowledgments
Supported by the Debora Kogan Lyda Ovarian Cancer Memorial Fund. Funding support was also provided in part by (NIH): 5K12HD0012580-15, U10 C180855 and 3P30CA013148-43S3 to Charles A Leath.
Footnotes
Presented at the Society for Maternal-Fetal Medicine 38th Annual Pregnancy Meeting, Dallas, TX, January 29-February 3, 2018.
Financial Disclosure
Each author has indicated that he or she has met the journal’s requirements for authorship.
The authors did not report any potential conflicts of interest.
References
- 1.American Cancer Society. [Retrieved September 25 2017];Ovarian cancer. Available at: http://www.cancer.org/cancer/ovariancancer/detailedguide/ovarian-cancer-key-statistics.
- 2.Lowe KA, Chia VM, Taylor A, O’Malley C, et al. An international assessment of ovarian cancer incidence and mortality. Gynecol Oncol. 2013;130:107–14. doi: 10.1016/j.ygyno.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 3.Kurman RJ, Shih I. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34:433–43. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erickson BK, Conner MG, Landen CN., Jr The Role of the fallopian tube in the origin of ovarian cancer. Am J Obstet Gynecol. 2013;209:409–14. doi: 10.1016/j.ajog.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crum CP. Intercepting pelvic cancer in the distal fallopian tube: theories and realities. Mol Oncol. 2009;3:165–70. doi: 10.1016/j.molonc.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kindelberger DW, Lee Y, Miron A, Hirsch MS, et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: Evidence for a causal relationship. Am J Surg Pathol. 2007;31:161–9. doi: 10.1097/01.pas.0000213335.40358.47. [DOI] [PubMed] [Google Scholar]
- 7.Dilley SE, Straughn JM, Jr, Leath CA., III The Evolution of and Evidence for Opportunistic Salpingectomy. Obstet Gynecol. 2017;130:814–24. doi: 10.1097/AOG.0000000000002243. [DOI] [PubMed] [Google Scholar]
- 8.Walker JL, Powell CB, Chen L, Carter J, et al. Society of Gynecology Oncology recommendations for the prevention of ovarian cancer. Cancer. 2015;121:2108–20. doi: 10.1002/cncr.29321. [DOI] [PubMed] [Google Scholar]
- 9.Salpingectomy for ovarian cancer prevention. Committee Opinion No. 620. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2015;125:279–81. doi: 10.1097/01.AOG.0000459871.88564.09. [DOI] [PubMed] [Google Scholar]
- 10.Lawrie TA, Kulier R, Nardin JM. Techniques for the interruption of tubal patency for female sterilization. Cochrane Database Syst Rev. 2016;(8):CD003034. doi: 10.1002/14651858.CD003034.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwon JS, McAlpine JN, Hanley GE, Finlayson SJ, et al. Costs and benefits of opportunistic salpingectomy as an ovarian cancer prevention strategy. Obstet Gynecol. 2015;125:338–45. doi: 10.1097/AOG.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 12.Dilley SE, Havrilesky LJ, Bakkum-Gamez J, Cohn D, et al. Cost-effectiveness of opportunistic salpingectomy for ovarian cancer prevention. Gynecol Oncol. 2017;146(2):373–379. doi: 10.1016/j.ygyno.2017.05.034. [DOI] [PubMed] [Google Scholar]
- 13.Hanley GE, McAlpine JN, Pearce CL, Miller D. The performance and safety of bilateral salpingectomy for ovarian cancer prevention in the United States. Am J Obstet Gynecol. 2017;216:270.e1–9. doi: 10.1016/j.ajog.2016.10.035. [DOI] [PubMed] [Google Scholar]
- 14.Garcia C, Martin M, Tucker LY, Lyon L, et al. Experience with opportunistic salpingectomy in a large, community-based health system in the United States. Obstet Gynecol. 2016;128:277–83. doi: 10.1097/AOG.0000000000001531. [DOI] [PubMed] [Google Scholar]
- 15.McAlpine JN, Hanley GE, Woo MM, Tone AA, et al. Opportunistic salpingectomy: uptake, risks, and complications of a regional initiative for ovarian cancer prevention. Am J Obstet Gynecol. 2014;210:471.e1–11. doi: 10.1016/j.ajog.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Morelli M, Venturella R, Mocciaro R, Di Cello, et al. Prophylactic salpingectomy in premenopausal low-risk women for ovarian cancer: primum non nocere. Gynecol Oncol. 2013;129:448–51. doi: 10.1016/j.ygyno.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 17.Vorwergk J, Radose MP, Nicolaus K, Baus N, et al. Prophylactic bilateral salpingectomy (PBS) to reduce ovarian cancer risk incorporated in standard premenopausal hysterectomy: complications and re-operation rate. J Cancer Res Clin Oncol. 2014;140:859–65. doi: 10.1007/s00432-014-1622-6. [DOI] [PubMed] [Google Scholar]
- 18.Minig L, Chuang L, Patrono MG, Cardenas-Rebollo JM, et al. Surgical outcomes and complications of prophylactic salpingectomy at the time of benign hysterectomy in premenopausal women. J Minim Invasive Gynecol. 2015;22:653–7. doi: 10.1016/j.jmig.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Venturella R, Lico D, Borelli M, Inbrogno MG, et al. 3 to 5 years later: long-term effects of prophylactic bilateral salpingectomy on ovarian function. J Minim Invasive Gynecol. 2017;24:145–150. doi: 10.1016/j.jmig.2016.08.833. [DOI] [PubMed] [Google Scholar]
- 20.Findley AD, Siedhoff MT, Hobbs KA, Steege JF, et al. Short-term effects of salpingectomy during laparoscopic hysterectomy on ovarian reserve: a pilot randomized controlled trial. Fertil Steril. 2013;100:1704–8. doi: 10.1016/j.fertnstert.2013.07.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shinar S, Blecher Y, Alpern S, Many A, et al. Total bilateral salpingectomy versus partial bilateral salpingectomy for permanent sterilization during cesarean delivery. Arch Gynecol Obstet. 2017;295(5):1185–89. doi: 10.1007/s00404-017-4340-x. [DOI] [PubMed] [Google Scholar]
- 22.Danis RB, Della Badia CR, Richard SD. Postpartum permanent sterilization: could bilateral salpingectomy replace bilateral tubal ligation? J Minim Invasive Gynecol. 2016;23(6):928–32. doi: 10.1016/j.jmig.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Ganer HH, Gluck O, Keidar R, Kerner R. Ovarian reserve following cesarean section with salpingectomy vs. tubal ligation: a randomized trial. Am J Obstet Gynecol. 2017 doi: 10.1016/j.ajog.2017.04.028. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]