Abstract
Objective
To evaluate the relationship between maternal self-reported race/ethnicity and persistent wheezing illness in former high-risk, extremely low gestational age newborns (ELGAN), and to quantify the contribution of socioeconomic, environmental, and biological factors on this relationship.
Study design
We assessed persistent wheezing illness determined at 18–24 months corrected (for prematurity) age in survivors of a randomized trial. Parents/caregivers were surveyed for wheeze and inhaled asthma medication use quarterly to 12 months, and at 18 and 24 months. We used multivariable analysis to evaluate the relationship of maternal race to persistent wheezing illness, and identified mediators for this relationship via formal mediation analysis.
Results
Of 420 infants (25.2±1.2 weeks’ gestation and 714±166 grams at birth, 57% male, 34% maternal black race), 189 (45%) had persistent wheezing illness. After adjustment for gestational age, birth weight and sex, infants of black mothers had increased odds of persistent wheeze compared with infants of non-black mothers (OR=2.9, 95% CI 1.9, 4.5). Only bronchopulmonary dysplasia, breast milk diet, and public insurance status were identified as mediators. In this model, the direct effect of race accounted for 69% of the relationship between maternal race and persistent wheeze, while breast milk diet, public insurance status, and bronchopulmonary dysplasia accounted for 8%, 12%, and 10%, respectively.
Conclusions
Among former high-risk ELGAN, infants of black mothers have increased odds of developing persistent wheeze. A substantial proportion of this effect is directly accounted for by race, which may reflect unmeasured environmental influences, and acquired and innate biological differences.
Keywords: asthma, socioeconomic factors, prematurity
Long-term respiratory morbidity is a common adverse outcome of prematurity; premature infants with and without bronchopulmonary dysplasia (BPD) remain at increased risk for respiratory disease throughout childhood 1, 2. Many of these children suffer from wheezing disorders or asthma, associated with recurrent hospitalizations and long-term medication requirements 3, 4. Children born very preterm are at highest risk, and those with early recurrent wheeze remain at increased risk for wheeze throughout early childhood 3, 5.
Racial differences in childhood asthma in the US are apparent; non-Hispanic black children are affected at higher rates than non-Hispanic white children 6. Even after consideration of socioeconomic status (SES) and environmental risk factors, black children have increased odds of wheeze and asthma compared with white children 7, 8. However, less is known about the relationship between race and wheezing illness among preterm infants, with several studies demonstrating increased occurrence in moderately preterm black children 9–13. Increased susceptibility to environmental pollutants and Vitamin D intake may be modifying factors 12, 13. Although studies have identified environmental risk factors, eg, young siblings or exposure to environmental tobacco smoke (ETS), few studies have quantified the effect of multiple SES and environmental factors on infant wheezing disorders, particularly among extremely preterm infants 3, 14. Beck et al found that approximately half of the relationship between race and asthma-related hospitalization in children could be explained by socioeconomic hardships 15, 16. This suggests that innate and acquired biological and genetic influences explain some of the racial disparity in wheezing disorders. Whether these factors relate to differences in lung structure, function or immunity remain undefined.
The aims of the current study were) to determine the relationship between maternal black race and persistent wheezing illness among former extremely low gestational age newborns (ELGAN), and to quantify, via mediation analysis, the indirect effects of socioeconomic, environmental, and innate and acquired pulmonary biological factors on the development of wheezing disorders among infants of black mothers. We hypothesized that black maternal race would be independently associated with persistent wheezing illness among former ELGAN.
Methods
This was a secondary analysis from the randomized controlled Trial of Late Surfactant (TOLSURF, ClinicalTrials.gov: NCT01022580), under Institutional Review Board approval at 25 US academic centers 17. Infants ≤28 0/7 weeks’ gestational age (GA), who were mechanically ventilated between 7–14 days of life, were randomized to late surfactant and inhaled nitric oxide (iNO) versus iNO-alone. Those with major anomalies, life expectancy <7 days, or active co-morbidities at time of enrollment were excluded from the trial. No difference was seen by treatment group for the primary outcome, survival without BPD at 36 weeks’ postmenstrual age (by physiological testing), although infants of black mothers were less likely to have BPD after receiving iNO 17, 18.
Perinatal characteristics and sociodemographic data were collected at enrollment. To identify maternal race/ethnicity, mothers selected Hispanic/Latino versus not Hispanic/Latino and (all that apply) White/Caucasian, Black/African American, Asian, American Indian/Alaska Native, and Native Hawaiian/Other Pacific Islander. For this study, race/ethnicity was dichotomized to black (non-Hispanic) versus non-black. Mothers who selected multiple races were included in the non-black group.
At discharge, parents/caregivers were surveyed for factors known to modify respiratory morbidity in former ELGAN and other children 19. Specifically, we collected data on the presence of additional children <5 years in the home, furry pets in the home, anticipated day care attendance and breast milk diet, maternal educational attainment, public insurance status, and parental history of asthma (Discharge Questionnaire, Appendix 2; available at www.jpeds.com). Potential ETS exposure was defined by factors previously shown to be associated with elevated cotinine levels in children: 1) allowing any smoking in the home, 2) having a parent or other household member who smokes, or 3) travelling regularly in a car with someone who smokes 20, 21.
Parent/caregiver questionnaires were administered at 3, 6, 9, 12, 18, and 24 months corrected (for prematurity) age to assess events related to respiratory health including exposure to inhaled medications (bronchodilators or corticosteroids), wheeze auscultated by a medical professional and diagnosis of respiratory syncytial virus (RSV) infection. At 18 and 24 months, we asked if the child had a physician diagnosis of asthma, eczema or hay fever. Infants were classified with a history of atopy if caregivers reported eczema or hay fever. Data collected by questionnaire were not further verified by medical record review.
We defined persistent wheezing illness as having one of the following: physician diagnosis of asthma at 18–24 months and wheezing or medication (inhaled bronchodilator or corticosteroid) exposure at any questionnaire; or medication in the first 12 months and wheeze in the second 12 months of life; or wheeze in the first 12 months and medication in the second 12 months; or medication exposure in both the first and second 12 months; or wheeze and medication exposure in the second 12 months of life reported at separate visits (18 and 24 months). These criteria were adapted from the 3-year definition used in the Vitamin D Antenatal Asthma Reduction Trial (on average, ELGAN are 21–27 months chronological age at 18–24 months corrected age) 22.
Statistical analyses
Our primary question was the effect of maternal race (black versus non-black) on persistent wheezing illness. All analyses were 2-sided (P < .05) and utilized Stata 14.0 (College Station, TX). Univariate analyses were analyzed by chi-square or t-test. Due to the clinical importance of GA, sex and birth weight, all multivariable models were adjusted for these characteristics. To evaluate the independent influence of maternal race on persistent wheezing illness, we considered other baseline characteristics that are published risk factors for respiratory illness in preterm infants (antenatal corticosteroids, birth weight percentile, product of multiple gestation, maternal age) as potential confounders (Figure 1, A), as well as TOLSURF treatment assignment (late surfactant versus control); a priori, we planned to adjust the mediation analysis for factors that changed the effect size of maternal black race by ≥20% 3. Generalized estimating equations accounted for non-independence between siblings (exchangeable correlation).
Figure 1.
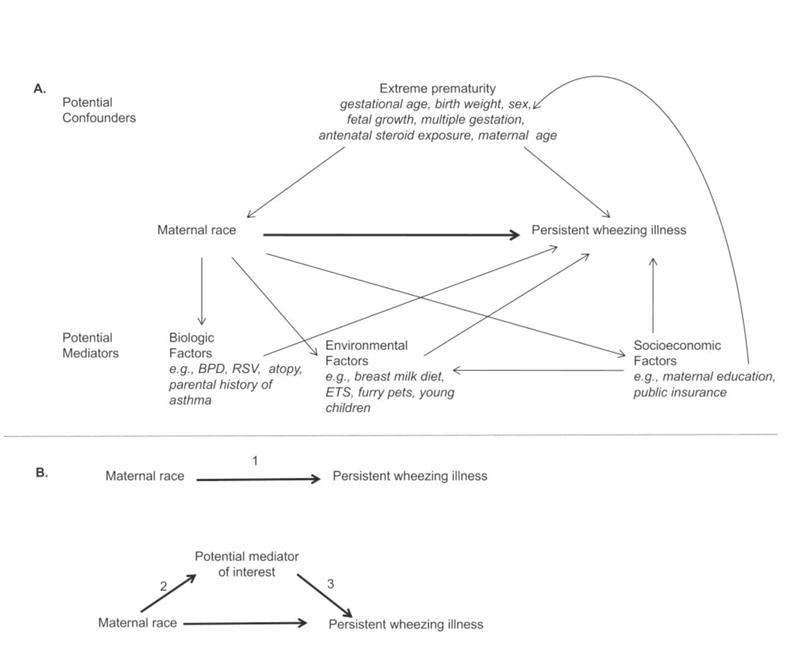
Relationships of confounders and mediators for the association of maternal race and persistent wheeze. 1A, Diagram representing complex interactions between potential confounders and possible mediators of the relationship between maternal race and persistent wheeze. 1B, Mediation framework for statistical conditions that must be satisfied to be considered a mediator. In formal mediation analysis:
(1) represents the direct effect of maternal race on persistent wheezing illness
(2 and 3) represents the indirect effect of maternal race through a mediator.
We proceeded step-wise with mediation analysis. We evaluated if BPD, as well as additional sociodemographic characteristics, environmental and other exposures after hospital discharge, and family or personal history of asthma/atopy satisfied the statistical conditions required to be a mediator between maternal race and persistent wheezing illness by the following steps (Figure 1, B) 23, 24: (1) Establish that race is significantly associated with the outcome, persistent wheezing illness, (2) Establish that race is significantly associated with the potential mediator of interest, and (3) establish that the potential mediator of interest is independently associated with persistent wheezing illness, after controlling for race. If all 3 conditions were met, variables were considered mediators of the relationship between maternal race and persistent wheezing illness.
Finally, we used multivariable mediation analysis with dichotomous variables, while adjusting for baseline characteristics, to quantify the percent of the relationship between race and persistent wheeze explained by the pathway through each mediator (indirect effect) versus the percent explained by the pathway through race (direct effect, Figure 1, B). As this methodology does not account for interdependence of siblings, sensitivity analyses were performed to assess the robustness of our findings. First, we selected a single sibling by random sampling, and second, we excluded all siblings.
Results
Patients were enrolled in TOLSURF from January 2010 to September 2013, with follow up conducted through January 2016. Of 455 infants discharged alive, 420 (92%) could be classified for persistent wheezing illness (Figure 2; available at www.jpeds.com). This extremely preterm cohort was predominantly male, mean gestation ~25 weeks’ and birth weight 700 grams (Table I), similar to the overall cohort available for 12-month follow up 19. When compared with those who were classified for wheezing illness, baseline characteristics of infants and their mothers who were unable to be classified (n = 29) differed somewhat from those who could be classified; they were less likely to be male (8/29, 28%; P = 0.002), with younger mothers (25.4 ± 5.7 years; P = 0.003) who were somewhat more likely to report black race (14/29, 48%; P = 0.13).
Figure 2.
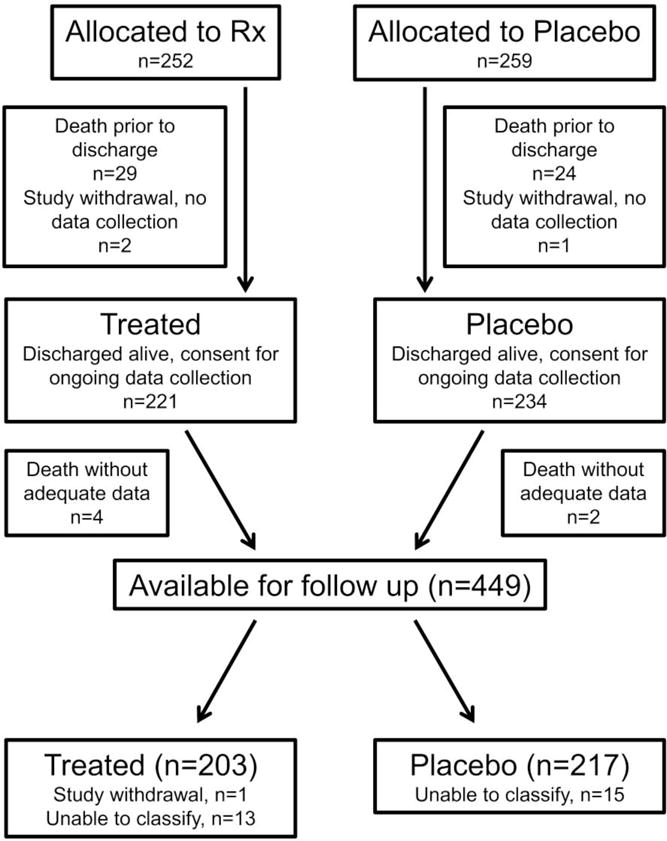
online. Patient flow diagram. Deaths and study withdrawals prior to neonatal discharge detailed in Ballard et al, 2016 17.
Table 1.
Cohort characteristics and univariate relationships with persistent wheezing illnessa
| Total cohort (N=420) | No persistent wheezing illness (N=231) | Persistent wheezing illness (N=189) | P-value | |
|---|---|---|---|---|
| Infant characteristics | ||||
| Gestational age (weeks) | 25.2 ± 1.2 | 25.2 ± 1.2 | 25.4 ± 1.2 | 0.1 |
| Birth weight (grams) | 714 ± 166 | 697 ± 157 | 734 ± 175 | 0.02 |
| Birth weight percentileb | 41.5 ± 28 | 41.2 ± 28 | 42 ± 27 | 0.78 |
| Male sex | 240 (57) | 115 (50) | 125 (66) | 0.001 |
| Product of multiple gestation | 130 (31) | 83 (36) | 47 (25) | 0.02 |
| Antenatal steroid exposure | 366 (88) | 212 (97) | 154 (82) | 0.002 |
| Randomized to late surfactant | 203 (48) | 112 (48) | 91 (48) | 0.95 |
| Maternal characteristics | ||||
| Maternal age (years) | 29.1 ± 6.4 | 29.4 ± 6.5 | 28.7 ± 6.3 | 0.31 |
| Maternal race | <0.001 | |||
| White, Non-hispanic | 209 (50) | 134 (58) | 75 (40) | |
| White, Hispanic | 49 (12) | 30 (13) | 19 (10) | |
| Black | 144 (34) | 57 (25) | 87 (46) | |
| Otherc | 18 (4) | 10 (4) | 8 (4) | |
Data reported as mean ± SD or N (%). P-value by chi square or t-test.
Corresponds to Step 1 from mediation analysis.
Percentile as determined by Fenton reference curves (2013)
Other includes Asian, American Indian/Alaska Native, Native Hawaiian/Pacific Islander, multiple race, and unknown
Overall, 189 (45%) infants had persistent wheezing illness. Infants with persistent wheeze were less likely to have received antenatal steroids and be a product of multiple gestation, but were more likely to be male, weigh more at birth, and be infants of black mothers. Late surfactant had no effect on persistent wheeze, so we did not further consider treatment assignment. After dichotomizing infants by maternal race (black versus non-black), infants of black mothers had 2.6 (95% CI 1.7, 4.0; p<0.001) times the odds of developing persistent wheezing illness compared with those of non-black mothers. The odds ratio increased modestly (2.9, 95% CI 1.9, 4.5; p<0.001) with adjustment for GA, sex, and birth weight.
We evaluated for the inclusion of other baseline covariates that might affect the association between maternal race and persistent wheeze. Although antenatal steroid exposure was associated with persistent wheeze while adjusting for maternal race (OR 0.47, 95% CI 0.24, 0.91; p=0.03), none of the covariates substantially changed the effect of black race, and were therefore not included in subsequent analyses (Table 2; available at www.jpeds.com).
Table 2.
online. The effect of maternal race on persistent wheezing illness, adjusted for selected baseline characteristics
| Variable | Odds ratio (95% CI) | P-value |
|---|---|---|
| Birth weight percentilea | 1.0 (0.97, 1.0) | 0.32 |
| Black | 2.9 (1.8, 4.5) | <0.001 |
|
| ||
| Antenatal steroids | 0.47 (0.24, 0.91) | 0.03 |
| Black | 2.9 (1.8, 4.5) | <0.001 |
|
| ||
| Product of multiple gestation | 0.65 (0.40, 1.0) | 0.08 |
| Black | 2.8 (1.8, 4.4) | <0.001 |
|
| ||
| Maternal age | 1.0 (0.96, 1.0) | 0.82 |
| Black | 2.9 (1.8, 4.5) | <0.001 |
P-value by generalized estimating equation. All analyses adjusted for birth weight, sex, and gestational age
Percentile as determined by Fenton reference curves (2013)
Next, we sought to determine the socioeconomic (maternal education, public insurance status), environmental (breast milk diet, ETS, young child or furry pet in home) and biological (BPD, caregiver report of RSV infection, atopy, and parent history of asthma) factors that may mediate the relationship between maternal race and persistent wheeze (data on prevalence and unadjusted relationships with persistent wheezing illness are provided, Table 3; available at www.jpeds.com). As only two infants were anticipated to attend daycare, this variable was not evaluated further. Black maternal race had no significant association with potential ETS exposure, young siblings in the home, RSV, or parent history of asthma (Table 4). Although maternal black race was associated with increased odds of infant history of eczema/hay fever, this relationship was not statistically significant (p=0.08). Additionally, although maternal black race was associated with decreased odds of furry pets in the home and lower educational attainment, these 2 variables were not independently associated with persistent wheeze when adjusted for maternal race, and therefore were not considered mediators in this analysis. Black maternal race was positively associated with public insurance status and negatively associated with breast milk diet and BPD, and these variables were independently associated with persistent wheeze, while adjusting for maternal race. Thus, public insurance status, breast milk diet, and BPD were identified as mediators of the relationship between maternal black race and persistent wheezing (Table 4). Adjusted effects of potential mediators not independently associated with black race are provided (Table 5; available at www.jpeds.com).
Table 3.
online. Prevalence of potential mediators and univariate analyses for relationship with persistent wheezing illness
| Potential mediator | Total cohort (N=420) | No persistent wheezing illness (N=231) | Persistent wheezing illness (N=189) | P-value |
|---|---|---|---|---|
| Potential ETS exposure | 97 (24) | 44 (20) | 53 (28) | 0.04 |
| Child <5y in home | 242 (62) | 124 (58) | 118 (66) | 0.12 |
| Eczema and/or hay fever | 189 (45) | 88 (38) | 101 (53) | 0.002 |
| RSV | 66 (15) | 27 (12) | 39 (21) | 0.01 |
| Parent history of asthma | 70 (17) | 31 (13) | 39 (21) | 0.05 |
| Furry pet in home | 171 (44) | 97 (46) | 74 (42) | 0.46 |
| Anticipated breast milk diet | 187 (45) | 118 (53) | 69 (37) | 0.001 |
| Public insurance | 243 (59) | 114 (51) | 129 (69) | <0.001 |
| BPD | 279 (66) | 139 (60) | 140 (74) | 0.003 |
| Maternal education | 0.03 | |||
| Less than high school | 54 (13) | 29 (12) | 25 (13) | |
| High school graduate/some college | 206 (49) | 101 (44) | 105 (56) | |
| College graduate/graduate school | 160 (38) | 101 (44) | 59 (31) |
Data reported as mean ± SD or N (%). P-value by chi square or t-test.
BPD – bronchopulmonary dysplasia; ETS – environmental tobacco smoke; RSV – respiratory syncytial virus
Table 4.
Step-wise assessment of potential mediators between race and persistent wheezing illness
| Is race associated with the potential mediator?a | Are race and the potential mediator independently associated with persistent wheezing illness?b | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Potential mediator | ORc | 95% CI | P-value | Variable | ORd | 95% CI | P-value |
| Potential ETS exposure | 1.2 | (0.76, 2.0) | 0.40 | – | – | – | – |
|
| |||||||
| Child <5 years in home | 1.4 | (0.87, 2.1) | 0.18 | – | – | – | – |
|
| |||||||
| Eczema and/or hay fever | 1.5 | (0.95, 2.2) | 0.08 | – | – | – | – |
|
| |||||||
| RSV | 1.0 | (0.58, 1.8) | 0.99 | – | – | – | – |
|
| |||||||
| Parent history of asthma | 1.5 | (0.88, 2.5) | 0.15 | – | – | – | – |
|
| |||||||
| Furry pet in home | 0.09 | (0.05, 0.17) | <0.001 | Furry pet in home | 1.2 | (0.72, 1.9) | 0.51 |
| Black | 3.3 | (1.9, 5.5) | <0.001 | ||||
|
| |||||||
| Maternal education: college graduate/graduate school | 0.21 | (0.14, 0.36) | <0.001 | Maternal education: college graduate/graduate school | 0.82 | (0.52, 1.3) | 0.40 |
| Black | 2.7 | (1.7, 4.3) | <0.001 | ||||
|
| |||||||
| Anticipated breast milk diet | 0.37 | (0.24, 0.58) | <0.001 | Anticipated breast milk diet | 0.64 | (0.41, 0.98) | 0.04 |
| Black | 2.7 | (1.7, 4.2) | <0.001 | ||||
|
| |||||||
| Public insurance | 5.4 | (3.2, 9.1) | <0.001 | Public insurance | 1.7 | (1.0, 2.6) | 0.03 |
| Black | 2.5 | (1.5, 4.0) | <0.001 | ||||
|
| |||||||
| BPD | 0.54 | (0.36, 0.82) | 0.02 | BPD | 2.4 | (1.6, 3.8) | <0.001 |
| Black | 3.5 | (2.2, 5.6) | <0.001 | ||||
Corresponds to Step 2 from mediation analysis.
Corresponds to Step 3 from mediation analysis.
OR for black versus non-black race as a predictor of the potential mediator
ORs for potential mediator and black race when variables adjusted for each other.
All analyses adjusted for gestational age, birth weight, and sex
BPD – bronchopulmonary dysplasia; ETS – environmental tobacco smoke, RSV – respiratory syncytial virus
Table 5.
online. The effect of maternal race on persistent wheezing illness, adjusted for socioeconomic, biological, and environmental factors that did not meet criteria as mediators for the relationship.
| Variable | OR | 95% CI | P-value |
|---|---|---|---|
| ETS exposure | 1.5 | (0.89, 2.4) | 0.14 |
| Black | 2.9 | (1.8, 4.5) | <0.001 |
|
| |||
| Child <5 years in home | 1.3 | (0.82, 2.0) | 0.28 |
| Black | 2.8 | (1.7, 4.4) | <0.001 |
|
| |||
| Eczema and/or hay fever | 1.6 | (1.1, 2.4) | 0.03 |
| Black | 2.8 | (1.8, 4.4) | <0.001 |
|
| |||
| RSV | 2.0 | (1.2, 3.5) | 0.02 |
| Black | 2.9 | (1.9, 4.6) | <0.001 |
|
| |||
| Parent history of asthma | 1.7 | (0.98, 3.1) | 0.06 |
| Black | 2.8 | (1.8, 4.4) | <0.001 |
P-value by generalized estimating equation. All analyses adjusted for birth weight, sex and gestational age
ETS – environmental tobacco smoke; RSV – respiratory syncytial virus
We then tested these three variables in a mediation analysis model (Figure 1, B) to determine the percent of the relationship between maternal race and persistent wheeze explained through direct versus indirect pathways. In this model (n=410 infants with complete data), 69% of the relationship is explained directly by maternal black race (Table 6). Despite the known importance of SES and environmental exposures on wheezing disorders, only 8% of the relationship between maternal black race and persistent wheeze is mediated via breast milk diet and 12% via public insurance status. BPD explained 10% of the relationship between maternal black race and persistent wheezing illness. Interestingly, the coefficient for BPD was negative, indicating that the effect of maternal black race would be underestimated if BPD were not included in the analysis. These data are consistent with an increase in the odds ratio for black race and persistent wheezing illness after adjustment for BPD (from 2.9 to 3.5), along with the expected relationship of increased odds for BPD and persistent wheezing illness (Table 4).
Table 6.
Contribution of direct and indirect effects on the relationship of black maternal race and persistent wheezing illness
| Full cohort (N=410) | Sibling selected at random (N=366) | |||
|---|---|---|---|---|
| Coefficient, 95% CI | % mediated | Coefficient, 95% CI | % mediated | |
| Direct pathway | ||||
| Black race | 0.24, (0.12, 0.34) | 69% | 0.27, (0.15, 0.39) | 72% |
| Indirect pathways, via mediator | ||||
| Public insurance | 0.04, (−0.02, 0.10) | 12% | 0.04, (−0.01, 0.09) | 12% |
| Anticipated breast milk diet | 0.03, (0.001, 0.07) | 8% | 0.02, (−0.01, 0.06) | 6% |
| BPD | −0.04, (−0.08, −0.02) | 10% | −0.04, (−0.09, −0.02) | 10% |
All analyses adjusted for gestational age, birth weight and sex
BPD – bronchopulmonary dysplasia
Sensitivity analyses were performed to evaluate the effect of siblings; 96/420 (23%) of infants had a sibling included in the mediation analysis. When a single sibling was selected at random (n=366), changes in effects were modest (Table 6). The direct effect of maternal black race explained 72% of the relationship, and estimates of indirect effects of breast milk diet, public insurance status, and BPD were minimally altered. Similarly, when all siblings were excluded from the analysis (n=317), the direct effect of maternal black race remained at 72%.
Discussion
In this cohort of former high-risk ELGAN, we quantified the increased odds of persistent wheezing illness by 24 months corrected age for infants of black mothers, compared with infants of non-black mothers. Furthermore, we demonstrated that although breast milk diet, public insurance status, and BPD all partially mediate the relationship between maternal race and wheezing illness, a substantial proportion of the relationship is directly mediated through maternal race.
Our findings in ELGAN are consistent with previously described racial disparities in risk of wheezing disorders. In recent (2001–13) population-based US data, annual asthma prevalence among non-Hispanic black children (0–17 years) ranged from 1.3–2.1 times that of white children 6. Similarly, Gold et al found that black children (7–14 years) had increased odds (OR 1.47, 95% CI 1.25, 1.74) for persistent wheeze compared with white children7. Among preterm infants, several studies have demonstrated increased rates of wheezing illness for African American children compared with white children, although data on the extremely preterm are limited 9–13. In infants ≥32 weeks’ GA, African-American children had increased odds of recurrent wheeze by age 3 years (OR 1.39, 95% CI 1.24, 1.56), similar to findings at 1 year corrected age for moderately preterm infants 10, 13. In the current study, our adjusted odds ratio of 2.9 (95% CI 1.9, 4.5) suggests that the racial disparity we have documented is valid, even among high-risk ELGAN treated with iNO and classified into wheezing phenotypes as early as 24 months corrected age. Although we collected only maternal race in this study, relatedness of genetic ancestry is common among couples, so maternal race is likely a good surrogate for the child 25. Notably, in a prior study, there was no differential effect of iNO on bronchodilator use by maternal race in ELGAN, despite the suggestion that infants of black mothers might have less BPD following iNO therapy 26–28.
Although studies have sought to determine the relationship between socioeconomic and environmental risk factors and wheezing disorders, the interrelationships of these factors and race have not been extensively studied. Beck et al demonstrated that 49% of the relationship between black race and asthma-related hospital admission in children (1–17 years) was attributable to financial and social hardship and SES (measured by income and educational attainment) 15. Similarly, in a follow up study including data on environmental exposures, access to care, disease management, and biological disease-related factors, socioeconomic factors explained 53% of the racial disparity in admissions, with 80% of the race-related variability resolving after inclusion of these additional data 16. However, hospitalization may be more impacted by measures of lower SES for children with respiratory illness compared with other health outcomes, although data are inconsistent 29–32. Regardless, in the current study, we did not collect comprehensive data to assess SES, social stressors, and all possible environmental exposures. We did consider inclusion of maternal age and educational attainment, and a number of potential exposures that can be related to SES 20, 21, 33–36. As only public insurance status remained in our final mediation analysis, our available data and methodology may underestimate the explanatory role of social factors in the observed racial disparity. Other differences with prior studies may be due to our select study population of high-risk ELGAN, among which GA and birth weight could explain variability due to sociodemographic characteristics, as there are also racial disparities in preterm birth (Figure 1,A) 37, 38. Regardless, race consistently explains a substantial proportion of disparities observed in wheezing disorders across varied patient populations. Investigators have stated that social and environmental factors must be accounted for prior to attributing those disparities to innate biological and/or genetic differences, which remain unmeasured in many studies; some differences (e.g., lung function) have been mapped to specific genomic regions 39–42. Although we showed race directly explained about two-thirds of the relationship between race and persistent wheeze, this effect likely represents a combination of both innate genetic differences and acquired biological changes (lung structure, function and/or immunity) due to social racial disparities not specifically accounted for by our socioeconomic status variables.
Breast milk diet after discharge was negatively associated with both maternal black race and persistent wheezing illness, and met criteria as a mediator in this study. Infants continuing on breast milk after discharge likely had greater exposure to breast milk during hospitalization (usually 3–4 months) as well. Prolonged breast milk diet (≥3 months) is considered protective against childhood wheezing illness and asthma, although associations are variable 33, 34, 43. Proposed mechanisms include developmental effects on immunity and atopy. These protective effects may be greatest in atopic and young children (for which early respiratory infection is likely important). Prolonged breastfeeding is also related to socioeconomic factors, with decreased breast milk exposure associated with lower family income and maternal educational attainment, and increased rates of exposure to tobacco smoke before and after birth 33. Thus, our findings related to breast milk diet may capture both biological effects of breast milk on development of lung immunity, and the impact of socioenvironmental exposures (Figure 1, A). Exposure to pre- and postnatal tobacco smoke increases risk and severity of childhood wheezing disorders, with young children most sensitive to this exposure 20, 35, 36. In utero and postnatal smoke exposure alter lung structure and diminish function, and higher proportions of African ancestry place active smokers at greatest risk for loss of function 44–46. Although we have documented that ~25% of our population was at risk for postnatal ETS exposure, we did not assess gestational smoke exposure nor measure cotinine levels. However, other models assessing post-discharge respiratory outcomes in ELGAN did not demonstrate substantial differences in effect for reports of gestational and post-discharge smoke exposure 47. In our analysis, potential ETS exposure was not significantly associated with maternal black race, nor was it significantly associated with persistent wheezing illness after adjustment for race. This lack of effect in our cohort may reflect our broad definition of ETS or the shared socioeconomic factors influencing preterm birth and wheezing disorders; among former ELGAN with BPD, even children who do not report household smokers can have detectable cotinine levels, biasing against an effect of reported ETS in this population 20, 21, 36.
Persistent/recurrent wheeze incidence depends on its definition. In predominantly term-born infants, incidence was 25–30% at 3 years (selected for parental history of asthma/atopy), ~15% at 2 years, and ~20% at 1 year 14, 22, 48–50. However, Escobar et al recorded recurrent wheeze rates of 6.5% and 3% for late preterm and term-born infants at 3 years, using more stringent criteria, and Hibbs et al documented incidence of 46% at 1 year in moderately preterm infants 10, 13. Our rates of persistent wheeze in high-risk ELGAN at 2 years are consistent with a published meta-analysis (OR 2.5–3 for children born <32 weeks’ GA) 3. As study outcome data were by caregiver recall, there is potential for misclassification. However, caregivers can provide reliable and accurate responses related to respiratory illness in young children, with a short recall interval of one year demonstrating good agreement with medical records 51–53. Various strengths and limitations of sociodemographic variables considered in this study have been discussed, but ultimately we cannot assess the specific contribution of unmeasured characteristics and exposures due to limitations of the data that were collected, which may lead to overestimation of the direct effect of maternal race. We did not collect data about specific viral infections other than RSV, which may have differential effects by race 54. In addition, we are not able to calculate confidence intervals around the proportions of direct and indirect effects of mediation, given the limitations of binary mediation. Further, although there is likely a biological basis (differences in lung structure, function and immune function) for a proportion of the racial disparity we describe by accepted methodologies, the complex interactions of SES, social stressors, environment, and race preclude a conclusion based on genetic factors alone, particularly given the sociodemographic background of preterm birth 37, 38, 41.
In conclusion, we quantify, for the first time, a similar influence of maternal black race on persistent wheeze among former high-risk ELGAN as that previously shown for substantial prematurity alone, suggesting that black race exacerbates the risk of wheezing illness due to extreme prematurity. As ELGAN have decreased lung function compared with term-born controls, those born to black mothers are at risk for further decrements in lung function with age 55. Although we found breast milk diet and insurance status mediate the relationship between maternal race and persistent wheezing illness, a substantial proportion of the relationship is explained via the direct effect, which would be underestimated from this cohort of iNO-treated ELGAN if the diagnosis of BPD were not considered. This suggests that innate biological risk factors explain some proportion of the racial disparity observed. Future studies should better characterize and phenotype persistent wheezing illness in former preterm infants and describe the underlying innate and acquired biological and genetic causes.
Acknowledgments
We thank Karin L. Knowles, for managing the administrative and regulatory aspects of the study; Dr Carol Blaisdell from NHLBI and the NHLBI Data Safety Monitoring Board (Drs Avroy Fanaroff, Marilee Allen, Traci Clemons, Leonard Glantz, David Reboussin, and Betty Vohr) for their service; Dr Michael A. Kohn for his input and help with the statistical approach; the neonatal nurses, nurse practitioners, residents, fellows, and respiratory therapists who made this study possible; and to the families and infants who participated in the study. Ms Knowles received salary support from NHLBI for her role in conduct of TOLSURF.
TOLSURF was funded through cooperative agreements with NHLBI (U01 HL094338 and U01 HL094355). Consistent with this, Dr Carol Blaisdell, the NHLBI Scientific Officer and an employee of NHLBI was present and participated in all TOLSURF Steering Committee meetings as a non-voting member. K.W. was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI (UL1 TR000004). ONY, Inc provided Infasurf and IKARIA, Inc provided inhaled nitric oxide and its delivery system for the conduct of the TOLSURF. Neither company was involved in study design, data collection, analysis or interpretation, manuscript writing, or the decision to submit any manuscript for publication.
Abbreviations
- BPD
bronchopulmonary dysplasia
- ELGAN
extremely low gestational age newborns
- ETS
environmental tobacco smoke
- GA
gestational age
- iNO
inhaled nitric oxide
- RSV
respiratory syncytial virus
- SES
socioeconomic status
- TOLSURF
Trial of Late Surfactant
Appendix 1
Additional members of the TOLSURF Study:
UCSF Benioff Children’s Hospital, San Francisco, CA:
Suzanne Hamilton Strong RN, Jill Immamura-Ching RN, Margaret Orfanos-Villalobos RN, Cassandra Williams RN
Alta Bates Summit Medical Center, Berkeley, CA, and UCSF Benioff Children’s Hospital Oakland, Oakland, CA:
David J. Durand MD, Jeffrey D. Merrill MD, Dolia Horton RRT, Loretta Pacello RCP, April Willard RN Children’s Mercy Hospital, Kansas City, MO:
William E. Truog MD, Cheryl Gauldin RN, Anne Holmes RN, Patrice Johnson RRT, Kerrie Meinert RRT Women and Children’s Hospital of Buffalo, Buffalo, NY:
Anne Marie Reynolds MD Janine Lucie NNP, Patrick Conway, Michael Sacilowski, Michael Leadersdorff RRT, Pam Orbank RRT, Karen Wynn NNP
Anne and Robert H. Lurie Children’s Hospital/Northwestern University, Chicago, IL:
Robin H. Steinhorn MD, Maria deUngria, MD, Janine Yasmin Khan MD, Karin Hamann RN, Molly Schau RN, Brad Hopkins RRT, James Jenson RRT
Texas Children’s Hospital, Houston, TX:
Carmen Garcia RN
Stony Brook University Hospital, Stony Brook, NY:
Aruna Parekh MD, Jila Shariff MD, Rose McGovern RN, Jeff Adelman RRT, Adrienne Combs RN, Mary Tjersland RRT
University of Washington, Seattle, WA:
Dennis E. Mayock MD, Elizabeth Howland, Susan Walker RN, Jim Longoria RRT, Holly Meo RRT University of Texas Health Science Center, Houston, TX:
Amir Khan MD, Georgia McDavid RN, Katrina Burson RN BSN, Richard Hinojosa BSRT RRT, Christopher Johnson MBA RRT, Karen Martin RN BSN, Sarah Martin RN BSN, Shawna Rogers RN BSN, Sharon Wright, MT
University of Florida College of Medicine, Jacksonville, UF Health Shands Hospital, and Wolfson Children’s Hospital, Jacksonville, FL:
Mark L. Hudak MD, Kimberly Barnette RRT, Amanda Kellum RRT, Michelle Burcke RN, Christie Hayes RRT, Stephanie Chadwick RN, Danielle Howard RN, Carla Kennedy RRT, Renee Prince RN
Wake Forest School of Medicine and Forsyth Medical Center, Winston Salem, NC:
Jennifer Helderman MD, T. Michael O’Shea MD, Beatrice Stefanescu MD, Kelly Warden RN, Patty Brown RN, Jennifer Griffin RRT, Laura Conley RRT
University of Minnesota Amplatz Children’s Hospital, Minneapolis, MN:
Catherine M. Bendel MD, Michael Georgieff MD, Bridget Davern, Marla Mills RN, Sharon Ritter RRT
Medical University of South Carolina, Charleston, SC:
Carol Wagner MD, Rita M. Ryan MD, Deanna Fanning RN, Jimmy Roberson RRT
Children’s Hospitals and Clinics of Minnesota, St. Paul, MN:
Mark C. Mammel MD, Andrea Lampland MD, Pat Meyers RRT, Angela Brey RRT
Children’s Hospitals and Clinics of Minnesota, Minneapolis, MN:
Ellen M. Bendel-Stenzel MD, Neil Mulrooney MD, Cathy Worwa RRT, Pam Dixon RN, ANM, Gerald Ebert RRT-NPS, Cathy Hejl RRT, Molly Maxwell RT, Kristin McCullough, RN
University of Tennessee Health Science Center, Memphis, TN:
Ramasubbareddy Dhanireddy MD, Mohammed T. El Abiad MD, Ajay Talati MD, Sheila Dempsey RN, Kathy Gammage RRT MBA, Gayle Gower RN, Kathy James RRT, Pam LeNoue RN
All Children’s Hospital, St. Petersburg, FL:
Victor J. McKay MD, Suzi Bell DNP, Dawn Bruton RN BSN CCRP, Michelle Beaulieu DNP, Richard Williams RRT
Florida Hospital for Children, Orlando, FL:
Rajan Wadhawan MD, Robin Barron-Nelson RN, Shane Taylor RRT
Arkansas Children’s Hospital and University of Arkansas Medical Sciences, Little Rock, AK:
Sherry E. Courtney MD, Carol Sikes RN, Gary Lowe RRT, Betty Proffitt RRT
Clinical Coordinating Center:
University of California San Francisco, Department of Pediatrics:
Elizabeth E. Rogers MD, Cheryl Chapin, Hart Horneman, Karin Hamann RN, Susan Kelley RRT, Karin Knowles, Nancy Newton RN MS
Data Coordinating Center:
University of California San Francisco, Department of Epidemiology and Biostatistics:
Eric Vittinghoff PhD, Jean Hietpas, Laurie Denton, Lisa Palermo MS, Lucy Wu
Appendix 2
TOLSURF
BREATHING OUTCOME QUESTIONNAIRE
This information to be collected from parent/guardian at time of discharge from study hospital.
-
1
Information received from (list primary caregiver): (select one)
Mother Father Grandparent Foster Parent Other -
2
How many people normally live in your home including your baby (for at least 6 months of the year)? (select one)
2 – 3 4 – 6 7 – 10 > 10 -
2.a
Are there any children < 5 years of age (other than your baby) that live in the home?
None 1 – 2 3 – 5 6 – 8 > 8 -
3
Do you have any pets? (select all that apply)
None Dog Cat Other furry animals Fish Birds Other -
4Infant feeds: (select one)
- Breast milk only
- Formula only
- Breast milk and formula
- No enteral feedings
- Other
-
5
Will your child receive any care outside the home in the next year?
Yes No Unknown -
5.a
Who will provide care?(select all that apply)
Relatives Daycare Friends Other -
5.b
Will other children that are not siblings be present at outside care site?
Yes No Unknown -
6
Please describe the situation regarding smoking in your child’s home:
-
6.aWhich one of the following statements best describes the situation regarding smoking in your child’s home? (select one)
- Smoking is allowed in any room in the home
- Smoking is limited to part of the house where the child will rarely go
- Occasionally there is smoking inside the house (visitor, family member)
- There is no smoking inside the house at all
- Other
-
6.b
Does either parent smoke?
Yes No Unknown -
6.b.i
Estimated number of cigarettes per day:
< 5 5 – 10 11 – 20 > 1 pack/day Unknown -
6.c
All together, how many people who live in the home smoke?
None 1 – 2 > 2 Unknown -
6.d
Will your child travel regularly (at least once a week) in a vehicle (car or truck) that someone smokes in, even when the child is not in the car?
Yes No Unknown -
7
Please tell us what breathing and allergy problems run in the family.
-
7.aBiological parents - one or both: (select all that apply)
- Asthma/recurrent lung infections
- Allergies (allergies/hayfever)
- Medication allergies
- Eczema
- Other
-
7.bGrandparents - one or both: (select all that apply)
- Asthma/recurrent lung infections
- Allergies (allergies/hayfever)
- Medication allergies
- Eczema
- Other
-
7.cSiblings - one or both: (select all that apply)
- Asthma/recurrent lung infections
- Allergies (allergies/hayfever)
- Medication allergies
- Eczema
- Other
-
8
Please tell us more about your baby’s background:
-
8.aMaternal education: (select one)
- Some education, High School not completed
- High School graduate
- Some College
- College graduate
- Graduate study
- Unknown/Unavailable
-
8.bPaternal education: (select one)
- Some education, High School not completed
- High School graduate
- Some College
- College graduate
- Graduate study
- Unknown/Unavailable
-
8.c
How will your child’s health care be paid for?
Private Insurance Medicaid/Public No Insurance (self pay)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
Portions of this study were presented as an abstract at the Pediatric Academic Societies annual meeting, May 6–9, San Francisco, California.
Trial Registration: ClinicalTrials.gov NCT01022580.
References
- 1.Harju M, Keski-Nisula L, Georgiadis L, Raisanen S, Gissler M, Heinonen S. The burden of childhood asthma and late preterm and early term births. J Pediatr. 2014;164:295–9.e1. doi: 10.1016/j.jpeds.2013.09.057. [DOI] [PubMed] [Google Scholar]
- 2.Astle V, Broom M, Todd DA, Charles B, Ringland C, Ciszek K, et al. Respiratory outcomes study (RESPOS) for preterm infants at primary school age. J Asthma. 2015;52:40–5. doi: 10.3109/02770903.2014.952436. [DOI] [PubMed] [Google Scholar]
- 3.Been JV, Lugtenberg MJ, Smets E, van Schayck CP, Kramer BW, Mommers M, et al. Preterm birth and childhood wheezing disorders: a systematic review and meta-analysis. PLoS Med. 2014;11:e1001596. doi: 10.1371/journal.pmed.1001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards MO, Kotecha SJ, Lowe J, Richards L, Watkins WJ, Kotecha S. Management of Prematurity-Associated Wheeze and Its Association with Atopy. PLoS One. 2016;11:e0155695. doi: 10.1371/journal.pone.0155695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Escobar GJ, Masaquel AS, Li SX, Walsh EM, Kipnis P. Persistent recurring wheezing in the fifth year of life after laboratory-confirmed, medically attended respiratory syncytial virus infection in infancy. BMC Pediatr. 2013;13:97. doi: 10.1186/1471-2431-13-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akinbami LJ, Simon AE, Rossen LM. Changing Trends in Asthma Prevalence Among Children. Pediatrics. 2016;137 doi: 10.1542/peds.2015-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gold DR, Rotnitzky A, Damokosh AI, Ware JH, Speizer FE, Ferris BG, Jr, et al. Race and gender differences in respiratory illness prevalence and their relationship to environmental exposures in children 7 to 14 years of age. Am Rev Respir Dis. 1993;148:10–8. doi: 10.1164/ajrccm/148.1.10. [DOI] [PubMed] [Google Scholar]
- 8.Islam JY, Keller RL, Aschner JL, Hartert TV, Moore PE. Understanding the Short- and Long-Term Respiratory Outcomes of Prematurity and Bronchopulmonary Dysplasia. Am J Respir Crit Care Med. 2015;192:134–56. doi: 10.1164/rccm.201412-2142PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar R, Yu Y, Story RE, Pongracic JA, Gupta R, Pearson C, et al. Prematurity, chorioamnionitis, and the development of recurrent wheezing: a prospective birth cohort study. J Allergy Clin Immunol. 2008;121:878–84.e6. doi: 10.1016/j.jaci.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Escobar GJ, Ragins A, Li SX, Prager L, Masaquel AS, Kipnis P. Recurrent wheezing in the third year of life among children born at 32 weeks’ gestation or later: relationship to laboratory-confirmed, medically attended infection with respiratory syncytial virus during the first year of life. Arch Pediatr Adolesc Med. 2010;164:915–22. doi: 10.1001/archpediatrics.2010.177. [DOI] [PubMed] [Google Scholar]
- 11.Robison RG, Kumar R, Arguelles LM, Hong X, Wang G, Apollon S, et al. Maternal smoking during pregnancy, prematurity and recurrent wheezing in early childhood. Pediatr Pulmonol. 2012;47:666–73. doi: 10.1002/ppul.22501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strickland MJ, Klein M, Flanders WD, Chang HH, Mulholland JA, Tolbert PE, et al. Modification of the effect of ambient air pollution on pediatric asthma emergency visits: susceptible subpopulations. Epidemiology. 2014;25:843–50. doi: 10.1097/EDE.0000000000000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hibbs AM, Babineau DC, Wang X, Redline S. Race differences in the association between multivitamin exposure and wheezing in preterm infants. J Perinatol. 2015;35:192–7. doi: 10.1038/jp.2014.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taveras EM, Camargo CA, Jr, Rifas-Shiman SL, Oken E, Gold DR, Weiss ST, et al. Association of birth weight with asthma-related outcomes at age 2 years. Pediatr Pulmonol. 2006;41:643–8. doi: 10.1002/ppul.20427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beck AF, Huang B, Simmons JM, Moncrief T, Sauers HS, Chen C, et al. Role of financial and social hardships in asthma racial disparities. Pediatrics. 2014;133:431–9. doi: 10.1542/peds.2013-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beck AF, Huang B, Auger KA, Ryan PH, Chen C, Kahn RS. Explaining Racial Disparities in Child Asthma Readmission Using a Causal Inference Approach. JAMA Pediatr. 2016;170:695–703. doi: 10.1001/jamapediatrics.2016.0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ballard RA, Keller RL, Black DM, Ballard PL, Merrill JD, Eichenwald EC, et al. Randomized Trial of Late Surfactant Treatment in Ventilated Preterm Infants Receiving Inhaled Nitric Oxide. J Pediatr. 2016;168:23–9.e4. doi: 10.1016/j.jpeds.2015.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wai KC, Kohn MA, Ballard RA, Truog WE, Black DM, Asselin JM, et al. Early Cumulative Supplemental Oxygen Predicts Bronchopulmonary Dysplasia in High Risk Extremely Low Gestational Age Newborns. J Pediatr. 2016;177:97–102.e2. doi: 10.1016/j.jpeds.2016.06.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keller RL, Eichenwald EC, Hibbs AM, Rogers EE, Wai KC, Black DM, et al. The Randomized, Controlled Trial of Late Surfactant: Effects on Respiratory Outcomes at 1-Year Corrected Age. J Pediatr. 2017;183:19–25.e2. doi: 10.1016/j.jpeds.2016.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wakefield M, Banham D, Martin J, Ruffin R, McCaul K, Badcock N. Restrictions on smoking at home and urinary cotinine levels among children with asthma. Am J Prev Med. 2000;19:188–92. doi: 10.1016/s0749-3797(00)00197-5. [DOI] [PubMed] [Google Scholar]
- 21.Collaco JM, Aherrera AD, Breysse PN, Winickoff JP, Klein JD, McGrath-Morrow SA. Hair nicotine levels in children with bronchopulmonary dysplasia. Pediatrics. 2015;135:e678–86. doi: 10.1542/peds.2014-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Litonjua AA, Carey VJ, Laranjo N, Harshfield BJ, McElrath TF, O’Connor GT, et al. Effect of Prenatal Supplementation With Vitamin D on Asthma or Recurrent Wheezing in Offspring by Age 3 Years: The VDAART Randomized Clinical Trial. JAMA. 2016;315:362–70. doi: 10.1001/jama.2015.18589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–82. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 24.Ehrenkranz RA, Das A, Wrage LA, Poindexter BB, Higgins RD, Stoll BJ, et al. Early nutrition mediates the influence of severity of illness on extremely LBW infants. Pediatr Res. 2011;69:522–9. doi: 10.1203/PDR.0b013e318217f4f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zou JY, Park DS, Burchard EG, Torgerson DG, Pino-Yanes M, Song YS, et al. Genetic and socioeconomic study of mate choice in Latinos reveals novel assortment patterns. Proc Natl Acad Sci U S A. 2015;112:13621–6. doi: 10.1073/pnas.1501741112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ballard RA, Truog WE, Cnaan A, Martin RJ, Ballard PL, Merrill JD, et al. Inhaled Nitric Oxide in Preterm Infants Undergoing Mechanical Ventilation. N Engl J Med. 2006;355:343–53. doi: 10.1056/NEJMoa061088. [DOI] [PubMed] [Google Scholar]
- 27.Ballard RA. Inhaled nitric oxide in preterm infants–correction. N Engl J Med. 2007;357:1444–5. doi: 10.1056/NEJMc076350. [DOI] [PubMed] [Google Scholar]
- 28.Hibbs AM, Walsh MC, Martin RJ, Truog WE, Lorch SA, Alessandrini E, et al. One-year respiratory outcomes of preterm infants enrolled in the Nitric Oxide (to prevent) Chronic Lung Disease trial. J Pediatr. 2008;153:525–9. doi: 10.1016/j.jpeds.2008.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wynne J, Hull D. Why are children admitted to hospital? Br Med J. 1977;2:1140–2. doi: 10.1136/bmj.2.6095.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watson JP, Cowen P, Lewis RA. The relationship between asthma admission rates, routes of admission, and socioeconomic deprivation. Eur Respir J. 1996;9:2087–93. doi: 10.1183/09031936.96.09102087. [DOI] [PubMed] [Google Scholar]
- 31.Alpern ER, Stanley RM, Gorelick MH, Donaldson A, Knight S, Teach SJ, et al. Epidemiology of a pediatric emergency medicine research network: the PECARN Core Data Project. Pediatr Emerg Care. 2006;22:689–99. doi: 10.1097/01.pec.0000236830.39194.c0. [DOI] [PubMed] [Google Scholar]
- 32.Franklin JA, Anderson EJ, Wu X, Ambrose CS, Simoes EA. Insurance Status and the Risk of Severe Respiratory Syncytial Virus Disease in United States Preterm Infants Born at 32–35 Weeks Gestational Age. Open Forum Infect Dis. 2016;3:ofw163. doi: 10.1093/ofid/ofw163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dell S, To T. Breastfeeding and asthma in young children: findings from a population-based study. Arch Pediatr Adolesc Med. 2001;155:1261–5. doi: 10.1001/archpedi.155.11.1261. [DOI] [PubMed] [Google Scholar]
- 34.Gdalevich M, Mimouni D, Mimouni M. Breast-feeding and the risk of bronchial asthma in childhood: a systematic review with meta-analysis of prospective studies. J Pediatr. 2001;139:261–6. doi: 10.1067/mpd.2001.117006. [DOI] [PubMed] [Google Scholar]
- 35.Akinbami LJ, Kit BK, Simon AE. Impact of environmental tobacco smoke on children with asthma, United States, 2003–2010. Acad Pediatr. 2013;13:508–16. doi: 10.1016/j.acap.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Howrylak JA, Spanier AJ, Huang B, Peake RW, Kellogg MD, Sauers H, et al. Cotinine in children admitted for asthma and readmission. Pediatrics. 2014;133:e355–62. doi: 10.1542/peds.2013-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeFranco E, Moravec W, Xu F, Hall E, Hossain M, Haynes EN, et al. Exposure to airborne particulate matter during pregnancy is associated with preterm birth: a population-based cohort study. Environ Health. 2016;15:6. doi: 10.1186/s12940-016-0094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Collins JW, Jr, Rankin KM, David RJ. African American women’s lifetime upward economic mobility and preterm birth: the effect of fetal programming. Am J Public Health. 2011;101:714–9. doi: 10.2105/AJPH.2010.195024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burchard EG, Silverman EK, Rosenwasser LJ, Borish L, Yandava C, Pillari A, et al. Association between a sequence variant in the IL-4 gene promoter and FEV(1) in asthma. Am J Respir Crit Care Med. 1999;160:919–22. doi: 10.1164/ajrccm.160.3.9812024. [DOI] [PubMed] [Google Scholar]
- 40.Burchard EG, Ziv E, Coyle N, Gomez SL, Tang H, Karter AJ, et al. The importance of race and ethnic background in biomedical research and clinical practice. N Engl J Med. 2003;348:1170–5. doi: 10.1056/NEJMsb025007. [DOI] [PubMed] [Google Scholar]
- 41.Cheng TL, Goodman E. Race, ethnicity, and socioeconomic status in research on child health. Pediatrics. 2015;135:e225–37. doi: 10.1542/peds.2014-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hernandez-Pacheco N, Flores C, Oh SS, Burchard EG, Pino-Yanes M. What Ancestry Can Tell Us About the Genetic Origins of Inter-Ethnic Differences in Asthma Expression. Curr Allergy Asthma Rep. 2016;16:53. doi: 10.1007/s11882-016-0635-4. [DOI] [PubMed] [Google Scholar]
- 43.Wright AL, Holberg CJ, Taussig LM, Martinez FD. Relationship of infant feeding to recurrent wheezing at age 6 years. Arch Pediatr Adolesc Med. 1995;149:758–63. doi: 10.1001/archpedi.1995.02170200048006. [DOI] [PubMed] [Google Scholar]
- 44.Stocks J, Dezateux C. The effect of parental smoking on lung function and development during infancy. Respirology. 2003;8:266–85. doi: 10.1046/j.1440-1843.2003.00478.x. [DOI] [PubMed] [Google Scholar]
- 45.Aldrich MC, Kumar R, Colangelo LA, Williams LK, Sen S, Kritchevsky SB, et al. Genetic ancestry-smoking interactions and lung function in African Americans: a cohort study. PLoS One. 2012;7:e39541. doi: 10.1371/journal.pone.0039541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McEvoy CT, Schilling D, Clay N, Jackson K, Go MD, Spitale P, et al. Vitamin C supplementation for pregnant smoking women and pulmonary function in their newborn infants: a randomized clinical trial. JAMA. 2014;311:2074–82. doi: 10.1001/jama.2014.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keller RL, Feng R, DeMauro SB, Ferkol T, Hardie W, Rogers EE, et al. Bronchopulmonary Dysplasia and Perinatal Characteristics Predict 1-Year Respiratory Outcomes in Newborns Born at Extremely Low Gestational Age: A Prospective Cohort Study. J Pediatr. 2017;187:89–97.e3. doi: 10.1016/j.jpeds.2017.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gold DR, Burge HA, Carey V, Milton DK, Platts-Mills T, Weiss ST. Predictors of repeated wheeze in the first year of life: the relative roles of cockroach, birth weight, acute lower respiratory illness, and maternal smoking. Am J Respir Crit Care Med. 1999;160:227–36. doi: 10.1164/ajrccm.160.1.9807104. [DOI] [PubMed] [Google Scholar]
- 49.Ly NP, Gold DR, Weiss ST, Celedon JC. Recurrent wheeze in early childhood and asthma among children at risk for atopy. Pediatrics. 2006;117:e1132–8. doi: 10.1542/peds.2005-2271. [DOI] [PubMed] [Google Scholar]
- 50.Mallol J, Garcia-Marcos L, Sole D, Brand P. International prevalence of recurrent wheezing during the first year of life: variability, treatment patterns and use of health resources. Thorax. 2010;65:1004–9. doi: 10.1136/thx.2009.115188. [DOI] [PubMed] [Google Scholar]
- 51.Pless CE, Pless IB. How well they remember. The accuracy of parent reports. Arch Pediatr Adolesc Med. 1995;149:553–8. doi: 10.1001/archpedi.1995.02170180083016. [DOI] [PubMed] [Google Scholar]
- 52.Boggs E, Minich N, Hibbs AM. Performance of commonly used respiratory questionnaire items in a cohort of infants born preterm. Open J Pediatr. 2013;3:260–5. doi: 10.4236/ojped.2013.33045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.D’Souza-Vazirani D, Minkovitz CS, Strobino DM. Validity of maternal report of acute health care use for children younger than 3 years. Arch Pediatr Adolesc Med. 2005;159:167–72. doi: 10.1001/archpedi.159.2.167. [DOI] [PubMed] [Google Scholar]
- 54.Torgerson DG, Giri T, Druley TE, Zheng J, Huntsman S, Seibold MA, et al. Pooled Sequencing of Candidate Genes Implicates Rare Variants in the Development of Asthma Following Severe RSV Bronchiolitis in Infancy. PLoS One. 2015;10:e014264. doi: 10.1371/journal.pone.0142649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kotecha SJ, Edwards MO, Watkins WJ, Henderson AJ, Paranjothy S, Dunstan FD, et al. Effect of preterm birth on later FEV1: a systematic review and meta-analysis. Thorax. 2013;68:760–6. doi: 10.1136/thoraxjnl-2012-203079. [DOI] [PubMed] [Google Scholar]


