Abstract
Objectives
To determine whether infant cases with craniofacial microsomia (CFM) evidence poorer neurodevelopmental status than demographically-similar infants without craniofacial diagnoses (“controls”), and to examine cases’ neurodevelopmental outcomes by facial phenotype and hearing status.
Study design
Multi-center, observational study of 108 cases and 84 controls aged 12 to 24 months. Participants were assessed by the Bayley Scales of Infant and Toddler Development-Third Edition (Bayley-III) and the Preschool Language Scales, Fifth Edition (PLS-5). Facial features were classified with the Phenotypic Assessment Tool for Craniofacial Microsomia.
Results
After adjustment for demographic variables, there was little difference in Bayley-III or PLS-5 outcomes between cases and controls. Estimates of mean differences ranged from −0.23 to 1.79 corresponding to standardized effect sizes of −0.02 to 0.12 (P values from .30 to .88). Outcomes were better among females and those with higher socioeconomic status. Among cases, facial phenotype and hearing status showed little to no association with outcomes. Analysis of individual test scores indicated that 21% of cases and 16% of controls were developmentally delayed (OR=0.68, 95% CI 0.29 to 1.61).
Conclusions
Although learning problems have been observed in older children with CFM, we found no evidence of developmental or language delay among infants. Variation in outcomes across prior studies may reflect differences in ascertainment methods and CFM diagnostic criteria.
Keywords: hemifacial, neurocognitive, facial phenotype
Craniofacial microsomia (CFM), also known as hemifacial microsomia, is a complex congenital condition typically involving underdevelopment of the mandible and ear (1, 2). CFM occurs in approximately 1 in 3,500 to 5,600 live births (3), with higher prevalence among Hispanic and Native American families (4, 5). CFM has been characterized as a spectrum of phenotypic anomalies ranging from isolated unilateral microtia to bilateral malformations of the ear, mandible, facial soft tissue, and orbit (2). Other cranial and extra-cranial malformations may co-occur (eg, lateral oral clefts, vertebral anomalies, cardiac defects).
Among the several functional problems associated with CFM (2), neurodevelopmental delays are perhaps the least understood and most difficult to recognize clinically. Although such deficits can strongly affect children’s quality of life (6, 7), their impact can be mitigated by early evaluation and intervention (8, 9). The few existing studies of neurodevelopment in the CFM population, mostly involving older children, have suggested that severe intellectual disability is rare, but mild to moderate neurodevelopmental delays and learning problems may occur at higher rates than those found in the general population (10–13). Given the heterogeneity of the CFM phenotype (2), and the theorized embryological mechanisms underlying the development of face and brain (14), it is possible that neurodevelopmental outcomes may vary in relation to the severity and pattern of anomalies observed in this condition (eg, microtia with or without microphthalmia or vertebral defects). Although previous investigations have examined neurodevelopmental outcomes by one or more selected features (e.g., presence or absence of extracranial anomalies) (10, 11), a consistent pattern of association between phenotype and developmental outcomes has yet to emerge.
Based on the findings from previous neurodevelopmental studies, we hypothesized that infants with CFM would show lower test scores on average and a higher frequency of developmental delay than controls. In secondary analyses, we examined neurodevelopmental outcomes by (a) facial phenotype using a modified version of the pictorial Orbit, Mandible, Ear, Nerve, and Soft tissue (OMENS) scoring system, which includes ratings for orbital asymmetry, mandibular hypoplasia, ear anomalies, facial nerve involvement, and soft-tissue deficiency (15, 16) and (b) the presence and severity of hearing impairment. We also estimated the effects of relevant covariates on neurodevelopmental status, including sex, socioeconomic status (SES), ethnicity, and bilingual home environment.
METHODS
Infants between the ages of 12 and 24 months were recruited to participate in an ongoing observational, longitudinal, multi-center project called Craniofacial Microsomia: Longitudinal Outcomes in Children Pre-Kindergarten (CLOCK), which tracks the neurodevelopmental, speech and hearing outcomes, and phenotypic features of infants with and without CFM (“cases” and “controls,” respectively). Participants were enrolled between 2012 and 2017 from one of 6 craniofacial centers: Children’s Hospital of Los Angeles (CHLA), Children’s Hospital of Philadelphia (CHOP), Seattle Children’s Hospital (SCH), University of Illinois-Chicago (UIC, including Shriners Hospital for Children, Chicago), and University of North Carolina (UNC) at Chapel Hill. This research was approved by the institutional review boards at all participating centers. All parents gave informed consent for their infant to participate in the study.
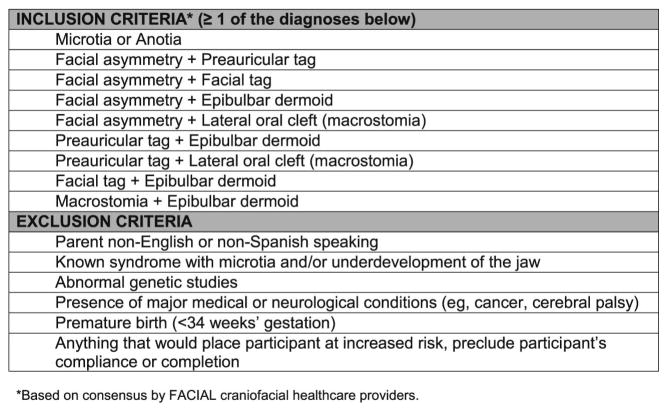
Cases were recruited from each site’s hospital-based craniofacial centers, hospital-based clinics seeing infants or young children with CFM (e.g. hearing screening programs, ENT programs), and research study websites (eg, https://www.clinicaltrials.gov). To be eligible, cases had to have (1) at least one of the CFM inclusion criteria developed by the Facial Asymmetry Collaborative for Interdisciplinary Analysis and Learning (FACIAL) network (Figure; available at www.jpeds.com); (2) an age between 12–24 months (corrected for prematurity, when applicable, for children born between 34–36 weeks’ gestational age); and (3) a legal guardian who was able to provide informed written consent and be willing to participate for the duration of the study. Exclusion criteria for cases included: (1) diagnosis of a known syndrome (eg, Townes-Brocks, Treacher Collins, branchio-oto-renal, or Nager syndromes); (2) presence of an abnormal karyotype or major medical or neurological conditions (e.g., cancer, cerebral palsy); (3) premature birth (less than 34 weeks’ gestation); (4) any circumstance that would preclude the family’s ability to participate fully in the research; (5) a sibling already participating in the CLOCK study, or (6) a consenting parent who did not speak English or Spanish. Of the 219 potentially eligible cases that were approached, 108 (49%) were enrolled. Most non-participating families declined passively by not responding to study invitations.
Figure 1.
Eligibility Criteria for the CLOCK study
We identified eligible participants with demographic characteristics that met our frequency–matching criteria for the case cohort; these included infant age and sex, family SES, and language spoken in the home (English or Spanish). Exclusion criteria for controls included: (1) meeting one or more of the exclusionary criteria for cases; and (2) diagnosis or history of any disorder, condition, or injury that would affect facial features (e.g., craniofacial malformation or deformation; facial surgery or trauma). Of the 148 potentially eligible controls that were approached, 84 (57%) were enrolled.
Measures
We used the Bayley Scales of Infant and Toddler Development–Third Edition (Bayley-III) to assess cognitive and motor skills (17). The Bayley-III yields composite scores for both cognition and motor ability and subscale scores for fine and gross motor development. Raw scores are converted to norm-referenced standard scores (mean = 100, SD = 15) for composite scales and scaled scores (mean = 10, SD = 3) for the motor subscales. The Preschool Language Scale–Fifth Edition (PLS-5) was used to assess expressive and receptive language, using either the English or Spanish version of this norm-referenced, validated test (18). We selected the PLS-5 over the Bayley-III language scales as the latter are available only in English, and the Spanish version of the PLS-5 includes culturally relevant items with norms from a large sample of monolingual and bilingual Spanish-speaking children (19). The PLS-5 yields two scale scores, auditory comprehension (PLS-AC) and expressive communication (PLS-EC), as well as a total language score (PLS-TL), all based on a combination of child performance, examiner observations, and caregiver reports.
For both tests, gestational age was calculated using family report of due date and birth date. We corrected Bayley-III scores for prematurity for children born between 34 and 36 weeks’ gestation. Testing was completed in English (69%), Spanish (13%), or a combination of English and Spanish (18%) determined by families’ reports of their language use across settings, as well as examiners’ observations of participants’ language use during the study visit. Trained bilingual psychometrists used verbal Bayley-III prompts that were translated into Spanish for consistent use across sites. The PLS-5 has standardized versions in both English and Spanish. All assessments were videotaped and about 20% were double-scored for reliability by three of the authors (BC, KKS, and MS). Average level of agreement for item-by-item scoring was 97%, with agreement levels for single test administrations ranging from 70% to 100%.
Cases’ hearing status was based primarily on audiological information obtained as part of routine clinical care. Ninety-one (84%) of cases had such data available for review. Infants with audiology data were considered to have hearing loss when they demonstrated a greater than 20 decibel Pure Tone Average (PTA) over 4 frequencies (500, 1000, 20000, 4000 Hz; PTAs were calculated if at least 3 frequencies were present). For those cases without audiograms, hearing status was based on the absence of the external ear canal (i.e. aural atresia) or external ear canal stenosis, both of which result in conductive hearing loss. In a prior investigation, 98% of children with aural atresia or external ear canal stenosis had PTA of > 20 dB hearing loss (20). This allowed us to create the following categories of hearing status for all cases: (1) “No hearing loss” was defined as the absence of atresia and a negative audiometric finding; (2) “Unilateral hearing loss” referred to single-sided hearing loss based on audiometry or the presence of unilateral atresia; and (3) “Bilateral hearing loss” was assigned to cases meeting at least one of the following criteria: (a) presence of bilateral atresia, (b) audiological evidence of hearing loss in both ears, and/or (c) the presence of unilateral atresia plus audiological evidence of hearing loss in the contralateral ear.
Phenotypic classifications were based on the integration of data collected from a health history interview with parent, medical record abstraction, and analysis of multiple craniofacial photographs taken of each participant. A modified version of the Phenotypic Assessment Tool for Craniofacial Microsomia (PAT-CFM (21–22)) was used by two craniofacial dysmorphologists (CH and DL) to classify the presence/absence and severity of facial features associated with CFM (for details see Luquetti et al (23)). Both phenotype coders were blinded to neurodevelopmental test scores. We categorized the presence of microtia according to the classification scheme developed by Marx and modified by Rogers (24,25). This is the classification used in the modified pictorial OMENS form and one of the most frequently used systems(16). Distinguishing the degree of mandibular and/or facial soft tissue deficiency on photographs is challenging and the two features are often associated (26, 27). Therefore, we used the term “mandibular hypoplasia” to include the presence of hypoplasia of either the facial soft tissue or mandible.
Three phenotypic subgroups were identified: 1) microtia only (in the absence of mandibular hypoplasia, epibulbar dermoids, lateral clefts, preauricular or facial tags, small and/or displaced orbit, nerve palsies); 2) microtia and mandibular hypoplasia; and 3) other combinations of CFM-associated malformations (two or more were required). We also developed phenotypic groups based on the laterality of features (e.g., right unilateral microtia plus left facial tag), the laterality of external ear involvement, the maximum grade of ear involvement, and the presence of extracranial anomalies (e.g., congenital heart defect).
Statistical Analyses
Group differences in neurodevelopment were estimated using linear regression with robust standard error estimates. Controls served as the referent for all analyses. Estimates were adjusted for age of the assessment (continuous), sex (male vs. female), SES (continuous), study site (categorical), race (white vs. non-white), and testing language (any Spanish vs. exclusively English). We also examined characteristics of cases and controls by the presence/absence of neurodevelopmental delay, defined as >1 standard deviation below the normative test mean for any one or more of the following: total PLS-5 scores and Bayley-III cognitive and motor scale composite scores. Logistic regression was used to estimate the odds of delay and adjusted for all demographic variables included in the primary analyses. We also conducted 2 post hoc sensitivity analyses: (1) excluding CHLA (which tested predominantly in Spanish) and (2) restricted to participants who had assessments in English only.
We conducted several secondary analyses to evaluate differences by cases’ phenotype, using controls as the referent category and adjusting for the same demographic factors as those listed above. We estimated differences across three categories of phenotype (microtia only, microtia plus mandibular hypoplasia, and other CFM-associated features), by overall laterality of features (unilateral or bilateral), by external ear involvement (unilateral or bilateral, excluding cases with no ear involvement), by grade of external ear involvement (0–2 and 3–4), and by the presence or absence of extracranial anomalies. We also evaluated whether cases’ neurodevelopmental outcomes differed by hearing status (no hearing loss, unilateral hearing loss, and bilateral hearing loss).
RESULTS
Demographic characteristics of cases and controls are provided in Table I. Cases were more likely than controls to be male, to be Hispanic or Latino, and to undergo their assessment in Spanish or a combination of Spanish and English. There was no evidence for differences between cases and controls in PLS-5 or Bayley-III outcomes after adjustment for demographic confounding variables. Estimates of mean differences from the linear regression models were small, ranging from −0.23 to 1.79 (p-values ranged from 0.30 to 0.88) (Table 2). Examination of covariates in the adjusted models revealed that outcome scores were higher in females than males and with increasing SES. PLS-5 and Bayley-III cognitive scores were also higher among infants from families who spoke some Spanish relative to those who spoke English exclusively. There was also evidence for differences by study site, with SCH participants consistently scoring higher and UIC participants consistently scoring lower compared with CHLA participants.
Table 1.
Characteristics of infants with and without craniofacial microsomia
| Characteristic | Cases | Controls | ||
|---|---|---|---|---|
| N | % | N | % | |
| Total | 108 | 100.0 | 84 | 100.0 |
| Sex | ||||
| Male | 67 | 62.0 | 37 | 44.0 |
| Female | 41 | 38.0 | 47 | 56.0 |
| Age at visit | ||||
| Mean, SD | 14.5 | 2.8 | 14.4 | 2.7 |
| <13 months | 32 | 29.6 | 32 | 38.1 |
| 13–14 months | 41 | 38.0 | 26 | 31.0 |
| >14 months | 35 | 32.4 | 26 | 31.0 |
| Gestational age | ||||
| Mean, SD | 39.0 | 1.5 | 39.5 | 1.4 |
| 34–37 weeks | 13 | 12.0 | 11 | 13.1 |
| >37–40 weeks | 73 | 67.6 | 48 | 57.1 |
| >40 weeks | 22 | 20.4 | 25 | 29.8 |
| SES* | ||||
| Mean, SD | 35.2 | 14.2 | 37.5 | 15.0 |
| I | 16 | 14.8 | 15 | 17.9 |
| II | 20 | 18.5 | 17 | 20.2 |
| III | 28 | 25.9 | 23 | 27.4 |
| IV | 29 | 26.9 | 19 | 22.6 |
| V | 15 | 13.9 | 9 | 10.7 |
| Hispanic or Latino* | ||||
| No | 49 | 45.4 | 51 | 60.7 |
| Yes | 59 | 54.6 | 31 | 36.9 |
| Race* | ||||
| White | 80 | 74.1 | 48 | 57.1 |
| Black/African American | 3 | 2.8 | 5 | 6.0 |
| Asian | 7 | 6.5 | 0 | 0.0 |
| American Indian/AK Native | 3 | 2.8 | 3 | 3.6 |
| Other race | 3 | 2.8 | 4 | 4.8 |
| Multiracial | 10 | 9.3 | 23 | 27.4 |
| Insurance | ||||
| Private | 50 | 46.3 | 44 | 52.4 |
| Medicaid | 58 | 53.7 | 40 | 47.6 |
| Testing language (PDP) | ||||
| 100% English | 69 | 63.9 | 63 | 75.0 |
| 100% Spanish | 19 | 17.6 | 7 | 8.3 |
| Combination English/Spanish | 20 | 18.5 | 14 | 16.7 |
| Study site | ||||
| CHLA | 40 | 37.0 | 8 | 9.5 |
| CHOP | 3 | 2.8 | 6 | 7.1 |
| SCH | 38 | 35.2 | 57 | 67.9 |
| UNC | 16 | 14.8 | 7 | 8.3 |
| UIC | 11 | 10.2 | 6 | 7.1 |
| Phenotype | ||||
| Microtia only | 30 | 27.8 | 0 | 0.0 |
| Microtia + Mandibular hypoplasia | 59 | 54.6 | 0 | 0.0 |
| Other | 19 | 17.6 | 0 | 0.0 |
| Atypical/No discernible anomaly | 0 | 0.0 | 84 | 100.0 |
| Hearing loss* | ||||
| None | 5 | 4.6 | 76 | 97.4 |
| Unilateral | 67 | 62.0 | 2 | 2.6 |
| Bilateral | 36 | 33.3 | 0 | 0.0 |
| Hearing aid use | ||||
| No | 53 | 49.1 | 84 | 100.0 |
| Yes (current or past use) | 55 | 50.9 | 0 | 0.0 |
| Hearing aid use (% based on those with hearing aid use) | ||||
| <2 hrs/day | 12 | 25.0 | - | - |
| 2–6 hrs/day | 20 | 41.7 | - | - |
| >6 hrs/day | 16 | 33.3 | - | - |
| Intervention services | ||||
| Occupational therapy | 7 | 6.5 | 0 | 0 |
| Physical therapy | 13 | 12.0 | 1 | 1.19 |
| Speech, hearing, or language services | 59 | 54.6 | 0 | 0.0 |
| Other developmental services | 8 | 7.4 | 0 | 0 |
| Any services | 70 | 64.8 | 1 | 1.2 |
Missingness: SES (1 control); ethnicity (2 controls); race (1 control, 2 cases); hearing loss (6 controls)
Table 2.
Comparison of mean neurodevelopmental scores for children with and without CFM
| Test | Measure | Controls N | Cases N | Unadjusted | Adjusted* | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean Difference | 95% CI | p-value | Mean Difference | 95% CI | p-value | ||||||
| PLS-5 | Total | 82 | 108 | −0.89 | −3.60 | 1.81 | 0.52 | 0.80 | −2.07 | 3.67 | 0.58 |
| Auditory Comprehension | 82 | 108 | −0.22 | −2.80 | 2.36 | 0.87 | 1.20 | −1.48 | 3.88 | 0.38 | |
| Expressive Communication | 82 | 108 | −1.48 | −4.49 | 1.54 | 0.34 | 0.34 | −2.93 | 3.61 | 0.84 | |
| Bayley-III | Cognitive | 82 | 108 | −3.44 | −6.71 | −0.17 | 0.04 | −0.23 | −3.30 | 2.84 | 0.88 |
| Motor Composite | 81 | 107 | −1.37 | −4.66 | 1.93 | 0.42 | 1.79 | −1.61 | 5.19 | 0.30 | |
| Fine Motor | 82 | 108 | −0.14 | −0.87 | 0.60 | 0.71 | 0.30 | −0.51 | 1.11 | 0.47 | |
| Gross Motor | 81 | 107 | −0.32 | −0.93 | 0.28 | 0.30 | 0.24 | −0.36 | 0.84 | 0.44 | |
Adjusted for age (months), SES (continuous), sex, study site (categorical), race (white/non-white), language (English/Spanish or combo)
The criterion for developmental delay was met in one or more areas by 13 (16%) of controls and 23 (21%) of cases (Table 3). Delayed cases were more likely to be male. In both cases and controls, delayed participants were of lower SES and more likely to have Medicaid insurance. After adjustment for demographic factors there was little evidence for case-control differences in the odds of developmental delay (OR=0.68, 95% CI 0.29 to 1.61).
Table 3.
Characteristics of infants with craniofacial microsomia by delay
| Characteristic | Not delayed | Delayed | ||
|---|---|---|---|---|
| N | % | N | % | |
| Total | 85 | 100.0 | 23 | 100.0 |
| Sex | ||||
| Male | 50 | 58.8 | 17 | 73.9 |
| Female | 35 | 41.2 | 6 | 26.1 |
| Age at visit | ||||
| Mean, SD | 14.3 | 2.4 | 15.3 | 4.0 |
| <13 months | 22 | 25.9 | 10 | 43.5 |
| 13–14 months | 36 | 42.4 | 5 | 21.7 |
| >14 months | 27 | 31.8 | 8 | 34.8 |
| SES | ||||
| Mean, SD | 37.0 | 14.1 | 28.9 | 12.7 |
| I | 15 | 17.6 | 1 | 4.3 |
| II | 17 | 20.0 | 3 | 13.0 |
| III | 23 | 27.1 | 5 | 21.7 |
| IV | 21 | 24.7 | 8 | 34.8 |
| V | 9 | 10.6 | 6 | 26.1 |
| Hispanic or Latino | ||||
| No | 41 | 48.2 | 8 | 34.8 |
| Yes | 44 | 51.8 | 15 | 65.2 |
| Race* | ||||
| White | 63 | 74.1 | 17 | 73.9 |
| Black/African American | 2 | 2.4 | 1 | 4.3 |
| Asian | 5 | 5.9 | 2 | 8.7 |
| American Indian/AK Native | 2 | 2.4 | 1 | 4.3 |
| Other race | 3 | 3.5 | 0 | 0.0 |
| Multiracial | 10 | 11.8 | 0 | 0.0 |
| Insurance | ||||
| Private | 43 | 50.6 | 7 | 30.4 |
| Medicaid | 42 | 49.4 | 16 | 69.6 |
| Testing language (PDP) | ||||
| 100% English | 57 | 67.1 | 12 | 52.2 |
| 100% Spanish | 14 | 16.5 | 5 | 21.7 |
| Combination English/Spanish | 14 | 16.5 | 6 | 26.1 |
| Phenotype | ||||
| Microtia only | 20 | 23.5 | 10 | 43.5 |
| Microtia + Mandibular hypoplasia | 50 | 58.8 | 9 | 39.1 |
| Other | 15 | 17.6 | 4 | 17.4 |
| Study site | ||||
| CHLA | 27 | 31.8 | 13 | 56.5 |
| CHOP | 2 | 2.4 | 1 | 4.3 |
| SCH | 36 | 42.4 | 2 | 8.7 |
| UNC | 12 | 14.1 | 4 | 17.4 |
| UIC | 8 | 9.4 | 3 | 13.0 |
Missingness: race (1 case with delay)
Phenotype differences
There was little evidence for differences by CFM phenotype. Relative to controls, cases with microtia only and those with microtia with mandibular hypoplasia scored slightly higher than controls for PLS-5 total language and auditory comprehension, but estimates included the null and were inconsistent in direction for other measures (Table 4). Mean differences for cases with other CFM features were inconsistent and imprecise across measures. Cases with bilateral CFM features scored lower than controls on all 7 measures and cases with unilateral involvement consistently scored higher than controls; however, effect sizes were modest and confidence intervals included the null for all estimates (Table 5; available at www.jpeds.com). A similar pattern was observed for laterality of external ear involvement (Table 6; available at www.jpeds.com). There was little evidence for differences by grade of ear involvement (Table 7; available at www.jpeds.com). Nineteen percent of cases had extracranial malformations and these cases scored lower than unaffected controls across all measures, although estimates were imprecise and included the null (Table 8; available at www.jpeds.com). Cases without extracranial malformations had modestly higher scores than controls, but all estimates included the null.
Table 4.
Comparison of mean neurodevelopmental scores for children with and without CFM, by phenotype
| Test | Measure | Controls N=84 |
Cases | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Microtia only N=30 |
Microtia + Mandibular Hypoplasia N=59 |
Other CFM-associated features N=19 |
||||||||||||
| Mean Difference* | 95% CI | p-value | Mean Difference* | 95% CI | p-value | Mean Difference* | 95% CI | p-value | ||||||
| PLS-5 | Total | Ref | 1.20 | −2.90 | 5.30 | 0.57 | 1.52 | −1.81 | 4.84 | 0.37 | −2.03 | −7.04 | 2.98 | 0.43 |
| Auditory Comprehension | Ref | 2.62 | −0.74 | 5.98 | 0.13 | 1.52 | −1.51 | 4.56 | 0.33 | −1.53 | −6.54 | 3.48 | 0.55 | |
| Expressive Communication | Ref | −0.59 | −5.74 | 4.56 | 0.82 | 1.39 | −2.39 | 5.16 | 0.47 | −2.05 | −7.37 | 3.27 | 0.45 | |
| Bayley-III | Cognitive | Ref | −1.75 | −6.48 | 2.99 | 0.47 | 0.00 | −3.59 | 3.59 | 0.99 | 0.75 | −3.89 | 5.38 | 0.75 |
| Motor Composite | Ref | −0.39 | −5.43 | 4.64 | 0.88 | 2.86 | −1.06 | 6.78 | 0.15 | 0.71 | −6.86 | 8.29 | 0.85 | |
| Fine Motor | Ref | 0.43 | −0.74 | 1.61 | 0.47 | 0.46 | −0.42 | 1.35 | 0.31 | −0.38 | −1.93 | 1.16 | 0.63 | |
| Gross Motor | Ref | −0.60 | −1.53 | 0.33 | 0.21 | 0.48 | −0.21 | 1.16 | 0.17 | 0.43 | −0.91 | 1.78 | 0.53 | |
Adjusted for age (months), SES (continuous), sex, study site (categorical), race (white/non-white), language (English/Spanish or combo)
Table 5.
Comparison of mean neurodevelopmental scores for children with and without CFM, by overall laterality of involved features
| Test | Measure | Controls N=84 |
Cases* | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unilateral N=66 |
Bilateral N=42 |
|||||||||
| Mean Difference** | 95% CI | p-value | Mean Difference** | 95% CI | p-value | |||||
| PLS-5 | Total | Ref | 2.70 | −0.37 | 5.78 | 0.08 | −2.05 | −5.85 | 1.75 | 0.29 |
| Auditory Comprehension | Ref | 2.83 | 0.02 | 5.63 | 0.05 | −1.25 | −4.77 | 2.28 | 0.49 | |
| Expressive Communication | Ref | 2.24 | −1.30 | 5.77 | 0.21 | −2.50 | −6.92 | 1.91 | 0.26 | |
| Bayley-III | Cognitive | Ref | 0.54 | −3.15 | 4.23 | 0.77 | −1.38 | −5.12 | 2.35 | 0.47 |
| Motor Composite | Ref | 3.57 | −0.13 | 7.28 | 0.06 | −0.98 | −5.96 | 4.00 | 0.70 | |
| Fine Motor | Ref | 0.69 | −0.21 | 1.59 | 0.13 | −0.28 | −1.33 | 0.78 | 0.61 | |
| Gross Motor | Ref | 0.50 | −0.16 | 1.15 | 0.14 | −0.16 | −1.06 | 0.73 | 0.72 | |
Includes external ear, mandible, soft tissue, ear canal, coloboma, nerves, dermoids, tags, lateral cleft, cleft lip, and orbits (size + displacement)
Adjusted for age (months), SES (continuous), sex, study site (categorical), race (white/non-white), language (English/Spanish or combo)
Table 6.
Comparison of mean neurodevelopmental scores for children with and without CFM, by external ear involvement
| Test | Measure | Controls N=84 |
Cases* | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unilateral N=72 |
Bilateral N=31 |
|||||||||
| Mean Difference** | 95% CI | p-value | Mean Difference** | 95% CI | p-value | |||||
| PLS-5 | Total | Ref | 2.26 | −0.83 | 5.35 | 0.15 | −1.26 | −5.43 | 2.91 | 0.55 |
| Auditory Comprehension | Ref | 2.30 | −0.63 | 5.23 | 0.12 | −0.83 | −4.32 | 2.66 | 0.64 | |
| Expressive Communication | Ref | 1.99 | −1.50 | 5.48 | 0.26 | −1.67 | −6.77 | 3.44 | 0.52 | |
| Bayley-III | Cognitive | Ref | 0.52 | −2.96 | 4.01 | 0.77 | −1.66 | −5.92 | 2.59 | 0.44 |
| Motor Composite | Ref | 3.65 | 0.06 | 7.24 | 0.05 | −3.58 | −8.71 | 1.54 | 0.17 | |
| Fine Motor | Ref | 0.76 | −0.11 | 1.63 | 0.09 | −0.66 | −1.73 | 0.40 | 0.22 | |
| Gross Motor | Ref | 0.38 | −0.26 | 1.03 | 0.25 | −0.55 | −1.50 | 0.41 | 0.26 | |
Excludes 5 controls without ear involvement
Adjusted for age (months), SES (continuous), sex, study site (categorical), race (white/non-white), language (English/Spanish)
Table 7.
Comparison of mean neurodevelopmental scores for children with and without CFM, by maximum external ear grade
| Test | Measure | Controls N=84 |
Cases | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grades 0–2 N=38 |
Grades 3–4 N=70 |
|||||||||
| Mean Difference* | 95% CI | p-value | Mean Difference* | 95% CI | p-value | |||||
| PLS-5 | Total | Ref | −1.22 | −4.76 | 2.32 | 0.50 | 2.16 | −0.98 | 5.30 | 0.18 |
| Auditory Comprehension | Ref | 0.78 | −2.62 | 4.18 | 0.65 | 1.48 | −1.43 | 4.38 | 0.32 | |
| Expressive Communication | Ref | −2.83 | −6.81 | 1.15 | 0.16 | 2.48 | −1.12 | 6.08 | 0.18 | |
| Bayley-III | Cognitive | Ref | −0.27 | −3.75 | 3.21 | 0.88 | −0.21 | −3.93 | 3.52 | 0.91 |
| Motor Composite | Ref | 0.55 | −3.72 | 4.83 | 0.80 | 2.65 | −1.30 | 6.61 | 0.19 | |
| Fine Motor | Ref | 0.21 | −0.78 | 1.20 | 0.68 | 0.37 | −0.58 | 1.31 | 0.44 | |
| Gross Motor | Ref | −0.03 | −0.79 | 0.74 | 0.95 | 0.42 | −0.28 | 1.13 | 0.23 | |
Adjusted for age (months), SES (continuous), sex, study site (categorical), race (white/non-white), language (English/Spanish)
Table 8.
Comparison of mean neurodevelopmental scores for children with and without CFM, by presence of extracranial malformations
| Test | Measure | Controls* N=83 |
Cases | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Absent N=87 |
Present N=21 |
|||||||||
| Mean Difference** | 95% CI | p-value | Mean Difference** | 95% CI | p-value | |||||
| PLS-5 | Total | Ref | 1.97 | −1.02 | 4.96 | 0.20 | −3.15 | −7.85 | 1.55 | 0.19 |
| Auditory Comprehension | Ref | 1.85 | −1.01 | 4.71 | 0.20 | −1.18 | −4.88 | 2.52 | 0.53 | |
| Expressive Communication | Ref | 1.89 | −1.46 | 5.24 | 0.27 | −4.79 | −10.69 | 1.11 | 0.11 | |
| Bayley-III | Cognitive | Ref | 0.34 | −3.03 | 3.70 | 0.84 | −2.29 | −6.78 | 2.19 | 0.31 |
| Motor Composite | Ref | 3.21 | −0.36 | 6.77 | 0.08 | −4.75 | −9.60 | 0.09 | 0.05 | |
| Fine Motor | Ref | 0.59 | −0.25 | 1.43 | 0.16 | −1.04 | −2.26 | 0.18 | 0.09 | |
| Gross Motor | Ref | 0.40 | −0.25 | 1.05 | 0.23 | −0.55 | −1.51 | 0.41 | 0.26 | |
Excludes 1 control with an extracranial malformation
Adjusted for age (months), SES (continuous), sex, study site (categorical), race (white/non-white), language (English/Spanish)
Hearing
Among cases, 33% had bilateral hearing loss, 62% had unilateral hearing loss, and 5% had no evidence for hearing loss. Cases with hearing loss and without use of a hearing aid had higher scores than controls on most measures, in particular PLS-5 auditory comprehension and Bayley-III fine and composite motor scores, although all estimates included the null (mean differences ranged from −0.47 to 3.13, p-values ranged from 0.12 to 0.98; Table 9; available at www.jpeds.com). There was little evidence for differences in cases with hearing loss and hearing aid use relative to controls. Cases with no hearing loss and no hearing aid use had lower scores across most outcomes, although only one, Bayley-III gross motor, included the null (estimates ranged from −4.80 to 0.97, p-values ranged from 0.002 to 0.88).
Table 9.
Comparison of mean neurodevelopmental scores for children with and without CFM, by hearing loss and hearing aid use
| Test | Measure | Controls * N=76 |
Cases* | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No hearing loss, no hearing aid use N=4 |
Hearing loss + hearing aid use N=36 |
Hearing loss w/out hearing aid use N=61 |
||||||||||||
| Mean Difference** | 95% CI | p-value | Mean Difference** | 95% CI | p-value | Mean Difference** | 95% CI | p-value | ||||||
| PLS-5 | Total | Ref | −4.06 | −10.87 | 2.75 | 0.24 | 1.48 | −2.78 | 5.74 | 0.50 | 0.83 | −2.34 | 4.00 | 0.61 |
| Auditory Comprehension | Ref | −2.68 | −9.60 | 4.24 | 0.45 | 0.59 | −3.11 | 4.28 | 0.76 | 2.05 | −1.04 | 5.13 | 0.20 | |
| Expressive Communication | Ref | −4.80 | −12.14 | 2.54 | 0.20 | 2.23 | −2.82 | 7.27 | 0.39 | −0.47 | −3.99 | 3.06 | 0.80 | |
| Bayley-III | Cognitive | Ref | 0.37 | −4.41 | 5.15 | 0.88 | 1.25 | −3.60 | 6.11 | 0.61 | −0.04 | −3.60 | 3.52 | 0.98 |
| Motor Composite | Ref | −2.56 | −10.04 | 4.91 | 0.50 | −0.32 | −5.73 | 5.09 | 0.91 | 3.13 | −0.81 | 7.07 | 0.12 | |
| Fine Motor | Ref | 0.97 | −0.42 | 2.37 | 0.17 | 0.11 | −1.06 | 1.28 | 0.85 | 0.37 | −0.56 | 1.31 | 0.43 | |
| Gross Motor | Ref | −1.91 | −3.13 | −0.70 | 0.002 | −0.26 | −1.24 | 0.71 | 0.60 | 0.60 | −0.08 | 1.29 | 0.09 | |
Cases who used a hearing aid for <2 hrs/day were considered non-users; cases without documented hearing loss but who reported using a hearing aid were considered to have hearing loss. Excludes 7 cases with missing information on time spent using a hearing aid, 2 controls with unilateral hearing loss, and 6 controls missing information on hearing loss.
Adjusted for age (months), SES (continuous), sex, study site (categorical), race (white/non-white), language (English/Spanish or combo)
DISCUSSION
In this case-control study we found no evidence for neurodevelopmental differences between infants with and without CFM in the first to second year of life. Group differences in mean test scores were negligible before and after statistical adjustment for socio-demographic variables that may affect neurodevelopment, including age, sex, SES, ethnicity, and the language in which testing was conducted (English or Spanish). Null findings were consistently observed across tested development domains, including cognition, language, and fine and gross motor skills. These results were only modestly influenced by facial phenotype, including the graded severity of ear anomalies. The presence of extracranial anomalies was associated with lower test scores across all measured domains, although these effects were also modest. There was no indication that hearing loss or use of hearing aids was related to developmental outcomes among infants with CFM, a finding that is likely due to their limited exposure to hearing intervention at this age. Positive intervention effects are more likely to be observed when these children are assessed at age 3.
Our findings regarding group differences are largely discrepant from those observed in previous neurodevelopmental investigations of children with CFM. The most notable differences are observed in comparisons with the Cohen et al (10) report of 24 infants and preschoolers with CFM (average age 26 months). Standardized developmental tests were given as part of a clinical work-up, including an earlier version of the Bayley exam (28) than the one we used. Nearly 60% of the Cohen sample scored more than two standard deviations below the mean in one or more developmental domains and developmental status varied with phenotypic presentation as indicated by substantially lower test performance among patients with bilateral (vs. unilateral) anomalies, low muscle tone, and cervical spine abnormalities.
Two other studies published previously followed participants in a longitudinal cohort recruited by Werler et al (7). Neurobehavioral assessments were administered in childhood (average age 7) and adolescence (average age 13). Testing at age 7 indicated that children with CFM were 2 to 3 times more likely than children without craniofacial anomalies to score in the delayed range on tests of receptive vocabulary and visual-motor skills (11). In the adolescent follow-up (12), average scores on tests of IQ and academic achievement were lower in cases than controls, with group differences most prominent in language functions. However, the magnitude of most differences was small (standardized effect sizes, −0.04 to −0.2). Analyses by facial phenotype showed that case and control group differences were slightly larger for cases with both microtia and mandibular hypoplasia than either condition alone, but with small effect sizes. As in the present study, cases’ hearing status unexpectedly had little effect on these outcomes, which the investigators attributed to the use of non-standardized hearing assessments.
Such discrepancies among studies may be due to variation in ascertainment methods (e.g., recruitment from audiology vs. genetics clinics), case sample definitions in relation to the CFM spectrum, and exclusions of related or other medical conditions. For example, nearly 20% of the patients examined by Cohen et al (10) had anomalies identified by brain imaging, a subgroup that generated an average IQ of 65. In contrast, Werler et al (7) observed < 5% of their sample to have central nervous system (CNS) anomalies (as indicated by medical records) and these participants were excluded from their case to control comparisons. In the current study, we also found < 5% of cases to have documented indications of CNS involvement. In another example, Collett et al (2011)(11) excluded children who had unilateral microtia without other features of CFM, but in the current sample we included these cases because isolated unilateral microtia represents an important clinical and theoretical endpoint for the CFM spectrum. This is suggested by the overlapping epidemiology and associated extracranial anomalies (e.g. renal and cervical spine anomalies) between this subgroup and the rest of the CFM spectrum and the resulting potential for misclassification of individuals with CFM-features as having “isolated” microtia (29–33). Wide discrepancies in neurodevelopmental (and other) outcomes will likely persist until investigators adopt the same diagnostic and exclusionary criteria in their definitions of CFM case samples.
The lack of differences observed here between cases and controls and among case subgroups may be due in large part to the age range within which our baseline assessment was conducted (12 to 24 months). In the second year of life, CFM risk factors such as compromised speech and expressive language have yet to influence substantially cognitive development (e.g., assessment of problem-solving skills at this age is largely based on nonverbal functions), but may affect outcomes at later ages. Moreover, given the challenge of obtaining specific vocal or verbal responses from infants in a testing situation, it is difficult to measure speech and language reliably at this age, necessitating reliance upon parents’ recall of language functions. We will be assessing this cohort again at age 3, when we will have a better opportunity to observe directly the impact of speech and expressive language on overall development
Our finding of equivalent developmental status among infants and toddlers with and without CFM may indicate that the academic problems observed among older children with CFM might be influenced by post-infancy risk factors, such as the impact of hearing loss on language acquisition and later, the effects of speech dysfluency or anomalous facial appearance on socialization and achievement motivation. However, the design of this study cannot rule out the possibility that differences might not be accurately assessed or present at this age, or that prenatal neuropathology also contributes to the school-age outcomes of children with CFM (14).
Test scores in the current study were examined categorically using a common criterion for developmental delay (34, 35). Only slightly more cases than controls met this criterion in one or more areas of functioning (21% vs. 16% respectively). Nevertheless, it is important clinically to understand which variables within the case group may distinguish delayed from non-delayed infants. Although we had too few delayed cases to evaluate such differences statistically, the descriptive data in Table 4 suggest that lower SES may be a distinguishing variable. Over 60% of delayed infants with CFM were in families from the lowest two categories of SES (vs. 35% of non-delayed cases). Correspondingly, 70% of delayed cases received Medicaid funding for their medical services (vs. 49% of non-delayed cases).
Consistent with prior epidemiological studies of CFM(5), over half the case families in this cohort (55%) were of Hispanic/Latino ancestry, with 63% of parents in this subgroup speaking Spanish exclusively or a combination of Spanish and English. These demographic characteristics required a bilingual approach to infant assessment, including the use of English and Spanish versions of the PLS-5 as well as examiners fluent in both English and Spanish for Bayley-III administration. Incorporation of bilingual assessment is a complex process that introduces several methodological complications and potential sources of error. For example, different language versions of the same test differ slightly in item content, different normative groups are involved, bilingual test administrations can vary and inter-tester reliability may differ by language. We sought to attenuate the impact of these issues through careful test selection, written protocols to standardize bilingual test administrations, recruitment of frequency-matched controls, and inclusion of related covariates in statistical analyses (eg, test language). In our view, the methodological complications of bilingual assessment are far outweighed by the benefits of having a representative, diverse sample of children with CFM.
Our study does have limitations. First, like prior studies, our sample size was limited, particularly for subgroup analyses, and that could have led to an inability to detect differences. Second, only about half of identified eligible case families consented to participate, which could limit the study’s generalizability of findings to the broader population of children with CFM. However, comparisons of participating and nonparticipating eligible cases indicated that these two groups were reasonably similar with respect to both demographics and phenotype, although some exceptions were observed (eg,, participants were slightly more likely to be male and Hispanic; see Luquetti et al(21) for details). Finally, we were unable to obtain complete audiological data for ~ 15% of our cohort. Consequently, we classified the hearing status of these individuals in relation to the documented presence of aural atresia or external ear canal stenosis. Although not ideal, we have confidence in this classification method, as previous research has shown that nearly all children with either of these conditions have definite hearing loss, as determined by audiological evaluations (20).
CFM is a highly variable diagnosis that has multiple risk factors potentially influencing children’s development. The learning problems observed at school age and in adolescence(11, 12) cannot be understood fully without the longitudinal tracking of development beginning in infancy. This study represents an important first step in elucidating the trajectory of functional outcomes among young children with CFM and opportunities for intervention. We plan to follow this CFM cohort longitudinally. In this baseline assessment of we found no evidence for neurodevelopmental differences among infants with CFM between the ages of one and two. This suggests that the academic problems observed in some older children with CFM might not be accurately assessed or present at this age, or might be influenced by the increasing toll of CFM-related risk factors on post-infancy development (e.g., hearing and speech impairments). As many older children with CFM show average or above average achievement(11, 12), it is important to understand which variables in early childhood best distinguish delayed from non-delayed cases. These issues will provide the investigative framework for our next evaluation of this sample at age 3.
Acknowledgments
Supported by the National Institute of Dental and Craniofacial Research (R01 DE 022438) and the Center for Clinical and Translational Research at Seattle Children’s Research Institute (UL1 TR000423). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors declare no conflicts of interest.
We thank the families who so generously volunteered their time to participate in our study. We also acknowledge the support from advocacy organizations for their assistance with identifying study participants. Additional members of the CLOCK study team (CLOCK = Craniofacial microsomia: Longitudinal Outcomes in Children pre-Kindergarten) include: Seattle Children’s Hospital (coordination center): Craig Birgfeld, MD; Sara Kinter, MA, CCC-SLP; Susan Norton, PhD; Jessica Mendoza, PhD PT; Amber Sand, BA; Kathleen Sie, MD; Babette Siebold, PhD; Laura Stueckle, MPH; Alison Paolozzi, BA CSP. Children’s Hospital of Los Angeles: Mark Urata, MD, DDS; Art Fahradyan, MD; Amanda Tyree, MA. Children’s Hospital of Philadelphia: Leanne Mage PhD; Scott Bartlett, MD; University of Illinois at Chicago and Shriners Hospital for Children, Chicago: Janine Rosenberg, PhD; Claudia Crilly Bellucci, MS; Jody Coppersmith, MA; Stephanie McConville, PhD; Suzel Bautista, BA; Amanda Lossia, MS. University of North Carolina: Amelia Drake, MD; Marina Pastore Rampazzo, DDS; Daniela Vivaldi, DDS. University of Pittsburgh: Jeff Cohen, PhD. The Robotics Institute, Carnegie Mellon University: Zakia Hammel, PhD. New York University: Harriett Oster, PhD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gorlin RJ, Cohen MJ, Hennekan RCM. Syndromes of the head and neck. 4. New York: Oxford University Press; 2001. [Google Scholar]
- 2.Heike CL, Luquetti DV, Hing AV. Craniofacial microsomia overview. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Mefford HC, et al., editors. GeneReviews((R)) Seattle (WA): University of Washington, Seattle; 2014. GeneReviews is a registered trademark of the University of Washington, Seattle. All rights reserved. [PubMed] [Google Scholar]
- 3.Barisic I, Odak L, Loane M, Garne E, Wellesley D, Calzolari E, et al. Prevalence, prenatal diagnosis and clinical features of oculo-auriculo-vertebral spectrum: A registry-based study in Europe. Eur J Hum Genet. 2014 Aug;22:1026–33. doi: 10.1038/ejhg.2013.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris J, Kallen B, Robert E. The epidemiology of anotia and microtia. Journal of Medical Genetics. 1996;33:809–13. doi: 10.1136/jmg.33.10.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luquetti DV, Leoncini E, Mastroiacovo P. Microtia-anotia: A global review of prevalence rates. Birth Defects Res A Clin Mol Teratol. 2011 Sep;91:813–22. doi: 10.1002/bdra.20836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khetani MA, Collett BR, Speltz ML, Werler MM. Health-related quality of life in children with hemifacial microsomia: Parent and child perspectives. J Dev Behav Pediatr. 2013 Nov-Dec;34:661–8. doi: 10.1097/DBP.0000000000000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Werler MM, Starr JR, Cloonan YK, Speltz ML. Hemifacial microsomia: From gestation to childhood. J Craniofac Surg. 2009 Mar;20(Suppl 1):664–9. doi: 10.1097/SCS.0b013e318193d5d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guralnick MJ. Why early intervention works: A systems perspective. Infants Young Child. 2011 Jan 1;24:6–28. doi: 10.1097/IYC.0b013e3182002cfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guralnick MJ. Early intervention for children with intellectual disabilities: An update. J Appl Res Intellect Disabil. 2016 Jan 13; doi: 10.1111/jar.12233. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 10.Cohen MS, Samango-Sprouse CA, Stern HJ, Custer DA, Vaught DR, Saal HM, et al. Neurodevelopmental profile of infants and toddlers with oculo-auriculo-vertebral spectrum and the correlation of prognosis with physical findings. Am J Med Genet. 1995 Dec 18;60:535–40. doi: 10.1002/ajmg.1320600610. [DOI] [PubMed] [Google Scholar]
- 11.Collett BR, Speltz ML, Cloonan YK, Leroux BG, Kelly JP, Werler MM. Neurodevelopmental outcomes in children with hemifacial microsomia. Arch Pediatr Adolesc Med. 2011 Feb;165:134–40. doi: 10.1001/archpediatrics.2010.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Speltz ML, Wallace ER, Collett BR, Heike CL, Luquetti DV, Werler MM. Intelligence and academic achievement of adolescents with craniofacial microsomia. Plast Reconstr Surg. 2017 Sep;140:571–80. doi: 10.1097/PRS.0000000000003584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kesser BW, Krook K, Gray LC. Impact of unilateral conductive hearing loss due to aural atresia on academic performance in children. Laryngoscope. 2013 Sep;123:2270–5. doi: 10.1002/lary.24055. [DOI] [PubMed] [Google Scholar]
- 14.Kjaer I. Human prenatal craniofacial development related to brain development under normal and pathologic conditions. Acta Odontol Scand. 1995 Jun;53:135–43. doi: 10.3109/00016359509005963. [DOI] [PubMed] [Google Scholar]
- 15.Vento AR, LaBrie RA, Mulliken JB. The O.M.E.N.S. classification of hemifacial microsomia. Cleft Palate Craniofac J. 1991 Jan;28:68–76. doi: 10.1597/1545-1569_1991_028_0068_tomens_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 16.Gougoutas AJ, Singh DJ, Low DW, Bartlett SP. Hemifacial microsomia: Clinical features and pictographic representations of the OMENS classification system. Plast Reconstr Surg. 2007 Dec;120:112e–20e. doi: 10.1097/01.prs.0000287383.35963.5e. [DOI] [PubMed] [Google Scholar]
- 17.Bayley N. Bayley scales of infant and toddler development. 3. San Antonio, TX: Harcourt Assessment; 2006. [Google Scholar]
- 18.Zimmerman IL, Steiner VG, Pond RE. Preschool language scales. 5. San Antonio, TX: Pearson; 2011. [Google Scholar]
- 19.Zimmerman IL, Steiner VG, Pond RE. Preschool language scales. 5. San Antonio, TX: Pearson; 2012. Spanish. [Google Scholar]
- 20.Mitchell RM, Saltzman BS, Norton SJ, Harrison RG, Heike CL, Luquetti DV, et al. Hearing loss in children with craniofacial microsomia. Cleft Palate Craniofac J. 2017 Nov;54:656–63. doi: 10.1597/15-348. [DOI] [PubMed] [Google Scholar]
- 21.Birgfeld CB, Luquetti DV, Gougoutas AJ, Bartlett SP, Low DW, Sie KC, et al. A phenotypic assessment tool for craniofacial microsomia. Plast Reconstr Surg. 2011 Jan;127:313–20. doi: 10.1097/PRS.0b013e3181f95d15. [DOI] [PubMed] [Google Scholar]
- 22.Birgfeld CB, Heike CL, Saltzman BS, Leroux BG, Evans KN, Luquetti DV. Reliable classification of facial phenotypic variation in craniofacial microsomia: A comparison of physical exam and photographs. Head Face Med. 2016 Mar 31;12:1–13. doi: 10.1186/s13005-016-0109-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luquetti DL, Speltz ML, Wallace ER, Siebold B, Collett BR, Drake AF, et al. Methods and challenges in a cohort study in infants and toddlers with craniofacial microsomia: The CLOCK study. doi: 10.1177/1055665618821014. Manuscript submitted for publication, April, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marx H. Die missbildungen des ohres. In: ODAK, editor. Handbuch der Spez Path Anatomie Histologie. Berlin, Germany: Springer; 1926. p. 131. [Google Scholar]
- 25.Tanzer RC. Microtia. Clin Plast Surg. 1978 Jul;5:317–36. [PubMed] [Google Scholar]
- 26.Poon CC, Meara JG, Heggie AA. Hemifacial microsomia: Use of the OMENS-plus classification at the Royal Children’s Hospital of Melbourne. Plast Reconstr Surg. 2003 Mar;111:1011–8. doi: 10.1097/01.PRS.0000046245.44567.D6. [DOI] [PubMed] [Google Scholar]
- 27.Tuin AJ, Tahiri Y, Paine KM, Paliga JT, Taylor JA, Bartlett SP. Clarifying the relationships among the different features of the OMENS+ classification in craniofacial microsomia. Plast Reconstr Surg. 2015 Jan;135:149e–56e. doi: 10.1097/PRS.0000000000000843. [DOI] [PubMed] [Google Scholar]
- 28.Bayley N. The Bayley scales of infant development. 2. San Antonio, TX: The Psychological Corporation; 1993. [Google Scholar]
- 29.Vendramini-Pittoli S, Kokitsu-Nakata NM. Oculoauriculovertebral spectrum: Report of nine familial cases with evidence of autosomal dominant inheritance and review of the literature. Clin Dysmorphol. 2009 Apr;18:67–77. doi: 10.1097/MCD.0b013e328323a7dd. [DOI] [PubMed] [Google Scholar]
- 30.Tasse C, Majewski F, Bohringer S, Fischer S, Ludecke HJ, Gillessen-Kaesbach G, et al. A family with autosomal dominant oculo-auriculo-vertebral spectrum. Clin Dysmorphol. 2007 Jan;16:1–7. doi: 10.1097/MCD.0b013e328010d313. [DOI] [PubMed] [Google Scholar]
- 31.Goodin K, Prucka S, Woolley AL, Kohlhase J, Smith RJ, Grant J, et al. Familial transmission of oculoauriculovertebral spectrum (Goldenhar syndrome) is not due to mutations in either EYA1 or SALL1. Am J Med Genet A. 2009 Mar;149A:535–8. doi: 10.1002/ajmg.a.32673. [DOI] [PubMed] [Google Scholar]
- 32.Bennun RD, Mulliken JB, Kaban LB, Murray JE. Microtia: A microform of hemifacial microsomia. Plastic & Reconstructive Surgery. 1985 Dec;76:859–65. [PubMed] [Google Scholar]
- 33.Keogh IJ, Troulis MJ, Monroy AA, Eavey RD, Kaban LB. Isolated microtia as a marker for unsuspected hemifacial microsomia. Arch Otolaryngol Head Neck Surg. 2007 Oct;133:997–1001. doi: 10.1001/archotol.133.10.997. [DOI] [PubMed] [Google Scholar]
- 34.Johnson S, Moore T, Marlow N. Using the Bayley-III to assess neurodevelopmental delay: which cut-off should be used? Pediatr Res. 2014 May;75:670–4. doi: 10.1038/pr.2014.10. [DOI] [PubMed] [Google Scholar]
- 35.Spencer-Smith MM, Spittle AJ, Lee KJ, Doyle LW, Anderson PJ. Bayley-III cognitive and language scales in preterm children. Pediatrics. 2015 May;135:e1258–65. doi: 10.1542/peds.2014-3039. [DOI] [PubMed] [Google Scholar]