Abstract
Glucagon-like peptide 1 (GLP-1) neurons of the caudal brainstem project to many brain areas, including the lateral septum (LS), which has a known role in stress responses. Previously, we showed that endogenous GLP-1 in the LS plays a physiologic role in the control of feeding under non-stressed conditions, however, central GLP-1 is also involved in behavioral and endocrine responses to stress. Here, we asked whether LS GLP-1 receptors (GLP-1R) contribute to stress-induced hypophagia. Male rats were implanted with bilateral cannulas targeting the dorsal subregion of the LS (dLS). In a within-subjects design, shortly before the onset of the dark phase, rats received dLS injections of saline or the GLP-1R antagonist Exendin (9-39) (Ex9) prior to 30 min restraint stress. Food intake was measured continuously for the next 20 h. The stress-induced hypophagia observed within the first 30 minutes of dark was not influenced by Ex9 pretreatment, but Ex9 tended to blunt the effect of stress as early as 1 and 2 h into the dark phase. By 4–6 h, there were significant stress X drug interactions, and Ex9 pretreatment blocked the stress-induced suppression of feeding. These effects were mediated entirely through changes in average meal size; stress suppressed meal size while dLS Ex9 attenuated this effect. Using a similar design, we examined the role of dLS GLP-1R in the neuroendocrine response to acute restraint stress. As expected, stress potently increased serum corticosterone, but blockade of dLS GLP-1Rs did not affect this response. Together, these data show that endogenous GLP-1 action in the dLS plays a role in some but not all of the physiologic responses to acute stress.
1. Introduction
Central glucagon-like peptide 1 is produced in hindbrain GLP-1 neurons in both the nucleus of the solitary tract (NTS) and the medullary reticular formation (MRF) [1,2]. GLP-1 neurons project widely throughout the brain [3], and are activated by incoming signals from the vagus nerve. Most research on the role of brain GLP-1 has focused on its contribution to food intake control. This is supported by findings that intracerebroventricular (ICV) GLP-1 effectively suppresses food intake, and more importantly, that feeding is increased when endogenous GLP-1 receptor (GLP-1R) stimulation is reduced by the GLP-1R antagonist, Exendin 9-39 (Ex9) or through GLP-1 or GLP-1R mRNA knockdown [4–7]. These loss-of-function studies support the idea that central GLP-1 is essential to the physiologic control of energy balance.
In addition to its effects under normal, non-stressed conditions, it is well established that central GLP-1 signaling plays a role in behavioral and endocrine stress responses [8–10], and central GLP-1 has been shown to modulate hypothalamic-pituitary-adrenal (HPA) axis activity [11–14]. Previous studies have established that acute stress activates GLP-1 neurons in the NTS and MRF in rats [2,15]. Furthermore, central administration of GLP-1 induces c-fos expression in corticotropin-releasing hormone (CRH)-producing neurons in the paraventricular nucleus (PVN) of the hypothalamus and leads to an increase in plasma adrenocorticotropic hormone (ACTH) and corticosterone (CORT) [11]. GLP-1 neurons have also been shown to contribute to stress-induced hypophagia [15]. Recent data demonstrate that central GLP-1R antagonism, delivered globally through ICV injection, reduced the ability of acute stress to suppress chow intake early in the dark phase [2]. Although the specific central site of action was not determined in that paper, the bed nucleus of the stria terminalis (BNST) was identified as a candidate based on patterns of c-fos expression induced by stress and modulated by Ex9 treatment. The PVN population of GLP-1Rs has also been suggested as a potential mediator for some of these effects, and site-specific intra-PVN injection of GLP-1 increases HPA axis activity [9]. Recent studies using mice that lack PVN GLP-1Rs provide evidence that these receptors play a role in the neuroendocrine and sympathetic nervous system responses to acute and chronic stress, as well as in anxiety-like behavior [10]. Here, we examine the potential role of another GLP-1R population, those in the dorsal lateral septum (dLS).
Previous research on the LS supports a role for this nucleus in reward and stress-related behaviors [16–20]. Stimulation of LS corticotropin-releasing factor2 receptors (CRF2R) promotes anorexia and increases stress-like behaviors [21]. Blockade of CRF2Rs in the LS attenuates restraint-stress-induced anorexia [22]. Furthermore, inhibition of LS neurons can reduce the anorectic effects of stress and increase sucrose intake in rats submitted to weekly cycles of stress [23,24]. This area has also been shown to play a role in modulation of HPA axis activity [20]. Until recently, the LS had not been considered a major player in the central control of food intake. However, more recent studies have demonstrated a role for the LS in the physiologic control of feeding. Sweeney and colleagues showed that an excitatory pathway from the ventral hippocampus to the LS suppresses feeding, as does an inhibitory pathway from the LS to the lateral hypothalamus (LH) [25,26]. GLP-1 fibers and receptors are present in the LS [3,27,28], as well, and recent experimental data from our laboratory showed that GLP-1 in the LS influences food intake [29]. In a series of studies, we demonstrated that stimulation of GLP-1R in the LS suppresses feeding and blockade of these receptors increases food intake in a variety of feeding tests, and that GLP-1R in the dorsal subregion of the LS, in particular, mediated effects on both food intake and motivation for food [29]. Our data support the idea that endogenous GLP-1 signaling in the dLS plays a physiologic role in limiting food intake. Based on these findings and the well-established role of the LS in stress responses [18,20], we sought to determine whether dLS GLP-1 receptors play a role in stress-induced hypophagia.
Here, we hypothesized that after 30 minutes of restraint stress, endogenously released GLP-1 in the dLS acts on GLP-1R-bearing neurons in this nucleus to suppress chow intake in rats. To test this hypothesis, we blocked GLP-1R in the dLS using the antagonist Ex9, with the expectation that Ex9 treatment would attenuate stress-induced hypophagia. Because the LS can modulate neuroendocrine stress responses and central GLP-1 is also known to influence HPA axis activity, we hypothesized that endogenous GLP-1 signaling in this region plays a role in the neuroendocrine response to acute stress. To test this hypothesis, we determined whether the CORT response to acute restraint stress is attenuated by blockade of dLS GLP-1Rs.
2. Methods
2.1. Subjects
Naïve male Wistar rats (Harlan, Indianapolis, IN) were maintained individually in temperature-controlled vivariums on a 12:12-h light-dark cycle in plastic cages. Rats had ad libitum access to distilled water and rat chow (Purina 5001), except where otherwise noted. All experimental procedures were approved by the Florida State University Institutional Animal Care and Use Committee and conformed to the standard of the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996).
2.2. Surgery
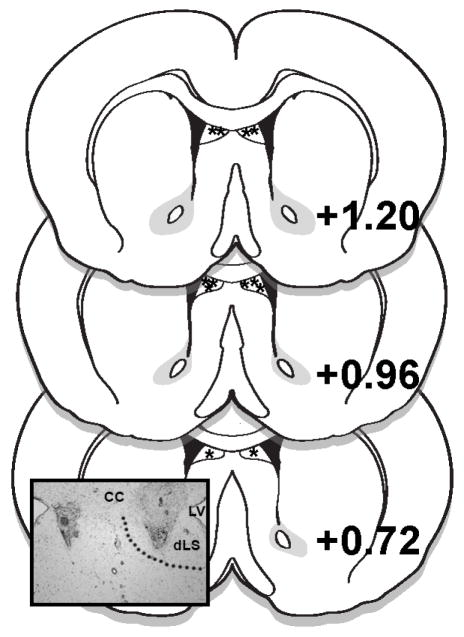
Rats were implanted with bilateral 26 G guide cannulas under 2–4% isoflurane delivered at a rate of 1 l/min. Bilateral cannulas targeted the dLS (0.6 mm lateral to midline, 1.0 mm anterior to bregma, and 3.0 mm ventral to skull surface). Injectors (33G) extending 2.0 mm below the end of the guide cannulas were used. Rats were given 1 week to recover from surgery prior to the start of experimentation. Cannula placements were verified histologically after the conclusion of the experiment. Injection sites within the boundaries of the dorsal subdivision of the LS, as drawn in the atlas of Paxinos and Watson [30], were considered correct, and data from rats with accurate bilateral cannula placements were included in analyses (70% hit rate) (Fig. 1).
Figure 1.
Diagram of LS injection placements based on the atlas of Paxinos and Watson [30]. Additional subjects’ injection sites were identified in similar locations at points between the anterior-posterior levels displayed here. The photomicrograph inset shows a representative injection site. CC = corpus callosum; LV = lateral ventricle; dLS = dorsal lateral septum.
2.3. General methods for behavioral experiments
Before the start of testing, rats were habituated to all experimental procedures. For habituation to intra-dLS injection procedures, rats received a 0.25 μl injection of sterile 0.9% saline at a rate of 0.25 μl/min, delivered to each hemisphere, for a total volume of 0.5 μl distributed across the two dLS sites; injections into each hemisphere were given simultaneously. Injectors were then left in place for an additional minute before removal. Body weights were recorded daily, and all drug treatments were separated by a minimum of 48 h.
2.4. Study 1: effects of dLS GLP-1R blockade on stress-induced hypophagia
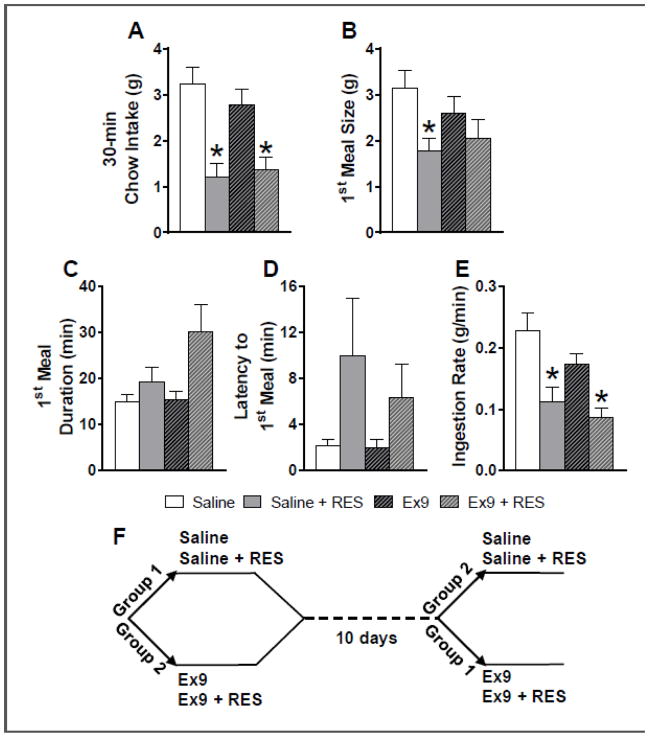
Naïve male Wistar rats with bilateral cannulas targeting the dLS (n = 12) were housed in the BioDAQ continuous food intake monitoring system (Research Diets, New Brunswick, NJ), which uses sensitive load cells to record the weight of food hoppers with a resolution of 1 second and 0.02 g. Rats had ad libitum access to distilled water and rat chow (Purina 5001) except where otherwise noted. On test days, access to food was removed 4 h before dark onset, and 45 min before dark onset, all rats received intra-dLS injections. This study utilized a within-subjects, crossover design in which rats were divided into two groups (Fig 2F). Each group received conditions presented in counterbalanced order. Rats in group 1 received intra-dLS saline with no stress, and intra-dLS saline plus 30 minutes of restraint stress (RES) (n = 6); group 2 received intra-dLS Ex9 (5 μg total) with no stress, and intra-dLS Ex9 plus RES (n =6). The 5 μg dose was evenly divided between the two hemispheres (i.e., 2.5 μg on each side). This dose of Ex9 was selected based on a pilot study in which we found that it was subthreshold for a feeding effect early in the dark cycle when delivered bilaterally to the dLS (data not shown). Ten days after the second brain injection, the crossover occurred and rats received the other two conditions in counterbalanced order. For the RES conditions, rats were restrained for 30 min in a rodent restraint cone, and released just prior to dark onset. While the rats were in the restraint cone, they were placed in individual plastic holding cages (not their home cage), and when they were removed from the cone, rats were placed back into their home cages. We chose to use non-home cage holding during restraint to minimize movement of home cages, because rats in this experiment were housed in the BioDAQ system. Because daily maintenance of this equipment requires that rats be temporarily moved to holding cages, subjects in this study were accustomed to this non-home cage location. For the no-stress conditions, rats were placed back into their holding cages after intra-dLS injections were made. In all conditions, chow was returned immediately before dark onset and subsequent intake was monitored by the BioDAQ system for 20 h.
Figure 2.
A: At 30 min post-dark onset, restraint stress significantly suppressed chow intake after both intra-dLS saline and Ex9 treatment (*p < 0.01 vs. respective no stress condition). B: The size (g) of the first meal after dark onset was significantly suppressed under dLS saline conditions (*p < 0.01 relative to saline). C: The duration of the first meal (min) tended to be increased by stress, but this was not statistically significant. D: Latency to begin the first meal (min) tended to be increased by stress but this was not statistically significant. E: Ingestion rate during the first meal was suppressed by stress (*p < 0.01 vs. respective no stress condition). All data are shown as mean ± SEM. n = 12. F: Timeline for the order and timing of the drug treatments and crossover trials for studies 1 and 2.
Food intake and meal pattern analyses were conducted with the Data Viewer software (Research Diets, New Brunswick, NJ). We first examined cumulative chow intake 30 min after dark onset, because this is the period during which others [2] have found RES to most profoundly suppress food intake. We also assessed cumulative intake in hourly intervals up to 20 h. Based on our cumulative chow intake findings that the effect of stress on feeding was expressed most prominently within the first half of the dark phase, we only analyzed meal pattern variables for the first 6 h of the dark phase. A single meal was defined as the consumption of at least 0.25 g of chow separated by ≥15 min from subsequent intake. We examined latency to begin the first meal, meal size, number of meals, meal duration, ingestion rate and inter-meal intervals (IMI). Latency to begin the first meal was defined as the number of min between the time when chow access resumed and the start of the first meal. Ingestion rate was calculated as g consumed during the meal divided by meal duration. IMI was defined as the time (sec) between the end of one meal and the start of the subsequent meal.
2.5. Study 2: effects of dLS GLP-1R blockade on the CORT response to stress
A second cohort of naïve male Wistar rats (n = 6) were implanted with bilateral cannulas targeting the dLS. In the mid-light phase (beginning at 4 h post-light onset), 15 min before intra-dLS injection, initial 200 μl blood samples were taken via tail snip to provide baseline CORT levels (timepoint -60). Fifteen minutes after baseline, all rats received intra-dLS injections of saline vehicle or 5 μg Ex9 (2.5 μg on each side, as described above). Fifteen minutes after dLS injection, for the RES condition, rats were restrained for 30 min in a rodent restraint cone. At the end of the 30 min of restraint, tail blood was again taken (timepoint 0); blood was again taken 15, 30, 60, and 120 min after the rat had been removed from the restraint cone. This study utilized the same within-subjects, crossover design described above, such that all rats received all conditions presented in counterbalanced order with a minimum of 10 days between stress conditions (Fig 2F).
Serum CORT concentrations were determined using a competitive enzyme immunoassay (Immunodiagnostic Systems Ltd., Fountain Hills, AZ), according to the manufacturer’s instructions. Assay sensitivity for CORT was 0.55 ng/ml, with synthetic CORT recovery of 98.60 ± 7.04% and linearity of 99.67 ± 4.79%. All blood samples were collected into 1.5 ml polypropylene microcentrifuge tubes. Blood was allowed to clot undisturbed at room temperature for 30 min. Samples were then centrifuged (14000 rpm, 20 min, 4°C), and serum was removed and stored at −80°C until processing.
Statistical Analysis
Data are reported as mean ± SE. Effects were evaluated by three-way or two-way repeated measures ANOVA, as appropriate. Pairwise planned comparisons were made with Holm-Bonferroni tests. P values of <0.05 were taken as significant.
3. Results
3.1. Study 1: effects of LS GLP-1R blockade on stress-induced hypophagia
3.1.1. Chow intake
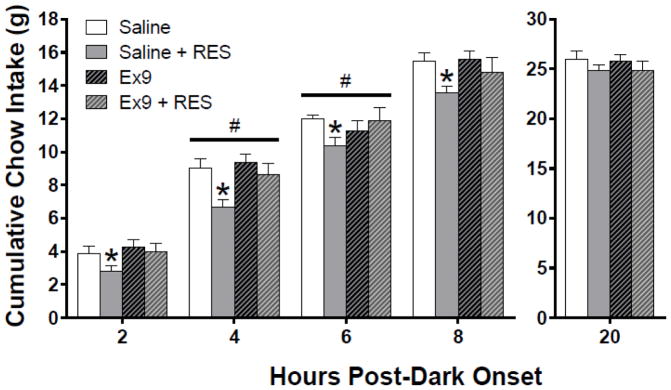
On test days, two-way repeated measures ANOVA revealed a main effect of stress to suppress chow intake at 30 min post-dark onset [30-min: F (1, 11) = 30.67, p < .00001]; at this timepoint, pairwise comparisons demonstrated that restraint stress significantly suppressed chow intake after both intra-dLS saline and Ex9 treatment (p < 0.01) (Fig 2A). Statistics for the main effect of stress were similar for cumulative intake from hours 1 – 8 h post-dark onset [e.g., 4h: F (1, 11) = 8.82, p< .05]; and at these hourly timepoints, pairwise comparisons showed that restraint stress significantly suppressed chow intake only following intra-dLS saline (p < 0.01). We present intake in 2-h bins for visual clarity (Fig 3). Beginning at 1 and 2 h post-dark onset, GLP-1R blockade tended to reduce the ability of acute stress to suppress chow intake (pairwise comparisons showing no effect of stress after dLS Ex9 treatment); however, there was not a significant stress by drug interaction at these time points. At 4 and 6 h, there were significant stress x drug interactions [4h: F (1, 11) = 7.51, p < 0.05], [6h: F (1, 11) = 5.31, p < 0.05]; Ex9 treatment significantly attenuated the effect of stress-induced hypophagia (Fig 3). Starting at 9 h post-dark onset, we found a significant main effect of stress on cumulative intake, but pairwise comparisons revealed no significant differences across conditions, and by 20 h, there was no longer a significant main effect of stress. Based on this pattern of results, we focused our meal pattern analysis on the first half of the dark phase, where the effects of stress and Ex9 were evident.
Figure 3.
At 2, 4, 6, and 8 h post-dark onset, restraint stress significantly suppressed cumulative intake relative to the saline no stress condition. At 4 and 6 h there was a significant stress by drug interaction; Ex9 treatment significantly attenuated the effect of stress-induced hypophagia. (*p < 0.05 relative to saline, #p < 0.05 stress x drug interaction). No effects were observed for cumulative intake at 20 h. All data are shown as mean ± SEM. n = 12.
Responses to stress can change with repeated stress experience, and our experiment used a crossover design in which rats did experience 30 min restraint stress twice, separated by 10 days. Therefore, we looked for a possible effect of condition order in our feeding data. In group 1, which received intra-dLS saline conditions first, we found that restraint stress suppressed dark-onset cumulative chow intake by 60% ± 8% during the first 30 min. In group 2 (rats that received the dLS saline conditions after the crossover), we found that restraint stress suppressed dark-onset cumulative chow intake by 70% ± 14% during the same time period [t (10) =0.59, NS]. The suppressive effect of stress on food intake throughout the dark phase was similar in group 1 and group 2, despite group 2 rats having previously experienced 30 min restraint once before (e.g., suppression of chow intake at 4h: group 1, 23% ± 10% vs. group 2, 23% ± 7% [t (10) =0.14, NS] and at 6h: group 1, 18% ± 8% vs. group 2, 10% ± 5% [t (10) =0.94, NS]). This suggests that rats did not show significant adaptation of the feeding response to stress within this experiment.
3.1.2. Meal pattern analyses
Because the effect of stress to suppress intake was most profound during the first 30 min of the dark phase, when the first meal of the day is typically consumed, we examined first meal size and related variables. Two-way repeated measures ANOVA revealed a main effect of stress to suppress the size of the first meal [F (1, 11) = 6.46, p < 0.05]; pairwise comparisons demonstrated that restraint stress significantly suppressed first meal size after intra-dLS saline, but not following dLS Ex9 treatment (Fig 2B). There was a trend for a stress x drug interaction, but this failed to reach significance [F (1, 11) = 4.55, p = 0.056]. We also found a significant main effect of stress on first meal duration [F (1, 11) = 8.69, p < 0.05], but pairwise comparisons did not reveal significant differences across conditions (Fig 2C). Stress tended to increase latency to begin the first meal [main effect of stress F (1, 11) = 5.73, p < 0.05], but there was a high degree of variability and pairwise comparisons revealed no significant differences across conditions (Fig 2D). Two-way repeated measures ANOVA revealed a main effect of stress to suppress ingestion rate during the first meal [F (1, 11) = 18.56, p < 0.01], with no effect of drug and no interaction. Pairwise comparisons demonstrated that restraint stress significantly suppressed average ingestion rate during the first meal after intra-dLS saline and also following dLS Ex9 treatment. (p < 0.001) (Fig 2E).
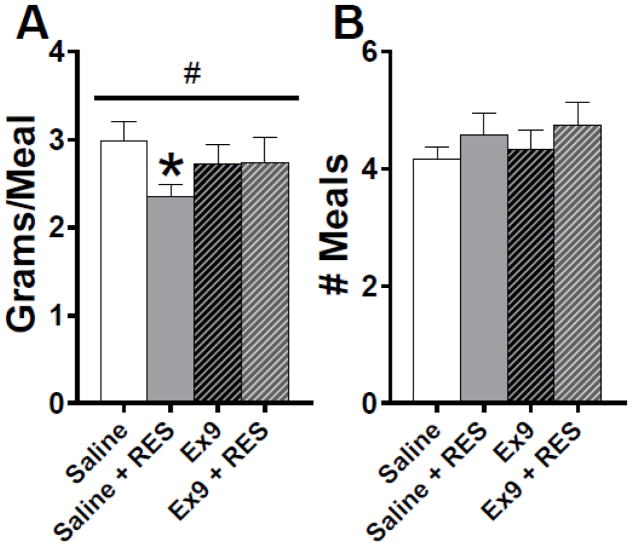
When we examined average meal size over the first 6 hours of the dark phase, two-way repeated measures ANOVA revealed a stress x drug interaction [F (1, 11) = 7.38, p < 0.05]. Restraint stress significantly suppressed average meal size in the dLS saline conditions (p < 0.01), and this effect of stress to was eliminated by dLS GLP-1R blockade (Fig 4A). During this period, there were no effects on the number of meals consumed (Fig 4B), average meal duration or IMI (Table 1).
Figure 4.
A: Acute restraint stress reduced averaged meal size and this effect was attenuated by Ex9 (*p < 0.01 relative to saline, #p < 0.05 stress x drug interaction). B: There was no difference in the number of meals consumed over the first 6 h of the dark cycle across all treatment groups. All data are shown as mean ± SEM. n = 12.
Table 1.
| Meal pattern variable | Saline | Saline + RES | Ex9 | Ex9 + RES |
|---|---|---|---|---|
| 6 h average meal duration, min | 19.09 ± 2.60 | 20.41 ± 6.10 | 16.78 ± 1.53 | 19.75 ± 2.69 |
| 6 h average IMI, min | 73.37 ± 4.89 | 65.95 ± 5.35 | 70.01 ± 7.36 | 67.09 ± 6.64 |
3.2. Study 2: effects of dLS GLP-1R blockade on the CORT response to stress
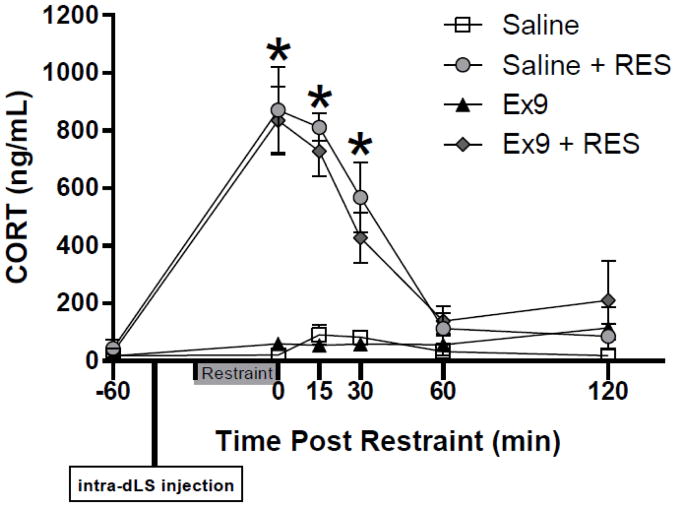
Stress strongly increased serum CORT regardless of drug treatment. Three-way ANOVA yielded significant main effects of time [F(5, 25) = 38.62, p < 0.001] and stress [F(5, 25) = 53.06, p < 0.001]; there was also a significant time x stress interaction [F(5, 25) = 37.1, p < 0.001]. We used two-way ANOVA to examine stress effects in detail at individual time points. There were no differences in serum CORT at timepoint -60 (baseline). Significant main effects of stress were observed immediately after the restraint (timepoint 0) [F(1, 5) = 53.56, p < 0.001], 15 min post-stress [F (1, 5) = 125.82, p < 0.0001], and 30 min post-stress [F (1, 5) = 22.01, p < 0.01]. Pairwise comparisons showed that restraint increased CORT after both intra-dLS saline and Ex9 (p < 0.01) (Fig 5). At 60 and 120 min post-stress, CORT was no longer significantly elevated (Fig 5). At no point were there any effects of dLS Ex9. There was a significant main effect of stress on integrated area under curve (AUC) serum CORT response [F (1, 5) = 45.05, p< 0.001]. Restraint stress significantly increased CORT AUC after both intra-dLS saline and Ex9 treatment (p < 0.001): AUC after saline, 6534.33 ± 1579.83 ng/ml vs. saline + RES, 66503.5 ± 10579.15 ng/ml vs. Ex9, 10865 ± 5629.16 ng/ml vs. Ex9 + RES, 65255.67 ± 12415.07 ng/ml.
Figure 5.
Stress strongly increased serum CORT regardless of intra-dLS treatment. Serum CORT was significantly elevated immediately following restraint (timepoint 0), 15 and 30 min post-stress (*p < 0.01 vs. respective no stress condition). At 60 and 120 min post-stress, CORT was no longer significantly elevated. Data are shown as mean ± SEM. n = 6.
4. Discussion
Our results provide direct evidence that LS GLP-1R are involved in coordinating the hypophagic response to acute stress. Here, we used a pharmacologic approach to determine the relationship between GLP-1 signaling in the dLS and the hypophagic and CORT responses to an acute stressor. Intra-dLS infusion of saline followed by restraint stress suppressed dark-onset cumulative chow intake by 62% during the first 30 min and by 36% during the first hour of the dark cycle. This finding is consistent with previous work demonstrating that restraint stress reliably reduces food intake [31–33], and the largest effect of stress to reduce intake occurs within the first 30 min of the dark phase [2]. We found that at 30 min after dark onset, blockade of dLS GLP-1R had no impact on the ability of stress to suppress feeding, but later in the dark cycle, dLS GLP-1R blockade did significantly attenuate stress-induced hypophagia. This effect of Ex9 began to emerge by 2 h post-dark onset and the interaction between stress and Ex9 was statistically significant at 4 and 6 h post-dark. This time course suggests that the initial hypophagic response to stress is not mediated by dLS GLP-1R, but that these receptors play a role in sustaining that hypophagia for a longer duration by reducing compensatory feeding. Given the ability of lateral ventricle Ex9 to block stress-induced hypophagia within the first 30 min of the dark phase, GLP-1R in other brain regions (e.g., the BNST) are likely to play a role in the early anorexic response to stress [2], and the initiation of stress-induced hypophagia could be at least partially mediated through other, non-GLP-1R, mechanisms. Rats eventually compensated for the stress-induced suppression of intake under both saline and Ex9 conditions, but blocking dLS GLP-1R caused an earlier return to non-stressed levels of cumulative chow intake. These results support a role for dLS GLP-1Rs in the feeding response to stress, but also suggest that other neural circuitry must contribute to stress-induced hypophagia, as well.
Meal pattern analyses can provide insight into the behavioral mechanism of the cumulative feeding effects of stress and dLS GLP-1R blockade. Cumulative intake is the product of the number of meals taken and the size of those meals. Stress and dLS Ex9 could be acting on either or both of those variables, and they do not necessarily have to act on the same variable. For example, a reduction in meal size caused by stress could be compensated for by an increase in meal number caused by Ex9. However, we observed that the effect of stress to suppress feeding was entirely mediated by reduction in average meal size, and that dLS Ex9 blocked this effect, with no influence on meal number. This result demonstrates that stress and Ex9 are both influencing feeding through the same behavioral mechanism, and further support the idea that stress is acting to suppress feeding in part through dLS GLP-1Rs.
Detailed examination of the temporal pattern of feeding also helps us understand how stress suppressed cumulative intake so strongly during the first 30 min of the dark. We saw that the first meal size was suppressed by stress under dLS saline conditions, and there was a tendency for longer latency to begin eating after restraint stress under dLS saline and Ex9 conditions, though this did not reach statistical significance. Latency to begin the first meal after stress was highly variable across subjects, as shown by the large error bars in Fig. 2D. Particularly for those rats that took longer to begin their first meal, this in combination with a tendency toward longer meal durations means that in the stress conditions, several subjects did not complete their first meals of the dark phase within the first 30 minutes. By contrast, under the no-stress conditions, all but 1 rat completed their first meals within the first 30 min. Therefore, although stress effects on latency to begin the meal and meal duration were not statistically significant in pairwise comparisons, we suggest that the initial effect of stress on food intake may involve a combination of these factors with an effect on the size of that first meal.
In addition to examining stress-induced hypophagia here, we also assessed the CORT response to restraint stress. Activation of the HPA axis is modulated by complex networks of neural circuits that converge on CRH cells in the PVN, and changes in these neural pathways can lead to altered HPA activity [34]. The LS can modulate neuroendocrine stress responses [20], and central GLP-1 signaling has been shown to activate HPA axis stress responses [11–14]. Therefore, we hypothesized GLP-1Rs in the LS may play a role in modulating activation of the HPA axis. Specifically, we hypothesized that dLS GLP-1R blockade would reduce stress-induced CORT release. As expected, restraint stress potently increased CORT levels under dLS saline conditions, but, contrary to our hypothesis, CORT was equally stimulated by stress after dLS pretreatment with Ex9. These data suggest that GLP-1R in the dLS may not have a role in modulating HPA axis activity. In contrast, Ghosal and colleagues [10] have recently demonstrated that loss of GLP-1R function in the PVN attenuates the neuroendocrine response to both chronic variable stress and acute restraint stress. Specifically, they found that the plasma ACTH and CORT responses to restraint stress were suppressed in in mice that lack PVN GLP-1Rs. Together, these results suggest that endogenous GLP-1 in the PVN, but not the dLS contributes to the neuroendocrine response to acute stress. Interestingly, although Ghosal and colleagues [10] did not examine stress-induced hypophagia as we did here, they found no evidence of altered feeding behavior under non-stressed conditions in the mice lacking PVN GLP-1Rs. These dissociations between effects on feeding and HPA responses highlight the complexity of the neural circuitry that mediates responses to stress.
Close examination of the timing of the feeding and CORT responses to stress observed here provide additional clues about the underlying mechanisms. Within the first 30 min after dark onset (and the end of the restraint period), dLS Ex9 had no impact on the feeding response to stress, but rather interacted with stress significantly later on in the dark phase. The increase in CORT following restraint was essentially contained entirely within 60 min post-restraint, with the peak appearing immediately after the restraint period ended. Together, these data suggest that the ability of LS GLP-1R to attenuate stress-induced hypophagia during the later hours of the dark phase is independent of the CORT response to stress.
In conclusion, together with previous studies, our behavioral data suggest that dLS-projecting GLP-1 neurons play a role in both inhibiting intake during normal, non-stressed feeding conditions as well as in the response to acute restraint stress. However, this pathway does not appear to influence the neuroendocrine stress response. Overall, these findings suggest that the roles of GLP-1 neurons and brain GLP-1R populations in behavioral and physiological responses to stress are heterogeneous, and further research to determine which GLP-1R populations contribute to which responses is warranted.
Highlights.
Endogenous activation of GLP-1R in the dLS contributes to stress-induced hypophagia
Blockade of dLS GLP-1R attenuates acute restraint stress-induced hypophagia
Blockade of these receptors does not impact the CORT response to restraint
Acknowledgments
Grants
This work was funded by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (NIH R01-DK095757) to D.L.W and (NIH F31-DK115102) to S.J.T. We wish to thank the Society for the Study of Ingestive Behavior, the Sakai family and other donors to the Randall R. Sakai New Investigator Travel Award, which supported S.J.T.’s travel to the 2017 annual meeting to present some of the data included in this manuscript.
Footnotes
Disclosures
The authors have no conflicts of interest to declare.
Author Contributions
SJT and DLW developed the project ideas; SJT and CBM performed the experiments; SJT and DLW analyzed the data; SJT and DLW wrote the manuscript; and all authors contributed to editing the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Larsen PJ, Tang-Christensen M, Holst JJ, Ørskov C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience. 1997;77:257–270. doi: 10.1016/S0306-4522(96)00434-4. [DOI] [PubMed] [Google Scholar]
- 2.Maniscalco JW, Zheng H, Gordon PJ, Rinaman L. Negative Energy Balance Blocks Neural and Behavioral Responses to Acute Stress by “Silencing” Central Glucagon-Like Peptide 1 Signaling in Rats. J Neurosci. 2015;35:10701–10714. doi: 10.1523/JNEUROSCI.3464-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rinaman L. Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Res. 2010;1350:18–34. doi: 10.1016/j.brainres.2010.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrera JG, Jones KR, Herman JP, D’Alessio Da, Woods SC, Seeley RJ. Hyperphagia and increased fat accumulation in two models of chronic CNS glucagon-like peptide-1 loss of function. J Neurosci. 2011;31:3904–13. doi: 10.1523/JNEUROSCI.2212-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turton MD, O’Shea D, Gunn I, Beak SA, Edwards CMB, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JPH, Smith DM, Ghatei MA, Herbert J, Bloom SR. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- 6.Skibicka KP. The central GLP-1: implications for food and drug reward. Front Neurosci. 2013;7:181. doi: 10.3389/fnins.2013.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanoski SE, Hayes MR, Skibicka KP. Glp-1 and Weight Loss: Unraveling the Diverse Neural Circuitry. Am J Physiol - Regul Integr Comp Physiol. 2016 doi: 10.1152/ajpregu.00520.2015. ajpregu.00520.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghosal S, Myers B, Herman JP. Role of central glucagon-like peptide-1 in stress regulation. Physiol Behav. 2013;122:201–207. doi: 10.1016/j.physbeh.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kinzig KP, D’Alessio Da, Herman JP, Sakai RR, Vahl TP, Figueiredo HF, Murphy EK, Seeley RJ. CNS glucagon-like peptide-1 receptors mediate endocrine and anxiety responses to interoceptive and psychogenic stressors. J Neurosci. 2003;23:6163–6170. doi: 10.1523/JNEUROSCI.23-15-06163.2003. 23/15/6163 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghosal S, Packard AEB, Mahbod P, McKlveen JM, Seeley RJ, Myers B, Ulrich-Lai Y, Smith EP, D’Alessio DA, Herman JP. Disruption of glucagon-like peptide 1 signaling in Sim1 neurons reduces physiological and behavioral reactivity to acute and chronic stress. J Neurosci. 2017;37:184–193. doi: 10.1523/JNEUROSCI.1104-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larsen PJ, Tang-Christensen M, Jessop DS. Central administration of glucagon-like peptide-1 activates hypothalamic neuroendocrine neurons in the rat. Endocrinology. 1997;138:4445–4455. doi: 10.1210/en.138.10.4445. [DOI] [PubMed] [Google Scholar]
- 12.Lachey JL, D’Alessio DA, Rinaman L, Elmquist JK, Drucker DJ, Seeley RJ. The role of central glucagon-like peptide-1 in mediating the effects of visceral illness: Differential effects in rats and mice. Endocrinology. 2005;146:458–462. doi: 10.1210/en.2004-0419. [DOI] [PubMed] [Google Scholar]
- 13.Rinaman L. Interoceptive stress activates glucagon-like peptide-1 neurons that project to the hypothalamus. Am J Physiol. 1999;277:R582–R590. doi: 10.1152/ajpregu.1999.277.2.R582. [DOI] [PubMed] [Google Scholar]
- 14.Gil-Lozano M, Pérez-Tilve D, Alvarez-Crespo M, Martís A, Fernandez AM, Catalina PAF, Gonzalez-Matias LC, Mallo F. GLP-1(7-36)-amide and exendin-4 stimulate the HPA axis in rodents and humans. Endocrinology. 2010;151:2629–2640. doi: 10.1210/en.2009-0915. [DOI] [PubMed] [Google Scholar]
- 15.Maniscalco JW, Kreisler AD, Rinaman L. Satiation and Stress-Induced Hypophagia: Examining the Role of Hindbrain Neurons Expressing Prolactin-Releasing Peptide or Glucagon-Like Peptide 1. Front Neurosci. 2013;6:1–17. doi: 10.3389/fnins.2012.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol. 1954;47:419–427. doi: 10.1037/h0058775. http://psycnet.apa.orgjournals/com/47/6/419. [DOI] [PubMed] [Google Scholar]
- 17.Luo AH, Tahsili-Fahadan P, Wise Ra, Lupica CR, Aston-Jones G. Linking context with reward: a functional circuit from hippocampal CA3 to ventral tegmental area. Science. 2011;333:353–7. doi: 10.1126/science.1204622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anthony TE, Dee N, Bernard A, Lerchner W, Heintz N, Anderson DJ. Control of stress-induced persistent anxiety by an extra-amygdala septohypothalamic circuit. Cell. 2014;156:522–536. doi: 10.1016/j.cell.2013.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sartor GC, Aston-Jones GS. A septal-hypothalamic pathway drives orexin neurons, which is necessary for conditioned cocaine preference. J Neurosci. 2012;32:4623–31. doi: 10.1523/JNEUROSCI.4561-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singewald GM, Rjabokon A, Singewald N, Ebner K. The modulatory role of the lateral septum on neuroendocrine and behavioral stress responses. Neuropsychopharmacology. 2011;36:793–804. doi: 10.1038/npp.2010.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bakshi VP, Newman SM, Smith-Roe S, Jochman Ka, Kalin NH. Stimulation of lateral septum CRF2 receptors promotes anorexia and stress-like behaviors: functional homology to CRF1 receptors in basolateral amygdala. J Neurosci. 2007;27:10568–77. doi: 10.1523/JNEUROSCI.3044-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohata H, Shibasaki T. Involvement of CRF2 receptor in the brain regions in restraint- induced anorexia. Neuroreport. 2011;22:494–8. doi: 10.1097/WNR.0b013e3283487467. [DOI] [PubMed] [Google Scholar]
- 23.Mitra A, Lenglos C, Timofeeva E. Inhibition in the lateral septum increases sucrose intake and decreases anorectic effects of stress. Eur J Neurosci. 2014:1–14. doi: 10.1111/ejn.12798. [DOI] [PubMed] [Google Scholar]
- 24.Mitra A, Lenglos C, Timofeeva E. Activation of GABA A and GABA B receptors in the lateral septum increases sucrose intake by differential stimulation of sucrose licking activity. Behav Brain Res. 2014;273:82–88. doi: 10.1016/j.bbr.2014.07.035. [DOI] [PubMed] [Google Scholar]
- 25.Sweeney P, Yang Y. An excitatory ventral hippocampus to lateral septum circuit that suppresses feeding. Nat Commun. 2015;6:10188. doi: 10.1038/ncomms10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sweeney P, Yang Y. An Inhibitory Septum to Lateral Hypothalamus Circuit That Suppresses Feeding. J Neurosci. 2016;36:11185–11195. doi: 10.1523/JNEUROSCI.2042-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Göke R, Larsen PJ, Mikkelsen JD, Sheikh SP. Distribution of GLP-1 binding sites in the rat brain: Evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur J Neurosci. 1995;7:2294–2300. doi: 10.1111/j.1460-9568.1995.tb00650.x. [DOI] [PubMed] [Google Scholar]
- 28.Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999;403:261–80. doi: 10.1002/(sici)1096-9861(19990111)403:2<261::aid-cne8>3.0.co;2-5. http://www.ncbi.nlm.nih.gov/pubmed/9886047. [DOI] [PubMed] [Google Scholar]
- 29.Terrill SJ, Jackson CM, Greene HE, Lilly N, Maske CB, Vallejo S, Williams DL. Role of Lateral Septum Glucagon-Like Peptide 1 Receptors in Food Intake. Am J Physiol - Regul Integr Comp Physiol. 2016;311:R124–R132. doi: 10.1152/ajpregu.00460.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic; New York: 2007. [DOI] [PubMed] [Google Scholar]
- 31.Rybkin II, Zhou Y, Volaufova J, Smagin GN, Ryan DH, Harris RB. Effect of restraint stress on food intake and body weight is determined by time of day. Am J Physiol. 1997;273:R1612–R1622. doi: 10.1152/ajpregu.1997.273.5.R1612. [DOI] [PubMed] [Google Scholar]
- 32.Calvez J, Fromentin G, Nadkarni N, Darcel N, Even P, Tomé D, Ballet N, Chaumontet C. Inhibition of food intake induced by acute stress in rats is due to satiation effects. Physiol Behav. 2011;104:675–683. doi: 10.1016/j.physbeh.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 33.Krahn DD, Gosnell BA, Grace M, Levine AS. CRF antagonist partially reverses CRF- and stress-induced effects on feeding. Brain Res Bull. 1986;17:285–289. doi: 10.1016/0361-9230(86)90233-9. [DOI] [PubMed] [Google Scholar]
- 34.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]