Abstract
Background
Healthy lifestyle choices including participation in regular physical activity may improve health outcomes in survivors of childhood cancer. We aimed to evaluate the efficacy of a web-delivered, physical activity intervention among adolescent survivors to increase moderate-to-vigorous physical activity (MVPA) and improve fitness, neurocognitive and health-related quality of life (HRQoL) over 24-weeks.
Procedure
This randomized controlled trial was conducted among survivors (aged ≥ 11 to < 15 years) treated at a single institution. Participants were randomized to either a physical activity intervention delivered over the internet or a control group. The intervention group received educational materials, an activity monitor and access to an interactive website designed to motivate increased physical activity via rewards; the control group received an activity monitor and educational materials. Physical activity, fitness, neurocognitive and HRQoL outcomes were assessed at baseline and 24-weeks. Mean changes were compared between groups using paired t-tests.
Results
Of 97 survivors enrolled, 78 completed the study, mean age was 12.7 (SD 1.1), 80% were white, and 55.1% were female. Fifty-three survivors were assigned to the intervention and 25 to the control group. While survivors in the intervention group increased, and those in the control group decreased (4.7±119.9 vs. −24.3±89.7 minutes) weekly MVPA, this difference was not significant (p=0.30). However, hand grip strength, number of sit-ups and pushups, neurocognitive function, and HRQoL outcomes improved in the intervention, but not in the control group.
Conclusions
An interactive, rewards based intervention designed to increase MVPA is feasible in adolescent survivors of childhood cancer.
Keywords: physical activity, web-based intervention, pediatric cancer survivor, health related quality of life
INTRODUCTION
Survivors of childhood cancer are at increased risk of poor health outcomes like obesity and metabolic syndrome1 that could be potentially remediated with healthy lifestyle choices including regular physical activity. However, over half of childhood cancer survivors do not meet physical activity guidelines.2 Small studies of supervised in-hospital exercise interventions indicate that it is possible to improve cardiovascular fitness, muscle strength and flexibility among young survivors. More generalizable interventions designed to increase engagement in independent physical activity have had less success.3 Creative and engaging interventions may help facilitate survivors to initiate and maintain physical activity. The purpose of this study was to evaluate the initial efficacy of a web-delivered, interactive, rewards-based physical activity intervention among adolescent cancer survivors to increase moderate to vigorous physical activity (MVPA) and improve fitness, neurocognitive and health-related quality of life (HRQoL) outcomes over 24 weeks.
METHODS
Participants
This randomized controlled trial included survivors (of any diagnosis) who were treated at St. Jude Children’s Research Hospital (SJCRH); were ≥ 11 to < 15 years of age; were in active follow-up (not currently undergoing active treatment for cancer); did not meet the Centers for Disease Control and Prevention’s (CDC) physical activity guidelines at enrollment (e.g. ≥ 60 minutes of activity a day, seven days a week); and had internet access and a computer with software compatible with the study activity monitor. Participants were randomized to either a rewards based intervention or a control group (2:1 randomization). The study protocol was approved by the St. Jude Children’s Research Hospital Institutional Review Board. Informed consent (from the guardian) and assent (from the child) was given prior to enrollment on study.
Intervention
Participants in the intervention group received educational materials, an activity monitor and access to an interactive website designed to encourage physical activity via rewards. When participants first logged into the website, they created an avatar (a character used to represent the participant). Participants uploaded individual physical activity data from their monitor to the website, accumulating points based on daily activity levels. The goal was to progress the avatar through various levels on the website using the points earned. Points could also be redeemed for small prizes (e.g. t-shirts, stickers) and/or gift cards. Participants in the control group received an activity monitor and educational materials only.
Outcome Measures
Physical Activity
Physical activity was assessed using accelerometry at baseline, 12 and 24-weeks. The triaxial accelerometer (wGT3X-BT; ActiGraph, Pensacola FL) detects and records the magnitude of acceleration as counts per preset epoch, predefined as 60 seconds. Participants wore the device for seven days. In addition, physical activity was continuously monitored with intervention study device throughout the 24-weeks. Data was automatically transferred to the website administrator when the device was connected to the computer for both the intervention and control groups. The control group connected the device to transmit data and charge the device but did not have access to the intervention website.
Fitness
Hand grip strength in kilograms was measured using a Jamar hand held dynamometer (Sammons Preston Rolyan, Nottinghamshire, UK), with the participant in a seated position and the shoulder at 0-10° and the elbow in 90° of flexion. Each participant completed three trials and the average was used for analysis.4 To assess proximal muscle strength, participants were asked to do full sit ups and either a knee or full pushup for 30 seconds, with the number completed recorded and used for analysis. Handgrip, sit-ups and pushups were assessed at baseline and 24-weeks only.
Neurocognitive
General intelligence was assessed using the Wechsler Abbreviated Scale of Intelligence (WASI).5 which provides a Full Scale Intelligence Quotient (IQ), based on tests of vocabulary and visual-spatial construction. Cognitive flexibility was assessed using the Delis-Kaplan Executive Function System (D-KEFS).6 Both measures were administered at baseline and 24-weeks.
Health Related Quality of Life
Health related quality of life was measured using the Pediatric Quality of Life Inventory (PedsQL) v4.0.7 The PedsQL provides an overall general quality of life score, and five subscales that characterize domains of psychosocial, physical, emotional, social and school functioning. HRQoL was collected at baseline and 24-weeks.
Statistical Approach
Descriptive statistics were calculated and compared between groups using t-tests, chi-square tests and Fishers exact tests where appropriate. Change in each respective outcome was assessed by calculating the difference between baseline and 24-week follow-up. Change from baseline to 24-week follow up in the intervention group was compared to change in the control group using t-tests for MPVA and fitness, neurocognitive and HRQoL outcomes. All analysis were conducted in SAS v9.4 (Cary, NC).
RESULTS
Participant recruitment is described in the CONSORT diagram (Fig. 1). Of the 189 survivors eligible for recruitment, 97 survivors enrolled and 78 completed the study. The mean age was 12.7 (SD 1.1), 80% were white, and 55.1% were female (Table 1). Fifty-three survivors were assigned to the intervention and 25 to the control group. Baseline average weekly MVPA was 175 minutes (SD 101.9) for the intervention and 200 (SD 90.6) for the control group. The intervention and control groups did not differ by demographics, treatment modalities, diagnosis type, or baseline MVPA (Table 1). In addition, there were no differences in characteristics between those who completed the intervention and those who withdrew or were lost to follow up after randomization. The completion rate for the intervention group was slightly higher than the completion rate in the control group (84.2% vs 80.6%, respectively – see Fig. 1). Of the 92 survivors who did not enroll in the study, 2 (2.8%) indicated they were already physically active; 6 (8.4%) had parents who reported medical or behavioral issues; 21 (29.6%) reported being either too busy, did not want to miss school to travel for follow-up appointment at 6 months or just did not want to commit to a 6 month intervention; 4 (5.6%) did not want to wear the activity monitor for 6 months; 8 (11.3%) were not interested; and 30 (42.3%) did not give a specific reason. Of the 16 who were randomized and did not complete the intervention, 8 (50.0%) were lost to follow up; 7 (43.8%) did not give a specific reason for withdrawing; and 1 (6.2%) was overwhelmed with school commitments. A total of 4 participants never accessed the website, of which 3 withdrew and 1 completed the intervention. Of those that completed the intervention, roughly 62% progressed their avatar to at least one level, with 20% progressing through level 6 and beyond.
Figure 1.
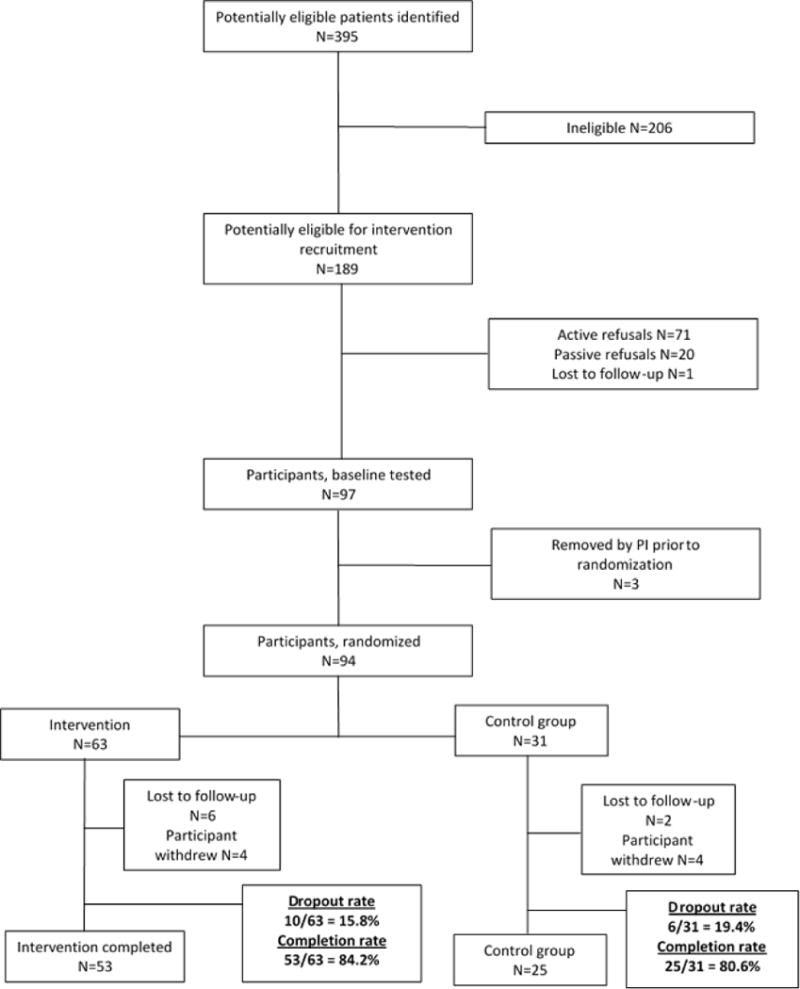
CONSORT diagram describing participant flow for the study. Of the 189 potentially eligible survivors for recruitment, 94 were randomized, with 63 assigned to the intervention and 31 assigned to the control group. The completion rate was 84.2% (n=53) for the intervention group and 80.6% (n=25) for the control group.
Table 1.
Characteristics of the study population by study status and randomization status
| Completed Study N=78 |
Withdrew N=16 |
P | Intervention Group N=53 |
Control Group N=25 |
P | |
|---|---|---|---|---|---|---|
| Current Age, Median (Range) | 12.7 (11.0-15.0) | 12.5 (11.3-14.9) | 0.96a | 12.8 (11.1-14.9) | 12.4 (11.0-15.0) | 0.21a |
| Age at Diagnosis, Median (Range) | 2.6 (0.0-11.3) | 4.9 (0.5-11.3) | 0.09a | 2.5 (0.0-11.3) | 3.1 (0.3-9.4) | 0.72a |
| Survival time, Median (Range) | 9.3 (2.4-14.3) | 7.8 (1.2-13.7) | 0.16a | 9.9 (2.4-14.3) | 8.7 (3.0-13.7) | 0.52a |
| Gender, N (%) | ||||||
| Female | 43 (55.1) | 4 (25.0) | 0.05b | 29 (54.7) | 14 (56.0) | 0.92c |
| Male | 35 (44.9) | 12 (75.0) | 24 (45.3) | 11 (44.0) | ||
| Race, N (%) | ||||||
| Black | 13 (16.7) | 5 (31.3) | 0.35b | 7 (13.2) | 6 (24.0) | 0.49b |
| White | 62 (79.5) | 11 (68.8) | 44 (83.0) | 18 (72.0) | ||
| Other | 3 (3.9) | 0 (0.0) | 2 (3.8) | 1 (4.0) | ||
| Diagnosis, N (%) | ||||||
| Acute lymphoblastic leukemia | 18 (23.1) | 6 (37.6) | 0.93b | 12 (22.6) | 6 (24.0) | 0.43b |
| Acute myeloid leukemia | 1 (1.3) | 0 (0.0) | 0 (0.0) | 1 (4.0) | ||
| CNS Tumors | 17 (21.8) | 3 (18.8) | 14 (26.4) | 3 (12.0) | ||
| Ewing Sarcoma | 1 (1.3) | 1 (6.2) | 1 (1.9) | 0 (0.0) | ||
| Germ cell tumor | 1 (1.3) | 0 (0.0) | 1 (1.9) | 0 (0.0) | ||
| Hodgkin lymphoma | 2 (2.5) | 0 (0.0) | 1 (1.9) | 1 (4.0) | ||
| Neuroblastoma | 6 (7.7) | 2 (12.6) | 3 (5.7) | 3 (12.0) | ||
| Non-Hodgkin lymphoma | 6 (7.7) | 1 (6.2) | 3 (5.7) | 3 (12.0) | ||
| Retinoblastoma | 11 (14.1) | 1 (6.2) | 9 (17.0) | 2 (8.0) | ||
| Rhabdomyosarcoma | 4 (5.1) | 1 (6.2) | 3 (5.7) | 1 (4.0) | ||
| Soft tissue sarcoma | 1 (1.3) | 0 (0.0) | 1 (1.9) | 0 (0.0) | ||
| Wilms tumor | 6 (7.7) | 1 (6.2) | 4 (7.6) | 2 (8.0) | ||
| Other malignancy | 4 (5.1) | 0 (0.0) | 1 (1.9) | 3 (12.0) | ||
| Treatment, N (%) | ||||||
| Surgery | 73 (93.6) | 15 (93.8) | 0.98c | 49 (92.5) | 24 (96.0) | 0.55c |
| Chemotherapy | 63 (80.8) | 13 (81.3) | 0.96c | 43 (81.1) | 20 (80.0) | 0.91c |
| Radiation | 29 (37.2) | 7 (43.8) | 0.62c | 19 (35.9) | 10 (40.0) | 0.72c |
| Physical Activity | ||||||
| Baseline Weekly MVPA, Mean minutes (SD) | 183.0 (98.5) | 158 (113.5) | 0.37d | 175.0 (101.9) | 200.2 (90.6) | 0.29d |
| Baseline Weekly MVPA, Median minutes (Range) | 168.6 (0.0-366.3) | 146.6 (0.0-336.7) | 0.39a | 161.7 (0.0-361.2) | 193.4 (0.0-366.3) | 0.28a |
Wilcoxon test
Fishers exact test
Chi-square test
T-test
Survivors who were enrolled in the intervention increased their MVPA and maintained that increase over time (Fig. 2), while survivors in the control group steadily decreased their weekly MVPA. There was no statistical difference between groups for mean change in weekly MVPA after 24-weeks (4.7 (SD 119.9) minutes intervention group, −24.3 (SD 89.7) control group, p=0.30). Mean changes (Table 2) in fitness measures (hand grip strength, number of sit-ups and pushups), two neurocognitive outcomes (e.g. Full Scale IQ and Inhibitory control), and HRQoL outcomes assessed using the Pediatric Quality of Life Inventory v48 (e.g. overall score, and physical functioning subscale) statistically improved over time in the intervention group, but not in the control group. However, this result could be due to sample size since randomization was 2:1 (intervention vs. control allotment). In addition, the change in fitness, neurocognitive outcomes and HRQoL between the intervention group and control group did not differ statistically at 6 month follow-up (see Comparison of Change results in Table 2). Intervention efficacy did not differ by level of avatar advancement among participants in the intervention group.
Figure 2.
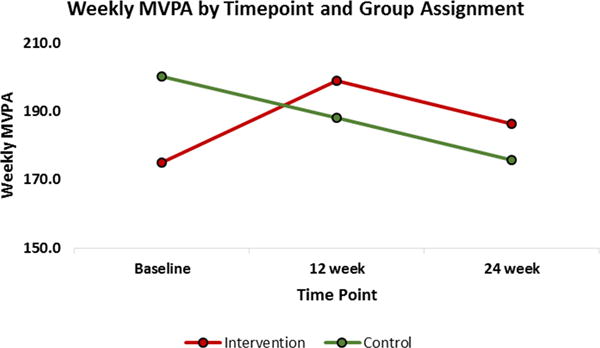
Weekly moderate to vigorous physical activity (MVPA) by intervention group and time point. Survivors in the intervention group increased MVPA from baseline to 12-weeks, and then decreased slightly from 12-weeks to 24-weeks but still maintained an increase across time compared to baseline MVPA. The control group steadily declined in MVPA across time.
Table 2.
Changes in fitness, neurocognitive and HRQoL outcomes by intervention and control group from baseline to 24-week follow-up
| Intervention Group | Control Group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Baseline to 24 week follow up | Baseline to 24 week follow up | Comparison of Change – Intervention vs. Control | |||||||||
|
| |||||||||||
| Fitness Outcomes | N | Baseline Value (SD) | Mean Change | T-statistica | p | N | Baseline Value (SD) | Mean Change | T-statistica | p | p |
| Hand grip, kg | 50 | 19.95 (6.00) | 1.13 | 2.65 | 0.01 | 24 | 22.27 (7.21) | 1.14 | 1.27 | 0.22 | 0.99 |
| Sit ups, number in 30 sec | 49 | 15.08 (5.71) | 2.49 | 3.27 | 0.002 | 22 | 15.70 (5.91) | 2.5 | 2.13 | 0.05 | 0.99 |
| Pushups, number in 30 sec | 48 | 11.08 (5.83) | 2.67 | 3.58 | 0.0008 | 21 | 11.61 (6.46) | 2.14 | 1.52 | 0.14 | 0.72 |
| Neurocognitive Outcomes | |||||||||||
| WASI Full Scale IQ Z-Score b | 51 | −0.18 (0.80) | 0.13 | 2.07 | 0.04 | 24 | −0.28 (0.51) | 0.05 | 0.44 | 0.67 | 0.55 |
| D-KEFS Inhibition Z-Score c | 50 | −0.04 (0.84) | 0.23 | 2.82 | 0.007 | 22 | −0.10 (0.70) | 0.12 | 0.81 | 0.43 | 0.50 |
| Quality of Life Outcomesd | |||||||||||
| PEDS QL4 Total Mean | 52 | 74.19 (16.02) | 3.83 | 2.63 | 0.01 | 24 | 75.32 (17.98) | 1.71 | 0.70 | 0.49 | 0.44 |
| PEDS QL4 Psychosocial Summary Mean | 52 | 73.83 (16.40) | 2.83 | 1.82 | 0.07 | 24 | 75.20 (16.99) | 1.18 | 0.64 | 0.53 | 0.53 |
| PEDS QL4 Physical Functioning Mean | 52 | 74.88 (19.21) | 5.65 | 2.69 | 0.01 | 24 | 75.54 (23.41) | 2.70 | 0.58 | 0.57 | 0.57 |
| PEDS QL4 Emotional Functioning Mean | 52 | 75.72 (15.23) | 2.64 | 1.85 | 0.07 | 24 | 78.80 (19.00) | −0.63 | −0.22 | 0.83 | 0.25 |
| PEDS QL4 Social Functioning Mean | 52 | 76.54 (21.09) | 3.87 | 1.92 | 0.06 | 24 | 76.80 (21.64) | 4.58 | 1.40 | 0.18 | 0.85 |
| PEDS QL4 School Functioning Mean | 52 | 69.23 (20.85) | 1.92 | 0.86 | 0.39 | 24 | 70.00 (21.21) | −0.42 | −0.14 | 0.89 | 0.55 |
SD: standard deviation, HRQoL: health related quality of life
paired T-test
Wechsler Abbreviated Scale of Intelligence, full scale IQ score converted to a z-score (higher score indicates better function)
Delis-Kaplan Executive Function system, Color-Word Interference Test (higher score indicates better function)
Pediatric Quality of Life Inventory (PedsQL) v4.0 (higher score indicates better quality of life)
DISCUSSION
Although changes in MVPA did not differ significantly between the intervention and control group, variability in our sample was high (pooled SD: 110.9 minutes per week), indicating insufficient power to detect a difference in MVPA across 24-weeks. The a priori hypothesis was to detect a difference of 4 minutes of MVPA between the two groups (28-minutes per week). This null result with a small sample is consistent with a previous study9 that found similar results in adolescent cancer survivors (14-18 years) using an activity monitor and a virtual support group to increase MVPA. Replicating this intervention in a larger cohort of survivors of childhood cancer would provide the power needed to test the hypothesis adequately. This is warranted since no large scale multi-site portable and affordable physical intervention activity studies have been conducted in the young adolescent cancer survivor population.
The decrease we saw in the control group’s MVPA over the course of the 6 month period is consistent with previous publications.10,11 For instance, children ages 9 to 15 years in the general population decrease their daily MVPA an average of 37 minutes a day per year.11 This reduction could also be due to the large variability in MVPA at baseline. When we examined mean changes in fitness, neurocognitive function and HRQoL by group, participants in the intervention group significantly improved while those in the control group did not. Improvements in grip strength, and number of sit-ups and pushups, are parallel with findings from a study that found improvements in upper body strength in adolescent cancer survivors over a 10-week physical activity intervention.12 The modest gains we saw in two neurocognitive outcomes are similar to an association found by Paxton et al13 between self -reported cognition and leisure time activity in adolescent cancer survivors. Riggs et al found that aerobic exercise training increased white matter, hippocampal volume and cognitive reaction times in children survivors of brain tumors after a 12 week training period, with increases still observed at 24 weeks14. Additionally, in a non-cancer population, Hillman et al15 reported that healthy preadolescent children with higher levels of fitness (aerobic capacity, muscle strength and flexibility) had higher amplitudes of P3 (a component of event-related brain potentials that has been associated with increased attention16). Exercise and improvements in HRQoL have been reported previously in childhood cancer survivors3,12,17–19. Exercise may improve physical function specific HRQoL through improvements in cardiovascular fitness, while overall HRQoL improvement may be driven mainly by improvements in physical function HRQoL13 or by general improvements in mood (e.g. increases in serotonin) and increases in positive body image20.
Although fitness and neurocognitive measures improved over time in the controls, the differences were not statistically different from baseline to 24-week follow-up. This improvement could be due to practice effects21 or potentially age-related increases in fitness and neurocognitive outcomes. It is important to note that the change in the fitness measures in the intervention group were of similar magnitude to the change in the control group, indicating that the improvements we observed in the intervention group may be due to differences in sample size between the two groups. These preliminary findings indicate that increasing MVPA may have positive effects on fitness, neurocognitive and HRQoL outcomes in adolescent survivors of pediatric cancer; however, further investigation in a larger population and over a longer intervention period is warranted.
This pilot study was conducted to gather preliminary data for a larger trial. After conducting the pilot, the authors concluded that: (1) due to MVPA variability, we would need to recruit a larger sample to adequately test the original study hypothesis or identify an alternate primary outcome measure; (2) testing this intervention in a homogenous group of survivors would lend specific information regarding a focused disease group; and (3) evaluating and subsequently implementing the intervention at multiple sites would test the generalizability and portability of the intervention outside of SJCRH. To this end, the authors are currently leading an effort to expand the intervention across multiple Children’s Oncology Group (COG; https://www.childrensoncologygroup.org) sites, with the addition of more outcome measures and a specific focus on one diagnosis group, acute lymphoblastic leukemia (ALL). We will conduct a two arm randomized study to evaluate the effects of this intervention on cardiopulmonary fitness, muscle strength, cardiovascular disease risk factors (blood pressure, obesity, lipids, insulin and glucose), fatigue, quality of life, and school attendance (ClinicalTrials.gov Trial Number: NCT03223753).
This expanded trial will be achieved by recruiting children 8-15 years of age, diagnosed with ALL, who received treatment at a COG site, are in remission, and are within the first 3 months after completion of curative therapy. Outcome measures will be assessed at enrollment (prior to randomization), at 6 months (end of intervention) and one year off therapy. We planned study implementation for multiple sites with an approach that minimizes staff and institutional burden, with the investigators overseeing training, standardization of all of the measures, and quality control checks. The evaluations can be done by a clinical research associate, research nurse or other designated staff member. A training video with accompanying training manual that teaches standardized procedures for collecting the outcome measures and instructions for use of the study device will be sent to participating sites.
Overall, the pilot study presented here provides the foundation for the development of an easily disseminated and affordable physical activity intervention in the childhood cancer survivor population. While potentially challenging to scale up, the intervention is convenient, portable, cost-effective and feasible. Since the intervention is home- and internet-based, it is easier to disseminate than intensive, clinic based interventions that require staff to deliver the intervention components. Similar convenient and portable physical activity interventions such as active video games have reported encouraging results in improving physical activity among youth.22,23 Because prior behavior patterns predict future behavior,24 the use of a rewards-based intervention to help encourage activity may help to promote lifelong physical activity,25,26–28 as these children and adolescents enter a period of growing autonomy and independence from parents.29,30 Importantly, web-based interactive physical activity interventions for young cancer survivors may provide opportunities to minimize long-term morbidities.
Acknowledgments
Funding Sources: This work was supported by National Institutes of Health National Cancer Institute Cancer Center Core Grant CA 21765 and by the American Lebanese Syrian Associated Charities; devices, website access and study support provided by HopeLab.
Abbreviations Key
- MVPA
Moderate to vigorous physical activity
- HRQoL
Health related quality of life
- SD
Standard deviation
- ALL
Acute lymphoblastic leukemia
- COG
Children’s Oncology Group
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
References
- 1.Barnea D, Raghunathan N, Friedman DN, Tonorezos ES. Obesity and Metabolic Disease After Childhood Cancer. Oncology (Williston Park) 2015;29(11):849–855. [PMC free article] [PubMed] [Google Scholar]
- 2.Ness KK, Leisenring WM, Huang S, et al. Predictors of inactive lifestyle among adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Cancer. 2009;115(9):1984–1994. doi: 10.1002/cncr.24209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang TT, Ness KK. Exercise interventions in children with cancer: a review. Int J Pediatr. 2011;2011:461512. doi: 10.1155/2011/461512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mathiowetz V, Weber K, Volland G, Kashman N. Reliability and validity of grip and pinch strength evaluations. J Hand Surg Am. 1984;9(2):222–226. doi: 10.1016/s0363-5023(84)80146-x. [DOI] [PubMed] [Google Scholar]
- 5.Wechsler D. Manual for the Wechsler abbreviated intelligence scale (WASI) San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- 6.Delis DC, Kaplan E, Kramer JH. Delis-Kaplan executive function system (D-KEFS) Psychological Corporation; 2001. [Google Scholar]
- 7.Varni JW, Seid M, Knight TS, Uzark K, Szer IS. The PedsQLTM 4.0 Generic Core Scales: Sensitivity, responsiveness, and impact on clinical decision-making. J Behav Med. 2002;25(2):175–193. doi: 10.1023/a:1014836921812. [DOI] [PubMed] [Google Scholar]
- 8.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39(8):800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Mendoza JA, Baker KS, Moreno MA, et al. A Fitbit and Facebook mHealth intervention for promoting physical activity among adolescent and young adult childhood cancer survivors: A pilot study. Pediatr Blood Cancer. 2017 doi: 10.1002/pbc.26660. [DOI] [PubMed] [Google Scholar]
- 10.Sallis JF. Epidemiology of physical activity and fitness in children and adolescents. Crit Rev Food Sci Nutr. 1993;33(4-5):403–408. doi: 10.1080/10408399309527639. [DOI] [PubMed] [Google Scholar]
- 11.Nader PR, Bradley RH, Houts RM, McRitchie SL, O’Brien M. Moderate-to-vigorous physical activity from ages 9 to 15 years. JAMA. 2008;300(3):295–305. doi: 10.1001/jama.300.3.295. [DOI] [PubMed] [Google Scholar]
- 12.Keats MR, Culos-Reed SN. A community-based physical activity program for adolescents with cancer (project TREK): program feasibility and preliminary findings. J Pediatr Hematol Oncol. 2008;30(4):272–280. doi: 10.1097/MPH.0b013e318162c476. [DOI] [PubMed] [Google Scholar]
- 13.Paxton RJ, Jones LW, Rosoff PM, Bonner M, Ater JL, Demark-Wahnefried W. Associations between leisure-time physical activity and health-related quality of life among adolescent and adult survivors of childhood cancers. Psychooncology. 2010;19(9):997–1003. doi: 10.1002/pon.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riggs L, Piscione J, Laughlin S, et al. Exercise training for neural recovery in a restricted sample of pediatric brain tumor survivors: a controlled clinical trial with crossover of training versus no training. Neuro Oncol. 2017;19(3):440–450. doi: 10.1093/neuonc/now177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hillman CH, Castelli DM, Buck SM. Aerobic fitness and neurocognitive function in healthy preadolescent children. Med Sci Sports Exerc. 2005;37(11):1967–1974. doi: 10.1249/01.mss.0000176680.79702.ce. [DOI] [PubMed] [Google Scholar]
- 16.Polich J, Ladish C, Burns T. Normal variation of P300 in children: age, memory span, and head size. Int J Psychophysiol. 1990;9(3):237–248. doi: 10.1016/0167-8760(90)90056-j. [DOI] [PubMed] [Google Scholar]
- 17.Gohar SF, Comito M, Price J, Marchese V. Feasibility and parent satisfaction of a physical therapy intervention program for children with acute lymphoblastic leukemia in the first 6 months of medical treatment. Pediatr Blood Cancer. 2011;56(5):799–804. doi: 10.1002/pbc.22713. [DOI] [PubMed] [Google Scholar]
- 18.San Juan AF, Chamorro-Vina C, Mate-Munoz JL, et al. Functional capacity of children with leukemia. Int J Sports Med. 2008;29(2):163–167. doi: 10.1055/s-2007-964908. [DOI] [PubMed] [Google Scholar]
- 19.Speyer E, Herbinet A, Vuillemin A, Briancon S, Chastagner P. Effect of adapted physical activity sessions in the hospital on health-related quality of life for children with cancer: a cross-over randomized trial. Pediatr Blood Cancer. 2010;55(6):1160–1166. doi: 10.1002/pbc.22698. [DOI] [PubMed] [Google Scholar]
- 20.Ortega FB, Ruiz JR, Castillo MJ, Sjostrom M. Physical fitness in childhood and adolescence: a powerful marker of health. Int J Obes (Lond) 2008;32(1):1–11. doi: 10.1038/sj.ijo.0803774. [DOI] [PubMed] [Google Scholar]
- 21.Bartels C, Wegrzyn M, Wiedl A, Ackermann V, Ehrenreich H. Practice effects in healthy adults: a longitudinal study on frequent repetitive cognitive testing. BMC Neurosci. 2010;11(1):118. doi: 10.1186/1471-2202-11-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barnett A, Cerin E, Baranowski T. Active Video Games for Youth: A Systematic Review. J Phys Act Health. 2011;8(5):724–737. doi: 10.1123/jpah.8.5.724. [DOI] [PubMed] [Google Scholar]
- 23.Biddiss E, Irwin J. Active video games to promote physical activity in children and youth: a systematic review. Arch Pediatr Adolesc Med. 2010;164(7):664–672. doi: 10.1001/archpediatrics.2010.104. [DOI] [PubMed] [Google Scholar]
- 24.Rhodes RE, Courneya KS, Jones LW. Personality and social cognitive influences on exercise behavior: adding the activity trait to the theory of planned behavior. Psychol Sport Exerc. 2004;5(3):243–254. [Google Scholar]
- 25.Dishman RK, Buckworth J. Increasing physical activity: a quantitative synthesis. Med Sci Sports Exerc. 1996;28(6):706–719. doi: 10.1097/00005768-199606000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Must A, Jacques PF, Dallal GE, Bajema CJ, Dietz WH. Long-term morbidity and mortality of overweight adolescents. A follow-up of the Harvard Growth Study of 1922 to 1935. N Engl J Med. 1992;327(19):1350–1355. doi: 10.1056/NEJM199211053271904. [DOI] [PubMed] [Google Scholar]
- 27.Dietz WH. Periods of Risk in Childhood for the Development of Adult Obesity — What Do We Need to Learn? J Nutr. 1997;127(9):1884S–1886S. doi: 10.1093/jn/127.9.1884S. [DOI] [PubMed] [Google Scholar]
- 28.Serdula MK, Ivery D, Coates RJ, Freedman DS, Williamson DF, Byers T. Do obese children become obese adults? A review of the literature. Prev Med. 1993;22(2):167–177. doi: 10.1006/pmed.1993.1014. [DOI] [PubMed] [Google Scholar]
- 29.Steinberg L. Adolescence. 9. New York: McGraw-Hill Humanities/Social Sciences/Languages; 2010. [Google Scholar]
- 30.Christie D, Viner R. Adolescent development. BMJ. 2005;330(7486):301–304. doi: 10.1136/bmj.330.7486.301. [DOI] [PMC free article] [PubMed] [Google Scholar]


