Abstract
Background
High-dose aspirin (HDA) is used with intravenous immunoglobulin (IVIg) in Kawasaki Disease (KD). Practice regarding HDA varies, and it is unclear whether HDA duration affects long-term course.
Methods
We retrospectively studied KD patients at our hospital over 10 years. Patients were categorized as having received HDA for 0, 1–7, or >7 days. Primary outcome was maximum coronary Z-score at diagnosis and follow-up; secondary outcomes included inflammatory markers.
Results
103 patients had HDA duration documented; 35 had coronary artery abnormalities (CAAs) at diagnosis. There was no difference in demographics or inflammatory markers between HDA groups, and no difference in HDA duration between patients with or without CAAs. Seventeen patients received no HDA; they had longer illness and defervescence duration before diagnosis and were less likely to receive IVIg. For CAAs, multivariate regression revealed that HDA duration did not predict coronary Z-score at 9–15 months. Higher Z-score at diagnosis was associated with higher Z-score at 9–15 months.
Conclusion
The only factor associated with coronary Z-score at 9–15 months was Z-score at diagnosis. At our institution, longer illness and defervescence duration and lack of IVIg administration were associated with not administering HDA. HDA duration did not affect clinically-relevant outcomes, particularly CAA persistence.
INTRODUCTION
Kawasaki Disease (KD) is an acute vasculitis syndrome first described by Dr. Tomisaku Kawasaki in 1967 (1). It most commonly affects children under the age of 5 years and is typically self-limited. Its classic symptoms include high fevers and signs of generalized inflammation of the skin, eyes, mouth, cervical lymph nodes, and palms and soles (2). The vasculitis affects medium-sized blood vessels, most notably coronary artery abnormalities (CAAs), and can cause chronic alterations in the coronary circulation. CAAs occur in up to 25% of untreated patients (3), with the most serious consequence being death from acute coronary artery thrombosis (4).
Intravenous immunoglobulin (IVIg) was first shown in 1984 to reduce the incidence of CAAs in KD (5). A number of dosing regimens were studied, and in 1991 the most effective of those was shown to be a single infusion of 2 g/kg administered over 12 hours (6). This dose was instituted in the 1993 American Heart Association (AHA) scientific statement on KD (7) and has been standardly used since then.
Acetylsalicylic acid or aspirin was first proposed as a therapeutic option for KD, given both its anti-inflammatory and anti-platelet effects, when thrombocytosis was recognized as a key feature of the disease 6 years after its initial description (8). At moderate (30–50 mg/kg/day) and high (80–100 mg/kg/day) doses, aspirin exerts anti-inflammatory effects, whereas at lower doses (3–5 mg/kg/day), the predominant effect is anti-platelet. The initial recommended dose of aspirin in the acute phase of KD in Japan was 30 mg/kg/day (9,10). Simultaneously, data published in the United States demonstrated that malabsorption of aspirin may occur in the acute phase, necessitating higher doses to achieve clinical effects (11,12). This notion was supported by data indicating that aspirin doses of 80–100 mg/kg/day could shorten the duration of fever (4) and, when compared to no aspirin therapy, were associated with a decreased incidence of CAAs (13).
These early data on the effect of aspirin on CAAs were questioned in 1992 when a multi-center study demonstrated no difference in the incidence of CAAs between patients treated with IVIg and high-dose aspirin (HDA), defined in that trial as 100 mg/kg/day, versus those treated with IVIg and low-dose aspirin (5–10 mg/kg/day) (14). The same conclusion was reached by a meta-analysis that showed no difference in the incidence of CAAs at 30 or 60 days after disease onset between the low- or high-dose aspirin groups, with high-dose aspirin being defined as > 80 mg/kg/day (15). A subsequent meta-analysis showed that the prevalence of CAAs was inversely related to the total dose of IVIg but was independent of the aspirin dose used (16).
With respect to effects of aspirin on the duration of fever, which can influence the length of hospitalization, a 1992 study found that HDA was associated with a shorter duration of fever (14); however, a more recent comparison of HDA and low-dose aspirin in the treatment of KD showed no difference in fever duration between the two groups (17).
The conflicting evidence regarding the effects of HDA on CAA incidence and fever duration is reflected in the guidelines for the treatment of KD. In 1988, a consensus statement prepared by North American participants of the Third International Kawasaki Disease Symposium recommended HDA of 80–100 mg/kg/day for 14 days in the management of KD (18). Five years later, the AHA statement on KD recommended HDA until the patient was afebrile, acknowledging, however, that some clinicians recommend HDA until the 14th day of illness (7). The 2004 and recently-published 2017 AHA statements on KD once again recognized differences in practices regarding HDA dosing among institutions (19,20). Both statements specifically note that HDA “does not appear to lower the frequency of the development of coronary abnormalities”, based on evidence evaluating the effect of HDA on CAAs at early follow-up - 30 and 60 days after diagnosis (15,21). The 2017 AHA statement recommends that “administration of moderate- (30–50 mg/kg/day) to high-dose (80–100 mg/kg/day) aspirin is reasonable until the patient is afebrile, although there is no evidence that it reduces coronary artery aneurysms” (20). Furthermore, there are no data to indicate superiority of either the 30–50 mg/kg/day dose used in Japan and Europe or the 80–100 mg/kg/day dose used in the United States (20).
The purpose of the present study was to evaluate a single center’s experience of demographic and illness factors that may affect clinician practices regarding HDA duration prescribed in acute KD, as well as the impact of HDA duration on key clinical outcomes, including long-term status of CAAs up to 15 months after diagnosis, IVIg resistance, duration of fever, and normalization of inflammatory markers.
METHODS
A retrospective study was performed at the University of Minnesota Masonic Children’s Hospital, a tertiary referral center. Medical records of all children with an International Classification of Diseases-9 (ICD-9) diagnosis of KD between September 2006 and December 2015 were reviewed. Of 126 patients who met the criteria of acute KD, 103 patients had HDA duration documented and were included in the study. This chart review was approved by the University of Minnesota Institutional Review Board.
During the decade of this study, there was no standardized approach to determine the duration of HDA therapy at our institution. Because numerous providers cared for these patients, there was variability in practice regarding HDA duration. The decision to change from HDA to low-dose aspirin appeared to be influenced by laboratory markers of inflammation or the presence of CAA, but the rationale for the timing of the change was not clearly documented in several cases.
Patients were categorized as having received one of three durations of HDA: 0 days, 1–7 days, or more than 7 days. Demographic and illness variables compared among these three groups included age, sex, race, days of illness before diagnosis, and classic versus incomplete KD status. Fever was defined as a temperature of 38°C or higher. Criteria for classic and incomplete KD were defined according to the 2017 AHA statement on KD (20). Coronary artery dimensions were determined by transthoracic echocardiography and were recorded as body surface area-adjusted Z-scores (22). The primary outcome measure was the maximum coronary artery Z-score. This measure, along with the secondary outcome measures of C-reactive protein (CRP) and platelet count were compared at the time of diagnosis, at 4–8 weeks after diagnosis, and at 9–15 months after diagnosis. CAAs were defined as a maximum coronary artery Z-score greater than or equal to 2.5 (2). Other secondary outcomes recorded included days of fever after diagnosis and IVIg resistance. IVIg-resistant patients were defined as those who received more than one dose of 2g/kg of IVIg and/or additional therapies including corticosteroids, biologic therapeutics, or other immunosuppressive agents, given at least 36 hours since the end of the first IVIg infusion.
Statistical analysis was performed using the SAS software package (version 9.3; SAS Institute Inc., Cary, NC). P-values were calculated using one-way analysis of variance for continuous variables and Fisher’s exact test for categorical variables. A P-value of less than 0.05 was considered statistically significant. The statistical significance of multiple comparisons was determined using the Bonferroni adjustment (23). To assess the impact of HDA duration on longitudinal variables, a linear mixed model was used to account for within-subject correlation with adjustment for age at diagnosis, sex and IVIg resistance. A linear regression model with the same adjustments was used to determine predictors of coronary artery Z-score at 9–15 months in patients who had developed CAAs at diagnosis.
RESULTS
Patient Characteristics
Of 126 patients diagnosed with acute KD in the study time frame, 103 patients had HDA duration documented and were included in the study. Among the included patients, 63 (61%) were male, 68 (66%) were white, and 72 (70%) were under the age of 5 years. Seventeen patients (17%) received 0 days of HDA, 63 (61%) received 1–7 days, and 23 (22%) received > 7 days. Of the 103 study patients, 35 (34%) of patients had CAAs at diagnosis. Only two of those patients had isolated left main coronary artery (LMCA) dilation; this is a location where Z-scores should be interpreted with caution due to frequent anatomic variations (20), but review of the echocardiograms in our two patients indicated obviously dilated vessels (maximum Z-score of 44.24 in one case and 3.54 in the other, with borderline ectasia of the LAD in the latter).
Clinical Correlates of High Dose Aspirin Duration
All Patients
Within the overall study population, three factors correlated with duration of HDA prescribed: illness duration prior to diagnosis, duration of defervescence prior to diagnosis, and administration of IVIg (Table 1). Specifically, illness duration and the duration of defervescence prior to diagnosis were both longer in the 0 day HDA group than in the other two groups (P < 0.001 for both variables). Among the 17 patients in the 0 day HDA group, 5 had fever at the time of diagnosis, and all 5 of them received IVIg. Overall, a significantly lower proportion of patients in the 0 day HDA group received IVIg compared to the other groups (68% vs 98% and 100%, P < 0.001). All but one patient who received any HDA received IVIg, while 6 of 17 patients in the 0 day HDA group (35% of group) did not receive IVIg. It is unusual for a patient to receive HDA but not IVIg; in this case, the primary care provider started HDA for KD based on recurrence of a peeling rash several days after an initial 5-day period of fever and rash. However, the consulting cardiologist did not recommend IVIg because the fever had resolved and the echocardiogram was normal; the HDA was then discontinued.
Table 1.
All-Patient Comparison of Clinical Correlates and Outcome Variables by high-dose aspirin (HDA) group
| HDA Duration Group | |||||
|---|---|---|---|---|---|
| Variable | Statistics/ Category | 0 day (N =17) | 1–7 day (N =63) | > 7 day (N =23) | P- value |
| Age at diagnosis, years | Mean(SD) | 6.56 (5.89) | 4.19 (3.61) | 4.24 (3.92) | 0.10 |
| Age range at diagnosis | < 1 year(%) | 3 (18) | 11 (17) | 3 (13) | 0.12 |
| 1–5 years(%) | 5 (29) | 34 (54) | 16 (70) | ||
| > 5 years(%) | 9 (53) | 18 (29) | 4 (17) | ||
| Sex | Male (%) | 11 (65) | 34 (54) | 18 (78) | 0.12 |
| Female(%) | 6 (35) | 29 (46) | 5 (22) | ||
| Race | White (%) | 11 (65) | 41 (65) | 16 (70) | 0.76 |
| Black (%) | 3 (18) | 6 (10) | 1 (4) | ||
| Asian (%) | 1 (6) | 6 (10) | 3 (13) | ||
| Hispanic (%) | 2 (12) | 8 (13) | 1 (4) | ||
| Other (%) | 0 (0) | 2 (3) | 2 (9) | ||
| Yes (%) | 10 (59) | 40 (69) | 11 (50) | ||
| Classic KD | No (%) | 7 (41) | 18 (31) | 11 (50) | 0.27 |
| Unknown | 0 | 5 | 1 | ||
| Illness duration before diagnosis, days | Mean (SD) | 23 (16.87)a,b | 8.23 (5)a | 10.86 (5)b | <0.001 |
| Defervescence duration before diagnosis, days | Mean (SD) | 11.76 (13.47)a,b | 0.46 (2.55)a | 0.24 (0.70)b | <0.001 |
| CAAs at diagnosis | Yes (%) | 7 (41) | 18 (29) | 10 (43) | 0.34 |
| No (%) | 10 (59) | 45 (71) | 13 (57) | ||
| CRP at diagnosis, g/dL | Mean (SD) | 6.87 (5.24) | 11.93 (9.15) | 9.76 (7.29) | 0.19 |
| Platelet count at diagnosis, x109/L | Mean (SD) | 543.1 (403.5) | d 373 (170.3)d | 485.5 (202.8)d | 0.03 |
| IVIg #1 given | Yes (%) | 11 (65)a,b | 62 (98)a | 23 (100)b | <0.001 |
| No (%) | 6 (35) | 1 (2) | 0 (0) | ||
| IVIg Resistance | Yes (%) | 5 (45) | 14 (23) | 10 (43) | 0.08 |
| No (%) | 6 (55) | 48 (77) | 13 (57) | ||
| Unknown | 6 | 1 | 0 | ||
| Fever duration after diagnosis, days | Mean(SD) | 0.13 (0.50) | 1.16 (3.30) | 2.41 (5.09) | 0.14 |
| CRP at 4–8 weeks, g/dL | Mean(SD) | 0.69 (0.58) | 0.32 (0.22) | 0.66 (0.61) | 0.05 |
| CRP at 9–15 months, g/dL | Mean(SD) | 0.96 (1.10) | 0.57 (0.13) | 0.34 (0.11) | 0.42 |
| Platelet count at 4–8 weeks, x 109/L | Mean (SD) | 319.9 (127.8) | 358.3 (115.2) | 394.3 (110.2) | 0.32 |
| Platelet count at 9–15 months, x 109/L | Mean (SD) | 388.3 (145.5) | 283.3 (106.5) | 264.4 (59.8) | 0.08 |
Superscripts denote pairwise comparisons of statistical significance for variables with P-values < 0.05, a for a significant difference between the 0 day HDA group and the 1–7 day HDA group, b for a significant difference between the 0 day HDA group and the > 7 day HDA group, c for a significant difference between the 1–7 day HDA group and the > 7 day HDA group, and d for no significant differences in pairwise comparison. Bonferroni’s adjustment for multiple comparisons is used, whereby a P-value threshold for statistical significance between pairs is 0.05/(number of pairwise comparisons) = 0.05/3 = 0.0167. CAA = Coronary Artery Abnormality, CRP = C-Reactive Protein, HDA = High-Dose Aspirin, IVIg = Intravenous Immunoglobulin, KD = Kawasaki Disease, SD = Standard Deviation.
There was no association between patient age, sex, or race and the duration of HDA in the overall study population. The proportions of patients with classic versus incomplete KD and with CAAs at the time of diagnosis were also not different between the three HDA groups (Table 1). The CRP at diagnosis was not associated with the duration of HDA. There was a statistically significant difference in platelet count at diagnosis between the three HDA groups, with the 0 days of HDA group having the highest value, followed by the >7 day group, then the 1–7 day group (Table 1).
Patients without CAAs at Diagnosis
Table 2 contains data regarding the subgroup of 68 patients without CAAs at the time of diagnosis. Within this group, 10 patients (15%) received 0 days of HDA, 45 (66%) received 1–7 days, and 13 (19%) received > 7 days. Four of the 10 patients who did not receive HDA also did not receive IVIg, whereas only one patient who received HDA did not receive IVIg (described above); pairwise comparison demonstrated a significant difference between the 0 day HDA group and the 1–7 day HDA group for IVIg administration (60% vs 98%, P = 0.003). As with the overall study population, illness duration and the duration of defervescence prior to diagnosis were longer in the 0 day HDA group than in the other two groups (P < 0.001 for both variables). The average age at diagnosis was higher in the 0 day HDA group (9.01 years) than in the other two groups (3.74 and 2.87 years, P < 0.001), whereas platelet count at diagnosis was higher in the > 7 day HDA group (522.5 x 109/L) compared to the other two groups (324.3 x 109/L and 352.7 x 109/L, P < 0.001). There was no association between patient sex, race, or KD status and the duration of HDA prescribed.
Table 2.
Comparison of Clinical Correlates and Clinical Outcome Variables by high-dose aspirin (HDA) group in patients with Kawasaki Disease (KD) and no coronary artery abnormalities (CAAs) at diagnosis
| HDA Duration Group | |||||
|---|---|---|---|---|---|
| Variable | Statistics/ Category | 0 day (N = 10) | 1–7 day (N = 45) | > 7 day (N = 13) | P- value |
| Age at diagnosis, years | Mean(SD) | 9.01 (6.37)a,b | 3.74 (2.99)a | 2.87 (1.50)b | <0.001 |
| Age range at diagnosis | < 1 year(%) | 1 (10) | 7 (16) | 1 (7.69) | 0.002 |
| 1–5 years(%) | 2 (20) | 27 (60) | 12 (92.31) | ||
| > 5 years(%) | 7 (70) | 11 (24) | 0 (0) | ||
| Sex | Male (%) | 9 (90) | 23 (51) | 9 (69.23) | 0.05 |
| Female (%) | 1 (10) | 22 (49) | 4 (30.77) | ||
| Race | White (%) | 6 (60) | 28 (62) | 8 (62) | 0.58 |
| Black (%) | 3 (30) | 4 (9) | 1 (8) | ||
| Asian (%) | 1 (10) | 6 (13) | 3 (23) | ||
| Hispanic (%) | 0 (0) | 5 (11) | 0 (0) | ||
| Other (%) | 0 (0) | 2 (4) | 1 (8) | 0.06 | |
| Classic KD | Yes (%) | 6 (60) | 33 (77) | 5 (42) | |
| No (%) | 4 (40) | 10 (23) | 7 (58) | ||
| Unknown | 0 | 2 | 1 | ||
| Illness duration before diagnosis, days | Mean(SD) | 18.70(13.95)a,b | 7.75 (4.19)a | 10.09 (3.78)b | <0.001 |
| Defervescence duration before diagnosis, days | Mean(SD) | 11 (13.31)a,b | 0.63 (3.04)a | 0 (0)b | <0.001 |
| CRP at diagnosis, g/dL | Mean(SD) | 7.18 (5.27) | 10.31 (7.84) | 5.23 (1.60) | 0.18 |
| Platelet count at diagnosis, x 109/L | Mean(SD) | 352.7 (212.9)b | 324.3 (93.89)c | 522.5 (149.6)b,c | <0.001 |
| IVIg #1 given | Yes (%) | 6 (60)a | 44 (98)a | 13 (100) | 0.003 |
| No (%) | 4 (40) | 1 (2) | 0 (0) | ||
| IVIg Resistance | Yes (%) | 2 (33) | 6 (14) | 4 (31) | 0.20 |
| No (%) | 4 (67) | 38 (86) | 9 (69) | ||
| Unknown | 4 | 1 | 0 | ||
| Fever duration after diagnosis, days | Mean(SD) | 0 (0) | 0.16 (0.47) | 0.42 (0.79) | 0.17 |
| CRP at 4–8 weeks, g/dL | Mean(SD) | 0.83 (0.62)a | 0.32 (0.21)a | 0.52 (0.33) | 0.03 |
| CRP at 9–15 months, g/dL | Mean(SD) | 0.48 (0.20) | 0.63 (0.18) | - | 0.99 |
| Platelet count at 4–8 weeks, x 109/L | Mean (SD) | 261.2 (71.86)b | 342.0 (104.7) | 417.3 (110.3)b | 0.03 |
| Platelet count at 9–15 months, x 109/L | Mean (SD) | 256.5 (89.80) | 309.1 (95.16) | 219.0 (35.36) | 0.42 |
Superscripts denote pairwise comparisons of statistical significance for variables with P-values < 0.05, a for a significant difference between the 0 day HDA group and the 1–7 day HDA group, b for a significant difference between the 0 day HDA group and the > 7 day HDA group, c for a significant difference between the 1–7 day HDA group and the > 7 day HDA group, and d for no significant differences in pairwise comparison. Bonferroni’s adjustment for multiple comparisons is used, whereby a P-value threshold for statistical significance between pairs is 0.05/(number of pairwise comparisons) = 0.05/3 = 0.0167. CAA = Coronary Artery Abnormality, CRP = C-Reactive Protein, HDA = High-Dose Aspirin, IVIg = Intravenous Immunoglobulin, KD = Kawasaki Disease, SD = Standard Deviation.
Patients with CAAs at Diagnosis
Table 3 contains data regarding the subgroup of 35 patients with CAAs at the time of diagnosis. Despite the presence of CAAs at diagnosis, 7 patients (20%) received 0 days of HDA; 18 (51%) received 1–7 days, and 10 (29%) received > 7 days. Two of the 7 patients who did not receive HDA also did not receive IVIg, whereas 100% of patients who received HDA also received IVIg. As with the overall study population, illness duration was significantly longer in the 0 day HDA group (29.14 days) compared to the other two HDA groups (9.35 and 11.70 days, P < 0.001), while the duration of defervescence was longer in the 0 day HDA group than in the 1–7 day HDA group (12.86 vs. 0.06 days, P = 0.002). The fraction of female patients was higher in the 0 day HDA group (71%) and lowest in the >7 day HDA group (10%).
Table 3.
Comparison of Clinical Correlates and Clinical Outcome Variables by high-dose aspirin (HDA) group in patients with Kawasaki Disease (KD) and with coronary artery abnormalities (CAAs) at diagnosis
| HDA Duration Group | |||||
|---|---|---|---|---|---|
| Variable | Statistics/ Category | 0 day (N = 7) | 1–7 day (N =18) | >7 day (N =10) | P-value |
| Age at diagnosis, years | Mean (SD) | 3.06 (2.73) | 5.31 (4.73) | 6.02 (5.32) | 0.42 |
| Age range at diagnosis | <1 year (%) | 2 (29) | 4 (22) | 2 (20) | 0.99 |
| 1–5 years(%) | 3 (43) | 7 (39) | 4 (40) | ||
| > 5 years (%) | 2 (29) | 7 (39) | 4 (40) | ||
| Sex | Male (%) | 2 (29)d | 11 (61)d | 9 (90)d | 0.04 |
| Female (%) | 5 (71) | 7 (39) | 1 (10) | ||
| Race | White (%) | 5 (71) | 13 (72) | 8 (80) | 0.90 |
| Black (%) | 0 (0) | 2 (11) | 0 (0) | ||
| Asian (%) | 0 (0) | 0 (0) | 0 (0) | ||
| Hispanic (%) | 2 (29) | 3 (17) | 1 (10) | ||
| Other (%) | 0 (0) | 0 (0) | 1 (10) | ||
| Classic KD | Yes (%) | 4 (57) | 7 (47) | 6 (60) | 0.90 |
| No (%) | 3 (43) | 8 (53) | 4 (40) | ||
| Unknown | 0 | 3 | 0 | ||
| Illness duration before diagnosis, days | Mean (SD) | 29.14 (19.79)a,b | 9.35 (5.54)a | 11.70 (5.27)b | <0.001 |
| Defervescence duration before diagnosis, days | Mean (SD) | 12.86 (14.68)a | 0.06 (0.24)a | 0.50 (0.97) | 0.002 |
| Coronary artery Z-score at diagnosis | Mean (SD) | 12.18 (5.58) | 7.07 (8.22) | 6.80 (4.45) | 0.21 |
| CRP at diagnosis, g/dL | Mean (SD) | 6.42 (5.97) | 16.54 (11.26) | 12.93 (8.10) | 0.20 |
| Platelet count at diagnosis, x 109/L | Mean (SD) | 765.2 (475.9) | 488.4 (245.9) | 452.7 (245.1) | 0.12 |
| IVIg #1 given | Yes (%) | 5 (71)d | 18 (100)d | 10 (100)d | 0.04 |
| No (%) | 2 (29) | 0 (0) | 0 (0) | ||
| IVIg Resistance | Yes (%) | 3 (60) | 8 (44) | 6 (60) | 0.71 |
| No (%) | 2 (40) | 10 (56) | 4 (40) | ||
| Unknown | 2 | 0 | 0 | ||
| Fever duration after diagnosis, days | Mean (SD) | 0.29 (0.76) | 3.67 (5.46) | 4.80 (6.92) | 0.24 |
| Persistent CAAs at 4–8 weeks | Yes (%) | 5 (100) | 6 (75) | 8 (89) | 0.58 |
| No (%) | 0 (0) | 2 (25) | 1 (11) | ||
| Unknown | 2 | 10 | 1 | ||
| Persistent CAAs at 9–15 months | Yes (%) | 3 (100) | 4 (33)c | 8 (89)c | 0.01 |
| No (%) | 0 (0) | 8 (67) | 1 (11) | ||
| Unknown | 4 | 6 | 1 | ||
| Coronary artery Z-score at 4–8 weeks | Mean (SD) | 14.48 (7.51) | 14.40 (13.87) | 10.20 (6.95) | 0.64 |
| Coronary artery Z-score at 9–15 months | Mean (SD) | 11.14 (12.77) | 6.25 (9.47) | 8.33 (7.50) | 0.69 |
| CRP at 4–8 weeks, g/dL | Mean (SD) | 0.42 (0.57) | 0.33 (0.27) | 0.84 (0.86) | 0.37 |
| CRP at 9–15 months, g/dL | Mean (SD) | 1.44 (1.63) | 0.50 (0.00) | 0.34 (0.11) | 0.30 |
| Platelet count at 4–8 weeks, x 109/l | Mean (SD) | 466.5 (130.8) | 389.1 (134.0) | 365.6 (110.4) | 0.60 |
| Platelet count at 9–15 months, x 109/L | Mean (SD) | 454.3 (123.4)a | 250.0 (118.3)a | 279.5 (60.47) | 0.02 |
Superscripts denote pairwise comparisons of statistical significance for variables with P-values < 0.05, a for a significant difference between the 0 day HDA group and the 1–7 day HDA group, b for a significant difference between the 0 day HDA group and the > 7 day HDA group, c for a significant difference between the 1–7 day HDA group and the > 7 day HDA group, and d for no significant differences in pairwise comparison. Bonferroni’s adjustment for multiple comparisons is used, whereby a P-value threshold for statistical significance between pairs is 0.05/(number of pairwise comparisons) = 0.05/3 = 0.0167. CAA = Coronary Artery Abnormality, CRP = C-Reactive Protein, HDA = High-Dose Aspirin, IVIg = Intravenous Immunoglobulin, KD = Kawasaki Disease, SD = Standard Deviation.
Similar to the entire study population, there was no association between patient age, race, or KD status and the duration of HDA among patients with CAAs at diagnosis. The maximum coronary artery Z-score value, CRP level, and platelet count at diagnosis were also not correlated with the duration of HDA prescribed.
Clinical Course and Outcomes Based on Duration of High Dose Aspirin
All Patients
Among the overall study population, there was no association between the duration of HDA prescribed and IVIg resistance, fever duration after diagnosis, CRP, or platelet count at 4–8 weeks or 9–15 months (Table 1).
Patients without CAAs at Diagnosis
Between the three HDA groups, univariate analysis of the 68 patients without CAAs at diagnosis (Table 2) demonstrated a higher CRP at 4–8 weeks in the 0 day HDA group compared to the 1–7 day HDA group (0.83 g/dL vs. 0.32 g/dL, P = 0.03) and a higher platelet count at 4–8 weeks in the > 7 day HDA group compared to the 0 day HDA group (417.3 x 109/L vs. 342.0 x 109/L, P = 0.03). However, neither of these variables was different between the HDA groups at 9–15 months. Similar to the overall study population, there was no association between the duration of HDA prescribed and IVIg resistance or fever duration after diagnosis. No patients without CAAs at the time of diagnosis subsequently developed CAAs.
Patients with CAAs at Diagnosis
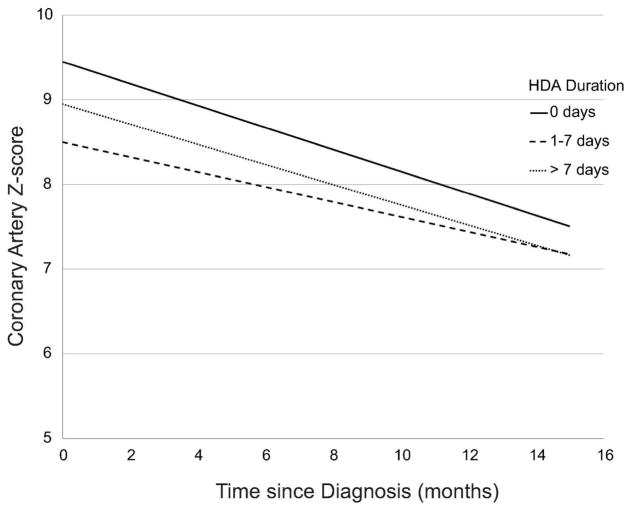
Among the patients with CAAs at the time of diagnosis, CAAs were still present at the 4–8 week timepoint in 19 of 35 patients and continued to persist even at the 9–15 month timepoint in 15 of them. (Table 3). Comparing the three HDA groups, univariate analysis demonstrated a lower incidence of persistent CAAs at 9–15 months in the 1–7 day HDA group compared to the > 7 day HDA group (33% versus 89%, P = 0.01). However, multivariate analysis adjusted for age, sex and IVIg resistance revealed no correlation between HDA duration and coronary artery dimensions at 9–15 months. Although the platelet count at 9–15 months was higher in the 0 day HDA group compared to the 1–7 day group in univariate analysis (454.3 x 109/L versus 250 x 109/L, P = 0.02), this difference did not persist in the multivariate analysis. Similar to the overall study population, there was no association between the duration of HDA prescribed and IVIg resistance, fever duration after diagnosis, or CRP at 4–8 weeks or 9–15 months. Longitudinal analysis adjusted for age, sex and IVIg resistance demonstrated no difference in the rate of decline of coronary Z-score (Figure 2), CRP, or platelet count between the HDA groups.
Figure 2. Rate of coronary artery Z-score decline among patients with coronary artery abnormalities (CAAs) at diagnosis, as categorized by high-dose aspirin (HDA) duration group.
A linear mixed model adjusted for age, sex and IVIg resistance was used to generate each line, accounting for within-patient correlation and assuming that Z-scores were linearly associated with time.
With respect to persistence versus resolution of CAAs at 9–15 months (Table 4), univariate analysis revealed that patients with persistent CAAs had longer duration of fever after diagnosis (6.07 versus 0.67 days, P = 0.03) and higher incidence of IVIg resistance (79% versus 11%, P = 0.003). After adjusting for age, sex, and IVIg resistance, the only factor associated with higher coronary Z-scores at 9–15 months was higher coronary Z-scores at diagnosis.
Table 4.
Comparison of Clinical Correlates and Clinical Outcome Variables by coronary artery abnormality (CAA) status at 9–15 months in patients with Kawasaki Disease (KD) and CAAs at diagnosis
| Variable | Statistics/ Category | Resolution of CAAs at 9–15 months (N = 9) | Persistence of CAAs at 9–15 months (N = 15) | P- value |
|---|---|---|---|---|
| Age at diagnosis, years | Mean (SD) | 6.46 (4.65) | 5.73 (5.40) | 0.61 |
| Age range at diagnosis | <1 year (%) | 1 (11) | 4 (27) | 0.86 |
| 1–5 years (%) | 3 (33) | 5 (33) | ||
| >5 years (%) | 5 (56) | 6 (40) | ||
| Sex | Male (%) | 6 (67) | 11 (73) | 0.99 |
| Female (%) | 3 (33) | 4 (27) | ||
| Race | White (%) | 7 (78) | 10 (67) | 0.47 |
| Black (%) | 1 (11) | 0 (0) | ||
| Asian (%) | 0 (0) | 0 (0) | ||
| Hispanic (%) | 1 (11) | 4 (27) | ||
| Other (%) | 0 (0) | 1 (7) | ||
| Classic KD | Yes (%) | 2 (29) | 10 (71) | 0.16 |
| No (%) | 5 (71) | 4 (29) | ||
| Unknown | 2 | 1 | ||
| Illness duration before diagnosis, days | Mean (SD) | 8.78 (4.29) | 13.71 (7.80) | 0.12 |
| Defervescence duration before diagnosis, days | Mean (SD) | 0.33 (1.00) | 2.07 (4.86) | 0.34 |
| CRP at diagnosis, g/dL | Mean (SD) | 15.23 (11.09) | 12.83 (7.68) | 0.60 |
| Platelet count at diagnosis, x 109/L | Mean (SD) | 438.4 (229.1) | 602 (374.7) | 0.38 |
| IVIg #1 given | Yes (%) | 9 (100) | 14 (93) | 0.99 |
| No (%) | 0 (0) | 1 (7) | ||
| HDA duration, days | Mean (SD) | 5.44 (5.64) | 10.47 (9.14) | 0.13 |
| IVIg Resistance | Yes (%) | 1 (11) | 11 (79) | 0.003 |
| No (%) | 8 (89) | 3 (21) | ||
| Unknown | 0 | 1 | ||
| Fever duration after diagnosis, days | Mean (SD) | 0.67 (1.00) | 6.07 (7.10) | 0.03 |
| CRP at 4–8 weeks, g/dL | Mean (SD) | 0.41 (0.32) | 0.66 (0.74) | 0.75 |
| CRP at 9–15 months, g/dL | Mean (SD) | 0.50 (0.00) | 0.33 (0.09) | 0.07 |
| Platelet count at 4–8 weeks, x 109/L | Mean (SD) | 332.3 (135.1) | 393.3 (127.2) | 0.43 |
| Platelet count at 9–15 months, x 109/L | Mean (SD) | 261.3 (80.56) | 280.1 (118.8) | 0.87 |
CAA = Coronary Artery Abnormality, CRP = C-Reactive Protein, HDA = High-Dose Aspirin, IVIg = Intravenous Immunoglobulin, KD = Kawasaki Disease, SD = Standard Deviation.
DISCUSSION
This retrospective study of 103 patients with KD was designed to understand variations in clinical practice around the duration of HDA therapy and how these different durations affect coronary artery outcomes beyond the acute and subacute phases of the disease. A relatively large fraction of patients in this study did not receive HDA. This group was characterized by a longer duration of illness before diagnosis and lack of administration of IVIg. Patients who did not receive HDA represent a subset of the KD population in whom defervescence typically occurred prior to diagnosis as part of a prolonged illness course. Review of the charts of these patients indicates that the rationale for not administering HDA was most commonly that the febrile phase of the illness had ended. However, 5 of 17 patients who had no HDA did have fever at the time of diagnosis; the reasons for not giving HDA in these 5 cases varied. Of note, low-dose aspirin was administered to 15 of the 17 patients who did not receive HDA; it was withheld in one patient due to elevation of liver enzymes and in the other due to practitioner concern about precipitating Reye syndrome. Most importantly, although these factors may influence clinical decisions regarding initiation of HDA therapy, the duration of HDA therapy itself had no impact on clinically-relevant outcome measures, particularly the late development of or persistence of CAAs and the duration of fever after diagnosis. After adjusting for IVIg resistance, a known risk factor for persistence of CAAs (2,20), the only factor associated with higher coronary artery Z-scores at 9–15 months was higher coronary artery Z-scores at diagnosis. This association has been described in early (24) and late (25) follow-up, and may serve as a prognostic predictor of CAA outcomes.
Similar data exist from the 1990s and early 2000s regarding the lack of effect of HDA administration on CAA evolution (14,15,16,21) and fever duration (17); however, the 2004 and 2017 AHA statements acknowledge that there is variability in practices regarding HDA dosing among institutions (19,20), and our study demonstrates a wide within-institution variability in practice. Additionally, these studies and newer ones (26) have largely evaluated the effect of HDA on short-term outcomes in the acute and subacute phases of the disease, and so it was important to determine whether a particular HDA duration conferred measurable clinical benefit in the convalescent phase and beyond. Based on our study, there was no apparent correlation between duration of HDA and long-term clinical outcomes, suggesting that HDA may not be necessary in the acute management of KD. Consistent with this suggestion, Dallaire and colleagues recently reported their findings from a retrospective study comparing HDA (80 mg/kg/day) to low-dose aspirin (3–5 mg/kg/day) in acute KD; they found no difference in the risk of CAA between these two groups, so concluded that low-dose aspirin is not inferior to HDA for reducing CAA risk (27). However, another recent retrospective report found that patients who received low-dose aspirin had three times higher odds of needing IVIG re-treatment compared to those who received HDA, but with no difference in duration of hospitalization or of CA aneurysms (28). Determining whether and how HDA should be used in the acute management of KD is important, because HDA has been associated with a number of adverse effects, including transaminitis (29) and rarely, Reye syndrome (30). Transaminitis was seen in two patients in our study necessitating early discontinuation of HDA, and no cases of Reye syndrome were observed.
Notably, our data also identified a small subset of patients with “missed” KD. In particular, three patients who initially had normal echocardiograms and were not treated for KD subsequently presented again and were diagnosed with KD with interval development of CAAs. All three patients had an illness duration of 14 days or longer, and two did not receive HDA. These cases highlight the importance of maintaining a low threshold for the diagnosis and treatment of KD based on previously established criteria (19,20), particularly in the setting of a prolonged illness course.
Our study also highlights the degree of practice variability in the management of KD at our center. In response to the findings presented here, we have now implemented a clinical practice guideline based on the 2017 AHA statement on KD (20).
Limitations of this study include its single-center and retrospective nature, a relatively small number of patients with CAAs, as well as loss of follow-up data. The latter is particularly true for patients with no CAAs at presentation or at 4–8 weeks, as well as for laboratory values that are occasionally not documented over time. Confounding by indication is common in retrospective studies such as this; indeed, it seems clear that the patients who received no HDA were at a later stage of disease than the other two groups. However, there were no clinically meaningful differences in the presenting symptoms, laboratory values, or coronary artery Z-scores between those patients who received 1–7 days of HDA compared to those that received >7 days of HDA in this study, suggesting that prolonged administration of HDA does not influence outcomes. Of note, 34% of the patients in this study had CAAs at the time of diagnosis. This is a higher percentage than in most centers and underscores the need for a high index of suspicion for KD in children, especially those with incomplete KD who are mostly outside the expected 1–5-year age range.
Overall, this study adds to a growing body of literature supporting the notion that the duration of HDA therapy does not influence meaningful long-term clinical outcomes in KD, particularly CAA persistence. These findings highlight an opportunity to design larger, multi-center prospective trials that can provide more generalizable data regarding the interplay between HDA duration and long-term coronary artery outcomes, including the question of whether the routine use of HDA is necessary in the acute management of KD.
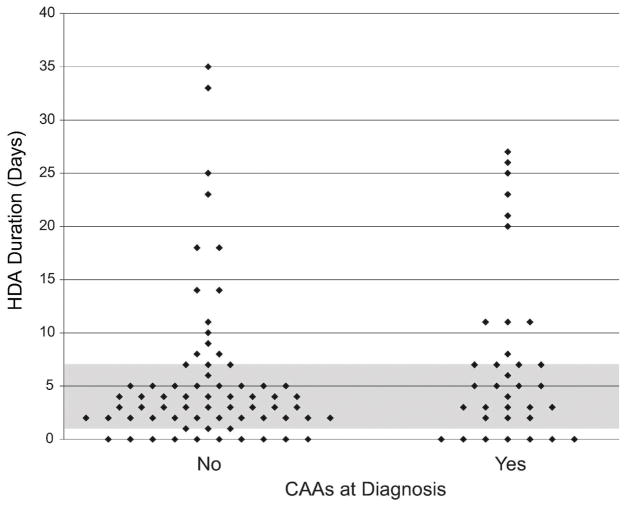
Figure 1. Categorization of study subjects based on presence or absence of coronary artery abnormalities (CAAs) at diagnosis and duration of high-dose aspirin therapy (HDA).
Comparison of HDA duration between patients without CAAs at diagnosis (left) and patients with CAAs at diagnosis (right). Each point indicates one patient. Among the 1–7 day HDA group, the days of HDA were a mean of 3.75, median 3, with an interquartile range (IQR) of 2–5 days. Among the >7 days HDA group, the days of HDA were a mean of 17.78, median 18, and IQR 11–25 days.
Acknowledgments
The authors would like to acknowledge Michael Muradian for assisting with initial data collection.
Footnotes
Disclosure Statement: The authors have no financial relationships or conflicts of interest relevant to this article to disclose.
Statement of Financial Support: Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Kawasaki T. Infantile acute febrile mucocutaneous lymph node syndrome with specific desquamation of the fingers and toes. Clinical observation of 50 cases. Jpn J Allerg. 1967:178–222. [PubMed] [Google Scholar]
- 2.Newburger JW, Takahashi M, Burns JC. Kawasaki Disease. J Am Coll Cardiol. 2016 Apr 12;67(14):1738–49. doi: 10.1016/j.jacc.2015.12.073. [DOI] [PubMed] [Google Scholar]
- 3.Newburger JW, Takahashi M, Burns JC, et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med. 1986 Aug 7;315(6):341–7. doi: 10.1056/NEJM198608073150601. [DOI] [PubMed] [Google Scholar]
- 4.Melish ME, Hicks RV, Reddy V. Kawasaki syndrome: an update. Hosp Pract (Hosp Ed) 1982 Mar;17(3):99–106. doi: 10.1080/21548331.1982.11702289. [DOI] [PubMed] [Google Scholar]
- 5.Furusho K, Kamiya T, Nakano H, et al. High-dose intravenous gammaglobulin for Kawasaki disease. Lancet. 1984 Nov 10;2(8411):1055–8. doi: 10.1016/s0140-6736(84)91504-6. [DOI] [PubMed] [Google Scholar]
- 6.Newburger JW, Takahashi M, Beiser AS, et al. A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. N Engl J Med. 1991 Jun 6;324(23):1633–9. doi: 10.1056/NEJM199106063242305. [DOI] [PubMed] [Google Scholar]
- 7.Dajani AS, Taubert KA, Gerber MA, et al. Diagnosis and therapy of Kawasaki disease in children. Circulation. 1993 May;87(5):1776–80. doi: 10.1161/01.cir.87.5.1776. [DOI] [PubMed] [Google Scholar]
- 8.Asai T, Kiguciii H, Nagai Y, et al. Analysis of cardiac involvement in 29 cases with MCLS. Jpn J Pediatr. 1973;26:824–836. [Google Scholar]
- 9.Kato H, Koike S, Yokoyama T. Kawasaki disease: effect of treatment on coronary artery involvement. Pediatrics. 1979 Feb;63(2):175–9. [PubMed] [Google Scholar]
- 10.Yokoyama T, Kato H, Ichinose E. Aspirin treatment and platelet function in Kawasaki disease. Kurume Med J. 1980;27(1):57–61. doi: 10.2739/kurumemedj.27.57. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs JC. Salicylate treatment of epidemic Kawasaki disease in New York City. Ther Drug Monit. 1979;1(1):123–30. doi: 10.1097/00007691-197901000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Koren G, MacLeod SM. Difficulty in achieving therapeutic serum concentrations of salicylate in Kawasaki disease. J Pediatr. 1984 Dec;105(6):991–5. doi: 10.1016/s0022-3476(84)80097-9. [DOI] [PubMed] [Google Scholar]
- 13.Koren G, Rose V, Lavi S, et al. Probable efficacy of high-dose salicylates in reducing coronary involvement in Kawasaki disease. JAMA. 1985 Aug 9;254(6):767–9. [PubMed] [Google Scholar]
- 14.Melish ME, Takahashi M, Shulman ST, et al. Comparison of low dose aspirin vs. high dose aspirin as an adjunct to intravenous gamma globulin in the treatment of Kawasaki syndrome [abstract] Pediatr Res. 1992;31:170A. [Google Scholar]
- 15.Durongpisitkul K, Gururaj VJ, Park JM, et al. The prevention of coronary artery aneurysm in Kawasaki disease: a meta-analysis on the efficacy of aspirin and immunoglobulin treatment. Pediatrics. 1995 Dec;96(6):1057–61. [PubMed] [Google Scholar]
- 16.Terai M, Shulman ST. Prevalence of coronary artery abnormalities in Kawasaki disease is highly dependent on gamma globulin dose but independent of salicylate dose. J Pediatr. 1997 Dec;131(6):888–93. doi: 10.1016/s0022-3476(97)70038-6. [DOI] [PubMed] [Google Scholar]
- 17.Saulsbury FT. Comparison of high-dose and low-dose aspirin plus intravenous immunoglobulin in the treatment of Kawasaki syndrome. Clin Pediatr (Phila) 2002 Oct;41(8):597–601. doi: 10.1177/000992280204100807. [DOI] [PubMed] [Google Scholar]
- 18.Pediatr Infect Dis J; Management of Kawasaki syndrome: a consensus statement prepared by North American participants of the Third International Kawasaki Disease Symposium; Tokyo, Japan. December, 1988; 1989. Oct, pp. 663–7. [PubMed] [Google Scholar]
- 19.Newburger JW, Takahashi M, Gerber MA, et al. Council on Cardiovascular Disease in the Young; American Heart Association; American Academy of Pediatrics. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young. American Heart Association. Circulation. 2004 Oct 26;110(17):2747–71. doi: 10.1161/01.CIR.0000145143.19711.78. [DOI] [PubMed] [Google Scholar]
- 20.McCrindle BW, Rowley AH, Newburger JW, et al. American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Surgery and Anesthesia; and Council on Epidemiology and Prevention. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals From the American Heart Association. Circulation. 2017 Mar 29; doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 21.Baumer JH, Love SJ, Gupta A, et al. Salicylate for the treatment of Kawasaki disease in children. Cochrane Database Syst Rev. 2006 Oct 18;(4):CD004175. doi: 10.1002/14651858.CD004175.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colan SD. Normal echocardiographic values for cardiovascular structures. In: Lai WW, Cohen MS, Geva T, Mertens L, editors. Echocardiography in Pediatric and Congenital Heart Disease. Wiley-Blackwell; West Sussex, UK: 2009. pp. 772–773. Appendix 1. [Google Scholar]
- 23.Bland JM, Altman DG. Multiple significance tests: the Bonferroni method. BMJ. 1995 Jan 21;310(6973):170. doi: 10.1136/bmj.310.6973.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCrindle BW, Li JS, Minich LL, et al. Pediatric Heart Network Investigators. Coronary artery involvement in children with Kawasaki disease: risk factors from analysis of serial normalized measurements. Circulation. 2007 Jul 10;116(2):174–9. doi: 10.1161/CIRCULATIONAHA.107.690875. Epub 2007 Jun 18. [DOI] [PubMed] [Google Scholar]
- 25.Friedman KG, Gauvreau K, Hamaoka-Okamoto A, et al. Coronary Artery Aneurysms in Kawasaki Disease: Risk Factors for Progressive Disease and Adverse Cardiac Events in the US Population. J Am Heart Assoc. 2016 Sep 15;5(9) doi: 10.1161/JAHA.116.003289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuo HC, Lo MH, Hsieh KS, et al. High-Dose Aspirin is Associated with Anemia and Does Not Confer Benefit to Disease Outcomes in Kawasaki Disease. PLoS One. 2015 Dec 10;10(12):e0144603. doi: 10.1371/journal.pone.0144603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dallaire F, Fortier-Morissette Z, Blais S, et al. Aspirin dose and prevention of coronary abnormalities in Kawasaki disease. Pediatrics. 2017 Jun;139(6):e20170098. doi: 10.1542/peds.2017-0098. [DOI] [PubMed] [Google Scholar]
- 28.Dhanrajani A, Chan M, Pau S, et al. Aspirin dose in Kawasaki disease – the ongoing battle. Arthritis Care Res. 2017 Dec 29; doi: 10.1002/acr.23504. doi:10.1002. [DOI] [PubMed] [Google Scholar]
- 29.Research Committee of the Japanese Society of Pediatric Cardiology and Cardiac Surgery Committee for Development of Guidelines for Medical Treatment of Acute Kawasaki Disease. Guidelines for medical treatment of acute Kawasaki disease: report of the Research Committee of the Japanese Society of Pediatric Cardiology and Cardiac Surgery (2012 revised version) Pediatr Int. 2014 Apr;56(2):135–58. doi: 10.1111/ped.12317. [DOI] [PubMed] [Google Scholar]
- 30.Lee JH, Hung HY, Huang FY. Kawasaki disease with Reye syndrome: report of one case. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi. 1992 Jan-Feb;33(1):67–71. [PubMed] [Google Scholar]