Abstract
Background
Poor adherence to immunosuppressive medications is a major cause of premature graft loss among children and young adults. Multicomponent interventions have shown promise, but have not been fully evaluated.
Study Design
Unblinded, parallel arm randomized trial to assess the efficacy of a clinic-based adherence-promoting intervention.
Setting & Participants
Prevalent kidney transplant recipients 11–24 years of age and ≥3 months post-transplantation at 8 kidney transplant centers in Canada and the United States (Feb. 2012 – May 2016) were included.
Intervention
Adherence was electronically monitored in all participants during a 3-month run-in, followed by a 12-month intervention. Participants assigned to the TAKE-IT intervention could choose to receive text message, email, and/or visual cue dose reminders, and met with a coach at 3-month intervals when adherence data from the prior 3 months were reviewed with the participant. ‘Action-Focused Problem-Solving’ was used to address adherence barriers selected as important by the participant. Participants assigned to the control group met with coaches at 3-month intervals but received no feedback about adherence data.
Outcomes
The primary outcomes were electronically-measured ‘taking’ adherence (the proportion of prescribed doses of immunosuppressive medications taken) and ‘timing’ adherence (the proportion of doses of immunosuppressive medications taken between 1 hour before and 2 hours after the prescribed time of administration) on each day of observation. Secondary outcomes included standard deviation of tacrolimus trough levels, self-reported adherence, acute rejection, and graft failure.
Results
There were 81 patients assigned to intervention (median age 15.5 years; 57% male) and 88 to the control group (median age 15.8 years; 61% male). Electronic adherence data were available for 64 intervention and 74 control participants. Participants in the intervention group had significantly greater odds of taking prescribed medications (OR, 1.66; 95% CI, 1.15–2.39]) and taking medications at or near the prescribed time (OR, 1.74; 95% CI, 1.21–2.50) than controls.
Limitations
Lack of electronic adherence data for some participants may have introduced bias. There was low statistical power for clinical outcomes.
Conclusions
The multicomponent TAKE-IT intervention resulted in significantly better medication adherence than the control condition. Better medication adherence may result in improved graft outcomes, but this will need to be demonstrated in larger studies.
Keywords: adherence, randomized trial, adolescent, young adult, intervention, kidney transplantation, renal transplant, teen, compliance, intervention, behavioural, immunosuppressant, graft survival, end-stage renal disease (ESRD)
INTRODUCTION
Adolescence and young adulthood is a high-risk period for kidney transplant recipients. Graft failure rates increase from about 11 years old, and are higher between 17 and 24 years than at any other age1. Poor adherence to immunosuppressive therapy is believed to be a major contributor to the high graft failure rates in this age group2–4, and is one of the most important factors limiting survival of renal allografts in any age group5.
Most poor medication adherence is ‘unintentional’6. Forgetting and poor organization and planning were the most commonly identified adherence barriers in young kidney transplant recipients7,8. Neurocognitive dysfunction, particularly disturbances in executive function and memory9–11, is common among individuals who experienced chronic kidney disease in childhood; this may also impair medication management abilities.
A 2008 NIH consensus conference highlighted the urgent need for randomized trials of adherence-promoting interventions for adolescent and young adult transplant recipients and, like a subsequent Cochrane review12, emphasized the superiority of multi-component interventions13. The innovative TAKE-IT intervention was developed to address common modifiable barriers to adherence with an individually-tailored approach that included a combination of electronic adherence monitoring and feedback, problem-solving skills training, goal-setting, and technology-based adherence support (text message dose reminders). These approaches showed the most promise in prior studies14,15–20, and subsequent studies and systematic reviews have reinforced TAKE-IT’s choice of intervention components21–23.
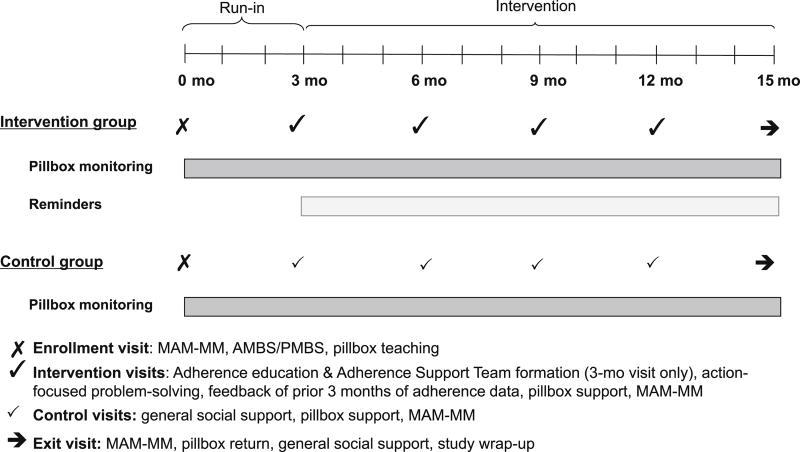
The aim of this study was to test the efficacy of TAKE-IT, a novel, multicomponent adherence-promoting intervention, compared with an attention control condition, in improving medication adherence among adolescent and young adult kidney transplant recipients. We hypothesized that those randomized to the TAKE-IT intervention would demonstrate significantly better medication adherence than those assigned to control. Our intervention, outlined in Figure 1, was geared to improve ‘implementation’ of the prescribed medication regimen, defined as “the extent to which a patient’s actual dosing corresponds to the prescribed dosing regimen24”.
Figure 1. Timeline of study procedures.
The schedule of intervention and control visits is shown. The first 3 months of the study constituted a run-in period during which no intervention was applied. Group allocation was concealed for the first 2 months of the run-in.
MAM-MM- Medical Adherence Measure- Medication Module
AMBS- Adolescent medication barriers scale
PMBS- Parent medication barriers scale
METHODS
Trial Design
This prospective, parallel arm, unblinded, randomized controlled trial was conducted at 8 pediatric transplant programs in Canada and the United States from Feb. 2012 to May 2016. The methods were previously described in detail25. TAKE-IT was approved by the Research Ethics Boards of all sites (Research Ethics Board approval number 10-365-PED). Written informed consent was obtained from all participants and parents (for those <18y).
Study Population
Prevalent kidney-only transplant recipients aged 11–24 years who were ≥3 months post-transplantation, had a functioning graft, and were expected to be followed-up in one of the participating centers for the 15-month study were eligible. Patients were excluded for impending graft failure, severe neurocognitive disabilities, lack of electronic pillbox connectivity, use of liquid immunuosuppressive medications, having a sibling participating in the study, participating in another adherence-promoting intervention study, or inability to communicate comfortably in English (or French -- Montreal site only).
Study overview
Participants were recruited and face-to-face study visits conducted every 3 months over 15 months (Figure 1), in conjunction with routine clinic visits, by a paid study ‘coach’ with no specific professional background, but with training in intervention and control procedures. Some coaches had an undergraduate psychology degree, some were psychology graduate students, and one was a nurse; all were trained clinical research professionals and also served as the study coordinator. There was one coach at each site who was not a member of, and did not interact with the treating team; the coach conducted both intervention and control visits. Clinical providers continued to deliver standard of care to all participants, including adherence assessment and promotion, but were not involved with delivery of the intervention. No information collected for study purposes was shared with the treating team.
Adherence assessment
At enrollment, all participants were given an electronic multi-dose pillbox in which all medications were stored. During the first 4–6 months of recruitment, participants received a Medminder pillbox (Medminder, Needham, MA). Due to technical difficulties with this device for some participants, subsequent participants were provided a SimpleMed device (Vaica Medical, Tel Aviv, Israel); the Medminder and Simplemed were similar, with the same types of adherence tracking and reminder functions. Both devices connected using cellphone technology; an internet connection was not needed. Prescribed dosing times were recorded in each participant’s web-based pillbox record. The date and time of each pillbox compartment opening was registered in the patient’s electronic pillbox record. Participants were asked to keep a log of medication dosing times when they took a dose from a source other than the pillbox26 (i.e. pocket, purse), and to inform study staff if they were not using the pillbox for a period (i.e. weekend or vacation travel).
Participants and their parent completed the validated adolescent (AMBS) and parent (PMBS) versions of the Medication Barriers Survey27, and a self-report adherence questionnaire, the Medical Adherence Measure Medication Module (MAM-MM)28 at enrollment.
A 3-month run-in period, during which no intervention was applied, followed the enrollment visit; this allowed habituation to the device and collection of baseline adherence data prior to intervention. Pillbox dose reminder functions were disabled for all participants during the run-in.
Randomization and blinding
The statistician randomized participants centrally by computer-generated sequence after enrollment, by site, 1:1 to intervention or control in age strata (11–13 y., 14–16 y., 17–19 y., 20–24 y.), in blocks of 4. Allocation to intervention or control was concealed from participants, clinical care providers, and study personnel during the first two months of the run-in. Group allocation was revealed to study personnel and participants one month prior to the first intervention to allow intervention participants to plan for a longer visit. Blinding was not feasible. The clinical team was not informed of group allocation.
Intervention
Figure 1 outlines timing and content of intervention sessions. The intervention was based on the self-management model29. At the first intervention visit, the patient and coach (together with the parent, for participants who chose this) formed an Adherence Support Team, and the responsibility of each team member for medication adherence was clarified30. The coach delivered standardized education on immunosuppressive medications via slide presentation, identified adherence barriers using the AMBS/PMBS27 and the last 3 months of electronic monitoring data and then used ‘Action-Focused Problem-Solving’ to address barriers selected as most important by the patient. The patient chose one or two barriers to address at each session. Action-focused problem-solving involved brainstorming possible solutions to a barrier, rating the potential solutions, and selecting one solution to implement. Action-focused problem-solving is distinguished from other problem-solving approaches by placing the solution in concrete terms in the form of an “If/when…then…” statement25. Intervention arm participants could also choose to receive text message, email, or visual cue dose reminders throughout the study. At subsequent sessions the coach, patient, and parent jointly reviewed the electronic adherence monitoring data from the prior 3 months to identify adherence patterns and guide the development and revision of action plans. Patients could continue to work on the same barrier(s), or select a new barrier to address.
Control condition
Control group study visits were conducted at the same intervals as intervention visits and consisted of the coach engaging in active listening and providing nonspecific support only. Adherence was NOT discussed with control participants.
Training and Treatment Integrity
All coaches underwent an intensive 2-day, in-person training session, including lectures and observed role-playing with feedback (Item S1). Intervention and control sessions were audio-recorded for assessment of treatment integrity25. Fidelity (i.e. was intended content delivered to intervention patients, but not control) was assessed for all recorded intervention and control sessions. For each coach, competency (i.e., quality of content delivery) was evaluated for all sessions for each of their first two participants and a random sample of 25% of their subsequent intervention sessions (overall 37% were rated for competency). Supervising psychologists reviewed sessions, rated competency, and provided feedback to Coaches via monthly teleconferences throughout the study.
Outcome Measures
The primary outcomes were daily ‘taking adherence” (proportion of prescribed doses taken) and “timing adherence” (proportion of prescribed doses taken within 1 hour before to 2 hours after the prescribed dosing time), as measured using electronic monitoring26,31–34. Because the majority of participants took immunosuppressives two times per day, and the risks associated with missing 1 of 2 doses are likely different that those associated with missing 2 of 2 doses, we wished to capture all possible dosing scenarios. Each day was scored as 0%, 50%, or 100%, depending on whether the patient took none, half, or all prescribed doses. Timing adherence scores could also take the values 0%, 50%, or 100%. Each participant had taking and timing adherence scores calculated for each day of the study to allow assessment of change over time. On days that the pillbox was not in use (turned off, not communicating with the server, or participant-reported non-use), no score was calculated (data missing). Additional analyses were conducted using electronic adherence data supplemented with data from logs of doses taken from a source other than the electronic pillbox.
Secondary adherence outcomes included standard deviation (SD) of tacrolimus trough levels and self-reported adherence. For the 6-month interval before intervention and the 12-month intervention interval, the SD of all tacrolimus trough levels obtained for clinical care (except during hospitalizations or illnesses) were calculated for participants with ≥3 levels. Self-reported taking and timing adherence, assessed using the MAM-MM, were scored as the proportion of doses taken and the proportion of doses taken up to 2 hours after the prescribed time respectively in the previous week. MAM-MM scores for each patient were summarized as the mean of the two scores in the run-in and the mean of the four scores post-intervention.
The estimated glomerular filtration rate (eGFR; Schwartz formula35 for children and CKD-EPI36 equation for young adults ≥18 years) was calculated at enrollment, intervention start, and study exit. Annualized change in eGFR over the intervention interval was calculated for each participant. Adverse event rates, including acute rejections (biopsy-proven or physician diagnosed) and graft failures were also determined.
Statistical analysis
We estimated a sample size of 75 participants per group to have 85% power to detect a 20% difference in taking adherence between groups, using 2–sided t-tests and setting alpha at 0.05, assuming a common standard deviation of 40%. Power calculations for repeated measures analyses are not standard; a repeated measures approach is expected to provide additional power. Targeted enrollment of 176 participants accounted for 15% drop-out.
Primary analyses were conducted using SAS (Version 9.2, SAS Institute Inc.), following intention-to-treat principles. All available electronic adherence data were used. The first 2 weeks of electronic data during the run-in were excluded to allow accommodation to the device37. We used unadjusted ordinal logistic regression with generalized estimating equations to account for repeated measures (i.e. score on each day) to compare taking and timing adherence between the intervention and control groups during the intervention interval. An additional ‘as-treated’ analysis considered participants who were assigned to intervention, but did not receive intervention, as controls. Comparisons of tacrolimus SD and self-report adherence were done using Wilcoxon rank-sum tests.
Several sensitivity analyses were performed. We used multivariable models to adjust for factors not well balanced between intervention and control. To determine which variables to include in the adjusted models, we calculated standardized differences between intervention and control38; all variables with effect size >0.2 were included. To estimate results including the expected contributions of the 17 patients assigned to intervention and the 14 assigned to control who had no pillbox data in the intervention interval, we used inverse probability weighting, based on their age (<17y vs. ≥17y), sex, race (Black vs. non-Black), and pre-intervention adherence (MAM score 100 vs. <100) profiles39,40.
Annualized change in eGFR over the intervention interval was compared between groups using a 2-sided, independent two samples t-test. Acute rejection, graft failure, and other adverse event rates were compared between intervention and control groups using Chi square.
RESULTS
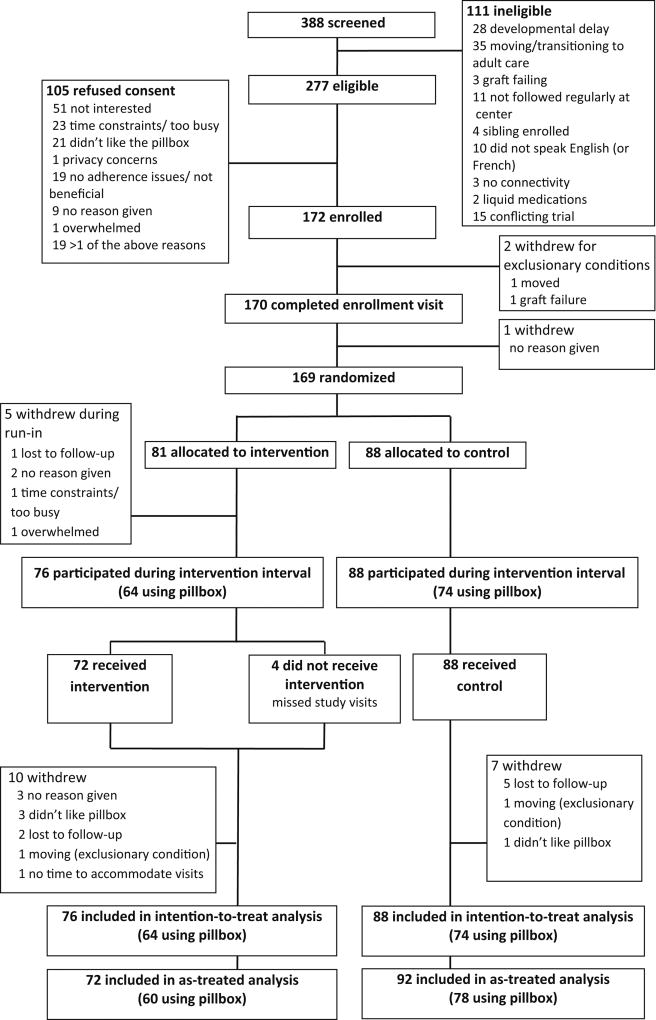
We screened 388 patients, representing virtually all patients in the target age range across sites. We identified 277 who were eligible, and enrolled 172 between Feb. 3, 2012 and Feb 1, 2015; 169 were randomized -- 81 to intervention and 88 to control (Figure 2). The characteristics of refusals and those without pillbox data in the intervention interval are shown in Tables S1 and S2. Baseline characteristics (Table 1) were similar between the two groups, though not perfectly balanced. Pre-intervention adherence and barrier burden by AMBS and PMBS were similar between groups. Intervention patients spent more time with the coach than controls. Additional details on participant characteristics are provided in Table S3.
Figure 2. Eligibility, randomization and follow-up.
There were 64 intervention and 74 control participants who had electronic adherence data available for analysis. Participants who declined to use the electronic pillbox were permitted to remain in the study and followed the same study procedures as patients using the pillbox, except that those in the intervention group could not receive dose reminders from the pillbox, nor were they able to review their adherence data with the coach.
Table 1.
Participant characteristics, pre-intervention adherence and Coach contact time
| Control (n = 88) |
Intervention (n = 81) |
|
|---|---|---|
|
| ||
| Demographics | ||
|
| ||
| Median age in | 15.8 (13.3 – 17.5) | 15.5 (13.2 – 17.4) |
|
| ||
| Male (%) | 54 (61) | 46 (57) |
|
| ||
| Race | ||
| White (%) | 57 (65) | 57 (70) |
| Black (%) | 11 (13) | 9 (11) |
| Asian (%) | 7 (8) | 4 (5) |
| Other (%) | 13 (15) | 11 (14) |
|
| ||
| Hispanic (%) | 5 (6) | 9 (11) |
|
| ||
| U.S. Study site (%) | 56 (64) | 54 (67) |
|
| ||
| Healthcare Insurer | ||
| U.S. Public | 20 (23) | 26 (32) |
| Private | 36 (41) | 28 (25) |
| Canadian provincial | 32 (36) | 27 (33) |
|
| ||
| Medication Insurer | ||
| U.S. Public | 24 (27) | 27 (33) |
| Private | 54 (61) | 42 (52) |
| Canadian provincial | 10 (11) | 12 (15) |
|
| ||
| Household income | ||
| Less Than $25,000 | 10 (11) | 19 (24) |
| $25,000 – $50,000 | 20 (23) | 17 (21) |
| $51,000 – $75,000 | 22 (25) | 17 (2) |
| $76,000 – $100,000 | 9 (10) | 6 (7) |
| Greater Than $100,000 | 16 (18) | 12 (15) |
| Prefer Not to Answer | 2 (2) | 5 (6) |
| Unknown | 9 (10) | 5 (6) |
|
| ||
| Disease characteristics | ||
|
| ||
| Median years post-Tx | 3.0 (0.8 – 7.2) | 3.7 (0.7 – 7.9) |
|
| ||
| Total no. of Tx | ||
| 1 | 81 (92) | 74 (91) |
| 2 | 7 (8) | 7 (9) |
|
| ||
| Donor source | ||
| Living | 51 (58) | 37 (46) |
| Deceased | 37 (42) | 44 (54) |
|
| ||
| Median total lifetime duration of dialysis, mo | 5.0 (0 – 12.0) | 10.0 (0.1 – 19.0) |
|
| ||
| Median age at Tx in years | 12.0 (8.3 – 15.2) | 11.5 (6.8 – 14.8) |
|
| ||
| Primary disease | ||
| CAKU (%) | 41 (47) | 29 (36) |
| Glomerulonephritis (%) | 5 (6) | 9 (11) |
| FSGS (%) | 6 (7) | 14 (17) |
| Other (%) | 36 (41) | 29 (36) |
|
| ||
| Number of past acute rejections | ||
| 0 (%) | 76 (86) | 59 (73) |
| 1 (%) | 8 (9) | 16 (20) |
| ≥2 (%) | 4 (5) | 6 (7( |
|
| ||
| Comorbidities | ||
| None | 43 (49) | 41 (51) |
| ≥1* | 45 (51) | 40 (49) |
|
| ||
| Median eGFR at baseline (ml/min/1.73m2; IQR) | 70.2 (56.0 – 85.9) | 69.4 (56.8 – 82.5) |
|
| ||
| Treatment characteristics: | ||
|
| ||
| No. of doses of immunosuppressives per day (%) | ||
| 1 | 6 (7) | 6 (7) |
| 2 | 82 (93) | 75 (93) |
|
| ||
| Median total number of medications (IQR) | 7.0 (5.0 – 9.0) | 7.0 (5.0 – 9.0) |
|
| ||
| Pre-intervention adherence and barriers | ||
|
| ||
| Run-in electronic adherence data | ||
| Days with 100% taking adherencea | 75% | 73% |
| Days with 100% timing adherence a | 67% | 65% |
|
| ||
| SD of Tac levels in the 6 mo before intervention | ||
| Once per day formulation* | 1.0 ± 0.4 | 1.1 ± 0.5 |
| Twice per day formulation** | 2.0 ± 1.7 | 2.2 ± 1.9 |
|
| ||
| Self-reported adherence (MAM; enrollment) | ||
| Taking adherence | 97.6 ± 7.1 | 98.8 ± 3.6 |
| Timing adherence | 92.9 ± 15.1 | 94.3 ± 12.2 |
|
| ||
| AMBS† | ||
| Total score | 38.1 ± 9.8 | 37.5 ± 11.2 |
| Disease frustration/ adolescent issues subscale | 19.5 ± 6.0 | 18.4 ± 6.6 |
| Ingestions issues subscale | 10.3 ± 3.3 | 10.3 ±3.5 |
| Regimen tasks/ cognitive subscale | 8.3 ± 2.8 | 8.7 ± 3.3 |
| Number of barriers reported | 3.2 ± 2.5 | 3.4 ± 2.7 |
| Proportion with ≥3 barriers (%) | 46 (52) | 43 (53) |
|
| ||
| PMBS✧ | ||
| Total score | 37.5 ± 10.3 | 34.0 ± 9.7 |
| Disease frustration/ adolescent issues subscale | 16.5 ± 5.3 | 14.4 ± 4.9 |
| Ingestions issues subscale | 6.3 ± 2.4 | 5.7 ± 2.4 |
| Regimen tasks/ cognitive subscale | 12.0 ± 4.6 | 10.9 ± 4.2 |
| Parent reminder subscale | 2.7 ± 1.4 | 3.0 ± 1.5 |
| Number of barriers reported | 3.8 ± 2.9 | 3.1 ± 2.4 |
| Proportion with ≥3 barriers (%) | 46 (58) | 37 (51) |
|
| ||
| Time in study and coach contact time | ||
|
| ||
| Run-in interval | ||
|
| ||
| Median patient-months | 3.1 (2.8 – 3.5) | 3.2 (2.9 – 3.6) |
|
| ||
| Proportion of days with no adherence score | 4.6% (0.0% – 22.7%) | 3.7% (0% – 14.1%) |
|
| ||
| Median time spent with coach at enrolment visit (min) | 45.0 (40.0 – 60.0) | 45.0 (40.0 – 60.0) |
|
| ||
| Intervention interval | ||
|
| ||
| Median patient-months | 12.4 (11.4 – 13.0) | 12.2 (11.3 – 13.3) |
|
| ||
| Proportion of days with no adherence score | 4.5% (1.1% – 10.7%) | 5.5% (2.3% – 15.0%) |
|
| ||
| Proportion of days on Medminder | 19.8% | 32.6% |
|
| ||
| Median time spent with coach (min) | ||
| 1st Intervention visit (at 3 mo) | 26.5 (22.0 – 33.5) | 80.0 (62.5 – 96.0) |
| 6-mo visit | 25.0 (20.0 – 30.0) | 40.0 (35.0 – 55.0) |
| 9-mo visit | 23.5 (20.0 – 30.0) | 35.0 (30.0 – 44.6) |
| 12-mo visit | 21.6 (20.0 – 27.0) | 31.5 (27.0 – 40.0) |
| 15-mo visit | 30.0 (25.0 – 40.0) | 35.0 (30.0 – 45.0) |
|
| ||
| Proportion of visits at which a caregiver was present | 100% (68% – 100%) | 100% (60% – 100%) |
SD, standard deviation; AMBS, adolescent-reported adherence barriers score; PMBS, parent-reported adherence barriers; Tac, tacrolimus; Tx, transplantation
Except as indicated, categorical data presented as count (percentage), continuous data as median [interquartile range] or mean +/− SD.
9 control and 6 intervention patients with ≥3 trough levels available to calculate SD
55 control and 47 intervention patients with ≥3 trough levels available to calculate SD
A higher AMBS score represents a greater burden of barriers. The possible range for total score is 17–85; subscale scores range from 7–35 for Disease frustration/adolescent issues, 6–30 for Ingestion issues, and 4–20 for Regimen adaptation/ Cognitive.
A higher PMBS score represents a greater burden of barriers. The possible range for total score is 16–80; subscale scores range from 7–35 for Disease frustration/adolescent issues, 3–15 for Ingestion issues, 5–25 for Regimen adaptation/ Cognitive, and 1–5 for Parent reminder.
all prescribed doses taken
Fidelity & competency assessments
Across intervention and control sessions, 96% of the content elements were delivered. Competency ratings, scored from 1 (poor) to 3 (highest quality), were high across interventionists and stable throughout the study. Mean scores ranged from 2.49–3.00 across each component rated.
Primary outcomes
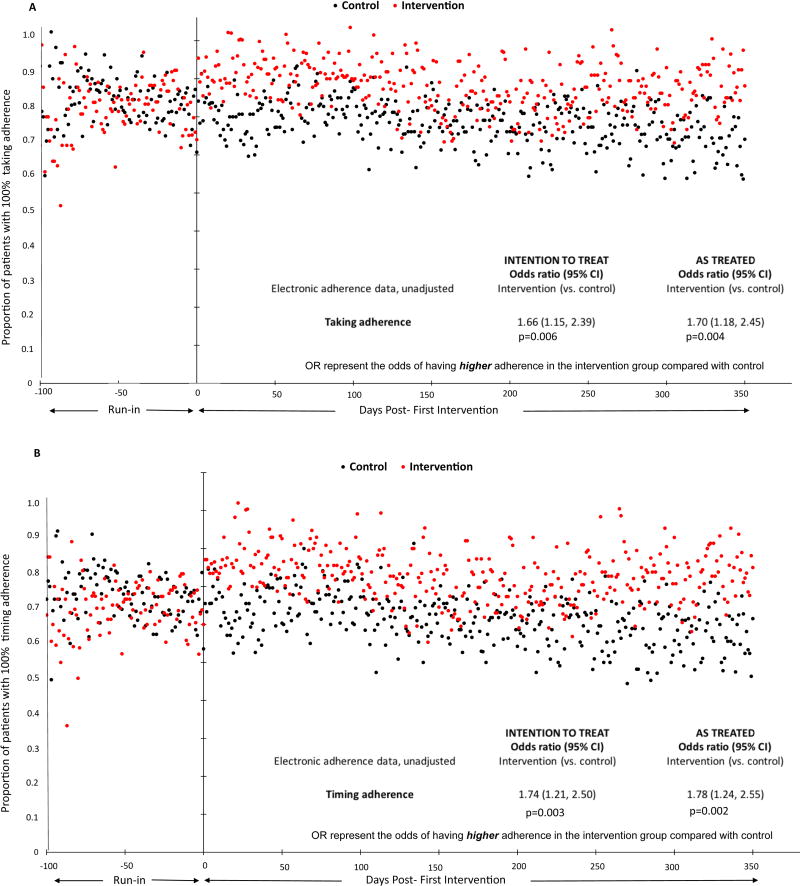
Medication-taking adherence improved among intervention participants immediately following the first intervention visit and remained fairly stable thereafter (Figure 3A). In contrast, there was no change in taking adherence among control participants. During the intervention interval, taking adherence was 100% on 78% of days for intervention participants compared with 68% for controls. The likelihood of better taking adherence was significantly greater among intervention participants than controls (OR, 1.66; 95% confidence interval (CI), 1.15–2.39; p=0.006). Timing of medication adherence also improved immediately after the first intervention (Figure 3B). Intervention participants had 100% timing adherence on 73% of days during the intervention interval, and had significantly greater odds of higher timing adherence scores (OR, 1.74; 95%CI, 1.21–2.50; p=0.003) than controls, who had 100% timing adherence on 61% of days. Results were unchanged after supplementing electronic adherence data with patients’ adherence logs, after adjustment for potential confounders, and in reweighted analyses (Table 2). Table S4 shows the proportions of days for which intervention and control participants had 0% and 50% taking and timing adherence.
Figure 3. Comparison of (A) taking and (B) timing adherence between intervention and control groups.
The proportion of intervention (red) and control (black) participants with 100% (a) taking and (b) timing adherence on each day of observation are shown. Intention to treat analyses included the 64 intervention and 74 control participants with pillbox data in the intervention interval. As treated analyses included the 60 participants assigned to intervention who received the intervention and had pillbox data in the intervention interval, and the 78 participants who did not receive the intervention and had pillbox data in the intervention interval. The odds ratios represent the odds of having higher adherence in the intervention group compared with control.
Table 2.
Sensitivity analyses
| OR (95% CI), Intervention vs. control | ||
|---|---|---|
|
| ||
| INTENTION TO TREAT | AS TREATED | |
|
| ||
| Electronic adherence data, adjusted* | ||
|
| ||
| Taking | 2.06 (1.07, 3.95) | 2.13 (1.10, 4.10) |
| Timing | 2.39 (1.31, 4.35) | 2.47 (1.35, 4.51) |
|
| ||
| Electronic adherence data supplemented with logs, unadjusted | ||
|
| ||
| Taking | 1.71 (1.16, 2.50) | 1.75 (1.20, 2.57) |
| Timing | 1.81 (1.24, 2.64) | 1.86 (1.27, 2.71) |
|
| ||
| Electronic adherence data supplemented with logs, adjusted | ||
|
| ||
| Taking | 1.95 (1.01, 3.80) | 2.02 (1.03, 3.96) |
| Timing | 2.18 (1.19, 4.00) | 2.25 (1.23, 4.15) |
|
| ||
| Reweighted to include those who withdrew or stopped using pillbox during run-in** | ||
|
| ||
| Taking | 1.63 (1.13, 2.36) | 1.67 (1.15, 2.42) |
| Timing | 1.70 (1.18, 2.46) | 1.74 (1.20, 2.52) |
OR, odds ratio; CI, confidence interval
Intention to treat analyses included the 64 intervention and 74 control participants with pillbox data in the intervention interval. As treated analyses included the 60 participants assigned to intervention who received the intervention and had pillbox data in the intervention interval, and the 78 participants who did not receive the intervention and had pillbox data in the intervention interval. The ORs represent the odds of having higher adherence in the intervention group compared with control.
Adjusted analysis compared intervention with control, adjusting for medication insurer, ethnicity, self-reported taking adherence pre-intervention, total score of the parent version of the medication barriers scale, primary disease, number of prior rejections, number of doses of immunosuppressive medications per day, donor source, and pillbox type (Simplemed vs. Medminder).
Reweighted analyses used the age, sex, race, and pre-intervention self-reported adherence profiles of those who were randomized but contributed no electronic adherence data in the intervention interval to weight the contributions of others with the same profiles in the same treatment group to effectively include the contributions of these missing individuals in the analyses.
Secondary outcomes
There was no difference between groups in the SD of tacrolimus trough levels (Table 3). Self-reported taking and timing adherence were high, and did not differ between groups. There were no graft failures. Acute rejection rates were numerically lower in the intervention than control group, but the difference was not statistically significant. There was no difference in annualized change in eGFR over the intervention interval by group. There were no differences in adverse event rates between intervention and control groups, except cytomegalovirus infection, which was higher in the intervention group.
Table 3.
Secondary outcomes and Adverse events
| Control | Intervention | p-value | |
|---|---|---|---|
|
| |||
| Secondary adherence outcomes | |||
|
| |||
| SD of Tac (2x/d formulation) trough levels during intervention interval† | 1.4 (0.9 to 2.1) | 1.6 (0.9 to 2.5) | 0.5 |
|
| |||
| SD of Tac (1x/d formulation) trough levels during intervention interval†† | 1.2 (0.8 to 1.4) | 1.2 (0.9 to 2.1) | 0.8 |
|
| |||
| Mean self-reported adherence during intervention interval | |||
| Taking adherence | 97.1 ± 6.0 | 98.3 ± 4.5 | 0.2 |
| Timing adherence | 92.9 ± 9.3 | 95.0 ± 7.9 | 0.2 |
|
| |||
| Graft outcomes | |||
|
| |||
| Graft failure rate | 0 | 0 | -- |
|
| |||
| Acute rejection rate (events /100 patient-months) | 1.69 | 1.06 | 0.3 |
|
| |||
| Annualized change in eGFR* (ml/min/1.73m2) | −3.3 (−7.7 to 3.7) | −2.3 (−10.6 to 2.3) | 0.5 |
|
| |||
|
Adverse events (events per 100 patient-months)
| |||
| All adverse events | 12.7 | 12.9 | 0.9 |
|
| |||
| Post-Tx lymphoproliferative disorder | 0.20 | 0.0 | 0.3 |
|
| |||
| Epstein-Barr virus infection | 0.0 | 0.0 | --- |
|
| |||
| Cytomegalovirus infection | 0.0 | 0.59 | 0.02 |
|
| |||
| BK virus nephropathy | 0.0 | 0.0 | --- |
|
| |||
| Influenza | 0.50 | 0.35 | 0.7 |
|
| |||
| Other infection | 3.09 | 2.36 | 0.4 |
|
| |||
| Vomiting/diarrhea | 0.30 | 0.59 | 0.4 |
|
| |||
| Surgery/ procedure | 0.60 | 0.83 | 0.6 |
|
| |||
| Other** | 2.59 | 3.19 | 0.5 |
|
| |||
| Hospitalizations | 5.38 | 4.96 | 0.7 |
Unless otherwise indicated, values shown are median [interquartile range] or mean +/− SD. Tx, transplantation; Tac, tacrolimus; SD, standard deviation; eGFR, estimated glomerular filtration rate
63 control and 58 intervention patients with ≥3 trough levels available to calculate SD
10 control and 9 intervention patients with ≥3 trough levels available to calculate SD
Annualized change in eGFR was calculated as eGFR at study exit minus eGFR at start of the intervention interval, normalized to a 1-year period. A negative value indicates a decline in eGFR and a positive value indicates an increase in eGFR.
Other adverse events included: headache, abdominal pain, alopecia, fracture, dehydration, urinary obstruction, fever, constipation, asthma, minor pains, anemia, seizures, diabetes, mood disorder, accidental medication overdose, arrhythmia, hypertension, renal contusion, pneumonitis, neutropenia, and hemoptysis. No adverse event was judged to be related to the intervention.
DISCUSSION
This multicenter randomized trial of a clinic-based, multi-component adherence-promoting intervention showed significantly better adherence, as measured by electronic monitoring, among young kidney transplant recipients assigned to intervention than control. It is not possible to determine which components of the TAKE-IT intervention were most powerful in promoting adherence. Prior studies, both in transplant and other chronic disease populations, suggested that reminders, problem-solving, and action-planning may each have benefits16,19,20,41,42. Different patients may benefit more or less from different components of the intervention – a reason multicomponent interventions are preferred. A systematic review showed that feedback of adherence data may be particularly powerful21. A shorter trial in adult heart, liver, and lung20,34 transplant recipients of a similar multicomponent intervention showed benefit of comparable magnitude, as did an intervention in adult kidney recipients that included only dose reminders and feedback20. Whether all intervention components would have the same impact in adolescents and young adults as in older adults is not known. As in a prior study34, we also observed improved adherence immediately following the first intervention visit. We speculate that patients left the first intervention visit motivated to improve, explaining the rapid effect; our intervention was designed for sustained effect, with continuous support via dose reminders and ‘booster’ intervention sessions every 3 months.
We found no differences between treatment groups in adherence measured by self-report or by SD of tacrolimus levels. Self-reported adherence was very high for both groups, likely reflecting the well-known under-reporting of missed and late doses43–45 and the short timeframe for reporting (1 week). Failure to detect group differences in self-reported adherence may be due to a ceiling effect, whereby variability is not captured so differences are not observed. The observed lack of difference in SD of tacrolimus levels between groups has several possible explanations. First, the patients for whom ≥3 tacrolimus levels were available may represent a biased sample of the most adherent patients. Second, patients may have better adherence in the days preceding a planned blood level measurement. Third, a high SD of tacrolimus trough levels may not always reflect erratic intake but rather variability in drug exposure for other reasons46. Fourth, we must consider the possibility that adherence did not actually differ between the groups, and that the differences observed between groups in electronically-monitored adherence represent more assiduous use of the pillbox by those in the intervention group. Participants may have taken meds without opening the pillbox or may have opened the pillbox without ingesting the medication every time. Future studies should consider including patient-reported outcomes.
The study has limitations. The longer duration of visits for intervention patients compared with controls may have resulted in some attention bias. The patients who withdrew or ceased to use the pillbox may introduce bias if they represented patients who were more or less adherent than those who continued to use the pillbox. A sensitivity analyses in which the adherence data of other participants with the same group allocation, age, sex, race, and pre-intervention adherence profile were weighted to account for these missing values39,40 returned similar results. However, it is likely that if non-pillbox users assigned to intervention had mostly poor adherence, whereas non-pillbox users assigned to control had mostly good adherence, the effect of intervention would be substantially attenuated. Given random assignment to treatment group, such a scenario is unlikely, but not impossible. The weighted analysis described above provides the best estimate of the contributions of non-pillbox users. In addition, the primary outcome of adherence rather than a clinical outcome is a limitation.
Given the relatively short study duration, the expected numbers of graft failures and acute rejections were so low, and the expected decline in eGFR so small (and variable), that it was not feasible to power the study to assess these outcomes. Powering the study to assess de novo donor specific antibodies (dnDSA) was also not feasible. Although an estimated 25–36% of pediatric patients develop dnDSA47,48, as many as half of these may develop dnDSA within the first 3–6 months post-transplant48. Due to the relatively small number of new transplant procedures each year in the target age group, it was necessary to enroll prevalent patients. If development of DSA were the outcome, prevalent patients with existing DSA would have to be excluded. Not only would this severely curtail the number of potentially eligible patients, but may select for the most adherent patients, since development of DSA is strongly associated with poor adherence49,50. Furthermore, because detection of DSA may fluctuate over time, with subsequently undetectable DSA in up to 47% who were once positive48, it would be difficult to accurately determine true DSA status at intervention initiation. Therefore, it was necessary to evaluate an intermediate outcome. There is a strong theoretical basis, as well as empirical evidence, for an association between poor adherence and poor graft outcomes5,50.
The generalizability of the study may be limited due to the relatively small proportion of participants who were black. Another issue potentially limiting generalizability was the unwillingness of some participants to use the electronic pillbox – which was central to the intervention. Self-selection of patients into an adherence trial (62% consent rate) may also limit generalizability if patients more motivated to improve their adherence participated. Adherence is a complex issue; it is unlikely that any single intervention will be appropriate for all patients.
The study also has important strengths. TAKE-IT is one of the largest adherence trials ever conducted in the kidney transplant population. Identification of an approach that is effective in promoting adherence to chronic daily medication represents a major advance. How to effectively integrate the TAKE-IT intervention into clinical practice is the subject of a new study (TAKE-IT TOO; https://www.takeittoo.org). We expect that transplant nurses or other allied health professionals could learn the action-focused problem-solving approach relatively quickly from a clinical psychologist. The time required to deliver the intervention may require additional resources; based on the time needed for each session during the trial, a program following 60 adolescents would spend ~150 hours per year delivering the intervention. Given that clinical care teams already devote some effort to promoting adherence, extra time required may be less. The cost of electronic monitoring, with reminders, and ability to feed-back objective adherence data to patients remains high. However, technology advances and recognition of benefits may lead to more widespread use resulting in lower prices, or in coverage of this technology by insurers. The TAKE-IT TOO study aims to develop a more usable electronic monitoring system to potentially improve the ability of care providers to efficiently assess adherence, and improve acceptance by patients. However, not all patients will accept electronic monitoring.
The significantly better adherence observed in the intervention group may lead to better graft outcomes. The costs associated with the TAKE-IT intervention may be offset by savings due to lower rejection and graft failure rates, and fewer biopsies and hospitalizations51, however this question requires study in a formal cost-benefit analysis.
Supplementary Material
Acknowledgments
We would like to thank the study coaches and the patients and families who participated in the study.
Support: The study was funded by the American National Institutes of Health, National Institutes of Diabetes, Digestive and Kidney diseases (NIDDK; R01DK092977). Dr. Foster, a member of the Research Institute of the McGill University Health Centre, was supported by a Fonds de recherche du Quebec Santé Chercheur-boursier clinicien award. The funder had no role in study design, data collection, analysis, interpretation of data, writing the report, or and the decision to submit the report for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial registration: Registered at Clinicaltrials.gov with study number NCT01356277.
Authors’ Contributions: Research idea and study design: BJF, SF, AP, NZ, BK, JS, VRD, LB, DH; data acquisition: BJF, SF, AP, JS, VRD, DM, DH, VP, NZ, CH; data analysis/interpretation: RR, HZ, BJF, SF, AP, SA; statistical analysis: RR, HZ. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Financial Disclosure: Dr. Foster is a co-investigator on an investigator-initiated study funded by Astellas Canada.
The remaining authors declare that they have no relevant financial interests.
References
- 1.Foster BJ, Dahhou M, Zhang X, Platt RW, Samuel SM, Hanley JA. Association Between Age and Graft Failure Rates in Young Kidney Transplant Recipients. Transplantation. 2011;92(11):1237–1243. doi: 10.1097/TP.0b013e31823411d7. [DOI] [PubMed] [Google Scholar]
- 2.Dobbels F, Ruppar T, De Geest S, Decorte A, Van Damme-Lombaerts R, Fine RN. Adherence to the immunosuppressive regimen in pediatric kidney transplant recipients: a systematic review. Pediatr Transplant. 2010;14(5):603–613. doi: 10.1111/j.1399-3046.2010.01299.x. [DOI] [PubMed] [Google Scholar]
- 3.Dew MA, Dabbs AD, Myaskovsky L, et al. Meta-analysis of medical regimen adherence outcomes in pediatric solid organ transplantation. Transplantation. 2009;88(5):736–746. doi: 10.1097/TP.0b013e3181b2a0e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinsky BW, Takemoto SK, Lentine KL, Burroughs TE, Schnitzler MA, Salvalaggio PR. Transplant outcomes and economic costs associated with patient noncompliance to immunosuppression. Am J Transplant. 2009;9(11):2597–2606. doi: 10.1111/j.1600-6143.2009.02798.x. [DOI] [PubMed] [Google Scholar]
- 5.Sellares J, de Freitas DG, Mengel M, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2011;12(2):388–399. doi: 10.1111/j.1600-6143.2011.03840.x. [DOI] [PubMed] [Google Scholar]
- 6.Griva K, Davenport A, Harrison M, Newman SP. Non-adherence to immunosuppressive medications in kidney transplantation: intent vs. forgetfulness and clinical markers of medication intake. Ann Behav Med. 2012;44(1):85–93. doi: 10.1007/s12160-012-9359-4. [DOI] [PubMed] [Google Scholar]
- 7.Simons LE, McCormick ML, Mee LL, Blount RL. Parent and patient perspectives on barriers to medication adherence in adolescent transplant recipients. Pediatr Transplant. 2009;13(3):338–347. doi: 10.1111/j.1399-3046.2008.00940.x. [DOI] [PubMed] [Google Scholar]
- 8.Zelikovsky N, Schast AP, Palmer J, Meyers KE. Perceived barriers to adherence among adolescent renal transplant candidates. Pediatr Transplant. 2008;12(3):300–308. doi: 10.1111/j.1399-3046.2007.00886.x. [DOI] [PubMed] [Google Scholar]
- 9.Gerson AC, Butler R, Moxey-Mims M, et al. Neurocognitive outcomes in children with chronic kidney disease: Current findings and contemporary endeavors. Ment Retard Dev Disabil Res Rev. 2006;12(3):208–215. doi: 10.1002/mrdd.20116. [DOI] [PubMed] [Google Scholar]
- 10.Haavisto A, Korkman M, Holmberg C, Jalanko H, Qvist E. Neuropsychological profile of children with kidney transplants. Nephrol Dial Transplant. 2012;27(6):2594–2601. doi: 10.1093/ndt/gfr650. [DOI] [PubMed] [Google Scholar]
- 11.Reed-Knight B, Lee JL, Cousins LA, Mee LL. Intellectual and academic performance in children undergoing solid organ pretransplant evaluation. Pediatr Transplant. 2015;19(2):229–234. doi: 10.1111/petr.12389. [DOI] [PubMed] [Google Scholar]
- 12.Nieuwlaat R, Wilczynski N, Navarro T, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2014;(11) doi: 10.1002/14651858.CD000011.pub4. CD000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fine RN, Becker Y, De Geest S, et al. Nonadherence consensus conference summary report. Am J Transplant. 2009;9(1):35–41. doi: 10.1111/j.1600-6143.2008.02495.x. [DOI] [PubMed] [Google Scholar]
- 14.De Bleser L, Matteson M, Dobbels F, Russell C, De Geest S. Interventions to improve medication-adherence after transplantation: a systematic review. Transpl Int. 2009;22(8):780–797. doi: 10.1111/j.1432-2277.2009.00881.x. [DOI] [PubMed] [Google Scholar]
- 15.Bonner S, Zimmerman BJ, Evans D, Irigoyen M, Resnick D, Mellins RB. An individualized intervention to improve asthma management among urban Latino and African-American families. J Asthma. 2002;39(2):167–179. doi: 10.1081/jas-120002198. [DOI] [PubMed] [Google Scholar]
- 16.Rapoff MA, Belmont J, Lindsley C, Olson N, Morris J, Padur J. Prevention of nonadherence to nonsteroidal anti-inflammatory medications for newly diagnosed patients with juvenile rheumatoid arthritis. Health Psychol. 2002;21(6):620–623. doi: 10.1037//0278-6133.21.6.620. [DOI] [PubMed] [Google Scholar]
- 17.Nansel TR, Iannotti RJ, Simons-Morton BG, Plotnick LP, Clark LM, Zeitzoff L. Long-term maintenance of treatment outcomes: diabetes personal trainer intervention for youth with type 1 diabetes. Diabetes Care. 2009;32(5):807–809. doi: 10.2337/dc08-1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kahana S, Drotar D, Frazier T. Meta-analysis of psychological interventions to promote adherence to treatment in pediatric chronic health conditions. J Pediatr Psychol. 2008;33(6):590–611. doi: 10.1093/jpepsy/jsm128. [DOI] [PubMed] [Google Scholar]
- 19.Miloh T, Annunziato R, Arnon R, et al. Improved adherence and outcomes for pediatric liver transplant recipients by using text messaging. Pediatrics. 2009;124(5):e844–850. doi: 10.1542/peds.2009-0415. [DOI] [PubMed] [Google Scholar]
- 20.Reese PP, Bloom RD, Trofe-Clark J, et al. Automated Reminders and Physician Notification to Promote Immunosuppression Adherence Among Kidney Transplant Recipients: A Randomized Trial. Am J Kidney Dis. 2017;69(3):400–409. doi: 10.1053/j.ajkd.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 21.Demonceau J, Ruppar T, Kristanto P, et al. Identification and assessment of adherence-enhancing interventions in studies assessing medication adherence through electronically compiled drug dosing histories: a systematic literature review and meta-analysis. Drugs. 2013;73(6):545–562. doi: 10.1007/s40265-013-0041-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mistry N, Keepanasseril A, Wilczynski NL, et al. Technology-mediated interventions for enhancing medication adherence. J Am Med Inform Assoc. 2015;22(e1):e177–193. doi: 10.1093/jamia/ocu047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nevins TE, Nickerson PW, Dew MA. Understanding Medication Nonadherence after Kidney Transplant. J Am Soc Nephrol. 2017;28(8):2290–2301. doi: 10.1681/ASN.2017020216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vrijens B, De Geest S, Hughes DA, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012;73(5):691–705. doi: 10.1111/j.1365-2125.2012.04167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foster BJ, Pai A, Zhao H, Furth S, Group T-IS. The TAKE-IT study: aims, design, and methods. BMC Nephrol. 2014;15:139. doi: 10.1186/1471-2369-15-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Denhaerynck K, Steiger J, Bock A, et al. Prevalence and risk factors of non-adherence with immunosuppressive medication in kidney transplant patients. Am J Transplant. 2007;7(1):108–116. doi: 10.1111/j.1600-6143.2006.01611.x. [DOI] [PubMed] [Google Scholar]
- 27.Simons LE, Blount RL. Identifying barriers to medication adherence in adolescent transplant recipients. J Pediatr Psychol. 2007;32(7):831–844. doi: 10.1093/jpepsy/jsm030. [DOI] [PubMed] [Google Scholar]
- 28.Zelikovsky N, Schast AP. Eliciting accurate reports of adherence in a clinical interview: development of the Medical Adherence Measure. Pediatr Nurs. 2008;34(2):141–146. [PubMed] [Google Scholar]
- 29.Modi AC, Pai AL, Hommel KA, et al. Pediatric self-management: a framework for research, practice, and policy. Pediatrics. 2012;129(2):e473–485. doi: 10.1542/peds.2011-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pai AL, Gray E, Kurivial K, Ross J, Schoborg D, Goebel J. The Allocation of Treatment Responsibility scale: a novel tool for assessing patient and caregiver management of pediatric medical treatment regimens. Pediatr Transplant. 2010;14(8):993–999. doi: 10.1111/j.1399-3046.2010.01391.x. [DOI] [PubMed] [Google Scholar]
- 31.De Geest S, Schafer-Keller P, Denhaerynck K, et al. Supporting medication adherence in renal transplantation (SMART): a pilot RCT to improve adherence to immunosuppressive regimens. Clin Transplant. 2006;20(3):359–368. doi: 10.1111/j.1399-0012.2006.00493.x. [DOI] [PubMed] [Google Scholar]
- 32.Kuypers DR, Peeters PC, Sennesael JJ, et al. Improved adherence to tacrolimus once-daily formulation in renal recipients: a randomized controlled trial using electronic monitoring. Transplantation. 2013;95(2):333–340. doi: 10.1097/TP.0b013e3182725532. [DOI] [PubMed] [Google Scholar]
- 33.Schafer-Keller P, Steiger J, Bock A, Denhaerynck K, De Geest S. Diagnostic accuracy of measurement methods to assess non-adherence to immunosuppressive drugs in kidney transplant recipients. Am J Transplant. 2008;8(3):616–626. doi: 10.1111/j.1600-6143.2007.02127.x. [DOI] [PubMed] [Google Scholar]
- 34.Dobbels F, De Bleser L, Berben L, et al. Efficacy of a medication adherence enhancing intervention in transplantation: The MAESTRO-Tx trial. J Heart Lung Transplant. 2017;36(5):499–508. doi: 10.1016/j.healun.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz GJ, Munoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20(3):629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Denhaerynck K, Schafer-Keller P, Young J, Steiger J, Bock A, De Geest S. Examining assumptions regarding valid electronic monitoring of medication therapy: development of a validation framework and its application on a European sample of kidney transplant patients. BMC Med Res Methodol. 2008;8:5. doi: 10.1186/1471-2288-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang D, Dalton JE. A unified approach to measuring the effect size between two groups using SAS®. 2012 [Google Scholar]
- 39.Langkamp DL, Lehman A, Lemeshow S. Techniques for handling missing data in secondary analyses of large surveys. Acad Pediatr. 2010;10(3):205–210. doi: 10.1016/j.acap.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res. 2013;22(3):278–295. doi: 10.1177/0962280210395740. [DOI] [PubMed] [Google Scholar]
- 41.Gross R, Bellamy SL, Chapman J, et al. Managed problem solving for antiretroviral therapy adherence: a randomized trial. JAMA Intern Med. 2013;173(4):300–306. doi: 10.1001/jamainternmed.2013.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quittner AL, Modi AC, Roux AL. Psychosocial challenges and clinical interventions for children with cystic fibrosis: A developmental approach. In: Brown R, editor. Handbook of Pediatric Psychology in School Settings. New Jersey: Lawrence Erlbaum Associates; 2003. pp. 333–361. [Google Scholar]
- 43.Quittner AL, Modi AC, Lemanek KL, Ievers-Landis CE, Rapoff MA. Evidence-based assessment of adherence to medical treatments in pediatric psychology. J Pediatr Psychol. 2008;33(9):916–936. doi: 10.1093/jpepsy/jsm064. discussion 937–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi L, Liu J, Fonseca V, Walker P, Kalsekar A, Pawaskar M. Correlation between adherence rates measured by MEMS and self-reported questionnaires: a meta-analysis. Health Qual Life Outcomes. 2010;8:99. doi: 10.1186/1477-7525-8-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stirratt MJ, Dunbar-Jacob J, Crane HM, et al. Self-report measures of medication adherence behavior: recommendations on optimal use. Transl Behav Med. 2015;5(4):470–482. doi: 10.1007/s13142-015-0315-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sapir-Pichhadze R, Wang Y, Famure O, Li Y, Kim SJ. Time-dependent variability in tacrolimus trough blood levels is a risk factor for late kidney transplant failure. Kidney Int. 2014;85(6):1404–1411. doi: 10.1038/ki.2013.465. [DOI] [PubMed] [Google Scholar]
- 47.Ginevri F, Nocera A, Comoli P, et al. Posttransplant de novo donor-specific hla antibodies identify pediatric kidney recipients at risk for late antibody-mediated rejection. Am J Transplant. 2012;12(12):3355–3362. doi: 10.1111/j.1600-6143.2012.04251.x. [DOI] [PubMed] [Google Scholar]
- 48.Kim JJ, Balasubramanian R, Michaelides G, et al. The clinical spectrum of de novo donor-specific antibodies in pediatric renal transplant recipients. Am J Transplant. 2014;14(10):2350–2358. doi: 10.1111/ajt.12859. [DOI] [PubMed] [Google Scholar]
- 49.Wiebe C, Gibson IW, Blydt-Hansen TD, et al. Rates and Determinants of Progression to Graft Failure in Kidney Allograft Recipients With De Novo Donor-Specific Antibody. Am J Transplant. 2015;15(11):2921–2930. doi: 10.1111/ajt.13347. [DOI] [PubMed] [Google Scholar]
- 50.Wiebe C, Nevins TE, Robiner WN, Thomas W, Matas AJ, Nickerson PW. The Synergistic Effect of Class II HLA Epitope-Mismatch and Nonadherence on Acute Rejection and Graft Survival. Am J Transplant. 2015;15(8):2197–2202. doi: 10.1111/ajt.13341. [DOI] [PubMed] [Google Scholar]
- 51.Axelrod DA, Schnitzler MA, Xiao H, et al. The Changing Financial Landscape of Renal Transplant Practice: A National Cohort Analysis. Am J Transplant. 2017;17(2):377–389. doi: 10.1111/ajt.14018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.