Abstract
Background
In high-resource settings, the HIV-attributable risk of myocardial infarction (MI) is higher among women than among men. The extent to which unique mechanisms contribute to MI risk among women vs. men with HIV remains unclear.
Methods
Subclinical coronary atherosclerotic plaque characteristics – including high-risk morphology plaque features – were compared among 48 HIV-infected women (48 [41, 54] years) and 97 HIV-infected men (48 [42, 52] years) on stable antiretroviral therapy (ART) without known cardiovascular disease. These individuals had previously completed coronary computed tomography angiography and metabolic/immune phenotyping as part of a prospective study.
Results
Extending prior analyses, now focusing exclusively on ART-treated participants, we found that HIV-infected women had a lower prevalence of any subclinical coronary atherosclerotic plaque (35% vs. 62%, P=0.003) and a lower number of segments with plaque (P=0.01), compared to HIV-infected men. We also report for the first time that ART-treated HIV-infected women had a lower prevalence of high-risk positively remodeled plaque (25% vs. 51%, P=0.003) and a lower number of positively remodeled plaque segments (P=0.002). In models adjusting for cardiovascular risk factors, we further showed that male sex remained associated with any coronary plaque (OR 3.8, 95%CI [1.4, 11.4]) and with positively remodeled plaque (OR 3.7, 95%CI [1.4, 10.9]).
Conclusions
ART-treated HIV-infected women (vs. HIV-infected men) had a lower prevalence and burden of subclinical coronary plaque and high-risk morphology plaque. Thus, unique sex-specific mechanisms beyond subclinical plaque may drive the higher HIV-attributable risk of MI among women vs. men.
Keywords: HIV, Women, Sex, Atherosclerosis, Cardiovascular Disease
INTRODUCTION
Studies conducted in high-resource settings suggest that the HIV-attributable risk of myocardial infarction (MI) is higher among women than among men1,2,3,4. In the present study, we endeavored to assess whether the mechanisms predisposing to MI among individuals with HIV differ by sex. To do so, we performed new analyses focused on ART-treated HIV-infected individuals without known cardiovascular disease (CVD) who had been previously recruited to undergo coronary computed tomography angiography (CTA)5,6,7.
Prior studies from our group provide critical context for the current investigation. Initial studies focused on men revealed that asymptomatic HIV-infected men (vs. men without HIV) had a significantly increased prevalence of subclinical coronary atherosclerotic plaque5 and an increased prevalence and burden of coronary plaque with high-risk morphology8. High risk-morphology plaque – that is, plaque with features of low attenuation or positive remodeling9 – is prone to rupture, resulting in MI10. Next, studies led by Fitch et al. illustrated that asymptomatic HIV-infected women (vs. women without HIV) had an increased proportion of non-calcified plaque, and that those HIV-infected women with non-calcified plaque had the highest levels of systemic immune activation7. In a secondary four-group comparison of women and men with and without HIV, Fitch et al. also showed a significant difference in overall plaque prevalence across groups, with plaque prevalence highest among HIV-infected men7.
In the present study, we built on the aforementioned findings. For the first time, we systematically compared plaque characteristics – including high-risk morphology plaque – among HIV-infected women and men, controlling for differences between groups. For this purpose, we generated novel data on subclinical high-risk morphology plaque characteristics among asymptomatic HIV-infected women. In order to maximize the generalizability and clinical relevance of our findings on sex differences in subclinical coronary atherosclerotic plaque, we focused our analyses on those HIV-infected women and men who reported stable use of ART. Strong imperatives exist to examine whether mechanisms fueling MI risk among ART-treated HIV-infected individuals may differ by sex. Indeed, without rigorous sex differences research, mechanistic insights from studies enrolling all-male or predominantly-male cohorts may be misapplied to women.
METHODS
Study Design and Participants
In this study, data on subclinical coronary atherosclerotic plaque – including high-risk morphology plaque – were compared among previously recruited HIV-infected women and men without known CVD. These participants (ages 18-60 years) had been previously recruited from the greater Boston area to undergo coronary CTA and detailed metabolic/immune phenotyping5,6,7. Recruitment took place between September 2006 and October 2012. Data on general and high risk coronary plaque characteristics had been previously assessed among HIV-infected men5,6,8 and data on general coronary plaque characteristics had been previously assessed among HIV-infected women7. Data on high-risk coronary plaque characteristics among HIV-infected women were newly generated for the purpose of these analyses. Consonant with the current HIV standard of care, the analyses we present were restricted to those HIV-infected women and men on stable ART (> 3 months) who had successfully completed coronary CTA procedures. All participants provided informed consent. Approval for the study was obtained from the Institutional Review Boards of the Massachusetts General Hospital and the Massachusetts Institute of Technology.
Study Procedures
Immune and Metabolic Phenotyping
Participants underwent history and physical examination. Select HIV-specific parameters – including time since HIV diagnosis, ART regimen, total duration of ART, and nadir CD4+ T-cell count – were participant-reported. Fasting total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglycerides, and hemoglobin A1c were quantified using standard techniques. Flow cytometry was performed to assess CD4+ T-cell counts and HIV viral load was quantified with ultrasensitive reverse-transcription polymerase chain reaction (Roche COBAS Amplicor). ELISAs were used to assess levels of soluble CD14 (sCD14) (R&D Systems) and soluble CD163 (sCD163) (Trillium). Abdominal visceral (VAT) and subcutaneous adipose tissue (SAT) area was determined using single slice computed tomography at the level of the fourth lumbar vertebra.
Coronary CTA and Coronary Atherosclerotic Plaque Analysis
Coronary CTA was performed using a 64-slice CT scanner or 64-slice dual-source CT scanner (Siemens Medical Solutions), as previously detailed5,6,7,8. Experienced cardiovascular imagers blinded to participants’ clinical status assessed plaque parameters including: any plaque, plaque type (calcified, non-calcified), plaque with high-risk morphology features (positive remodeling, low attenuation), and obstructive plaque (≥ 70% stenosis). With respect to plaque morphology, high-risk plaque features were defined as follows: low attenuation plaque had a mean minimum attenuation < 40 Hounsfield Units; positively remodeled plaque had a [plaque segment diameter/reference segment diameter] > 1.05 indicating eccentric volume gain8. Examples of select plaque characteristics are depicted in Figure 1. Plaque data on women vs. men were read by a limited number of experienced imagers, who had received similar training and employed the same diagnostic criteria.
Figure 1. Examples of Select Plaque Characteristics on Coronary Computed Tomography Angiography.
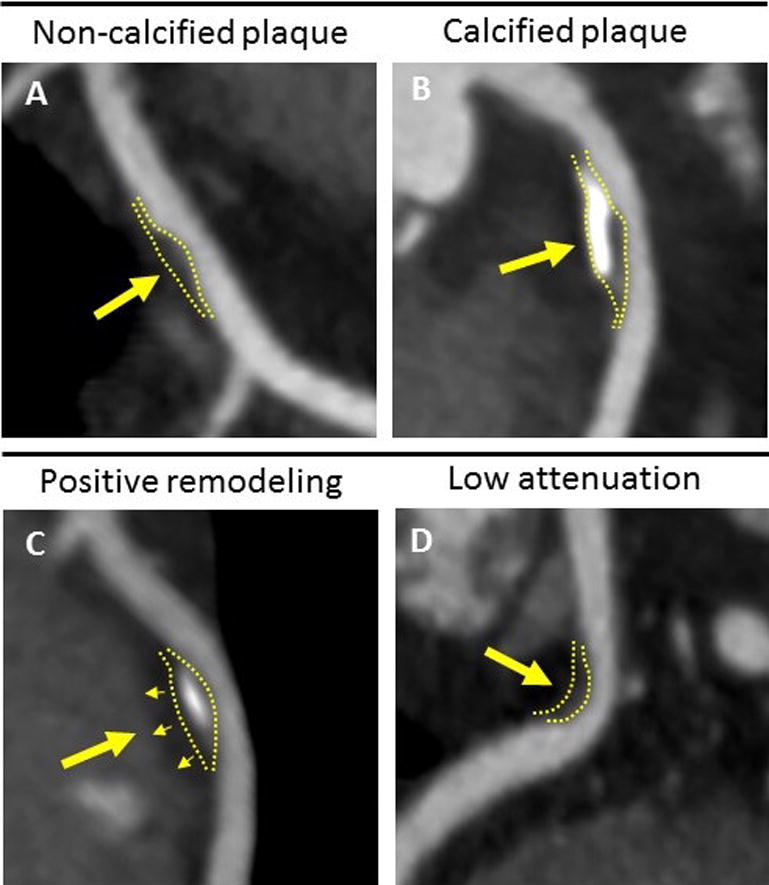
A) Non-calcified plaque without calcified components. B) Predominantly calcified plaque with large calcifications. C) Positively remodeled plaque with eccentric expansion into perivascular tissue. D) Low attenuation plaque.
Statistical Analysis
The outcomes of interest in our analysis were differences in prevalence and burden of any plaque and of high-risk morphology plaque between HIV-infected women and men. Normality of variables was determined using the Shapiro-Wilk test and confirmed by visual assessment. Normally distributed variables are shown as mean ± standard deviation (SD) and non-normally distributed variables are shown as median [interquartile range (IQR)]. For data on plaque segments, both mean ± SD and median [IQR] are presented descriptively. Demographic, immunologic, and metabolic parameters were compared between HIV-infected women and men. Plaque characteristics were then compared between HIV-infected women and men in the sample overall, as well as in the subset of participants with any plaque. For these analyses, student’s two-tailed t-test was used for normally distributed continuous variables, Wilcoxon rank-sum test was used for non-normally distributed continuous variables, and chi-square test was used for categorical variables. Multivariable logistic regression models were constructed to identify independent relationships of sex with presence of any plaque and presence of high-risk morphology plaque while adjusting for relevant traditional and HIV-specific CVD risk factors that differed between women and men. Adjusted odds ratios are reported with 95% confidence intervals. Statistical significance was defined as P ≤ 0.05. Statistical analyses were performed using JMP Pro software (version 12.0, SAS Institute, Cary, North Carolina, USA).
RESULTS
Participant Characteristics
The participants studied consisted of 48 ART-treated HIV-infected women and 97 ART-treated HIV-infected men. Fifty percent of women were postmenopausal as defined by no menses for at least one year. Six percent of women were taking hormonal contraceptives. Demographic and clinical characteristics of study participants are compared in Table 1. Women and men had similar age (48 [41, 54] vs. 48 [42, 52] years, P = 0.75), as well as similar time since HIV diagnosis (14.6 ± 5.9 vs. 13.8 ± 6.5 years, P = 0.46), total duration of ART (8.9 [3.9, 11.8] vs. 8.0 [4.5, 11.0] years, P = 0.51), CD4+ T-cell count (535 [411, 759] vs. 462 [303, 744] cells/mm3, P = 0.10), and frequency of virologic suppression (84% vs. 85%, P = 0.83). Women and men had comparable nucleoside reverse transcriptase inhibitor and protease inhibitor use, whereas non-nucleoside reverse transcriptase inhibitor use was lower in women compared to men (17% vs. 49%, P < 0.0001).
Table 1.
Baseline Characteristics Among ART-Treated HIV-Infected Women and Men Overall
| HIV-Infected Women (n = 48) |
HIV-Infected Men (n = 97) |
P-value | |
|---|---|---|---|
| Demographic and Traditional Cardiovascular Disease Risk Parameters | |||
| Age, y | 48 [41, 54] | 48 [42, 52] | 0.75 |
| Race/Ethnicity, % | < 0.0001 | ||
| White | 25 | 65 | |
| Black/African American | 60 | 21 | |
| Hispanic | 8 | 10 | |
| Current hypertension, % | 13 | 27 | 0.04 |
| Current diabetes mellitus, % | 19 | 7 | 0.05 |
| Current smoking, % | 50 | 40 | 0.24 |
| History of IVDU, % | 25 | 20 | 0.46 |
| History of cocaine use, % | 56 | 71 | 0.08 |
| History of heroin use, % | 27 | 14 | 0.07 |
| Current alcohol use, % | 61 | 59 | 0.86 |
| Current statin use, % | 10 | 17 | 0.28 |
| Statin duration (current users), y | 0.3 [0.3, 4.9] | 2.0 [0.5, 3.0] | 0.50 |
| Total Framingham Point Score | 11 [7, 14] | 9 [6, 11] | N/A1 |
| Total cholesterol, mg/dL | 181 [156, 210] | 175 [155, 203] | 0.35 |
| LDL-C, mg/dL | 105 ± 37 | 101 ± 31 | 0.51 |
| HDL-C, mg/dL | 57 [44, 72] | 45 [38, 55] | 0.0001 |
| Triglycerides, mg/dL | 91 [71, 116] | 119 [83, 184] | 0.006 |
| Hemoglobin A1c, % | 5.6 [5.4, 5.8] | 5.3 [5.0, 5.7] | 0.0005 |
| BMI, kg/m2 | 27.5 ± 5.2 | 26.2 ± 4.6 | 0.17 |
| VAT, cm2 | 75 [34, 119] | 138 [80, 258] | < 0.0001 |
| SAT, cm2 | 278 [195, 406] | 166 [103, 233] | < 0.0001 |
| Immunologic Parameters | |||
| Time since HIV diagnosis, y | 14.6 ± 5.9 | 13.8 ± 6.5 | 0.46 |
| NRTI use, % | 94 | 97 | 0.38 |
| NNRTI use, % | 17 | 49 | < 0.0001 |
| PI use, % | 63 | 55 | 0.37 |
| Total duration of ART, y | 8.9 [3.9, 11.8] | 8.0 [4.5, 11.0] | 0.51 |
| CD4+ T cell count, cells/mm3 | 535 [411, 759] | 462 [303, 744] | 0.10 |
| Nadir CD4+ T-cell count, cells/mm3 | 199 [64, 260] | 165 [54, 264] | 0.82 |
| Viral load undetectable, % | 84 | 85 | 0.83 |
| Hepatitis C co-infection, % | 29 | 23 | 0.40 |
| CMV IgG seropositivity, % | 94 | 90 | 0.43 |
| Systemic Monocyte Activation Markers | |||
| sCD163, ng/mL | 1509 [1084, 2457] | 1062 [693, 1548] | 0.0003 |
| sCD14, ng/mL | 2023 [1312, 2661] | 307 [157, 443] | < 0.0001 |
Normally distributed variables are presented as mean ± standard deviation (SD); non-normally distributed data are presented as median [interquartile range (IQR)]. P-values were determined by student’s two-tailed t-test, Wilcoxon rank- sum test, and chi-square test for normally distributed, non-normally distributed, and categorical variables, respectively.
ART, antiretroviral therapy; BMI, body mass index; CMV, cytomegalovirus; HDL-C, high-density lipoprotein cholesterol; HIV, human immunodeficiency virus; IgG, immunoglobulin G; IVDU, intravenous drug use; LDL-C, low-density lipoprotein cholesterol; NNRTIs, non-nucleoside reverse transcriptase inhibitors; NRTIs, nucleoside reverse transcriptase inhibitors; PIs, protease inhibitors; SAT, subcutaneous adipose tissue; sCD14, soluble CD14; sCD163, soluble CD163; VAT, visceral adipose tissue.
Total Framingham Point Score not directly comparable between sexes as sex factors into score.
With respect to traditional CVD risk factors, ART-treated HIV-infected women and men had similar body mass index (BMI) (27.5 ± 5.2 vs. 26.2 ± 4.6 kg/m2, P = 0.17), smoking status (50% vs. 40%, P = 0.24), and history of intravenous drug use (25% vs. 20%, P = 0.46). Despite comparable BMI, body composition differed between sexes with lower VAT (75 [34, 119] vs. 138 [80, 258] cm2, P < 0.0001) and higher SAT (278 [195, 406] vs. 166 [103, 233] cm2, P < 0.0001) in women. In our sample, women had a lower prevalence of hypertension (13% vs. 27%, P = 0.04), but a higher prevalence of diabetes mellitus (19% vs. 7%, P = 0.05). While HDL-C also was higher in women compared to men (57 [44, 72] vs. 45 [38, 55] mg/dL, P = 0.0001), LDL-C was comparable between sexes (105 ± 37 vs. 101 ± 31 mg/dL, P = 0.51). With respect to systemic monocyte activation markers, levels of sCD163 (1509 [1084, 2457] vs. 1062 [693, 1548] ng/mL, P = 0.0003) and sCD14 (2023 [1312, 2661] vs. 307 [157, 443] ng/mL, P < 0.0001) were higher in women.
Coronary Plaque Characteristics Among Participants Overall
Coronary plaque prevalence and characteristics among HIV-infected women and men are shown in Table 2A. In new analyses among ART-treated participants, we show that HIV-infected women had a lower prevalence of any subclinical coronary atherosclerotic plaque compared to HIV-infected men (35% vs. 62%, P = 0.003) (Figure 2). Women also had a lower number of vascular segments with plaque (P = 0.01) and a lower prevalence of obstructive plaque (≥ 70% stenosis) (0% vs. 5%, P = 0.05). Despite the lower prevalence and burden of any coronary plaque among women, there was no significant difference in the prevalence of non-calcified plaque or number of non-calcified plaque segments between groups. In terms of high-risk morphology plaque features, women had a lower prevalence of positively remodeled plaque (25% vs. 51%, P = 0.003) (Figure 2) and a lower number of positively remodeled plaque segments (P = 0.002) compared to men. There was no significant difference in the prevalence of low attenuation plaque or the number of low attenuation plaque segments between groups.
Table 2.
Coronary Plaque Burden and Characteristics Among ART-Treated HIV-Infected Women and Men
| A) Overall Sample | |||
|---|---|---|---|
| HIV-Infected Women (n = 48) |
HIV-Infected Men (n = 97) |
P-value | |
| Prevalence of coronary plaque, % | 35 | 62 | 0.003 |
| Prevalence of non-calcified plaque, % | 33 | 47 | 0.12 |
| Prevalence of calcified plaque, % | 4 | 14 | 0.07 |
| Prevalence of positively remodeled plaque, % | 25 | 51 | 0.003 |
| Prevalence of low attenuation plaque, % | 15 | 23 | 0.24 |
| Prevalence of high plaque burden (≥ 4 segments), % | 15 | 24 | 0.18 |
| Prevalence of obstructive plaque (≥ 70% stenosis), % | 0 | 5 | 0.05 |
| No. segments with plaque | 1.3 ± 2.3 (0 [0, 2]) | 2.1 ± 2.5 (1 [0, 3]) | 0.01 |
| No. segments with non-calcified plaque | 0.9 ± 1.5 (0 [0, 2]) | 1.0 ± 1.6 (0 [0, 1]) | 0.30 |
| No. segments with calcified plaque | 0.04 ± 0.2 (0 [0, 0]) | 0.2 ± 0.7 (0 [0, 0]) | 0.08 |
| No. segments with positively remodeled plaque | 0.5 ± 1.1 (0 [0, 1]) | 1.2 ± 1.5 (1 [0, 2]) | 0.002 |
| No. segments with low attenuation plaque | 0.2 ± 0.5 (0 [0, 0]) | 0.4 ± 0.8 (0 [0, 0]) | 0.21 |
| B) Individuals with Plaque | |||
|---|---|---|---|
|
HIV-Infected Women (n = 17) |
HIV-Infected Men (n = 60) |
P-value | |
| Prevalence of non-calcified plaque, % | 94 | 76 | 0.07 |
| Prevalence of calcified plaque, % | 12 | 22 | 0.33 |
| Prevalence of positively remodeled plaque, % | 71 | 82 | 0.33 |
| Prevalence of low attenuation plaque, % | 41 | 37 | 0.74 |
| Prevalence of high plaque burden (≥ 4 segments), % | 41 | 39 | 0.87 |
| Prevalence of obstructive plaque (≥ 70% stenosis), % | 0 | 8 | 0.10 |
| No. segments with plaque | 3.7 ± 2.6 (3 [2, 6]) | 3.4 ± 2.4 (2 [1, 5]) | 0.57 |
| No. segments with non-calcified plaque | 2.5 ± 1.5 (2 [2, 4]) | 1.7 ± 1.7 (1 [1, 2]) | 0.02 |
| % Plaque non-calcified | 75 ± 28 (75 [67, 100]) | 53 ± 38 (50 [11, 100]) | 0.03 |
| No. segments with calcified plaque | 0.1 ± 0.3 (0 [0, 0]) | 0.4 ± 0.8 (0 [0, 0]) | 0.31 |
| % Plaque calcified | 3 ± 8 (0 [0, 0]) | 8 ± 18 (0 [0, 0]) | 0.34 |
| No. segments with positively remodeled plaque | 1.3 ± 1.4 (1 [0, 2]) | 1.9 ± 1.6 (2 [1, 3]) | 0.09 |
| % Plaque positively remodeled | 33 ± 33 (33 [0, 50]) | 62 ± 37 (67 [43, 100]) | 0.004 |
| No. segments with low attenuation plaque | 0.5 ± 0.7 (0 [0, 1]) | 0.6 ± 1.0 (0 [0, 1]) | 0.97 |
| % Plaque low attenuation | 17 ± 28 (0 [0, 33]) | 17 ± 27 (0 [0, 33]) | 0.88 |
Continuous data are expressed as both mean ± standard deviation (SD) and median [interquartile range (IQR)] for descriptive purposes. P-values were determined using Wilcoxon rank-sum test for continuous variables and chi-square test for categorical variables.
Figure 2. Prevalence of Any Plaque and Positively Remodeled Plaque Among ART-Treated HIV-Infected Women and Men.
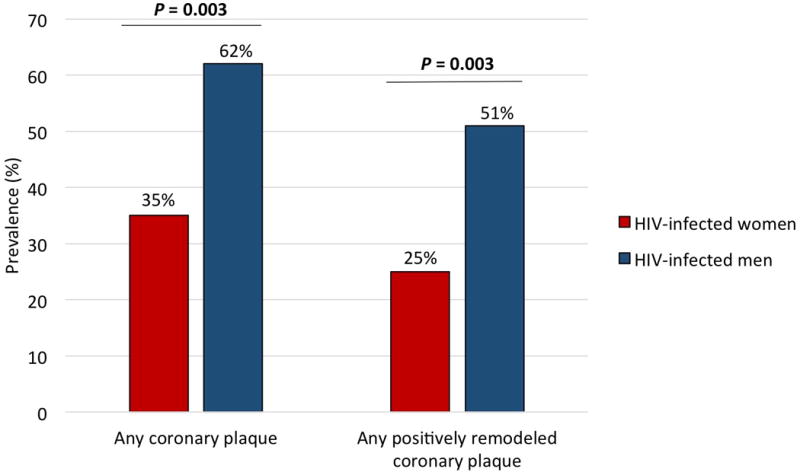
HIV-infected women had a lower prevalence of any coronary plaque (35% vs. 62%, P = 0.003) and of positively remodeled plaque (25% vs. 51%, P = 0.003) compared to HIV-infected men. P-values were determined using chi-square test.
Multivariable Analyses of Presence of Plaque and Presence of Positively Remodeled Plaque Among Participants Overall
We next sought to investigate whether the lower prevalence of any plaque or of positively remodeled plaque among ART-treated HIV-infected women compared to men would persist when controlling for pertinent traditional and HIV-specific CVD risk factors that differed between groups (Table 3). We found that male sex was associated with markedly increased odds of coronary plaque (OR 3.8, 95% CI [1.4, 11.4]) in a multivariable analysis that controlled for age, race, hypertension, diabetes mellitus, HDL-C, VAT, and ART class. Likewise, male sex conferred nearly four-fold increased odds of positively remodeled plaque (OR 3.7, 95% CI [1.4, 10.9]) in a model adjusting for these covariates. The relationships of sex with any plaque and positively remodeled plaque were consistent in a model that adjusted for ART regimen (e.g., NNRTI-based, PI-based, Other) rather than each ART class individually (Supplemental Table 1).
Table 3.
Multivariable Models Relating Sex to Presence of Plaque and Presence of Positively Remodeled Plaque Among ART-Treated HIV-Infected Women and Men (n = 142)
| Presence of Any Plaque | Presence of Positively Remodeled Plaque | |||
|---|---|---|---|---|
| Odds Ratio [95% CI] | P-value | Odds Ratio [95% CI] | P-value | |
| Age, y | 6.6 [3.0, 16.0] | < 0.0001 | 3.4 [1.7, 7.2] | 0.0007 |
| Male sex | 3.8 [1.4, 11.4] | 0.01 | 3.7 [1.4, 10.9] | 0.01 |
| White race | 5.2 [0.8, 49.7] | 0.11 | 2.8 [0.5, 24.4] | 0.29 |
| Black/African American race | 5.2 [0.8, 48.0] | 0.10 | 2.2 [0.4, 18.4] | 0.41 |
| Hispanic race/ethnicity | 0.7 [0.07, 9.0] | 0.81 | 0.5 [0.04, 5.6] | 0.54 |
| Current hypertension | 1.6 [0.6, 5.0] | 0.38 | 1.4 [0.6, 3.8] | 0.45 |
| Current diabetes mellitus | 0.8 [0.2, 2.9] | 0.71 | 0.7 [0.2, 2.5] | 0.63 |
| HDL-C, mg/dL | 0.9 [0.7, 1.1] | 0.28 | 1.0 [0.8, 1.3] | 0.97 |
| VAT, cm2 | 1.0 [0.9, 1.0] | 0.30 | 1.0 [0.9, 1.0] | 0.18 |
| NRTI use | 1.2 [0.1, 9.9] | 0.88 | 1.4 [0.2, 12.5] | 0.77 |
| NNRTI use | 1.1 [0.4, 3.6] | 0.82 | 1.0 [0.3, 3.1] | 0.99 |
| PI use | 0.6 [0.2, 1.8] | 0.36 | 0.8 [0.3, 2.2] | 0.64 |
Odds ratios and P-values were determined by multivariable logistic regression. Odds ratios for continuous variables are reported per a 10-unit change in the variable of interest. Odds ratios for categorical variables compare odds of presence versus absence of parameter.
HDL-C, high-density lipoprotein cholesterol; NNRTIs, non-nucleoside reverse transcriptase inhibitors; NRTIs, nucleoside reverse transcriptase inhibitors; PIs, protease inhibitors; VAT, visceral adipose tissue.
Coronary Plaque Characteristics Among Participants with Plaque
From the overall ART-treated HIV-infected sample, 17 women (35%) and 60 men (62%) had plaque on coronary CTA. Among this subset of participants, sex-specific comparisons of coronary plaque burden and plaque characteristics are shown in Table 2B. The number of vascular segments with plaque was comparable between groups. While the prevalence of obstructive plaque (≥ 70% stenosis) tended to be lower in women compared to men, this difference was not significant (0% vs. 8%, P = 0.10). In contrast, ART-treated HIV-infected women with plaque had an increased number of non-calcified plaque segments (P = 0.02). These women also had an increased proportion of non-calcified plaque (75 ± 28% vs. 53 ± 38%, P = 0.03), consistent with previous reports7. As for high-risk morphology plaque features, there was no sex difference in the prevalence of positively remodeled plaque (71% vs. 82%, P = 0.33). However, women had a lower proportion of plaque segments with positive remodeling (33 ± 33% vs. 62 ± 37%, P = 0.004), and tended to have a lower number of positively remodeled segments (P = 0.09). The prevalence and extent of low attenuation plaque were comparable between groups.
DISCUSSION
Our study revealed significant sex differences in coronary atherosclerotic plaque among asymptomatic HIV-infected individuals on ART. Male sex conferred nearly four-fold increased odds of having any subclinical coronary atherosclerotic plaque and high-risk morphology subclinical coronary atherosclerotic plaque characterized by positive remodeling. These findings held even after controlling for traditional and HIV-specific CVD risk factors that differed between groups. Moreover, compared with asymptomatic HIV-infected men, HIV-infected women had a lower prevalence of obstructive coronary artery disease and a lower number of vascular segments affected by plaque despite having lower rates of statin use. Given that epidemiologic studies have shown an increased HIV-attributable risk of MI among women vs. men1,2,3,4, our findings imply that mechanisms other than gradual epicardial vessel occlusion and/or sudden macroscopic plaque rupture may contribute to MI among women with HIV.
Among our sample of asymptomatic ART-treated HIV-infected individuals, women (vs. men) had a lower prevalence of coronary atherosclerotic plaque, a lower prevalence of obstructive plaque, and a reduced plaque burden (number of coronary segments with plaque). Sex differences in coronary plaque prevalence remained significant even after controlling for CVD risk factors that differed between groups with male sex conferring markedly increased odds of plaque. These findings are consonant with data from several general population studies exploring sex differences in coronary atherosclerotic plaque. For example, in a large study of asymptomatic individuals undergoing coronary CTA, male sex was strongly associated with prevalence of any plaque in adjusted models (OR 5.21, 95% CI [3.20, 8.49])11. Furthermore, several large studies of individuals undergoing coronary CTA to evaluate stable angina suggested that symptomatic women had a lower prevalence of any plaque12,13 and/or obstructive plaque12,13,14 compared with symptomatic men. The number of segments with calcified and/or mixed coronary atherosclerotic plaque also was shown to be lower in symptomatic women compared to men14. Our finding that HIV-infected women had a lower prevalence of obstructive plaque and reduced plaque burden compared to men is potentially clinically relevant, as plaque burden/extent of disease has been shown in general population studies to portend adverse cardiovascular events15.
Our investigation introduces the first assessment of high-risk morphology plaque features among asymptomatic HIV-infected women. We found that asymptomatic ART-treated HIV-infected women had a lower prevalence of positively remodeled plaque than asymptomatic HIV-infected men, even after controlling for relevant CVD risk factors. Positively remodeled plaque represents plaque which prompts the affected arterial segment to balloon outward, eccentrically16. Such plaque is identified by coronary CTA when the affected segment diameter is noted to exceed the reference segment diameter9. Pathologic studies have shown that coronary atherosclerotic plaques with a large, necrotic lipid core tend to cause positive remodeling whereas fibrous plaques do not17. Positive remodeling is widely believed to characterize coronary atherosclerotic plaques that provoke ischemia18 and unstable angina19, and that are prone to rupture, resulting in acute coronary syndrome10,20.
Our finding that asymptomatic ART-treated HIV-infected women had a lower prevalence of positively remodeled plaque compared with asymptomatic HIV-infected men is novel and clinically relevant. This finding also extends previous work demonstrating that asymptomatic HIV-infected men had an increased prevalence and burden of positively remodeled plaque compared to non-HIV-infected men8,21. Of interest, while general population studies have not previously reported on sex differences in positively remodeled plaque prevalence among asymptomatic individuals, an intravascular ultrasound study performed on symptomatic individuals undergoing cardiac catheterization did suggest sex differences. In particular, women in this prior study were noted to have a significantly lower remodeling index than men22.
The reduced prevalence of coronary plaque and positively remodeled plaque among asymptomatic ART-treated HIV-infected women suggests that mechanisms other than gradual luminal occlusion and/or plaque rupture may contribute to ischemic heart disease among this group. General population literature on sex differences in coronary atherosclerotic plaque provides key context in this regard. Women (vs. men) who survived an MI were less likely to exhibit obstructive plaque on coronary angiography23 or culprit plaque rupture on coronary intravascular ultrasound24. Analogously, women (vs. men) who experienced sudden cardiac death were less likely to exhibit rupture of a thin-capped fibroatheroma (thin fibrous cap/large necrotic core) on coronary artery pathologic analyses at autopsy25. Among these women, erosion of a more fibrotic plaque type rich in smooth muscle cells and proteoglycans was more commonly observed in coronary artery sections25. Thus, among HIV-infected women, one pathway to MI may be repeated erosion of (initially) non-obstructive plaque.
A second potential pathway to ischemia among women – and particularly, women with HIV – may be microvascular disease. A highly informative general population study by Taqueti et al. has provided support for this concept. In this study, excess CVD risk among women was attributable, not to obstructive epicardial artery disease, but rather to reduced coronary flow reserve – an integrated measure of functionally significant pathology in large and small vessels26. Among HIV-infected women, heightened systemic immune activation/inflammation7 may be expected to contribute uniquely to endothelial dysfunction/microvascular disease and downstream comorbidities27,28.
Our finding that HIV-infected women had a lower prevalence of high-risk, rupture prone plaque than HIV-infected men also dovetails with recent findings by Crane et. al. characterizing the types of MI experienced by HIV-infected women and men29. Assessing all MIs experienced by HIV-infected individuals in the Centers for AIDS Research Network of Integrated Clinical Systems (CNICS) system between 1996 and 2014, Crane et al. showed that HIV-infected women had a predilection towards type II MI (resulting from mismatch of oxygen supply and demand) whereas HIV-infected men had a predilection towards type I MI (resulting from instability of coronary atherosclerotic plaques)29. Taken together, these data suggest that MI mechanisms differ among HIV-infected women and men and reinforce the need to tailor diagnostic/therapeutic strategies to the dominant sex-specific pathophysiologic processes at play.
Of note, among those asymptomatic ART-treated HIV-infected women in our sample who did demonstrate subclinical coronary atherosclerosis, plaque burden was not low and plaque characteristics were not benign. HIV-infected women (vs. men) had a similar number of vascular segments with plaque, prevalence and extent of low attenuation plaque, and prevalence of positively remodeled plaque – although the extent of positively remodeled plaque remained higher in men. Moreover, those ART-treated HIV-infected women with subclinical coronary atherosclerotic plaque had an increased number of noncalcified plaque segments. This subgroup also had an increased proportion of noncalcified plaque, consistent with previous findings by Fitch et al. in a mixed group of ART-treated and untreated women7. General population studies have revealed that symptomatic women had a greater percentage of non-calcified plaque than symptomatic men14. Moreover, among symptomatic individuals with any plaque, non-calcified plaque correlated more closely with major adverse cardiovascular events than did calcified plaque15. Thus, while an absence of macroscopic plaque among asymptomatic HIV-infected women may not necessarily be reassuring, the presence of plaque in this group could be particularly worrisome.
Study limitations include a relatively small sample of HIV-infected individuals recruited from one geographic area, precluding our ability to disentangle whether differences in use of specific antiretroviral agents influenced differences in atherogenesis. Of note, however, our analysis represents one of the largest and most comprehensive coronary CTA-based assessments of plaque morphology among HIV-infected women on ART. Although the women and men in this study were not matched explicitly, the groups were comparable with respect to age, key traditional CVD risk factors (e.g. BMI, smoking status, LDL-C) and important HIV-specific CVD risk factors (e.g. time since HIV diagnosis, total duration of ART, CD4+ T-cell count, and viral load). Moreover, the lower prevalence of any plaque and of positively remodeled plaque among women vs. men remained significant even after adjusting for relevant parameters that differed between sexes. Select HIV-specific CVD risk factors (e.g. time since HIV diagnosis, total duration of ART, nadir CD4+ T-cell count) were participant-reported and could not be specifically verified. Plaque data on women vs. men were read by a limited number of cardiovascular imagers – all blinded to participant status – who received similar training and employed the same diagnostic criteria. Lastly, our sample was limited to HIV-infected women and men without clinical CVD, and thus, by design, we lack information about coronary plaque characteristics among HIV-infected individuals with anginal symptoms. However, our findings on sex differences in coronary plaque in an asymptomatic group of HIV-infected participants were consonant with those from large-scale general population studies enrolling symptomatic patients without HIV.
Overall, we found that HIV-infected women on ART had a lower prevalence of subclinical coronary atherosclerotic plaque and positively remodeled plaque as well as a lower burden of overall plaque and positively remodeled plaque, compared with HIV-infected men on ART. However, those HIV-infected women who did have coronary atherosclerotic plaque had a reduced extent of positively remodeled plaque but an increased extent of non-calcified plaque (vs. men). Additional research is needed to explain why the HIV-attributable risk of MI is higher among women vs. men1,2,3,4 despite a lower prevalence of macroscopic subclinical atherosclerosis among women. The REPRIEVE trial (Randomized Trial to Prevent Vascular Events in HIV) and embedded mechanistic CTA substudy will provide critical data in this regard. Only by understanding distinct mechanisms of HIV-associated CVD at play in women can we deliver “precision” preventive care to this population of 17 million worldwide30.
Supplementary Material
Acknowledgments
We thank the participants in this study and the Nursing Staff of the MGH and MIT Clinical Research Centers.
Financial Support: This work was supported by an investigator-initiated grant from Bristol Myers Squibb, Inc, and by National Institutes of Health (NIH) Grants M01RR01066, 1UL1RR025758, and 8UL1TR000170 to the Harvard Clinical and Translational Science Center. Support was also received from NIH P30DK040561, Nutrition and Obesity Research Center at Harvard. LTF received support from NIHT32DK007028. TGN received support from the Kohlberg Foundation, the American Heart Association Fellow to Faculty Award 12FTF12060588, NIH 1R01HL130539-01A1, NIH 1R01HL137562-01A1, and NIH/Harvard Center for AIDS Research P30AI060354. MT received support from the Harvard Clinical and Translational Science Center 5KL2TR001100-05. SS received support from NIH K23 HL136262. SEL received support from NIH 1R01 AI123001-01, the MGH Executive Committee on Research (Claflin Award), the William F. Connell Family and the Yvonne L. Munn Center for Nursing Research (Connell Extension Grant). JL received support from NIH F32 HL088991 and NIH K23 HL092792. MVZ received support from NIH 1R01 AI123001-01, 1R01HL137562 - 01A1, and NIH/Harvard Center for AIDS Research P30AI060354.
Footnotes
Disclosures: BF, LTF, MTL, MM, BS, TGN, JEH, THB, ESL, LAS, MT, SS, and KVF have nothing to declare. SEL is a non-paid Board member of the community non-profit organization Healing Our Community Collaborative, and received one-time compensation for CME educational offerings sponsored by the Physician Research Network, Dartmouth Hitchcock Medical Center, and a one-time speaker’s fee from the Association of Nurses in AIDS Care. JL has served on Medical Affairs Advisory Board for Gilead Sciences and has received study drug donation from Shire for an NIH-funded study. MVZ has participated in a Scientific Advisory Board Meeting for Roche Diagnostics and has received research funding for an investigatory-initiated study from Gilead to her institution. All disclosures are unrelated to this manuscript.
References
- 1.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007 Jul;92(7):2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lang S, Mary-Krause M, Cotte L, et al. Increased risk of myocardial infarction in HIV-infected patients in France, relative to the general population. AIDS. 2010 May 15;24(8):1228–1230. doi: 10.1097/QAD.0b013e328339192f. [DOI] [PubMed] [Google Scholar]
- 3.Durand M, Sheehy O, Baril JG, Lelorier J, Tremblay CL. Association between HIV infection, antiretroviral therapy, and risk of acute myocardial infarction: a cohort and nested case-control study using Quebec’s public health insurance database. J Acquir Immune Defic Syndr. 2011 Jul 01;57(3):245–253. doi: 10.1097/QAI.0b013e31821d33a5. [DOI] [PubMed] [Google Scholar]
- 4.Hanna DB, Ramaswamy C, Kaplan RC, et al. Trends in Cardiovascular Disease Mortality Among Persons With HIV in New York City, 2001-2012. Clin Infect Dis. 2016 Oct 15;63(8):1122–1129. doi: 10.1093/cid/ciw470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lo J, Abbara S, Shturman L, et al. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS. 2010 Jan 16;24(2):243–253. doi: 10.1097/QAD.0b013e328333ea9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burdo TH, Lo J, Abbara S, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis. 2011 Oct 15;204(8):1227–1236. doi: 10.1093/infdis/jir520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fitch KV, Srinivasa S, Abbara S, et al. Noncalcified coronary atherosclerotic plaque and immune activation in HIV-infected women. J Infect Dis. 2013 Dec 1;208(11):1737–1746. doi: 10.1093/infdis/jit508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zanni MV, Abbara S, Lo J, et al. Increased coronary atherosclerotic plaque vulnerability by coronary computed tomography angiography in HIV-infected men. AIDS. 2013 May 15;27(8):1263–1272. doi: 10.1097/QAD.0b013e32835eca9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maurovich-Horvat P, Ferencik M, Voros S, Merkely B, Hoffmann U. Comprehensive plaque assessment by coronary CT angiography. Nat Rev Cardiol. 2014 Jul;11(7):390–402. doi: 10.1038/nrcardio.2014.60. [DOI] [PubMed] [Google Scholar]
- 10.Motoyama S, Sarai M, Harigaya H, et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol. 2009 Jun 30;54(1):49–57. doi: 10.1016/j.jacc.2009.02.068. [DOI] [PubMed] [Google Scholar]
- 11.Rivera JJ, Nasir K, Cox PR, et al. Association of traditional cardiovascular risk factors with coronary plaque sub-types assessed by 64-slice computed tomography angiography in a large cohort of asymptomatic subjects. Atherosclerosis. 2009 Oct;206(2):451–457. doi: 10.1016/j.atherosclerosis.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 12.Schulman-Marcus J, Hartaigh BO, Gransar H, et al. Sex-Specific Associations Between Coronary Artery Plaque Extent and Risk of Major Adverse Cardiovascular Events: The CONFIRM Long-Term Registry. JACC Cardiovasc Imaging. 2016 Apr;9(4):364–372. doi: 10.1016/j.jcmg.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grunau GL, Ahmadi A, Rezazadeh S, et al. Assessment of sex differences in plaque morphology by coronary computed tomography angiography–are men and women the same? J Womens Health (Larchmt) Feb. 2014;23(2):146–150. doi: 10.1089/jwh.2013.4496. [DOI] [PubMed] [Google Scholar]
- 14.Nasir K, Gopal A, Blankstein R, et al. Noninvasive assessment of gender differences in coronary plaque composition with multidetector computed tomographic angiography. Am J Cardiol. 2010 Feb 15;105(4):453–458. doi: 10.1016/j.amjcard.2009.09.053. [DOI] [PubMed] [Google Scholar]
- 15.Hou ZH, Lu B, Gao Y, et al. Prognostic value of coronary CT angiography and calcium score for major adverse cardiac events in outpatients. JACC Cardiovasc Imaging. 2012 Oct;5(10):990–999. doi: 10.1016/j.jcmg.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006 Apr 18;47(8 Suppl):C13–18. doi: 10.1016/j.jacc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 17.Burke AP, Kolodgie FD, Farb A, Weber D, Virmani R. Morphological predictors of arterial remodeling in coronary atherosclerosis. Circulation. 2002 Jan 22;105(3):297–303. doi: 10.1161/hc0302.102610. [DOI] [PubMed] [Google Scholar]
- 18.Park HB, Heo R, ó Hartaigh B, et al. Atherosclerotic plaque characteristics by CT angiography identify coronary lesions that cause ischemia: a direct comparison to fractional flow reserve. JACC Cardiovasc Imaging. 2015 Jan;8(1):1–10. doi: 10.1016/j.jcmg.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schoenhagen P, Ziada KM, Kapadia SR, Crowe TD, Nissen SE, Tuzcu EM. Extent and direction of arterial remodeling in stable versus unstable coronary syndromes: an intravascular ultrasound study. Circulation. 2000 Feb 15;101(6):598–603. doi: 10.1161/01.cir.101.6.598. [DOI] [PubMed] [Google Scholar]
- 20.Puchner SB, Liu T, Mayrhofer T, et al. High-risk plaque detected on coronary CT angiography predicts acute coronary syndromes independent of significant stenosis in acute chest pain: results from the ROMICAT-II trial. J Am Coll Cardiol. 2014 Aug 19;64(7):684–692. doi: 10.1016/j.jacc.2014.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller PE, Haberlen SA, Metkus T, et al. HIV and coronary arterial remodeling from the Multicenter AIDS Cohort Study (MACS) Atherosclerosis. 2015 Aug;241(2):716–722. doi: 10.1016/j.atherosclerosis.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hara H, Yajima J, Kirigaya H, et al. Sex differences in coronary atherosclerosis: coronary angiography and intravascular ultrasonography. J Cardiol. 2004 May;43(5):215–221. [PubMed] [Google Scholar]
- 23.Chokshi NP, Iqbal SN, Berger RL, et al. Sex and race are associated with the absence of epicardial coronary artery obstructive disease at angiography in patients with acute coronary syndromes. Clin Cardiol. 2010 Aug;33(8):495–501. doi: 10.1002/clc.20794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lansky AJ, Ng VG, Maehara A, et al. Gender and the extent of coronary atherosclerosis, plaque composition, and clinical outcomes in acute coronary syndromes. JACC Cardiovasc Imaging. 2012 Mar;5(3 Suppl):S62–72. doi: 10.1016/j.jcmg.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Farb A, Burke AP, Tang AL, et al. Coronary plaque erosion without rupture into a lipid core. A frequent cause of coronary thrombosis in sudden coronary death. Circulation. 1996 Apr 1;93(7):1354–1363. doi: 10.1161/01.cir.93.7.1354. [DOI] [PubMed] [Google Scholar]
- 26.Taqueti VR, Shaw LJ, Cook NR, et al. Excess Cardiovascular Risk in Women Relative to Men Referred for Coronary Angiography Is Associated With Severely Impaired Coronary Flow Reserve, Not Obstructive Disease. Circulation. 2017 Feb 7;135(6):566–577. doi: 10.1161/CIRCULATIONAHA.116.023266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raghavan A, Rimmelin DE, Fitch KV, Zanni MV. Sex Differences in Select Non-communicable HIV-Associated Comorbidities: Exploring the Role of Systemic Immune Activation/Inflammation. Curr HIV/AIDS Rep. 2017 Dec;14(6):220–228. doi: 10.1007/s11904-017-0366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stone L, Looby SE, Zanni MV. Cardiovascular disease risk among women living with HIV in North America and Europe. Curr Opin HIV AIDS. 2017 Nov;12(6):585–593. doi: 10.1097/COH.0000000000000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crane HM, Paramsothy P, Drozd DR, et al. Types of Myocardial Infarction Among Human Immunodeficiency Virus-Infected Individuals in the United States. JAMA Cardiol. 2017 Mar 1;2(3):260–267. doi: 10.1001/jamacardio.2016.5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.UNAIDS. Global Epidemiology Report. 2015 http://www.who.int/hiv/data/en/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


