Abstract
Background
Left ventricular diastolic dysfunction (DD) is an independent risk factor for mortality in sickle cell anemia (SCA) and is associated with increased extracellular volume (ECV) on cardiac MRI (CMR). Exercise impairment is common in SCA, but its causes and prognostic value are not well understood.
Objective
To study the effects of DD and ECV on cardiopulmonary exercise test (CPET) in patients with SCA.
Methods and Results
As part of a prospective study to characterize the cardiomyopathy of SCA (NCT02410811), twenty children and adults with SCA underwent CMR, echocardiography and cycle ergometer CPET (age range 8–43 years). Maximum exercise was reached in 18 patients and 17 (94%) had reduced exercise capacity (%predicted VO2 less than 80%). Six patients had DD and none had systolic dysfunction. Patients with DD had lower exercise capacity compared to patients with normal diastolic function (%predicted VO2 48.2 ± 9.1% vs 61.2 ± 11.7%; p=0.01). The z-score of left ventricular lateral E/e' ratio, which is a marker of DD, was negatively associated with %predicted VO2 (r=−0.61, p=0.01). All patients with moderate-to-severe exercise impairment (%predicted VO2 < 60%) had lateral E/e’ z-score >2. In a multivariate analysis, lateral E/e’ z-score was independently associated with %predicted VO2 (p=0.02). All participants had elevated ECV but the degree of elevation was not associated with exercise parameters.
Conclusion
Left ventricular DD is associated with decreased exercise capacity in SCA. Interventions to prevent or delay DD could improve exercise capacity, quality of life, and long-term outcomes in SCA.
Keywords: Sickle cell anemia, diastolic dysfunction, exercise impairment, left atrial pressure, myocardial fibrosis, exercise echocardiogram
Introduction
Approximately 1 in 700 African-Americans has sickle cell anemia (SCA), and as many as 100,000 individuals are affected in the United States.1 Cardiac complications are leading causes of mortality and morbidity in SCA.2, 3 Diastolic dysfunction (DD) and pulmonary hypertension are known cardiopulmonary complications of SCA and are independent risk factors for early mortality in SCA.4–6 DD is associated with microscopic myocardial fibrosis in SCA mice and with diffuse myocardial fibrosis, assessed by cardiac MRI (CMR), in humans with SCA.3, 7, 8
Exercise capacity, defined by oxygen uptake at peak exercise (peak VO2) during cardiopulmonary exercise test (CPET), is a determinant of mortality and a treatment target in patients with DD in other clinical settings.9 Peak VO2 is decreased in a significant proportion of children and young adults with SCA compared to normal controls even after controlling for anemia.10–12 The ventilation-to-carbon dioxide production slope at maximum exercise (VE/VCO2 slope) is another important exercise measure that assesses ventilation efficiency and has been shown to correlate with left ventricular filling pressures and mortality in patients with DD.9, 10, 13, 14 The VE/VCO2 slope has also been reported to be abnormal in patients with SCA.10
Several factors could lead to the exercise abnormalities seen in patients with SCA, but the effects of cardiac disease in SCA on exercise capacity using CPET have not been elucidated. While adult SCA patients with DD had shorter 6-minute walk distance compared to patients without DD, the cardiopulmonary effects of DD on functional capacity are not clear.15–18 Specifically, the effects of DD and myocardial fibrosis on CPET-derived measures of exercise capacity that have prognostic value in other cardiac diseases have not been studied in SCA.16 We sought to determine the impact of DD and myocardial fibrosis on functional capacity of SCA patients using CPET.
Methods
Participants and study design
Participants with SCA were enrolled in a prospective, longitudinal CMR study to characterize SCA-related cardiomyopathy (ClinicalTrials.gov NCT02410811). Participants who had a CMR and a resting echocardiogram during this study were approached to participate in a voluntary CPET and exercise echocardiogram at their study exit visit. The main exclusion criteria were chronic transfusion therapy, glomerular filtration rate <60 mL/min/1.73 m2, and any contraindication to CPET. The study was approved by the Institutional Review Board of Cincinnati Children’s Hospital Medical Center. Informed consent was obtained from adults or parents of minor participants. Participants were monitored for the development of adverse events in the 30 days following CPET. Baseline laboratory testing was obtained at the time of CMR.
CMR protocol and image analysis
CMR was performed on a 1.5 T scanner (Philips Ingenia, Best, Netherlands). ECV was measured from T1-maps acquired with a modified Look-Locker inversion recovery (MOLLI) sequence in the short and long axis planes before and 10 minutes post-contrast as previously described.8 All planimetric and T1 analyses were done with CMR42 (Circle Imaging; Alberta, Canada). ECV was calculated using the formula:
Echocardiography and diastolic classification
Transthoracic echocardiography was performed with a Philips iE-33 system (Philips Electronics; Andover, MA). Measurements were analyzed using Syngo Dynamics (Siemens Healthcare, Germany). Pulsed-wave Doppler was used to measure mitral and tricuspid inflow peak velocity at early (E) and late filling (A). Tissue Doppler imaging was used to determine mitral and tricuspid valve annular velocities in early (e’) and late diastole (a’) at both the septal and lateral annulus. Continuous-wave Doppler sampling of the peak tricuspid regurgitation velocity (TRV) was used.8
DD was determined according to the recently published American Society of Echocardiography and European Association of Cardiovascular Imaging guidelines.19 The criteria were modified to account for potential anemia-related changes or changes that may not be exclusively attributed to DD in SCA as previously described.8 Briefly, patients with left atrial (LA) enlargement, abnormal e’, and abnormal E/e’ ratio were considered to have DD. Patients with LA enlargement and only one additional abnormality out of 3 (abnormal e’, E/e’ ratio, or TRV ≥ 2.8 m/s) were considered to have inconclusive results. Patients with inconclusive results were further classified based on exercise echocardiography (see below). Patients who had a resting echocardiogram with normal e’, normal E/e’ ratio and TRV < 2.8 m/s were considered to have no DD. Age, body size, and sex corrected z-scores were used to adjust for echocardiographic variables which is the standard of care in pediatric echocardiography laboratories. 8, 19–22
All patients underwent an exercise echocardiogram immediately after maximum exercise according to the American Society of Echocardiography guidelines.18 Similar to the echocardiographic evaluation at rest, we evaluated systolic and diastolic function at peak exercise while the patients were lying down immediately after peak exercise.23 In case of fusion of early and late diastolic Doppler waves (E and A and/or e′ and a′) at high heart rates, imaging was delayed until the separation of these waves. Patients with inconclusive classification who had exercise mitral annular e’ velocity < 7 cm/s, and E/e’ > 14 were considered to have DD.17 Patients with inconclusive resting echocardiogram who did not meet these criteria on exercise echocardiogram were classified as having no DD. The correlations between measures of diastolic function using resting echocardiogram and exercise parameters on CPET were studied.
CPET
A maximal cardiopulmonary exercise was performed using a previously calibrated cycle ergometer (Corival Load Cycle 400). The ramp protocol was used in which the test starts with an initial work rate based on patient’s body surface area and proceeds with linear increases every minute with a goal to reach peak exercise after ten minutes.24 A maximal exercise test was judged to be reached if two of the following three criteria were met: respiratory exchange ratio (RER) >1.1, maximal heart rate ≥85% of the age-predicted maximal heart rate, or maximal rating of perceived exertion >18. Gas exchange at rest, during exercise, and during recovery was analyzed to determine measures of oxygen uptake (VO2), carbon dioxide output (VCO2), and ventilation (VE), and VE/VCO2 slope at maximum exercise was calculated.10, 25, 26 Since peak VO2 is influenced by age, sex, and body weight, %predicted VO2 was used to account for these variables in our study.27–29 The prognostic value of %predicted VO2 in patients with cardiac dysfunction has been shown in previous studies.28 %predicted VO2 was used previously to in SCA and was found to be lower in patients with SCA who also had restrictive lung disease.15
Reduced exercise capacity was defined as %predicted VO2 <80%. Mild impairment of exercise capacity was defined as %predicted VO2 60–80% while moderate-to-severe impairment was defined as %predicted VO2 <60%.28 Spirometry was performed before CPET. Restrictive lung disease (RLD) was defined as FVC <80% while obstructive lung disease was defined as FEV1/FVC ratio <80%.30
Statistical analysis
A student t-test or Mann-Whitney U test was used to compare 2 groups of continuous parametric or non-parametric variables, respectively, or Fisher’s exact test for categorical variables. Associations between normally distributed variables were calculated using the Pearson correlation coefficient. All P-values were two-tailed and differences were considered significant when P<0.05. Because of the significant impact of anemia and RLD on exercise capacity, multivariate regression models were derived to determine independent predictors of %predicted VO2. Statistical analyses were performed using JMP®, Version 12 from SAS Institute Inc. (Cary, NC).
Results
Patient characteristics and exercise performance
Twenty patients with SCA (homozygous HbSS) participated in the study (median age 21 years, age range 8–43 years). No adverse events were reported within 30 days of CPET. The baseline clinical and laboratory characteristics of the patients are summarized in table 1. Two patients did not reach maximum exercise due to muscular fatigue. As expected, patients with SCA had significant exercise impairment (mean VO2 = 21.6± 6.1 ml/kg/min and mean %predicted VO2=57±12.4%). Of the 18 patients who reached maximum exercise, 17 (94%) had reduced exercise capacity defined as %predicted VO2 < 80%; 6 of them (29%) had mild impairment (%predicted VO2 60–80%) and 11 (71%) had moderate-to-severe impairment (%predicted VO2 <60%).
Table 1.
Baseline clinical and laboratory characteristics of study participants.
| Characteristic | Value |
|---|---|
| Age (yr) | 22.9 ± 9 |
| BSA (m2) | 1.67± 0.37 |
| Female, n (%) | 12 (60) |
| Receiving hydroxyurea, n (%) | 15 (75) |
| Baseline Heart rate (bpm) | 73 ± 14 |
| Systolic blood pressure (mmHg) | 118 ± 12 |
| Diastolic blood pressure (mmHg) | 67 ± 8 |
| White blood cell count (103/mm3) | 9.6 ± 3.7 |
| Hemoglobin (g/dL) | 9.9 ± 1.6 |
| Hematocrit (%) | 28.1 ± 4.3 |
| Reticulocyte count (%) | 6.3 (4.8–10.8) |
| Platelet count (103/mm3) | 350 ± 96 |
| Bilirubin (mg/dL) | 2.1 (1.5–2.8) |
| AST (unit/L) | 49 ± 29 |
| LDH (unit/L) | 560 ± 275 |
| Plasma free hemoglobin (mg/dL) | 21.1(11.1–90.1) |
| Nucleated RBC (cell/100 WBC) | 0.5(0.1–3.75) |
| Fetal hemoglobin (%) | 16.8 ± 13.6 |
| Mean corpuscular volume (fL) | 92.4 ± 19.8 |
| Absolute neutrophil count (K/uL) | 4.8 ± 2.5 |
| Creatinine (mg/dL) | 0.56 ± 0.17 |
| Cystatin C (mg/L) | 0.64 ± 0.14 |
| GFR (mL/min/1.73 m2) | 145 ± 38 |
| NT-proBNP (pg/mL) | 41(22–131) |
| FEV1 (%) | 82 ± 14 |
| FVC (%) | 88 ± 13 |
| FEV1/FVC (%) | 93 ± 9 |
| RLD (FVC<80%), n (%) | 6(30%) |
| OLD (FEV1/FVC<80%), n (%) | 1(5%) |
| Native T1 (ms) | 1001 ± 68 |
| ECV | 0.43 ± 0.08 |
The values are reported as mean ± standard deviation or median (interquartile range). AST: aspartate aminotransferase, ECV: extracellular volume, fl: femtoliter, FEV1: forced expiratory volume in the first second, FVC: forced vital capacity, GFR: glomerular filtration rate, LDH: lactate dehydrogenase, OLD: obstructive lung disease, RBC: red blood cell, RLD: Restrictive lung disease, VO2: oxygen consumption, VCO2: carbon dioxide production, Yr: Year.
Association between diastolic dysfunction and exercise capacity
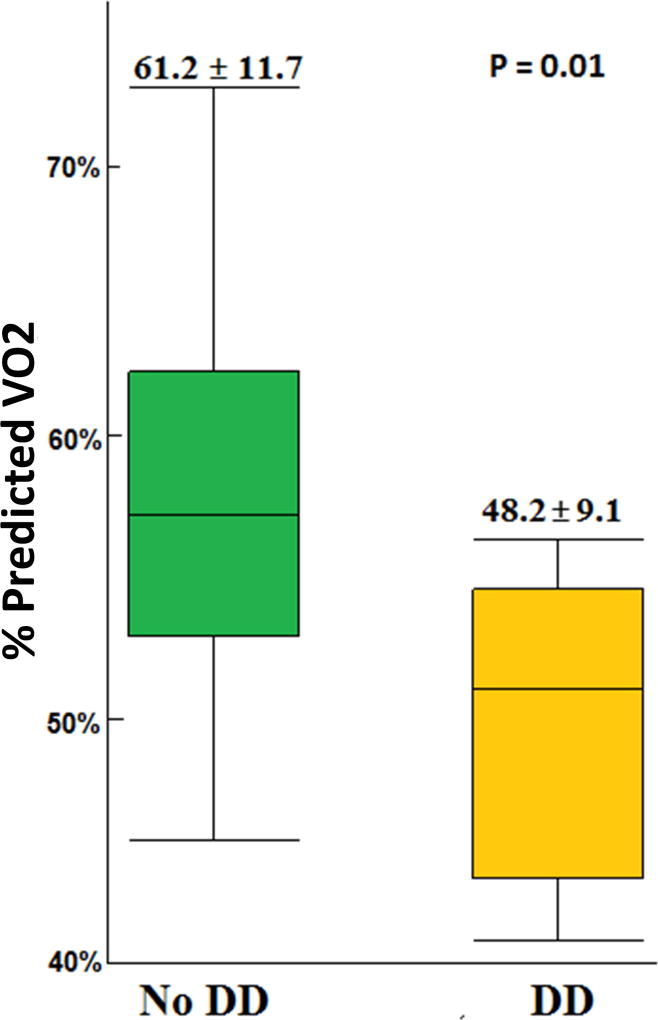
Six participants (30%) met the definition of DD on resting echocardiogram. Five patients who had inconclusive classification on resting echocardiogram did not meet DD criteria on exercise echocardiogram so were considered not to have DD. Patients with DD had lower exercise capacity compared to patients without DD (%predicted VO2 48.2 ± 9.1% versus 61.2 ± 11.7%, p=0.01) (Figure1). All patients with DD had moderate-to-severe exercise impairment compared to 58% (7/12) of patients without DD (P=0.04) (Table 2, Figure 2). Patients with DD had higher VE/VCO2 slope (35.7 ± 8.9 versus 27.8 ± 3.6, p=0.04) indicating lower ventilation efficiency. Patients with DD had lower hemoglobin concentration compared to patients without DD (8.7 ± 0.9 versus 10.6 ± 1.6 g/dL, p=0.02) (Table2).
Figure 1.
Table 2.
Clinical, laboratory and exercise parameters of study participants based on diastolic function.
| Characteristic | Diastolic dysfunction (n=6) |
No diastolic dysfunction (n=12) |
p Value |
|---|---|---|---|
| Age (yr) | 25 ± 12.4 | 20 ± 7.6 | 0.43 |
| Body surface area (m2) | 1.67 ± 0.27 | 1.64 ± 0.32 | 0.68 |
| Gender (female) | 4(66%) | 7(58%) | 0.5 |
| Receiving hydroxyurea, n (%) | 5 (83) | 9 (75) | 0.34 |
| Hemoglobin (g/dL) | 8.7 ± 0.9 | 10.6 ± 1.6 | 0.02 |
| Plasma free hemoglobin | 99 ± 137 | 24 ± 28 | 0.21 |
| Hemoglobin F (%) | 9 ± 6 | 21 ± 16 | 0.08 |
| Cardiac Index (liter/minute/m2) | 9.2 ± 1.5 | 9.5 ± 2.5 | 0.66 |
| Heart rate at exercise cessation (BPM) | 165 ± 11 | 176 ± 16 | 0.15 |
| Exercise duration (minute) | 7.9 ± 2 | 8.2 ± 1.2 | 0.68 |
| Peak work rate (Watt) | 191 ± 137 | 352 ± 339 | 0.17 |
| Respiratory exchange rate | 1.4 ± 0.14 | 1.4 ± 0.15 | 0.82 |
| VO2 at maximum exercise (ml/kg/min) | 18.3 ± 4 | 23.1 ± 6.4 | 0.4 |
| % predicted VO2 at maximum exercise | 48.2 ± 9.1 | 61.2 ± 11.7 | 0.01 |
| VE/VCO2 slope at maximum exercise | 35.7 ± 8.9 | 27.8 ± 3.6 | 0.04 |
| Moderate-to-severe exercise impairment, n (%) | 6/6 (100) | 7/12 (58) | 0.04 |
| VE/VO2 at maximum exercise | 47.7 ± 10.7 | 38 ± 7.3 | 0.04 |
| Native T1(ms) | 1035 ± 79 | 976 ± 58 | 0.09 |
| ECV | 0.46 ± 0.08 | 0.39 ± 0.05 | 0.05 |
| NT-Pro BNP (pg/mL) | 183 ± 289 | 48 ± 45 | 0.12 |
| FEV1 (%) | 73 ± 13 | 88 ± 13 | 0.03 |
| FVC (%) | 81 ± 13.9 | 94 ± 11.3 | 0.07 |
| FEV1/FVC (%) | 88 ± 11 | 96 ± 8.2 | 0.1 |
| RLD (FVC<80%), n (%) | 3 (50) | 2 (16) | 0.29 |
ECV: extracellular volume, FEV1: forced expiratory volume in the first second, FVC: forced vital capacity, RLD: Restrictive lung disease, VO2: oxygen consumption, VCO2: carbon dioxide production, Yr: Year.
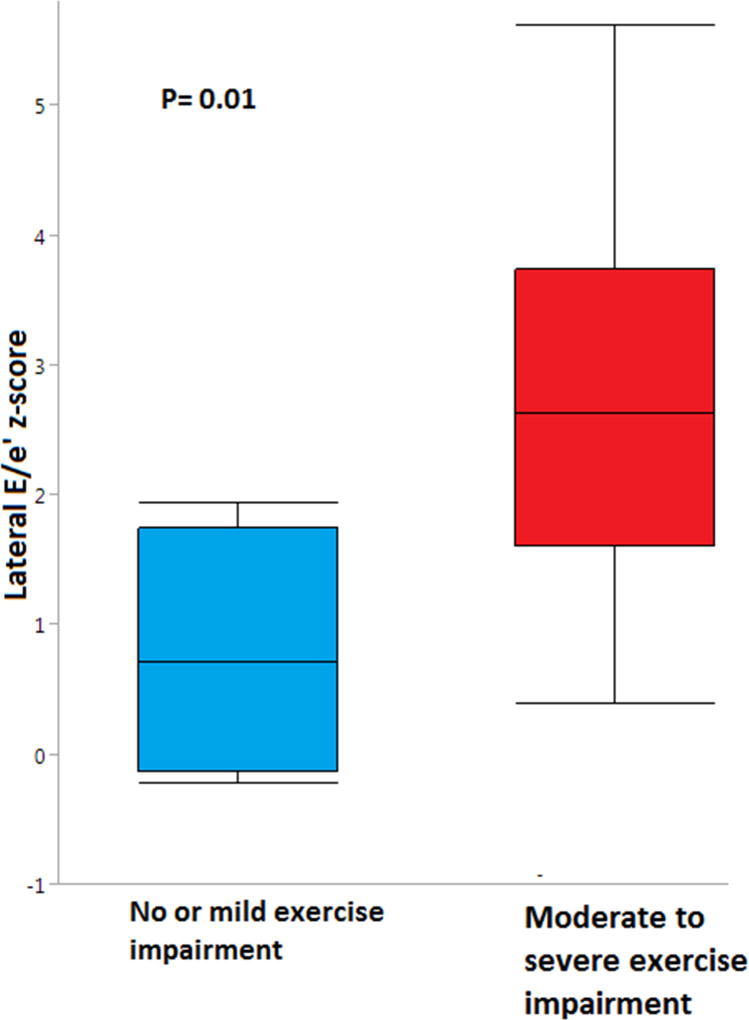
Figure 2.
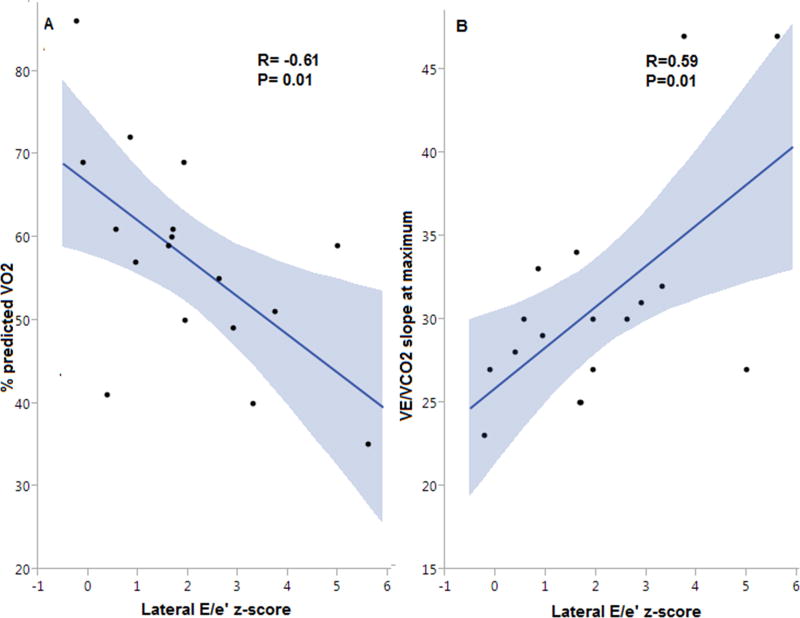
Of the echocardiographic diastolic measures, the lateral E/e’ z-score, which correlates with left atrial pressure, had the strongest association with %predicted VO2 (r=−0.61, p=0.01) (Figure 3). In addition, the z-scores of lateral and septal E/e’ ratios were positively associated with VE/VCO2 slope while lateral e’ z-score was negatively associated with VE/VCO2 slope. Moreover, the z-scores of septal E/e’ ratio and lateral e’ had a trend toward a negative and a positive association with %predicted VO2, respectively (r=−0.45, p=0.05 and r=0.42, p=0.07).TRV and LA volume indices did not have a significant association with exercise capacity or VE/VCO2 slope (Supplemental Table S1). Similarly, there was no association between echocardiographic or CMR measures of systolic function and either %predicted VO2 or VE/VCO2 slope. Patients with DD had numerically higher native T1 and ECV values compared to patients without DD (Table 2). ECV was elevated in all patients 0.43 ± 0.08 compared to our institutional normal control values 0.26±0.02, p < 0.001.8 Although all participants had abnormally elevated ECV, there was no association between ECV and either %predicted VO2 or VE/VCO2 slope (Table 3).
Figure 3.
Table 3.
Univariate predictors of % predicted VO2.
| Characteristic | R or difference in means | P Value |
|---|---|---|
| FVC | 0.59 | 0.009 |
| FEV1 | 0.03 | 0.12 |
| E/e’ z score | −0.61 | 0.01 |
| e’ z score | 0.42 | 0.07 |
| Creatinine | −0.45 | 0.038 |
| Bilirubin | −0.45 | 0.041 |
| Hemoglobin (g/dL) | 0.47 | 0.048 |
| Restrictive lung disease | −15.78 | 0.022 |
| Diastolic dysfunction | −13.3 | 0.01 |
| Reticulocyte count | −0.56 | 0.017 |
| ECV | −0.03 | 0.2 |
| Tricuspid regurgitation velocity | 0.001 | 0.9 |
ECV: extracellular volume, FEV1: forced expiratory volume in the first second, FVC: forced vital capacity.
Patients with moderate-to-severe exercise impairment had higher lateral E/e’ z-scores compared to patients with no or mild exercise impairment (2.7 ± 1.6 vs 0.8 ± 0.9, p=0.01) (Figure 2). Receiver operating characteristic curve for lateral E/e’ z-score to predict moderate-to-severe exercise dysfunction showed an area under the curve of 0.85. Lateral E/e’ z-score >2 had a sensitivity of 64% and a specificity of 100% to predict moderate-to-severe exercise impairment. All patients who had lateral E/e’ z-score >2 had moderate-to-severe exercise impairment.
Clinical and laboratory predictors of exercise capacity
Multiple clinical and laboratory factors correlated with %predicted VO2. In a univariate analysis, hemoglobin concentration was positively associated with %predicted VO2, while reticulocyte count, total bilirubin and serum creatinine were negatively associated with %predicted VO2, as well as the presence of DD or RLD (Table 3). This reflects the multifactorial etiology of exercise impairment in this patient population.
In a linear multivariate regression model that included lateral E/e’ z-score, hemoglobin concentration, and presence of RLD, the lateral E/e’ z-score was independently associated with %predicted VO2 (p=0.014). (Supplemental Table S2)
Discussion
Left ventricular DD is an independent risk factor for mortality in SCA that is associated with diffuse myocardial fibrosis.4, 8 Exercise capacity assessed by CPET is an important predictor of survival in patients with DD in other settings.28 This study confirmed that exercise impairment is extremely common in SCA and demonstrated an association between DD and impaired exercise capacity in patients with SCA, including young individuals. Lateral E/e’ z-score is an independent predictor of reduced exercise capacity and ventilation efficiency in SCA. Diffuse myocardial fibrosis is elevated in SCA and appears to predate DD, but the degree of elevation was not directly associated with exercise capacity in our study.
Exercise impairment is common in children and young adults with SCA. Our results are consistent with the findings by Liem and colleagues who showed that patients with SCA have lower peak VO2 compared to normal controls, even after controlling for hemoglobin concentration, suggesting that factors other than anemia are contributing to impaired functional capacity.10 These findings were replicated in other studies that confirmed exercise impairment and demonstrated the safety of performing CPET in individuals with SCA.31, 32 The data on association between cardiopulmonary disease and CPET variables is limited.32 Van Beers et al. found no association between TRV and exercise capacity in 44 patients who underwent CPET and echocardiography.32 Similarly, we did not find an association between TRV and CPET variables in this study; however, both our study and Van Beers’ had a limited range of TRV and did not include patients with right heart catheterization-proven pulmonary hypertension or patients with high TRV (>3 m/s). The effects of DD on CPET variables, however, were not evaluated in previous studies.
Left ventricular DD is common in SCA.4, 33 In a recent meta-analysis, the prevalence of DD was 11–77% in SCA patients depending on the diagnostic criteria.4, 33 In a population study that included older individuals with SCA, both TRV and lateral E/e’ were independent predictors of the 6-minute walk distance.16 The 6-minute walk distance is an easy test to perform, but its results are more variable, not always consistent with CPET results and do not allow for investigation into the causes of impaired functional capacity.34, 35 In addition, CPET-derived measures, especially peak VO2 and VE/VCO2 slope, have additional prognostic value in many cardiac diseases, particularly DD, and remain the gold standard to evaluate exercise capacity.35, 36 Our study demonstrates the association between DD and both variables (%predicted VO2 and VE/VCO2 slope) and reveals a significant functional impairment in SCA patients who have DD. The increase in VE/VCO2 slope and VE/VO2 may reflect a hyperventilation response to exercise in patients with DD. This could be related to increase in dead space ventilation due to pulmonary edema, lung stiffness related to chronic congestion, or decreased muscle mass in patients with DD.37, 38 Markers of ventilatory efficiency on CPET have been associated with elevated pulmonary pressure resulting from left sided heart disease, which could result from DD in SCA.39, 40 Of note lateral E/e’ z-score was an independent predictor of %predicted VO2 while septal E/e’ z-score showed only a trend toward association with %predicted VO2. As compared to septal E/e’, lateral E/e’ correlates better with left ventricular filling pressures which may explain the stronger association with exercise parameters for lateral E/e’ in our study.19, 41–44 Furthermore both e’ and E/e’ are important echocardiographic criteria for DD. However, each of these measures has its own limitations, and several hemodynamic factors affect their measurement.17 Therefore, diastolic measures should not be used in isolation to make a diagnosis of DD.19 Nonetheless, E/e’ correlates best with left ventricular filling pressures in previous studies and may reflect the interaction between the preload which is increased in SCA and DD.41 E/e’ was associated with mortality in SCA patients while e’ alone did not.4
Exercise training for 3 months in patients with DD without SCA led to an improvement in exercise capacity and E/e’ ratio in previous studies.45, 46 Although the benefits of exercise training was not studied in SCA, recent literature confirmed the feasibility and safety of exercise training in SCA.47 Treatments of DD, such as spironolactone, also improve exercise capacity in patients without SCA.48 Measures of DD and exercise capacity are potential measurable end-points for therapies of DD in SCA. Our findings support the importance of echocardiographic screening for DD in SCA.
The association between diffuse myocardial fibrosis and DD has been demonstrated in our previous studies.8, 49 All participants in this study had abnormally elevated ECV, indicating diffuse myocardial fibrosis.8 Although DD correlated with exercise capacity, we did not find an association between ECV and exercise capacity in this study. While this could be due to the relatively small sample size and lack of study participants with normal ECV measurements, it could also suggest that increased ECV precedes the development of DD and exercise impairment. This finding was also suggested by the presence of abnormal ECV values in young children who did not have echocardiographic evidence of DD yet.8
Our study has several limitations. First, this is a relatively small sample with a wide age range that may limit the interpretation of the correlations between variables and the generalizability of the findings. Despite the small sample, however, the findings of this study are novel and can be the basis of a larger confirmatory study. Second, classification of DD is less clear in children. In this study, we used age-, size-, and sex-corrected z-scores to account for these variations and incorporated exercise echocardiography results in accordance with the most recent DD guidelines to improve the accuracy of the classification.19 However, there are limited data to inform the diagnosis of DD in SCA and in the pediatric population. Third, not all patients reached maximum exercise, which further limited our sample size and may have resulted in selection bias. Evaluating sub-maximum exercise measures may be valuable in these settings. Fourth the diagnosis of RLD was made by spirometry without a comprehensive pulmonary function test which is the gold standard for diagnosis as spirometry cannot measure residual lung volumes.
In summary, despite the small sample size this study showed for the first time that DD is associated with poor exercise capacity assessed by %predicted VO2 and VE/VCO2 slope in children and adults with SCA. Lateral E/e’ z-score is an independent predictor of exercise capacity after correcting for anemia and RLD. The association of DD with clinical outcomes of SCA and the therapeutic targeting of DD should be explored to ameliorate cardiac disease and improve outcomes in SCA. Furthermore, as the mechanism of DD is likely distinct in SCA compared to DD due to other reasons, SCA-directed therapies effect on DD should be investigated. Exercise programs may also improve exercise capacity and quality of life in SCA.
Supplementary Material
Acknowledgments
This study is supported by the NIH-NHLBI Excellence in Hemoglobinopathy Research Award (EHRA) program (U01HL117709) (PM and CTQ). ON and TA were recipients of the U01HL117709 Translational Research Scholar Award. TA was the recipient of Cincinnati Children’s Strauss Research Award. We thank Sandy Knecht, Katie Lehmkuhl, Stephanie Stewart, Lauren Longshore and Kaylee Wright who performed the echocardiogram and exercise stress testing. We also thank Courtney Little, Eileen Beckman and Amy Shova for assistance with recruitment of participants and collection of clinical data.
Abbreviation
- A
Atrial contraction (Late filling)
- A’
Mitral annular late diastolic velocity
- CMR
Cardiac Magnetic Resonance Imaging
- CPET
Cardiopulmonary exercise test
- DD
Diastolic dysfunction
- E
Early filling
- ECV
Extracellular volume
- E’
Mitral annular early diastolic velocity
- FEV1
Forced expiratory volume
- FVC
Forced vital capacity
- LA
Left atrium
- MOLLI
Modified Look-Locker Inversion Recovery
- RLD
Restrictive lung disease
- RER
Respiratory exchange ratio
- SCA
Sickle cell anemia
- TRV
Tricuspid regurgitation Velocity
- VE
Ventilation
- VCC2
Carbon dioxide production
- VO2
Oxygen uptake
Footnotes
Relationship with industry: No relationships with industry
Conflict of Interest Statement: None
References
- 1.Hassell KL. Population estimates of sickle cell disease in the U.S. Am J Prev Med. 2010;38:S512–21. doi: 10.1016/j.amepre.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 2.Fitzhugh CD, Lauder N, Jonassaint JC, Telen MJ, Zhao X, Wright EC, Gilliam FR, De Castro LM. Cardiopulmonary complications leading to premature deaths in adult patients with sickle cell disease. American journal of hematology. 2010;85:36–40. doi: 10.1002/ajh.21569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakeer N, James J, Roy S, Wansapura J, Shanmukhappa SK, Lorenz JN, Osinska H, Backer K, Huby AC, Shrestha A, Niss O, Fleck R, Quinn CT, Taylor MD, Purevjav E, Aronow BJ, Towbin JA, Malik P. Sickle cell anemia mice develop a unique cardiomyopathy with restrictive physiology. Proc Natl Acad Sci U S A. 2016 doi: 10.1073/pnas.1600311113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sachdev V, Machado RF, Shizukuda Y, Rao YN, Sidenko S, Ernst I, St Peter M, Coles WA, Rosing DR, Blackwelder WC, Castro O, Kato GJ, Gladwin MT. Diastolic dysfunction is an independent risk factor for death in patients with sickle cell disease. Journal of the American College of Cardiology. 2007;49:472–9. doi: 10.1016/j.jacc.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Machado RF, Anthi A, Steinberg MH, Bonds D, Sachdev V, Kato GJ, Taveira-DaSilva AM, Ballas SK, Blackwelder W, Xu X, Hunter L, Barton B, Waclawiw M, Castro O, Gladwin MT, Investigators MSH N-terminal pro-brain natriuretic peptide levels and risk of death in sickle cell disease. JAMA. 2006;296:310–8. doi: 10.1001/jama.296.3.310. [DOI] [PubMed] [Google Scholar]
- 6.Gladwin MT, Sachdev V, Jison ML, Shizukuda Y, Plehn JF, Minter K, Brown B, Coles WA, Nichols JS, Ernst I, Hunter LA, Blackwelder WC, Schechter AN, Rodgers GP, Castro O, Ognibene FP. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. The New England journal of medicine. 2004;350:886–95. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- 7.Riesenkampff E, Messroghli DR, Redington AN, Grosse-Wortmann L. Myocardial T1 mapping in pediatric and congenital heart disease. Circulation Cardiovascular imaging. 2015;8:e002504. doi: 10.1161/CIRCIMAGING.114.002504. [DOI] [PubMed] [Google Scholar]
- 8.Niss O, Fleck R, Makue F, Alsaied T, Desai P, Towbin JA, Malik P, Taylor MD, Quinn CT. Association between diffuse myocardial fibrosis and diastolic dysfunction in sickle cell anemia. Blood. 2017;130:205–213. doi: 10.1182/blood-2017-02-767624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holland DJ, Kumbhani DJ, Ahmed SH, Marwick TH. Effects of treatment on exercise tolerance, cardiac function, and mortality in heart failure with preserved ejection fraction. A meta-analysis. Journal of the American College of Cardiology. 2011;57:1676–86. doi: 10.1016/j.jacc.2010.10.057. [DOI] [PubMed] [Google Scholar]
- 10.Liem RI, Reddy M, Pelligra SA, Savant AP, Fernhall B, Rodeghier M, Thompson AA. Reduced fitness and abnormal cardiopulmonary responses to maximal exercise testing in children and young adults with sickle cell anemia. Physiol Rep. 2015;3 doi: 10.14814/phy2.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watson AM, Liem RI, Lu Z, Saville B, Acra S, Shankar S, Buchowski M. Longitudinal differences in aerobic capacity between children with sickle cell anemia and matched controls. Pediatric blood & cancer. 2015;62:648–53. doi: 10.1002/pbc.25383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liem RI, Young LT, Lay AS, Pelligra SA, Labotka RJ, Thompson AA. Reproducibility of tricuspid regurgitant jet velocity measurements in children and young adults with sickle cell disease undergoing screening for pulmonary hypertension. American journal of hematology. 2010;85:741–5. doi: 10.1002/ajh.21793. [DOI] [PubMed] [Google Scholar]
- 13.Guazzi M, Myers J, Arena R. Cardiopulmonary exercise testing in the clinical and prognostic assessment of diastolic heart failure. Journal of the American College of Cardiology. 2005;46:1883–90. doi: 10.1016/j.jacc.2005.07.051. [DOI] [PubMed] [Google Scholar]
- 14.Klaassen SHC, Liu LCY, Hummel YM, Damman K, van der Meer P, Voors AA, Hoendermis ES, van Veldhuisen DJ. Clinical and Hemodynamic Correlates and Prognostic Value of VE/VCO2 Slope in Patients With Heart Failure With Preserved Ejection Fraction and Pulmonary Hypertension. Journal of cardiac failure. 2017;23:777–782. doi: 10.1016/j.cardfail.2017.07.397. [DOI] [PubMed] [Google Scholar]
- 15.Liem RI, Nevin MA, Prestridge A, Young LT, Thompson AA. Functional capacity in children and young adults with sickle cell disease undergoing evaluation for cardiopulmonary disease. American journal of hematology. 2009;84:645–9. doi: 10.1002/ajh.21507. [DOI] [PubMed] [Google Scholar]
- 16.Sachdev V, Kato GJ, Gibbs JS, Barst RJ, Machado RF, Nouraie M, Hassell KL, Little JA, Schraufnagel DE, Krishnamurti L, Novelli EM, Girgis RE, Morris CR, Rosenzweig EB, Badesch DB, Lanzkron S, Castro OL, Taylor JGt, Hannoush H, Goldsmith JC, Gladwin MT, Gordeuk VR, Walk PI. Echocardiographic markers of elevated pulmonary pressure and left ventricular diastolic dysfunction are associated with exercise intolerance in adults and adolescents with homozygous sickle cell anemia in the United States and United Kingdom. Circulation. 2011;124:1452–60. doi: 10.1161/CIRCULATIONAHA.111.032920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Alexandru Popescu B, Waggoner AD, Houston T, Oslo N, Phoenix A, Nashville T, Hamilton OC, Uppsala S, Ghent, Liege B, Cleveland O, Novara I, Rochester M, Bucharest R and St, Louis M. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17:1321–1360. doi: 10.1093/ehjci/jew082. [DOI] [PubMed] [Google Scholar]
- 18.Pellikka PA, Nagueh SF, Elhendy AA, Kuehl CA, Sawada SG, American Society of E American Society of Echocardiography recommendations for performance, interpretation, and application of stress echocardiography. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2007;20:1021–41. doi: 10.1016/j.echo.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 20.Eidem BW, McMahon CJ, Cohen RR, Wu J, Finkelshteyn I, Kovalchin JP, Ayres NA, Bezold LI, O'Brian Smith E, Pignatelli RH. Impact of cardiac growth on Doppler tissue imaging velocities: a study in healthy children. J Am Soc Echocardiogr. 2004;17:212–21. doi: 10.1016/j.echo.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Kawel-Boehm N, Maceira A, Valsangiacomo-Buechel ER, Vogel-Claussen J, Turkbey EB, Williams R, Plein S, Tee M, Eng J, Bluemke DA. Normal values for cardiovascular magnetic resonance in adults and children. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2015;17:29. doi: 10.1186/s12968-015-0111-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez L, Colan SD, Frommelt PC, Ensing GJ, Kendall K, Younoszai AK, Lai WW, Geva T. Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2010;23:465–95. doi: 10.1016/j.echo.2010.03.019. quiz 576-7. [DOI] [PubMed] [Google Scholar]
- 23.Obokata M, Kane GC, Reddy YN, Olson TP, Melenovsky V, Borlaug BA. Role of Diastolic Stress Testing in the Evaluation for Heart Failure With Preserved Ejection Fraction: A Simultaneous Invasive-Echocardiographic Study. Circulation. 2017;135:825–838. doi: 10.1161/CIRCULATIONAHA.116.024822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takken T, Blank AC, Hulzebos EH, van Brussel M, Groen WG, Helders PJ. Cardiopulmonary exercise testing in congenital heart disease: equipment and test protocols. Neth Heart J. 2009;17:339–44. doi: 10.1007/BF03086280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gitt AK, Wasserman K, Kilkowski C, Kleemann T, Kilkowski A, Bangert M, Schneider S, Schwarz A, Senges J. Exercise anaerobic threshold and ventilatory efficiency identify heart failure patients for high risk of early death. Circulation. 2002;106:3079–84. doi: 10.1161/01.cir.0000041428.99427.06. [DOI] [PubMed] [Google Scholar]
- 26.Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, Forman D, Franklin B, Guazzi M, Gulati M, Keteyian SJ, Lavie CJ, Macko R, Mancini D, Milani RV, American Heart Association Exercise CR, Prevention Committee of the Council on Clinical C, Council on E, Prevention, Council on Peripheral Vascular D, Interdisciplinary Council on Quality of C and Outcomes R Clinician's Guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122:191–225. doi: 10.1161/CIR.0b013e3181e52e69. [DOI] [PubMed] [Google Scholar]
- 27.Arena R, Myers J, Abella J, Pinkstaff S, Brubaker P, Moore B, Kitzman D, Peberdy MA, Bensimhon D, Chase P, Forman D, West E, Guazzi M. Determining the preferred percent-predicted equation for peak oxygen consumption in patients with heart failure. Circulation Heart failure. 2009;2:113–20. doi: 10.1161/CIRCHEARTFAILURE.108.834168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stelken AM, Younis LT, Jennison SH, Miller DD, Miller LW, Shaw LJ, Kargl D, Chaitman BR. Prognostic value of cardiopulmonary exercise testing using percent achieved of predicted peak oxygen uptake for patients with ischemic and dilated cardiomyopathy. Journal of the American College of Cardiology. 1996;27:345–52. doi: 10.1016/0735-1097(95)00464-5. [DOI] [PubMed] [Google Scholar]
- 29.Cooper DM, Weiler-Ravell D, Whipp BJ, Wasserman K. Aerobic parameters of exercise as a function of body size during growth in children. J Appl Physiol Respir Environ Exerc Physiol. 1984;56:628–34. doi: 10.1152/jappl.1984.56.3.628. [DOI] [PubMed] [Google Scholar]
- 30.Johnson JD, Theurer WM. A stepwise approach to the interpretation of pulmonary function tests. American family physician. 2014;89:359–66. [PubMed] [Google Scholar]
- 31.Hostyn SV, Carvalho WB, Johnston C, Braga JA. Evaluation of functional capacity for exercise in children and adolescents with sickle-cell disease through the six-minute walk test. Jornal de pediatria. 2013;89:588–94. doi: 10.1016/j.jped.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 32.van Beers EJ, van der Plas MN, Nur E, Bogaard HJ, van Steenwijk RP, Biemond BJ, Bresser P. Exercise tolerance, lung function abnormalities, anemia, and cardiothoracic ratio in sickle cell patients. American journal of hematology. 2014;89:819–24. doi: 10.1002/ajh.23752. [DOI] [PubMed] [Google Scholar]
- 33.Niss O, Quinn CT, Lane A, Daily J, Khoury PR, Bakeer N, Kimball TR, Towbin JA, Malik P, Taylor MD. Cardiomyopathy With Restrictive Physiology in Sickle Cell Disease. JACC Cardiovasc Imaging. 2016 doi: 10.1016/j.jcmg.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laboratories ATSCoPSfCPF. ATS statement: guidelines for the six-minute walk test. American journal of respiratory and critical care medicine. 2002;166:111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 35.Guazzi M, Dickstein K, Vicenzi M, Arena R. Six-minute walk test and cardiopulmonary exercise testing in patients with chronic heart failure: a comparative analysis on clinical and prognostic insights. Circ Heart Fail. 2009;2:549–55. doi: 10.1161/CIRCHEARTFAILURE.109.881326. [DOI] [PubMed] [Google Scholar]
- 36.Cahalin LP, Chase P, Arena R, Myers J, Bensimhon D, Peberdy MA, Ashley E, West E, Forman DE, Pinkstaff S, Lavie CJ, Guazzi M. A meta-analysis of the prognostic significance of cardiopulmonary exercise testing in patients with heart failure. Heart failure reviews. 2013;18:79–94. doi: 10.1007/s10741-012-9332-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clark AL, Volterrani M, Swan JW, Coats AJ. The increased ventilatory response to exercise in chronic heart failure: relation to pulmonary pathology. Heart. 1997;77:138–146. doi: 10.1136/hrt.77.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guazzi M, Cahalin LP, Arena R. Cardiopulmonary exercise testing as a diagnostic tool for the detection of left-sided pulmonary hypertension in heart failure. Journal of cardiac failure. 2013;19:461–7. doi: 10.1016/j.cardfail.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 39.Arena R, Owens DS, Arevalo J, Smith K, Mohiddin SA, McAreavey D, Ulisney KL, Tripodi D, Fananapazir L, Plehn JF. Ventilatory efficiency and resting hemodynamics in hypertrophic cardiomyopathy. Med Sci Sports Exerc. 2008;40:799–805. doi: 10.1249/MSS.0b013e31816459a1. [DOI] [PubMed] [Google Scholar]
- 40.Andersen OS, Smiseth OA, Dokainish H, Abudiab MM, Schutt RC, Kumar A, Sato K, Harb S, Gude E, Remme EW, Andreassen AK, Ha JW, Xu J, Klein AL, Nagueh SF. Estimating Left Ventricular Filling Pressure by Echocardiography. Journal of the American College of Cardiology. 2017;69:1937–1948. doi: 10.1016/j.jacc.2017.01.058. [DOI] [PubMed] [Google Scholar]
- 41.Park HS, Naik SD, Aronow WS, Visintainer PF, Das M, McClung JA, Belkin RN. Differences of lateral and septal mitral annulus velocity by tissue Doppler imaging in the evaluation of left ventricular diastolic function. The American journal of cardiology. 2006;98:970–2. doi: 10.1016/j.amjcard.2006.04.044. [DOI] [PubMed] [Google Scholar]
- 42.Rivas-Gotz C, Manolios M, Thohan V, Nagueh SF. Impact of left ventricular ejection fraction on estimation of left ventricular filling pressures using tissue Doppler and flow propagation velocity. The American journal of cardiology. 2003;91:780–4. doi: 10.1016/s0002-9149(02)03433-1. [DOI] [PubMed] [Google Scholar]
- 43.Kasner M, Westermann D, Steendijk P, Gaub R, Wilkenshoff U, Weitmann K, Hoffmann W, Poller W, Schultheiss HP, Pauschinger M, Tschope C. Utility of Doppler echocardiography and tissue Doppler imaging in the estimation of diastolic function in heart failure with normal ejection fraction: a comparative Doppler-conductance catheterization study. Circulation. 2007;116:637–47. doi: 10.1161/CIRCULATIONAHA.106.661983. [DOI] [PubMed] [Google Scholar]
- 44.Edelmann F, Bobenko A, Gelbrich G, Hasenfuss G, Herrmann-Lingen C, Duvinage A, Schwarz S, Mende M, Prettin C, Trippel T, Lindhorst R, Morris D, Pieske-Kraigher E, Nolte K, Dungen HD, Wachter R, Halle M, Pieske B. Exercise training in Diastolic Heart Failure (Ex-DHF): rationale and design of a multicentre, prospective, randomized, controlled, parallel group trial. European journal of heart failure. 2017;19:1067–1074. doi: 10.1002/ejhf.862. [DOI] [PubMed] [Google Scholar]
- 45.Edelmann F, Gelbrich G, Dungen HD, Frohling S, Wachter R, Stahrenberg R, Binder L, Topper A, Lashki DJ, Schwarz S, Herrmann-Lingen C, Loffler M, Hasenfuss G, Halle M, Pieske B. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: results of the Ex-DHF (Exercise training in Diastolic Heart Failure) pilot study. Journal of the American College of Cardiology. 2011;58:1780–91. doi: 10.1016/j.jacc.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 46.Liem RI, Akinosun M, Muntz DS, Thompson AA. Feasibility and safety of home exercise training in children with sickle cell anemia. Pediatric blood & cancer. 2017 doi: 10.1002/pbc.26671. [DOI] [PubMed] [Google Scholar]
- 47.Kosmala W, Rojek A, Przewlocka-Kosmala M, Wright L, Mysiak A, Marwick TH. Effect of Aldosterone Antagonism on Exercise Tolerance in Heart Failure With Preserved Ejection Fraction. Journal of the American College of Cardiology. 2016;68:1823–1834. doi: 10.1016/j.jacc.2016.07.763. [DOI] [PubMed] [Google Scholar]
- 48.Bakeer N, James J, Roy S, Wansapura J, Shanmukhappa SK, Lorenz JN, Osinska H, Backer K, Huby AC, Shrestha A, Niss O, Fleck R, Quinn CT, Taylor MD, Purevjav E, Aronow BJ, Towbin JA, Malik P. Sickle cell anemia mice develop a unique cardiomyopathy with restrictive physiology. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:E5182–91. doi: 10.1073/pnas.1600311113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.