Abstract
Background
Although studies suggest decreased incident hepatocellular carcinoma (HCC) after direct-acting antivirals (DAA), data are conflicting regarding HCC recurrence and aggressiveness in patients who have a history of HCC with complete response.
Aim
Characterize HCC recurrence patterns after DAA therapy.
Methods
Two reviewers searched MEDLINE and SCOPUS from January 2015 to December 2017 and identified studies evaluating HCC recurrence patterns following DAA therapy. A pooled estimate was calculated using the DerSimonian and Laird method for a random effects model. The study was conducted in accordance with PRISMA guidelines.
Results
Among 24 studies (n=1820 patients), the proportion of patients with HCC recurrence following DAA therapy ranged from 0% to 59% (pooled estimate 24.4%; 95%CI 18.4 – 30.4%). Among 11 full text manuscripts, pooled HCC recurrence was 21.9% (95%CI 16.2% – 28.3%). Factors associated with recurrence included history of prior HCC recurrence and a shorter interval between HCC complete response and DAA initiation. Nine studies comparing DAA-treated and interferon-treated or untreated patients found similar recurrence among DAA-treated patients. Most (77.8%) patients with HCC recurrence were detected at an early tumor stage, of whom 64.7% received curative treatment. Study limitations included heterogeneous cohorts, potential misclassification of HCC absence prior to DAA, ascertainment bias for recurrence, and short durations of follow-up.
Conclusion
Current data suggest acceptable HCC recurrence rates after DAA therapy, particularly if DAA therapy is delayed at least 6 months after HCC complete response. However, data characterizing HCC recurrence after DAA therapy are of limited quality, highlighting the need for high-quality prospective studies.
Keywords: liver cancer, hepatitis C, recurrence, direct acting antiviral therapy
INTRODUCTION
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related death worldwide, with chronic hepatitis C (HCV) being the most common underlying etiology in the United States and Europe.1 Liver transplantation, surgical resection, and local ablative therapy are potentially curative and associated with excellent long-term survival but are limited by tumor recurrence exceeding 50% at 5 years.2
Prior attempts at adjuvant pharmacologic therapy for HCC have failed to improve survival or reduce recurrence, with the exception of anti-viral therapy.3 Interferon-based therapy was associated with a significant reduction in HCC incidence in patients with HCV-related cirrhosis as well as HCC recurrence after curative therapy.4 The mechanism for reduction in HCC recurrence is unclear – whether related to sustained viral response (SVR) or direct interferon-related immune-mediated antitumor effect.5 This debate has become more relevant with the introduction of direct acting antiviral (DAA) therapy, which has replaced interferon-based therapy for HCV.6 Although studies have demonstrated improvements in fibrosis and portal hypertension as well as decreased risk of incident HCC in patients with HCV-related cirrhosis, some observational studies suggest an increased risk and aggressiveness of HCC recurrence after DAA therapy.7–10
This issue remains a topic of debate, leading to uncertainty if or when to treat HCV-infected patients with a history of HCC.11, 12 These data have created fear among patients receiving DAA therapy and prompted some providers to withhold HCV treatment from patients with a history of HCC. The European Medicine Agency’s Pharmacovigilance Risk Assessment Committee has required high quality data evaluating this association. Given the lack of randomized data evaluating this association, we are forced to rely on cohort studies and indirect comparisons across studies. The aim of this systematic review is to characterize HCC recurrence patterns following DAA-based therapy.
METHODS
Literature search and Study Selection
We searched MEDLINE and SCOPUS databases from January 1, 2015 through December 1, 2017 using search terms: (liver ca$ or hepatocellular ca$ or hcc or hepatoma) and (hepatitis C or HCV) and (interferon-free or direct-acting antiviral or DAA). We also manually searched abstracts from the 2016 and 2017 American Association for the Study of Liver Diseases, European Association for the Study of the Liver, and International Liver Cancer Association annual conferences. Additional studies that may have been missed by the electronic search were identified through manual searching of reference lists from applicable studies and consultation with experts in the field. If the applicability of an article could not be determined by title or abstract alone, the full text was reviewed. Articles and abstracts were independently evaluated for possible inclusion by two authors (N.S. and A.S.) and any uncertainties were resolved through discussion with another reviewer (N.P.). The study was conducted in accordance with Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines.13
Studies were included for analysis if they evaluated HCC recurrence after DAA therapy in a cohort of patients with chronic HCV infection and a history of HCC with complete response. We included studies with complete response from surgical, local ablative, or locoregional therapies; however, we excluded cohorts focusing exclusively on post-transplant HCC recurrence and studies that included interferon-based regimens in combination with DAAs. Complete response in studies was typically defined using modified Response Evaluation Criteria in Solid Tumors (mRECIST), i.e. disappearance of arterial enhancement in all HCC lesions. Studies may have been retrospective or prospective but were required to report the proportion of patients with recurrence after DAA therapy. Additional exclusion criteria included 1) non-English language, 2) non-human data, and 3) lack of original data. If publications reported data using the same cohort of patients, the study with more complete data was included.
Data extraction
Two authors (N.S. and A.S.) independently reviewed and extracted required information from eligible studies using standardized forms. A third investigator (N.P.) was available to resolve any discrepancies between the sets of extracted data. The data extraction form included the following study design items: geographical location and date of study, study design (prospective vs. retrospective), characteristics and size of study cohort, inclusion and exclusion criteria, HCC treatment regimen, median time from HCC treatment to DAA treatment, and duration of follow-up. In addition, the extraction form recorded the following primary data: proportion of patients with HCC recurrence and patterns of recurrence (i.e. tumor burden and HCC-directed treatment). Two authors (N.S. and A.S.) independently assessed study quality using a modified checklist based upon a modified version of the Newcastle-Ottawa quality assessment scale, with discrepancies resolved by consensus.14 Studies with less than one year of follow-up or loss to follow-up exceeding 5% were regarded as high risk of bias.
Statistical analysis
The aim of this study was to characterize HCC recurrence patterns after DAA therapy. For each individual study, the proportion of patients with HCC recurrence with 95% confidence intervals was calculated. Estimates of effect were pooled using a binomial-normal model and the DerSimonian and Laird method for a random effects model. Heterogeneity was initially evaluated graphically by examination of forest plots and statistically by the inconsistency index (I2), with values >50% consistent with the possibility of substantial heterogeneity.15 Subgroup analyses were planned for HCC detection for predefined subsets of studies based on (i) prospective vs. retrospective study design, (ii) study location, (iii) duration of follow-up (iv) timing of DAA initiation, and (v) availability of full text manuscripts. We assessed for presence of heterogeneity between subgroups, with statistical significance defined as p<0.05. We also performed a subgroup analysis among studies reporting early HCC recurrence after DAA initiation. We performed univariate and multivariate meta-regression analyses to evaluate the potential association between study-level covariates (study location, study design, sample size, proportion of patients with early HCC, timing of DAA initiation, and length of follow-up) and HCC recurrence. A secondary analysis among studies reporting relative recurrence rates between DAA-treated and untreated patients, in which we determined a pooled estimate for the relative risk of recurrence using a random effects model. Publication bias was initially evaluated graphically by funnel plot analysis. Analyses were performed using STATA 14.0 (StataCorp, College Station, TX, USA).
RESULTS
Literature search
Upon review of the 827 titles identified by the search strategy, 63 abstracts were further examined. Twenty-three publications underwent full-text review to determine their eligibility for the meta-analysis and four were excluded. The remaining 16 studies were selected after meeting all applicable inclusion criteria. Overall, 20 studies were excluded because they presented data on HCC occurrence but not recurrence, 11 had insufficient data for abstraction, 5 had overlapping cohorts, 4 exclusively characterized post-transplant HCC recurrence, and 7 studies were excluded for lack of original data (Supplemental Figure). Finally, review of American Association for the Study of Liver Diseases, European Association for the Study of the Liver, and International Liver Cancer Association conference abstracts identified 7 additional studies and recursive literature searches identified 1 full-length manuscript and 2 letters to the editor that met inclusion criteria, producing a total of 26 studies.16–39 The study by the ANRS collaborative study group included two distinct cohorts,24 yielding a total of 27 cohorts. Two included studies reported relative HCC recurrence rates compared to interferon-treated or untreated patients but did not report absolute HCC recurrence rates,41,42 yielding 24 studies with 25 cohorts characterizing the primary outcome of absolute HCC recurrence rates after DAA initiation.
Study Characteristics
Characteristics of the 24 studies (i.e. 25 cohorts) reporting the absolute proportion of patients with HCC recurrence after DAA therapy are described in Table 1. The majority of cohorts were retrospective (n=19) and only available as abstracts or letters to the editor (n=14). Thirteen cohorts were conducted in Europe, ten in Asia, and two in the United States. Studies included a total of 1820 DAA-treated patients, although most cohorts (n=19) included less than 100 patients, resulting in imprecise estimates for HCC recurrence with wide confidence intervals. Most patients had achieved HCC complete response after resection or ablation; however, 25-50% of patients in some studies had received non-curative therapies including transarterial chemoembolization (TACE). Duration of follow-up to assess recurrence ranged from 3 to 36 months, although some studies defined recurrence from date of HCC treatment and others evaluated recurrence from date of DAA initiation. Although most studies only included single-arm cohorts, 7 of the studies included a comparator arm of interferon-treated and/or untreated patients. Two additional studies did not report the absolute proportion of patients with HCC recurrence but compared relative recurrence between DAA-treated patients and interferon-treated or untreated patients, yielding a total of 9 comparative studies (Table 2).
Table 1.
Characteristics of studies reporting HCC recurrence rates after DAA therapy
| Author Year | Study location | Study Design | Number DAA-HCC patients (% early) | AFP level prior to HCC treatment | Type of HCC treatment | Time from HCC treat to DAA | Follow-up Start | Follow-up Period | Recurrence (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Bielen 201716* | Belgium | Retrospective | 41 (83%) |
46 ± 70 | Transplant 51% Resection 24% Ablation 22% TACE 3% |
12 months | End of DAA treatment | 32 months | 14.6 (5.6 – 29.2) |
| Cabibbo 201717* | Italy | Prospective | 143 (100%) | 18 ± 26 | Resection 36% Ablation 46% TACE 18% |
11 months | Start of DAA treatment | 8.7 months | 20.3 14.0 - 27.8 |
| Ikeda 201718* | Japan | Retrospective | 177 (NR) |
17 (2 – 4730) |
Resection 31% Ablation 46% TACE 20% Radiation 3% |
10.7 months | Start of DAA treatment | 20.7 months | 34.6 27.9 - 41.7 |
| Nagata 201719* | Japan | Retrospective | 83 (100%) |
NR | Resection or ablation | NR | HCC treatment | 27.6 months | 27.1 18.2 – 36.9 |
| Ogawa 201720* | Japan | Prospective | 152 (95%) |
7 (5 – 16) |
Resection 40% Ablation 32% TACE 15% Multimodal 12% Radiation 1% |
14.4 months | Start of DAA treatment | 17 months | 17.5 (11.9-23.9) |
| Reig 201721* | Spain | Retrospective | 77 (97%) |
10 (1 - 369) |
Resection 36% Ablation 53% TACE 10% |
11.2 months | Start of DAA treatment | 8.2 months | 27.3 (17.7 - 38.6) |
| Virlogeux 201722* | France | Retrospective | 23 (91%) |
13 (2 - 170) |
Resection 26% Ablation 61% Other 13% |
7.1 months | Start of DAA treatment | 35.7 months | 47.8 (26.8 - 69.4) |
| Conti 201623* | Italy | Retrospective | 59 (98%) |
NR | Resection 32% Ablation 41% TACE 8% Multimodal 17% |
12.4 months | End of DAA treatment | 5.5 months |
28.8 (17.8 - 42.1) |
| ANRS 201624* | France | Retrospective | 189 (NR) |
NR | NR | NR | Start of DAA treatment | 20.2 months | 12.7 (8.3 - 18.3) |
| ANRS 201624* | France | Retrospective | 13 (NR) |
NR | NR | >3 months | Start of DAA treatment | 21.3 months | 7.6 (0.2 - 36.0) |
| Rinaldi 201625* | Italy | Retrospective | 15 (100%) |
NR | Resection 14% Ablation 86% |
11.3 months | Start of DAA treatment | 2.8 months | 6.7 (0.2 - 32.0) |
| Zavaglia 201726 | Italy | Retrospective | 31 (84%) |
10 (2 - 278) |
Resection 42% Ablation 19% TACE 13% Multimodal 26% |
19.3 months | Start of DAA treatment | 8 months | 3.2 (0 - 16.7) |
| Zeng 201627 | China | Retrospective | 10 (100%) |
NR | Ablation 100% | NR | End of DAA Treatment | 15 months | 0 (0 - 25.9) |
| Torres 201628 | USA | Prospective | 8 (NR) |
51 (6 - 7258) |
Resection 50% Ablation 38% Proton therapy 12% |
7.5 months | Start of DAA treatment | 12 months | 0 (0 - 31.2) |
| Gheoghe 201729 | Romania | Retrospective | 20 (NR) |
NR | NR | NR | DAA treatment | 6 months | 20.0 (5.7 – 43.7) |
| Granata 201730 | Italy | Prospective | 65 (83%) |
NR | NR | 9 months | Start of DAA treatment | 18 months | 27.7 (17.3 - 40.2) |
| Kolly 201731 | Germany, Belgium, Switzerland | Retrospective | 56 (NR) |
NR | Ablation, resection or TACE | NR | HCC treatment | 21 months | 1-year 19% 2-year 44% |
| Yasui 201732 | Japan | Retrospective | 46 (NR) |
NR | NR | NR | End of DAA treatment | 6 months | 14.3 (5.4 – 28.5) |
| Minami 201733 | Japan | Retrospective | 163 (91%) |
NR | Resection 14 Ablation 147 Radiotherapy 1 TACE 1 |
8 months | Start of DAA treatment | 14.5 months | 47.9 (40.0 - 55.8) |
| Ohki 201734 | Japan | Retrospective | 20 (100%) |
13 (7 – 25) |
Ablation 100% | 7.6 months | HCC treatment | 24 months | 35.0 (15.4 - 59.2) |
| Sangiovanni 201735 |
Italy | Prospective | 101 (98%) |
NR | Resection 28% Ablation 48% TACE 10% Multi-modality 14% |
12 months | Start of DAA treatment | 11.1 months | 32.7 (23.7 - 42.7) |
| Singal 201737 | USA | Retrospective | 207 (87%) |
16 (7 – 52) |
Resection 19% Ablation 27% TACE 37% Multi-modality 16% |
7.2 months | HCC treatment | 22.7 months | 45.9 (39.0 - 52.9) |
| Urabe 201736 | Japan | Prospective | 63 (NR) |
NR | NR | 24.3 months | Start of DAA treatment | 10.9 months | 38.5 (26.2 - 51.2) |
| Tokoro 201638 | Japan | Retrospective | 22 (NR) |
NR | NR | NR | Start of DAA treatment | 16.2 months | 59.1 (36.4 - 79.3) |
| Tsuda 201639 | Japan | Retrospective | 36 (NR) |
NR | NR | NR | Start of DAA treatment | 11.4 months | 25.0 (12.1 - 42.2) |
DAA – direct acting antiviral; HCC – hepatocellular carcinoma; NR – not reported; TACE – transarterial chemoembolization
available as full length manuscript
Table 2.
Characteristics of studies with direct comparisons of HCC recurrence rates
| Author Year | Study location | Study Arms | Number of patients | Comparison of Recurrence |
|---|---|---|---|---|
| Virlogeux 201722 | France | DAA-treated and untreated | 23/45 | 1.7 vs. 4.2/100 person-months in DAA-treated and untreated patients (adjusted HR 0.24, 95%CI 0.10 – 0.55) |
| ANRS 201624 | France | DAA-treated and untreated | 189/78 | 0.73 vs. 0.66/100 person-months in DAA-treated and untreated patients (adjusted HR 1.04, 95%CI 0.53 – 2.07) |
| ANRS 201624 | France | DAA-treated and untreated | 13/66 | 1.1 vs. 1.7/100 person-months in DAA-treated and untreated patients (adjusted HR 0.40, 95%CI 0.05 – 3.03) |
| Granata 201730 | Italy | DAA-treated and untreated | 65/186 | Time to recurrence: 23.2 vs. 23.0 months in DAA-treated and untreated patients |
| Yasui 201732 | Japan | DAA-treated and IFN-treated | 46/26 | Proportion: 14% vs. 22% in DAA-treated and IFN-treated (p=0.50) |
| Joko 201741 | Japan | DAA-treated and IFN-treated | 368/148 | No difference in early recurrence between DAA-treated and IFN-treated patients |
| Ohki 201734 | Japan | DAA-treated, IFN-treated, and untreated | 20/20/20 | 35% vs. 55% vs. 55% in DAA-treated, IFN-treated, and untreated patients (p=0.38) |
| Singal 201737 | USA | DAA-treated and untreated | 207/127 | Proportion: 46% vs. 50% in DAA-treated and untreated patients (adjusted OR 0.80, 95%CI 0.48-1.35) |
| Tanaka 201742 | Japan | DAA-treated, IFN-treated, and untreated | 16/16/119 | Lower recurrence in DAA-treated than untreated group (adjusted HR 0.56, 95%CI 0.26-0.97) |
DAA – direct acting antiviral; IFN - interferon
HCC Recurrence following DAA Therapy
The pooled point estimate for HCC recurrence following DAA therapy was 25.1% (95%CI 19.4 – 31.2%); however, there was significant statistical heterogeneity (I2 = 86%) (Figure 1). The proportion of patients with HCC recurrence varied widely between studies, ranging from 0% to 59% within 2 years (Table 1). In subgroup analyses, pooled estimates for HCC recurrence were similar between prospective and retrospective cohort studies (23.9% vs. 25.6%, p=0.76), studies with duration of follow-up shorter or longer than 12 months (21.9% vs. 27.6%, p=0.29), and studies in which HCC recurrence was assessed starting at DAA initiation vs. other times (24.6% vs. 26.0%, p=0.84). However, HCC recurrence was higher in studies conducted in the United States compared to Europe and Asia (43.3% vs. 22.1% vs. 28.9%, p<0.001). There continued to be significant heterogeneity, with I2 values remaining greater than 70%, in all subgroup analyses. Among the 11 studies with data regarding “early” recurrence, the proportion with recurrence within 6 months ranged from 5% - 29%, with a pooled proportion of 10.3% (95%CI 6.3 – 14.4%; I2 = 49%).
Figure 1.
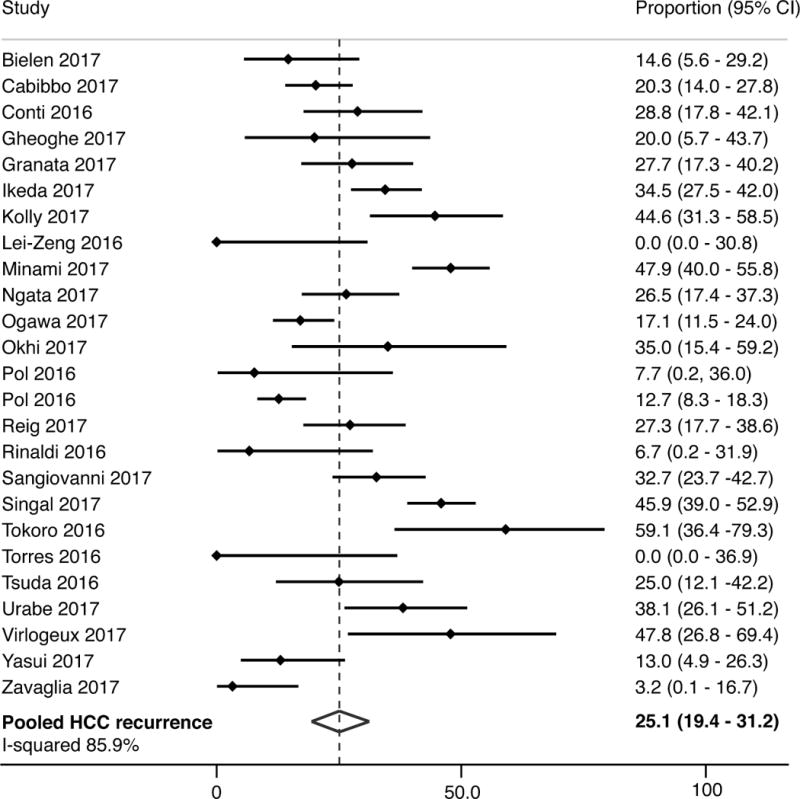
Proportion of patients with recurrence of hepatocellular carcinoma following direct-acting antiviral therapy
Among the 11 studies available as a full text manuscript, the pooled HCC recurrence rate was 21.9% (95%CI 16.2% - 28.3%), although there continued to be statistical heterogeneity with I2 >75%. In subgroup analyses, pooled estimates for HCC recurrence were similar between prospective and retrospective cohort studies (18.6% vs. 22.9%), studies with duration of follow-up shorter or longer than 12 months (22.6% vs. 22.0%), studies in which HCC recurrence was assessed starting at DAA initiation vs. other times (22.7% vs. 21.1%), and studies conducted in Europe and Asia (20.1% vs. 25.7%). There was no association between HCC recurrence and study location, study design, sample size, the proportion of patients with early HCC, median time from HCC treatment to DAA initiation, or median length of follow-up in meta-regression analyses.
The most commonly reported factor associated with increased recurrence within included studies was the interval between HCC complete response and DAA initiation. Reig and colleagues found higher recurrence (41% vs. 23%) in patients treated within 4 months of HCC complete response,21 whereas Ogawa reported this association using a cut-off of 1 year (HR 0.31, 95%CI 0.10 – 0.77),20 and Minimi et al using a cut-off of 2 years (HR 0.34).40 Similarly, Kolly et al found patients with a longer timeframe between HCC treatment and DAA therapy had significantly lower hazards of recurrence (HR 0.91, 95%CI 0.85 – 0.96).31 A history of prior HCC recurrence was also associated with increased risk of HCC recurrence following DAA therapy (HR 2.2-2.3), as reported by Minami, Cabibbo, and Ikeda.17, 18, 40 Although many studies reported no difference in HCC recurrence by classic risk factors including degree of liver dysfunction, tumor burden, or alpha fetoprotein (AFP) levels, each was found to be associated with higher recurrence in one study. Conti described higher recurrence in those with increased liver stiffness (OR 1.19, 95%CI 1.01 – 1.39),23 Ogawa and Cabibbo found higher recurrence in those with a history of multifocal HCC (HR 2.34, 95%CI 1.05 – 5.39)20 and larger HCC lesions (HR 2.73, 95%CI 1.23 – 6.06)17 respectively, Minami reported higher recurrence in those with AFP-L3 > 15% (HR 3.08) or DCP > 40 mAU/mL (HR 2.0),40 and Ogawa found higher recurrence in patients who underwent non-curative procedures such as TACE (HR 2.31, 95%CI 1.04 – 5.15).20
Nine studies compared HCC recurrence in DAA-treated (n=947) patients to interferon-treated (n=210) and/or untreated (n=641) patients (Table 2). 22, 24, 30, 32, 34, 37, 41, 42 Five studies reported no significant difference in HCC recurrence between DAA-treated and untreated patients in multivariable analyses, while two studies found significantly lower HCC recurrence among DAA-treated patients. Among the five studies reporting relative risk of recurrence with 95% confidence intervals, DAA-treated patients had a lower pooled recurrence risk than untreated patients (OR 0.55, 95%CI 0.25 – 0.85). All three studies comparing DAA-treated and interferon-treated patients reported no difference in HCC recurrence between the two groups; however, only unadjusted analyses were reported.
HCC Recurrence Patterns
Patterns of HCC recurrence including tumor burden and HCC-directed treatment after DAA therapy are detailed in Table 3. The majority of patients (77.8%, 95%CI 72.9-82.1) with HCC recurrence across studies were detected at an early stage, and two-thirds of those found at an early stage (64.7%, 95%CI 57.9-71.2) received curative treatment with liver transplant, resection or ablation. Only three studies described response to HCC therapy.18, 21, 37 Reig and colleagues reported that 32% of patients had tumor progression within 6 months of recurrence,21 whereas Singal et al reported 17% had progressive disease after HCC-directed therapy37 and Ikeda et al observed rapid tumor progression in only 5% of patients.18
Table 3.
Patterns of HCC recurrence after DAA therapy
| Author Year | Number with HCC recurrence | Tumor burden at time of recurrence | HCC-directed Treatment |
|---|---|---|---|
| Bielen 201716 | 6 | 33% BCLC A, 33% BCLC B, 17% BCLC C, 17% unknown | 17% resection 33% TACE and 50% supportive care |
| Cabibbo 201717 | 29 | 62% BCLC A, 21% BCLC B, 7% BCLC C, 10% BCLC D | 38% resection or ablation 45% TACE and 7% systemic therapy |
| Reig 201721 | 21 | 90% BCLC A, 10% BCLC B | 38% transplant, resection or ablation 19% TACE and 29% systemic therapy |
| Conti 201623 | 17 | 56% unifocal and 70% diameter <2 cm | Not reported |
| Rinaldi 201625 | 1 | 100% BCLC A | Patient treated with ablation |
| Granata 201730 | 18 | 76% monofocal, 12% multifocal, 12% advanced | Not reported |
| Yasui 201732 | 6 | 100% BCLC A | Not reported |
| Minami 201733 | 78 | 86% BCLC A, 13% BCLC B, 13% BCLC C | 85% resection or ablation 14% TACE and 1% supportive care |
| Ohki 201734 | 7 | 100% BCLC A | 86% ablation 14% TACE |
| Sangiovanni 201735 | 33 | 79% BCLC A, 6% BCLC B, 6% BCLC C, 9% unknown | 52% transplant, resection or ablation 42% TACE and 6% systemic therapy |
| Singal 201737 | 95 | 74% within Milan Criteria | 27% transplant, resection or ablation 54% TACE and 7% systemic therapy |
| Tokoro 201638 | 13 | Diameter 1.2 cm (0.7 – 2.0) | Not reported |
| Tsuda 201639 | 9 | 56% unifocal, 22% 2-3 nodules, 22% >3 nodules | Not reported |
BCLC – Barcelona Clinic Liver Cancer; HCC – hepatocellular carcinoma
Quality Assessment
Quality assessment of included studies is provided in Table 4. Most studies had appropriate representativeness of the cohort and exposure ascertainment. There was heterogeneity in cohorts with several studies including patients with non-early stage HCC, high AFP levels, multiple prior HCC recurrences, or treatment with locoregional therapies who are all at higher risk of HCC recurrence than their counterparts. There was also heterogeneity in the length of time between HCC complete response and DAA initiation, which is associated with risk of HCC recurrence. Whereas some studies required patients to have HCC complete response for at least 3-6 months, other studies included patients who were treated immediately after HCC-directed treatment. Most studies ascertained HCC recurrence through medical records but only 7 excluded patients with any suspicious nodules prior to DAA initiation and only 5 included prospective surveillance protocols. There were an additional 11 studies that explicitly stated they required documented absence of HCC (but not suspicious nodules) on imaging prior to DAA initiation, and 8 retrospective studies reported a recommended surveillance protocol after HCC complete response but were regarded as medium risk of bias given potential for underuse of surveillance in clinical practice. Seven studies did not explicitly state they excluded HCC prior to DAA initiation and 12 studies did not describe their surveillance protocol after HCC complete response so were regarded as having a high risk of bias. Most studies had short follow-up periods less than one year as well as high (>10%) or unknown loss to follow-up. Although comparative studies performed multivariable or propensity-score matched analyses, there were remaining issues with confounders including degree of liver dysfunction and immortal time bias.
Table 4.
Quality assessment of studies by checklist
| Author Year | Representative cohort | Ascertainment of exposure | Outcome not present at start | Assessment of outcome | Sufficient follow-up period | Adequacy of follow-up |
|---|---|---|---|---|---|---|
| Bielen 201716 | * | * | High | High | * | High |
| Cabibbo 201717 | * | * | * | * | High | Unknown |
| Ikeda 201718 | * | * | Medium | Medium | * | * |
| Ngata 201719 | * | * | High | Medium | * | Unknown |
| Ogawa 201720 | * | * | * | * | * | Unknown |
| Reig 201721 | * | * | * | Medium | High | * |
| Virlogeux 201722 | * | * | * | Medium | * | High |
| Conti 201623 | * | * | Medium | Medium | High | * |
| ANRS 201624 | * | * | Medium | High | * | Unknown |
| ANRS 201624 | * | * | Medium | High | * | Unknown |
| Rinaldi 201625 | * | * | * | Medium | High | Unknown |
| Zavaglia 201726 | * | * | * | High | High | High |
| Zeng 201627 | * | * | High | Medium | * | * |
| Torres 201628 | * | * | Medium | * | High | Unknown |
| Gheoghe 201729 | * | * | Medium | High | High | Unknown |
| Granata 201730 | * | * | * | * | * | Unknown |
| Kolly 201731 | * | * | High | High | * | Unknown |
| Yasui 201732 | High | * | High | High | High | Unknown |
| Minami 201733 | * | * | Medium | High | * | High |
| Ohki 201734 | * | * | High | High | * | Unknown |
| Sangiovanni 201735 | * | * | Medium | * | High | High |
| Singal 201737 | * | * | Medium | High | * | Unknown |
| Urabe 201736 | * | * | Medium | High | High | Unknown |
| Tokoro 201638 | * | * | High | Medium | * | Unknown |
| Tsuda 201639 | * | * | Medium | High | High | Unknown |
High = high risk of bias; Medium = intermediate risk of bias;
= low risk of bias
DISCUSSION
There is ongoing uncertainty about the potential risks and benefits of DAA therapy in patients with a history of HCC. In this comprehensive systematic review, we found a pooled estimate of 24.4% for HCC recurrence after DAA therapy, although this point estimate must be interpreted in the context of its wide confidence intervals, clinical heterogeneity within and between studies, and methodological limitations of current data. While there are few comparative studies with interferon-treated or untreated patients, these data suggest DAA-treated patients have similar if not lower recurrence than interferon-treated or untreated patients. Most patients with HCC recurrence across studies were found at an early stage and underwent curative treatments, suggesting recurrence following DAA therapy is not aggressive; however, few data characterized treatment response or post-recurrence prognosis. Current studies have notable limitations including risk for misclassification of HCC complete response prior to DAA initiation and ascertainment bias, precluding definitive conclusions about the risk of HCC recurrence and highlighting the need for higher quality data.
Providers and patients must weigh the potential for increased early HCC recurrence against demonstrated long-term benefits of DAA therapy. Patients treated for HCC are at risk for both early and late recurrence, with early recurrence typically related to intrinsic tumor factors, while late recurrence is associated with cirrhosis-related factors such as active viremia and degree of liver dysfunction.43,44 It has been hypothesized that rapid decrease in HCV viral load with DAAs results in decreased immune surveillance of microscopic HCC tumor clones and hence an increased risk of early HCC recurrence.21,45 Conversely, as seen with interferon-based therapies, successful treatment with DAAs can result in fibrosis regression and improvements in portal hypertension and liver dysfunction, shown to be the major driver of death in patients with HCC complete response and untreated HCV infection.46 Further, DAA therapy may reduce risk of late HCC recurrence, by decreasing HCV viremia and improving liver function.10, 47 Unfortunately, current studies are limited by short durations of follow-up, precluding adequate characterization of early vs. late recurrence, highlighting a need for studies with longer durations of follow-up.
Identifying predictors for increased early recurrence may identify subgroups in whom DAA therapy should be avoided. Few predictors for early recurrence were consistently reported except history of prior HCC recurrence and the interval between HCC complete response and DAA initiation, with shorter intervals being associated with higher risk of recurrence. Delaying DAA therapy may allow for longer duration of immune surveillance of existing microscopic HCC clones. Delaying DAA treatment can also create a longer time to verify HCC complete response, thereby minimizing the chance of misclassification bias. The sensitivity of one-time CT or MRI for small HCC lesions is low, with sensitivities of only 40-50% for subcentimeter lesions and 60-70% for 1-2 cm lesions.48 Given the lack of urgency for HCV therapy after HCC complete response, it appears prudent to wait at least 6 months after HCC complete response to initiate DAA therapy, which would typically allow for 2-3 interim multi-phase CT or MRI scans to confirm durable HCC response.
Notably, most studies to date are single-arm, retrospective cohort studies with clinical heterogeneity in tumor burden, HCC treatments leading to complete response, and follow-up periods. When interpreting the proportion of patients with HCC recurrence in these single arm studies, it is important to consider the natural history of HCC after complete response, in which many patients will have HCC recurrence independent of DAA therapy. HCC recurrence after complete response can vary substantially depending on which HCC-directed treatment received. While surgical resection and local ablative therapies are considered curative, recurrence rates approach 25-35% within the first year and 50-60% within 2 years.44, 49, 50 Further, up to 25-50% of patients in some studies received TACE, which is typically not curative and associated with a high risk of recurrence.51 Further, patients with high-risk tumor characteristics (e.g. multifocal HCC or elevated AFP) have higher recurrence rates than their counterparts; however specific data regarding these risk factors and subgroup analyses were not reported in many studies.
In addition to concerns about clinical heterogeneity within study populations, we noted potential for misclassification and ascertainment biases. Several studies did not exclude patients with suspicious nodules prior to DAA treatment or included patients with HCC complete response for short periods of time. Therefore, it is likely some patients already had recurrent HCC at the time of DAA initiation, which would lead to an overestimation of post-DAA HCC recurrence. On the other hand, most studies did not include a standardized surveillance protocol to assess for HCC recurrence. Prior studies have demonstrated underuse of HCC surveillance in patients with cirrhosis and the same issue may plague post-treatment patients, leading to an underestimation of post-DAA recurrence.52, 53 These limitations highlight the need for high-quality prospective studies with strict inclusion criteria and a standardized surveillance protocol. Although comparative studies suggest recurrence rates among DAA-treated patients may be similar to interferon-treated and untreated patients, these analyses are limited by potential for confounders such as degree liver dysfunction. These studies can also be limited by immortal time bias, as patients with early recurrence would typically not receive DAA treatment and therefore would be over-represented in the untreated group. Given the high prevalence of limitations in study design, we propose minimum criteria for future studies in this area (Table 5).
Table 5.
Minimum reporting recommendations for future studies
| 1. Presence of cirrhosis and Child Pugh score at baseline |
| 2. Baseline tumor burden and median AFP prior to HCC treatment |
| 3. Number of prior HCC recurrences |
| 4. Types of HCC treatment leading to complete response |
| 5. Confirmation of HCC complete response immediately preceding DAA therapy |
| 6. Time from HCC treatment to DAA initiation |
| 7. Time from last HCC complete response assessment to HCC recurrence |
| 8. HCC surveillance protocol and adherence |
| 9. Tumor burden at time of recurrence, treatment of recurrence, and response to treatment |
| 10. Predictors of HCC recurrence after DAA therapy |
A prior systematic review comparing HCC occurrence and recurrence following interferon-and DAA-based therapy only included 10 studies characterizing recurrence after DAA therapy,54 whereas we identified 26 studies – including several recent prospective studies. Waziry and colleagues also included cohorts evaluating post-transplant recurrence, but we excluded these studies given post-transplant recurrence could be driven by a different mechanism. Finally, our study also characterized patterns of HCC recurrence and highlighted limitations of available data, which informed our recommendations for minimum reporting criteria.
Although our study provides a comprehensive summary of current literature, limitations of available data hindered our ability to make strong conclusions about the potential association between DAA therapy and HCC recurrence. In fact, only three studies did not have high risk of bias for at least one category of the Newcastle-Ottawa quality assessment scale. There was also significant heterogeneity between studies, including different patient selection criteria, timing of DAA therapy, and durations of follow-up. Second, an individual-level meta-analysis would be more powerful, but we did not have patient-level data to perform subgroup analyses by degree of tumor burden, type of HCC treatment, or time between HCC complete response and DAA initiation. Given most data were obtained from small single-center cohorts, included studies may suffer from reporting bias. Factors associated with recurrence were based on descriptive review of included studies, with differential reporting and meta-analysis of these factors was not possible. In addition, we excluded non-English studies due to practical considerations, which may further bias the results.
In summary, there are conflicting data about the potential for increased HCC recurrence after DAA therapy; however most studies have methodological limitations. Ongoing multi-center retrospective and prospective studies should provide some insight into this controversial issue; we highlighted some suggested minimum reporting requirements to avoid the pitfalls of current studies. While awaiting these data, it is likely prudent to wait at least 6 months after HCC complete response before initiating DAA therapy.
Supplementary Material
Supplemental Figure. Map of literature search and study selection
Acknowledgments
Financial support: This work was conducted with support from NCI RO1 CA212008 and RO1 CA222900. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- AFP
alpha fetoprotein
- BCLC
Barcelona Clinic Liver Cancer
- CT
computed tomography
- DAA
Direct acting antiviral
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- MRI
magnetic resonance imaging
- RESICT
response evaluation criteria in solid tumors
- TACE
transarterial chemoembolization
Footnotes
DR NEEHAR PARIKH (Orcid ID : 0000-0002-5874-9933)
DR AMIT G SINGAL (Orcid ID : 0000-0002-1172-3971)
Conflicts of Interests: Amit G. Singal is on the speakers’ bureau for Gilead and has a research grant from Abbvie.
Author Contributions
Neema Saraiya was involved in acquisition of data, interpretation of data, and critical revision of manuscript for important intellectual content.
Adam Yopp was involved in interpretation of data and critical revision of manuscript for important intellectual content.
Nicole Rich was involved in interpretation of data and critical revision of manuscript for important intellectual content.
Mobolaji Odewole was involved in critical revision of manuscript for important intellectual content.
Neehar Parikh was involved in interpretation of data and critical revision of manuscript for important intellectual content.
Amit G. Singal was involved in study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of manuscript for important intellectual content, and study supervision. He is the guarantor of the article.
All authors approved the final version of the manuscript.
References
- 1.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–1273 e1. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reddy SK, Steel JL, Chen HW, DeMateo DJ, Cardinal J, Behari J, Humar A, Marsh JW, Geller DA, Tsung A. Outcomes of curative treatment for hepatocellular cancer in nonalcoholic steatohepatitis versus hepatitis C and alcoholic liver disease. Hepatology. 2012;55:1809–19. doi: 10.1002/hep.25536. [DOI] [PubMed] [Google Scholar]
- 3.Samuel M, Chow PK, Chan Shih-Yen E, Machin D, Soo KC. Neoadjuvant and adjuvant therapy for surgical resection of hepatocellular carcinoma. Cochrane Database Syst Rev. 2009:CD001199. doi: 10.1002/14651858.CD001199.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singal AG, Volk ML, Jensen D, Di Bisceglie AM, Schoenfeld PS. A sustained viral response is associated with reduced liver-related morbidity and mortality in patients with hepatitis C virus. Clin Gastroenterol Hepatol. 2010;8:280–8. 288 e1. doi: 10.1016/j.cgh.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 5.Singal AG, Marrero JA. Interferon therapy and prevention of hepatocellular carcinoma in hepatitis C. Dig Dis Sci. 2012;57:832–4. doi: 10.1007/s10620-012-2069-8. [DOI] [PubMed] [Google Scholar]
- 6.Liang TJ, Ghany MG. Current and future therapies for hepatitis C virus infection. N Engl J Med. 2013;368:1907–17. doi: 10.1056/NEJMra1213651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ioannou GN, Green PK, Berry K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J Hepatol. 2017 doi: 10.1016/j.jhep.2017.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of Hepatocellular Cancer in HCV Patients Treated With Direct-Acting Antiviral Agents. Gastroenterology. 2017;153:996–1005 e1. doi: 10.1053/j.gastro.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Reig M, Boix L, Bruix J. The impact of direct antiviral agents on the development and recurrence of hepatocellular carcinoma. Liver Int. 2017;37(Suppl 1):136–139. doi: 10.1111/liv.13321. [DOI] [PubMed] [Google Scholar]
- 10.Foster GR, Irving WL, Cheung MC, Walker AJ, Hudson BE, Verma S, McLauchlan J, Mutimer DJ, Brown A, Gelson WT, MacDonald DC, Agarwal K. Impact of direct acting antiviral therapy in patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;64:1224–31. doi: 10.1016/j.jhep.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 11.Trotter JF. Pro: Direct-acting antivirals are associated with occurrence and recurrence of hepatocellular carcinoma. Liver Transpl. 2017;23:1593–1595. doi: 10.1002/lt.24960. [DOI] [PubMed] [Google Scholar]
- 12.Mehta N, Yao FY. Con: Treating hepatitis C virus With direct-acting antivirals: Fear not the perceived threat of hepatocellular carcinoma. Liver Transpl. 2017;23:1596–1600. doi: 10.1002/lt.24959. [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9, W64. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 14.Wells GA, Shea B, O’Connell D, Peterson J. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 3rd Symposium on Systematic Reviews Beyond the Basics. 2000 [Google Scholar]
- 15.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bielen R, Moreno C, Van Vlierberghe H, Bourgeois S, Mulkay JP, Vanwolleghem T, Verlinden W, Brixco C, Decaestecker J, de Galocsy C, Janssens F, Van Overbeke L, Van Steenkiste C, D’Heygere F, Cool M, Wuyckens K, Nevens F, Robaeys G. The risk of early occurrence and recurrence of hepatocellular carcinoma in hepatitis C-infected patients treated with direct-acting antivirals with and without pegylated interferon: A Belgian experience. J Viral Hepat. 2017;24:976–981. doi: 10.1111/jvh.12726. [DOI] [PubMed] [Google Scholar]
- 17.Cabibbo G, Petta S, Calvaruso V, Cacciola I, Cannavo MR, Madonia S, Distefano M, Larocca L, Prestileo T, Tine F, Bertino G, Giannitrapani L, Benanti F, Licata A, Scalisi I, Mazzola G, Cartabellotta F, Alessi N, Barbara M, Russello M, Scifo G, Squadrito G, Raimondo G, Craxi A, Di Marco V, Camma C. Is early recurrence of hepatocellular carcinoma in HCV cirrhotic patients affected by treatment with direct-acting antivirals? A prospective multicentre study. Aliment Pharmacol Ther. 2017;46:688–695. doi: 10.1111/apt.14256. [DOI] [PubMed] [Google Scholar]
- 18.Ikeda K, Kawamura Y, Kobayashi M, Kominami Y, Fujiyama S, Sezaki H, Hosaka T, Akuta N, Saitoh S, Suzuki F, Suzuki Y, Arase Y, Kumada H. Direct-Acting Antivirals Decreased Tumor Recurrence After Initial Treatment of Hepatitis C Virus-Related Hepatocellular Carcinoma. Dig Dis Sci. 2017;62:2932–2942. doi: 10.1007/s10620-017-4739-z. [DOI] [PubMed] [Google Scholar]
- 19.Nagata H, Nakagawa M, Asahina Y, Sato A, Asano Y, Tsunoda T, Miyoshi M, Kaneko S, Otani S, Kawai-Kitahata F, Murakawa M, Nitta S, Itsui Y, Azuma S, Kakinuma S, Nouchi T, Sakai H, Tomita M, Watanabe M. Effect of interferon-based and -free therapy on early occurrence and recurrence of hepatocellular carcinoma in chronic hepatitis C. J Hepatol. 2017;67:933–939. doi: 10.1016/j.jhep.2017.05.028. [DOI] [PubMed] [Google Scholar]
- 20.Ogawa E, Furusyo N, Nomura H, Dohmen K, Higashi N, Takahashi K, Kawano A, Azuma K, Satoh T, Nakamuta M, Koyanagi T, Kato M, Shimoda S, Kajiwara E, Hayashi J. Short-term risk of hepatocellular carcinoma after hepatitis C virus eradication following direct-acting anti-viral treatment. Aliment Pharmacol Ther. 2017 doi: 10.1111/apt.14380. [DOI] [PubMed] [Google Scholar]
- 21.Reig M, Boix L, Marino Z, Torres F, Forns X, Bruix J. Liver Cancer Emergence Associated with Antiviral Treatment: An Immune Surveillance Failure? Semin Liver Dis. 2017;37:109–118. doi: 10.1055/s-0037-1601349. [DOI] [PubMed] [Google Scholar]
- 22.Virlogeux V, Pradat P, Hartig-Lavie K, Bailly F, Maynard M, Ouziel G, Poinsot D, Lebosse F, Ecochard M, Radenne S, Benmakhlouf S, Koffi J, Lack P, Scholtes C, Uhres AC, Ducerf C, Mabrut JY, Rode A, Levrero M, Combet C, Merle P, Zoulim F. Direct-acting antiviral therapy decreases hepatocellular carcinoma recurrence rate in cirrhotic patients with chronic hepatitis C. Liver Int. 2017;37:1122–1127. doi: 10.1111/liv.13456. [DOI] [PubMed] [Google Scholar]
- 23.Conti F, Buonfiglioli F, Scuteri A, Crespi C, Bolondi L, Caraceni P, Foschi FG, Lenzi M, Mazzella G, Verucchi G, Andreone P, Brillanti S. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol. 2016;65:727–33. doi: 10.1016/j.jhep.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 24.ANRS Collaborative Study Group. Lack of evidence of an effect of direct-acting antivirals on the recurrence of hepatocellular carcinoma: Data from three ANRS cohorts. J Hepatol. 2016;65:734–40. doi: 10.1016/j.jhep.2016.05.045. [DOI] [PubMed] [Google Scholar]
- 25.Rinaldi L, Di Francia R, Coppola N, Guerrera B, Imparato M, Monari C, Nevola R, Rosato V. Hepatocellular carcinoma in HCV cirrhosis after viral clearance with direct acting antiviral therapy: preliminary evidence and possible meanings. World Cancer Research Journal. 2016;3:e748. [Google Scholar]
- 26.Zavaglia C, Okolicsanyi S, Cesarini L, Mazzarelli C, Pontecorvi V, Ciaccio A, Strazzabosco M, Belli L. Is the risk of neoplastic recurrence increased after prescribing direct-acting antivirals for HCV patients whose HCV was previously cured? Journal of Hepatology. 2017;66 doi: 10.1016/j.jhep.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 27.Zeng Q, Li Z, Liang H, Xu G, Li C, Zhang D, Li W, Sun C, Wang F, Yu Z. Unexpected high incidence of hepatocellular carcinoma in patients with hepatitis C in the era of DAAs: too alarming? Journal of Hepatology. 2016;65:1068–9. doi: 10.1016/j.jhep.2016.07.029. [DOI] [PubMed] [Google Scholar]
- 28.Torres HA, Vauthey JN, Economides MP, Mahale P, Kaseb A. Hepatocellular carcinoma recurrence after treatment with direct-acting antivirals: First, do no harm by withdrawing treatment. J Hepatol. 2016;65:862–4. doi: 10.1016/j.jhep.2016.05.034. [DOI] [PubMed] [Google Scholar]
- 29.Gheorghe L, Iacob M, Grasu M, Dumitru R, Iacob R, Lupescu I, Gheorghe C. Development of de novo and recurrent hepatocellular carcinoma in a Romanian cohort of compensated HCV genotype 1b liver cirrhosis with SVR after 3D and ribavirin therapy. Journal of Hepatology. 2017;2007:S445. [Google Scholar]
- 30.Granata R, Di Costanzo GG, Zamparelli MS, Guarino M, Cordone G, Tortora R. Hepatocellular carcinoma recurrence rate in HCV infected patients treated with direct antiviral agents. A single center experience. Journal of Hepatology. 2017;66:S717. [Google Scholar]
- 31.Kolly P, Waidmann O, Vermehren J, Moreno C, Berg T, Semela D, Zeuzem S, Dufour JF. Hepatocellular carcinoma recurrence after direct antiviral agent treatment: a European multicentric study. Journal of Hepatology. 2017;66:S621. doi: 10.1016/j.jhep.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Yasui Y, Kurosaki M, Wang W, Okada M, Kubota Y, Goto T, Komiyama Y, Higuchi M. Direct acting antivirals did not increase early recurrences after curative treatment of HCV related hepatocellular carcinoma in comparison with IFN-based treatment. Journal of Hepatology. 2017;66:S748. [Google Scholar]
- 33.Minami T, Tateishi R, Wake T, Nishibatake M, Nakagomi R, Sato M, Uchino K, Enooku K. Hepatocellular carcinoma recurrence after curative treatments in patients with chronic hepatitis C who underwent direct-acting antiviral therapy. Hepatology. 2017;66:760A–761A. [Google Scholar]
- 34.Ohki T, Yoshida H, Goto E, Sato T, Imamura J, Akamatsu M, Sato S, Obi S, Koike Y. Direct acting antiviral therapy after curative treatment of hepatocellular carcinoma improved recurrence free survival rate. Hepatology. 2017;66:759A. [Google Scholar]
- 35.Sangiovanni A, Alimenti E, Biganzoli E, Borgio G, Brunacci M, Brunetto M, D’Ambrosio R, Fargion S. IFN-free DAA treatment of cirrhotic HCV patients with or without history of HCC: a multi-center prospective trial in Italy. Hepatology. 2017;66:734A–735A. [Google Scholar]
- 36.Urabe A, Sakamori R, Tatsumi T, Yamada R, Tahata Y, Imai Y, Yamada A. Effects of IFN-free therapy for hepatitis C virus after hepatocellular carcinoma treatment on early HCC recurrence compared to IFN-based therapy. Hepatology. 2017;66:843A–844A. [Google Scholar]
- 37.Singal AG, Hoteit MA, John B, Kulik L, Eswaran S, Jou JH, Tran T, Wong R. Direct acting antiviral therapy is associated with shorter time to HCC recurrence but not increased risk of recurrence. Hepatology. 2017;66:729A. [Google Scholar]
- 38.Tokoro M, Seike M, Iwao M, Arakawa M, Endo M, Oribe J, Honda K, Murakami K. The features of hepatocellular carcinoma after IFN free treatment. Hepatology. 2016;64:671A. [Google Scholar]
- 39.Tsuda Y, Nishikawa T, Nakmura K, Yokohama K, Ohama H, Sujishi T, Tsuchimoto T. The effect of interferon-free therapy on tumor recurrence in HCV patients with treatment history of hepatocellular carcinoma. Hepatology. 2016;64:666A–667A. [Google Scholar]
- 40.Minami T, Tateishi R, Nakagomi R, Fujiwara N, Sato M, Enooku K, Nakagawa H, Asaoka Y, Kondo Y, Shiina S, Koike K. The impact of direct-acting antivirals on early tumor recurrence after radiofrequency ablation in hepatitis C-related hepatocellular carcinoma. J Hepatol. 2016;65:1272–1273. doi: 10.1016/j.jhep.2016.07.043. [DOI] [PubMed] [Google Scholar]
- 41.Joko K, Mashiba T, Kurosaki M, Ochi H, Izumi N. Does Interfeon-free direct-acting antiviral therapy for hepatitis C lead to unexpected recurrences of hepatocellular carcinoma? Real-world nation-wide multicenter study by the Japanese Red Cross Hospital Liver Study Group. Hepatology. 2017;66:742A. doi: 10.1371/journal.pone.0194704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanaka S, Tamori A, Takemura S, Shinkawa H, Ito T, Kawada N, Kubo S. Effects of an adjuvant direct-acting antiviral drug therapy-induced sustained virological response after hepatic resection hepatitis C virus-related solitary hepatocellular carcinoma. Hepatology. 2017;66:747A–748A. [Google Scholar]
- 43.Portolani N, Coniglio A, Ghidoni S, Giovanelli M, Benetti A, Tiberio GA, Giulini SM. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg. 2006;243:229–35. doi: 10.1097/01.sla.0000197706.21803.a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poon RT, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer. 2000;89:500–7. [PubMed] [Google Scholar]
- 45.Nault JC, Colombo M. Hepatocellular carcinoma and direct acting antiviral treatments: Controversy after the revolution. J Hepatol. 2016;65:663–5. doi: 10.1016/j.jhep.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 46.Cabibbo G, Petta S, Barbara M, Attardo S, Bucci L, Farinati F, Giannini EG, Negrini G, Ciccarese F, Rapaccini GL, Di Marco M, Caturelli E, Zoli M, Borzio F, Sacco R, Virdone R, Marra F, Mega A, Morisco F, Benvegnu L, Gasbarrini A, Svegliati-Baroni G, Foschi FG, Olivani A, Masotto A, Nardone G, Colecchia A, Persico M, Craxi A, Trevisani F, Camma C. Hepatic decompensation is the major driver of death in HCV-infected cirrhotic patients with successfully treated early hepatocellular carcinoma. J Hepatol. 2017;67:65–71. doi: 10.1016/j.jhep.2017.01.033. [DOI] [PubMed] [Google Scholar]
- 47.Deterding K, Honer Zu, Siederdissen C, Port K, Solbach P, Sollik L, Kirschner J, Mix C, Cornberg J, Worzala D, Mix H, Manns MP, Cornberg M, Wedemeyer H. Improvement of liver function parameters in advanced HCV-associated liver cirrhosis by IFN-free antiviral therapies. Aliment Pharmacol Ther. 2015;42:889–901. doi: 10.1111/apt.13343. [DOI] [PubMed] [Google Scholar]
- 48.Roberts LR, Sirlin CB, Zaiem F, Almasri J, Prokop LJ, Heimbach JK, Murad MH, Mohammed K. Imaging for the diagnosis of hepatocellular carcinoma: A systematic review and meta-analysis. Hepatology. 2017 doi: 10.1002/hep.29487. [DOI] [PubMed] [Google Scholar]
- 49.Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg. 2015;261:947–55. doi: 10.1097/SLA.0000000000000710. [DOI] [PubMed] [Google Scholar]
- 50.Shiina S, Tateishi R, Arano T, Uchino K, Enooku K, Nakagawa H, Asaoka Y, Sato T, Masuzaki R, Kondo Y, Goto T, Yoshida H, Omata M, Koike K. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol. 2012;107:569–77. doi: 10.1038/ajg.2011.425. quiz 578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jeong SO, Kim EB, Jeong SW, Jang JY, Lee SH, Kim SG, Cha SW, Kim YS, Cho YD, Kim HS, Kim BS, Kim YJ, Goo DE, Park SY. Predictive Factors for Complete Response and Recurrence after Transarterial Chemoembolization in Hepatocellular Carcinoma. Gut Liver. 2017;11:409–416. doi: 10.5009/gnl16001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singal AG, Tiro J, Li X, Adams-Huet B, Chubak J. Hepatocellular Carcinoma Surveillance Among Patients With Cirrhosis in a Population-based Integrated Health Care Delivery System. J Clin Gastroenterol. 2017;51:650–655. doi: 10.1097/MCG.0000000000000708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singal AG, Yopp A, C SS, Packer M, Lee WM, Tiro JA. Utilization of hepatocellular carcinoma surveillance among American patients: a systematic review. J Gen Intern Med. 2012;27:861–7. doi: 10.1007/s11606-011-1952-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Waziry R, Hajarizadeh B, Grebely J, Amin J, Law M, Danta M, George J, Dore GJ. Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: A systematic review, meta-analyses, and meta-regression. J Hepatol. 2017;67:1204–1212. doi: 10.1016/j.jhep.2017.07.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure. Map of literature search and study selection


