Abstract
Objective
To determine the percentage of children with nonalcoholic fatty liver disease (NAFLD) in whom intervention for low density lipoprotein-cholesterol (LDL-C) or triglycerides (TG) was indicated based upon National Heart, Lung, and Blood Institute (NHLBI) guidelines.
Study design
Multi-center, longitudinal cohort study of children with NAFLD enrolled in the NIDDK Nonalcoholic Steatohepatitis Clinical Research Network. Fasting lipid profiles were obtained at diagnosis. Standardized dietary recommendations were provided. After 1 year, lipid profiles were repeated and interpreted according to NHLBI Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction. Main outcomes were meeting criteria for clinically actionable dyslipidemia at baseline, and either achieving lipid goal at follow up or meeting criteria for ongoing intervention.
Results
There were 585 participants, mean age 12.8 years. Prevalence of children warranting intervention for LDL-C at baseline was 14%. After 1 year of recommended dietary changes, 51% achieved goal LDL-C, 27% qualified for enhanced dietary and lifestyle modifications, and 22% met criteria for pharmacologic intervention. Elevated TG were more prevalent, with 51% meeting criteria for intervention. At 1 year, 25% achieved goal TG with diet and lifestyle changes, 38% met criteria for advanced dietary modifications, and 37% qualified for antihyperlipidemic medications.
Conclusion
Over half of children with NAFLD met intervention thresholds for dyslipidemia. Based on the burden of clinically relevant dyslipidemia, lipid screening in children with NAFLD is warranted. Clinicians caring for children with NAFLD should be trained in lipid management.
Keywords: Pediatric, NAFLD, Dyslipidemia, Cardiovascular, Diet, Statin
Atherosclerotic coronary artery disease is the primary cause of mortality in adults in the United States, and is responsible for nearly 400,000 deaths annually.1 The process of atherosclerosis begins in childhood and is associated with dyslipidemia.2,3 Therefore, both the American Academy of Pediatrics and the American Heart Association recommend identifying children at risk for premature cardiovascular disease. In 2011, the revised National Heart, Lung, and Blood Institute (NHLBI) Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction4 reinforced the importance of lipid screening in childhood and provided a pathway for lifestyle and pharmacologic interventions. In addition, they identified subgroups requiring specialized attention but did not address children with nonalcoholic fatty liver disease (NAFLD).
NAFLD is an emerging clinical problem in children and adolescents. With an estimated 9.6% prevalence, NAFLD is the most common cause of chronic liver disease in children,5 and the leading cause of liver transplantation in young adults.6,7,8 Cardiovascular disease is a serious extra-hepatic comorbidity in patients with NAFLD, and is the most common cause of death in adults with this condition.9,10,11 In children, NAFLD is also associated with multiple cardiovascular risk factors such as obesity, insulin resistance, hypertension, and dyslipidemia.12,13,14 Recent pediatric NAFLD guidelines include monitoring of lipids.15 However, the treatment of hypercholesterolemia and/or hypertriglyceridemia in children with NAFLD has not been addressed. Thus it is unknown how many children with NAFLD require intervention for dyslipidemia. Moreover, there are no longitudinal data on lipids in children with NAFLD in response to intervention. Therefore, the primary study aim was to apply the NHLBI guidelines to children with NAFLD. Specific goals were to determine the frequency of clinical intervention needed for elevated plasma low density lipoprotein (LDL-C) or triglycerides (TG) and the outcomes on lipid profiles following one year of dietary standard of care.
METHODS
The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) NASH Clinical Research Network (NASH CRN) enrolled children in longitudinal cohort studies (Database and Database 2; NCT01061684) and clinical trials (TONIC; NCT00063635 and CyNCh; NCT01529268). These studies have been described16,17 and were performed at each participating pediatric clinical center across the United States (see appendix). The studies were approved by the institutional review boards at the participating institutions. Written, informed consent was obtained from a parent or guardian for all participants, and written assent was obtained from children. For this analysis, we included children who were ages 9–18 years with biopsy-confirmed NAFLD. Those without weight, height, blood pressure, or fasting lipid panels measured at the initial study visit were excluded from this analysis. Those who were already taking a statin, fibrate, or omega-3 fish oil at the time of enrollment in the study, or those who were subsequently prescribed one of these medications prior to 48 week follow up were excluded from the application of the guidelines as well.
Age and sex were recorded for all participants. The following medical background was obtained in all study participants: presence of diabetes mellitus type I and type II, chronic inflammatory disease (such as juvenile rheumatoid arthritis or lupus), renal disease and dysfunction, HIV status, organ transplant, and the use of any lipid lowering medications in the preceding 6 months.
Physical measurements included height, weight, systolic and diastolic blood pressures. Body mass index (BMI) was calculated as weight (kg) divided by height (m) squared. Blood pressure percentiles were computed according to The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents.18 At the initial visit, fasting laboratory assays included total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and triglycerides (TG). All laboratory studies were repeated at the 48 week follow-up.
A diagnosis of NAFLD was based on liver histology with at least 5% of hepatocytes containing macrovesicular fat, and exclusion of other causes of liver disease based upon clinical history, laboratory studies, and histology. Biopsy specimens were stained with hematoxylin-eosin and Masson’s trichrome stains, and reviewed and scored by the Pathology Committee of the NASH CRN according to the NASH CRN scoring system.19 Liver biopsies were diagnosed as NASH, borderline NASH, or NAFLD without NASH based upon the aggregate presence and degree of the individual features of NAFLD. The diagnosis of NASH, borderline NASH, or NAFLD was made as a consensus agreement of the NASH CRN pathology committee.
NHLBI Guidelines
The 2011 revised NHLBI guidelines were applied to our study cohort. Normal plasma lipid levels in children were defined based upon The National Cholesterol Education Program (NCEP) Expert Panel on Cholesterol levels in Children.20 The NHLBI guidelines provide a separate decision-tree for LDL-C and TG in order to determine the specific intervention required, based upon the degree of lipid derangement (Figures 1 and 2). By following the NHLBI algorithms, children who met thresholds for clinically actionable dyslipidemia were identified at baseline as being in the Target LDL-C or the Target TG group.
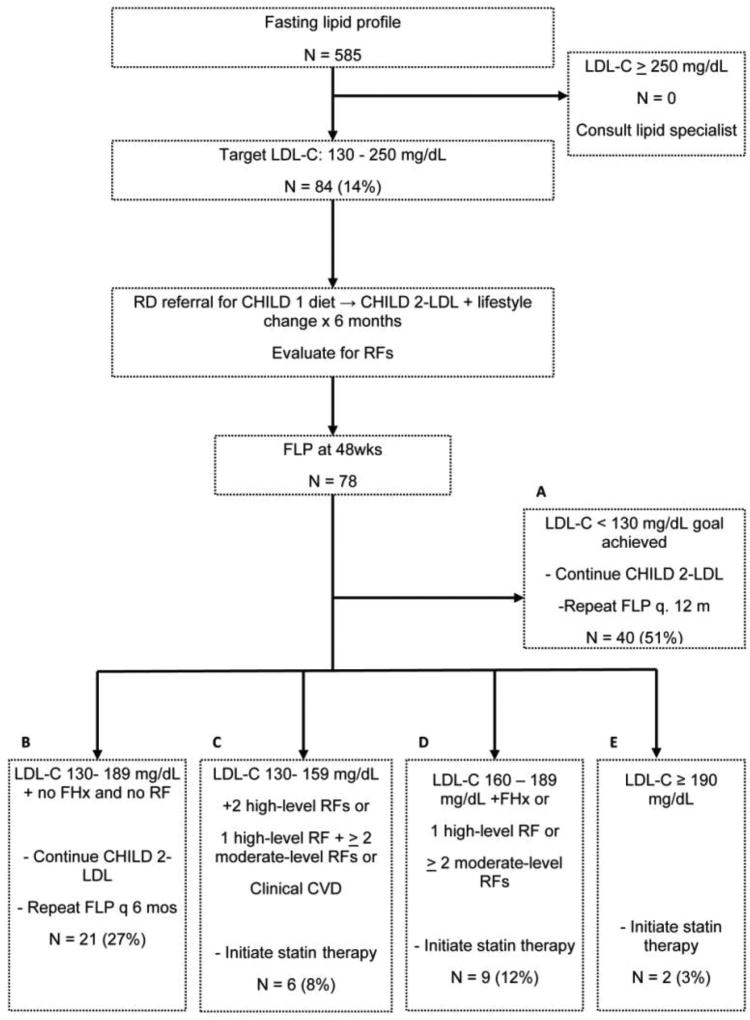
Figure 1.
The NHLBI guideline for elevated LDL-C. This algorithm and the superimposed numbers show the participants that met criteria for actionable LDL-C and after dietary interventions, those at follow up who met criteria for statin therapy, dietary intensification, or those that met goals.
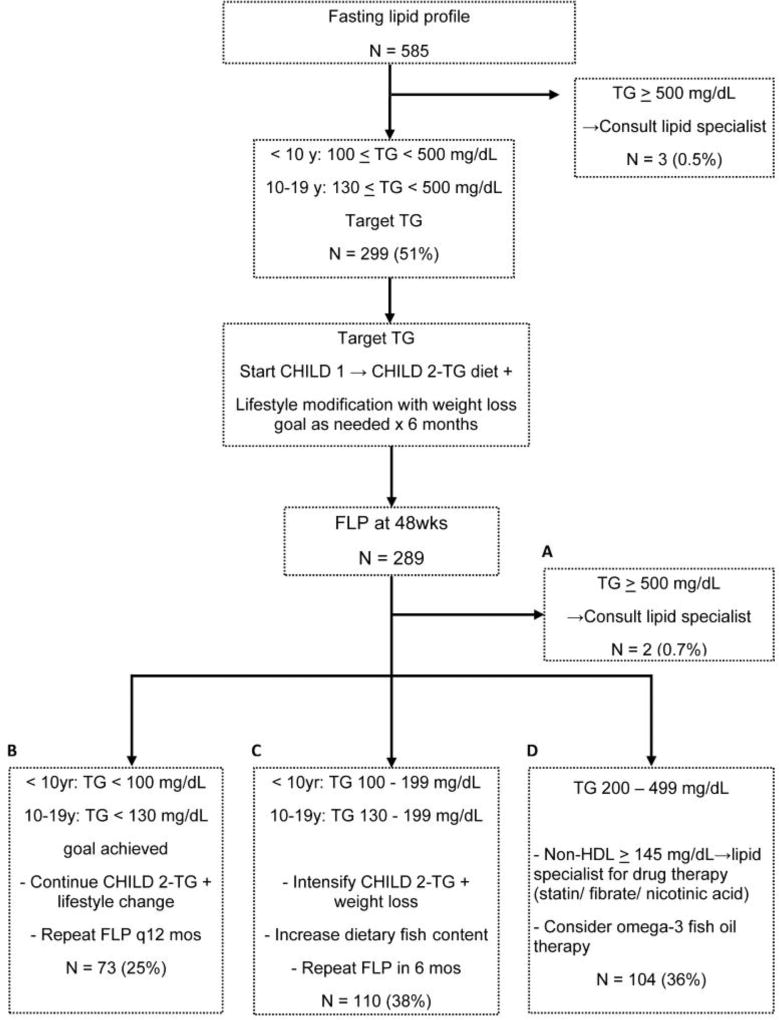
Figure 2.
The NHLBI guideline for elevated TG. This algorithm and the superimposed numbers show the participants that met criteria for actionable TG and after dietary interventions, those at follow up who met criteria for consideration of medications, dietary intensification, or those that met goals.
The Target LDL-C pathway was applied to those with baseline plasma LDL-C >130 mg/dL. After receiving dietary counseling, similar to that of the Cardiovascular Health Integrated Lifestyle Diet (CHILD-1) as described in the algorithm (online), a repeat fasting lipid profile was obtained at one year follow up. Taking into consideration the presence of high and moderate-risk factors (online), combined with the degree to which LDL-C remained elevated, children who did not achieve goal LDL-C would meet criteria for consideration of statin therapy or the intensification of a lipid-specific diet (online). The Target TG pathway was applied to children with baseline plasma TG with age specific cutoffs > 100 mg/dL for children < 10 years, and >130 mg/dL for children 10 years and older. In a similar fashion, children received dietary counseling and fasting lipid profiles were obtained at one year follow up. In children who did not achieve their goal TG, the guidelines provide instructions for initiation of the CHILD 2-TG diet (online) or the consideration of pharmacotherapy.
NASH CRN Standard of Care
The NASH CRN Standards of Care Committee developed a uniform set of practices to be applied by investigators in the NASH CRN to the care of pediatric patients with NAFLD. Recommendations for dietary counseling were based upon guidelines from the USDA; specifically, limiting percent of daily caloric intake from saturated fatty acids to < 10%, replacing these with mono and polyunsaturated fatty acids, and limiting dietary cholesterol intake to <300 mg.21 These recommendations were consistent with those outlined in the CHILD-1 diet in the NHLBI guidelines. However, the individual application of these recommendations and the specifics of caloric and cholesterol intake were not monitored by each participating site.
Statistical analyses
The demographic and clinical characteristics of all children included in this study were reported using standard descriptive statistics. Medians and ranges were reported for continuous variables and counts and percentages were given for categorical variables. Following the NHLBI guidelines for management of dyslipidemia in children, we calculated the frequency of actionable cases as patients flowed through the NHLBI branch-tree algorithm. Two separate algorithms were used to define dyslipidemia targeting LDL-C and TG levels independently. Subjects already on statins, fibrates, or omega-3 fish oil were excluded from analysis.
Study participants were pooled from four distinct NASH CRN protocols. Sensitivity analyses were performed to determine whether lipid levels at enrollment or follow up varied between protocols. R version 3.3.2 software (The R Foundation for Statistical Computing, Vienna, Austria) was used for all analyses.
RESULTS
There were 669 children enrolled in the NASH CRN, of whom 3 were excluded for missing data, 3 were excluded because they did not have NAFLD, and 40 were excluded because they were < 9 years of age. Thus, 623 children with NAFLD ages 9–18 were evaluated in this study; 25 were receiving pharmacologic intervention (statin, n =10; fish oil, n =15). During the course of the investigation, prior to the week 48 evaluation, an additional 13 children were prescribed antihyperlipidemic medications (statin, n=6; fish oil, n=7). Therefore, there were 585 children with NAFLD not taking antihyperlipidemic medications evaluated for their response to standard of care intervention at both baseline and 48 weeks. Demographic and clinical characteristics for these 585 children are shown in Tables 1 and 2. There was a male predominance of 72% (n=419) in our group. The mean age for all subjects was 12.8 (SD 2.4) years, and BMI 32.6 (SD 6.5) kg/m2. In the cohort, the median LDL-C was 97 (19, 212) mg/dL, and the median triglyceride concentration was 133 (18, 726) mg/dL. Using the NHLBI guidelines, 4% (n= 24) of participants met criteria for the target LDL-C pathway alone and 41% (n=239) met criteria for the TG pathway alone. An additional 10% (n= 60) of participants had lipid profiles that qualified them for both target pathways. The remaining 45% (n=262) did not qualify for either pathway.
Table 1.
Baseline demographic, laboratory, and clinical characteristics among children with NAFLD
| Variables N (%) or median (range) |
Target LDL-C only N=24 |
Target TG only N= 239 |
Target both N= 60 |
Not targeted N= 262 |
Overall N= 585 |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 16 (67%) | 164 (69%) | 39 (65%) | 200 (76%) | 419 (72%) |
| Female | 8 (33%) | 75 (31%) | 21 (35%) | 62 (24%) | 166 (28%) |
| BMI (kg/m2) | 34 (22, 48) | 33 (20, 59) | 31 (23, 54) | 31 (21, 68) | 32 (20, 68) |
| BMI Z-score | 2.4 (1.4, 2.8) | 2.4 (1.1, 3.2) | 2.4 (1.2, 2.8) | 2.3 (0.9, 3.4) | 2.3 (0.9, 3.4) |
| SBP (mm Hg) | 128 (105, 158) | 121 (85, 167) | 121 (98, 152) | 121 (74, 168) | 121 (74, 168) |
| DBP (mm Hg) | 68 (55, 91) | 68 (40, 114) | 70 (51, 95) | 67 (46, 105) | 68 (40, 114) |
| LDL-C (mg/dL) | 150 (131, 205) | 97 (19,128) | 143 (131, 213) | 89 (19, 128) | 97 (19, 213) |
| HDL-C (mg/dL) | 43 (31, 58) | 35 (15, 62) | 39 (27, 58) | 39 (23, 89) | 39 (15, 89) |
| Total cholesterol (mg/dL) | 209 (178, 275) | 170 (62, 298) | 228 (186, 309) | 147 (70, 360) | 166 (62, 360) |
| TG (mg/dL) | 97 (53, 133) | 169 (115, 434) | 221 (133, 443) | 86 (18, 726)* | 133 (18, 726)* |
| AST (U/L) | 76 (SD 54) | 66 (SD 50) | 81 (SD 57) | 57 (SD 44) | 64 (SD 49) |
| ALT (U/L) | 128 (SD 92) | 110 (SD 90) | 138 (SD 84) | 96 (SD 88) | 107 (SD 89) |
| GGT (U/L) | 58 (SD 39) | 45 (SD 34) | 69 (SD 53) | 38 (SD 28) | 45 (SD 35) |
| Fibrosis stage | |||||
| None | 10 (41%) | 77 (32%) | 12 (21%) | 94 (36%) | 193 (33%) |
| Zone 3, periportal | 3 (13%) | 45 (19%) | 10 (17%) | 28 (11%) | 86 (15%) |
| Zone 3, perisinusoidal | 9 (38%) | 9 (38%) | 24 (41%) | 95 (36%) | 217 (37%) |
| Bridging | 2 (8%) | 26 (11%) | 11 (19%) | 38 (14%) | 77 (13%) |
| Cirrhosis | 0 (0%) | 1 (0%) | 1 (2%) | 7 (3%) | 9 (2%) |
| Diagnosis | |||||
| NAFLD, not NASH | 9 (38%) | 64 (27%) | 13 (22%) | 80 (30%) | 166 (28%) |
| Borderline NASH | 8 (33%) | 105 (44%) | 29 (48%) | 125 (48%) | 267 (46%) |
| Definite NASH | 7 (29%) | 70 (29%) | 18 (30%) | 57 (22%) | 152 (26%) |
per the NHLBI algorithm, participants with TG > 500 mg/dL were automatically referred to a lipid specialist for pharmacologic intervention and were therefore not included in the analysis of participants receiving dietary intervention and repeat fasting lipid panels
Table 2.
Follow up demographic, laboratory, and clinical characteristics among children with NAFLD
| Variables N (%) or median (range) |
Target LDL-C only N=24 |
Target TG only N= 239 |
Target both N= 60 |
|---|---|---|---|
| BMI (kg/m2) | 33 (23, 50) | 34 (19, 64) | 33 (24, 65) |
| BMI Z-score | 2.3 (1.5, 2.8) | 2.4 (0.6, 3.4) | 2.3 (1.2, 2.8) |
| SBP (mm Hg) | 124 (95, 166) | 124 (93, 162) | 125 (97, 153) |
| DBP (mm Hg) | 69 (48, 85) | 69 (49, 99) | 68 (52, 99) |
| LDL-C (mg/dL) | 128 (85, 209) | 97 (27, 178) | 131 (73, 189) |
| HDL-C (mg/dL) | 39 (27, 66) | 35 (19, 66) | 35 (19, 58) |
| Total cholesterol (mg/dL) | 189 (139, 275) | 166 (81, 271) | 213 (124, 286) |
| TG (mg/dL) | 124 (44, 381) | 159 (44, 514) | 213 (80, 709) |
| AST (U/L) | 57 (SD 59) | 55 (SD 42) | 62 (SD 55) |
| ALT (U/L) | 93 (SD 101) | 94 (SD 86) | 110 (SD 97) |
| GGT (U/L) | 49 (38) | 39 (SD 25) | 63 (SD 64) |
LDL-cholesterol
As shown in Figure 1, 14% (n=84) of our study participants had levels that met criteria for intervention in the Target LDL-C pathway. The median LDL-C of those targeted was 145 (131, 212) mg/dL. After one year, fasting lipid panels were available for 78 participants with targeted LDL-C. Of these, 51% (n= 40) achieved goal levels as shown in Figure 1, Box A. Achieving goal level of LDL-C at one year follow up was significantly associated with a decrease in BMI Z-score compared with those that did not meet goal (−0.07 (−0.91, 0.13) vs. 0.01 (−0.34, 0.31), p= 0.018). Additionally, achieving goal LDL-C at follow up was also significantly associated with a decrease in ALT compared with those not achieving goal (−57 (−242, 126) vs. 0 (−223, 203), p=0.003)
An additional 27% (n=21) of participants with targeted LDL-C met criteria for enhanced dietary modifications with a lipid-specific diet (Figure 1, Box B). As shown in Figure 1,, application of the guidelines including risk factors (as shown in supplemental data) shows that 22% (n= 17) of those originally with targeted LDL-C met criteria for statin at 1 year. Thus, the combination of children already taking a statin at baseline or reaching criteria for initiation of a statin at one year represented 5.3% (33/623) of the entire cohort.
Triglycerides
As shown in Figure 2, 51% (n=299) of the cohort met criteria for intervention in the Target Triglyceride pathway. The median triglyceride concentration of those targeted was 138 (38, 330) mg/dL in children <10 years and 147 (20, 727) mg/dL for those 10–18 years. Three participants (0.5%) had baseline triglyceride concentrations that warranted referral to a lipid specialist as shown in Figure 2, Box A. After one year, fasting lipid panels were available for 289 participants with targeted triglycerides. Of these, 25% (n=73) achieved goal triglyceride levels as shown in Figure 2, Box B. Achieving goal TG at one year follow up was associated with a significantly greater decrease in BMI Z-score compared with those that did not achieve goal levels of TG (−0.08 (−1.37, 0.24) vs. −0.02 (−1.42, 0.39), p= 0.033). There were no significant differences between groups with respect to change in ALT (−15 (−305, 642) vs. −16 (−416, 203), p= 0.958
However, the large majority of subjects met criteria for continued interventions at one year; 38% (n=110) had triglyceride levels between 130–199 mg/dL, and thus warranted intensification of the CHILD-2- TG diet as shown in Figure 2, Box C. There were 36% (n=104) of participants that met criteria for antihyperlipidemic medications as shown in Figure 2, Box D.
Lipid values and histology
As shown in Table 1, there was no significant difference in meeting criteria for target LDL-C, TG, both, or neither by fibrosis stage (p= 0.098) or diagnosis of NASH (p= 0.334)
Sensitivity analysis
In both sets of sensitivity analysis, the percent of participants from each of the four studies meeting criteria for application of the LDL and TG algorithms was not statistically significant (P = .094 and p=0.341, respectively). Furthermore, comparison between the 4 groups and the percentages of those achieving various LDL and TG-related outcomes also did not differ statistically (p= 0.484 and p= 0.787, respectively).
DISCUSSION
We studied the prevalence of clinically relevant lipid derangement in a large, multicenter cohort of children with NAFLD from pediatric centers across the United States. Two thirds of the cohort met criteria for intervention based on either LDL-C or TG elevation. Abnormalities in TG concentration was the more common lipid derangement. Following one year of care, approximately half of children in the target LDL-C group and one fourth of children in the target TG group reached the recommended lipid level as per the NHLBI guidelines. Thus, a substantial number of participants, despite receiving standard of care recommendations, met criteria for additional therapeutic intervention in the form of nutrition and/or medication. Approximately 30% of children in the target LDL-C group and 40% of children in the target TG group met thresholds for a lipid-specific CHILD-2 diet and continued lipid monitoring. Furthermore, one fifth of the cohort with targeted LDL-C and close to 40% of participants with targeted TG met criteria for consideration of antihyperlipidemic medications.
In children with NAFLD, there is a higher prevalence of clinically actionable dyslipidemia than in the general population. Based upon data from NHANES 1999–2012, 6.5% of adolescents aged 12–17 years in the general population met target LDL-C values compared with 15% of our cohort of children with NAFLD.22 Notably, our study included longitudinal data and demonstrated that 5.3% of the children with NAFLD in our cohort were either already prescribed or reached criteria for use of a statin at follow up. There are no available longitudinal data for comparison. However, using cross-sectional data only, indications for statin use were met in less than 1% of adolescents ages 12–17 and up to 2.5% of youths 17–21 years in the general population.23 Furthermore, the prevalence of hypertriglyceridemia meeting target TG levels is 12% in the general population24 and 25% in children with obesity.25, 26 Thus the fact that 51% of our cohort of children with NAFLD had target TG levels necessitating intervention and indicates the clinical relevance of dyslipidemia in pediatric NAFLD. Based upon these findings, lipid screening is warranted in all children with NAFLD.
Children with NAFLD and dyslipidemia should be considered to be at increased risk for premature cardiovascular disease. The association between NAFLD and dyslipidemia in children has been shown to be strong and independent of weight.13, 27 In NAFLD, the predominant pattern observed is combined dyslipidemia; characterized by an elevation in LDL-C, TG, and a decrease in HDL-C.28 Combined dyslipidemia is highly atherogenic and predictive of future cardiovascular disease.29, 30 Notably, atherosclerosis is a progressive process that begins in childhood31, 32 and is associated with dyslipidemia.33, 34 In addition to dyslipidemia, NAFLD itself may increase the risk of atherosclerosis. In an autopsy study of children ages 2 to 19 years the prevalence of atherosclerosis was increased by a factor of 2 in those children with NAFLD.35 In children with NAFLD, carotid intimal media thickness has been shown to be greater than in matched controls, i.e. children with obesity but without NAFLD.36, 37, 38 Therefore, although coronary artery disease and stroke tend to be conditions of later adulthood, this evidence suggests that children with NAFLD may be at an increased risk of cardiovascular morbidity and mortality, perhaps at a younger age than the general population. Screening for dyslipidemia in childhood, as recommended by the NHLBI guidelines, allows for both early detection of cardiovascular disease risk and the potential for intervention.
The management of dyslipidemia in children is rooted in nutritional intervention. In a randomized controlled, multi-center trial of children with dyslipidemia, the Dietary Intervention Study in Children (DISC), dietary counseling by a registered nutritionist produced a significant reduction in intake of saturated fats and cholesterol. This resulted in a reduction of plasma LDL-C compared with those who received routine educational materials on heart-healthy diet. These findings were sustained over 3 years.39 In the present study, the initiation of a CHILD-2 TG or LDL-C specific diet was the most common intervention required. With standard of care dietary recommendations, half of the targeted LDL-C cohort and one fourth of the targeted TG cohort achieved their lipid goals at one year follow up. However, a substantial percentage of those with targeted lipid levels met criteria for ongoing nutritional intervention. Although it was not known how many children had their counseling done by a physician versus a registered dietician, prior studies have shown that dietary education from a registered dietician can be more effective for long term LDL-C reduction.40 Nonetheless, pediatric gastroenterologists have identified inadequate access to registered dieticians and lack of insurance coverage for such referrals as substantial barriers to care.41 Therefore, strong consideration should be given to making access to a registered dietician a standard part of care in children with NAFLD.
Statins are the mainstay of therapy for individuals in whom dietary interventions fail to achieve goal LDL-C levels and should also be considered for cardiovascular risk reduction even in patients with NAFLD. However, given their potential adverse effect profile, these medications are often under-utilized. It is known that statins may cause elevations in serum aminotransferases42, and are thus often avoided in patients with elevated aminotransferases or preexisting liver disease. The AASLD practice guidelines concluded that in adults “statins are safe in patients with liver disease and there is no evidence that patients with chronic liver disease including NAFLD and NASH are at higher risk for serious liver injury than those without liver disease.”43 In children, the prevailing data on the efficacy and safety of statin use has been in the population with familial hypercholesterolemia. In a meta-analysis of these studies, statins were shown to be efficacious for lipid lowering and were not associated with an increased risk of liver-related adverse events.44 However, there is a gap in knowledge regarding efficacy and safety in children with underlying liver disease, such as NAFLD. Although the NHLBI guidelines recommend their use in adverse lipid profiles refractory to diet and lifestyle changes, additional studies on the long term benefits and safety of statins in children with NAFLD would be beneficial.
A strength of this study includes a large, multi-center cohort that was broadly representative of children with clinical NAFLD. Subjects were recruited by the NASH CRN, which has geographic diversity as well as a detailed, rigorous protocol to phenotype the cohort. Furthermore, the longitudinal nature of this study adds novel information about the treatment of hypercholesterolemia and hypertriglyceridemia in children with NAFLD including how interventions such as dietary modifications influence this over time. Our study had excellent retention at 48 week follow up.
A limitation of this study is that although recommendations in the standard of care documents on daily caloric intake from saturated fatty acids and cholesterol were similar to those in the NHLBI guidelines, they were not identical. Furthermore, the individual counseling provided by physicians versus registered dieticians at study sites was not monitored, and specific recommendations were undocumented. Although all participants received some form of dietary counseling, it is possible that there may be variation between study sites in terms of specific counseling that was offered. Although a lack of rigid control in the dietary implementation limited the homogeneity of response, the implementation across centers is more reflective of the real world and thus is likely to be a more generalizable result with respect to the rates of change in this patient population. Additionally, we may have under-estimated children with hypercholesterolemia who would meet criteria for consideration of medication, based on incomplete family history of CVD. Lastly, our study evaluated children ages 9–18, as this is the recommended age for universal screening of lipids, per the NHLBI guidelines. However, this highlights a gap in the knowledge about the cardiovascular risk in younger children with NAFLD, and future studies are needed to better evaluate this age group.
In summary, NAFLD is a common clinical problem that is frequently associated with clinically actionable dyslipidemia. Targeted dietary interventions can be effective for a subset of this population. The number of children who will require more aggressive intervention including medications is substantial. This strongly supports the new pediatric NAFLD guidelines15 and merits consideration of pediatric NAFLD as a risk factor for clinically significant dyslipidemia in future pediatric lipid guidelines. Future studies should prospectively address safety of statin usage specifically in children with NAFLD and identify optimal levels of LDL-C and triglycerides to reduce cardiovascular disease risk in children with NAFLD.
Acknowledgments
The Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (U01DK061718, U01DK061728, U01DK061731, U01DK061732, U01DK061734, U01DK061737, U01DK061738, U01DK061730, U01DK061713). Additional support was received from the National Center for Advancing Translational Sciences (NCATS) (UL1TR000439, UL1TR000077, UL1TR000436, UL1TR000150, UL1TR000424, UL1TR000006, UL1TR000448, UL1TR000040, UL1TR000100, UL1TR000004, UL1TR000423, UL1TR000058, UL1TR000454).
Abbreviations used in this paper
- NAFLD
nonalcoholic fatty liver disease
- LDL-C
low density lipoprotein-cholesterol
- TG
triglycerides
- NHLBI
National Heart, Lung, and Blood Institute
- NIDDK
National Institute of Diabetes and Digestive and Kidney Diseases
- NASH CRN
Nonalcoholic Steatohepatitis Clinical Research Network
- BMI
Body mass index
- SD
standard deviation
APPENDIX
Additional members of the Nonalcoholic Steatohepatitis Clinical Research Network Pediatric Clinical Centers
Baylor College of Medicine, Houston, TX: Stephanie H. Abrams, MD, MS (2007–2013); Sarah Barlow, MD; Ryan Himes, MD; Rajesh Krisnamurthy, MD; Leanel Maldonado, RN (2007–2012); Rory Mahabir, BS
Cincinnati Children’s Hospital Medical Center, Cincinnati, OH: April Carr, BS, CCRP; Kimberlee Bernstein, BS, CCRP; Kristin Bramlage, MD; Kim Cecil, PhD; Stephanie DeVore, MSPH (2009–2011); Rohit Kohli, MD; Kathleen Lake, MSW (2009–2012); Daniel Podberesky, MD (2009–2014); Alex Towbin, MD;
Columbia University, New York, NY: Gerald Behr, MD; Jay H. Lefkowitch, MD; Ali Mencin, MD; Elena Reynoso, MD
Emory University, Atlanta, GA: Adina Alazraki, MD; Rebecca Cleeton, MPH, CCRP; Maria Cordero; Albert Hernandez; Saul Karpen, MD, PhD; Jessica Cruz Munos (2013–2015); Nicholas Raviele (2012–2014);
Indiana University School of Medicine, Indianapolis, IN: Molly Bozic, MD; Oscar W. Cummings, MD; Ann Klipsch, RN; Emily Ragozzino; Kumar Sandrasegaran, MD; Girish Subbarao, MD; Laura Walker, RN
Johns Hopkins Hospital, Baltimore, MD: Kimberly Kafka, RN; Ann Scheimann, MD
Northwestern University Feinberg School of Medicine/Ann & Robert H. Lurie Children’s Hospital of Chicago: Joy Ito, RN; Mark H. Fishbein, MD; Saeed Mohammad, MD; Cynthia Rigsby, MD; Lisa Sharda, RD; Peter F. Whitington, MD
Saint Louis University, St Louis, MO: Sarah Barlow, MD (2002–2007); Theresa Cattoor, RN; Jose Derdoy, MD (2007–2011); Janet Freebersyser, RN; Debra King, RN (2004–2015); Jinping Lai, MD; Pat Osmack; Joan Siegner, RN (2004–2015); Susan Stewart, RN (2004–2015); Susan Torretta; Kristina Wriston, RN (2015)
University at Buffalo, Buffalo, NY: Susan S. Baker, MD, PhD; Diana Lopez-Graham; Sonja Williams; Lixin Zhu, PhD
University of California San Diego, San Diego, CA: Hannah Awai, MD; Craig Bross; Jennifer Collins; Janis Durelle;Michael Middleton, MD, PhD; Melissa Paiz; Claude Sirlin, MD; Patricia Ugalde-Nicalo, MD; Mariana Dominguez Villarreal
University of California San Francisco, San Francisco, CA: Bradley Aouizerat, PhD; Jesse Courtier, MD; Linda D. Ferrell, MD; Natasha Feier, MS; Ryan Gill, MD, PhD; Camille Langlois, MS; Emily Rothbaum Perito, MD; Patrika Tsai, MD
University of Washington Medical Center and Seattle Children’s Hospital, Seattle, WA:
Kara Cooper; Simon Horslen, MB, ChB; Evelyn Hsu, MD; Karen Murray, MD; Randolph Otto, MD; Matthew Yeh, MD, PhD; Melissa Young
Washington University, St. Louis, MO: Elizabeth M. Brunt, MD (2002–2015); Kathryn Fowler, MD (2012–2015)
Resource Centers
National Cancer Institute, Bethesda, MD: David E. Kleiner, MD, PhD
National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD: Sherry Brown, MS; Edward C. Doo, MD; Jay H. Hoofnagle, MD; Patricia R. Robuck, PhD, MPH (2002–2011); Averell Sherker, MD; Rebecca Torrance, RN, MS
Johns Hopkins University, Bloomberg School of Public Health (Data Coordinating Center), Baltimore, MD: Jeanne M. Clark, MD, MPH; Michele Donithan, MHS; Erin Hallinan, MHS; Milana Isaacson, BS; Kevin P. May, MS; Laura Miriel, BS; Alice Sternberg, ScM; James Tonascia, PhD; Mark Van Natta, MHS; Laura Wilson, ScM; Katherine Yates, ScM
Table 3.
Cardiovascular Health Integrated Lifestyle Diet (CHILD-1)
| 2–21 years | ||
| Primary beverage: Fat-free unflavored milk | ||
| Limit/avoid sugar-sweetened beverages; encourage water | ||
| Fat content: | ||
| • Total fat 25–30% of daily kcal/EER | ||
| • Saturated fat 8–10% of daily kcal/EER | ||
| • Avoid trans fat as much as possible | ||
| • Cholesterol < 300 mg/d | ||
| Encourage high dietary fiber intake from foods. Naturally fiber-rich foods are recommended (fruits, vegetables, whole grains); fiber supplements are not advised. Limit refined carbohydrates (sugars, white rice, white bread). | ||
| Supportive actions: | ||
| • Teach portions based on EER for age/gender/activity | ||
| • Encourage moderately increased energy intake during periods of rapid growth and/or regular moderate-to-vigorous physical activity | ||
| • Encourage dietary fiber from foods: Age plus 5 g/d | ||
| • Limit naturally sweetened juice (no added sugar) to 4 oz/d | ||
| • Limit sodium intake | ||
| • Support DASH-style eating plan | ||
Table 4.
Risk factor definitions for dyslipidemia algorithm
| Definition | |
|---|---|
| Family History | myocardial infarction, angina, coronary artery bypass graft/stent/angioplasty, sudden cardiac death in parent, grandparent, aunt, or uncle male < 55 years, female <65 years |
| High Level Risk Factors | |
| Hypertension requiring drug therapy (BP > 99th percentile+ 5mmHg) | |
| Current cigarette smoker | |
| BMI > 97th percentile | |
| Presence of high risk conditions | Diabetes mellitus type I and II, chronic renal disease/ end stage renal disease/ post renal transplant, post orthotopic heart transplant, Kawasaki disease with current aneurysms |
| Moderate Level Risk Factors | |
| Hypertension not requiring drug therapy | |
| BMI > 95th and < 97th percentile | |
| HDL-C < 40 mg/dL | |
| Presence of moderate risk conditions | Kawasaki disease with regressed coronary aneurysms, chronic inflammatory disease (systemic lupus erythematosus, juvenile rheumatoid arthritis), Human immunodeficiency virus, nephrotic syndrome |
Table 5.
Cardiovascular Health Integrated Lifestyle Diet, LDL-C and TG specific (CHILD-2)
| Elevated LDL-C: CHILD 2-LDL | ||
| 2–21 years | ||
| Refer to a registered dietician for family medical nutrition therapy | ||
| • 25–30% of daily calories from fat, ≤ 7% from saturated fat, ~ 10% from monounsaturated fat | ||
| • < 200 mg/dL of cholesterol | ||
| • Avoid trans fats as much as possible | ||
| Supportive actions | ||
| • Plant sterol esters and/or plant stanol esters* up to 2 g/d as replacement for usual fat sources can be used after age 2 years in children with familial hypercholesterolemia. | ||
| • Plant stanol esters as part of a regular diet are marketed directly to the public. Short-term studies show no harmful effects in healthy children. | ||
| • The water-soluble fiber psyllium can be added to a low-fat, low saturated fat diet as cereal enriched with psyllium at a dose of 6 g/d for children 2–12 years, and 12 g/d for those ≥ 12 years. | ||
| • As in all children, 1 hour/day (h/d) of moderate-to-vigorous physical activity and < 2 h/d of sedentary screen time are recommended. | ||
| Elevated TG: CHILD 2-TG | ||
| 2–21 years | ||
| Refer to a registered dietician for family medical nutrition therapy | ||
| • 25–30% of daily calories from fat, ≤ 7% from saturated fat, ~ 10% from monounsaturated fat | ||
| • < 200 mg/dL of cholesterol | ||
| • Avoid trans fats as much as possible | ||
| Decrease sugar intake | ||
| • Replace simple with complex carbohydrates | ||
| • No sugar-sweetened beverages | ||
| Increase dietary fish to increase omega-3 fatty acids | ||
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
Portions of this study were presented as an abstract at the World Congress of Pediatric Gastroenterology, Hepatology and Nutrition, Montreal, Quebec, October 5–8 2016.
References
- 1.Heart Disease Facts & Statistics [Internet] [accessed March 9, 2017];The Center for Disease Control. Available from: https://www.cdc.gov/heartdisease/facts.htm.
- 2.Newman W, Freedman D, Voors A, Gard P, Srinivasan S, Cresanta J, Williamson G, et al. Relation of Serum Lipoprotein Levels and Systolic Blood Pressure to Early Atherosclerosis. N Engl J Med. 1986;314:138–144. doi: 10.1056/NEJM198601163140302. [DOI] [PubMed] [Google Scholar]
- 3.McGill HC, McMahan CA. Determinants of atherosclerosis in the young. Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Am J Cardiol. 1998;82:30T–36T. doi: 10.1016/s0002-9149(98)00720-6. [DOI] [PubMed] [Google Scholar]
- 4.Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents, National Heart, Lung, and Blood Institute. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents: Summary Report. Pediatrics. 2011;128:S213–S256. doi: 10.1542/peds.2009-2107C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–1393. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 6.Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and Outcomes of Liver Transplantation for Nonalcoholic Steatohepatitis in the United States. Gastroenterology. 2011;141:1249–1253. doi: 10.1053/j.gastro.2011.06.061. [DOI] [PubMed] [Google Scholar]
- 7.Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, et al. Nonalcoholic Steatohepatitis Is the Second Leading Etiology of Liver Disease Among Adults Awaiting Liver Transplantation in the United States. Gastroenterology. 2015;148:547–555. doi: 10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 8.Banini BA. NASH surpasses HCV as top etiology for adults listed for liver transplant; Presented at: American College of Gastroenterology Annual Scientific Meeting; Oct. 14–19, 2016; Las Vegas, NV. [Google Scholar]
- 9.Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: Natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617–649. doi: 10.3109/07853890.2010.518623. [DOI] [PubMed] [Google Scholar]
- 10.Byrne CD, Targher G. NAFLD: A multisystem disease. J Hepatol. 2015;62:S47–S64. doi: 10.1016/j.jhep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 11.Dunn W, Xu R, Wingard DL, Rogers C, Angulo P, Younossi SM, et al. Suspected Nonalcoholic Fatty Liver Disease and Mortality Risk in a Population-Based Cohort Study. Am J Gastroenterol. 2008;103:2263–2271. doi: 10.1111/j.1572-0241.2008.02034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newton KP, Hou J, Crimmins NA, Lavine JE, Barlow SE, Xanthakos SA, et al. Prevalence of Prediabetes and Type 2 Diabetes in Children With Nonalcoholic Fatty Liver Disease. JAMA Pediatr. 2016;170:e161971. doi: 10.1001/jamapediatrics.2016.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwimmer JB, Pardee PE, Lavine JE, Blumkin AK, Cook S. Cardiovascular risk factors and the metabolic syndrome in pediatric nonalcoholic fatty liver disease. Circulation. 2008;118:277–283. doi: 10.1161/CIRCULATIONAHA.107.739920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwimmer JB, Zepeda A, Newton KP, Xanthakos SA, Behling C, Hallinan EK, et al. Longitudinal assessment of high blood pressure in children with nonalcoholic fatty liver disease. PLoS One. 2014;9:e112569. doi: 10.1371/journal.pone.0112569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vos MB, Abrams SH, Barlow SE, Caprio S, Daniels SR, Kohli R, et al. NASPGHAN Clinical Practice Guideline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease in Children. J Pediatr Gastroenterol Nutr. 2017;64:319–334. doi: 10.1097/MPG.0000000000001482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, Rosenthal P, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011;305:1659–1668. doi: 10.1001/jama.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwimmer JB, Lavine JE, Wilson LA, Neuschwander-Tetri BA, Xanthakos SA, Kohli R, et al. In Children With Nonalcoholic Fatty Liver Disease, Cysteamine Bitartrate Delayed Release Improves Liver Enzymes but Does Not Reduce Disease Activity Scores. Gastroenterology. 2016;151:1141–1154.e9. doi: 10.1053/j.gastro.2016.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 19.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 20.National Cholesterol Education Program (NCEP): Highlights of the Report of the Expert Panel on Blood Cholesterol Levels in Children and Adolescents. Pediatrics. 1992;89:495–501. [PubMed] [Google Scholar]
- 21.Dietary Guidelines for Americans, 2010. [cited March 9, 2017];The U.S. Department of Agriculture and U.S. Department of Health and Human Services. Available from: https://health.gov/dietaryguidelines/dga2010/dietaryguidelines2010.
- 22.McCrindle BW, Tyrrell PN, Kavey RW. Will obesity increase the proportion of children and adolescents recommended for a statin? Circulation. 2013;128:2162–2165. doi: 10.1161/CIRCULATIONAHA.113.002411. [DOI] [PubMed] [Google Scholar]
- 23.Gooding HC, Rodday AM, Wong JB, Gillman MW, Lloyd-Jones DM, Leslie LK, et al. Application of Pediatric and Adult Guidelines for Treatment of Lipid Levels Among US Adolescents Transitioning to Young Adulthood. JAMA Pediatr. 2015;169:569–574. doi: 10.1001/jamapediatrics.2015.0168. [DOI] [PubMed] [Google Scholar]
- 24.Kit BK, Carroll MD, Lacher DA. Trends in Serum Lipids Among US Youths Aged 6 to 19 Years, 1988–2010. JAMA. 2012;308:1650–1656. doi: 10.1001/jama.2012.9136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cook S, Auinger P, Li C, Ford ES. Metabolic syndrome rates in United States adolescents, from the National Health and Nutrition Examination Survey, 1999–2002. J Pediatr. 2008;152:165–170. doi: 10.1016/j.jpeds.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Cruz ML, Goran MI. The metabolic syndrome in children and adolescents. Curr Diab Rep. 2004;4:53–62. doi: 10.1007/s11892-004-0012-x. [DOI] [PubMed] [Google Scholar]
- 27.Manco M, Bottazzo G, DeVito R, Marcellini M, Mingrone G, Nobili V. Nonalcoholic fatty liver disease in children. J Am Coll Nutr. 2008;27:667–676. doi: 10.1080/07315724.2008.10719744. [DOI] [PubMed] [Google Scholar]
- 28.Cali AMG, Zern TL, Taksali SE, De Oliveira A, Dufour S, Otvos J, et al. Intrahepatic fat accumulation and alterations in lipoprotein composition in obese adolescents: a perfect proatherogenic state. Diabetes Care. 2007;30:3093–3098. doi: 10.2337/dc07-1088. [DOI] [PubMed] [Google Scholar]
- 29.Kavey RW. Combined dyslipidemia in childhood. J Clin Lipidol. 2015;9:S41–S56. doi: 10.1016/j.jacl.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 30.Katsiki N, Mikhailidis DP, Mantzoros CS. Non-alcoholic fatty liver disease and dyslipidemia: An update. Metabolism. 2016;65:1109–1123. doi: 10.1016/j.metabol.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Pacifico L, Nobili V, Anania C, Verdecchia P, Chiesa C. Pediatric nonalcoholic fatty liver disease, metabolic syndrome and cardiovascular risk. World J Gastroenterol. 2011;17:3082–3091. doi: 10.3748/wjg.v17.i26.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stary HC. Evolution and progression of atherosclerotic lesions in coronary arteries of children and young adults. Arteriosclerosis. 1989;9:I19–32. [PubMed] [Google Scholar]
- 33.Berenson GS, Srinivasan SR, Nicklas TA. Atherosclerosis: a nutritional disease of childhood. Am J Cardiol. 1998;82:22T–29T. doi: 10.1016/s0002-9149(98)00719-x. [DOI] [PubMed] [Google Scholar]
- 34.McGill HC, McMahan CA, Herderick EE, Malcom GT, Tracy RE, Strong JP. Origin of atherosclerosis in childhood and adolescence. Am J Clin Nutr. 2000;72:1307S–1315S. doi: 10.1093/ajcn/72.5.1307s. [DOI] [PubMed] [Google Scholar]
- 35.Schwimmer JB, Deutsch R, Behling C, Lavine JE. Fatty liver as a determinant of atherosclerosis. Hepatology. 2005;42:610A. doi: 10.1002/hep.20842. [DOI] [PubMed] [Google Scholar]
- 36.Demircioğlu F, Koçyiğit A, Arslan N, Cakmakçi H, Hizli S, Sedat AT. Intima-Media Thickness of Carotid Artery and Susceptibility to Atherosclerosis in Obese Children With Nonalcoholic Fatty Liver Disease. J Pediatr Gastroenterol Nutr. 2008;47:68–75. doi: 10.1097/MPG.0b013e31816232c9. [DOI] [PubMed] [Google Scholar]
- 37.Pacifico L, Cantisani V, Ricci P, Osborn JF, Schiavo E, Anania C, et al. Nonalcoholic Fatty Liver Disease and Carotid Atherosclerosis in Children. Pediatr Res. 2008;63:423–427. doi: 10.1203/PDR.0b013e318165b8e7. [DOI] [PubMed] [Google Scholar]
- 38.Singh GK, Vitola BE, Holland MR, Sekarski T, Patterson BW, Magkos F, et al. Alterations in ventricular structure and function in obese adolescents with nonalcoholic fatty liver disease. J Pediatr. 2013;162:1160–8. 1168.e1. doi: 10.1016/j.jpeds.2012.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kwiterovich PO, Hartmuller G, Van Horn L, Christoffel K, Gernhoffer N, Gidding S, et al. Efficacy and Safety of Lowering Dietary Intake of Fat and Cholesterol in Children With Elevated Low-Density Lipoprotein Cholesterol. JAMA. 1995;273:1429–1435. doi: 10.1001/jama.1995.03520420045036. [DOI] [PubMed] [Google Scholar]
- 40.Henkin Y, Shai I, Zuk R, Brickner D, Zuilli I, Neumann L, et al. Dietary treatment of hypercholesterolemia: do dietitians do it better? a randomized, controlled trial. Am J Med. 2000;109:549–555. doi: 10.1016/s0002-9343(00)00566-0. [DOI] [PubMed] [Google Scholar]
- 41.Shapiro W, Yu E, Sanford J, Murray K, Ali S, Desai N, et al. Approach to the Management of Pediatric NAFLD by Pediatric Gastroenterologists across the United States. J Pediatr Gastroenterol Nutr. 2017;65:s244–245. [Google Scholar]
- 42.Thompson PD. What to Believe and Do About Statin-Associated Adverse Effects. JAMA. 2016;316:1969. doi: 10.1001/jama.2016.16557. [DOI] [PubMed] [Google Scholar]
- 43.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 44.Avis HJ, Vissers MN, Stein EA, Wijburg FA, Trip MD, Kastelein JJP, et al. A Systematic Review and Meta-Analysis of Statin Therapy in Children With Familial Hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2007;27:1803–1810. doi: 10.1161/ATVBAHA.107.145151. [DOI] [PubMed] [Google Scholar]