Abstract
Background
Accurate pathologic nodal staging improves early-stage non-small-cell lung cancer survival. In an ongoing implementation study, we measured the impact of a surgical lymph node specimen collection kit and a more thorough pathologic gross dissection method, on attainment of guideline-recommended pathologic nodal staging quality.
Methods
We prospectively collected data on curative-intent non-small cell lung cancer resections from 2009–2016 from 11 hospitals in 4 contiguous Dartmouth Hospital Referral Regions. We categorized patients into 4 groups based on exposure to the two interventions in our staggered implementation study design. We used Chi-squared tests to examine the differences in demographic and disease characteristics and surgical quality criteria across implementation groups.
Results
Of 2,469 patients, 1,615 (65%) received neither intervention; 167 (7%) received only the pathology intervention; 264 (11%) received only the surgery intervention; 423 (17%) had both. Rates of non-examination of lymph nodes reduced sequentially in the order of no intervention, novel dissection, kit, and combined interventions, including non-examination of: any lymph nodes, hilar/intrapulmonary and mediastinal nodes (p<0.001 for all comparisons). The rates of attainment of National Comprehensive Cancer Network, Commission on Cancer, American Joint Committee on Cancer, and American College of Surgeons Oncology Group guidelines increased significantly in the same sequential order (p<0.001 for all comparisons).
Conclusions
The combined effect of two interventions to improve pathologic lymph node examination has a greater effect on attainment of a range of surgical quality criteria than either intervention alone.
Graphical abstract
Thorough pathologic nodal staging is the most frequently missed component of oncologically-sound surgical resection of non-small cell lung cancer.1–3 Good-quality pathologic nodal staging is associated with improved survival.3–5 Attainment of the National Comprehensive Cancer Network (NCCN),3 American College of Surgeons Commission on Cancer (CoC),4 and the American Joint Committee on Cancer/International Union for Cancer Control (AJCC/UICC)5 lung cancer staging criteria have been associated with improved long-term survival. The American College of Surgeons Oncology Group (ACOSOG) Z0030 trial established the adequacy of rigorous systematic lymph node sampling for patients with early stage non-small cell lung cancer.6
The quality of pathologic nodal staging in ‘real-world practice’ is generally poor. Approximately 50% of resections for non-small cell lung cancer in large US databases fail to examine any mediastinal lymph nodes;7,8 12–18% of resections fail to examine any lymph nodes (pNX);9 10–20% have no hilar or intrapulmonary (N1) lymph nodes examined;2 and most resections for pN0 examine insufficient lymph nodes to accurately determine node-negativity.10 Analysis of surgeons’ and pathologists’ reports of the extent of mediastinal lymphadenectomy revealed significant discordance.11 Because accurate lymph node examination requires collaboration between surgery and pathology teams, sub-optimal lymph node examination probably arises from a combination of problems in surgical and pathologic practices.11–14
The quality deficit has been attributed to events in the operating room (suboptimal surgical harvest of hilar and mediastinal lymph node stations); poor communication between surgery and pathology teams (imprecise specimen labeling and insecure transfer); and events in the pathology laboratory (incomplete examination of lung resection specimens, inaccurate reporting).14 We estimated the quality improvement achieved by interventions aimed at surgery and pathology teams.
Material and Methods
With the approval of the Institutional Review Boards of all participating hospitals and the University of Memphis, with a waiver of the informed consent requirement for this low-risk quality improvement project, we conducted a prospective multi-institutional implementation study of two quality improvement processes in all eligible hospitals within our catchment area. We used a comprehensive and rigorous patient-level data abstraction to construct the Mid-South Quality of Surgical Resection database, a population-based dataset containing detailed information on all curative-intent lung cancer resections performed from January 1, 2004 onwards, in all eligible hospitals within 4 contiguous Dartmouth Hospital Referral Regions in North Mississippi, East Arkansas and West Tennessee, states with the 2nd, 3rd and 4th highest lung cancer incidence and mortality rates in the US.15,16 For this pragmatic ‘real-world’ population-based implementation study, eligible hospitals had 5 or more annual curative-intent lung cancer resections.
Inclusion criteria
We analyzed all non-small cell lung cancer resections performed from January 2009 to December 2016 (when complete information was available), excluding patients who received neoadjuvant therapy, because such therapy directly confounds lymph node retrieval, and patients with M1 disease.
Surgical intervention: deployment of a specimen collection kit
From 2010 – 2014, we pilot-tested a specimen collection kit at 3 institutions in metropolitan Memphis.17 From January 1, 2015, we began regional deployment of the kit, using a staggered implementation study design.18 We have previously demonstrated how this kit improves the quality of intraoperative hilar and mediastinal lymph node harvest, the precision of specimen labeling and the concordance between surgeon- and pathologist-reported hilar and mediastinal lymph node examination.17,19 In the current report, we sought to examine its impact on the attainment of specific survival-impactful quality measures in diverse settings.
Pathologic intervention: deployment of a novel lung gross dissection protocol
In response to evidence that standard gross dissection methods fail to retrieve up to 27% of intrapulmonary lymph nodes with metastasis, including 12% of pN0 resections,12 we developed a novel gross dissection method to improve the retrieval of intrapulmonary lymph nodes.20 This method entails a series of blunt dissections starting from the hilum outwards, with particular emphasis on the peri-bronchial region and areas of bronchial bifurcation. It was implemented in one pathology group (covering two hospitals in Metropolitan Memphis), starting in July 2012.20
Intervention groups
We compared the quality of pathologic nodal staging in patients who had surgery, without the kit and with the usual, standard gross dissection methods (non-intervention, [Group 1]) to patients whose surgery was performed: without the kit, but with the novel gross dissection method (pathologic intervention alone [Group 2]); with the kit but with the usual standard gross dissection (surgical intervention alone [Group 3]); with the kit and the novel gross dissection method (dual intervention cohort, [Group 4]).
Identification of intervention cases
We prospectively monitored use of the specimen collection kit in all cases by pre-notification and collection of a checklist enclosed within the kit by the research team.17,19 Deployment of the novel dissection method was assumed, without direct confirmation, from the implementation date (July 1, 2012) onward, at the two hospitals where the gross dissectors were trained on the new method.20
Nodal staging quality criteria
In the absence of a universal measure of pathologic nodal examination quality, we used several different criteria. These included specific markers of poor quality- non-examination of: any lymph nodes (the pNX rate),9 intrapulmonary lymph nodes (stations 11–14),12 N1 lymph nodes (stations 10–14),2 mediastinal lymph nodes,8 station 7,6 and station 10.6 Markers of good quality attainment were: a minimum of three mediastinal lymph node stations (recommended by the NCCN),22 aggregate NCCN surgical resection quality criteria (anatomic resection, negative margins, examination of at least 1 N1 lymph node, and a minimum of 3 mediastinal nodal stations),22 the CoC quality surveillance criterion (a minimum of 10 examined regional lymph nodes in stage IA-IIB resections),23 the AJCC/UICC criteria (examination of a minimum of three N1 and three mediastinal nodes or stations, including station 7),5 and nodal staging quality at least equivalent to that of the ACOSOG Z0030 systematic sampling criteria (examination of lymph nodes from stations 2R, 4R, 7 and 10R, for right-sided resections and 4L,5,6,7 and 10L for left-sided resections).6
Statistical analysis plan
We tested for differences in patient demographic and disease characteristics across the four intervention groups using chi-squared tests, or Fisher’s exact test when sample sizes were small, and analysis-of-variance F-tests. We examined the distribution of lymph nodes and stations postoperatively sampled across the cohorts using Kruskal-Wallis tests and detected pairwise differences with the Dwass, Steel, Critchlow-Fligner multiple comparison test.24,25
Rates of non-examination and attainment of quality criteria were also compared using Chisquared tests (or Fisher’s exact test) and pairwise differences with Tukey-Kramer adjustments.26 Factors associated with rates of attainment of quality criteria were identified with multivariable logistic models. Factors considered include intervention cohort, race, sex, age, insurance, use of any preoperative staging test, histology, grade, technique of resection, extent of resection, aggregate pathologic stage, surgeon, institution, and interaction of surgeon and institution to adjust for surgeon-institution crossover. Pathologic stage was excluded when modeling pNX, extent of resection was excluded when modeling the rates of attainment of anatomic resection and aggregate NCCN criteria. In additional analyses, we tested the impact of including preoperatively sampled lymph nodes in the analysis. All statistical analyses were assessed at the alpha=0.05 level and performed in SAS 9.4 (2013, SAS Institute Inc., Cary NC).
Results
Cohort characteristics across intervention groups
From 2009 to 2016, 2,469 resections were performed in the 11 hospitals contributing data to the Mid-South Quality of Surgical Resection database, of which 65% had neither intervention (Group 1), 7% had the pathology intervention only (Group 2), 11% had the surgical intervention only (Group 3), and 17% had both (Group 4). Differences in key demographic and clinical characteristics included age, insurance status, preoperative invasive staging, histology, tumor grade, the technique and extent of resection, pathologic N-category, and change in clinical to pathologic nodal status (Supplemental Table A1). Use of preoperative invasive mediastinal lymph node staging, although relatively infrequent, and robotically-assisted resections, were more common in patients with the pathology intervention (Supplemental Table A1). This pattern reflects surgical practice at the two institutions where the pathology intervention was deployed.
Regional lymph node yield
The total lymph node count increased significantly from Group 1, with a median of 6 (interquartile range 3–11), to 16 (10 – 21) in Group 4. The single intervention cohorts were similar in their total nodal counts, but the distribution of lymph node origin was very different (Table 1). The N1 nodal count was particularly affected by the novel gross dissection method, rising from a median of 3 (1 – 6) in Group 1, to 7 (2 – 13) in Group 2, and 7 (3 – 11) in Group 4. There was also a significant increase in the N1 count in Group 3 (median 6, interquartile range 3 – 9), partly because the kit mandates inclusion of hilar nodes (station 10). Mediastinal lymph node examination showed a striking increase with use of the kit, from 2 (0 – 5) in Group 1, to 6 (4 – 9) in Group 3, and 7 (5 – 12) in Group 4. This increase was mostly attributable to an increase in the number of lymph node stations examined, from a median of 2 (0– 3) in Group 1, to 4 (3 – 5) in Group 3 and 5 (4 – 6) in Group 4.
Table 1.
Distribution of lymph node examination and rates of non-examination of key nodal stationsa.
| Variable | Intervention Exposure Cohort | |||
|---|---|---|---|---|
| Neither (Group 1) |
Path Only (Group 2) |
Kit Only (Group 3) |
Both (Group 4) |
|
| N (%) | N (%) | N (%) | N (%) | |
| Total Number of Patients | 1615 (65) | 167 (7) | 264 (11) | 423 (17) |
| Distribution of Number of Lymph Nodes Sampledb | ||||
| Station 10 | 1 (0, 2)3,4 | 1 (0, 2)3,4 | 1 (1, 2)1,2,4 | 1 (1, 3)1,2,3 |
| Stations 11 – 14 | 2 (0, 4)2,3,4 | 5 (1, 11)1 | 4 (1, 7)1 | 4 (1, 9)1 |
| Total N1 | 3 (1, 6) 2,3,4 | 7 (2, 13)1 | 6 (3, 9)1 | 7 (3, 11)1 |
| Total mediastinal lymph nodes | 2 (0, 5)3,4 | 2 (0, 6) 3,4 | 6 (4, 8)1,2,4 | 8 (5, 12)1,2,3 |
| Total lymph nodes | 6 (3, 11)2,3,4 | 12 (5, 18)1,4 | 12 (8.5, 17)1,4 | 16 (10, 21)1,2,3 |
| Distributions of Stations Sampledb | ||||
| Stations 10–14 | 1 (1, 2)3,4 | 2 (1, 2)3,4 | 2 (1, 2)1,2 | 2 (2, 2)1,2 |
| Mediastinal lymph node stations | 2 (0, 3)3,4 | 1 (0, 3)3,4 | 4 (3, 5)1,2,4 | 5 (4, 6)1,2,3 |
All percentages rounded to the nearest whole number. Superscripts indicate significant differences (α=0.05 level) between the respective column and columns listed after adjusting for multiple comparisons;
Median (1st quartile, 3rd quartile).
Non-examination of key nodal stations
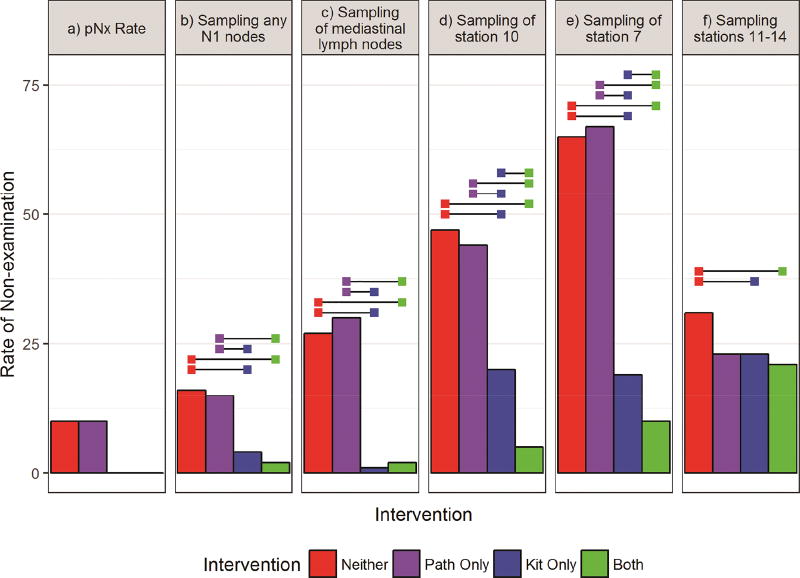
Overall, non-examination of lymph nodes decreased with use of the interventions (Figure 1; Supplemental Table A2). The pNX rate for resections in which the kit was not used was 10% while there were no pNX resections when the kit was used. The rate of non-examination of stations 11–14 was significantly higher in Group 1 (31%) compared to Groups 3 (23%) and 4 (21%). Excluding wedge resections (since the pathology intervention cannot be applied), the rates of non-examination of stations 11–14 were: 24%, 17%, 21%, and 18%, respectively. Resections without the kit (Groups 1 and 2) had significantly higher rates of non-examination of any N1, mediastinal, station 7 and 10 nodes. Group 4 had significantly lower rates of non-examination of hilar and specific mediastinal nodal stations than all other groups.
Figure 1.
Rates of non-examination of lymph nodes from: a) anywhere (pNX); b) N1 stations; c) mediastinal stations; d) station 10; e) station 7; f) stations 11–14. Horizontal bars indicate significantly different rates of attainment between intervention groups after Tukey adjustment for multiple comparisons (alpha=0.05).
Quality of nodal staging
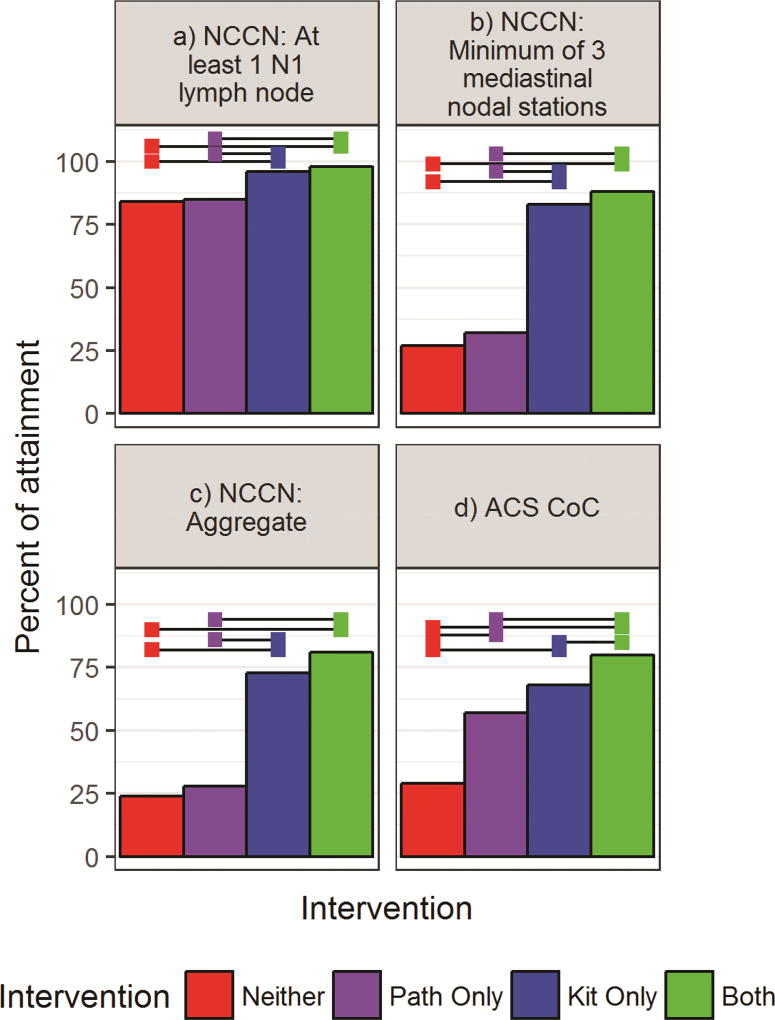
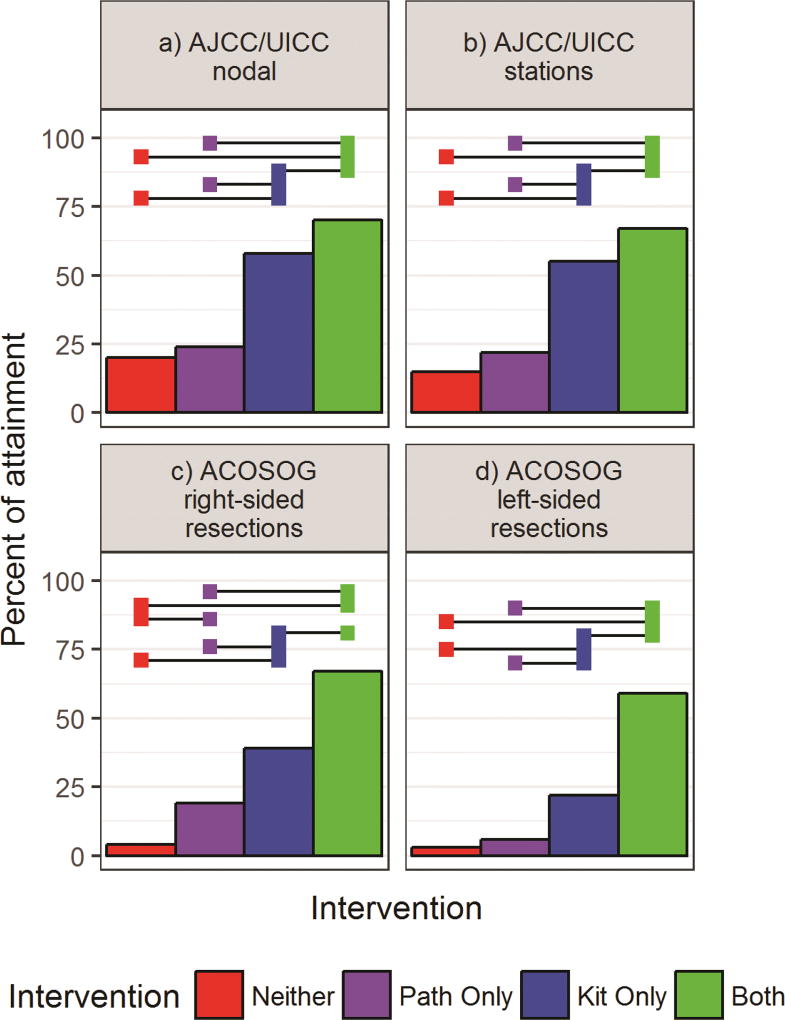
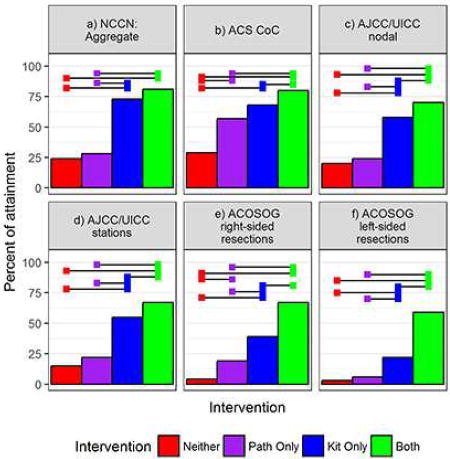
We found sequential improvement in the rate of attainment of all eight direct lymph node-related quality criteria in the comparisons from Group 1 to 4 (Figures 2 and 3; Supplemental Tables A3, A4, A5). Use of the kit (Groups 3 and 4) was associated with significantly higher rates of sampling any N1 lymph node, at least three mediastinal stations, and attainment of the aggregate NCCN quality compared to non-use of the kit (Figure 2; Supplemental Table A3; Supplemental Figure A1). Attainment of the CoC, AJCC and ACOSOG criteria also increased in sequential order from Group 1 to 4 (Figures 2 and 3; Supplemental Table A4).
Figure 2.
Left to right, top to bottom rates of attainment of: a) the National Comprehensive Cancer Network (NCCN) recommended examination of at least 1 N1 lymph node; b) ≥3 mediastinal nodal stations; c) the aggregate NCCN surgical resection quality criteria; d) the Commission on Cancer (CoC) quality surveillance criterion (a minimum of 10 examined regional lymph nodes in stage IA-IIB resections). Horizontal bars indicate significantly different rates of attainment between intervention groups after Tukey adjustment for multiple comparisons (alpha=0.05).
Figure 3.
Rates of attainment of: the American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) criteria , which requires examination of a minimum of three N1 and three mediastinal nodes (a) or stations, including station 7 (b); and American College of Surgeons’ Oncology Group (ACOSOG) Z0030 systematic sampling criteria, which include examination of lymph nodes from stations 2R, 4R, 7 and 10R, for right-sided resections (c) and 4L,5,6,7 and 10L for left-sided resections (d). Horizontal bars indicate significant differences between intervention groups after Tukey adjustment for multiple comparisons (alpha=0.05).
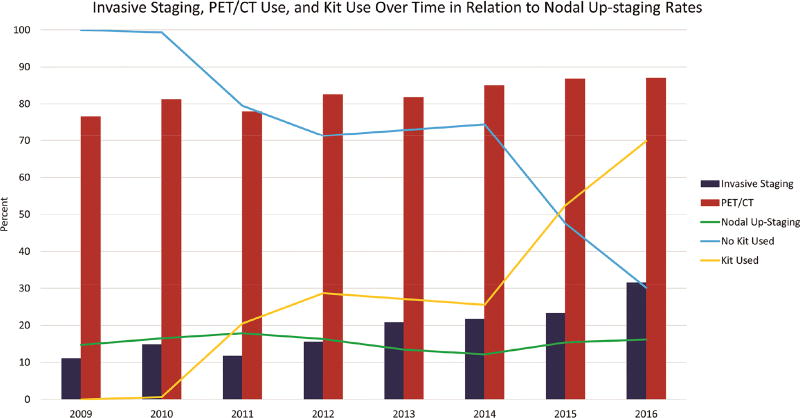
There was a significant difference in the rates of change in nodal staging between clinical and pathologic stage across the 4 exposure groups (p = 0.0096, Supplemental Table A1). Downstaging rates were higher in Groups 1 and 2, which had no exposure to the kit, and had higher rates of non-examination of specific nodal stations; upstaging rates were higher in Groups 3 and 4, which had exposure to the kit, and had evidence of more thorough nodal staging. In pair-wise comparisons, kit cases had significantly higher nodal upstaging rates (17% v 14%, p= 0.0013). This change occurred despite an increase in use of positron-emission/computerized tomographic (PET/CT) scans and preoperative invasive nodal staging during the era of kit deployment (Fig 4). The most striking contrast was between Groups 1 and 3, which both had similarly low rates of invasive preoperative staging (16%) and nodal upstaging rates of 14% v 21%, respectively (Supplemental Table A1).
Figure 4.
Evolution of preoperative staging PET/CT and invasive staging, kit use and nodal upstaging rates.
Factors associated with attainment of quality criteria
Characteristics such as large cell histology, use of video-assisted thoracoscopic surgery and sublobar resection were significant predictors of pNX resection (Supplemental Table A6a). Significant predictors of high nodal nonexamination rates, include intervention group, large cell histology, and sublobar resection. Patients in Group 1 had significantly higher adjusted odds of non-examination of stations 10–14, mediastinal lymph nodes and stations 7 and 10, compared to those in Groups 3 and 4.
Similar factors (non-intervention cohort, large cell histology, use of video-assisted thoracoscopic surgery, sublobar resection and pathologic stage) were associated with lower rates of attainment of NCCN quality criteria (Supplemental Table A6b). Compared to those in Group 1, patients in Group 3 had significantly higher adjusted odds of having a resection in which any N1 lymph node was sampled (2.9 [1.4, 6.1]), at least 3 mediastinal lymph node stations were sampled (14.0 [9.8, 20.0]) and the aggregate NCCN criteria were achieved (9.0 [6.6, 12.2]). The adjusted odds ratios were even greater for Group 4 compared to Group 1: 8.7 (3.9, 19.3), 11.3 (7.7, 16.6) and 8.4 (6.0, 11.9), respectively.
Factors associated with attaining the CoC, AJCC/UICC and ACOSOG Z0030 criteria were the intervention cohort, use of robotic-assisted technique and lobectomy rather than sublobar resection (Supplemental Table A6c). Across all sub-categories for these quality criteria, the adjusted odds of attainment were sequentially greater for patients in Groups 3 and 4, compared to those in Groups 1 and 2. Specifically, patients with both interventions (Group 4) or kit only (Group 3) had significantly higher adjusted odds ratios across all criteria compared to those with no intervention (Group 1) or those with the novel dissection only (Group 2). Group 4 had significantly higher adjusted odds of attaining the AJCC/UICC and ACOSOG Z0030 criteria, compared to Group 3. Group 2 had higher odds of attaining the CoC and right-sided ACOSOG Z0030 criteria than Group 1.
Impact of preoperative invasive nodal staging
Including preoperatively collected lymph nodes did not change the median and distribution of nodal or sampled-station counts.
Comment
In this population-based cohort from a high lung cancer mortality zone of the United States, the quality of pathologic nodal staging improved with implementation of well-designed interventions. The novel gross dissection protocol was independently associated with improved intrapulmonary lymph node yield and higher pathologic nodal staging quality levels, as indicated by higher CoC-defined quality attainment rates. The surgical lymph node specimen collection kit was associated with improved thoroughness of pathologic hilar and mediastinal nodal staging.
The best results were associated with the combination of interventions. This potential synergy between an intervention aimed at the surgery team, the nexus between surgery and pathology teams (the specimen collection kit) and another aimed directly at the pathology team (the novel gross dissection method) may suggest a collateral benefit in promoting healthy team-functioning among key members of the lung cancer care team by simplifying interdisciplinary interaction.27
Although the quality measures we evaluated varied in stringency and specificity, the relationship between intervention exposure and quality outcomes was consistent. Although we have not yet reported on survival, the link between the NCCN, CoC and UICC/AJCC guidelines and survival has been validated.3–5 Therefore, interventions that increase attainment of these guidelines may also improve survival. Other markers, such as the pNX rate,9 the rate of resections without mediastinal lymph node examination8 and station 10,3 although not previously promulgated by any recognized body, have also been strongly linked with survival. They are also relatively easy to measure. The ACOSOG Z0030 systematic sampling criteria, although stringent and possibly more difficult to measure, are nevertheless important because the survival equivalence of systematic sampling and mediastinal nodal dissection was established by these criteria.6
Limitations
We have not analyzed the survival impact at this time because we continue to expand the kit and novel gross dissection protocol across a diverse group of institutions within our region. We will report on survival when the dataset is more mature. Secondly, unlike the implementation of the surgical kit which was rigorously and prospectively verified, implementation of the novel gross dissection protocol was assumed to have occurred after dissector training, without verification. The quality of implementation probably varied over time and between gross dissectors. It seems likely that more rigorous oversight of the application of this method, with objective evaluation by a re-dissection protocol applied to remnant material, and feedback to gross dissectors, will increase the impact of the pathology intervention.12,20 Rigorous application of this novel gross dissection method is the objective of a proposed implementation study. Third, forces independent of our interventions may account for the observed patterns. Our staggered implementation study design allows us to control for the influence of such secular changes in practice.18
Fourth, there was temporal evolution in the thoroughness of preoperative staging practice (Fig 4), with increasing use of PET/CT scans and invasive preoperative nodal staging, which would be expected to have a confounding effect. More thorough preoperative staging, by independently increasing the detection of mediastinal nodal metastasis, could cause more patients to be triaged out of primary surgical resection and into neoadjuvant therapy pathways (and therefore be excluded from this analysis) or non-surgical treatment (and therefore be excluded from this database). Therefore, the finding of increased nodal upstaging in Groups 3 and 4 is even more remarkable. This point is reinforced by the sharp difference in nodal upstaging rates between groups 1 and 3, which had an identically low rate of preoperative invasive nodal staging.
Conclusions
We demonstrate the possibility of raising the quality of pathologic nodal staging in a diverse group of community-level institutions. The impact on attainment of pathologic staging quality measures is striking, as is the early evidence of impact on pathologic nodal stage distribution. Further exploration of deeper implementation of the novel gross dissection method, wider dissemination of both interventions, and rigorous examination of their potential impact across a more geographically and structurally diverse range of institutions is necessary to fully quantify their impact.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Little AG, Rusch VW, Bonner JA, et al. Patterns of surgical care of lung cancer patients. Ann Thorac Surg. 2005;80(6):2051–2056. doi: 10.1016/j.athoracsur.2005.06.071. [DOI] [PubMed] [Google Scholar]
- 2.Allen JW, Farooq A, O'brien TF, Osarogiagbon RU. Quality of surgical resection for nonsmall cell lung cancer in a US metropolitan area. Cancer. 2011;117(1):134–142. doi: 10.1002/cncr.25334. [DOI] [PubMed] [Google Scholar]
- 3.Osarogiagbon RU, Ray MA, Faris NR, et al. Prognostic value of national comprehensive cancer network lung cancer resection quality criteria. Ann Thorac Surg. 2017;103(5):1557–1565. doi: 10.1016/j.athoracsur.2017.01.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samson P, Crabtree T, Broderick S, et al. Quality measures in clinical stage I non-small cell lung cancer: Improved performance is associated with improved survival. Ann Thorac Surg. 2017;103(1):303–311. doi: 10.1016/j.athoracsur.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sobin LH, Gospodarowicz MK, Wittekind C, editors. TNM classification of malignant tumors. 7. New York: Wiley-Balcekwell; 2009. [Google Scholar]
- 6.Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non–small cell carcinoma: Results of the american college of surgery oncology group Z0030 trial. J Thorac Cardiovasc Surg. 2011;141:662–670. doi: 10.1016/j.jtcvs.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varlotto JM, Recht A, Nikolov M, Flickinger JC, DeCamp MM. Extent of lymphadenectomy and outcome for patients with stage I nonsmall cell lung cancer. Cancer. 2009;115:851–858. doi: 10.1002/cncr.23985. [DOI] [PubMed] [Google Scholar]
- 8.Osarogiagbon RU, Yu X. Mediastinal lymph node examination and survival in resected early-stage Non–Small-cell lung cancer in the surveillance, epidemiology, and end results database. J Thorac Oncol. 2012;7:1798–1806. doi: 10.1097/JTO.0b013e31827457db. [DOI] [PubMed] [Google Scholar]
- 9.Osarogiagbon RU, Yu X. Nonexamination of lymph nodes and survival after resection of non-small cell lung cancer. Ann Thorac Surg. 2013;96(4):1178–1189. doi: 10.1016/j.athoracsur.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 10.Osarogiagbon RU, Ogbata O, Yu X. Number of lymph nodes associated with maximal reduction of long-term mortality risk in pathologic node-negative non–small cell lung cancer. Ann Thorac Surg. 2014;97(2):385–393. doi: 10.1016/j.athoracsur.2013.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osarogiagbon RU, Allen JW, Farooq A, Wu JT. Objective review of mediastinal lymph node examination in a lung cancer resection cohort. J Thorac Oncol. 2012;7(2):390–396. doi: 10.1097/JTO.0b013e31823e5e2d. [DOI] [PubMed] [Google Scholar]
- 12.Ramirez RA, Wang CG, Miller LE, et al. Incomplete intrapulmonary lymph node retrieval after routine pathologic examination of resected lung cancer. J CliniOncol. 2012;30(23):2823–2828. doi: 10.1200/JCO.2011.39.2589. [DOI] [PubMed] [Google Scholar]
- 13.Osarogiagbon RU, Decker PA, Ballman K, Wigle D, Allen MS, Darling GE. Survival implications of variation in the thoroughness of pathologic lymph node examination in american college of surgeons oncology group Z0030 (alliance) Ann Thorac Surg. 2016;102(2):363–369. doi: 10.1016/j.athoracsur.2016.03.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butnor KJ, Asamura H, Travis WD. Node doubt: Rigorous surgical nodal procurement combined with thorough pathologic evaluation improves non-small cell lung carcinoma staging accuracy. Ann Thorac Surg. 2016;102(2):353–356. doi: 10.1016/j.athoracsur.2016.05.075. [DOI] [PubMed] [Google Scholar]
- 15.Faris NR, Smeltzer MP, Lu F, et al. Evolution in the surgical care of patients with Non–Small cell lung cancer in the mid-south quality of surgical resection cohort. Semin Thorac Cardiovasc Surg. 2017 doi: 10.1053/j.semtcvs.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Cancer Society. Cancer Facts & Figures 2016. Atlanta: American Cancer Society; 2016. [Google Scholar]
- 17.Osarogiagbon RU, Miller LE, Ramirez RA, et al. Use of a surgical specimen-collection kit to improve mediastinal lymph-node examination of resectable lung cancer. J Thorac Oncol. 2012;7(8):1276–1282. doi: 10.1097/JTO.0b013e318257fbe5. [DOI] [PubMed] [Google Scholar]
- 18.Hawkins NG, Sanson-Fisher RW, Shakeshaft A, D’Este C, Green LW. The multiple baseline design for evaluating population-based research. Am J Prev Med. 2007;33(2):162–168. doi: 10.1016/j.amepre.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 19.Osarogiagbon RU, Sareen S, Eke R, et al. Audit of lymphadenectomy in lung cancer resections using a specimen collection kit and checklist. Ann Thorac Surg. 2015;99(2):421–427. doi: 10.1016/j.athoracsur.2014.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Osarogiagbon RU, Eke R, Sareen S, et al. The impact of a novel lung gross dissection protocol on intrapulmonary lymph node retrieval from lung cancer resection specimens. Ann Diagn Pathol. 2014;18(4):220–226. doi: 10.1016/j.anndiagpath.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smeltzer MP, Faris N, Yu X, et al. Missed intrapulmonary lymph node metastasis and survival after resection of non-small cell lung cancer. Ann Thorac Surg. 2016;102(2):448–453. doi: 10.1016/j.athoracsur.2016.03.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Comprehensive Cancer Network clinical practice guidelines in oncology. [Accessed February 8, 2016];Non-small cell lung cancer. Available at: http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
- 23.Commission on Cancer. Cancer Programs Practice Profile Reports (CP3R) [Accessed February 8, 2016];Lung measure specifications. Available at: https://www.facs.org/w/media/files/quality%20programs/cancer/lungmeasuredocumentation_05272015.ashx.
- 24.Dwass M Some k-Sample Rank-Order Tests. In: Contributions to Probability and Statistics. Olkin I, Ghurye SG, Hoeffding W, Madow WG, Mann HB, editors. Stanford, CA: Stanford University Press; 1960. pp. 198–202. [Google Scholar]
- 25.Steel RG. A rank sum test for comparing all pairs of treatments. Technometrics. 1960;2(2):197–207. [Google Scholar]
- 26.Kramer CY. Extension of multiple range tests to group means with unequal numbers of replications. Biometrics. 1956;12(3):307–310. [Google Scholar]
- 27.Osarogiagbon RU, Rodriguez HP, Hicks D, et al. Deploying team science principles to optimize interdisciplinary lung cancer care delivery: Avoiding the long and winding road to optimal care. J Oncol Pract. 2016;12(11):983–991. doi: 10.1200/JOP.2016.013813. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.