Abstract
Background
Accurate prediction of longitudinal changes in cognitive function would potentially allow targeted intervention in those at greatest risk of cognitive decline. We sought to build a multivariate model using volumetric neuroimaging data alone to accurately predict cognitive function.
Methods
Volumetric T1-weighted neuroimaging data from virally suppressed HIV-positive individuals from the CHARTER cohort (n=139) were segmented into grey and white matter and spatially normalised before were entering into machine learning models. Prediction of cognitive function at baseline and longitudinally was determined using leave-one-out cross validation. Additionally, a multivariate model of brain ageing was used to measure the deviation of apparent brain age from chronological age and assess its relationship with cognitive function.
Results
Cognitive impairment, defined using the global deficit score, was present in 37.4%. However, it was generally mild and occurred more commonly in those with confounding comorbidities (p<0.001). Although multivariate prediction of cognitive impairment as a dichotomous variable at baseline was poor (AUC 0.59), prediction of the global T-score was better than a comparable linear model (adjusted R2=0.08, p<0.01 vs. adjusted R2=0.01, p=0.14). Accurate prediction of longitudinal changes in cognitive function was not possible (p=0.82).
Brain-predicted age exceeded chronological age by mean (95% confidence interval) 1.17 (-0.14-2.53) years, but was greatest in those with confounding comorbidities (5.87 [1.74-9.99] years) and prior AIDS (3.03 [0.00-6.06] years).
Conclusion
Accurate prediction of cognitive impairment using multivariate models using only T1-weighted data was not achievable, which may reflect the small sample size, heterogeneity of the data or that impairment was usually mild.
Keywords: HIV, cognitive impairment, neuroimaging, machine learning, multivariate analysis
Introduction
Care for HIV-positive individuals has changed markedly over the past three decades. Successfully treated HIV-infection is now the norm in Northern European settings, with over 90% of patients receiving antiretroviral therapy in the UK of whom 90% have sustained suppression of plasma HIV RNA.1 The rationale for universal treatment is clear following the publication of the INSIGHT START study.2 As such, treatment of long term comorbidities, such as cognitive impairment, is likely to make up a larger burden of care than opportunistic infections.
Cognitive impairment and accompanying neuroimaging abnormalities reportedly remain prevalent in the era of modern antiretrovirals.3,4 However, controlled data from exclusively virally suppressed patients suggests the prevalence may be lower than previously described.1,5,6 It is currently unclear whether virally suppressed patients experience further significant brain injury. Data from the Multicenter AIDS Cohort Study (MACS) and Central nervous system HIV Anti-Retroviral Therapy Effects Research (CHARTER) cohorts suggest the majority (>60%) of HIV-positive individuals remain stable cognitively over time.2,7,8 Approximately 10% experienced a decline in cognitive function, which was associated with ineffective antiretroviral therapy or treatment interruption. It should be noted that antiretroviral therapy was not universal in these cohorts and effective suppression of plasma HIV RNA was only evident in 70% and 41% respectively, which makes extrapolating these findings to successfully treated cohorts difficult.
Accurate prediction of cognitive impairment and longitudinal decline in cognitive function would be an important advance allowing targeted investigation and treatment in those at the greatest risk of ongoing brain injury. The richness and availability of T1-weighted neuroimaging data makes it an ideal candidate for assessing HIV-associated brain injury. Grey and white matter abnormalities have been reported in virally suppressed patients, however the relationship between these findings and HIV-associated cognitive impairment are inconsistent.3,4,6,9 Therefore, it is unclear if these changes have the specificity to discriminate between those with and without cognitive impairment with sufficient accuracy to be clinically useful.
These data have relied on the traditional approach of analysing neuroimaging data, namely to derive biologically-interpretable summary measures from regions of interest, which can then be analysed with analysis of variance or its variants. To assess spatial relationships a voxelwise ‘mass-univariate approach’ is commonly used. While these approaches have been the building blocks of modern neuroscience, they have limitations. This mass-univariate approach tests a model at each voxel based on data across a group of subjects resulting in inferences that are valid at a group level. However, these findings have limited application for prediction at the individual level, thereby limiting their clinical impact.10 A multivariate approach aims to do the reverse, namely use many voxels to predict a variable of interest.11 This approach is particularly suited to neuroimaging data, given its inherent high-dimensionality and multi-collinearity, and has potentially greater sensitivity to detect subtle, spatially distributed patterns of anatomy or activation allowing prediction at an individual level.10,12
Multivariate techniques, such as machine learning models, can broadly speaking be used for classification or regression problems. The difference between them is whether the outcome variable to be predicted is discrete (classification) or continuous (regression). Supervised learning using labelled training data has yielded success in predicting cognitive impairment in other disease areas,10 for example allowing the accurate classification of Alzheimer’s disease13 and white matter injury following traumatic brain injury (TBI).14 These multivariate approaches have barely been applied in HIV-disease but conceptually there is no difference from other diseases. Zhang et al15 used a multivariate classification model that was able to distinguish patients with HIV-associated neurocognitive dysfunction from HIV-negative individuals with mild cognitive impairment. In another study, Wade et al16 distinguished HIV-positive individuals from HIV-negative controls using a random forest classifier. However, in both of these studies viral suppression was not universal, which is likely to accentuate differences between HIV-positive and HIV-negative controls, therefore limiting the applicability of these findings to well-treated patients.
An alternative approach to building a model to predict a specific disease is to model how the ageing process itself influences brain structure. Deviation of brain age predicted using neuroimaging data from chronological age, or brain-predicted age difference (brain-PAD) may result from neurodegeneration secondary to multiple insults that share a final common pathophysiological pathway of atrophy. Brain-PAD has recently been shown to be a potential biomarker of brain ageing that relates to cognitive ageing, physiological ageing and risk of death.17 Using the same technique, accelerated ageing has been reported following traumatic brain injury,18 whereas study of HIV-infected individuals has suggested premature but not accelerated brain ageing.19 However, this method has not been tested in other HIV-positive cohorts and its ability to predict longitudinal cognitive function has not been determined.
Compared with prediction of cognitive impairment at baseline, the ability to be able to accurately predict longitudinal changes has the most clinical utility. Here, we sought to assess the prevalence of cognitive impairment and longitudinal decline in virally suppressed HIV-positive individuals. As a proof of principle, we aimed to build a generalisable, multivariate model using as inputs only T1-weighted MRI data and basic pre-processing to test two main hypotheses. Firstly, that cognitive function could be predicted from volumetric T1-weighted MRI data, and secondly, confirm that brain-predicted age exceeded chronological age in virally suppressed HIV-positive individuals and this deviation would be associated with longitudinal decline in cognitive function.
Methods
Participants
For these analyses, HIV-positive participants from the CHARTER cohort were included if they had plasma HIV RNA <50 copies/ml at the time of baseline neuroimaging data (n=139). The original CHARTER cohort and the neuroimaging sub-study are described in more detail in Heaton et al3 and Jernigan et al.4 Longitudinal cognitive function data was collected approximately every six months and was available for the majority of patients (n=111 with 391 separate assessments of cognitive function, mean 3.5 visits). Comorbid conditions were classified as per Antinori et al20 into ‘incidental’ (e.g. none or only mild TBI with no functional sequelae), ‘contributing’ (e.g. mild TBI with evidence of mild functional sequelae) and ‘confounding’ comorbidities (e.g. TBI without return to work/school).
Cognitive function
All participants completed a comprehensive neuropsychological test battery testing the domains of attention, executive function, learning, recall, speed of information processing, motor function and verbal fluency as previously described.3 Demographically adjusted T-scores, where higher scores represent better cognitive function, were converted to deficit scores and cognitive impairment was defined by a global deficit score (GDS) ≥0.5.21 Longitudinal changes in cognitive function were defined using regression based change scores using an independent normative sample (n=296) as previously described.8,22 Briefly, this method calculates standardised z-scores for each test measure at each interval. These were then averaged to create a summary regression change score (sRCS), which was used to determine the longitudinal change status. The sRCS corresponding to the top 5% of the independent normative sample defined the improve status in this patient group, whereas the bottom 5% defined the decline status, with the remaining 90% defined as stable. For binary outcome analysis those labelled as stable or improve were combined as non-decliners.
Neuroimaging acquisition and pre-processing
T1-weighted MRI data were collected with General Electric 1.5T scanners at Johns Hopkins University (n=30); Mt. Sinai School of Medicine (n=25); University of California at San Diego (n=47); University of Texas Medical Branch (n=29) and the University of Washington (n=8). 3D T1-weighted images were acquired sagittally with spoiled gradient recall (SPGR), TR = 20ms, TE = 6ms, flip angle = 30°, field-of-view = 240mm,124 slices of 1.3mm, in-plane resolution 0.94x0.94mm as previously described.4
3D T1 images were pre-processed as described previously using SPM12 (University College London, UK).6 Briefly, images were bias corrected, segmented into grey matter, white matter and cerebrospinal fluid (CSF), volumes calculated with the sum representing the total intracranial volume (TICV). Segmented images were then registered to a custom template, normalised to Montreal Neurological Institute (MNI) space using the DARTEL algorithm,23 modulated to retain the volumetric characteristics of the original data and smoothed with a 6mm full width half maximum kernel. To take advantage of the complementary data provided by grey and white matter, spatially normalised grey and white matter volumetric maps were spatially concatenated prior to multivariate analysis.
Multivariate analysis
Supervised classification and regression was performed with the Pattern Recognition for Neuroimaging Toolbox (PRoNTo)12 software package v.2.0 (Machine Learning and Neuroimaging Lab, University College London). For the purposes of classification, models were computed with support vector machines (SVM) and Gaussian processes classification (GPC) for cognitive impairment defined using the GDS as previously described. To assess the ability of structural imaging data to predict cognitive function in a continuous nature the global T-score was used as an outcome with Gaussian processes regression (GPR) and kernel ridge regression (KRR) models. Similarly, for longitudinal prediction, again using the processed baseline T1-weighted data as inputs, separate models were tested as before using longitudinal change status and sRCS as outcome variables.
Given the relatively small numbers in each group (n=52 with cognitive impairment), predictive models were assessed using leave-one-out cross-validation (LOOCV). For classification, class (i.e., cognitively impaired or not) accuracy and predictive value were calculated for each model. Additionally, the area under the receiver operator curve (AUC) and the balanced accuracy (mean of class accuracies, important if groups are unbalanced) were calculated. This gives an estimation of the generalisability of the model to new data. For regression, model accuracy was quantified with the correlation (Pearson’s r), total variance explained (R2), mean absolute error (MAE) and root mean squared error (RMSE). P-values were calculated using permutation testing with 1,000 repetitions.
Predicting brain-age using a normative sample
An alternative multivariate approach was also used whereby each participant’s apparent brain age was determined based on data from 2,001 healthy subjects ranging from 18-90 years as previously described (see Supplementary Digital Content 1 for further details of data sources).18,19 The advantage of this model is that the training data were acquired across a range of different scanner hardware and acquisition protocols, therefore making this method potentially less prone to site/scanner related effects. This GPR model accurately predicted chronological age in the normative training set (r= 0.94, R2=0.88, MAE=5.01 years). Brain-predicted age difference (brain-PAD), a measure of deviance from the normal ageing trajectory was calculated as follows: brain-predicted age – chronological age so that positive scores represented brains that appear ‘older’ than expected.
Statistical analysis
Univariate analyses were performed using Chi-squared, independent sample t-tests, Wilcoxon rank sum tests as appropriate and in the case of comorbidity using ANOVA with post-hoc testing performed using Tukey’s honest significant difference. Relationships between cognitive domains and brain-PAD were determined with multiple linear regression covarying for age, comorbidity status, scanner and TICV. Similarly, the relationship between brain-PAD and clinical variables was determined using multiple regression models with the same covariates. Both binary logistic regression and multiple linear regression were used to assess the relationship between longitudinal change status and sRCS with brain-PAD accounting for the same covariates. These analyses were performed using R v3.2.1 and p-values <0.05 (two-tailed) were considered significant.
Results
The baseline characteristics for the CHARTER participants (n=139) are described in Table 1. Potentially confounding medical problems were common, with 11.5% having ‘confounding’ and 25.2% having ‘contributing’ comorbidities. All participants had suppressed plasma HIV RNA but 2/121 (1.7%) had detectable CSF HIV RNA. Of these, one had CSF HIV RNA of 780 copies/ml and the other at the limit of detection at 50 copies/mL.
Table 1.
Baseline demographics
| Demographic | n=139 |
|---|---|
| Age (years), median (IQR) | 44 (44-50) |
| Male, n (%) | 110 (79.1%) |
| Ethnicity, n (%) | |
| White | 64 (46.0%) |
| Black | 61 (43.9%) |
| Hispanic | 13 (9.4%) |
| Other | 1 (0.7%) |
| Comorbidity, n (%) | |
| Incidental | 88 (63.3%) |
| Contributing | 35 (25.2%) |
| Confounding | 16 (11.5%) |
| Years of education, median (IQR) | 13.0 (12.0-15.0) |
| Weight (kg), median (IQR) | 77.7 (70.5-87.8) |
| Duration of HIV-infection (years), median (IQR) | 12.2 (6.3-15.8) |
| Current CD4+ (cells/μL), median (IQR) | 540 (353-698) |
| CD4+:CD8+ cell count ratio, median (IQR) | 0.60 (0.42-0.91) |
| Nadir CD4+ (cells/μL), median (IQR) | 121 (20-237) |
| Duration of antiretroviral therapy (years), median (IQR) | 6.3 (2.6-9.4) |
| Plasma HIV RNA <50 copies/mL, n (%) | 139 (100%) |
| Prior clinical AIDS, n (%) | 55 (39.6%) |
Cognitive function
Using the global deficit score, cognitive impairment was present in 52 (37.4%) participants at the time of MRI scanning, which was more frequent in those with comorbidities (25/88 [28%] of those with ‘incidental’ comorbidities, 14/35 [40%] with ‘contributing’ and 13/16 [81%] with ‘confounding’, =16.3, p<0.001). However, only 6 (4.3%) met the criteria for HIV-associated dementia. Using regression based change scores, 17 (14.5%) experienced cognitive decline, 83 (75.5%) remained stable and 10 (9.1%) improved (Fig. 1). There was no association between longitudinal cognitive outcome and comorbidity status (p=0.21) nor cognitive impairment at baseline (p=0.34).
Figure 1.
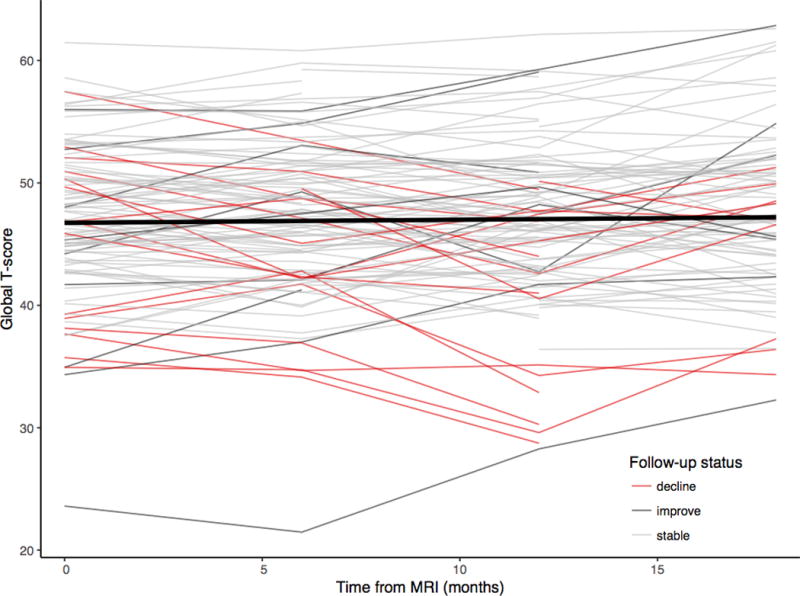
Spaghetti plot showing stability of global cognitive function over time.
Each line represents a participant’s longitudinal cognitive function. Red lines represent those with clinically significant cognitive decline. Thick black line represents the linear fit line across the entire group.
Multivariate prediction of cognitive function
Multivariate classification performance was not better than chance for both predicting baseline cognitive impairment nor patients who would experience longitudinal cognitive decline (Tables 2 & 3). Selecting subjects at random from the non-impaired or non-declining groups to ensure balanced subject numbers for training and testing the models yielded similar results (AUC for cross-sectional SVM: 0.55 and GPC 0.57; and for longitudinal changes SVM: 0.41 and GPC 0.43).
Table 2.
Prediction performance using structural imaging data for cognitive impairment defined by the global deficit score by model.
| Model | Accuracy (%) | p-value | PPV (%) | Accuracy (%) | p-value | PPV (%) | Balanced accuracy (%) | AUC | p-value |
|---|---|---|---|---|---|---|---|---|---|
|
Not impaired (n=87)
|
Impaired (n=52)
|
Overall
|
|||||||
| SVM | 72.4 | 0.33 | 65.0 | 34.6 | 0.25 | 42.9 | 53.5 | 0.56 | 0.22 |
| GPC | 77.0 | 0.56 | 65.7 | 32.7 | 0.10 | 46.0 | 60.4 | 0.59 | 0.14 |
|
Non-decliners (n=93)
|
Decliners (n=17)
|
Overall
|
|||||||
| SVM | 97.9 | 0.48 | 84.3 | 0.0 | 1.00 | 0.00 | 48.9 | 0.42 | 0.63 |
| GPC | 98.9 | 0.86 | 84.4 | 0.0 | 1.00 | 0.00 | 49.5 | 0.42 | 0.87 |
P-values calculated with permutation testing (1,000 repetitions). Abbreviations: AUC: area under the receiver operator curve; GPC: Gaussian process classification; PPV: positive predictive value; SVM: support vector machine.
Table 3.
Multivariate prediction performance of the global T-score using structural imaging data by model.
| Model (n=139) | Correlation | p-value | R2 | p-value | MSE | p-value | Norm MSE | p-value |
|---|---|---|---|---|---|---|---|---|
| GPR | 0.27 | 0.001 | 0.07 | 0.28 | 37.7 | 0.001 | 1.00 | 0.001 |
| KRR | 0.28 | <0.01 | 0.08 | 0.02 | 41.5 | 0.02 | 1.10 | 0.02 |
P-values calculated with permutation testing (1,000 repetitions). Abbreviations: GPC: Gaussian process classification; KRR: kernel ridge regression; MSE: mean squared error.
Using a multiclass GPC to predict longitudinal change status (i.e. stable, improve or decline) also yielded poor results with a balanced accuracy of only 28.4% (p=0.82) (decline: 70.6%, p=0.94, positive predictive value [PPV] 13.2%; stable 14.5%, p=0.31, PPV 63.2%; improve 0%, p=1.00, PPV=0%).
Both multivariate regression models predicted baseline global T-scores similarly well (KRR r=0.28, p=0.02; GPR r=0.27, p=0.001), with the KRR model explaining ~8% of the variance in global T-scores (Table 3). A comparable linear model using total grey and white matter volumes did not predict global T-scores (model adjusted R2=0.01, F2,136=1.99, p=0.14). Using KRR to predict longitudinal changes in cognitive function in a continuous manner (sRCS) was however unsuccessful (r= -0.07, p=0.62, R2=0.00, p=0.66).
Multivariate prediction of brain age
Using an alternative multivariate modelling approach, HIV-positive individual’s brain-predicted age exceeded chronological age compared to the normative controls by mean (95% CIs) 1.17 (-0.14-2.53) years, p=0.08. Both the patients with detectable CSF HIV RNA had an elevated brain-PAD: 7.1 and 7.0 years. Using multiple regression models, there was no association between brain-PAD and age nor duration of known HIV-infection (p=0.28 and p=0.87). Relative to those with incidental comorbid conditions, brain-PAD was greater in those with ‘contributing’ (3.24 [0.26-6.23] years, p=0.03) and ‘confounding’ (5.87 [1.74-9.99] years, p<0.01) comorbid conditions (Fig. 2). Additionally, there was no relationship between current CD4 cell count, CD4:CD8 ratios nor hepatitis C status and brain-PAD (p=0.56, p=0.62 and p=0.13 respectively). Those with prior AIDS had elevated brain-PAD (3.03 [0.00-6.06], p=0.05) and there was a trend for a negative association between nadir CD4 cell counts and brain-PAD (0.69 [-0.08-1.45] years per 100 cell/μL decrease, p=0.08).
Figure 2.
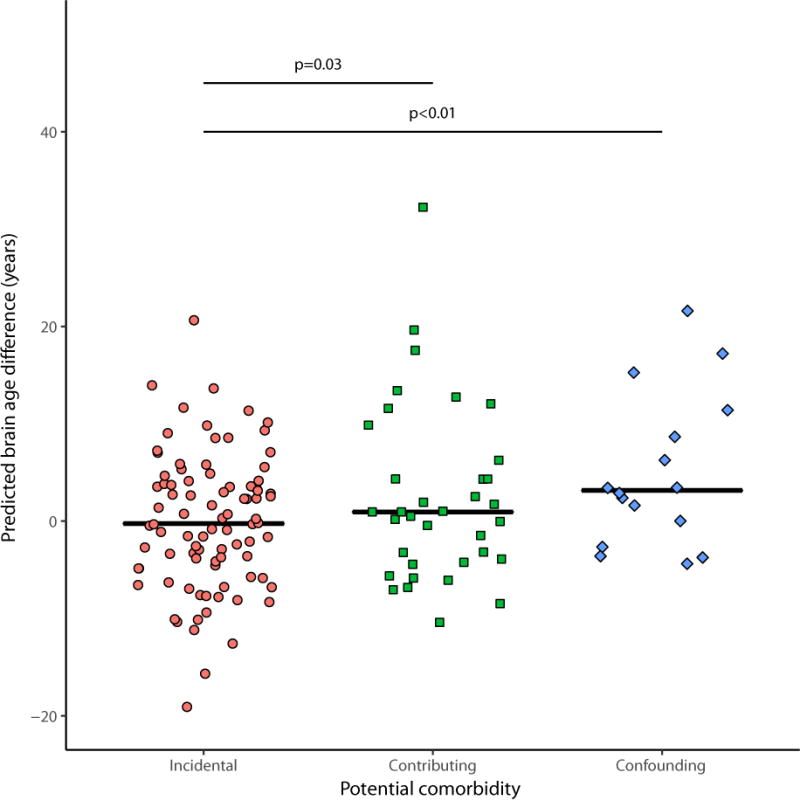
Jitterplot of brain-predicted age difference by comorbidity status.
Black lines represent the median for each group. Dotted line indicates brain-PAD in control population. P-values for comparisons between groups calculated using multiple regression models adjusted for age, intracranial volume and site/scanner.
Increased brain-PAD was independently associated with greater global deficit scores (regression estimate [95% CIs] 0.011 [0.001-0.022] years per unit increase in GDS, p=0.03). In univariate analysis, increased brain-PAD was negatively correlated with executive function, motor function and global T-scores with a negative trend for recall T-scores, however, after adjusting for potential confounders these associations were no longer significant (see Supplementary Digital Content 2 for details), nor was there a relationship with cognitive impairment at baseline (p=0.93). There was no association between brain-PAD and longitudinal changes in cognitive function (p=0.52), nor with longitudinal change status (see Supplementary Digital Content 3 for further details).
Discussion
Here, we show that cognitive decline occurred in 15% of HIV-positive individuals with sustained suppression of plasma viraemia. Whilst accurate discrimination between those with and without cognitive impairment was not possible using structural neuroimaging in this dataset, a multivariate model could predict baseline cognitive function as a continuous measure, while an equivalent linear model could not. Successfully treated HIV-positive individuals also had older appearing brains than expected confirming previous work. This increase in apparent brain age was not associated with duration of HIV infection nor age, which is suggests accentuated rather than accelerated ageing. None of the multivariate models could predict longitudinal changes in cognitive function.
Multivariate machine learning analyses of HIV-disease are sparse. Recently, Zhang et al15 reported a multivariate classification model that distinguished HIV-associated neurocognitive dysfunction from mild cognitive impairment in HIV-negative individuals longitudinal T1-weighted data. However, the number of patients with impairment in this study was small (n=15) and only 80% had undetectable plasma HIV RNA. Using a random forest classifier on subcortical morphometry data, Wade et al16 distinguished HIV-positive individuals from HIV-negative controls with an AUC of 0.72. However, only 38% of patients from this study had undetectable plasma viral loads, which is likely to accentuate differences from controls and again limits the applicability of these findings to well-treated patients.
One explanation for the inaccuracy of the present work compared to the previous studies may be that all the participants here were on fully suppressive antiretroviral therapy, which may limit the extent of progressive HIV-associated brain injury.24 Recent work from cohorts in North America25 and Europe26 has suggested that virally suppressed PLWH do not experience cerebral atrophy at an increased rate compared to HIV-negative controls despite evidence of reduced grey matter volume at study entry. Therefore, predicting HIV-associated longitudinal deteriorations in cognitive function on the basis of initial scans may be challenging. Furthermore, these data are consistent with our finding or stable cognitive function in the vast majority of virally suppressed PLWH. The limited classification performance reported here may also be reflective of the models not being sufficiently accurate and the relatively small sample size. However, using a regression approach cognitive function could be predicted with modest accuracy and for binary classification we used both SVM and GPC, which have been used successfully in other disease areas with comparable sample sizes.10 One interpretation of these data is that in contrast to Alzheimer’s disease, HIV-associated cognitive impairment is generally mild in treated patients and not associated with sufficient brain volume reduction to enable accurate delineation between those with and without cognitive impairment. This is supported by only 4.3% meeting the criteria for HIV-associated dementia. A potential limitation is that only the GDS method of defining cognitive impairment was tested. Other, more specific methods of defining HIV-associated cognitive impairment may be more clearly associated with brain injury. Another limitation is the multi-scanner nature of the data. This potentially increases variability unrelated to disease or cognitive function therefore limiting sensitivity and power. However, for a predictive neuroimaging model of cognitive impairment to be truly generalisable it would need to maintain accuracy across a reasonable range of acquisition protocols, scanner vendors and field strengths. As only 15% of well-treated patients experienced cognitive decline, accurate training of a classifier was not possible. Further study with a larger sample size studied over a longer time frame is necessary to determine whether predicting longitudinal cognitive decline in virally suppressed patients is possible. However, given the lack of any relationship between baseline volumetric data and longitudinal cognitive function, it is unlikely a larger sample would result in a sufficiently accurate model to be useful and other imaging modalities may be necessary to incorporate into the model.
The finding of older appearing brains in HIV-disease is in agreement with previous data with a similar, modest increase in apparent brain age.19 This study also showed an association of brain-PAD with cognitive data, in contrast to the findings presented here. In this subset of the CHARTER cohort, the relationship between brain-PAD and cognitive function was confounded by comorbidity status. CHARTER recruited patients with minimal exclusions, whereas the COBRA study recruited participants without potentially confounding comorbidities, which may explain the differences in results. The finding of increased brain-PAD complement those of Horvath et al27 who reported epigenetic changes suggestive of accelerated ageing in both the blood and brain of HIV-infected individuals. However, in contrast to the conclusion of that study, we found no evidence of accelerated brain ageing as age and duration of infection were not associated with increased brain-PAD. Also the modest increase in apparent brain age is less than that suggested by epigenetic changes.27 In contrast to Horvath et al27 all the patients presented here had suppressed plasma HIV RNA which is likely to ameliorate ongoing HIV-associated brain injury.
The cause of this accentuated or premature ageing is not clear. It is likely to reflect brain injury from HIV replication in the central nervous system, which is supported by the association of greater brain-PAD in those with prior AIDS as well as the negative trend with nadir CD4 cell count – both suggestive of greater brain injury in those with longer duration of untreated infection. However, it should be noted that comorbidity status was clearly associated with brain-PAD, had a larger effect size than HIV-status (when compared to the normative control dataset) and drove most of the associations with cognitive function given the attenuations of the associations after adjustment. This may reflect a final common pathway of pathology with many different types of neural insult converging in atrophy – mimicking the changes associated with age. These findings highlight the importance of accounting for comorbid conditions when it comes to correctly interpreting the aetiology of cognitive and neuroimaging abnormalities in PLWH. If these conditions are not accounted for in either study design or analysis the effect size of HIV may be overestimated. An important limitation, is that this model was designed to detect the changes associated with healthy brain ageing and therefore it may underestimate HIV-associated brain injury if it is distinct from the ageing process. Additionally, the lack of a study specific control group makes the interpretation of measures ‘in years’ less reliable despite the large normative sample used to train the model.
In conclusion, while superior to a comparable linear model, the performance of multivariate models to predict cognitive impairment and longitudinal cognitive decline fall well short of being clinically useful. Accounting for comorbidity is crucial when assessing brain injury in treated HIV-disease given the small proportion of virally suppressed HIV-positive individuals with severe cognitive impairment or who experience cognitive decline over time.
Supplementary Material
Supplementary digital content 1.docx Data sources for normative healthy brain age sample used to develop predictive model of brain ageing.
Supplementary digital content 2.docx Regression estimates showing the relationships between predicted brain age difference and cognitive function.
Supplementary digital content 3.docx Jitterplot of predicted brain age difference by change in cognitive function over time.
Acknowledgments
The CNS HIV Anti-Retroviral Therapy Effects Research was supported by awards N01 MH22005, HHSN271201000036C and HHSN271201000030C from the National Institutes of Health.
The CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) group is affiliated with Johns Hopkins University; the Icahn School of Medicine at Mount Sinai; University of California, San Diego; University of Texas, Galveston; University of Washington, Seattle; Washington University, St. Louis; and is headquartered at the University of California, San Diego and includes: Director: Igor Grant, M.D.; Co-Directors: Scott L. Letendre, M.D., Ronald J. Ellis, M.D., Ph.D., Thomas D. Marcotte, Ph.D.; Center Manager: Donald Franklin, Jr.; Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), J. Allen McCutchan, M.D.; Laboratory and Virology Component: Scott Letendre, M.D. (Co-P.I.), Davey M. Smith, M.D. (Co-P.I.).; Neurobehavioral Component: Robert K. Heaton, Ph.D. (P.I.), J. Hampton Atkinson, M.D., Matthew Dawson; Imaging Component: Christine Fennema-Notestine, Ph.D. (P.I.), Michael J Taylor, Ph.D., Rebecca Theilmann, Ph.D.; Data Management Component: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman; Statistics Component: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D.; Johns Hopkins University Site: Ned Sacktor (P.I.), Vincent Rogalski; Icahn School of Medicine at Mount Sinai Site: Susan Morgello, M.D. (Co-P.I.) and David Simpson, M.D. (Co-P.I.), Letty Mintz, N.P.; University of California, San Diego Site: J. Allen McCutchan, M.D. (P.I.); University of Washington, Seattle Site: Ann Collier, M.D. (Co-P.I.) and Christina Marra, M.D. (Co-P.I.), Sher Storey, PA-C.; University of Texas, Galveston Site: Benjamin Gelman, M.D., Ph.D. (P.I.), Eleanor Head, R.N., B.S.N.; and Washington University, St. Louis Site: David Clifford, M.D. (P.I.), Muhammad Al-Lozi, M.D., Mengesha Teshome, M.D.
The views expressed in this article are those of the authors and do not reflect the official policy or position of the United States Government.
Potential conflicts of interest
AW has received honoraria or research grants from or been a consultant or investigator in clinical trials sponsored by Abbott, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Janssen-Cilag, Roche, Pfizer and ViiV Healthcare.
References
- 1.England PH. HIV in the United Kingdom: 2014 Report. Public Health England; 2014. [Google Scholar]
- 2.INSIGHT START Study Group. Lundgren JD, Babiker AG, et al. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med. 2015;373(9):795–807. doi: 10.1056/NEJMoa1506816.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heaton RK, Clifford DB, Franklin DR, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jernigan TL, Archibald SL, Fennema-Notestine C, et al. Clinical factors related to brain structure in HIV: the CHARTER study. J Neurovirol. 2011;17(3):248–257. doi: 10.1007/s13365-011-0032-7.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Su T, Schouten J, Geurtsen GJ, et al. Multivariate normative comparison, a novel method for more reliably detecting cognitive impairment in HIV infection. AIDS. 2015;29(5):547–557. doi: 10.1097/QAD.0000000000000573.. [DOI] [PubMed] [Google Scholar]
- 6.Underwood J, Cole JH, Caan M, et al. Gray and White Matter Abnormalities in Treated Human Immunodeficiency Virus Disease and Their Relationship to Cognitive function. CLIN INFECT DIS. 2017 Apr; doi: 10.1093/cid/cix301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sacktor N, Skolasky RL, Seaberg E, et al. Prevalence of HIV-associated neurocognitive disorders in the Multicenter AIDS Cohort Study. Neurology. 2015 Dec; doi: 10.1212/WNL.0000000000002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heaton RK, Franklin DR, Deutsch R, et al. Neurocognitive change in the era of HIV combination antiretroviral therapy: the longitudinal CHARTER study. CLIN INFECT DIS. 2015;60(3):473–480. doi: 10.1093/cid/ciu862.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Su T, Caan MWA, Wit FWNM, et al. White matter structure alterations in HIV-1-infected men with sustained suppression of viraemia on treatment. AIDS. 2015 doi: 10.1097/QAD.0000000000000945. Publish Ahead of Print:1. [DOI] [PubMed] [Google Scholar]
- 10.Orrù G, Pettersson-Yeo W, Marquand AF, Sartori G, Mechelli A. Using Support Vector Machine to identify imaging biomarkers of neurological and psychiatric disease: A critical review. Neuroscience & Biobehavioral Reviews. 2012;36(4):1140–1152. doi: 10.1016/j.neubiorev.2012.01.004.. [DOI] [PubMed] [Google Scholar]
- 11.Pereira F, Mitchell T, Botvinick M. Machine learning classifiers and fMRI: a tutorial overview. NeuroImage. 2009;45(1 Suppl):S199–S209. doi: 10.1016/j.neuroimage.2008.11.007.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schrouff J, Rosa MJ, Rondina JM, et al. PRoNTo: pattern recognition for neuroimaging toolbox. Neuroinformatics. 2013;11(3):319–337. doi: 10.1007/s12021-013-9178-1.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vemuri P, Gunter JL, Senjem ML, et al. Alzheimer’s disease diagnosis in individual subjects using structural MR images: validation studies. NeuroImage. 2008;39(3):1186–1197. doi: 10.1016/j.neuroimage.2007.09.073.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hellyer PJ, Leech R, Ham TE, Bonnelle V, Sharp DJ. Individual prediction of white matter injury following traumatic brain injury. Ann Neurol. 2013;73(4):489–499. doi: 10.1002/ana.23824.. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Kwon D, Esmaeili-Firidouni P, et al. Extracting patterns of morphometry distinguishing HIV associated neurodegeneration from mild cognitive impairment via group cardinality constrained classification. Hum Brain Mapp. 2016 Aug; doi: 10.1002/hbm.23326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wade BSC, Valcour VG, Wendelken-Riegelhaupt L, et al. Mapping abnormal subcortical brain morphometry in an elderly HIV+ cohort. Neuroimage Clin. 2015;9:564–573. doi: 10.1016/j.nicl.2015.10.006.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cole JH, Ritchie SJ, Bastin ME, et al. Brain age predicts mortality. Mol Psychiatry. 2017;14:485. doi: 10.1073/pnas.081072698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cole JH, Leech R, Sharp DJ, for the Alzheimer’s Disease Neuroimaging Initiative Prediction of brain age suggests accelerated atrophy after traumatic brain injury. Ann Neurol. 2015;77(4):571–581. doi: 10.1002/ana.24367.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cole JH, Underwood J, Caan MWA, et al. Increased brain-predicted aging in treated HIV disease. Neurology. 2017 Mar; doi: 10.1212/WNL.0000000000003790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carey CL, Woods SP, Gonzalez R, et al. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol. 2004;26(3):307–319. doi: 10.1080/13803390490510031.. [DOI] [PubMed] [Google Scholar]
- 22.Cysique LA, Franklin D, Abramson I, et al. Normative data and validation of a regression based summary score for assessing meaningful neuropsychological change. J Clin Exp Neuropsychol. 2011;33(5):505–522. doi: 10.1080/13803395.2010.535504.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007.. [DOI] [PubMed] [Google Scholar]
- 24.Corrêa DG, Zimmermann N, Tukamoto G, et al. Longitudinal assessment of subcortical gray matter volume, cortical thickness, and white matter integrity in HIV-positive patients. J Magn Reson Imaging. 2016;44(5):1262–1269. doi: 10.1162/jocn.2009.21047.. [DOI] [PubMed] [Google Scholar]
- 25.Sanford R, Fellows LK, Ances BM, Collins DL. Association of Brain Structure Changes and Cognitive Function With Combination Antiretroviral Therapy in HIV-Positive Individuals. JAMA Neurol. 2018;75(1):72–79. doi: 10.1001/jamaneurol.2017.3036.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cole JH, Caan MWA, Underwood J, et al. No evidence for accelerated ageing-related brain pathology in treated HIV: longitudinal neuroimaging results from the Comorbidity in Relation to AI… - PubMed - NCBI. CLIN INFECT DIS. 2018 doi: 10.1093/cid/cix1124. [DOI] [PubMed] [Google Scholar]
- 27.Horvath S, Levine AJ. HIV-1 Infection Accelerates Age According to the Epigenetic Clock. Journal of Infectious Diseases. 2015;212(10):1563–1573. doi: 10.1093/infdis/jiv277.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary digital content 1.docx Data sources for normative healthy brain age sample used to develop predictive model of brain ageing.
Supplementary digital content 2.docx Regression estimates showing the relationships between predicted brain age difference and cognitive function.
Supplementary digital content 3.docx Jitterplot of predicted brain age difference by change in cognitive function over time.


