Abstract
The investigation of the corticomotor connectivity (CMC) to leg muscles is an emerging research area, and establishing reliability of measures is critical. This study examined the measurement reliability and the differences between bilateral soleus (SOL) and tibialis anterior (TA) CMC in 21 neurologically intact adults. Using single pulse transcranial magnetic stimulation (TMS), each muscle’s CMC was assessed twice (7 ± 2 days apart) during rest and active conditions. CMC was quantified using a standardized battery of eight measures (4/condition): resting motor threshold (RMT) during resting, motor evoked potential amplitude and latency (raw and normalized to height) in both conditions, contralateral silent period (CSP) during active. Using two reliability metrics (intraclass correlation coefficient and coefficient of variation of method error; good reliability: ≥0.75 and ≤15, respectively) and repeated-measures ANOVA, we investigated the reliability and Muscle X Body Side interaction. For both muscles, RMT, resting raw and normalized latencies, and active raw latency demonstrated good reliability, while CSP had good reliability only for TA. Amplitude did not demonstrate good reliability for both muscles. SOL CMC were significantly different from TA CMC for all measures but CSP; body side had no significant effect. Therefore, only certain measures may reliably quantify SOL and TA CMC while different CMC (except CSP) between SOL and TA suggests dissimilar corticospinal drive to each muscle regardless of the side.
Keywords: reliability, neuronavigation, soleus, tibialis anterior, transcranial magnetic stimulation, lower extremity
1. Introduction
The functional state of the neural connection between the motor cortex and a target muscle, corticomotor connectivity (CMC), can be assessed using transcranial magnetic stimulation (TMS)(Hallett, 2007). While most of the literature to date has focused mainly on CMC of the upper extremity muscles, a growing number of studies have gradually begun investigating the CMC of lower extremity muscles, including the soleus (SOL) and tibialis anterior (TA). Given the functional importance of SOL and TA in functional walking, several studies have investigated those muscles’ CMC either before, during, or after various tasks (Capaday et al., 1999, Mouthon et al., 2015, Obata et al., 2009) in various cohorts (Kumpulainen et al., 2015, Palmer et al., 2017, Thomas and Gorassini, 2005). However, despite the increased interest in examining SOL and TA CMC in different tasks and patient populations, gaps still exist regarding the CMC of these two ankle joint muscles in neurologically intact adults.
A thorough reliability analysis is required for both SOL and TA CMC during both rest (i.e., target muscle is not contracted) or active contraction (i.e., target muscle is actively contracted at a percentage of maximum isometric voluntary contraction, MVIC). The reliability (intra-rater, inter-rater, and test-retest) of these measures in neurologically intact adults has been inconsistently examined in the past decade, partly due to the variation in methodology and laboratory set-up. These studies have demonstrated that some SOL (Gray et al., 2017, Lewis et al., 2014) and TA (Cacchio et al., 2009, Chung and Mak, 2015, Forster et al., 2014, Forster et al., 2012, Souron et al., 2016, Tallent et al., 2012, van Hedel et al., 2007) CMC measures can be reliably assessed in healthy adults, including resting and active motor thresholds, amplitude and latency of the motor evoked potential (MEP), and cortical silent period (CSP). Despite the valuable information that these studies have provided to the field, several methodological considerations make compilation of the reliability data difficult. A need exists for a comprehensive reliability examination using a standardized methodology.
Inconsistencies in lower extremity reliability TMS studies include the following: 1) each of the reliability studies only investigated one muscle, either SOL or TA; therefore, the reliability assessment of one muscle was independently conducted from the other; and 2) the TMS-related methodological parameters, such as type of coil (e.g., circular coil, figure-of-eight coil, and double cone coil), stimulator type (e.g., monophasic, biphasic), use of neuronavigation software, muscle state (e.g., either rest or active), level of muscle state during active contraction (e.g., 10, 10-20, 30-80 %), and CMC measures of interest varied widely across studies. This substantial variability of testing parameters is of critical concern in the interpretation of reliability data (Beaulieu et al., 2017a), given their possible effects on the quantified CMC and the reproducibility of methods and results (Baker, 2016).
As the methodology for lower extremity TMS testing has evolved, it has become clear that upper extremity reliability findings cannot be simply extrapolated to the lower extremity. Compared to CMC hand muscle assessment, measuring the CMC of a distal leg muscle involves increased complexity due to the anatomical properties of the leg motor areas. These areas are located adjacent to the interhemispheric fissure at approximately 3-4 cm below the scalp surface, (Alkadhi et al., 2002, Conti et al., 2014, Terao and Ugawa, 2002) while the axons of the corticospinal neurons are oriented perpendicular to the medial cortical surface. In addition, these areas are relatively small and are less segregated than the hand muscle areas (Conti, Raffa, 2014, Saisanen et al., 2010). Therefore, precise activation of leg motor areas requires cautious selection of stimulation parameters (e.g., type of coil, finding the optimal hotspot of each muscle separately, use of neuronavigation) that may influence the measured CMC of a muscle (Ridding and Ziemann, 2010).
Given that each muscle has a different function and action for ankle mechanics, it is unclear whether CMC differs between these two muscles, as well as between the two legs (i.e., body side). The SOL and TA likely have a similar number of corticomotoneuronal connections, but the strength of these connections is weaker in SOL than in TA (Bawa et al., 2002). Weak responses of SOL to TMS might potentially be related to the contribution from other descending pathways (e.g., corticorubrospinal, corticoreticulospinal, etc.) to SOL activation (Nielsen and Petersen, 1995). Also, the different functional role that each muscle plays may explain this discrepancy in the strength of the measured CMC. Both muscles influence ankle motion during upright postural tasks and walking (Winter, 1991, Winter, 1995), but they differ in their primary function and action. SOL is an antigravity muscle designed to generate high force with small excursion of the muscle (Lieber and Friden, 2000), especially when the foot is in contact with the ground and sensory feedback is present (e.g., stance phase of walking and upright standing posture). In contrast, TA is less important for high force production and more functional for long muscle excursions (Lieber and Friden, 2000), especially when sensory feedback is minimum (e.g., swing phase of walking). Therefore, activation of SOL may not rely on the corticospinal tract to the same degree as TA given that other subdivisions of the nervous system may contribute to its activation (e.g. spinal modulation of sensory feedback). Moreover, given that leg dominance is not as robust as arm dominance and leg muscles are bilaterally active during upright static and dynamic motor tasks, it is crucial to assess whether the CMC of each muscle differs between legs. If the SOL or TA CMC differs between legs, this would indicate either stronger unilateral connectivity (i.e., indirect neurophysiological proxy of footedness) or a neurological insult along the neuromotor axis of the target muscle.
Therefore, the primary aim of this exploratory investigation was two-fold. First, we aimed to determine the intra-rater test-retest reliability, which was assessed using two measures (intraclass correlation coefficient – ICC and coefficient of variation of method error - CVME), for a comprehensive set of commonly reported SOL and TA CMC measures. Second, we aimed to determine if CMC differs between muscles (SOL and TA) and body side (left and right lower extremities). We completed this study in a group of neurologically intact adults using a battery of CMC measures calculated by automated methods, MRI-guided TMS leg-specific methodology, and two independent reliability metrics to maximize the experimental and data analysis rigor. By addressing these aims, we designed this experiment to provide the field with the first comprehensive battery of CMC assessment measures for SOL and TA, allowing for bilateral quantification of CMC properties and between-muscle or between-limb comparisons.
2. Materials and Methods
2.1 Participants
Twenty-one right-leg dominant neurologically intact adults (gender: 8 women; mean ± SD, age: 42 ± 11 years, height: 174.2 ± 11.7 cm, body mass: 74.9 ± 16.7 kg) participated in this study. We excluded individuals who had any history of brain injury or pre-existing neurological disorder, and/or contraindications to TMS (Rossi et al., 2009). All participants completed MRI (Shellock and Spinazzi, 2008) and TMS (Rossi et al., 2011) screening questionnaires to ensure safety and eligibility for MRI and TMS testing. Written informed consent was obtained from all participants prior to the experimental procedures, which were approved by the Medical University of South Carolina Institutional Review Board and adhered to the Declaration of Helsinki.
2.2 Experimental Organization
Figure 1 presents the experimental flow. Participants attended two experimental sessions (7 ± 2 days apart). Both sessions occurred at similar time of the day to eliminate any influence of diurnal variation on neural excitability (Castaingts et al., 2004). Given the potential effect of caffeine (Cerqueira et al., 2006) and alcohol (Conte et al., 2008) on CMC, we instructed participants to avoid consuming either substance for at least 3 hours prior to experimental procedures.
Figure 1.
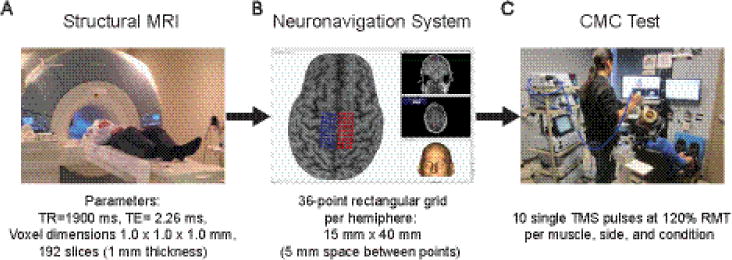
Flowchart of the experimental procedures prior to first session (A-B) and during both experimental sessions (C).
A) In a Siemens 3T TIM trio scanner (Siemens, Erlanger, Germany), participants were asked to keep still and wore earplugs to attenuate the scanner’s loud noise. B) Using Brainsight™ TMS frameless stereotaxy neuronavigation system, each participant’s MRI was co-registered to the anterior and posterior commissures, so each MRI could be mapped using the Montreal Neurological Institute atlas. Three-dimensional skin and curvilinear brain models were reconstructed. A skin model was used to identify the tip of the nose, nasion, and supratragic notch of the right and left ear, which were used to calculate the participant to image registration. A curvilinear brain model was used to manually identify the leg motor area on which a rectangular grid was overlaid below the cortical surface on each hemisphere. C) Single pulse TMS was applied bilaterally over the SOL and TA optimal hotspot using Brainsight™ while muscle responses were collected simultaneously from both legs during rest and active conditions. Both feet were secured in boots (7.5° of plantarflexion) to ensure a consistent lower extremity posture across participants and sessions, as well as ease of isometric ankle contractions. Additionally, to minimize any hip or knee motions, participants’ thighs were firmly strapped to the leg support pads.
2.3 Structural MRI and Neuronavigation System
To ensure accurate and precise positioning of the coil throughout the CMC testing and across visits, we used Brainsight™ (v2.2) neuronavigation system (Rogue Research Inc.; Montreal, Quebec, Canada) with the structural brain MRI of each participant. Before the first experimental session, participants attended a single structural MRI (magnetization-prepared rapid gradient-echo sequence) session (< 30 minutes) (Fig. 1A), and then shortly after, a Brainsight™ file for each participant was created (Fig. 1B).
2.4 EMG Recording
Following standard skin preparation and surface EMG (sEMG) electrode placement procedures (Hermens and Klug, 1999), sEMG electrodes (Motion Lab Systems; Baton Rouge, LN, USA) were attached over SOL (i.e., 2/3 of the line between the lateral femoral condyle to the lateral malleolus) and TA (i.e., 1/3 of the line between the tip of the fibula and the tip of the medial malleolus) bilaterally while a ground reference electrode (Natus Medical Incorporated; San Carlos, CA, USA) was placed on the patella. The signals were filtered (anti-alias filter of 1000 Hz), amplified (×2000) (Motion Lab Systems MA-300 system; Baton Rouge, LN, USA), sampled at 5 kHz (Cambridge Electronic Design Micro 1401-3; Cambridge, UK), and stored for offline analysis (Signal v5.11 and Spike2 v7.12, Cambridge Electronic Design; Cambridge, UK).
2.5 CMC assessment
Following standard recommended experimental and safety guidelines (Groppa et al., 2012, Rossini et al., 2015), all CMC related experimental procedures were replicated in both sessions across muscles and body sides to ensure the use of the exact same methodological procedures across participants. Participants sat in a comfortable position (trunk reclined ~5° from vertical) with both arms and shanks supported by armrests and lower limb support pads, respectively (Fig. 1C). In that posture, we assessed CMC of each muscle in two conditions. In the first condition (resting), the target muscle was fully relaxed. In the second condition (active contraction), the target muscle was slightly contracted at 15% MVIC. To determine MVIC, participants maximally contracted the target muscle 4 times (~5 second contractions separated by 1 minute). During each contraction, MVIC was determined using the EMG collected from the target muscle. The 15% MVIC was calculated from the highest value (i.e., average within a 100 ms window centered around the maximum rectified EMG) of the four contractions using Spike v7.15 (Cambridge Electronic Design; Cambridge, UK). During active contraction, we instructed participants to either slightly push down (plantarflexion) or pull against (dorsiflexion) the boot to match the moving line (which represented a rectified and low-pass filtered EMG of the target muscle) with the horizontal cursor (15% MVIC of the target muscle), and to sustain the contraction at that level for a few seconds. In both conditions, the muscle activity of all muscles was monitored by real-time visual feedback displayed on a computer screen located in front of the participant.
Single, monophasic TMS pulses (rise time: 100 μs; duration: 1ms) were applied using a double cone coil (120 mm outer wing diameter; maximum output: 1.4T), which induced a posteroanterior intracranial current. The coil was connected to a BiStim stimulator (The Magstim Company Limited; Whitland, UK) set at the standard mode (Sinclair et al., 2006). Throughout CMC testing, the time between stimuli ranged pseudo-randomly from 5-10 s to avoid stimulus anticipation and to minimize carry-over effects of the previous pulse (Awiszus, 2003). In each session, we used the neuronavigated 36-spot grid over each hemisphere (Fig. 1B) to locate bilateral optimal hotspots for each muscle using a suprathreshold intensity, which was determined by applying a single stimulus over the centered spot next to the medial fissure prior to hotspot search. We chose to use that single spot to achieve consistency between days and across participants, and because that spot is located at the locus of the leg motor area (Conti, Raffa, 2014). We always began at low intensitities (e.g., ~ 30% maximum stimulator output; MSO) and gradually increased the TMS intensity by 5% increments, until we reached the intensity that elicited three concecutive MEPs with peak-to-peak amplitudes greater than 50 μV in the contralateral target muscle. Next, we applied a single pulse over each each spot of the grid using that intensity; stimulation order was always the same. Then, the optimal hotspot was defined as the spot in the grid where a single stimulus applied over that spot elicited the largest response, which was quantified by the peak-to-peak amplitude. Thus, each optimal hotspot, which was the scalp position over the virtual spot of the neuronavigated grid, was used to determine the resting motor threshold (RMT) of each muscle and to apply 10 stimuli at 120% RMT of each muscle in both conditions.
We identified the RMT twice for each target muscle at rest using an adaptive threshold-hunting method, simple adaptive parameter estimation by sequential testing (SA-PEST) (Awiszus and Borckardt, 2011) (free software available at http://www.clinicalresearcher.org), and calculated the average RMT. We chose SA-PEST (starting intensity and initial step size: 45% and 6% MSO, respetively) over the relative frequency method (Groppa, Oliviero, 2012) because the former is more efficient (i.e., fewer stimuli) than the latter, even though both methods share similar precision (Silbert et al., 2013). The active motor threshold was not calculated due to the methodological limitations using an existing method (Rothwell et al., 1999) for lower extremities (e.g., background activity of leg muscles may exceed the 200-300 μV criterion). As active motor threshold is correlated with and lower than RMT (~ 82%)(Ngomo et al., 2012), a stimulus strength sufficient to generate a response under resting conditions is also sufficient to generate a response under active conditions. Therefore, to quantify all other metrics of CMC, 10 stimuli were applied at 120% RMT of each muscle in both conditions (i.e., 10 stimuli per muscle, side, and condition). The resting condition always preceded the active condition for methodological ease. During active, participants monitored and adjusted the contraction of the target muscle at the required 15% MVIC using real time visual biofeedback, which was the rectified and low-pass filtered EMG of the target muscle.
2.6 Data analysis
We quantified SOL and TA CMC by calculating eight measures; four measures per condition. Under both rest and active, we calculated the amplitude, raw latency, and normalized latency for each muscle. Additionally, RMT and CSP were measured only during rest and active, respectively. We reported RMT as the average of two measurements. For all other measures, we calculated the value from each MEP trace for all muscles and then averaged these 10 values to get a single value; Figure 2 presents schematically the MEP derived measures analysis. In addition to raw latency, normalized latency was calculated relative to each participant’s height (cm), as latency is influenced by distance to the target muscle ((msec/cm)*100) (Livingston et al., 2013). These were selected because each measure might reflect a different property of CMC. In general, it has been suggested that motor threshold reflects the general excitability of a muscle’s neuromotor axis, amplitude reflects the trans-synaptic activation of the corticomotoneuronal axons, and latency reflects the axonal conduction time from motor cortex to the target muscle (Ziemann et al., 2015). This group of measures thus characterize the global corticospinal control of the neuromotor axis. We also calculated CSP, a measure of intracortical control that reflects postsynaptic inhibitory mechanism in the primary motor cortex (Ziemann, Reis, 2015). We calculated all MEP derived measures using Matlab v8.1 (Mathworks, Inc.; Natick, MA, USA). To standardize the calculation of the MEP onset and offset in both conditions and the EMG resumption during active, we adapted previous published automated methods (Cacchio et al., 2011, Damron et al., 2008, Daskalakis et al., 2003).
Figure 2.
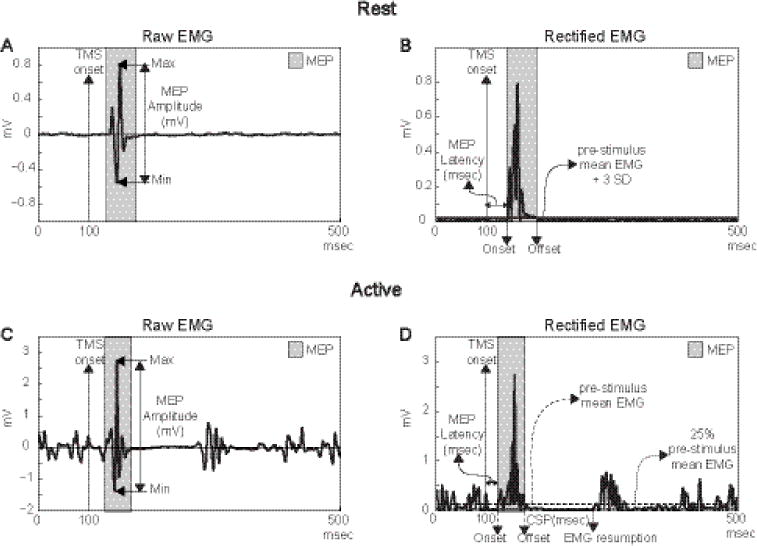
A schematic diagram of the MEP derived measures analysis during resting (A and B) and active (C and D) conditions.
In both conditions, MEP amplitude was defined as the largest difference between positive and negative peaks (A and C), while MEP latency was defined as the time between TMS onset and MEP onset (B and D). During active, CSP was also defined as the time between MEP offset and the resumption of baseline EMG (D).
2.7 Statistical analysis
As opposed to using only a single reliability metric, the intra-rater test-retest reliability for all SOL and TA CMC measures was determined based on two metrics: ICC and CVME. As it has been suggested and discussed in previous work (Beaulieu, Flamand, 2017a, Beaulieu et al., 2017b, Schambra et al., 2015), we chose this approach in order to assess two different measurement properties of reliability (reliabilityMP and measurement error) (Mokkink et al., 2010) using two metrics that are also independent from each other. As a metric of reliabilityMP (or relative reliability), ICC indicates the distinction between subjects within a sample; therefore, it can be used for diagnosis and/or prognosis (Schambra, Ogden, 2015). On the other hand, as a metric of measurement error (or absolute reliability), CVME reflects the discrepancy between repeated measurements (e.g., test and retest) within a sample (Portney and Watkins, 2009); therefore, it can quantify whether the target measure tracks changes well (e.g., before and after an intervention)(Atkinson and Nevill, 1998, Weir, 2005). To calculate ICC, we used the 2-way random model with absolute agreement type (A) and average measures form (k) and its 95% confidence interval (CI). To calculate CVME, we first calculated the method error (ME; ratio of the SD of the test-retest score differences to √2), and then we calculated CVME ((2ME/(Day 1 mean + Day 2 mean))*100) (Portney and Watkins, 2009). The advantage of using CVME instead of ME is that CVME is expressed as a percentage and has no units, similar to the ICC. ICC equal or above 0.75 and CVME equal or less than 15 usually suggests good reliability, yet no absolute standards exist (Portney and Watkins, 2009, Stokes, 1985). Given that we wanted to determine SOL and TA CMC measures that had both high reliability and low measurement error, we considered a CMC measure to have overall good reliability only if both ICC and CVME were ≥0.75 and ≤15, respectively.
In order to investigate whether CMC differs between muscles and body side, we performed a 2 × 2 repeated measures ANOVA using each CMC measure as a dependent variable, and MUSCLE (SOL vs. TA) and SIDE (Left vs. Right) as independent variables. For this analysis, we used data collected on day 2 (N=19).
IBM SPSS Statistics v24 (IBM Corp, Armonk, NY, USA) was used for all statistical tests and data is presented as means ± SD (CV - coefficient of variation).
3. Results
3.1 RMT: Responses and number of stimulations
Table 1 presents the number of stimuli used to determine the average RMT of each muscle and side in both days. Using the SA-PEST, fewer than 20 stimulation pulses were required on average to calculate RMT of both muscles. In 3 participants, SOL RMT could not be quantified, because measurable MEPs (greater than 50 μV) were inconsistently elicited using even maximal intensities (Day 1: 3 left, 2 right; Day 2: 2 left, 1 right). In contrast, measurable TA MEPs were consistently present. In one participant, the left TA RMT was not determined on day 1 due to technical issues.
Table 1.
Mean ± SD (CV) of number of stimuli required to determine bilaterally the average RMT of each muscle in both days using an adaptive threshold-hunting method.
| Muscle | Soleus | Tibialis Anterior | ||
|---|---|---|---|---|
| Body Side | Left | Right | Left | Right |
| Day 1 | 16 ± 8 (48) N (18) |
3 ± 5 (41) N (19) |
14 ± 4 (29) N (20) |
14 ± 6 (40) PN (21) |
| Day 2 | 16 ± 10 (64) N (19) |
15 ± 6 (41) N (20) |
14 ± 4 (28) N (21) |
13 ± 4 (33) N (21) |
Note: N denotes the number of subjects who had measurable MEPs per day, side, and muscle.
3.2 Reliability
Tables 2 and 3 present descriptive and reliability-related metrics for eight bilateral CMC measures of SOL (N=18) and TA (N=20), respectively, while figure 3 presents only the reliability-related metrics for all CMC measures of both muscles. In general, both RMT and latencies can reliably assess SOL CMC, whereas all measures except MEP amplitude can reliably assess TA CMC. For most SOL and TA CMC measures, both sides can be assessed and the muscle should preferably be at rest to maximize reliability.
Table 2.
Test-retest normative data (mean ± SD (CV)) and reliability (ICC and CVME) of eight SOL CMC measures
| Muscle State | Measure | Body Side | Day 1 Mean ± SD (CV) |
Day 2 Mean ± SD (CV) |
ICC95% CI | CVME |
|---|---|---|---|---|---|---|
| Rest | RMT (% MSO) |
Left |
53 ± 13 (24) |
52 ± 10 (19) |
0.93(0.82-0.97)¥ | 8† |
| Right |
52 ± 11 (22) |
52 ± 13 (25) |
0.93(0.82-0.98)¥ | 8† | ||
| Amplitude (mV) |
Left | 0.161 ± 0.187 (116) |
0.126 ± 0.066 (53) |
0.65(0.09-0.87) | 70 | |
| Right | 0.123 ± 0.062 (50) |
0.125 ± 0.057 (46) |
0.85(0.58-0.94)¥ | 25 | ||
| Latency (msec) |
Left |
37.8 ± 4.4 (12) |
37.2 ± 4.2 (11) |
0.95(0.86-0.98)¥ | 4† | |
| Right |
37.5 ± 4.4 (12) |
37.2 ± 4 (11) |
0.78(0.42-0.92)¥ | 7† | ||
| Normalized Latency ((msec/cm) *100) |
Left |
22 ± 2 (10) |
21 ± 2 (9) |
0.92(0.78-0.97)¥ | 4† | |
| Right | 22 ± 2 (10) |
21 ± 2 (11) |
0.72(0.24-0.90) | 7† | ||
| Active | CSP (ms) |
Left | 130.4 ± 47.0 (36) |
144.4 ± 47.9 (33) |
0.83(0.56-0.94)¥ | 18 |
| Right | 125.3 ± 45.4 (36) |
156.1 ± 41.7 (27) |
0.72(0.03-0.91) | 17 | ||
| Amplitude (mV) |
Left | 0.815 ± 0.511 (63) |
0.762 ± 0.508 (67) |
0.92(0.79-0.97)¥ | 25 | |
| Right | 0.766 ± 0.643 (84) |
0.864 ± 0.577 (67) |
0.93(0.83-0.98) ¥ | 27 | ||
| Latency (msec) |
Left | 32.6 ± 3.4 (10) |
34.5 ± 4.7 (13) |
0.64(0.1-0.86) | 8† | |
| Right |
35.2 ± 4.8 (14) |
33.6 ± 3.2 (10) |
0.78(0.40-0.92)¥ | 7† | ||
| Normalized Latency ((msec/cm) *100) |
Left | 19 ± 2 (10) |
20 ± 2 (9) |
0.52(−0.14-0.82) | 7† | |
| Right | 20 ± 2 (9) |
19 ± 2 (8) |
0.51(−0.21–0.81) | 7† |
Note: Bold text denotes that this measure for that side has an overall good reliability (i.e., both ICC and CVME were ≥0.75 and ≤15, respectively);
- denotes that the ICC for that measure was ≥0.75;
- denotes that the CVME for that measure was ≤ 15.
Table 3.
Test-retest normative data (mean ± SD (CV)) and reliability (ICC and CVME) of eight TA CMC measures
| Muscle State | Measure | Body Side | Day 1 Mean ± SD (CV) |
Day 2 Mean ± SD (CV) |
ICC(95% CI) | CVME |
|---|---|---|---|---|---|---|
| Rest | RMT (% MSO) |
Left |
52 ± 12 (26) |
50 ± 11 (23) |
0.90(0.74-0.96)¥ | 10† |
| Right |
48 ± 11 (23) |
48 ± 13 (23) |
0.89(0.73-0.96)¥ | 11† | ||
| Amplitude (mV) |
Left | 0.366 ± 0.315 (92) |
0.318 ± 0.168 (52) |
0.58(−0.07-0.83) | 57 | |
| Right | 0.466 ± 0.361 (81) |
0.385 ± 0.297 (80) |
0.84(0.60-0.94)¥ | 40 | ||
| Latency (ms) |
Left |
34.2 ± 2.5 (7) |
34.4 ± 3.6 (10) |
0.87(0.66-0.95)¥ | 4† | |
| Right |
33.7 ± 3.0 (9) |
34.3 ± 3.7 (10) |
0.93(0.82-0.97)¥ | 3† | ||
| Normalized Latency ((msec/cm) *100) |
Left | 20 ± 1 (5) |
20 ± 1 (8) |
0.70(0.21-0.88) | 4† | |
| Right |
19 ± 1 (5) |
20 ± 1 (6) |
0.77(0.43-0.91)¥ | 4† | ||
| Active | CSP (ms) |
Left | 153.8 ± 64.28 (42) | 168.9 ± 58.3 (35) | 0.90(0.74-0.96)¥ | 15† |
| Right | 145.7 ± 48.8 (33) |
143.9 ± 48.9 (34) |
0.79(0.46-0.92)¥ | 20 | ||
| Amplitude (mV) |
Left | 1.78 ± 0.85 (48) |
1.77 ± 0.99 (56) |
0.83(0.56-0.93)¥ | 29 | |
| Right | 1.81 ± 0.68 (38) |
1.67 ± 0.70 (42) |
0.82(0.56-0.93)¥ | 22 | ||
| Latency (msec) |
Left |
31.91 ± 2.98 (9) |
32.25 ± 2.85 (9) |
0.92(0.79-0.97)¥ | 4† | |
| Right |
31.94 ± 3.01 (9) |
32.45 ± 3.23 (10) |
0.93(0.82-0.97)¥ | 3† | ||
| Normalized Latency ((msec/cm) *100) |
Left | 18 ± 1 (7) |
19 ± 1 (6) |
0.71(0.25-0.88) | 5† | |
| Right | 19 ± 1 (6) |
19 ± 1 (6) |
0.69(0.21-0.88) | 4† |
Note: Bold text denotes that this measure for that side has an overall good reliability (i.e., both ICC and CVME were ≥0.75 and ≤15, respectively);
- denotes that the ICC for that measure was ≥0.75;
- denotes that the CVME for that measure was ≤ 15.
Figure 3.
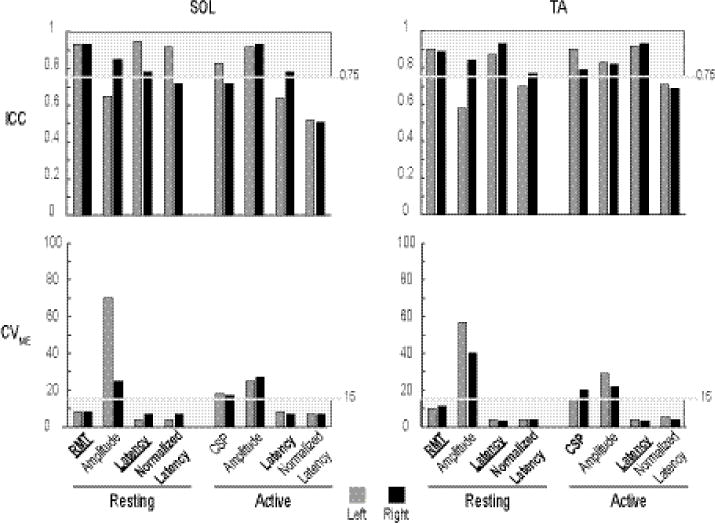
Bar plots of the reliability data (ICC – top row; CVME – bottom row) of SOL (left column) and TA (right column) of four measures per condition. Light grey shaded areas indicate the region of good reliability (ICC: 0.75-1; CVME: 0-15). Among the eight CMC measures of each muscle, only those indicated by the shaded area had good reliability (unilateral; bilateral).
SOL
During rest, good reliability was observed for RMT (Left – ICC: 0.93; CVME: 8, Right – ICC: 0.93; CVME: 8), latency (Left – ICC: 0.95; CVME: 4, Right – ICC: 0.78; CVME: 7), and normalized latency (Left – ICC: 0.92; CVME: 4). When active, only latency showed good reliability (Right - ICC: 0.78; CVME:7).
TA
During rest, RMT (Left – ICC: 0.90; CVME: 10, Right – ICC: 0.89; CVME: 11), latency (Left – ICC: 0.87; CVME: 4, Right – ICC: 0.93; CVME: 3), and normalized latency (Right – ICC: 0.77; CVME: 4) had good reliability. When active, CSP (Left −0.90; CVME: 15) and latency (Left −0.92; CVME: 4, Right – ICC: 0.93; CVME: 3) were the only measures that had good reliability.
3.3 Differences between muscle and body side
During rest, higher intensities were bilaterally required to elicit responses in SOL than in TA. In both conditions, bilateral amplitude was significantly lower in SOL than in TA, and both bilateral raw and normalized latencies were significantly longer in SOL than in TA. Interestingly, bilateral CSP was not significantly different between the two muscles. For both muscles, there was no difference between left and right leg for any CMC measure.
Resting (Fig. 4)
Figure 4.
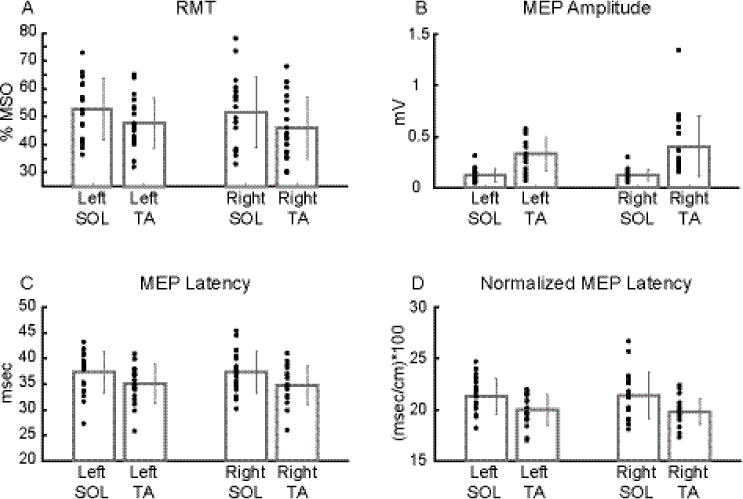
Average group data of each CMC measure between muscles and sides during rest.
Bilateral RMT (A), MEP latency (C), and normalized MEP latency (D) were significantly higher in SOL than TA. Bilateral MEP amplitude (B) was significantly lower in SOL than TA. Dark grey bar plots and error bars represent mean and ± 1 SD, respectively, while black dots indicate subject data (A: mean of two RMT values; B-D: mean of 10 MEP derived values).
No significant MUSCLE X SIDE interaction was detected for any measure (RMT: F1, 18 = 0.188, p = 0.669; amplitude: F1, 18 = 1.018, p = 0.326; latency: F1, 18 = 0.306, p = 0.587; normalized latency: F1, 18 = 0.448, p = 0.512). A main effect of MUSCLE was found for all four measures (RMT: F1, 18 = 20.092, p < 0.001; amplitude: F1, 18 = 41.948, p < 0.001; latency: F1, 18 = 16.970, p < 0.001; normalized latency: F1, 18 = 17.152, p < 0.001). No main effect of SIDE was detected for any measure (RMT: F1, 18 = 0.479, p = 0.498; amplitude: F1, 18 = 1.037, p = 0.322; latency: F1, 18 = 0.115, p = 0.739; normalized latency: F1, 18 = 0.068, p = 0.797).
Active (Fig. 5)
Figure 5.
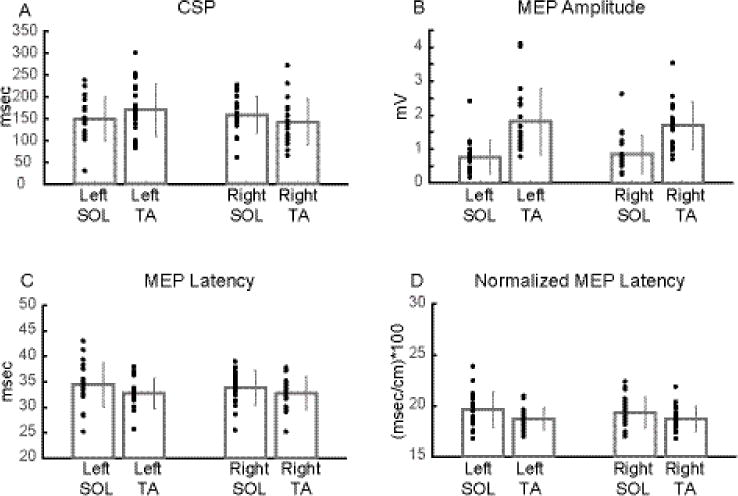
Average group data of each CMC measure between muscles and sides during active condition.
Bilateral CSP (A) did not significantly differ between muscles. MEP amplitude (B) was significantly lower in SOL than TA. Bilateral MEP latency (C) and normalized MEP latency (D) were significantly higher in SOL than TA. Dark grey bar plots and error bars represent mean and ± 1 SD, respectively, while black dots indicate subject data (A-D: mean of 10 MEP derived values).
There was a significant MUSCLE X SIDE interaction for CSP (F1, 18 = 9.491, p = 0.006) but not for the rest of the measures (amplitude: F1, 18 = 0.638, p = 0.435; latency: F1, 18 = 0.450, p = 0.511; normalized latency: F1, 18 = 0.134, p = 0.719). A main effect of MUSCLE was found for all measures (amplitude: F1, 18 = 51.381, p < 0.001; latency: F1, 18 = 17.390, p < 0.001; normalized latency: F1, 18 = 12.072, p = 0.003) but CSP (F1, 18 = 0.087, p = 0.771). Furthermore, no main effect of SIDE was detected for any measure (CSP: F1, 18 = 0.479, p = 0.189; amplitude: F1, 18 = 0.021, p = 0.887; latency: F1, 18 = 0.399, p = 0.536; normalized latency: F1, 18 = 0.370, p = 0.550).
4. Discussion
Using a robust methodological approach, the present study comprehensively assessed the bilateral reliability and muscle-specific differences in CMC of both SOL and TA in a neurologically intact cohort during rest and active contraction. Using two metrics that quantify the measurement properties of reliability and measurement error, our findings demonstrated that in general motor threshold and latencies at rest, as well as latency during active are highly reliable for both muscles, whereas CSP is reliable only for TA. Moreover, our findings demonstrated that SOL CMC is bilaterally different from TA CMC, based on all tested CMC measures (corticospinal control) except CSP (intracortical control). Interestingly, neither SOL nor TA CMC differ between body sides.
Depending on the measures used, SOL CMC can be reliably assessed in healthy adults. In particular, only RMT and latency could reliably assess SOL CMC. Because no prior study has investigated all of these SOL CMC measures, we cannot directly compare our findings with the results of previous research. However, two recent studies examined a few CMC measures of the SOL during active contraction in healthy adults. The first study quantified the unilateral test-retest reliability of three CMC measures of SOL (active motor threshold, amplitude, and area) in both healthy adults (randomly assigned side) and stroke participants (paretic side)(Lewis, Signal, 2014). Similar to our RMT results, the active motor threshold of SOL was reliable in the healthy cohort using both ICC (0.92) and typical percentage error (5), a metric calculated using a similar method as CVME. Additionally, the study’s results for MEP amplitude showed high ICC and relatively high typical percentage error, similar to our results. The second study examined only active motor threshold of the right SOL, which had an ICC of 0.95 (Gray, Sabatier, 2017). Though SOL CSP was feasibly measured in our healthy participants as in previous work (Ziemann et al., 1993), our results indicated that SOL CSP might not be as reliable as RMT and latencies. In summary, the bilateral RMT or resting latency can be used as reliable neurophysiological biomarkers of SOL CMC in healthy adults.
With an exception of amplitude, both resting and active, TA CMC can be reliably assessed using any of the CMC measure tested in the present study. In contrast to the limited prior investigations of SOL CMC reliability, more evidence on the reliability of TA CMC measures exists in healthy adults (Cacchio, Cimini, 2009, Chung and Mak, 2015, Forster, Limbart, 2014, Forster, Senft, 2012, Souron, Farabet, 2016, Tallent, Goodall, 2012, van Hedel, Murer, 2007). The majority of previous studies used only ICC to quantify reliability, with nearly all TA CMC measures demonstrating good reliability and similar ICC values to those reported presently. Despite the methodological differences (e.g., type of coil, use of neuronavigation, inter-session interval, etc.) between our study and the previous studies, the collective evidence suggests that RMT, resting and active latencies, and CSP are likely the best measures to assess TA CMC.
A critically important finding was that amplitude did not show good reliability for either muscle or condition when an ICC independent metric (i.e., CVME) was used. Since the introduction of TMS, amplitude has been considered one of the main CMC biomarkers (Bestmann and Krakauer, 2015). Previous work using either ICC or standard error of measurement suggested that MEP amplitude is reliable in healthy adults for both SOL (Lewis, Signal, 2014) and TA (Cacchio, Cimini, 2009). However, when CVME is used, as in the present study, the results are quite different, primarily because each metric reflects a different measurement property of reliability. For example, the resting amplitude of the right TA has ICC and CVME of 0.84 and 40, respectively. ICC is high mainly due to large variance in this metric across individual participants (see both SD and CV), yet CVME is relatively high, which reflects greater measurement error.
Since amplitude does not appear to have good reliability in the present study, use of amplitude as a primary metric of SOL or TA CMC in future studies should bear in mind two factors. The first factor is whether the poor reliability of amplitude is a reflection of methodological limitations (e.g., subtle movements of the coil during stimulation) or the actual underlying neurophysiology (e.g., desynchronization of TMS-evoked motor neuron discharges)(Wasserman et al., 2008). The second factor is whether amplitude should be used as a neurophysiological biomarker to assess the SOL and TA CMC. Based on our results using two independent reliability metrics, amplitude should interpreted with a caution, and perhaps other measures should be used for primary interpretation. For example, bilateral RMT was highly reliable in both muscles, and therefore could be used as the primary CMC outcome since it can be quickly determined (see Table 1). Furthermore, amplitude is often the main measure used in motor cortical mapping (Romero et al., 2011). Given the present results, future studies, whose aim will be to systematically track the plastic changes in the cortical motor areas of lower extremities, may consider using other measures. A recent study suggested that latencies could feasibly quantify the motor cortical representations of upper limb muscles (Kallioniemi et al., 2015). As latency of the tested leg muscles is highly reliable, future studies should test the possibility of quantifying the SOL and TA cortical representations by using latencies.
In the methodology employed in the present study, CMC (only the corticospinal component) differs between the two tested antagonistic ankle muscles. Our results are in agreement with early work that suggested TA is more cortically driven than SOL (Brouwer and Ashby, 1992, Brouwer and Qiao, 1995) and that CMC differs between the two muscles (Ackermann et al., 1991, Obata, Sekiguchi, 2009, Valls-Sole et al., 1994, Wochnik-Dyjas et al., 1998). This postulation was justified by our results, which showed that significantly higher intensities were required to induce motor responses, amplitudes were significantly lower, and latencies were significantly longer in SOL than TA. Moreover, SOL CMC was not assessed in all participants; TA CMC was assessed in everybody. Cumulatively, these results have both scientific and clinical significance. The difference in the corticospinal component of CMC between SOL and TA may be a reflection of the different functions of each muscle. For example, SOL is an antigravity muscle that needs to be activated with short spinal latencies during postural perturbation or to generate high force during the push-off phase of gait (Matsuyama and Drew, 2000, Matsuyama et al., 2004). Thus, this muscle may not be entirely reliant on motor cortical control, as other parts of the central nervous system may contribute to its activation. On the other hand, TA may require stronger reliance on corticospinal control to execute fine motor function. Interestingly, our results demonstrated that the intracortical component of CMC was not different between the two muscles. This result suggests that these two ankle antagonistic muscles may share similar intracortical mechanisms. Therefore, when the integrity of the descending motor pathways to either muscle is compromised due to a lesion in the central nervous system (e.g., stroke), the methodological parameters of targeting for the restoration of motor cortical excitability should be selected differently depending on the target muscle (SOL vs. TA) and component of the neuromotor axis (intracortical vs. corticospinal).
To the best of our knowledge, this is the first study that assessed bilaterally SOL and TA CMC in healthy adults. None of the CMC measures of either muscle differed between sides, hence there is not a unilateral CMC dominance for either muscle. Conversely, had there been a difference between sides for either muscle, this could have been an indication of either stronger unilateral connectivity, which might be used as an indirect neurophysiological proxy of footedness, or a unilateral impairment in the neuromotor axis of the target muscle (e.g., poststroke).
5. Limitations
A few methodological considerations are important for interpreting the results of this study. Numerous physiological or methodological factors might influence the effects of TMS over the neuromotor axis (Ridding and Ziemann, 2010, Wassermann, 2002). We sought to control these factors by asking participants not to consume alcohol or caffeine at least three hours prior to experiment. Similarly, we employed a data collection and analysis methodology that was consistent between muscles and sides, as well as across participants and days. The same sEMG electrodes, which were always placed by the same investigator using standard guidelines, were used for the same muscles between days and across participants. To reduce rater-related sources of reliability, the same investigator was the TMS operator in all sessions. Furthermore, we used automated methods to determine RMT twice for each muscle and to calculate all derived MEP measures. Use of neuronavigation with each participant’s structural MRI increased both the accuracy and precision of stimulating the hotspot, which was determined for each target muscle. Furthermore, the order of each condition was not randomized. Though the resting condition always preceded the active condition, there was a short break between the conditions for each muscle. Therefore, the effect of the fixed order should be minimal. Additionally, the ten stimuli collected for each muscle per condition may not be large enough or optimal for assessing the reliability of CMC measure. It has been recently reported that greater than 10 stimuli (e.g., 20-30) might be required to adequately assess the intra- and inter-session reliability of the lower and upper extremity CMC measure (Cavaleri et al., 2017, Goldsworthy et al., 2016). Given the present protocol, this number of stimuli might not be feasible. Nevertheless, future studies should investigate what is the optimal number of stimuli required to reliably quantify the CMC of a lower extremity muscle. Lastly, though we tried to control as many factors as possible, there were still some physiological (e.g., presence of BDNF Val66Met polymorphism), anatomical (e.g., distance between skull and surface of cerebral cortex), and methodological factors (e.g., thickness of adipose tissue at the location EMG electrodes were attached) that we did not control for, and they could potentially have influenced our results.
6. Conclusion
To the best to our knowledge, this is the first attempt to comprehensively assess simultaneously the bilateral SOL and TA CMC in healthy adults using rigorous standardized data collection and analysis methodology. Our findings provided certain insights about SOL and TA CMC. First, motor threshold and latency can reliably assess SOL and TA CMC, whereas caution should be exercised in using MEP amplitude to assess the CMC of either muscle, particularly for tracking changes. Second, CMC of SOL and TA differ only at the corticospinal level but not at the intracortical level quantified by CSP. Therefore, for the conditions tested in the present study, the corticospinal contributions to each muscle is dissimilar whereas the intracortical mechanisms might be the same. Third, each muscle’s CMC is not different between sides; therefore, a difference between sides may indicate an impaired unilateral CMC to either muscle.
Acknowledgments
The authors thank Dr. Jing Nong Liang for data collection assistance. This work was supported by a VA Career Development Award-2 RR&D N0787-W (MGB) and Institutional Development Award from the National Institute of General Medical Sciences of the NIH under grant number P20-GM109040 (MGB). The contents do not represent the views of the Department of Veterans Affairs or the United States Government.
Biography
Dr. Charalambous received his PhD from the Medical University of South Carolina. Under the supervision of Drs. Bowden and Dean, he investigated the cortical pathways and neuromechanics of ankle muscles after stroke. He then went on to complete postdoctoral training under the supervision of Drs. Reisman and Morton at the University of Delaware, where he investigated the behavioral and neurophysiological mechanisms of locomotor learning after stroke. He is currently a postdoctoral fellow at the New York University Langone under the supervision of Dr. Schambra and is developing neurophysiological approaches to probe and modulate subcortical pathways that may play a role in stroke recovery.

Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackermann H, Scholz E, Koehler W, Dichgans J. Influence of posture and voluntary background contraction upon compound muscle action potentials from anterior tibial and soleus muscle following transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1991;81:71–80. doi: 10.1016/0168-5597(91)90106-8. [DOI] [PubMed] [Google Scholar]
- Alkadhi H, Crelier GR, Boendermaker SH, Golay X, Hepp-Reymond MC, Kollias SS. Reproducibility of primary motor cortex somatotopy under controlled conditions. AJNR Am J Neuroradiol. 2002;23:1524–32. [PMC free article] [PubMed] [Google Scholar]
- Atkinson G, Nevill AM. Statistical methods for assessing measurement error (reliability) in variables relevant to sports medicine. Sports medicine (Auckland, NZ) 1998;26:217–38. doi: 10.2165/00007256-199826040-00002. [DOI] [PubMed] [Google Scholar]
- Awiszus F. TMS and threshold hunting. Supplements to Clinical neurophysiology. 2003;56:13–23. doi: 10.1016/s1567-424x(09)70205-3. [DOI] [PubMed] [Google Scholar]
- Awiszus F, Borckardt JJ. TMS Motor Threshold Assessment Tool (MTAT 1.0) 2011 [Google Scholar]
- Baker M. 1,500 scientists lift the lid on reproducibility. Nature. 2016;533:452–4. doi: 10.1038/533452a. [DOI] [PubMed] [Google Scholar]
- Bawa P, Chalmers GR, Stewart H, Eisen AA. Responses of ankle extensor and flexor motoneurons to transcranial magnetic stimulation. J Neurophysiol. 2002;88:124–32. doi: 10.1152/jn.2002.88.1.124. [DOI] [PubMed] [Google Scholar]
- Beaulieu LD, Flamand VH, Masse-Alarie H, Schneider C. Reliability and minimal detectable change of transcranial magnetic stimulation outcomes in healthy adults: A systematic review. Brain stimulation. 2017a;10:196–213. doi: 10.1016/j.brs.2016.12.008. [DOI] [PubMed] [Google Scholar]
- Beaulieu LD, Masse-Alarie H, Ribot-Ciscar E, Schneider C. Reliability of lower limb transcranial magnetic stimulation outcomes in the ipsi- and contralesional hemispheres of adults with chronic stroke. Clin Neurophysiol. 2017b;128:1290–8. doi: 10.1016/j.clinph.2017.04.021. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Krakauer JW. The uses and interpretations of the motor-evoked potential for understanding behaviour. Exp Brain Res. 2015;233:679–89. doi: 10.1007/s00221-014-4183-7. [DOI] [PubMed] [Google Scholar]
- Brouwer B, Ashby P. Corticospinal projections to lower limb motoneurons in man. Exp Brain Res. 1992;89:649–54. doi: 10.1007/BF00229889. [DOI] [PubMed] [Google Scholar]
- Brouwer B, Qiao J. Characteristics and variability of lower limb motoneuron responses to transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1995;97:49–54. doi: 10.1016/0924-980x(94)00265-9. [DOI] [PubMed] [Google Scholar]
- Cacchio A, Cimini N, Alosi P, Santilli V, Marrelli A. Reliability of transcranial magnetic stimulation-related measurements of tibialis anterior muscle in healthy subjects. Clin Neurophysiol. 2009;120:414–9. doi: 10.1016/j.clinph.2008.11.019. [DOI] [PubMed] [Google Scholar]
- Cacchio A, Paoloni M, Cimini N, Mangone M, Liris G, Aloisi P, et al. Reliability of TMS-related measures of tibialis anterior muscle in patients with chronic stroke and healthy subjects. J Neurol Sci. 2011;303:90–4. doi: 10.1016/j.jns.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Capaday C, Lavoie BA, Barbeau H, Schneider C, Bonnard M. Studies on the corticospinal control of human walking. I. Responses to focal transcranial magnetic stimulation of the motor cortex. J Neurophysiol. 1999;81:129–39. doi: 10.1152/jn.1999.81.1.129. [DOI] [PubMed] [Google Scholar]
- Castaingts V, Martin A, Van Hoecke J, Perot C. Neuromuscular efficiency of the triceps surae in induced and voluntary contractions: morning and evening evaluations. Chronobiology international. 2004;21:631–43. doi: 10.1081/cbi-120039207. [DOI] [PubMed] [Google Scholar]
- Cavaleri R, Schabrun SM, Chipchase LS. The number of stimuli required to reliably assess corticomotor excitability and primary motor cortical representations using transcranial magnetic stimulation (TMS): a systematic review and meta-analysis. Systematic reviews. 2017;6:48. doi: 10.1186/s13643-017-0440-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqueira V, de Mendonca A, Minez A, Dias AR, de Carvalho M. Does caffeine modify corticomotor excitability? Neurophysiologie clinique = Clinical neurophysiology. 2006;36:219–26. doi: 10.1016/j.neucli.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Chung LH, Mak MK. Reliability of transcranial magnetic stimulation related measurements of tibialis anterior muscle in healthy subjects and subjects with Parkinson’s disease. Brain stimulation. 2015;8:418. [Google Scholar]
- Conte A, Attilia ML, Gilio F, Iacovelli E, Frasca V, Bettolo CM, et al. Acute and chronic effects of ethanol on cortical excitability. Clin Neurophysiol. 2008;119:667–74. doi: 10.1016/j.clinph.2007.10.021. [DOI] [PubMed] [Google Scholar]
- Conti A, Raffa G, Granata F, Rizzo V, Germano A, Tomasello F. Navigated transcranial magnetic stimulation for “somatotopic” tractography of the corticospinal tract. Neurosurgery. 2014;10(Suppl 4):542–54. doi: 10.1227/NEU.0000000000000502. discussion 54. [DOI] [PubMed] [Google Scholar]
- Damron LA, Dearth DJ, Hoffman RL, Clark BC. Quantification of the corticospinal silent period evoked via transcranial magnetic stimulation. Journal of neuroscience methods. 2008;173:121–8. doi: 10.1016/j.jneumeth.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Molnar GF, Christensen BK, Sailer A, Fitzgerald PB, Chen R. An automated method to determine the transcranial magnetic stimulation-induced contralateral silent period. Clin Neurophysiol. 2003;114:938–44. doi: 10.1016/s1388-2457(03)00038-5. [DOI] [PubMed] [Google Scholar]
- Forster MT, Limbart M, Seifert V, Senft C. Test-retest reliability of navigated transcranial magnetic stimulation of the motor cortex. Neurosurgery. 2014;10(Suppl 1):51–5. doi: 10.1227/NEU.0000000000000075. discussion 5-6. [DOI] [PubMed] [Google Scholar]
- Forster MT, Senft C, Hattingen E, Lorei M, Seifert V, Szelenyi A. Motor cortex evaluation by nTMS after surgery of central region tumors: a feasibility study. Acta neurochirurgica. 2012;154:1351–9. doi: 10.1007/s00701-012-1403-4. [DOI] [PubMed] [Google Scholar]
- Goldsworthy MR, Hordacre B, Ridding MC. Minimum number of trials required for within- and between-session reliability of TMS measures of corticospinal excitability. Neuroscience. 2016;320:205–9. doi: 10.1016/j.neuroscience.2016.02.012. [DOI] [PubMed] [Google Scholar]
- Gray WA, Sabatier MJ, Kesar TM, Borich MR. Establishing between-session reliability of TMS-conditioned soleus H-reflexes. Neurosci Lett. 2017;640:47–52. doi: 10.1016/j.neulet.2017.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groppa S, Oliviero A, Eisen A, Quartarone A, Cohen LG, Mall V, et al. A practical guide to diagnostic transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol. 2012;123:858–82. doi: 10.1016/j.clinph.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007;55:187–99. doi: 10.1016/j.neuron.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Hermens HJ, Freriks B, Merletti R, Stegeman D, Blok J, Rau G, Disselhorst-Klug C, Hagg G. European Recommendations for Surface ElectroMyoGraphy: Results of the Seniam Project (SENIAM) 2nd. Enschede, Netherlands: Roessingh Research and Development; 1999. [Google Scholar]
- Kallioniemi E, Pitkanen M, Saisanen L, Julkunen P. Onset Latency of Motor Evoked Potentials in Motor Cortical Mapping with Neuronavigated Transcranial Magnetic Stimulation. The open neurology journal. 2015;9:62–9. doi: 10.2174/1874205X01509010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumpulainen S, Avela J, Gruber M, Bergmann J, Voigt M, Linnamo V, et al. Differential modulation of motor cortex plasticity in skill- and endurance-trained athletes. Eur J Appl Physiol. 2015;115:1107–15. doi: 10.1007/s00421-014-3092-6. [DOI] [PubMed] [Google Scholar]
- Lewis GN, Signal N, Taylor D. Reliability of lower limb motor evoked potentials in stroke and healthy populations: how many responses are needed? Clin Neurophysiol. 2014;125:748–54. doi: 10.1016/j.clinph.2013.07.029. [DOI] [PubMed] [Google Scholar]
- Lieber RL, Friden J. Functional and clinical significance of skeletal muscle architecture. Muscle Nerve. 2000;23:1647–66. doi: 10.1002/1097-4598(200011)23:11<1647::aid-mus1>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Livingston SC, Friedlander DL, Gibson BC, Melvin JR. Motor evoked potential response latencies demonstrate moderate correlations with height and limb length in healthy young adults. The Neurodiagnostic journal. 2013;53:63–78. [PubMed] [Google Scholar]
- Matsuyama K, Drew T. Vestibulospinal and reticulospinal neuronal activity during locomotion in the intact cat. I. Walking on a level surface. J Neurophysiol. 2000;84:2237–56. doi: 10.1152/jn.2000.84.5.2237. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Mori F, Nakajima K, Drew T, Aoki M, Mori S. Locomotor role of the corticoreticular-reticulospinal-spinal interneuronal system. Prog Brain Res. 2004;143:239–49. doi: 10.1016/S0079-6123(03)43024-0. [DOI] [PubMed] [Google Scholar]
- Mokkink LB, Terwee CB, Patrick DL, Alonso J, Stratford PW, Knol DL, et al. The COSMIN study reached international consensus on taxonomy, terminology, and definitions of measurement properties for health-related patient-reported outcomes. Journal of clinical epidemiology. 2010;63:737–45. doi: 10.1016/j.jclinepi.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Mouthon A, Ruffieux J, Walchli M, Keller M, Taube W. Task-dependent changes of corticospinal excitability during observation and motor imagery of balance tasks. Neuroscience. 2015;303:535–43. doi: 10.1016/j.neuroscience.2015.07.031. [DOI] [PubMed] [Google Scholar]
- Ngomo S, Leonard G, Moffet H, Mercier C. Comparison of transcranial magnetic stimulation measures obtained at rest and under active conditions and their reliability. Journal of neuroscience methods. 2012;205:65–71. doi: 10.1016/j.jneumeth.2011.12.012. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Petersen N. Evidence favouring different descending pathways to soleus motoneurones activated by magnetic brain stimulation in man. J Physiol. 1995;486(Pt 3):779–88. doi: 10.1113/jphysiol.1995.sp020853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata H, Sekiguchi H, Nakazawa K, Ohtsuki T. Enhanced excitability of the corticospinal pathway of the ankle extensor and flexor muscles during standing in humans. Exp Brain Res. 2009;197:207–13. doi: 10.1007/s00221-009-1874-6. [DOI] [PubMed] [Google Scholar]
- Palmer JA, Zarzycki R, Morton SM, Kesar TM, Binder-Macleod SA. Characterizing differential poststroke corticomotor drive to the dorsi- and plantarflexor muscles during resting and volitional muscle activation. J Neurophysiol. 2017;117:1615–24. doi: 10.1152/jn.00393.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portney LG, Watkins MP. Foundations of Clinical Research: Applications to Practice: Pearson/ Prentice Hall; 2009. [Google Scholar]
- Ridding MC, Ziemann U. Determinants of the induction of cortical plasticity by non-invasive brain stimulation in healthy subjects. J Physiol. 2010;588:2291–304. doi: 10.1113/jphysiol.2010.190314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero JR, Ramirez DM, Aglio LS, Gugino LD. Brain mapping using transcranial magnetic stimulation. Neurosurgery clinics of North America. 2011;22:141–52, vii. doi: 10.1016/j.nec.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Screening questionnaire before TMS: an update. Clin Neurophysiol. 2011;122:1686. doi: 10.1016/j.clinph.2010.12.037. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A, Safety of TMSCG Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–39. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol. 2015;126:1071–107. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell JC, Hallett M, Berardelli A, Eisen A, Rossini P, Paulus W. Magnetic stimulation: motor evoked potentials. The International Federation of Clinical Neurophysiology. Electroencephalography and clinical neurophysiology Supplement. 1999;52:97–103. [PubMed] [Google Scholar]
- Saisanen L, Kononen M, Julkunen P, Maatta S, Vanninen R, Immonen A, et al. Non-invasive preoperative localization of primary motor cortex in epilepsy surgery by navigated transcranial magnetic stimulation. Epilepsy research. 2010;92:134–44. doi: 10.1016/j.eplepsyres.2010.08.013. [DOI] [PubMed] [Google Scholar]
- Schambra HM, Ogden RT, Martinez-Hernandez IE, Lin X, Chang YB, Rahman A, et al. The reliability of repeated TMS measures in older adults and in patients with subacute and chronic stroke. Frontiers in cellular neuroscience. 2015;9:335. doi: 10.3389/fncel.2015.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shellock FG, Spinazzi A. MRI safety update 2008: part 2, screening patients for MRI. AJR Am J Roentgenol. 2008;191:1140–9. doi: 10.2214/AJR.08.1038.2. [DOI] [PubMed] [Google Scholar]
- Silbert BI, Patterson HI, Pevcic DD, Windnagel KA, Thickbroom GW. A comparison of relative-frequency and threshold-hunting methods to determine stimulus intensity in transcranial magnetic stimulation. Clin Neurophysiol. 2013;124:708–12. doi: 10.1016/j.clinph.2012.09.018. [DOI] [PubMed] [Google Scholar]
- Sinclair C, Faulkner D, Hammond G. Flexible real-time control of MagStim 200(2) units for use in transcranial magnetic stimulation studies. Journal of neuroscience methods. 2006;158:133–6. doi: 10.1016/j.jneumeth.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Souron R, Farabet A, Millet GY, Lapole T. Reliability of the functional measures of the corticospinal pathways to dorsiflexor muscles during maximal voluntary contractions. J Neurol Sci. 2016;369:368–74. doi: 10.1016/j.jns.2016.09.003. [DOI] [PubMed] [Google Scholar]
- Stokes M. Reliability and repeatability of methods for measuring muscle in physiotherapy. Physiotherapy Theory and Practice. 1985;1:71–6. [Google Scholar]
- Tallent J, Goodall S, Hortobagyi T, St Clair Gibson A, French DN, Howatson G. Repeatability of corticospinal and spinal measures during lengthening and shortening contractions in the human tibialis anterior muscle. PLoS One. 2012;7:e35930. doi: 10.1371/journal.pone.0035930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terao Y, Ugawa Y. Basic mechanisms of TMS. J Clin Neurophysiol. 2002;19:322–43. doi: 10.1097/00004691-200208000-00006. [DOI] [PubMed] [Google Scholar]
- Thomas SL, Gorassini MA. Increases in corticospinal tract function by treadmill training after incomplete spinal cord injury. J Neurophysiol. 2005;94:2844–55. doi: 10.1152/jn.00532.2005. [DOI] [PubMed] [Google Scholar]
- Valls-Sole J, Alvarez R, Tolosa ES. Responses of the soleus muscle to transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1994;93:421–7. doi: 10.1016/0168-5597(94)90148-1. [DOI] [PubMed] [Google Scholar]
- van Hedel HJ, Murer C, Dietz V, Curt A. The amplitude of lower leg motor evoked potentials is a reliable measure when controlled for torque and motor task. J Neurol. 2007;254:1089–98. doi: 10.1007/s00415-006-0493-4. [DOI] [PubMed] [Google Scholar]
- Wasserman E, Epstein CM, Ziemann U. The Oxford handbook of transcranial stimulation Oxford. New York: Oxford University Press; 2008. [Google Scholar]
- Wassermann EM. Variation in the response to transcranial magnetic brain stimulation in the general population. Clin Neurophysiol. 2002;113:1165–71. doi: 10.1016/s1388-2457(02)00144-x. [DOI] [PubMed] [Google Scholar]
- Weir JP. Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. Journal of strength and conditioning research/National Strength & Conditioning Association. 2005;19:231–40. doi: 10.1519/15184.1. [DOI] [PubMed] [Google Scholar]
- Winter DA. The biomechanics and motor control of human gait: Normal, Elderly and Pathological. 2nd. Waterloo, Ont: University of Waterloo Press; 1991. [Google Scholar]
- Winter DA. A.B.C (anatomy Biomechanics and Control) of Balance During Standing and Walking. Waterloo Biomechanics. 1995 [Google Scholar]
- Wochnik-Dyjas D, Glazowski C, Niewiadomska M. Peculiarity of soleus motor potentials evoked by transcranial magnetic stimulation and electrical stimulation of tibial nerve. Electroencephalogr Clin Neurophysiol. 1998;109:369–75. doi: 10.1016/s0924-980x(98)00032-0. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Netz J, Szelenyi A, Homberg V. Spinal and supraspinal mechanisms contribute to the silent period in the contracting soleus muscle after transcranial magnetic stimulation of human motor cortex. Neurosci Lett. 1993;156:167–71. doi: 10.1016/0304-3940(93)90464-v. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Reis J, Schwenkreis P, Rosanova M, Strafella A, Badawy R, et al. TMS and drugs revisited 2014. Clin Neurophysiol. 2015;126:1847–68. doi: 10.1016/j.clinph.2014.08.028. [DOI] [PubMed] [Google Scholar]


