Abstract
While previous research has identified a number of metabolic, neural, and hormonal events that could serve as potential satiety signals, the mechanisms that enable satiety signals to suppress food-seeking and eating behavior remain poorly specified. Here we investigate the idea that the inhibitory power of satiety signals is derived, at least in part, from their ability to signal that foods and food-related stimuli will not be followed by reinforcing postingestive consequences. Viewed in this way, the signaling relationship in which satiety cues are embedded defines what is known in Pavlovian conditioning as a “serial feature negative” (sFN) discrimination problem. In this problem a “negative feature” cue precedes the presentation of a “target” cue on trials without reinforcement. In contrast, the target is reinforced on trials when the negative feature cue is not presented. Satiety cues can be seen as paralleling the function of negative feature cues in that they signal when food-related target cues will be nonreinforced. We conducted two experiments with rats that assessed if satiety signals functioned like negative feature stimuli. Experiment 1 explicitly pretrained satiety cues as negative feature stimuli, irrelevant stimuli, or under conditions where their ability to serve as negative feature stimuli would be attenuated. Control by satiety cues was highly sensitive to these experimental contingencies, with the best performance exhibited by rats given sFN pretraining. This sFN pretraining also transferred to enhance performance during subsequent training on another sFN problem with both external and internal negative feature cues. We also found that discriminative control by satiety cues blocked the development of that control by external cues. Experiment 2 evaluated whether a manipulation known to impair sFN performance with external negative feature cues (i.e., maintenance on a western diet) would also impair sFN performance when satiety cues were trained as negative feature stimuli. The results showed that compared to standard chow, WD intake impaired sFN performance similarly with both types of stimuli. These experiments provide evidence that an associative mechanism, like that underlying sFN performance, is involved with the control of appetitive behavior by satiety cues.
Keywords: ingestive behavior, Western diet, state cues, learning, memory
1. Introduction
When food and stimuli associated with food are encountered these cues can evoke strong appetitive and consummatory responding on some occasions, but little responding at other times. To explain this pattern of behavioral modulation, it has been proposed that animals engage in appetitive and eating behavior until they become satiated and then refrain from making these responses until satiety wanes (e.g., for reviews see Blundell et al, 2010; Amin & Mercer, 2016). This perspective has generated a great deal of research aimed at discovering the neural and physiological events that give rise to the interoceptive sensory stimulation that defines satiety It is clear from this work that food satiety is likely the product of complex and interacting metabolic, hormonal, gastric, and other processes (e.g., Moran & Ladenheim, 2016; Clemmensen et al., 2017).
The goal of this paper is not to identify the origins of satiety signals. Rather, our focus is on the question of how satiety inhibits, and the absence of satiety promotes, appetitive behavior. Researchers have usually begged this question by assuming that satiety suppresses appetitive behavior without specifying how it produces that effect (e.g., Zanchi et al., 2017). For example, while many models have provided detailed descriptions of the complex physiological mechanisms that may lead to satiety, the links between those physiological mechanisms and behavior have often been described in motivational terms. However the mechanisms linking motivational constructs to behavior have been elusive, and as a result they have often been specified by no more than a few lines or arrows in a diagram (e.g., Andermann & Lowell, 2017; Sternson & Eiselt, 2017). Similarly, implicit in some of these accounts is that the suppression of intake is the result of some unconditioned or reflexive, and also largely unspecified, properties of satiety (e.g., Chaudhri et al, 2006; see Woods & Begg, 2016 for related discussion). However, the assumption that a response is unconditioned or “not learned” is supportable only to the extent that evidence that the response is learned is absent. We should note here that previous research has provided evidence that learning and other cognitive processes can contribute to the manifestation of satiety states (e.g., see Brunstrom, 2014; Martin, 2016). However, this is not the same as describing how the manifestation of satiety inhibits appetitive and consummatory behavior.
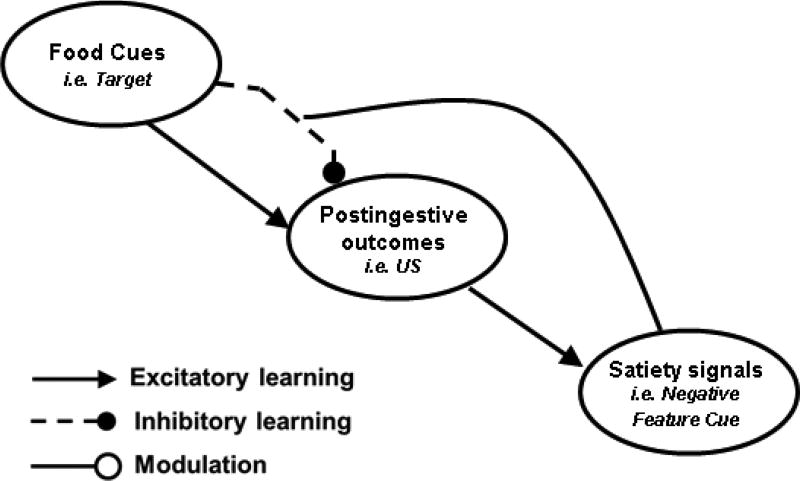
The present research investigates the hypothesis that what has been described as the unconditioned or motivational effects of satiety may be explained by the operation of learning and memory mechanisms. Previously, we theorized that energy intake and body weight regulation depend on what animals learn about environmental food cues, the postingestive sensory consequences associated with those cues, and the ability of their interoceptive satiety cues to predict when those food cues will be followed by those consequences (Davidson et al., 2007; Davidson et al., 2014a). Specifically, as depicted in Figure 1, cues related to the sight, aroma, taste, and texture of foods are thought to enter into simple direct associations with postingestive stimulation produced by eating. These environmental food cues can develop excitatory associations with postingestive consequences when those outcomes are satisfying or rewarding. However, the same food cues that have been associated with rewarding or reinforcing postingestive stimulation are also present when the postingestive consequences of eating become nonrewarding or even aversive. For example, the postingestive stimuli associated with food cues that are presented at the beginning of a large meal are likely to be qualitatively different (e.g., hedonically positive) compared to the postingestive stimuli those same cues would be paired with at the end of a meal.
Figure 1.
An associative model of energy regulation. This figure is a visual representation of a model of the associative mechanisms underlying energy and body weight regulation and how that model is similar to feature negative paradigm. Environmental food cues act as target cues and become embedded in both excitatory and inhibitory relationships with postingestive outcomes (the US). Satiety signals gate the activation of the inhibitory association between food cues and postingestive outcomes by acting as negative feature cues, thereby suppressing appetitive behavior (adapted from Davidson, Sample, & Swithers, 2014b).
Figure 1 shows that, according to our model, this relationship results in the formation of an inhibitory association that antagonizes the ability of the concurrent excitatory association to retrieve the memory of the rewarding postingestive consequences of intake. In the face of these two conflicting associations, animals must rely on other information to determine when to eat and when to refrain from eating. Our model proposes that satiety cues are a primary source of this information. Figure 1 shows that satiety cues influence the strength of appetitive behavior by gating or activating the inhibitory association that antagonizes the ability of the excitatory association to retrieve the memory of postingestive reward. Satiety stimuli weaken the response-evoking power of environmental food cues by suppressing retrieval of the memory of rewarding postingestive consequences of intake, which reduces the ability of food-related cues to initiate appetitive and ingestive behaviour.
The relationships among environmental food cues, postingestive outcomes, and satiety signals described in Figure 1 are also represented in what is known as the serial feature negative (sFN) discrimination problem. In this problem, one stimulus (a negative feature) precedes and signals that another stimulus (a target) will not be followed by a reinforcer, whereas presentation of the target without the negative feature is reinforced (e.g., Holland, 1989; Swartzentruber, 1995). The negative feature cue exhibits control over responding in that rats learn to respond less when the negative feature precedes the target compared to when the target is presented alone. As diagrammed in Figure 1, satiety signals fit the description of negative feature stimuli in that they predict that their target (food-related stimuli) will not be followed by a postingestive reinforcer, whereas the rewarding postingestive consequence does occurs in the absence of satiety.
Previous research in our lab showed that rats could solve discrimination problems when cues arising from 4-hr and 0-hr or from 24-hr and 0-hr levels of food deprivation served as relevant cues for sucrose reinforcement. Rats were also able to solve these discrimination problems when 4-hr or 24-hr food deprivation signaled reinforcement and 0-hr food deprivation did not and also when these discriminative contingencies were reversed (e.g., Sample et, 2016; Kanoski et al., 2007). The results of these studies showed that stimulus control by interoceptive satiety cues be learned, and can therefore based on more than any unconditioned suppressive effects that satiety may have on appetitive behavior. A report by Schepers & Bouton (2017) also provided evidence that satiety cues operate as memorial contextual cues in a manner independent of any unconditioned response suppressing properties they may possess. Another study in the report by Schlepers & Bouton showed that interoceptive contextual cues associated with hunger and satiety can enter into the learned control of behavior more readily than external contextual cues. Similarly, we found that interoceptive cues produced by food deprivation and satiation procedures fare well in direct competition with external cues for the discriminative control of appetitive (Sample et al, 2015; 2016) or aversive (Davidson, Flynn & Jarrard, 1992) behavior when both types of cues are equally predictive of reward and nonreward.
In the experiments reported here, satiety cues produced by free access to food for at least 24 hrs (0-hr food deprivation) will be viewed as negative feature stimuli that are informative about the nonreinforcement of external (e.g., auditory) cues. We considered satiety cues to be largely absent when testing occurred following a period of 4 or more hours without food (e.g., 4-hr or 24-hr food deprivation). Here, the absence of satiety cues signaled that the external food cues would be reinforced. We chose this deprivation level and contingency because of their similarity to what animals encounter during normal free-feeding. Because the “reversed” contingency (reinforced under 0-hr but not under 4-hr or 24-hr food deprivation) is unlikely to be encountered as part of the normal feeding ecology of rats or humans, it was not included in the current studies.
2. Experiment 1
Our first experiment examined (a) whether the ability of 0-hr (i.e., satiety) and 4-hr food deprivation (i.e., the absence of satiety) to modulate appetitive responding to external cues associated with sucrose reinforcement depends on the associative structure in which satiety cues are embedded; (b) if differences in these signaling relationships transfer to impact the acquisition of a sFN discrimination problem in which both satiety cues and external cues serve as compound negative feature stimuli; and (c) the relative salience of satiety and external cues when both are trained as negative feature stimuli.
To do so, we trained three separate groups of rats under different pretraining contingencies that were designed to vary the extent to which their internal satiety cues could serve as signals that a target external cue would be nonreinforced (i.e. negative feature cues). Table 1 shows that Group Deprivation Contingent (DepC) was explicitly pretrained under conditions in which satiety cues signaled the nonreinforcement of a compound external target cue (i.e. clicker and noise) and the absence of satiety was associated with reinforcement of that same compound target stimulus.
Table 1.
Experiment 1 Design. 0= 0-hr food deprived (food sated), 4-hr food deprived; A and B = external clicker or noise counterbalanced across groups; L=external light, T=external tone; + = sucrose reward, - = absence of sucrose reward.
| Group | Pretraining | Transfer Training | Test 1-Light | Test 2-0h |
|---|---|---|---|---|
| Deprivation Contingent (DepC) | 0→AB-, 4AB+ | 0L→T-, 4T+ | 4L→T+, 0L→T-, 4T+ | 0→T-, 0L→T-, 4T+ |
| Deprivation Non-Contingent (DepN) | 0/4A-, 0/4B+ | 0L→T-, 4T+ | 4L→T+, 0L→T-, 4T+ | 0→T-, 0L→T-, 4T+ |
| Deprivation Attenuated (DepAtt) | 0A/B-, 4A/B+ | 0L→T-, 4T+ | 4L→T+, 0L→T-, 4T+ | 0→T-, 0L→T-, 4T+ |
Group Deprivation Attenuated (DepAtt), like Group DepC, was pretrained with satiety cues as signals for nonreinforcement and the absence of satiety cues as signals for the reinforcement of external target cues. However, rather than being trained with a single compound target cue, like Group DepC, Group DepAtt was trained with the clicker as the target cue on half of the trials and the white noise as the target on the remaining half. Thus, for Group DepAtt, each of the two external target cues received only half as much training as did the single compound target cue that was trained for Group DepC. This reduced amount of reinforced training to each of the target cues was expected to attenuate discriminative responding for Group DepAtt by (a) reducing the degree to which each cue could evoke excitatory conditioned responses and (b) reducing the amount of inhibition each cue accrued, in part, because each cue also received a reduced number of nonreinforced trials relative to Group DepC. Based on previous findings that negative feature stimuli control appetitive responding by gating the activation of reward memories that are evoked by their target cues (Rescorla, 1985; Holland, 1989; 1992), reduced training of each target cue was expected to weaken the ability of satiety cues to act like negative feature cues, thereby reducing their ability to modulate responding evoked by those targets.
Unlike Groups DepC and DepAtt, Group Deprivation Non-Contingent (DepN) was not pretrained under conditions in which the nonreinforcement of external target cues was predicted by the presence of satiety cues. Rather for Group DepN reinforcement was correlated with the presentation of external discriminative stimuli, but varied noncontingently with the animal’s deprivation state. Thus, for Group DepN, satiety cues were not pretrained as negative feature stimuli. Differences in discriminative responding under 0- and 4-h food deprivation among these three groups would provide evidence that animals are sensitive to the associative relationships in which their satiety cues are embedded.
Next, all groups received transfer training on a sFN task where they could use either their internal satiety cues, an external visual (light) cue, or both types of cues as negative feature stimuli to determine if the target stimulus would not be reinforced. When prior learning about one set of stimuli promotes subsequent learning about another set of stimuli, positive transfer is said to occur. Conversely, if such prior learning interferes with subsequent learning, this represents negative transfer.
Learning about one discrimination problem can transfer positively to facilitate learning about a second discrimination problem to the extent that the solution to both problems is based on learning the same underlying associative relationships. Alternatively, positive transfer is much less likely when the two problems require learning different types of associative relations (Swartzentruber, 1991). Experiment 1 exploited these principles to assess the extent to which prior training with satiety signals as negative feature cues in a sFN discrimination problem would facilitate learning (i.e., yield positive transfer) in another sFN discrimination problem with both satiety cues and light cues compound negative feature stimuli. Superior transfer performance following sFN discrimination training, compared to prior training with satiety cues as either attenuated negative feature stimuli, or non-predictive cues would provide evidence that rats used their satiety cues as negative feature stimuli during sFN pretraninig. Thus, for Group DepC, pretraining with satiety cues as negative feature stimuli would be expected to enhance the ability of those cues to suppress responding to the tone target cue during transfer training. Similarly, to the extent that pretraining established a weaker negative feature cue for Group DepAtt compared to Group DepC, Group DepAtt should also exhibit weaker positive transfer than Group DepC. For Group DepN, because satiety cues were not trained as negative feature stimuli, little positive transfer would be expected during subsequent sFN training in which those internal cues served in compound with the light as feature negative stimuli.
Larger positive transfer from pretraining should increase the amount of interference when learning about a novel cue that shares similar associative relationships with reward. Previous research shows that pretraining one stimulus (A) as a signal for reinforcement will reduce or prevent learning about another stimulus (B) when A and B are subsequently trained in compound with the same signalling relationship and reinforcement that was originally used to train stimulus A. This type of “blocking” of learning about stimulus B by stimulus A is thought to occur to the extent that stimulus B provides no new information, relative to stimulus A, about the likelihood of receiving reinforcement (e.g., Kamin, 1968; Swartzentruber, 1991; Holland and Fox, 2003).
Following sFN training with satiety and light cues as compound negative feature stimuli, the final tests of Experiment 1 evaluated the extent to which satiety cues blocked the ability of the light cue to become a negative feature stimulus. Weak inhibitory control by the light negative feature cue would indicate greater blocking of learning about the light by satiety cues. Strong inhibitory control by the light would indicate that satiety cues failed to block learning about the light. If blocking of the light depends on the degree to which satiety cues were established as negative feature cues during pre-training, less learning about the light would be expected to accrue for DepC compared to Group DepAtt and DepN, with Group DepN exhibiting the most learning about the light. For all three groups, Test 1 measured the power of the light to suppress responding to the tone target in the absence of satiety cues. Test 2 assessed the power of satiety cues to suppress responding to the tone target when the light cue was absent.
3. Experiment 1 Methods
3.1 Subjects
Subjects were 24 naïve, male Sprague-Dawley rats weighing approximately 300–350g upon arrival (Harlan, Indianapolis). Rats were housed individually in hanging-wire stainless steel cages and given ad libitum access to water and standard rodent pellet laboratory chow except where noted. Rats were maintained on laboratory chow (Lab Diets 5001) which had a caloric density of 3.0 kcal/g (approximately 13% kcal from fat, 56% kcal from carbohydrates). The animal room was maintained on a 10:14 light: dark cycle with lights on at 1430-hr and off at 0030-hr. All procedures for the care and treatment of the rats were approved by the American University Institutional Animal Care and Use Committee.
3.2 Apparatus
All training and testing trials were conducted in 8 identical conditioning chambers housed within sound- and light-resistant shells. The chambers consisted of aluminum end walls and Plexiglas sidewalls, measuring 59.7 × 34.3 × 26.35 cm (Lafayette Instruments). Stainless steel rods measuring .48 cm in diameter and 1.07 cm apart made up the floors of the chamber. A recessed food magazine where sucrose pellets (45 mg Research Diets) were dispensed was located in the center of one end wall. An infrared photo transmitter and receiver were located on each side wall directly in front of the recessed food magazine. When the rat approached the food magazine, the beam was broken. Beam breaks were recorded by a computer-operated, infrared monitoring system.
3.3 Procedures
All of the rats were run in squads of 8 animals that were counterbalanced with respect to group assignment and conditioning chamber. Training trials always began at 1430h, but did not occur every day (approximately 5 training trials per week) in order to prevent the reward from being delivered on a single-alternating schedule. On each trial behavioral monitoring took place for 4 min. This period began with the onset of an external cue and terminated with external cue offset followed by the delivery of 5 sucrose pellets on reinforced trials, but no sucrose pellets on nonreinforced trials. The 4 min period of behavioral monitoring was subdivided into twenty-four 10s intervals. The percentage of these intervals in which the photo beam was broken served as the index of discriminative responding. On both reinforced and nonreinforced trials, the rats were removed from the conditioning chambers and returned to their home cages approximately 2min after the end of behavioral monitoring. Rats were given only one trial per day; therefore, rewarded and nonrewarded trials occurred on separate days. Table 1 contains the basic design of Experiment 1 and the contingencies each group received. Each phase is described in detail below.
3.3.1 Pretraining
Prior to the start of pretraining, rats were assigned to three groups (n=8 each) that were matched on body weight (M ± SD= DepC: 324.25±10.58g; DepN: 324.63±12.66g; DepAtt: 324.75±11.29g). For all rats, food deprivation levels at the beginning of each trial alternated everyday between 0-hr and 4-hr food deprived. Rats were given ad libitum access to food for approximately 24-hr before a trial on a 0-hr day and were not given any access to food for approximately 4-hr before a trial on 4-hr days. Pretraining trials consisted of a 4 min presentation of a discrete auditory cue, which was either a clicker sound or a 3 Hz noise (Lafayette Instruments). For the DepC group, rats were rewarded under 4-hr deprivation but received no pellets under 0-hr deprivation. The auditory cue was a combination of the clicker and the noise, such that rats in the DepC group were given the same amount of exposure to both external auditory cues as the other two groups. The auditory cue served as a consistent target stimulus that was both rewarded and nonrewarded, such that animals had to rely on their deprivation state to discern when the target would be rewarded or nonrewarded. For the DepAtt group, rats were rewarded under 4-hr deprivation but received no pellets under 0-hr deprivation. For this group, the clicker served as the target cue on half of the trials and the 3Hz noise was the target on the remaining half. Finally, rats in Group DepN were rewarded in the presence of one auditory cue but not the other. For this group, reinforcement was contingent only on the auditory cue as the rats were rewarded and non-rewarded an equal number of times under each deprivation level. Thus, Group DepN rats could only solve the discrimination by learning about the auditory cues.
3.3.2 Transfer Training
After the DepC and DepAtt groups achieved significant discrimination based on deprivation level for one block of two 4-hr (rewarded) and two 0-hr (nonrewarded) trials, all rats were switched to the same training contingencies. Food deprivation continued to vary between 0-hr and 4-hr, with 0-hr deprivation signaling no reward and 4-hr deprivation signaling sucrose reward for all groups. In addition to satiety cues, transfer training trials also consisted of either a 4 min light that was followed by a discrete 3000 Hz tone (Lafayette Instruments) which played during the last 10s of the light, or the absence of the light for 4 min and the same discrete tone played during the last 10s of darkness. Rats were exposed to sFN contingencies, such that trials under 4-hr food deprivation co-varied with no light while 0-hr food deprivation co-varied with the presentation of the light. When rats were under 4-hr deprivation and the light was absent, the tone was followed by the delivery of 5 sucrose pellets (4T+ trials). When rats were 0-hr food deprivation (i.e., food sated) and the light was present, the tone was followed by no sucrose pellets (0L→T- trials). On these trials, the light and 0-hr food deprivation cues could serve as a compound serial negative feature stimulus which signaled when the tone would not be reinforced. Thus, rats could use cues arising from the satiety state, the discrete cue provided by the 4 min light, or both satiety cues and the light cue to solve the transfer sFN discrimination problem. The ability of the satiety cue/external cue compound to serve as a negative feature stimulus was compared for all groups by recording the number of beam breaks that occurred during the 10 sec target cue.
3.3.3 Test 1-Light
After significant discrimination was achieved by all of the groups for one block of two rewarded and two nonrewarded transfer training trials, the relative capacities of the light and satiety cue to serve as serial negative feature stimuli was tested. During Test 1, responding during the 10 sec presentation of the tone target cue was assessed when that cue was preceded by the 4 min light in the absence of satiety cues (i.e., 4L→T+). Responding to the tone target on this test trial was compared to that on 4T+ and 0L→T- trials, like those used in transfer training. During Test 1, all rats received two trials with each of these trial types (i.e., two 4L→T+ two 0L→T-, and two 4T+ trials) over a total of six test days.
3.3.4 Test 2- 0hr food deprivation
Test 2 assessed the capacity of satiety cues to suppress responding to the tone target cue when the light stimulus was absent (0→T-). For all rats, responding to the tone target during this type of test trial was compared to responding on 0L→T- and 4T+ trials, like those used in transfer training. Similar to Test 1, in Test 2 the rats received two 0→T-, two 0L→T-, and two 4T+ trials over a period of six consecutive days.
3.4 Statistical Analysis
For the pretraining phase, percent of beam breaks during each 4min trial was analyzed using a repeated measures ANOVA with Trials, Deprivation Level (0-hr and 4-hr), and Block (consisting of two trials under 0-hr and two trials under 4-hr food deprivation) as within-subjects factors, and Group (DepC, DepAtt, and DepN) as a between-subjects factor. For the training and testing phases, number of responses during the 10s target (tone) was analyzed using a repeated measures ANOVA with Trials, +/− trial type during training (+ being rewarded and − being nonrewarded), Block (four trials made up of two + and two − trials), and Trial Type during testing (0L→T-,4+, 4L→T+, and 0→T-) as within-subjects factors, and Group (Contingent, DepAtt, and DepN) as a between-subjects factor. Alpha level was set a p < .05. Significant main effects and interactions were further analyzed with Bonferroni and Newman-Keuls post-hoc tests. One rat in the DepN group died half-way through the experiment and his data was excluded from all analyses.
4. Experiment 1 Results
4.1 Pretraining
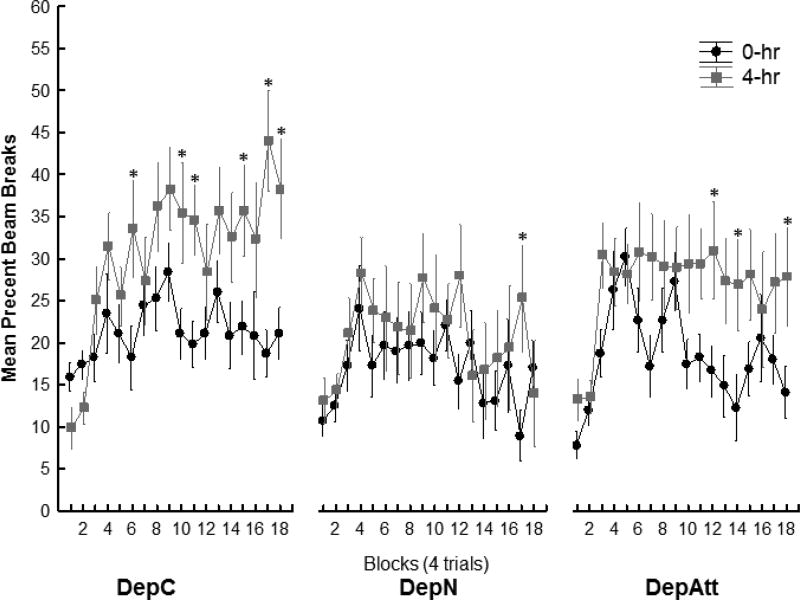
By the end of pretraining, both the DepC and DepAtt groups learned to use their interoceptive cues to discriminate between rewarded and nonrewarded trials. In contrast, Group DepN, for which food deprivation and reward availability varied noncontingently, showed little evidence of discriminative responding (Figure 2). A repeated measures ANOVA confirmed these impressions by yielding a significant Group × Deprivation Level × Block interaction (F(34,340) = 2.21, p < .001). Separate ANOVAs were used to compare the magnitude of discrimination for each group. Significant Group × Deprivation Level × Block interactions were obtained when Group DepC was compared directly to Group DepN (F(17, 221) = 2.38, p < .01) and to Group DepAtt (F(17,238) = 2.07, p < .01) respectively. This outcome indicates that the magnitude of the difference between the 4- and 0-hr deprivation levels was larger for Group DepC compared to both Groups DepN and DepAtt. Similarly, a significant Group × Deprivation Level × Block interaction was also obtained when Group DepAtt was compared directly to Group DepN (F(17, 221) = 2.22, p < .01). This indicates that discrimination performance for Group DepAtt also exceeded that shown by Group DepN. Neuman-Keuls tests were used to assess the effects of Deprivation Level on each Block for each Group. Group DepC responded significantly more under 4-hr food deprivation than under 0 hr deprivation on Blocks 6, 10, 11,15, 17, 18 (all ps<.05). For Group DepAtt this difference due to deprivation level was significant on Blocks 12, 14, and 18 (ps < .05), while for Group DepN the difference between 4- and 0-hr deprivation was significant on Block 17. An analysis of the last block of pretraining revealed a significant Group × Deprivation level interaction (F(2,20) = 7.04, p <.01). Newman-Keuls post-hoc tests showed that the DepC (p<.01) and the DepAtt (p<.05) groups responded significantly more on 4- compared to 0-hr food deprivation (ps < .05), whereas this difference was not significant for Group DepN (p > .74). There was no significant difference between the DepC and DepAtt groups in discrimination performance on the last block of pre-training (p = .59).
Figure 2.
Experiment 1 pretraining acquisition. Mean percent magazine entries during 4m period of behavior monitoring. The groups differed in their ability to discriminate using their deprivation cues (Group × Deprivation Level × Block interaction (F(34,340) = 2.21, p < .001) with Groups DepC and DepAtt exhibiting better discrimination performance compared to Group DepN on several blocks, including the last block of pretraining (ps <.05). See results for detailed analysis.
4.2 sFN Transfer Training
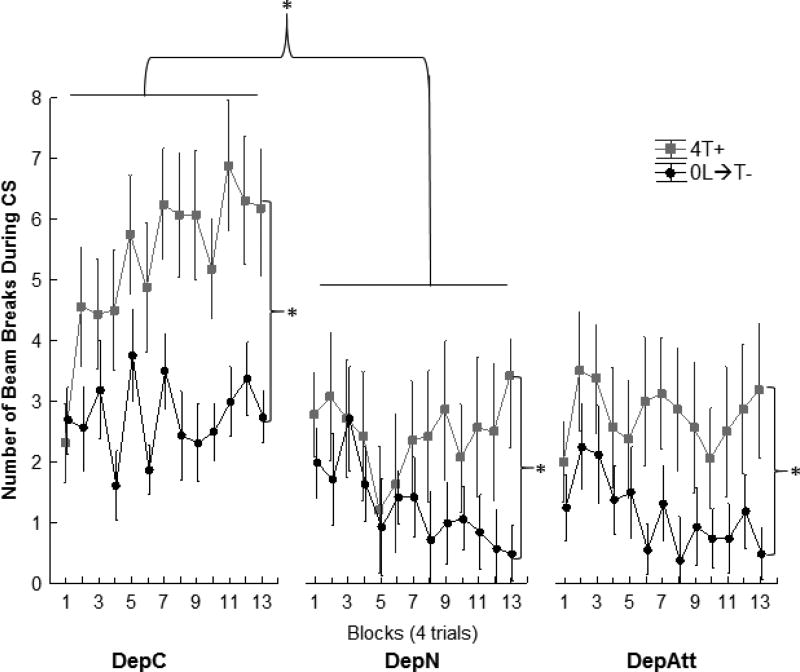
After the conclusion of pretraining, all rats received transfer training trials on the sFN discrimination problem in which both satiety cues and the external light could serve as compound negative feature stimuli (i.e., 0L→T-, 4T+). Figure 3 shows that while all groups learned to respond more on the rewarded (4T+) than on nonrewarded (0L→T-) trials, the rate of discrimination acquisition and the magnitude of the difference between the 4T+ and 0L→T- trials was somewhat greater for Group DepC than for either Group DepN or DepAtt, which differed little from each other. A repeated measures ANOVA analysis yielded main effects of +/− (F(1,20)=48.3, p<.0001) and Group (F(2,20)=4.22, p<.05) and a +/− × Block interaction ( F(12,240)=3.15, p<.001). However, there was no significant +/− × Group (F(2,20)=2.57, p=.10) or +/− × Block × Group (F( 23,240) < 1) interaction when all three groups were compared.
Figure 3.
Experiment 1 sFN transfer training acquisition. Mean ± SEM number of magazine entries during the 10s CS (tone). The rats in all groups learned the sFN discrimination (F(1,20)=48.3, p<.0001). Additional analyses found that Group DepC and DepN differed significantly (p< .05) whereas neither of these groups differed significantly from Group DepAtt. See results for detailed analysis.
Based on the significant Group interaction on the last block of pretraining we performed additional analyses that directly compared sFN discrimination performance between all of the groups. These ANOVAs found a significant main effect of +/− (F(1,13) = 45.46, p < .01) and a significant +/− × Group interaction (F(1,13) = 6.41, p < .05) that did not vary significantly as a function of Block (F (12, 15) < 1) when Group DepC was compared with Group DepN. Although a significant +/− × Group interaction indicates that the difference in responding on 4T+ trials compared to 0L→T- trials was larger for Group DepC than for Group DepN, Newman-Keuls tests showed that both Group DepC and DepN exhibited significant sFN discrimination performance across transfer training (ps. < .05). In contrast, while the ANOVA comparing Group DepN to Group DepAtt also obtained a significant effect of +/− (F(1, 13)=16.57, p. < .01), neither +/− × Group (F(1, 13) < 1) nor the +/− × Group × Block (F(12, 156) < 1) interactions achieved significance. Furthermore, an ANOVA comparing Groups DepC and DepAtt found a significant main effect of +/− (F(1,14)=42.36, p< .0001), but did not find significant +/− × Group (F(1, 14) < 1 nor the +/− × Group × Block (F(12, 168) < 1. These finding indicate that, across transfer training, serial feature negative discrimination performance for Group DepAtt did not differ from Groups DepN or DepC, but Groups DepC and DepN differed from each other. All groups were responding more on rewarded 4T+ trials than on nonrewarded 0L→T- trials.
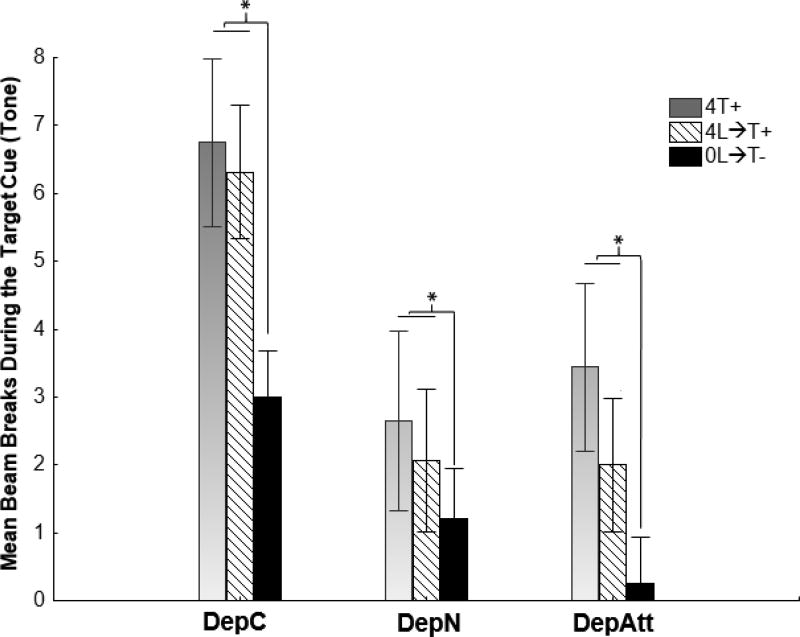
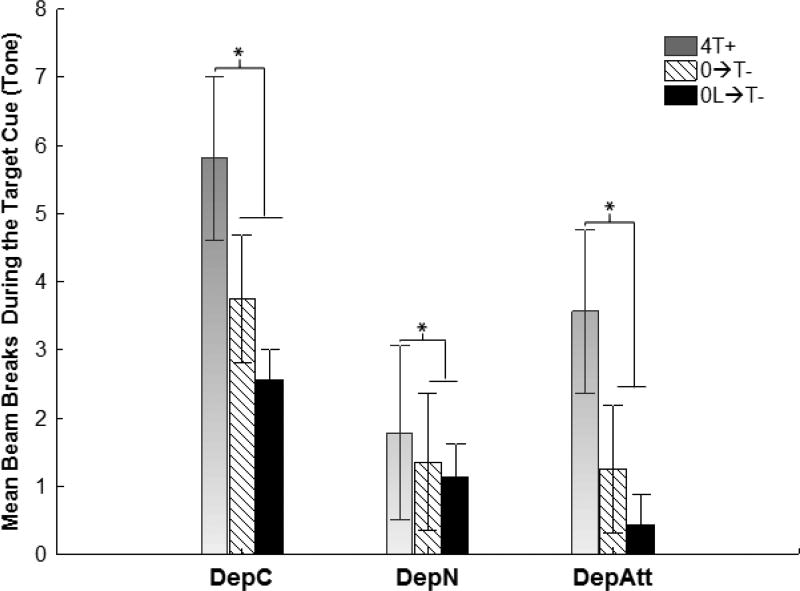
4.3 Test 1-Light
In the preceding transfer training phase, the light and satiety cues, produced by 0-hr food deprivation, were trained as a compound negative feature cue. To assess which of these cues was exerting the most control over discriminative responding, we tested the ability of the light to inhibit responding to the tone target under 4-hr food deprivation (i.e., on trials when the 0-hr satiety cue was absent). We compared performance on these 4L→T+ trials to performance on 0L→T- and 4T+ trials, like those presented during transfer training. Figure 4 shows that, as was the case during transfer training DepC differed from DepN and DepAtt with respect to overall levels of responding. However, the general pattern of responding on each type of trial was substantially the same for each group. This pattern indicates that during Test 1, the capacity of the light to suppress responding to the tone target in the absence of 0-hr satiety cues was limited. An overall repeated measures ANOVA obtained significant main effects of Group (F(2,20)=5.22, p<.05) and Trial Type (F(2,40)=15.39, p<.0001), but no Trial Type × Group interaction (F(4,40)=1.35), p=.27).
Figure 4.
Experiment 1 Test 1-light. The light exhibited little ability to inhibit responding to the tone target in the absence of 0-hr satiety cues. Responding to tone differed as a function of Trial Type (F(2,40)=15.39, p<.0001), but this effect did not vary by Group. As in transfer training, all groups responded less when the tone was presented with the light and 0-hr satiety cues (on 0L→T- trials) compared to when the tone was presented alone on 4T+ trials (p < .05) However, responding to the tone on trials with the light but with no 0-hr cues (i.e., 4L→T-) did not significantly differ from that a 4T+ trials. For further analysis see results section.
Post-hoc Bonferroni tests confirmed that the difference between responding on 4T+ and 0L→T- trials was significant (p < .01). This showed that the sFN discrimination established during transfer training was retained during Test 1. In contrast, responding on 4L→T+ test trials was not significantly different from that obtained on 4T+ trials (p > .35). This result indicates that the light had acquired little capacity to suppress responding to the tone target, when 0-hr cues were absent. The comparison of responding on 4L→T+ and 0L→T- trials is also consistent with this conclusion. Responding to the target cue was significantly less when the 0-hr deprivation cues was combined with the light compared to when the 0-hr cue was absent (p. < .01).
4.4 Test 2- 0-hr food deprivation
Test 2 assessed the ability of the 0-hr cue to suppress responding to the tone target without the presence of the light. As was the case for Test 1, Figure 5 shows that while the groups differed in overall level of responding, the pattern of responding to each type of trial in Test 2 was similar for all groups. Of special importance, all groups tended to respond more on 4T+ than both 0→T- and 0L→T- trials. This pattern of responding was especially pronounced for Groups DepC and DepAtt compared to Group DepN.
Figure 5.
Experiment 1 Test 2- 0-hr food deprivation. 0-hr satiety cues exhibited the ability to inhibit responding to the tone target in the absence of light cues. Similar to Test 1, responding to the tone differed by Trial Type (F(2,40) = 10.58, p. < .001), and this effect did not vary by Group. All groups responded more on 4T+ trials than on 0→T- or 0L→T- trials (ps<.01). See results for detailed analysis.
An ANOVA obtained a significant main effect of Trial Type (F(2,40) = 10.58, p. < .001), but no significant main effect of Group (F(2, 20) = 3.4, p = .054) or Group × Trial Type interaction (F(4, 40) = 1.36, p. >.26). Post-hoc Bonferroni tests confirmed that the difference between responding on 4T+ and 0L→T- trials was significant (p < .001). Thus, the sFN discrimination established during transfer training was retained during Test 2. Furthermore the responding on 0→T- without the light was also significantly less than that recorded on 4T+ trials (p < .01), while the difference in responding between 0→T- trials without the light and 0L→T- trials with the light was not significant (p. > .45). In agreement with the results of Test 1, the results of Test 2 indicate that light component of the compound negative feature stimulus used in transfer training, contributed little to the inhibition of responding to the tone target, relative to the 0-hr food cues.
5. Experiment 1 Discussion
The results of pretraining showed that stimulus control by food deprivation cues was sensitive to the experimental contingencies that were employed. Specifically, cues arising from 4- and 0-hr food deprivation exhibited little control over appetitive behavior in the absence of a predictive relationship between the presence of those cues and the delivery of sucrose reinforcement (Group DepN). In contrast, the rats in both Groups DepC and DepAtt showed significant discrimination performance when food deprivation level was correlated with reinforcement. The results provide evidence that the performance of Groups DepC and DepAtt was not based solely on any unconditioned activating or suppressive effects of 4- and 0-hr food deprivation, as the exposure of these two groups to each level of food deprivation was the same as that for Group DepN, which showed little evidence of differential responding under the 0- and 4-hr deprivation levels. Rather, the difference between the groups in pretraining appeared to be based on the discriminative contingencies to which they were exposed.
Furthermore, during pretraining Group DepAtt exhibited weaker stimulus control by deprivation state cues relative to Group DepC. This outcome indicates that discriminative responding was not based solely on a direct correlation between deprivation state and the delivery of reinforcement, because this correlation was the same for both groups. That is, both groups were pretrained with the same food deprivation level reinforcement contingency such that cues produced by 4-hr food deprivation signaled that a 4 min external target cue would terminate with reinforcement and cues arising from 0-hr food deprivation signaled nonreinforcement of that target cue.
However, the finding that Group DepC exhibited greater discriminative control by 0- and 4-hr food deprivation cues compared to Group DepAtt indicates that the power of food deprivation cues to control appetitive responding depended on differences between the groups in the strength of association of their target cues with reinforcement. Group DepC was pretrained with a compound target cue comprised of both a clicker and a white noise that was presented on every trial. For Group DepAtt the clicker served as the target cue on half of the trials and the white noise served as the target cue on the remaining trials. Thus, the single compound target cue for Group DepC received more rewarded and nonrewarded pretraining and presumably stronger excitatory and inhibitory associations with sucrose reward than did either of the respective clicker and white noise target cues for Group DepAtt.
This pattern of results is largely consistent with the model depicted in Figure 1. That model proposes that cues arising from different levels of food deprivation are not directly associated with the rewarding consequences of intake, but instead influence appetitive behavior by altering the activation of direction associations of external cues with those consequences. Accordingly, the ability of food deprivation cues to either excite or inhibit behavior will depend on the strength of the excitatory and inhibitory associations between external food cues and rewarding postingestive outcomes.
Following pretraining, all groups were given sFN training in which satiety cues produced by 0-hr food deprivation and an external light stimulus were trained in compound as negative feature stimuli that signaled the nonreinforcement of a 10-sec external tone. The results showed that all groups solved this sFN problem. However, discrimination performance was not equal for all groups. Discriminative responding for Group DepC was greater than for Group DepN, which did not differ significantly for Group DepAtt. In other words, performance during transfer testing was greatest for the group that exhibited the strongest discriminative control by deprivation cues during pre-training. This type of transfer would be expected to the extent that 0-hr food deprivation cues also functioned as negative feature stimuli during pretraining. If 0-hr food deprivation cues had been established as negative feature stimuli during pretraining this would potentially enable them to block or overshadow the development of the light as negative feature cue during transfer training. The results of Experiment 1 are also consistent with this interpretation. When discriminative control by 0-hr cues and the light was assessed independently, the findings indicated that the 0-hr satiety cues, and not the light, functioned as negative feature stimuli by inhibiting the ability of the tone target cue to evoke responding.
Despite differences in performance during pretraining and transfer training, Groups DepC, DepAtt, and DepN each exhibited much stronger inhibitory control by 0-hr food deprivation stimuli compared to the light cue during final testing. The basis of this finding merits some attention. Satiety cues produced by 0-hr food deprivation are distinct from the external light cue used in the present experiment in that only the satiety cues were present both in and outside of the experimental apparatus. Because of this, learning that satiety cues predict the nonreinforcement of food cues could occur in the home cage prior to any programmed experimental manipulation. In contrast, there was no opportunity for similar extra-experimental learning about the light cue because this stimulus was not encountered outside of the conditioning chambers. As a consequence, prior learning that satiety cues signaled nonreinforcement of food cues would be expected to transfer positively from the home cage to the experimental context, where those cues similarly signaled the nonreinforcement of external cues in the apparatus. There is no reason to assume that Groups DepC, DepAtt, and DepN differed in what they learned about their satiety signals prior to the experiment. Given this assumption, it would not be surprising if this prior learning about satiety cues enabled 0-hr deprivation stimuli to block the establishment of the light cue as negative feature stimulus for all three groups during sFN transfer training, regardless of pretraining manipulation.
6. Experiment 2
Our second study compared the effects of consuming a high-fat, high-sugar Western diet (WD) on sFN performance when external cues served as negative feature stimuli relative to when satiety cues (0-hr food deprivation) served that role. Specifically, we showed previously that discriminative performance in a sFN task is adversely affected by WD consumption (Kanoski et al., 2010; Davidson et al., 2012; Davidson et al., 2013) and these effects have been observed within 10 days of diet initiation (Davidson et al., 2013). Similarly, rapid impairment on several spatial learning tasks has been reported by our laboratory and by several other investigators (Murray, et al., 2009; Kanoski & Davidson, 2010; Beilharz, et al., 2014; Hargrave, et al., 2016). Based on this evidence, we were interested in the short term effects of WD on the ability of rats to use internal state cues and external visual cues as feature negative stimuli. If the mechanisms that underlie performance in a sFN task with conventional stimuli are similar to those that underlie discriminative control of behavior by interoceptive satiety cues, the decremental effects of WD intake on sFN performance with conventional stimuli would be expected to likewise occur when satiety cues served as negative feature stimuli.
To test this hypothesis, two groups of rats received sFN discrimination training. The Internal group (i.e., Int) was trained with a satiety cue (produced by 0-h food deprivation) as a signal that an auditory cue (tone) would be nonreinforced (i.e., the 0-hr cue served as a negative feature cue), while the External group (i.e., Ext) was trained to use a visual stimulus (light) to signal that the tone would be nonreinforced (i.e., the light would be a negative feature stimulus). After both groups acquired the discrimination, the Int and Ext groups were further subdivided such that half of the rats in each group were placed on ad libitum WD while the other half were maintained on ad libitum chow.
The effect of WD intake on behavioral control by these internal and external negative feature stimuli was indexed by the extent to which the suppressive power of those cues transferred to a previously trained and extinguished target cue (e.g. Rescorla, 1985; Davidson & Rescorla, 1986). This procedure involved reinforcing with sucrose reward (i.e., building an excitatory association) a simple conditioned cue (clicker), and then partially extinguishing that cue by removing the sucrose reward (i.e., building an inhibitory association). Then the rats were trained with either their internal cue (0-hr) or an external cue (light) serving as negative feature stimuli (described above). After training, probe tests were used to assess the extent to which the suppressive power of their negative feature cue (0-hr deprivation state or light) transferred to modulate responding evoked by the pretrained target stimulus (clicker). The use of this transfer target ensured that (a) the Internal and External groups were matched with respect to the level of responding to the transfer target and that (b) responding during the test phase was not a consequence of the establishment of a configural cue arising from the joint presentation of the negative feature stimulus and target cue during sFN training.
We expected that both the Int and Ext groups that remained on chow during probe testing would show greater transfer of discriminative control by their negative feature stimuli to the pretrained target cue, compared to WD-fed rats in each of these groups. If the effects of consuming WD on behavioral control were similar for both the Int and Ext groups, this would suggest that similar mechanisms underlie stimulus control by both internal and external feature negative cues.
7. Experiment 2 Methods
7.1 Subjects
Subjects were 40 naïve, male Sprague-Dawley rats of the same description as those used in Experiment 1. Rats were housed individually in exhaust ventilated plastic cages (Optirat) and given ad libitum access to water. Prior to testing, all rats were maintained on the laboratory chow described in Experiment 1. The animal room was maintained on a 12:12 light: dark cycle with lights on at 1000-hr and off at 2200-hr. All procedures for the care and treatment of the rats were approved by the American University Institutional Animal Care and Use Committee.
7.2 Diet s
WD was a pelleted, lard-based diet high in saturated fat and dextrose (Harlan, Teklad, TD 10768). The WD had a caloric density of approximately 4.4 kcal/g (42% kcal from fat, 37% kcal from carbohydrates, 19% kcal from protein) and contained the following (g/kg): 270g casein, 220.5g dextrose, 120g maltodextrin, 170g lard, 15g saf-flower oil, 15g soybean oil, 80g corn starch, and 50g cellulose. Approximately half of the rats were control animals that remained on laboratory chow (as described in section 3.1) until the completion of the experiment. Rats were given ad libitum access to diet between probe test trials. Probe test deprivation levels are described below.
7.3 Apparatus
The apparatus and reinforcers were the same as those described in section 3.2.
7.4 Procedures
All of the rats were run in squads of 8 animals that were counterbalanced with respect to group assignment and conditioning chamber. Training and testing trials always began at 1000h and followed the same training schedule as used in Experiment 1 (detailed in section 3.3). On each trial, rats were exposed to either a 4 min light or 0-hr food deprivation on nonreinforced trials or the absence of these cues (no light or 24-hr food deprived) on reinforced trials. During the last 10s of each of the 4min trials, rats were presented with an auditory target cue. Number of interruptions of a photobeam inside the recessed food magazine were recorded during the 10s period in which the target cue was presented. The termination of the target was immediately followed by the delivery of five sucrose pellets on reinforced trials, whereas no sucrose pellets were delivered on nonreinforced trials. On both reinforced and nonreinforced trials, the rats were removed from the conditioning chambers and returned to their home cages approximately 2 min after the target cue was terminated. Rats were given only one trial per day; therefore, rewarded and nonrewarded trials occurred on separate days. Table 2 describes the design of Experiment 2 and the contingencies each group received.
Table 2.
Experiment 2 Design. 95% = maintained at 95% of free feeding weight, 0= 0-hr food deprived (food sated), 4-hr food deprived; C=clicker, T=tone, L=light; + = sucrose reward, - = absence of sucrose reward.
| Group | Transfer Target Training |
Serial Feature Negative Discrimination Training |
New Groups: Diet |
Probe Transfer Cue Test (4d,12d) |
|---|---|---|---|---|
| Internal (Int) | 95% C+ 95%C- | 0→T-, 24T+ | Internal Modulator Chow | 0→C-, 24C+ |
| 95% C+ 95% C- | 0→T-, 24T+ | Internal Modulator WD | 0→C-, 24C+ | |
| External (Ext) | 0C+ 0C- | 95% L→T-, T+ | External Modulator Chow | 95%, L→C-, C+ |
| 0C+ 0C- | 95% L→T-, T+ | External Modulator WD | 95% L→C-, C+ |
7.4.1 Transfer Target Training
Prior to the start of transfer target training, rats were assigned to the Int and Ext groups (n=20 each) that were matched on body weight (M ± SEM = Int: 317.65 ± 10.42g; Ext: 318.85 ± 11.43). Rats in Ext group were given ad libitum access to standard chow. Because this group would never experience 0-hr food deprivation during any subsequent phases of the study, learning that accrued to 0-hr deprivation during transfer cue pretraining was not expected to affect subsequent performance. Rats in the Int group were fed chow and deprived to 95% of their ad libitum weight throughout transfer cue training. Rats in this group were maintained on 95% food deprivation during transfer cue training because, unlike the Ext group rats, these rats would have their deprivation state contingent with reward during training and testing. Because internal cues were meant to signal the nonreinforcement of the target cue during subsequent training and testing, learning about the 0-hr deprivation cue during transfer cue pretraining could affect performance during testing. Pretraining with a food deprivation regimen that maintained the rats at 95% of their free-feeding weight was designed to avoid this possibility.
The first half of transfer cue pretraining consisted of 4min rewarded trials in the conditioning apparatus. During the last 10s of this period a 10s clicker (Lafayette Instruments) was presented. The termination of the clicker was followed immediately by the delivery of 5 sucrose pellets. After both groups Int and Ext achieved asymptotic acquisition performance (indicated by two consecutive trials with similar levels of responding), all rats were given nonrewarded, extinction trials in which presentation of the clicker was not followed by sucrose reinforcement. Extinction continued until responding to the clicker was approximately half of that at the end of acquisition. Previous research with external cues showed that transfer control by negative feature cues is optimized with targets that have a history of training followed by extinction. This type of training is thought to embed transfer targets in concurrent excitatory and inhibitory associations with its unconditioned stimulus (US; e.g., sucrose). As noted previously, negative feature stimuli are thought to modulate responding to their targets by gating the activation of the inhibitory target→US association (Rescorla, 1985; Bouton & Swartzentruber, 1986).
7.4.2 SFN Discrimination Training
After both groups reduced their appetitive responding to the clicker by approximately half relative to their terminal acquisition performance, all rats were switched to their training contingencies. For Group Ext, deprivation level was maintained at 95% of ad libitum weight. Group Int’s deprivation level alternated between 0-hr and 24-hr food deprived. Training trials for the Ext group consisted of a 4min of light concluding with the 10s presentation of a 3000 Hz tone on nonreinforced trials (L→T-trials). On reinforced trials, no light was presented and 5 sucrose pellets were delivered immediately following termination of the 10 sec tone (T+ trials). Under these training conditions, rats in the Ext group could learn to use the external light to signal that the tone would not be reinforced (i.e. the light was trained as the negative feature cue).
Training trials for the Int group consisted of a 10s presentation of the tone target stimulus, but cues produced by 0-hr food deprivation replaced the light as a negative feature stimulus that signaled that the tone target cue would be nonreinforced (0→T-trials). When the 10 sec tone was presented and the rats were food deprived for 24-h (i.e., satiety cues were absence) its termination was followed immediately by the delivery of 5 sucrose pellets (24T+ trials). With these training contingencies, rats in the Int group could use their internal satiety cues as a negative feature stimulus to signal when the tone would not be reinforced.
7.4.3 Probe Testing with the Transfer Target Cue
After significant discrimination was achieved for four consecutive trials (two rewarded and two nonrewarded) in both groups, rats were further subdivided into 4 groups. Approximately half of the rats in the Ext group and half of the rats in the Int group were placed on WD (n= 12 rats per group). The remaining rats in each group were maintained on standard chow (n=8 rats per group). These groups were all matched on body weight (M ±SD= Int Chow: 390.5±20.28g; Int WD: 394.5±27.12g; Ext Chow: 400.63±25.78g; Ext WD: 398±22.05g), terminal performance with the previously trained transfer cue (clicker), and terminal sFN discrimination performance. All rats were then probe tested 4d and 12d after WD initiation. During probe testing, the Ext group was maintained at 95% of ad libitum weight, while the Int group’s deprivation level varied between 0-hr and 24-hr food deprived. All rats were given trials that were exactly like those presented during training, except that the presentation of transfer target stimulus (the clicker) was substituted for the presentation of the tone (Group Ext received C+ L→C-; Group Int received C +, 0→C-). The ability of the previously trained negative feature stimulus to inhibit responding to the transfer target was compared for the Int and Ext groups as a function of Diet (WD or chow) during probe testing. Each probe test consisted of four trials, two rewarded trials and two nonrewarded trials, which lasted for a total of four days (i.e., one trial per day).
7.5 Statistical Analysis
The dependent measure for pretraining, training, and testing was the number of beam breaks during the 10s target. Transfer cue training was analyzed using a repeated measures ANOVA with Phase (acquisition or extinction) and Trials as within-subjects factors and Group (Int or Ext, in the subsequent training phase) as a between-subjects factor. Training data was analyzed using a repeated measures ANOVA with Trials, +/− trial type (+ being rewarded and − being nonrewarded trials), and Block (four trials consisting of two + and two − trials) as within-subjects factors, and Group (Int and Ext) as a between-subjects factor. Testing data were analyzed using a 2 × 2 repeated measures factorial ANOVA with Trials, +/− trial type, and Block (four trials) as within-subjects factors and Group (Int and Ext) and Diet (WD or Chow) as between-subjects factors. The alpha level was set at .05, and significant main effects and interactions were further analyzed with Newman-Keuls post-hoc tests. The alpha level was set a p < .05.
7.6 Experiment 2 Results
7.6.1 Transfer Target Pretraining
At the end of reinforced pretraining, the mean beam breaks and standard errors of the mean (SEM) recorded during the 10s target cue (clicker) was 5.4 ± 3.7 SEM for Group Int and 4.6 ± 3.1 SEM for Group Ext. At the end of extinction these levels dropped to 2.8 ± 3.0 SEM for Group Int and 2.6 ± 2.8 SEM for Group Ext. ANOVA comparing performance for each group at the end of reinforced training and extinction phases yielded only a significant main effect of Phase (F(1,38)=13.38, p<.001). The main effect of Group (F(1,38)=.41, p. =.52) was not significant nor was there a significant Group × Phase interaction (F(1,38)=.23, p=.63). This pattern of results shows that rats in both Groups Int and Ext reduced responding from the end of training to the end of extinction, and that the groups did not differ significantly in level of responding at the end of either the training or extinction phases.
7.6.2 sFN Discrimination Training
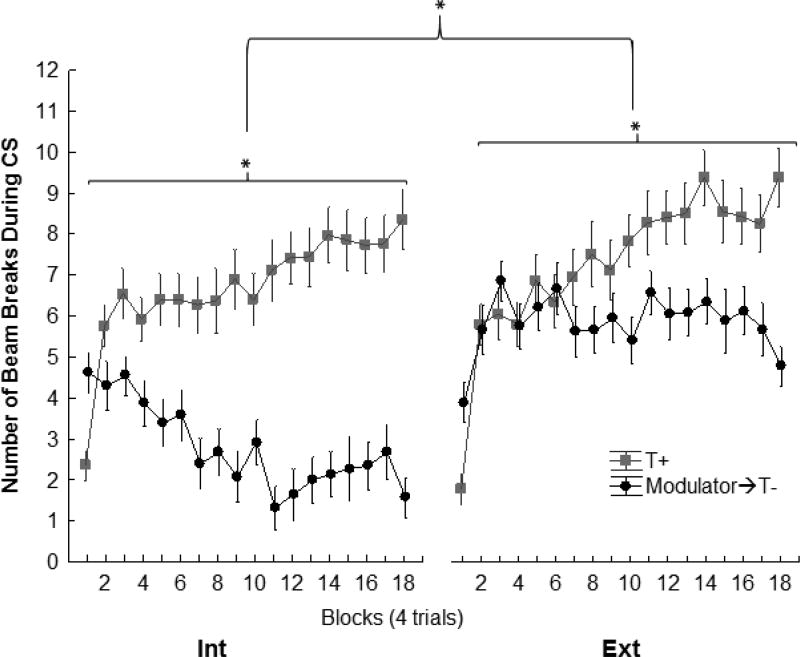
During training, rats in both the Ext and Int groups showed that they solved the sFN discrimination problem by responding more on reinforced T+ trials than on nonreinforced trials in which the negative feature stimulus preceded the tone target (0→T-; L→T-). Figure 6 shows that the magnitude of discrimination was greater for Group Int than for Group Ext, with responding on 0→T- trials for Group Int suppressed to a greater degree compared to L→T- trials for Group Ext. A repeated measures ANOVA confirmed these impressions by yielding a significant main effect of +/− (F(1,38) = 119.84, p<.0001) along with significant Group × +/− interaction (F(1,38) = 25.53, p. < .0001). Further analysis of the Group × +/− interaction using Newman-Kuels post-hoc found that, while both Groups Int (p<.001) and Ext (p<.001) were able to discriminate between rewarded and nonrewarded trials, Group Int was able to suppress responding on nonrewarded trials more efficiently than Group Ext (p<.001). It should be noted, that there was no significant difference in responding between groups on rewarded trials (p=.35); thus, the difference in discrimination performance for Groups Int and Ext during training is attributable to greater inhibition of responding on nonreinforced trials for Group Int compared to Group Ext.
Figure 6.
Experiment 2 serial feature negative discrimination training acquisition. sFN discrimination performance for Group Int exceeded that for Group Ext during sFN training. (Group × +/− interaction (F(1,38) = 25.53, p. < .0001). However, both Group Ext and Int learned to respond more on rewarded (+) trials with the tone alone (T+) than on nonrewarded (-) trials when their negative feature cue preceded presentation of the tone (negative featured→T-; ps < .01). See results for detailed analysis.
7.6.3 Probe Testing with Transfer Target Cue
By the last block of training, both groups were able to significantly discriminate between rewarded and nonrewarded trials and there were no group differences in discrimination (Figure 7a–b). A repeated measures ANOVA yielded a significant main effect of +/− (F(1,36)=144.44, p<.0001), but no +/− × Group (F(1,36) = 4, p = .05), +/− × Diet (F(1,36) = .01, p = .91), or +/− × Group × Diet (F(1,36) = 2.12, p = .15) interactions.
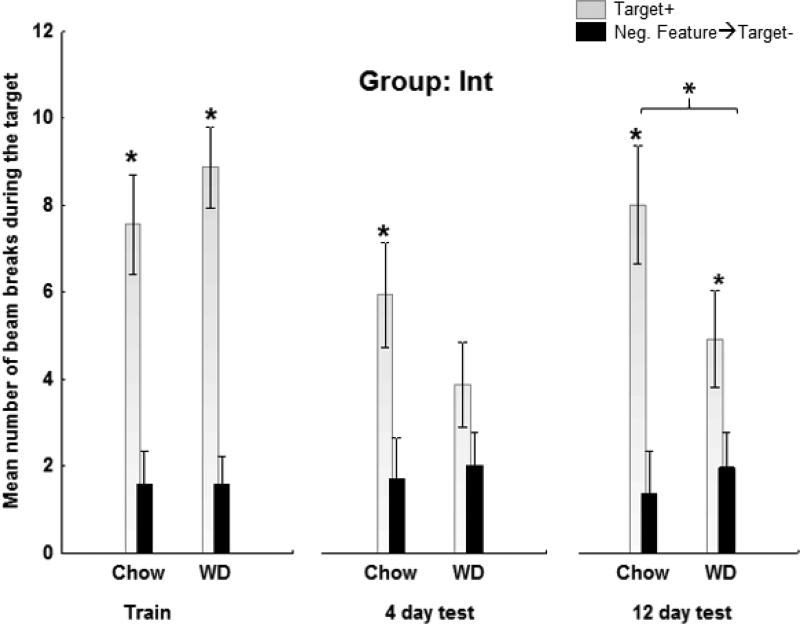
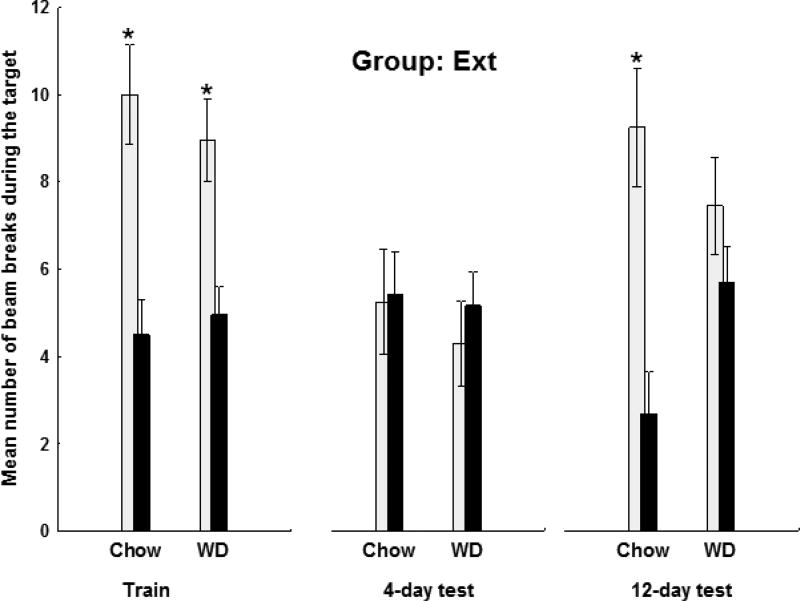
Figure 7.
a (Group Int) and b (Group Ext): Terminal training and probe testing. On the last block of training, both groups responded more on rewarded than on nonrewarded trials (F(1,36)=144.44, p<.0001) and the magnitude of this difference did not vary by group. For both Groups, discrimination performance during probe testing was significantly impaired for rats that were maintained on WD for 12 days compared to their chow-fed controls. This pattern of results yielded a +/− × Diet interaction (F(1,36) = 11.77, p< .01) that did not vary significantly by Group. See results for detailed analysis.
Figure 7a shows that the Chow-fed rats in Group Int exhibited strong discrimination performance on both the 4 day and 12 day probe tests, responding more on T+ trials than on 0→T- trials. However, for WD-fed rats in Group Int, discrimination performance on both probe days was considerably weaker. Figure 7b shows the comparable results for chow-fed and WD-rats in Group Ext. Discrimination performance was not apparent for either diet condition on the 4-day probe test. On the 12-day probe test clear discriminative responding emerged for the chow-fed rats but not for the WD-fed rats in Group Ext. An overall analysis of the 4-day and 12-day probe tests confirmed that the ability to discriminate between rewarded and nonrewarded trials varied as a function Diet, Group, and Test day. This analysis obtained a significant +/− × Diet (F(1,36) = 11.77, p< .01), but no +/− × Diet × Group interaction (F(1,36) = .03, p=.87), indicating that discrimination performance was weaker for rats fed WD compared to chow and this did not covary with Group. In addition, the +/− × Group (F(1, 36) = 6.32, p<.05) interaction was also significant, indicating the discrimination performance was stronger for Group Int than for Group Ext. Similar to the training phase, this difference was driven by the ability for rats in Group Int to suppress responding on the nonrewarded trials more effectively than rats in Group Ext (p<.01).
ANOVAs were then used to assess the effects of Group and Diet on discrimination performance on each test day separately. On the 4-day test, this analysis obtained only a significant +/– × Group interaction (F(1,36) = 10.75, p <.01), indicating that discrimination performance on the 4-day test was superior for Group Int relative to Group Ext.
The same analysis on the 12-day test yielded a significant main effect of +/– (F(1,36) = 61.75, p < .0001), and a significant +/– × Diet interaction (F(1,36) = 13.86, p< .001), but no significant interactions involving Group. These results confirm that on the 12-day probe test, rats fed WD exhibited impaired discrimination performance and this effect did not differ significantly by Group.
7.6.4 Body Weight
Immediately prior to diet initiation, mean weight for rats that would receive ad libitum WD during probe testing was 395.3g ± 5.03 and mean weight for rats the would be maintained on libitum chow was 395.6g ± 6.17. At the beginning of the Day 4 probe test, mean body weights were 462.3g ± 5.88 and 447.2g ± 7.20 for the WD-fed and chow-fed rats, respectively. At the beginning of the Day 12 probe test, mean weights for rats fed WD was 475.8g ± 6.03 and was 452.8g ±7.39 for rats fed chow. An ANOVA obtained a significant main effect of Day (F(1.4,50.31) = 1115.78, p < .0001). Newman-Keuls tests revealed that mean body weight was significantly higher on Day 12 compared to Day 4 and to the pretest (ps < .001) and was also significantly higher on Day 4 compared to the pretest (p < .001). The Diet × Day interaction was also significant (F(2,72) = 28.09, p < .0001). Newman-Keuls test showed the difference in mean body weight between WD-fed and chow-fed rats was significant on Day 12 (p = .04), but not on the pretest day (p = .97) or on Day 4 (p=.22). No main effect or interaction involving Group achieved significance.
8. Experiment 2 Discussion
The results showed that 12 days of WD maintenance impaired discrimination performance compared to chow-fed controls. Importantly, this WD-induced impairment was similar across negative feature cue type. In other words, WD had a similar disruptive effect on sFN performance for Group Int rats, trained with 0-hr deprivation, and Group Ext rats, trained with light stimulus, as negative feature cues.
Compared to Group Ext, Group Int exhibited superior discrimination performance during both the original sFN discrimination training and during probe testing, especially at the 4d probe test, with the transfer target cue. Moreover, weaker discrimination performance for Group Ext, relative to Group Int, during these phases appeared to be a consequence of weaker inhibitory control by the external light negative feature cue than by the internal negative feature cue produced by 0-hr food deprivation. This difference does not necessarily reflect distinct underlying mechanisms in stimulus control by each cue type. Rather, this finding is consistent with the hypothesis, noted previously, that satiety cues accrue extra-experimental associations as negative feature stimuli that positively transfer to facilitate performance with 0-hr food deprivation cues in the experimental context. In contrast, the light cue is not embedded in this signaling relationship outside of the experimental context and thus does not benefit from such positive transfer. This difference in discriminative responding during training complicates the interpretation of direct comparisons between Groups Int and Ext during probe testing. However, comparisons within each of these cue type groups across maintenance diet (WD versus chow) reduces this type of interpretive difficulty and establishes that discrimination performance for both groups was impaired for rats fed WD relative to rats fed standard chow.
9. General Discussion
We have proposed that deciding to eat or refrain from eating depends on an animal’s ability to use the presence or absence of satiety signals to predict when environmental food-related events (e.g., stimuli or responses) will be followed by rewarding postingestive outcomes. The level of appetitive responding depends on how strongly those environmental cues excite the retrieval of the memory of those rewarding postingestive consequences of intake. Satiety cues suppress appetitive and consummatory behaviors by inhibiting the retrieval of those postingestive reward memories. In the absence of satiety cues, the activation of those memories is above the necessary threshold to elicit food seeking and eating responses.
The results of Experiment 1 provide several lines of support for this general conceptualization. First, the results of the pretraining phase showed that the control of appetitive responding in the presence and absence of satiety cues was dependent on the type of discriminative contingency in which they were embedded. Strong control of discriminative responding developed rapidly when the presence and absence of satiety cues signaled the nonreinforcement and reinforcement, respectively, of a compound external cue associated with sucrose. When satiety cues were not predictive of reinforcement, little difference in responding in the presence and absence of satiety cues was observed. Furthermore, when satiety cues were perfect predictors of reinforcement, the discrimination performance did not depend solely on the contingency between the satiety cues and the delivery of sucrose pellets, but was determined, at least in part, by the amount of reinforced and nonreinforced training the rats received with external cues that were associated with sucrose. This pattern of results supports the idea, as noted above, that the ability of satiety and other types of negative feature stimuli to modulate responding depends on the degree to which external cues are embedded in excitatory and inhibitory associations with the memory of reward.
Second, the results of Experiment 1 showed that when all groups were subsequently trained to solve common sFN discrimination problems in which both satiety cues and an exteroceptive light stimulus could serve as negative feature stimuli, transfer test performance depended on the degree of discriminative control exhibited by satiety cues during pretraining. Previous studies indicate that conventional auditory and visual cues trained as feature cues in one sFN discrimination readily transfer their ability to modulate responding to a novel target stimulus that was also trained within a sFN problem (Bouton & Nelson, 1994). In Experiment 1, satiety cues exhibited this type of positive transfer to the extent that they were previously established as negative feature stimuli during pretraining.
Despite differing levels of positive transfer observed during sFN training, all rats, regardless of pretraining conditions, were able to show robust stimulus control by their satiety cues. That is, all groups were able to use satiety cues to suppress responding on nonrewarded trials similarly in the absence or presence of the light, but failed to suppress responding in the presence of the light when satiety cues were absent. This outcome suggests that satiety cues may be more salient than external cues when both are trained as negative feature stimuli. This relatively higher salience is also consistent with the possibility that satiety cues are established as negative feature stimuli outside of the experimental setting, giving them an advantage when they are later trained in competition with external cues as negative feature stimuli within an experimental context.
Experiment 2 revealed a different type of correspondence between stimulus control by satiety cues and control by conventional cues that were explicitly trained as sFN stimuli. Specifically, Experiment 2 replicated the finding that WD consumption impairs performance on a sFN task when conventional cues served as negative feature stimuli (Kanoski et al., 2010; Davidson et al., 2012; Davidson et al., 2013), and further demonstrated that the ability of satiety cues to serve as negative feature stimuli was similarly impaired by a shift to WD. Performance on sFN discriminations has been shown to be hippocampal-dependent (Holland et al., 1999; Holland & Fox, 2003), and a variety of evidence indicates that even short-term intake of a Western diet can produce signs of hippocampal pathophysiology (Murray, et al., 2009; Hargrave, et al., 2015; Beilharz, et al., 2016; Jais, et al., 2016).
The similarity of WD-induced impairment across internal and external feature negative cues observed in Experiment 2 is consistent with the hypothesis that stimulus control by both types of cues depends on similar underlying associative and physiological mechanisms, rather than an alternative hypothesis that WD may specifically impact the internal satiety cue by changing intrinsic motivation. During both training and testing, satiety cues exhibited stronger inhibitory control of responding to the target stimulus compared to that exhibited by external negative feature cues. However, extra-experimental experience using satiety signals as negative feature cues in inhibitory control may contribute to these differences by positively transferring into the experimental context, whereas the external cues are not typically embedded in an extra-experimental stimulus-event relationship that would help establish them as negative feature cues.
The findings of these experiments add to previous research that support the main components of our theoretical model. For example, it is well-established that animals can associate tastes with appetitive (e.g., nutritive or caloric) postingestive stimuli (Sclafani, 1997). It is also clear that anima ls can learn to use stimuli arising from different levels of food deprivation as discriminative cues to signal when it is appropriate to make an appetitive response (e.g., Davidson et al., 2005). Other findings make it clear that discriminative control by food deprivation state cues is based on learning about their interoceptive stimulus properties (e.g., Benoit & Davidson, 1996; Kanoski, Walls, & Davidson, 2007). The results of the present experiments support the view that learning about the postingestive consequences of intake and about interoceptive food deprivation stimuli are integrated as part of the associative control of energy regulation.
This paper is based on the analogy we have drawn between external cues trained as negative feature stimuli that modulate appetitive responding to their target cues and interoceptive satiety signals that modulate appetitive responding to food-related stimuli. We recognize that several factors make this analogy imperfect. For example, external stimuli can be directly measured with respect to their amplitude, frequency, and duration. In contrast, it is largely the case that the sensory properties of interoceptive cues can only be inferred based on other indirect measures (e.g., hours of deprivation). Comparison is complicated further by the fact that animals are exposed throughout their lifetime to interoceptive cues related to satiety and the relationships in which those cues are embedded, whereas animals may have little or no prior experience with the external cues used in experimental settings. In addition, unlike punctate and discrete external cues, signals arising from the interoceptive milieu are diffuse with an onset and offset that is difficult to specify. While turning off a light has stimulus consequences about which an animal could learn, the degree to which those consequences are analogous to “turning off” a satiety signal by food deprivation can be questioned.
However, none of these differences preclude the possibility that the mechanisms underlying the ability of satiety cues to modulate food intake are analogous to those mechanisms that underlie the ability of external negative feature cues to modulate responding to other food-related stimuli. Moreover, in spite of these differences we found substantial correspondence between stimulus control by satiety cues and external cues that were trained explicitly as negative feature stimuli. Even our assumption that internal cues produced by either a 4-hr or 24-hr period of food deprivation are of little consequence other than to remove satiety cues has theoretical justification. It has been argued that in societies where food is abundant, little eating occurs in response to biological hunger or bodily need for food, but that eating is instead the result of encounters with environment stimuli that have become strong conditioned elicitors of appetitive behavior and eating (Schachter, 1968; Woods, 2004). Within this framework, energy intake and body weight regulation depends on the ability of physiological satiety signals, whatever the bodily origins, to terminate the behaviors that are elicited by these conditioned cues. What has been termed the obesogenic environment (Corsica & Hood, 2011) is thought to result when environmental stimuli that are associated with highly palatable, energy-dense food and beverages overwhelm the ability of satiety cues to control intake. Indeed, it appears that overeating is not the result of an excess biological need for food, but a weakness in the ability of satiety to curb intake. This view of energy regulation provides the foundation for the theoretical perspective on which the present experiments were based.
This theoretical perspective provides a largely associative explanation about how satiety suppresses, and how food-related environmental cues promote, food intake and appetitive behavior. In other words, the suppression of appetitive responding need not be the result of some unconditioned property of satiety, but depends instead on what animals learn about their interoceptive satiety signals. Moreover, because this explanation relies on a hippocampal-dependent learning and memory process, findings implicating the hippocampus in the control of food intake and appetitive behavior in both human (Hebben et al., 1985; Rozin et al., 1998 Stevenson & Francis, 2017) and nonhu-man animals (Davidson & Jarrard, 1993; Davidson et al., 2009; 2010; Henderson et al., 2013) lend credence to this associative view. For example, several recent reports with rats and mice indicate that the inhibition of feeding depends on the activation of excitatory pathways originating in the hippocampus (Sweeney and Yang, 2015, Hsu et al., 2017). It may be the activation of these pathways that inhibit food intake that is elicited by environmental cues is based on a hippocampal-dependent learning and memory process like the one that has been investigated in the present paper. These considerations support the idea that learning and memory processes might underlie what have often been described as the motivational effects of satiety on appetitive behavior.
Highlights.
This work addressed the question of “how” satiety inhibits appetitive behavior
Satiety cues can influence energy regulation via learned associative mechanisms
Satiety cues may modulate appetitive behavior by serving as negative feature cues
Western diet impairs stimulus control by satiety and external negative feature cues
Acknowledgments
This paper was based, in part, on research completed by Sabrina Jones in partial fulfilment of the requirements of a Master’s degree at American University, Washington DC. We thank Mr. Farris Dwider for assistance with data collection for Experiment 2. Support for this research was provided by NIH grants R01HD028792 and R01DK110412 to Terry L. Davidson.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amin T, Mercer JG. Hunger and satiety mechanisms and their potential exploitation in the regulation of food intake. Current Obesity Reports. 2016;5(1):106–112. doi: 10.1007/s13679-015-0184-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andermann ML, Lowell BB. Toward a wiring diagram understanding of appetite control. Neuron. 2017;95(4):757–778. doi: 10.1016/j.neuron.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beilharz JE, Maniam J, Morris MJ. Short exposure to a diet rich in both fat and sugar or sugar alone impairs place, but not object recognition memory in rats. Brain, Behavior, and Immunity. 2014;37:134–141. doi: 10.1016/j.bbi.2013.11.016. [DOI] [PubMed] [Google Scholar]
- Beilharz JE, Maniam J, Morris MJ. Short-term exposure to a diet high in fat and sugar, or liquid sugar, selectively impairs hippocampal-dependent memory, with differential impacts on inflammation. Behavioural Brain Research. 2016;306:1–7. doi: 10.1016/j.bbr.2016.03.018. [DOI] [PubMed] [Google Scholar]
- Benoit SC, Davidson TL. Interoceptive sensory signals produced by 24hr food deprivation, pharmacological glucoprivation, and lipoprivation. Behavioral Neuroscience. 1996;110(1):168. doi: 10.1037/0735-7044.110.1.168. [DOI] [PubMed] [Google Scholar]
- Blundell J, De Graaf C, Hulshof T, Jebb S, Livingstone B, Lluch A, Mela D, Salah S, Schuring E, Van Der Knaap H, Westerterp M. Appetite control: methodological aspects of the evaluation of foods. Obesity Reviews. 2010;11:251–270. doi: 10.1111/j.1467-789X.2010.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Swartzentruber D. Analysis of the associative and occasion-setting properties of contexts participating in a Pavlovian discrimination. Journal of Experimental Psychology: Animal Learning and Cognit ion. 1986;12(4):333–350. doi: 10.1037/0097-7403.12.4.333. [DOI] [Google Scholar]
- Bouton ME, Nelson JB. Context-specificity of a target versus feature inhibition in a feature negative discrimination. Journal of Experimental Psychology: Animal Behavior Processes. 1994;20:51–56. doi: 10.1037/0097-7403.20.1.51. [DOI] [PubMed] [Google Scholar]
- Brunstrom JM. Mind over platter: pre-meal planning and the control of meal size in humans. International Journal of Obesity. 2014;38:S9–S12. doi: 10.1038/ijo.2014.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri O, Small C, Bloom S. Gastrointestinal hormones regulating appetite. Philosophical Transactions of the Royal Society B: Biological Sciences. 2006;361(1471):1187–1209. doi: 10.1098/rstb.2006.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemmensen C, Müller TD, Woods SC, Berthoud HR, Seeley RJ, Tschöp MH. Gut-Brain Cross-Talk in Metabolic Control. Cell. 2017;168(5):758–774. doi: 10.1016/j.cell.2017.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsica J, Hood M. Eating disorders in an obesogenic environment. Journal of the American Dietetic Association. 2011;111(7):996–1000. doi: 10.1016/j.jada.2011.04.011. [DOI] [PubMed] [Google Scholar]
- Davidson TL, Rescorla RA. Transfer of facilitation in the rat. Animal Learning & behavior. 1986;14(4):380–386. doi: 10.3758/BF03200082. [DOI] [Google Scholar]
- Davidson TL, Flynn FW, Jarrard LE. Potency of food deprivation intensity cues as discriminative stimuli. Journal of Experimental Psychology: Animal Behavior Processes. 1992;18(2):174. doi: 10.1037/0097-7403.18.2.174. [DOI] [PubMed] [Google Scholar]
- Davidson TL, Jarrard LE. A role for hippocampus in the utilization of hunger signals. Behavioral and Neural Biology. 1993;59(2):167–171. doi: 10.1016/0163-1047(93)90925-8. [DOI] [PubMed] [Google Scholar]
- Davidson TL, Kanoski SE, Tracy AL, Walla EK, Clegg D, Benoit SC. The interoceptive cue properties of ghrelin generalize to cues produced by food deprivation. Peptides. 2005;26:1602–1610. doi: 10.1016/j.peptides.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Davidson TL, Kanoski SE, Schier LA, Clegg DJ, Benoit SC. A potential role of the hippocampus in energy intake and body weigh regulation. Current Opinion in Pharmacology. 2007;7(6):613–616. doi: 10.1016/j.coph.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TL, Chan K, Jarrard LE, Kanoski SE, Clegg DJ, Benoit SC. Contributions of the hippocampus and medial prefrontal cortex to energy and body weight regulation. Hippocampus. 2009;19(3):235–252. doi: 10.1002/hipo.20499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TL, Monnot A, Neal AU, Martin AA, Horton JJ, Zheng W. The effects of high-energy diet on hippocampal-dependent discrimination performance and blood-brain barrier integrity differ for diet-induced obese and diet-resistant rats. Physiology & Behavior. 2012;107:26–33. doi: 10.1016/j.physbeh.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TL, Hargrave SL, Swithers SE, Sample CH, Fu X, Kinzig KP, Zheng W. Inter-relationships among diet, obesity and hippocampal-dependent cognitive function. Neuroscience. 2013;253:110–122. doi: 10.1016/j.neuroscience.2013.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TL, Tracy A, Schier L, Swithers S. A view of obesity as a learning and memory disorder. Journal of Experimental Psychology: Animal Learning and Cognition. 2014a;40(3):261–279. doi: 10.1037/xan0000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TL, Sample CH, Swithers SE. An application of Pavlovian principles to the problems of obesity and cognitive decline. Neurobiology of learning and memory. 2014b;108:172–184. doi: 10.1016/j.nlm.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargrave SL, Davidson TL, Lee T-J, Kinzig KP. Brain and behavioral perturbations in rats following Western diet access. Appetite. 2015;93:35–43. doi: 10.1016/j.appet.2015.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargrave SL, Davidson TL, Zheng W, Kinzig KP. Western diets induce blood-brain barrier leakage and alter spatial strategies in rats. Behavioral Neuroscience. 2016;130(1):123. doi: 10.1037/bne0000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebben N, Cirkein S, Eichenbaum H, Shedlack L. Diminished ability to interpret and report internal states after bilateral medial temporal resection: case H.M. Behavioral Neuroscience. 1985;9(6):1031–1039. doi: 10.1037/0735-7044.99.6.1031. [DOI] [PubMed] [Google Scholar]
- Henderson YO, Smith GP, Parent MB. Hippocampal neurons inhibit meal onset. Hippocampus. 2013;23(1):100–107. doi: 10.1002/hipo.22062. [DOI] [PubMed] [Google Scholar]
- Holland PC. Transfer of negative occasion setting and conditioned inhibition across conditioned and unconditioned stimuli. Journal of Experimental Psychology: Animal Behavior Processes. 1989;15(4):311. doi: 10.1037/0097-7403.15.4.311. [DOI] [PubMed] [Google Scholar]
- Holland PC. Occasion setting in Pavlovian conditioning. Psychology of Learning and Motivation. 1992;28:69–125. doi: 10.1016/S0079-7421(08)60488-0. [DOI] [Google Scholar]
- Holland PC, Lamoureux JA, Han JS, Gallagher M. Hippocampal lesions interfere with Pavolovian negative occasion setting. Hippocampus. 1999;9:143–157. doi: 10.1002/(SICI)1098-1063(1999)9:2<143::AID-HIPO6>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Holland PC, Fox GD. Effects of hippocampal lesions in overshadowing blocking procedures. Behavioral Neuroscience. 2003;117(3):650. doi: 10.1037/0735-7044.117.3.650. [DOI] [PubMed] [Google Scholar]
- Hsu TM, Noble EE, Liu CM, Cortella AM, Konanur VR, Suarez AN, Reiner DJ, Hahn JD, Hayes MR, Kanoski SE. A hippocampus to pre-frontal cortex neural pathway inhibits food motivation through glucagon-like peptide-1 signaling. Molecular P sychiatry. 2017 doi: 10.1038/mp.2017.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jais A, Solas M, Backes H, Chaurasia B, Kleinridders A, Theurich S, Mauer J, Steculorum SM, Hampel B, Goldau J, Alber J, Forster CY, Eming SA, Schwaninger M, Ferrara N, Karsenty G, Brüning J. Myeloid-Cell-Derived VEGF maintains brain glucose uptake and limits cognitive impairment in obesity. Cell. 2016;166(5):1338–1340. doi: 10.1016/j.cell.2016.03.033. [DOI] [PubMed] [Google Scholar]
- Kamin LJ. “Attention-like” processes in classical conditioning. Miami Symposium on the Prediction of Behavior: Aversive Stimulation. 1968:9–13. [Google Scholar]
- Kanoski SE, Walls EK, Davidson TL. Interoceptive “satiety” signals produced by leptin and CCK. Peptides. 2007;28(5):988–1002. doi: 10.1016/j.peptides.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoski SE, Davidson TL. Different patterns of memory impairments accompany short- and longer-term maintenance on a high-energy diet. Journal of Experimental Psychology: Animal Learning and Cognition. 2010;36(2):313–319. doi: 10.1037/a0017228. [DOI] [PubMed] [Google Scholar]
- Martin AA. Why can’t we control our food intake? The downside of dietary variety on learned satiety responses. Physiology & Behavior. 2016;162:120–129. doi: 10.1016/j.physbeh.2016.04.010. [DOI] [PubMed] [Google Scholar]
- Moran TH, Ladenheim EE. Physiologic and neural controls of eating. Gastroenterology Clinics. 2016;45(4):581–599. doi: 10.1016/j.gtc.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray AJ, Knight NS, Cochlin LE, McAleese S, Deacon RM, Rawlins JNP, Clarke K. Deterioration of physical performance and cognitive function in rats with short-term high-fat feeding. The FASEB Journal. 2009;23(12):4353–4360. doi: 10.1096/fj.09-139691. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Facilitation and inhibition. In: Miller RR, Spear NE, editors. Information Processing in Animals: Conditioned Inhibition. Erlbaum; Hillsdale, NJ: 1985. pp. 299–326. [Google Scholar]
- Rozin P, Dow S, Moscovitch M, Rajarm S. What causes humans to begin and end a meal? A role for memory for what has been eaten, as evidence by a study of multiple meal eating in amnesic patients. Psychological Science. 1998;9(5):392–396. doi: 10.1111/1467-9280.00073. [DOI] [Google Scholar]
- Sample CH, Martin AA, Jones S, Hargrave SL, Davidson TL. Western-style diet impairs stimulus control by food deprivation state cues: Implications for obesogenic environments. Appetite. 2015;93:13–23. doi: 10.1016/j.appet.2015.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sample CH, Jones S, Hargrave SL, Jarrard LE, Davidson TL. Western diet and the weakening of the interoceptive stimulus control of appetitive behavior. Behavioural brain research. 2016;312:219–230. doi: 10.1016/j.bbr.2016.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A. Learned controls of ingestive behaviour. Appetite. 1997;29(2):153–158. doi: 10.1006/appe.1997.0120. [DOI] [PubMed] [Google Scholar]
- Schachter S. Obesity and eating. Science. 1968;161(3843):751–756. doi: 10.1126/science.161.3843.751. [DOI] [PubMed] [Google Scholar]
- Schepers ST, Bouton ME. Hunger as a context: Food seeking that is inhibited during hunger can renew in the context of satiety. Psychological Science. 2017;28(11):1640–1648. doi: 10.1177/0956797617719084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternson SM, Eiselt AK. Three pillars for the neural control of appetite. Annual Review of Physiology. 2017;79:401–423. doi: 10.1146/annurev-physiol-021115-104948. [DOI] [PubMed] [Google Scholar]
- Stevenson RJ, Francis HM. The hippocampus and the regulation of human food intake. Psychological bulletin. 2017;143(10):1011. doi: 10.1037/bul0000109. [DOI] [PubMed] [Google Scholar]
- Swartzentruber D. Blocking between occasion setters and contextual stimuli. Journal of Experimental Psychology: Animal Behavior Processes. 1991;17(2):163–173. doi: 10.1037/0097-7403.17.2.163. [DOI] [PubMed] [Google Scholar]
- Swartzentruber D. Modulatory mechanisms in Pavlovian conditioning. Animal Learning & Behavior. 1995;23(2):123–143. doi: 10.3758/BF03199928. [DOI] [Google Scholar]
- Sweeney P, Yang Y. An excitatory ventral hippocampus to lateral septum circuit that suppressed feeding. Nature Communications. 2015;6 doi: 10.1038/ncomms10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SC. Gastrointestinal satiety signals I. An overview of gastrointestinal signals that influence food intake. American Journal of Physiology. 2004;286:G7–G13. doi: 10.1152/ajpgi.00448.2003. [DOI] [PubMed] [Google Scholar]
- Woods SC, Begg DP. Regulation of the motivation to eat. Current Topics in Behavioral Neurosciences. 2016;27:15–34. doi: 10.1007/7854_2015_381. [DOI] [PubMed] [Google Scholar]
- Zanchi D, Depoorter A, Egloff L, Haller S, Mählmann L, Lang UE, Drewe J, Beglinger C, Schmidt A, Borgwardt S. The impact of gut hormones on the neural circuit of appetite and satiety: A systematic review. Neuroscience & Biobehav-ioral Reviews. 2017;80:457–475. doi: 10.1016/j.neubiorev.2017.06.013. [DOI] [PubMed] [Google Scholar]