Abstract
Compulsive binge eating is a hallmark of binge eating disorder and bulimia nervosa and is implicated in some obesity cases. Eating disorders are sexually dimorphic, with females more often affected than males. Animal models of binge-like eating based on intermittent access to palatable food exist; but, little is known regarding sex differences or individual vulnerability in these models with respect to the reinforcing efficacy of food, the development of compulsive- and binge-like eating, or associated changes in whole-body metabolism or body composition. Adolescent male (n=24) and female (n=32) Wistar rats were maintained on chow or a preferred, high-sucrose, chocolate-flavored diet in continuous or intermittent, extended access conditions. Body weight and composition, intake, fixed- and progressive-ratio operant self-administration, and whole body energy expenditure and respiratory exchange ratios were measured across an 11-week study period. Subgroup analyses were conducted to differentiate compulsive-like “high responder” intermittent access rats that escalated to extreme progressive-ratio self-administration performance vs. more resistant “low responders.” Female rats had greater reinforcing efficacy of food than males in all diet conditions and were more often classified as “high responders”. In both sexes, rats with intermittent access showed cycling of fuel substrate utilization and whole-body energy expenditure. Further, “high-responding” intermittent access female rats had especially elevated respiratory exchange ratios, indicating a fat-sparing phenotype. Future studies are needed to better understand the molecular and neurobiological basis of the sex and individual differences we have observed in rats and their translational impact for humans with compulsive, binge eating disorders.
Keywords: food addiction OR addictive eating OR eating addiction, fixed-ratio and progressive ratio schedules of food operant self-administration, compulsive eating OR compulsivity, respiratory exchange ratio OR respiratory quotient OR RER, energy expenditure OR heat, overweight OR obesity OR adiposity OR body composition OR body fat OR body weight
1. Introduction
Binge eating, or consuming excess food in a short period of time with a loss of control1, defines several eating disorders and imparts a severe personal and public health burden2–8. Compulsive binge eating is a hallmark of binge eating disorder and bulimia nervosa9–10 and is also implicated in some obesity cases11–16. The construct of compulsive eating distinguishes the etiology, biology, severity, prognosis, and appropriate treatment of cases of binge-disordered eating and obesity from those without compulsive eating 9,17–28.
Animal models of compulsive eating have utilized intermittent schedules of access to palatable food to understand better the neurobiology and therapeutic targeting of addictive-like, binge eating29–33. Palatable foods are used because of their putative role in eliciting neurobehavioral changes colloquially known as “food addiction” 19,22,34, often including the development of compulsive, binge intake20,35–36. Intermittent access schedules are used to model a common form of dieting, whereby a person abstains from energy-dense, palatable foods while trying to eat less energy dense, and often less palatable, foods. This eating practice can lead to “yo-yo” dieting, with intake cycling, and is an etiologic risk factor for binge eating and poor metabolic outcomes37–43. Parallels between cyclic overeating vs. abstinence from palatable food and use-abstinence cycles in drug addiction also have been noted44–45. Compulsive-like, binge intake in such animal models may represent dysfunctions in behavioral control, a key element in conceptualizations of human food addiction20,28,34.
Similar to drug access models46–48, intermittent access models recently have compared effects of short (e.g., 30 minutes) vs. long (e.g., 24 hours) periods of access to palatable food32. While both schedules elicit binge-like eating, only intermittent long access leads to lasting underconsumption (“rejection”) of non-preferred diets during abstinence29–30,32,49–52. Intermittent long access to a palatable diet in female rats also led to body weight cycling and reductions in brown adipose tissue in relation to weight lost during abstinence from the diet32. Individual differences in intake behavior (both overeating and “rejection”) among rats on intermittent access schedules are seen30,32,53–58; how these impact metabolic outcomes is unknown. Also unknown is whether individual differences in the reinforcing value of food or compulsivity of food-directed instrumental behavior develop, key questions given the putative role of compulsivity in disordered eating. Indeed, in humans, motivational measures (e.g., relative reinforcement) provide unique and sometimes more powerful predictors of outcome vs. simple intake measures59–63. Due to the female preponderance of binge-related eating disorders64–71, sexual dimorphism in rodent models of binge eating also has been assessed. Females rats have shown faster eating rates72, higher “proneness,” defined by consistently greater intake55–56,73, and, potentially, higher reward value of palatable food73 after intermittent long access to palatable food. Sex differences in the development of binge-like instrumental behavior, including the reinforcing value of food or the compulsivity of food self-administration, putatively key components of addictive-like binge eating disorders20, 34, are understudied. Likewise, sex differences in metabolic outcomes, including body composition, energy expenditure, and fuel utilization, remain unclear.
Using our rat model of binge eating, the present study aimed to fill these gaps in our understanding of sex differences and individual differences in palatable food reinforcement and metabolic outcomes. In comparison to ad lib standard chow controls, adolescent rats of both sexes were subjected to continuous or intermittent long access to a preferred, sucrose-rich, but nutritionally-complete, chocolate-flavored diet. Body weight and composition, intake, fixed- and progressive-ratio operant self-administration, and whole-body metabolism were assessed. Further subgroup analyses were conducted on those intermittent access rats, labeled “high responders,” that developed the highest progressive-ratio self-administration performance to determine whether there are metabolic differences in these most “compulsive-like” rats. We hypothesized that energy expenditure and substrate utilization would cycle, similar to intake and body weight, in rats with long intermittent access in both sexes and that these cycles would be exaggerated in “high responder”, compulsive-like rats. Further, we hypothesized that female rats would 1) have a greater reinforcing efficacy of the palatable diet under both continuous and intermittent access conditions, 2) be more often classified as “high responders”, and 3) show greater metabolic cycling in the intermittent access condition.
2. Materials and Methods
2.1 Animals
Following Kreisler et. al32, 6–8 week old adult female (n = 32, 125–175 g) and male (n = 24, 215–300 g) Wistar rats (Charles River) were pair-housed (same-sex) upon arrival, separated by clear Plexiglas to enable individual food measurement, in wire-topped plastic cages in a temperature-(22 °C) and humidity- (60%) controlled vivarium (12:12 h reverse light-dark cycle). Before experiments, rats had ad libitum chow (C) (45-mg pellets, 5TUM TestDiet, St. Louis MO) and water available. Body weights and food intake were recorded for 2–3 weeks before experiments. Procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by The Scripps Research Institute’s Institutional Care and Use Committee.
2.2 Diet Schedules
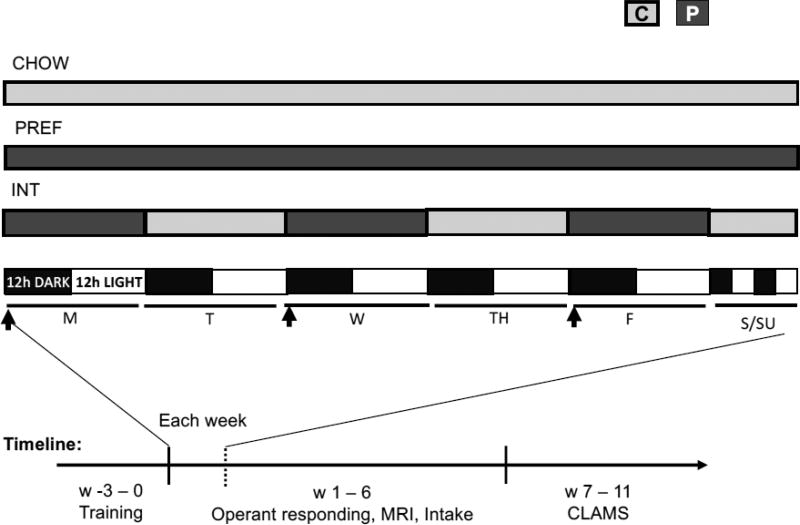
The “preferred” diet32,58 was sucrose-rich, chocolate-flavored and nutritionally-complete 45-mg pellets (P) (5TUL, Test Diets, St. Louis, MO) with similar macronutrient composition (~67% carbohydrates, 21% protein, and 13% fat by kcal) and caloric density (~3.44 kcal/g), compared to the chow (3.30 kcal/g). Rats were matched for baseline chow intake, body composition, and operant self-administration to 1 of 3 dietary groups: ad libitum access to the less-preferred grain chow (CHOW); ad libitum access to only the preferred, sucrose-rich chocolate-flavored diet (PREF); intermittent long access (INT) to the preferred diet for 24hr beginning at the dark cycle onset for 3 nonconsecutive days/week (Monday, Wednesday, Friday) with ad libitum chow otherwise. Days on which INT rats had access to the preferred diet are hereafter referred as “PREF” days, and days on which they only had access to the chow diet as “NON-PREF” days (Fig 1A).
FIG. 1.
Diet schedules and timeline. Schematic shows diet schedules for CHOW, INT, and PREF rats on chow (C) or preferred (P) diets in relation to a reverse light-dark cycle in one representative week during the diet schedules. The timeline at the bottom indicates the timing of behavioral tests. Diet schedule start at week 1. Arrows indicate time of operant self-administration sessions. Weekends are not shown to scale.
2.3 Self-administration apparatus
Operant food self-administration was performed in 30.5 × 25 × 30.5 cm chambers (Coulbourn Instruments, Whitehall, PA), in sound-attenuating, ventilated cubicles (Med Associates, Fairfax, VT), with Plexiglas ceiling and front and rear walls, stainless steel rod floors, and modular side walls. The right wall had two retractable levers, 2.1 cm above the floor and adjacent wall, with a pellet trough centered between and vertically aligned with the levers. Levers were extended throughout test sessions. Upon completion of a ratio requirement at an “active” lever, one 45-mg pellet was delivered 0.5 seconds afterward; responses at the other, “inactive” lever had no scheduled consequences. Following pellet delivery in all sessions, a 3.25-second post-reinforcement timeout, during which further active lever presses were recorded but had no consequences, was imposed to promote consumption of the previous pellet before successive deliveries. Ad libitum water was available via a bottle with sipper tube.
2.4 Body Composition Analysis
Whole body fat and lean mass were determined in awake rats by EchoMRI (Echo MRI-900, ACQ-SYS v.2008, Houston, TX). Measurements were taken one week prior to diet schedule start (BASELINE) and on 4 occasions during weeks 7–11 of the diet schedules. Measurements were also attempted during weeks 1–6 (not shown), but, due to technical failure, data were not collected for males; however, all subjects received similar experience in the MRI tube. Each analysis lasted ~5 min. Fat and lean mass were expressed as a % of body weight, measured prior to MRI.
2.5 Operant Self-Administration
All rats learned to self-administer chow (5TUM) food pellets over 3 weeks prior to experiment start as follows: (1) with their cage mates for 1 session for 24 hrs on a fixed-ratio 1 (FR) reinforcement schedule; (2) individually for one 12-hr FR session during the dark cycle; (3) one 3-hr FR session (4) five 30-min FR sessions (4) one progressive ratio (PR) session in which the response requirement increased exponentially with each pellet obtained per the progression described in Cottone, et al. 200851. PR sessions ended when rats failed to acquire another reinforcer within 14 min of the previous reinforcer, up to a maximum session duration of 2 hr. The “breakpoint” was defined as the last response requirement completed. Rats were then assigned to diet schedules. On days that INT rats had access to the preferred diet, all rats performed 1 weekly FR session and 1 weekly PR session (2 total sessions weekly), beginning at dark cycle onset, reinforced by their appropriate diet (i.e., CHOW received chow reinforcers and all others received preferred diet).
2.6 Indirect Calorimetry by Oxymax CLAMS
During the 3-week operant training, pre-experiment period, each rat was pre-acclimated for 48 hr to a test cage in our Oxymax Comprehensive Laboratory Animal Monitoring System (CLAMS) (Columbus Instruments, Columbus, OH). During weeks 7–11 of the diet schedule, rats, staggered as 8 per sex per week balanced across groups, were individually-housed in their respective test cages for indirect calorimetry measurement of respiratory exchange ratio (RER) (ratio of volume of CO2 expelled to O2 consumed) and energy expenditure (EE), calculated by the formula: [(3.815 + 1.232*RER)*VO2 (in liters)])74–75. VO2 was corrected to account for lean mass scaled to the 2/3 power76. Power-function normalization was used here, rather than covariate analysis, because of collinearity of sex with lean mass77. Each rat had a further 24 hr of reacclimation during an INT PREF day. Then, indirect calorimetry measurements were collected for the subsequent 48 hr over one full NON-PREF day and one full PREF day. Rats’ food was maintained on the appropriate diet schedule within the CLAMS in powdered form.
2.7 Experimental Timeline
After the three pre-experiment weeks of operant acquisition, baseline measurements, and CLAMS acclimation, rats were assigned to diet schedules, matched for body weight, percent body fat, and active lever operant responding. During weeks 1–6, rats performed operant sessions on two PREF food days (FR, PR once weekly at onset of dark cycle) and were placed in the EchoMRI at the onset of a third PREF food day (data were not obtained due to technical failure in weeks 1–6), had home cage food intake measured daily, and had body weights measured 2 times weekly at the end of a NON-PREF (Monday dark onset) vs. PREF (Thursday dark onset) food cycle. During weeks 7–11, rats continued on this schedule but with squads of 8 rats/sex staggered weekly through indirect calorimetry testing. All statistical analyses for intake, body weight, and operant performance were applied to data from weeks 1–6, before calorimetry testing began.
2.8 Statistical analyses
Behavior and intake measures were first analyzed by linear mixed-model restricted maximum likelihood analysis with a Type III test of fixed effects. Variables such as Group, Sex, and High/Low Responder Classification were between-subject factors, and Week, Hours and Phase were repeated measures. Due to non-sphericity of data, indirect calorimetry measures were analyzed by the general linear model with a Greenhouse-Geisser correction78. Following omnibus tests, higher order interactions were interpreted by comparison of lower-order interactions, main effects and pairwise comparisons, within the identified factors. Tukey’s pairwise tests were used for pairwise comparisons following GLM. To allow direct comparison between sexes, in some analyses, intake was normalized per body weight scaled to the 2/3 power76. Random effects, 2-way intraclass correlations of consistency were performed to assess stability of PR performance within a given subject79.. Key statistically significant effects for each figure are presented in Supplemental Table 1. Analyses were performed using GraphPad Prism 6.0 (GraphPad Software Inc., La Jolla, CA) and IBM SPSS Statistics v22 (IBM, Armonk, NY).
3. Results
3.1 Daily Intake
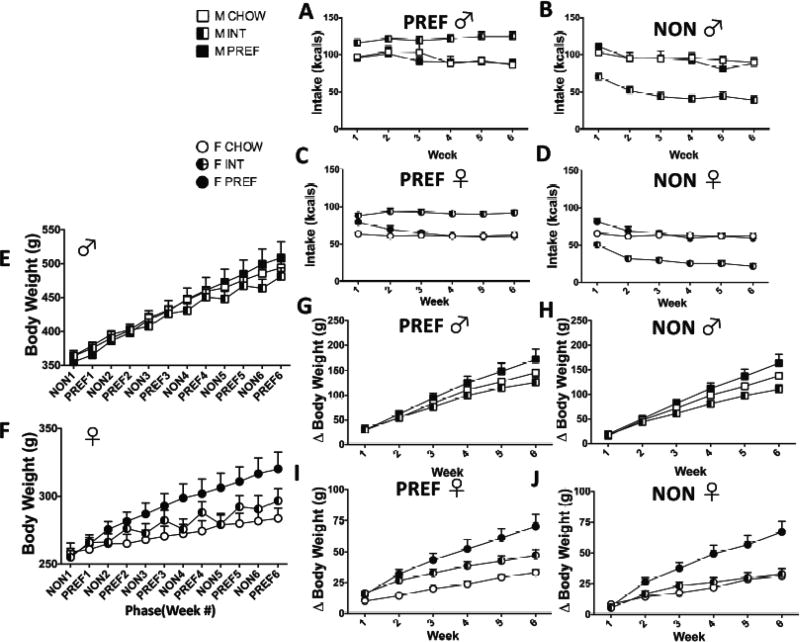
In the full model with both sexes, a significant Group X Day Interaction (F(2,550)=556.4, p<0.0001) indicated intake cycling in both sexes, wherein INT rats overconsumed on PREF days (FIG 2A & C; ps< 0.0001) and underconsumed on NON-PREF days (FIG 2B & D; ps<0.0001)). A Group X Sex X Day interaction (F(2,550)=5.7, p<0.005) indicated sex differences in the magnitude of this cycling, however. Specifically, a Sex x Group interaction on NON-PREF days (F(2,278)=12.3, p<0.0001), and not on PREF days, indicated that male INTs underrate more on NON-PREF days than females, whereas their overconsumption was comparable on PREF days . As expected, across diet groups, males had higher raw daily intake than females (Sex: F(1,550)=683.6, p<0.0001).
FIG 2.
Intake and body weight. On PREF food days (A) male and (C) female INTs ate more than PREFs and CHOWs, (ps<0.0001). Conversely, Male (B) and female (D) INTs ate much less than CHOWs and PREFs on NON-PREF food days (ps<0.0001). PREF rats increasingly weighed more than other diet groups (E & F) (Group X Week interaction, p<0.02). Measured at the end of PREF days (G), male INT rats gained less weight than male PREF rats (p<0.0001) and trended toward less weight gain than CHOW rats. Following NON-PREF days (H), male INT rats had gained less weight than PREF rats by Week 4 (p<0.04). Following PREF days, female (I) PREF rats gained significantly more weight than INTs and CHOW rats (ps<0.0001), but female INT rats also gained significantly more weight than female CHOW rats (p<0.0001). However, following underconsumption on NON-PREF days (J), female INT rats did not significantly differ from CHOW rats, while female PREF rats continued to gain more weight than both of these groups (ps<0.0001). Data show M+SEM. n = 6–12/group.
3.2 Body weight
A Group X Sex interaction indicated that diet schedule-related body weight differences were sex-dependent (Figs 2E &F; F(2, 513)=7.1, p<0.002). A Group X Week interaction indicated that PREF rats weighed more than INT or CHOW rats over time (F(10,190.6)=2.4, p<0.02). Regardless of diet schedule, as expected, male rats weighed more (F(1,513)=3724.4, p<0.0001). A Sex X Week X Group interaction following NON-PREF days (F(10,89)=2, p<0.05) and a Sex X Group interaction following PREF days (F(2,194)=9.6, p<0.0001) indicated sex differences in weight gain on the INT schedule, with respect to CHOW controls, as is elaborated below.
3.2.1 Males
Following PREF days (FIG 2G), male INT rats gained less weight than male PREF rats (p<0.0001) and trended toward less weight gain than CHOW rats (p<0.06). Following NON-PREF days (FIG 2H), male INT rats similarly had gained less weight than PREF rats by Week 4 (p<0.04). Male INT rats trended towards less weight gain than CHOWs following NON-PREF days as well, though this did not quite reach significance.
3.2.2 Females
Unlike in males, INT females never showed less weight gain than CHOW controls. Rather Group X Week interactions in females indicated earlier and greater weight gain in PREF rats than in CHOW females, with INT rats intermediate. Thus, following PREF food days (FIG 2I), PREF rats gained significantly more weight than both INTs and CHOW rats (ps<0.0001), but female INT rats also gained significantly more weight than female CHOW rats (p<0.0001). Following the underconsumption of NON-PREF days (FIG 2J), INT rats no longer had gained more than CHOW rats, while PREF rats still showed greater weight gain than both groups (ps<0.0001).
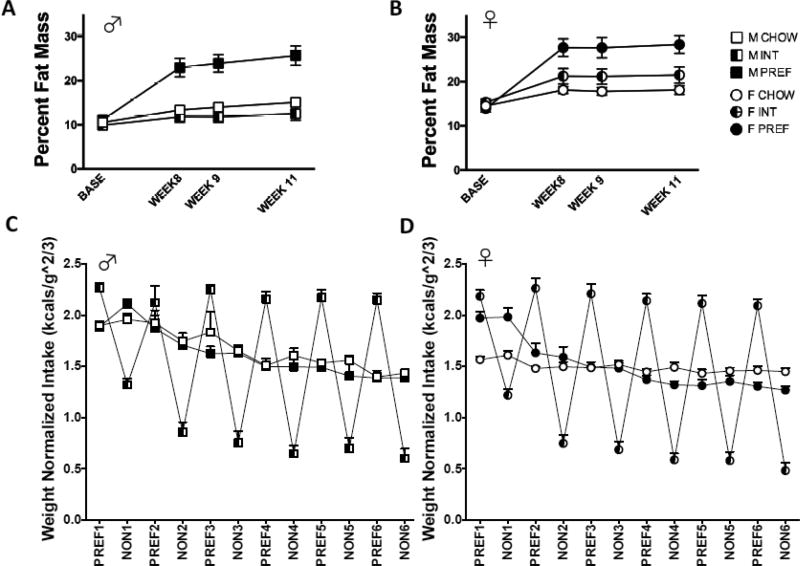
3.3 Body Composition
A Group X Sex interaction on body fat percentage (Fig. 3A & B; F(2,166)=7.3, p<0.002) reflected that female INT rats had steeper gains in body fat percentage than male INT rats. A Group X Week interaction reflected that PREF rats of both sexes developed greater percent body fat than INTs and CHOWs over time (F(6,105)=13.4, p<0.0001). Female INT rats also had significantly higher percent body fat than female CHOWs (p<0.02)—in contrast, male INTs trended toward lower percent body fat compared to male CHOWS (p<0.07). Finally, a Sex main effect (F(1,166)=71.7, p<0.0001) reflected greater percent body fat in females, as expected.
FIG 3.
Sex differences in body composition and weight-normalized intake on diet schedules. Female (A) rats have a greater percent body fat compared to male (B) rats (B)(p<0.0001). Female INT rats also had significantly higher percent body fat than female CHOWs (p<0.02) whereas male INTs trended toward lower percent body fat compared to male CHOWS. PREF rats of both sexes have significantly greater percent body fat than INTs and CHOWs over time (p<0.0001). When normalized for body weight (C), males still showed greater 24-hr energy intake per unit body weight than females (D)(p<0.0001). This sex difference was seen in both CHOW and PREF groups and in INT rats during the NON-PREF feeding phase. The sex difference was eliminated, however, in the PREF feeding phase, suggesting that relative to their respective controls, INT females overate more per body weight than INT males (Day X Group X Sex p<0.005). Data show M+SEM. n = 6–12/group.
For lean body mass percentage (Supplemental Fig 1), a Group X Sex interaction indicated that female INT rats showed disproportionately steeper loss of lean body mass percentage (vs. ad lib diet conditions) than did male INT rats (F(2,167)=8.9, p<0.0001). A Group X Week interaction showed that PREF rats of both sexes had less percent lean mass than INTs and CHOWs over time (F(6,106)=1.8, p<0.0001). Finally, across diet groups, males lost a greater percentage of lean body mass than did females (Sex X Week: F(1, 167)=302.5, p<0.0001).
3.4 Weight-normalized intake
After normalizing for body weight (FIG 3C & D), significant Sex X Group X Week (Overall: F(10,198)=2.0, p<0.04, PREF days: (F(10,103)=2.0, p<0.05) and Sex effects (Overall: F(1,484)=34.5, p<0.0001, PREF days: F(1,222)=12.6, p<0.0001) indicated that male CHOW and PREF rats initially normally ate more than their respective female counterparts, but that this sex difference was absent in INT rats on PREF days. In contrast, males still ate more than females across groups on NON-PREF food days (F(1, 222)=12.6, p<0.0001). Thus, female INT rats overconsume on PREF days to a greater degree than do male INT rats, thereby eliminating the normal sex difference seen in their ad lib fed counterparts.
3.5 Fixed-ratio self-administration
3.5.1 Active lever presses
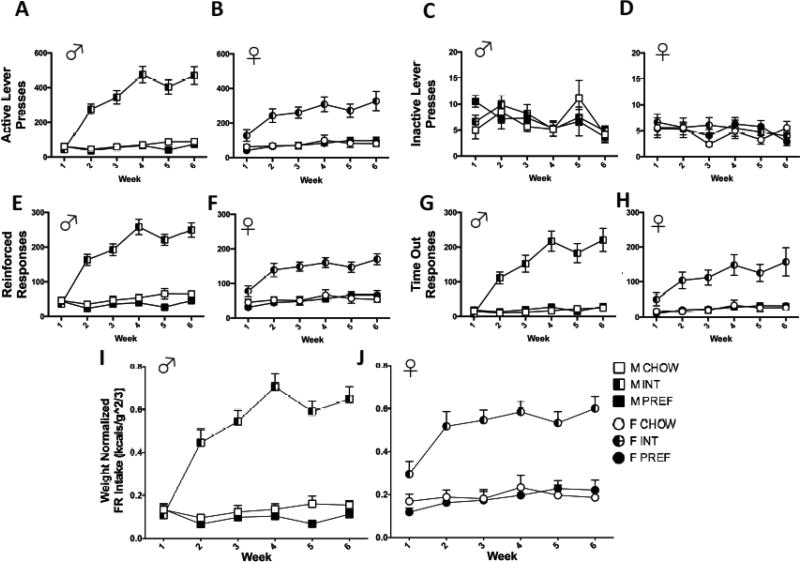
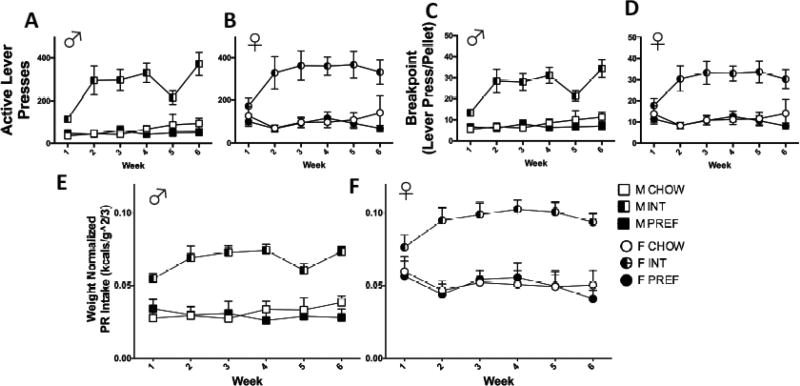
On a fixed-ratio-1 (FR) schedule of reinforcement, a Group X Sex X Week interaction (F(110,88)=2.6, p<0.01) on total active lever presses reflected that male INT rats showed larger escalation of responding than did females. This was most evident during weeks 4–6 when females appeared to plateau. Within both sexes, INTs made more active lever presses than CHOW and PREF rats by Week 2 (ps<0.0001) continuing through Week 6 (see Figs. 4A and B). There was no Sex main effect (p>0.3) on active lever presses.
FIG 4.
Fixed-ratio self-administration and weight-normalized FR comparison between sexes. Within both sexes, INTs made more active lever presses than CHOW and PREF rats by Week 2 (ps<0.0001) continuing through Week 6 (A and B). Males (C) have more inactive responses than females (D) among all groups (p<0.0005), but there were no effects of diet group on inactive lever presses collectively or within either sex. Both male (E) and female (F) INT rats obtained more reinforcers than CHOWs and PREFs by Week 2 (ps<00001), persisting through Week 6. Male (G) and female (H) INT rats of both sexes increased their timeout responding over time, vs CHOW and PREF rats (ps<0.0001). When both sexes’ 30-min session pellet consumption were weight-normalized (I & J), there was a significant effect of Sex (p<0.001) and a Group X Week interaction (p<0.0001), indicating that females (J) obtained disproportionately more reinforcers than males (I) across all diet groups and INT rats of both sexes similarly increased their self-administration across the study period. Data show M+SEM. n = 6–12/group.
3.5.2 Reinforced active lever presses
A Group X Sex X Week interaction (F(10,88)=3.3, p<0.002) similarly reflected that male INT rats showed greater increases across the study period than female INT rats (see Figs. 4E and F). Both male and female INT rats obtained more reinforcers than CHOWs and PREFs by Week 2 (ps<00001), persisting through Week 6. There was no Sex main effect on reinforcers earned (p>0.2).
3.5.3 Weight-normalized reinforcers earned
However, when pellets earned was normalized for body weight, results differed strikingly. Here, instead, a Sex main effect indicated that females obtained disproportionately more reinforcers than males across all diet groups (F(1,290)=11.6, p<0.001). A Group X Week interaction remained (F(10, 91) =8.7, p<0.0001), indicating that INT rats of both sexes similarly escalated their self-administration across the study period (see FIG 4I & J).
3.5.4 Non-reinforced “timeout” active lever presses
There were no effects (main or interaction) involving Sex on responding during the post-reinforcement “timeout” period. Group X Week interactions (F(10,89)=8.9, p<0.0001) indicated that INT rats of both sexes markedly and similarly increased their timeout responding over time vs. CHOW and PREF rats (ps<0.0001) (Figs. 4G and H). The mean ratio of timeout responses: reinforced responses was increased 1.8- and 2.3-fold in female and male INT rats, respectively, indicating a disproportionate increase in timeout responding.
3.5.5 Inactive lever presses
A Sex main effect (F(1,285)=13.0, p<0.0005) reflected that, across diet groups, males made more inactive lever presses than females. There were no main or interaction effects involving Group on inactive lever presses collectively or within either sex (FIG 4C & D).
3.6 Progressive ratio self-administration
Progressive ratio self-administration is a measure of the reinforcing efficacy of the diet for each group80. Sex main effects reflected that females had higher total active lever presses (F(1,265)<9.1, p<0.004) and breakpoints (F(1,274)=9.2, p<0.004) than males. There were no significant interactions involving both Sex and Group. Instead, Group X Week interactions (F(10,89)=3.5,p<0.002) indicated that INT rats of both sexes markedly and similarly increased their active lever presses (Fig. 5A) (F(10,89)=3.5, p<0.002) and breakpoints across PR sessions (Fig. 5B) (F(10,89)=3.1,p<0.002). Overall, independent of sex, INT schedule-induced increases in PR performance were significant vs. CHOW and PREF rats (ps<0.0001) (see Figs. 5A–D).
FIG 5.
Sex and intermittent access-related differences in the reinforcing efficacy of food. INT rats of both sexes increased their active lever presses (p<0.002) (A & B) and breakpoints (p<0.002) (C & D) compared to CHOWs and PREF rats (A & B) and breakpoints across PR sessions. There were significant Sex main effects (ps<0.004) such that females made more PR active lever presses and had higher breakpoints than males. When PR intake was normalized by body weight (E & F), an even stronger Sex main effect remained (p<0.0001) whereby females (F) had higher body weight-normalized PR intake than males (E). Data show M+SEM. n = 6–12/group.
When PR intake was normalized for body weight (FIG 5E & F), the Sex main effect was even greater (F(1,294)=77.0, p<0.0001), emphasizing that females had much greater body weight-normalized intake during effortful PR sessions than males. Intraclass correlations reveal strong, stable individual differences in PR performance in both males (ICC=0.79) and females (ICC=0.77).
3.7 High vs. low PR-responding INT rats
To address previously described stable individual differences in the development of compulsive eating phenotypes, and also observed here for PR performance, INT rats of both sexes were classified as high responders (HI INT) if on average between weeks 4–6 their average number of PR active lever presses was more than 2 standard deviations greater than the average of CHOW controls of both sexes. By this criterion (≥315 active lever presses), 50% of females vs. 36% of males were considered HI INT; the remainder were considered low-responders (LO INT).
3.7.1 Intake
Significant Class X Sex X Day interactions (F(3, 525)=7.2, p<0.0001) indicated sex differences in the degree to which intake of HI or LO INT rats cycled on PREF vs. NON-PREF feeding days. Furthermore, Class X Sex interactions were seen on both PREF (F(3, 261)=2.8, p<0.05) and NON-PREF days (F(3, 267)=9.5, p<0.0001), indicating that the sex differences in the effects of high- vs. low-responder Class were seen during both access phases, as detailed below.
3.7.1.1 Overeating on PREF days
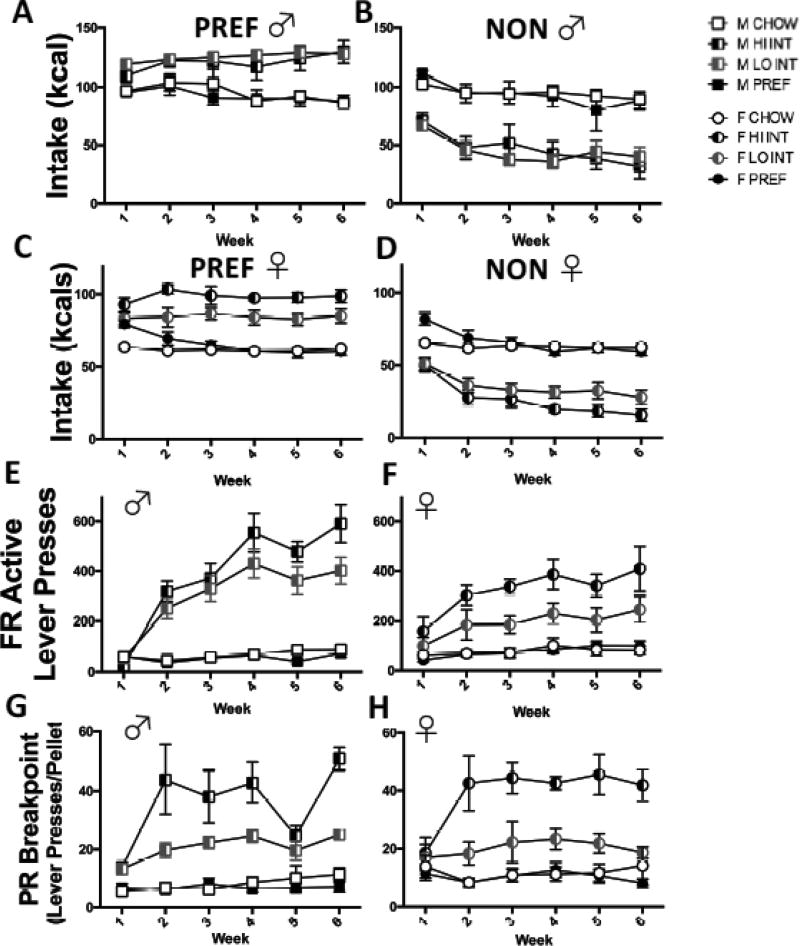
The Sex-related interactions reflected that unlike male INTs, who did not differ from one another (FIG 6A), HI INT females overate significantly more on PREF days than their LO INT counterparts (FIG 6C) (p<0.0001). LO and HI INT rats of both sexes ate more than their CHOW and PREF controls (ps<0.0001).
FIG 6.
Consummatory and instrumental behavior differences in high- vs. low-responding INT rats. On PREF days, male HI and LO INTs did not differ from one another (A), but HI INT females overate significantly more on PREF days than their LO INT counterparts (C) (p<0.0001). LO and HI INT rats of both sexes ate more than their CHOW and PREF controls (ps<0.0001). Male HI and LO INTs did not differ from one another (FIG 6B), but HI INT females underate significantly more on NON-PREF days than their LO INT counterparts (FIG 6D) (p<0.02) (see FIGS. 6B and 6D). In both sexes, LO and HI INTs both underate compared to CHOW and PREF rats (ps<0.0001). HI INT rats showed progressively greater active lever presses than LO INT rats, a difference that reached significance by week 4 in males (ps<0.05) (E) and by week 3 in females (ps<0.05) (F). In both sexes, HI INT rats escalated their breakpoints over time (especially from Week 1 to Week 2), whereas LO INT rats did not (G & H) Data show M+SEM. n = 4–10/group.
3.7.1.2 Undereating on NON-PREF days
Additionally, the Sex-related interactions reflected that again male INTs did not differ from one another (FIG 6B), but that HI INT females underate significantly more on NON-PREF days than their LO INT counterparts (FIG 6D) (p<0.02) (see FIGS. 6B and 6D). In both sexes, LO and HI INTs both underate compared to CHOW and PREF rats (ps<0.0001) when they did not have access to the preferred diet.
3.7.2 Fixed-ratio self-administration
A significant Sex X Class X Week interaction (F(15,83)=2.4, p<0.006) on FR active lever presses indicated that between sexes there were differences between high- and low-responder rats over time. In addition to all INT rats having elevated active lever presses compared to ad libitum fed rats by week 2 (ps<0.05), HI INT rats showed progressively greater active lever presses than LO INT rats. This difference that reached significance by week 4 in males (ps<0.05) and, slightly earlier, by week 3 in females (ps<0.05) (see FIGS 6E–6F).
3.7.3 Progressive-ratio self-administration
There were no significant interactions involving Sex and Class on PR performance. However, a significant Class X Week interaction (F(15,88.6)=3.9, p<0.0001) showed that, in both sexes, HI INT rats escalated their breakpoints over time (especially from Week 1 to Week 2), whereas LO INT rats did not (FIG 6G and H). As expected, a very large Class main effect (F(3, 263)=112.7, p<0.0001) confirmed the identified behavior difference between HI INT vs. LO INT rats, which differed significantly from one another from week 3 on (ps<0.05).
3.8 Indirect calorimetry
3.8.1 Respiratory exchange ratio
Respiratory exchange ratio (RER), the ratio of the volumes of carbon dioxide produced to oxygen consumed, is a proxy measure for whole-body relative fuel-substrate utilization. A high RER can indicate metabolism favoring carbohydrates as a fuel substrate and has been associated with positive energy balance and prospective obesity risk in humans79–80, whereas a low RER can indicate predominantly fat utilization81.
3.8.1.1 Diet schedule effects
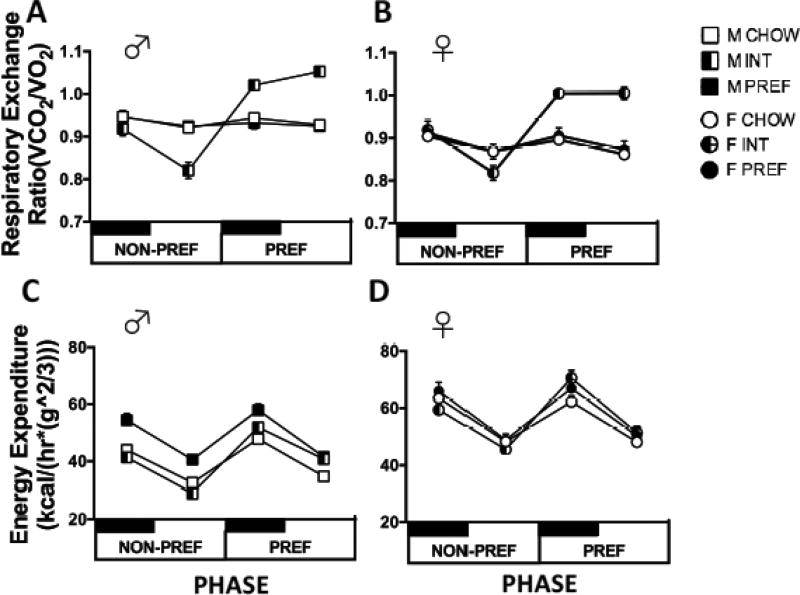
A Group X Phase X Light Cycle interaction was seen across all rats and within each sex (Overall: F(2,49)=33.8, p<0.0001, Male: F(2,20)=15.5, p<0.0001, Female: F(2,29)=17.9, p<0.0001) (see Figs. 7A and 7B). This interaction reflected cycling RER of INT rats. During the PREF food phase, INT rats of both sexes had significantly increased RERs vs. CHOW and PREF rats during both the light and dark cycle. In contrast, on NON-PREF days, INT rats had normal RER during the dark cycle, but significantly lower RERs than CHOW and PREF rats by the light cycle. Sex and Sex X Light Cycle effects indicated that RERs were lower in females, especially during the light cycle (F(1,49)=14.1, p<0.0001; F(1,49)=8.9, p<0.0001).
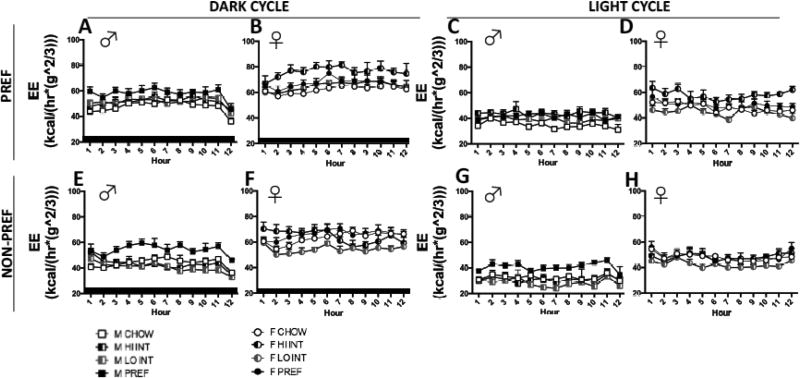
FIG 7.
Diet schedule- and sex-dependent differences in whole-body metabolism. During the PREF food phase, INT rats of both sexes (A & C) had significantly increased RERs compared to CHOW and PREF rats. On NON-PREF days, INT rats of both sexes (B & D) had normal RER during the dark cycle, but lower RERs than CHOW and PREF rats by the light cycle. Overall, RERs were lower in females than males, especially during the light cycle (p<0.0001). In both sexes, energy expenditure of INT rats, but not ad lib fed rats, cycled across diet access phases; with very reliably lower EE on NON-PREF days than on PREF days (p<0.0001) (C & D). INT rats had lower EE on NON-PREF access days than both CHOW and PREF rats (ps<0.02), and greater EE on PREF access days than CHOW rats (p<0.03). On PREF days, EE of INT females (D) increased more than that of males (C) during the dark cycle, and EE of INT males remained elevated during the light cycle more than that of INT females (p<0.02).
3.8.1.2 High vs. Low Responders
When INTs were subclassified as HI vs LO, Class significantly influenced RER in relation to the Diet Access Phase, Light Cycle and Hour (Class X Phase X Light Cycle X Hour: F(17, 266.2)=6.3; Class X Phase X Light Cycle: F(3,47)=24.1; Class X Phase X Hour: F(18.5, 289.9)=9.0; Class X Phase: F(3,47)=44.9; Class X Hour: F(18.6,291.3)=3.4, ps<0.0001). The Class effects on RER are elaborated below.
3.8.1.2.1 PREF phase
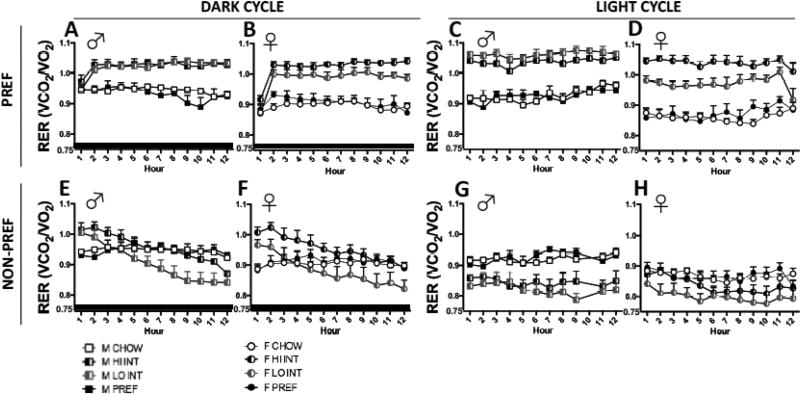
On PREF phase days (FIGS 8A, 8B), Class X Light Cycle effects (F(3,47)=10.0, p<0.0001) reflected that HI INT rats had higher RERs than LO INT rats. This difference was more apparent in females than males with a greater difference seen during the light cycle (Class X Sex X Light Cycle: F(3,47)=2.5, p<0.07). Accordingly there was a significant difference seen between HI vs. LO INT females (p<0.02), but not males (p>0.3). A Class X Light Cycle X Hour interaction (F(13.0, 203.1)=3.5, p<0.0001) indicated that HI INT and LOW INT rats of both sexes had significantly greater RERs than CHOW and PREF rats during hours 2–12 of the dark cycle and throughout the entire light cycle.
FIG 8.
HI vs LO INT compulsive-like classifications and respiratory exchange ratio (RER). During the PREF phase, HI INT rats had higher RERs than LO INT rats (p<0.0001). This difference was more apparent in females (B & D) than males (A &C) with a greater difference seen during the light cycle (p<0.07). There was a difference seen between HI vs. LO INT females (p<0.02), but not males (p>0.3). HI INT and LOW INT rats of both sexes had greater RERs than CHOW and PREF rats during the dark cycle and throughout the entire light cycle (p<0.0001).
During the NON-PREF phase, in both sexes, HI INT rats showed trends for greater RERs than LO INT rats across the entire dark cycle (E &F). RERs of both HI INT and LO INT rats, but not ad lib controls, decreased steadily across the dark cycle ( p<0.0001). HI INT rats had higher RERs than CHOWs and PREFs during the early of the dark cycle, and LO INT rats had lower RERs than ad lib controls in the late dark cycle, whereas RERs of HI INT rats did not drop significantly below those of controls. During the light cycle of the NON-PREF food phase (G & H), LO INT rats had lower RERs than ad lib controls from the beginning of the light cycle, but RERs of HI INT rats were not reduced immediately.
Data show M+SEM. n = 4–10/group.
3.8.1.2.1 NON-PREF phase
On NON-PREF food phase days (FIGS 8E, 8F), Class X Light Cycle X Time, Class X Light Cycle and Class X Time interactions were seen (F(17.1,267.8)=3.3; F(3,47)=15.5; F(14.7,230.4)=8.6, ps<0.0001). Across both sexes, HI INT rats showed trends for greater RERs than LO INT rats across the entire dark cycle, significantly so during hours 5–11. RERs of both HI INT and LO INT rats decreased steadily across the dark cycle (linear contrast: F(1,19)=76.9, p<0.0001) whereas no such change was seen in ad lib controls. As a result, HI INT rats had significantly higher RERs than CHOWs and PREFs during the first 3 hr of the dark cycle (vs. 2 hr for LO INT rats). Conversely, LO INT rats had lower RERs than ad lib controls beginning 7 hr into the dark cycle, whereas RERs of HI INT rats did not drop significantly below those of controls.
Similarly during the light cycle of the NON-PREF food phase (FIG 8G), LO INT rats showed significantly lower RERs than ad lib controls from the beginning of the light cycle, whereas RERs of HI INT rats were not significantly reduced until hour 4. Descriptively, reduced RER levels were slightly more apparent in INT males than females.
3.8.2 Energy expenditure
Energy expenditure (EE) as heat is expressed as kilocalories burned per hour, normalized for lean body mass.
3.8.2.1 Diet schedule effects
Overall and within each sex, there were highly significant Group X Phase interactions on EE (Figs. 7C, 7D; (F(2,49)=44.4; F(2,20)=31.0; F(2,29)=21.5, ps<0.0001). These interactions reflected that the EE of INT rats cycled across diet access phases. Specifically, INT rats very reliably had lower EE on NON-PREF days than on PREF days (F(1,21)=204.1, p<0.0001), a difference not evident in CHOW or PREF rats. Indeed, INT rats had significantly lower EE on NON-PREF access days than both CHOW and PREF rats (ps<0.02), a difference seen in both the dark (ps<0.03) and light cycle (ps<0.02). Conversely, INT rats had significantly greater EE on PREF access days than CHOW rats (p<0.03), a difference also seen in both the dark (ps<0.03) and light cycle (ps<0.02).
A smaller, but significant Group X Sex X Phase X Light Cycle interaction was seen as well (F(2,49)=6.0, p<0.006). This result reflected sex differences in the degree by and light cycle in which EE of INT rats increased on PREF days (Group X Sex X Light Cycle on access days: F(2,49)=4.6, p<0.02). Specifically, on PREF days, EE of INT females increased more than that of males during the dark cycle, whereas that of INT males remained elevated during the light cycle more than that of INT females (Sex X Phase X Light Cycle for Int rats: F(1,21)=8.1, p<0.02). Also contributing to this interaction was the finding that male, but not female, PREF rats had significantly elevated EE throughout both phases as compared to CHOW males.
3.8.2.2 High and Low Responders
When INTs were classified as HI vs LO responders, there were Class x Cycle x Phase x Sex (F(3, 47)=4.6, p=0.007) and Class X Phase interactions (F(3,47)=26.0, p<0.0001) as well as a Class main effect on EE (FIGS 9A,–9H) (F(3,47)=5.9, p<0.003). Direct comparison of HI INT vs. LO INT subjects revealed that LO INT rats had significantly lower EE than HI INT rats (F(1,19)=8.2, p<0.02). This was moderated by a Class X Access X Light Cycle trend (F(1,19)=4.2, p<0.057), whereby the EE of LOW INT rats was particularly lower than HI INT rats during the dark cycle of PREF days.
FIG 9.
HI vs LO INT classifications and energy expenditure (EE). During the PREF phase, HI INT females (B), but not males, (A) had significantly higher EE than both LO INTs(p<0.04) and CHOW controls (p<0.02) during the dark cycle. During the light cycle of the PREF phase, HI INT, but not LOW INT, males had greater EE than CHOWs (p<0.03) (C). HI INT females still had higher EE than both CHOW and LO INT rats (ps<0.02) (D). During the dark cycle of the NON-PREF phase, female (F), but not male (E) HI INT rats had higher EE initially than both LO INTs and CHOWs (p<0.0001). During the light cycle of the NON-PREF phase, LO INT males had lower EE than PREF males (p<0.004) (G). Females (H) had no significant Class-related effects at this time. Data show M+SEM. n = 4–10/group.
3.8.2.2.1 PREF food phase
Accordingly, during the PREF food phase, HI INT females had significantly higher EE than both LO INT females (F(1,10)=6.0, p<0.04) and CHOW controls (p<0.02) during the dark cycle (see FIG 9B), an effect not seen in males (p>0.9).
During the light cycle of the PREF food phase, HI INT females still had higher EE than both CHOW and LO INT rats (ps<0.02). HI INT, but not LOW INT, males had greater EE than CHOW rats then as well (p<0.03) (FIG 9C and D).
3.8.2.2.2 NON-PREF phase
During the dark cycle of the NON-PREF food phase, female HI INT rats had higher EE initially than both LO INTs and CHOWSs, resulting in a Class X Hour interaction (F(33,308)=2.7, p<0.0001). Finally, during the light cycle of the NON-PREF phase, LO INT males had lower EE than PREF males (q(19)=5.7, p<0.004). Females (FIG 9H), in contrast, showed no significant Class-related effects at this time.
4. Discussion
The present results show sex differences in in the reinforcing efficacy of food and individual differences in the vulnerability to develop escalated food reinforcement and associated consummatory, instrumental and metabolic phenotypes in a rat model of compulsive, binge-like eating. Females showed increased FR and PR food self-administration performance across multiple diet schedules, consistent with findings of greater self-administration of substances of abuse in fixed- and progressive-ratio operant paradigms84–85 Females also were more likely than males to show escalated, compulsive-like PR performance in the model of intermittent, extended access to highly preferred food. This result resonates with reports that females are more prone to develop increased intake upon renewed access to palatable food in other intermittent, extended access rat models56,73 While intermittent access to preferred food led to “yo-yo” cycling of whole-body fuel substrate utilization and energy expenditure (along with intake and body weight), these effects differed by both sex and the compulsive-like, high vs. low responder classification. The heightened food reinforcement in females may have implications for the female preponderance of compulsive eating86 and binge eating disorders in people64–71 . The findings also suggest sexually dimorphic vulnerability to develop increased food reinforcement (see relative reinforcement in humans59) when access to preferred food is withheld. Overall, the results also show that a compulsivity construct associates not only with food reinforcement, but also greater overeating and energy expenditure and, especially in females, decreased utilization of lipid as a fuel substrate in a rat binge-eating model.
4.1 Binge-like intake and operant self-administration
Similar to our previous study in free-feeding females with intermittent extended access32: both male and female INT rats showed cyclic daily (24-hr) overeating when they had access to the preferred diet vs. underconsumption of the less preferred diet to the point of weight loss when they did not (FIG 2A–D). When intake was normalized by body weight, a sex difference persisted whereby males receiving ad lib access to either diet still initially ate more than females per unit body weight. In contrast, females receiving intermittent access ate just as much as males when they had access to the preferred diet. Collectively, the results indicate that, relative to their respective controls, female INT rats overconsumed on PREF days to a greater degree than did male INT rats, thereby eliminating the normal sex difference seen in their ad lib fed counterparts. Unlike intermittent access, ad libitum access to the preferred diet did not lead to sustained overeating vs. CHOW rats in either sex, consistent with our previous findings32.
The reinforcing effect of preferred food increased markedly in INT rats. Under a fixed-ratio schedule of reinforcement, INT rats of both sexes developed much greater levels of active lever responding than CHOW or PREF rats by week 2 (FIGS 4A–H). Escalation of responding over time was larger in the INT males than in the INT females, particularly in weeks 4–6 when females appeared to plateau, while males continued to escalate. Furthermore, in INT rats of both sexes, an approximately 2-fold increased ratio of “timeout” to reinforced responses at the active lever was observed by week 6, with no change in responding at the inactive lever. These results, indicating persistent and disproportionately greater responding at the food-associated lever in INT rats despite periods of non-reinforcement, supports the compulsive, addiction-like phenotype of INT rats and is consistent with previous models of drug addiction87 and other intermittent access models to palatable food88. INT rats of both sexes also showed 2- to 5-fold increased breakpoints under a progressive ratio schedule of reinforcement compared to PREF and CHOW rats (FIGS 5A–D). The increased breakpoints may model addictive-like behavior in the framework of DSM-V criteria for substance use disorders, as INT rats spent prolonged time and effort to obtain the preferred diet89–90. Similarly, many animal models of addiction to substances of abuse have regarded heightened PR performance as an index of a loss of behavioral control, because seeking behavior continues despite decreasing reinforcement91–92. Supporting this interpretation, we have recently shown that high PR performance in INT rats correlates strongly with continued food self-administration despite contingent shock punishment93. These combined observations support the possibility that our model involves dysfunction of behavioral control. As such, it also may map onto the diagnostic criteria of “eating despite adverse consequences” observed in humans and theorized as a key element in the construct of compulsive eating20,34.
Sex differences in both basal and intermittent schedule-induced instrumental measures of food reinforcement also were seen. When considered as behavior emitted, INT male rats showed larger increases than INT females in active lever pressing; they earned more reinforcers and had more timeout responses. However, once intake from the 30-min fixed-ratio session was normalized for the different metabolic sizes of subjects, females actually self-administered more food per unit body mass than males across all 3 diet conditions (Fig. 4I). Moreover, females also showed greater progressive-ratio responding and breakpoints than males in all diet conditions, and these differences, become even stronger with weight normalization (FIGS 5A–E). Females also showed increased weight-normalized operant self-administration and even raw PR performance, even though their weight-normalized daily energy intake was lower than that of males. The results suggest a greater reinforcing effect of food on instrumental behavior in females. The greater reinforcing efficacy of food for females did not reflect non-specific activity, because male rats showed more inactive lever responding than females.
4.2 Sex differences in body mass and composition
Sex differences in weight gain and body composition also were seen. As expected, male rats weighed more than females, while females had a higher body fat percentage than males. Broadly speaking, females showed greater increases than males in body weight and relative adiposity as a result of access to the preferred diet. For example, female PREF rats, which received the sucrose-rich diet ad libitum, gained significantly more weight than chow controls, a difference that was not significant in males. Furthermore, female, but not male, INT rats also showed accelerated weight gain compared to CHOWs (FIG 2E–J). Also, whereas female INT rats showed cyclic body weight intermediate to ad libitum CHOW and PREF rats (replicating our previous finding32), male INT rats tended to weigh less than CHOW and PREF rats, especially after their non-preferred feeding cycles. Female INT rats also more steeply gained body fat percentage and lost lean mass percentage than male INTs. Collectively, these results are consistent with the greater female prevalence of obesity comorbid with disordered eating17 and class 3 obesity87,94–97 and further validate this model as a useful tool for modeling binge-like eating as a risk factor for overweight and fat accumulation in females.
4.3 Diet schedule-induced and sex differences in whole body metabolism
Sex differences in whole body metabolism under the different diet schedules were revealed using indirect calorimetry. First, male PREF rats had greater energy expenditure than CHOW controls under every studied condition, an effect noticeably absent in females (FIG 7C–D). The increased energy expenditure of male PREF rats may partly protect them from the excess body weight gain observed in female PREF rats and account for their reduced weight gain. Perhaps male PREF rats undergo a greater degree of diet-induced thermogenesis98–99; a hypothesis that warrants further study in men and women.
Intermittent access also led to marked changes in whole-body metabolism. Large, cyclic shifts in the fuel substrate utilization of Int rats were seen (FIG 7A–B); they showed very high RERs (~1.0) upon renewed access to the preferred diet, indicating almost complete reliance on carbohydrate, and low RERs (~0.8), indicating preference for fat utilization, during the light cycle of their non-preferred food phase (when they markedly reduced intake). Given the restrictive nature of the yo-yo -like cycling of intake and body mass, perhaps high RERs serve as an adaptation in INT rats to promote sparing of fat stores in preparation for abstinence from the PREF diet and then excess storing of fat when the preferred food becomes available. Accordingly, INT rats also consistently showed cycling of energy expenditure; they expended less energy than ad lib controls on days that the preferred food was unavailable, during which they decreased their intake.
Finally, some sex differences also were seen in the energy expenditure of INT rats. While INT rats of both sexes increased their EE when preferred food was available, female INT rats showed greater increases during the dark cycle when preferred access resumed. In contrast, male rats showed greater maintenance of elevated EE through the subsequent light cycle. This more protracted maintenance of heightened energy expenditure in male INT rats conceivably also may contribute to their blunted relative weight and fat gain as compared to females.
4.4 Intake and self-administration differences in high- vs. low-responding INT rats
We and others have previously reported stable individual differences in the vulnerability to develop overeating of preferred food and rejection of less preferred food in rats with long, intermittent access to palatable food.32,58 Accordingly, previous studies have characterized female rats as being binge-like-eating prone or resistant based on their degree of consistent overeating upon renewed access to the palatable diet.53,55–57,73 In humans, however, it has been shown that motivational measures, such as the relative reinforcing efficacy of food, may be more predictive of poor metabolic outcomes than intake alone100–101. Additionally, a key component of the definition of binge eating is the characteristic compulsive eating with loss of control, which is not adequately captured in measures of free-feeding intake. The PR schedule of reinforcement provides a proxy for the reinforcing efficacy and motivation to obtain food.80 Heightened PR performance has been used as evidence of compulsive-like responding for substances of abuse91–92. Therefore, we classified animals as high- vs. low-responders based on the “normal” (+2 standard deviations) active lever press performance of chow controls in the PR paradigm. Half (50%) of female vs. about one-third (36%) of male INT rats met criteria of high-responders, whereas no CHOW or PREF rats of either sex met this criterion. This higher percentage of INT high-responders being female is consistent with the greater prevalence of compulsive, addictive-like eating in women86 and provides evidence for a motivational aspect to the posited greater vulnerability of females to binge-like behavior56.
The high- vs. low-responder classification predicted greater daily overeating of the preferred diet and greater binge-like operant self-administration upon renewed access. Thus, by 4 weeks, high-responder females showed greater overeating on PREF days and greater undereating of CHOW on NON-PREF days than low-responder females. This increase in diet-cycling was not seen in males (FIG 6A–D). HI INT females also developed greater FR self-administration of the preferred food than LOW INT females by 3 weeks. The same difference was not evident between HI INT vs. LOW INT males until 4 weeks, suggesting a more rapid escalation of food self-administration in compulsive-like females (FIG 6E–F). Importantly, the individual difference in compulsive-like responding was not an antecedent, but rather developed with experience due to cryptic underlying reasons. That is, HI vs. LO INT rats showed very similar PR performance during Week 1, but then the HI INT group in both sexes showed marked escalation of PR responding from Week 1 to Week 2 (FIG 6G–H). These findings may translate to individual differences in vulnerability to binge eating-related disorders. The marked individual differences in INT rats and the stronger predictive nature of compulsive-like self-administration in females than males warrants further studies to understand better the neurobiological and genetic underpinnings of vulnerability to escalation of compulsive-like eating.
4.5 Metabolic differences in high- vs. low-responding INT rats
The compulsive-like high- vs. low-responding construct also differentiated the metabolism of subjects. Broadly speaking, high-responding female rats had especially elevated respiratory exchange ratios. Thus, only high-responding female, and not male, INT rats had higher RERs than their low-responding counterparts when preferred food was available (FIG 8A–D). Moreover, the elevated RERs of HI INT rats persisted longer into the non-preferred food phase of feeding than did those of LO INT rats. Conversely, LO INT rats had lower RERs than all other groups through much of the non-preferred phase of feeding (FIG 8E–H). Overall, the results suggest that female HI INT rats especially spare fat and utilize carbohydrate as a fuel source, whereas LO INT rats of both sexes especially rely on fat utilization during when preferred food is unavailable.
The high- vs. low-responding distinction also differentiated energy expenditure in a sex-dependent manner. Broadly speaking, HI INT rats expended more energy than LO INT rats and had increased energy expenditure vs. ad lib controls when the preferred food was available and early into the non-preferred feeding period. These differences were more apparent within females, especially in the dark phase when access to preferred food resumed. In contrast, LO INT rats had reduced energy expenditure during non- preferred feeding. The increased EE of (especially female) HI INT rats seems counterproductive insofar as these animals were burning more energy prior to the expected energy deficit of the non-preferred feeding phase. Perhaps the greater EE reflects greater thermogenesis or post-ingestive thermic effects secondary to their heightened food intake98–99 or, alternatively, activation/arousal due to the availability and recent intake of highly reinforcing food. Whatever the basis, perhaps there is a metabolic adaptation in these HI INT females that allows efficient utilization of carbohydrate as a fuel substrate, even persisting into periods when preferred food is not available, in order to “spare” body fat81–82.
An important subject for future study is whether the differences in whole body metabolism between HI vs. LO INT rats are an antecedent cause, unrelated correlate, or consequence of the compulsive-like phenotype. One could propose, for example, that energy homeostasis systems were altered, homeostatically or allostatically, by the dramatic, yo-yo like cycling of energy intake, expenditure, utilization and storage. Alternatively, the propensity to become a high-responder may be determined by individual differences in the body’s ability or strategy for adapting to these extreme energy states. As there were not behavioral differences observed at week 1 of the study between HI vs. LO INT rats, it can be hypothesized that the correlated behavioral and metabolic changes may evolve jointly over time. Additionally, it has been reported that palatable tastes can alter metabolism and increase bioavailability of the ingested food102–108, so motivational food stimuli can alter aspects of energy regulation. Perhaps this connection between compulsive-like behavior and fuel substrate utilization is due to shared neurobiological changes. For example, the cyclic food/energy stimuli may drive adaptations in circuits that coordinately subserve food reinforcement and regulate energy metabolism109 (e.g., ventral striatum, lateral hypothalamus). Clearly, this is an area for future mechanistic investigation.
5. Conclusions
In conclusion, we have identified sex differences in the development of binge-related instrumental behavior, including the reinforcing value of food or the compulsivity of food self-administration. Further, differences between sexes in metabolic responses to an intermittent extended access model of binge-like eating, including body composition, energy expenditure, and fuel utilization were identified. We observed cyclic substrate utilization and energy expenditure that were consistent with an energy- and fat-conserving strategy when preferred food was unavailable in both sexes in rats with long intermittent access to the preferred diet. Further, high-responding female rats had especially elevated respiratory exchange ratios indicating a fat-sparing phenotype. Additionally, female rats with intermittent access were more often classified as “high responders”, had a greater reinforcing efficacy of the palatable diet under both continuous and intermittent access conditions, and high-responder females showed more dramatic metabolic cycling in the intermittent access condition compared to chow-fed controls than males with the same designation. Further studies are needed to identify the molecular and neurobiological basis of the sex and individual differences we have observed, with the translational goal of better understanding compulsive, binge-like eating in people.
Supplementary Material
Highlights.
In both sexes, rats with intermittent access showed cycling fuel substrate utilization and energy expenditure.
The reinforcing efficacy of the palatable diet was greater in females than males in all access conditions.
Compulsive-like, high-responding female rats overate more and had especially elevated respiratory exchange ratios indicating a fat-sparing phenotype
Acknowledgments
Funding: Research reported in this publication was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under award P60 AA06420 as well as the Pearson Center for Alcoholism and Addiction Research, NIH/NIAAA Institutional Training Grant T32 AA007456, National Institutes of Health Clinical Translational Science Award (NIH CTSA) STSI TL1 training program, and the NIH-funded UCSD MSTP Summer Undergraduate Research Fellowship (SURF) Training Program R25 HL084692. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5. Washington, DC: 2013. [Google Scholar]
- 2.Agh T, Kovacs G, Pawaskar M, Supina D, Inotai A, Voko Z. Epidemiology, health-related quality of life and economic burden of binge eating disorder: a systematic literature review. Eating and weight disorders : EWD. 2015;20(1):1–12. doi: 10.1007/s40519-014-0173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erskine HE, Whiteford HA, Pike KM. The global burden of eating disorders. Current opinion in psychiatry. 2016;29(6):346–53. doi: 10.1097/YCO.0000000000000276. [DOI] [PubMed] [Google Scholar]
- 4.Agh T, Kovacs G, Supina D, Pawaskar M, Herman BK, Voko Z, Sheehan DV. A systematic review of the health-related quality of life and economic burdens of anorexia nervosa, bulimia nervosa, and binge eating disorder. Eating and weight disorders : EWD. 2016;21(3):353–64. doi: 10.1007/s40519-016-0264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hay P, Mitchison D, Collado AEL, Gonzalez-Chica DA, Stocks N, Touyz S. Burden and health-related quality of life of eating disorders, including Avoidant/Restrictive Food Intake Disorder (ARFID), in the Australian population. Journal of eating disorders. 2017;5:21. doi: 10.1186/s40337-017-0149-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hay P, Girosi F, Mond J. Prevalence and sociodemographic correlates of DSM-5 eating disorders in the Australian population. Journal of eating disorders. 2015;3:19. doi: 10.1186/s40337-015-0056-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schaumberg K, Welch E, Breithaupt L, Hubel C, Baker JH, Munn-Chernoff MA, Yilmaz Z, Ehrlich S, Mustelin L, Ghaderi A, Hardaway AJ, Bulik-Sullivan EC, Hedman AM, Jangmo A, Nilsson IAK, Wiklund C, Yao S, Seidel M, Bulik CM. The Science Behind the Academy for Eating Disorders’ Nine Truths About Eating Disorders. European eating disorders review : the journal of the Eating Disorders Association. 2017;25(6):432–450. doi: 10.1002/erv.2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kornstein SG. Epidemiology and Recognition of Binge-Eating Disorder in Psychiatry and Primary Care. The Journal of clinical psychiatry. 2017;78(Suppl 1):3–8. doi: 10.4088/JCP.sh16003su1c.01. [DOI] [PubMed] [Google Scholar]
- 9.Wiss DA, Brewerton TD. Incorporating food addiction into disordered eating: the disordered eating food addiction nutrition guide (DEFANG) Eating and weight disorders : EWD. 2017;22(1):49–59. doi: 10.1007/s40519-016-0344-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curtis C, Davis C. A qualitative study of binge eating and obesity from an addiction perspective. Eating disorders. 2014;22(1):19–32. doi: 10.1080/10640266.2014.857515. [DOI] [PubMed] [Google Scholar]
- 11.Palavras MA, Hay P, Filho CA, Claudino A. The Efficacy of Psychological Therapies in Reducing Weight and Binge Eating in People with Bulimia Nervosa and Binge Eating Disorder Who Are Overweight or Obese-A Critical Synthesis and Meta-Analyses. Nutrients. 2017;9(3) doi: 10.3390/nu9030299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCuen-Wurst C, Ruggieri M, Allison KC. Disordered eating and obesity: associations between binge-eating disorder, night-eating syndrome, and weight-related comorbidities. Annals of the New York Academy of Sciences. 2017 doi: 10.1111/nyas.13467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bak-Sosnowska M. Differential criteria for binge eating disorder and food addiction in the context of causes and treatment of obesity. Psychiatria polska. 2017;51(2):247–259. doi: 10.12740/PP/OnlineFirst/62824. [DOI] [PubMed] [Google Scholar]
- 14.Meany G, Conceicao E, Mitchell JE. Binge eating, binge eating disorder and loss of control eating: effects on weight outcomes after bariatric surgery. European eating disorders review : the journal of the Eating Disorders Association. 2014;22(2):87–91. doi: 10.1002/erv.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He J, Cai Z, Fan X. Prevalence of binge and loss of control eating among children and adolescents with overweight and obesity: An exploratory meta-analysis. The International journal of eating disorders. 2017;50(2):91–103. doi: 10.1002/eat.22661. [DOI] [PubMed] [Google Scholar]
- 16.de Zwaan M. Binge eating disorder and obesity. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2001;25(Suppl 1):S51–5. doi: 10.1038/sj.ijo.0801699. [DOI] [PubMed] [Google Scholar]
- 17.Gearhardt AN, Boswell RG, White MA. The association of “food addiction” with disordered eating and body mass index. Eating behaviors. 2014;15(3):427–33. doi: 10.1016/j.eatbeh.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pedram P, Sun G. Hormonal and dietary characteristics in obese human subjects with and without food addiction. Nutrients. 2014;7(1):223–38. doi: 10.3390/nu7010223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Avena NM, Bocarsly ME, Hoebel BG, Gold MS. Overlaps in the nosology of substance abuse and overeating: the translational implications of “food addiction”. Current drug abuse reviews. 2011;4(3):133–9. doi: 10.2174/1874473711104030133. [DOI] [PubMed] [Google Scholar]
- 20.Moore CF, Sabino V, Koob GF, Cottone P. Pathological Overeating: Emerging Evidence for a Compulsivity Construct. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2017;42(7):1375–1389. doi: 10.1038/npp.2016.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Randolph TG. The descriptive features of food addiction; addictive eating and drinking. Quarterly journal of studies on alcohol. 1956;17(2):198–224. [PubMed] [Google Scholar]
- 22.Rozin P, Levine E, Stoess C. Chocolate craving and liking. Appetite. 1991;17(3):199–212. doi: 10.1016/0195-6663(91)90022-k. [DOI] [PubMed] [Google Scholar]
- 23.Davis C. A commentary on the associations among ‘food addiction’, binge eating disorder, and obesity: Overlapping conditions with idiosyncratic clinical features. Appetite. 2017;115:3–8. doi: 10.1016/j.appet.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Davis C. An introduction to the Special Issue on ‘food addiction’. Appetite. 2017;115:1–2. doi: 10.1016/j.appet.2017.03.043. [DOI] [PubMed] [Google Scholar]
- 25.Davis C. Evolutionary and neuropsychological perspectives on addictive behaviors and addictive substances: relevance to the “food addiction” construct. Substance abuse and rehabilitation. 2014;5:129–37. doi: 10.2147/SAR.S56835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis C. From passive overeating to “food addiction”: a spectrum of compulsion and severity. ISRN obesity. 2013;2013:435027. doi: 10.1155/2013/435027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis C, Curtis C, Levitan RD, Carter JC, Kaplan AS, Kennedy JL. Evidence that ‘food addiction’ is a valid phenotype of obesity. Appetite. 2011;57(3):711–7. doi: 10.1016/j.appet.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 28.Parylak SL, Koob GF, Zorrilla EP. The dark side of food addiction. Physiology & behavior. 2011;104(1):149–56. doi: 10.1016/j.physbeh.2011.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parylak SL, Cottone P, Sabino V, Rice KC, Zorrilla EP. Effects of CB1 and CRF1 receptor antagonists on binge-like eating in rats with limited access to a sweet fat diet: lack of withdrawal-like responses. Physiology & behavior. 2012;107(2):231–42. doi: 10.1016/j.physbeh.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cottone P, Sabino V, Steardo L, Zorrilla EP. Opioid-dependent anticipatory negative contrast and binge-like eating in rats with limited access to highly preferred food. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33(3):524–35. doi: 10.1038/sj.npp.1301430. [DOI] [PubMed] [Google Scholar]
- 31.Davis JF, Melhorn SJ, Shurdak JD, Heiman JU, Tschop MH, Clegg DJ, Benoit SC. Comparison of hydrogenated vegetable shortening and nutritionally complete high-fat diet on limited access-binge behavior in rats. Physiology & behavior. 2007;92(5):924–30. doi: 10.1016/j.physbeh.2007.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kreisler AD, Garcia MG, Spierling SR, Hui BE, Zorrilla EP. Extended vs. brief intermittent access to palatable food differently promote binge-like intake, rejection of less preferred food, and weight cycling in female rats. Physiology & behavior. 2017;177:305–316. doi: 10.1016/j.physbeh.2017.03.039. [DOI] [PubMed] [Google Scholar]
- 33.Alboni S, Micioni Di Bonaventura MV, Benatti C, Giusepponi ME, Brunello N, Cifani C. Hypothalamic expression of inflammatory mediators in an animal model of binge eating. Behavioural Brain Research. 2017;320(Supplement C):420–430. doi: 10.1016/j.bbr.2016.10.044. [DOI] [PubMed] [Google Scholar]
- 34.Moore CF, Sabino V, Koob GF, Cottone P. Neuroscience of Compulsive Eating Behavior. Frontiers in Neuroscience. 2017;11:469. doi: 10.3389/fnins.2017.00469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hebebrand J, Albayrak O, Adan R, Antel J, Dieguez C, de Jong J, Leng G, Menzies J, Mercer JG, Murphy M, van der Plasse G, Dickson SL. “Eating addiction”, rather than “food addiction”, better captures addictive-like eating behavior. Neuroscience and biobehavioral reviews. 2014;47:295–306. doi: 10.1016/j.neubiorev.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 36.Koob GF. Theoretical frameworks and mechanistic aspects of alcohol addiction: alcohol addiction as a reward deficit disorder. Current topics in behavioral neurosciences. 2013;13:3–30. doi: 10.1007/7854_2011_129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldschmidt AB, Wall M, Loth KA, Le Grange D, Neumark-Sztainer D. Which Dieters Are at Risk for the Onset of Binge Eating? A Prospective Study of Adolescents and Young Adults. Journal of Adolescent Health. 51(1):86–92. doi: 10.1016/j.jadohealth.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mathes WF, Brownley KA, Mo X, Bulik CM. The biology of binge eating. Appetite. 2009;52(3):545–553. doi: 10.1016/j.appet.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Polivy J, Herman CP. Dieting and binging. A causal analysis. The American psychologist. 1985;40(2):193–201. doi: 10.1037//0003-066x.40.2.193. [DOI] [PubMed] [Google Scholar]
- 40.Cannon G. Dieting. Makes you fat? British Journal of Nutrition. 2007;93(4):569–570. doi: 10.1079/bjn20041382. [DOI] [PubMed] [Google Scholar]
- 41.Lowe MR. Dieting: proxy or cause of future weight gain? Obesity Reviews. 2015;16:19–24. doi: 10.1111/obr.12252. [DOI] [PubMed] [Google Scholar]
- 42.Lowe MR, Doshi SD, Katterman SN, Feig EH. Dieting and restrained eating as prospective predictors of weight gain. Frontiers in Psychology. 2013:4. doi: 10.3389/fpsyg.2013.00577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montani JP, Schutz Y, Dulloo AG. Dieting and weight cycling as risk factors for cardiometabolic diseases: who is really at risk? Obesity Reviews. 2015;16:7–18. doi: 10.1111/obr.12251. [DOI] [PubMed] [Google Scholar]
- 44.Volkow ND, Wang GJ, Tomasi D, Baler RD. Obesity and addiction: neurobiological overlaps. Obesity Reviews. 2013;14(1):2–18. doi: 10.1111/j.1467-789X.2012.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: Behavioral and neurochemical effects of intermittent, excessive sugar intake. Neuroscience & Biobehavioral Reviews. 2008;32(1):20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent Access to 20% Ethanol Induces High Ethanol Consumption in Long - Evans and Wistar Rats. Alcoholism: Clinical and Experimental Research. 2008;32(10):1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cohen A, Koob GF, George O. Robust Escalation of Nicotine Intake with Extended Access to Nicotine Self-Administration and Intermittent Periods of Abstinence. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37:2153. doi: 10.1038/npp.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wayner MJ, Greenberg I. Effects of hypothalamic stimulation, acclimation and periodic withdrawal on ethanol consumption. Physiology & behavior. 1972;9(5):737–740. doi: 10.1016/0031-9384(72)90043-1. [DOI] [PubMed] [Google Scholar]
- 49.Corwin RL, Wojnicki FH. Binge eating in rats with limited access to vegetable shortening. Current protocols in neuroscience. 2006 doi: 10.1002/0471142301.ns0923bs36. Chapter 9, Unit9.23B. [DOI] [PubMed] [Google Scholar]
- 50.Corwin RL. Binge-type eating induced by limited access in rats does not require energy restriction on the previous day. Appetite. 2004;42(2):139–42. doi: 10.1016/j.appet.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 51.Cottone P, Sabino V, Steardo L, Zorrilla EP. Intermittent access to preferred food reduces the reinforcing efficacy of chow in rats. American journal of physiology. Regulatory, integrative and comparative physiology. 2008;295(4):R1066–76. doi: 10.1152/ajpregu.90309.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cottone P, Sabino V, Roberto M, Bajo M, Pockros L, Frihauf JB, Fekete EM, Steardo L, Rice KC, Grigoriadis DE, Conti B, Koob GF, Zorrilla EP. CRF system recruitment mediates dark side of compulsive eating. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(47):20016–20. doi: 10.1073/pnas.0908789106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boggiano MM, Artiga AI, Pritchett CE, Chandler-Laney PC, Smith ML, Eldridge AJ. High intake of palatable food predicts binge-eating independent of susceptibility to obesity: an animal model of lean vs obese binge-eating and obesity with and without binge-eating. International journal of obesity (2005) 2007;31(9):1357–67. doi: 10.1038/sj.ijo.0803614. [DOI] [PubMed] [Google Scholar]
- 54.Klump KL, Culbert KM, Sisk CL. Sex Differences in Binge Eating: Gonadal Hormone Effects Across Development. Annual review of clinical psychology. 2017;13:183–207. doi: 10.1146/annurev-clinpsy-032816-045309. [DOI] [PubMed] [Google Scholar]
- 55.Hildebrandt BA, Klump KL, Racine SE, Sisk CL. Differential strain vulnerability to binge eating behaviors in rats. Physiology & behavior. 2014;127:81–6. doi: 10.1016/j.physbeh.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 56.Klump KL, Racine S, Hildebrandt B, Sisk CL. Sex differences in binge eating patterns in male and female adult rats. International Journal of Eating Disorders. 2013;46(7):729–736. doi: 10.1002/eat.22139. [DOI] [PubMed] [Google Scholar]
- 57.Klump KL, Suisman JL, Culbert KM, Kashy DA, Sisk CL. Binge eating proneness emerges during puberty in female rats: a longitudinal study. Journal of abnormal psychology. 2011;120(4):948–55. doi: 10.1037/a0023600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cottone P, Sabino V, Steardo L, Zorrilla EP. Consummatory, anxiety-related and metabolic adaptations in female rats with alternating access to preferred food. Psychoneuroendocrinology. 2009;34(1):38–49. doi: 10.1016/j.psyneuen.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gearhardt AN, Miller AL, Sturza J, Epstein LH, Kaciroti N, Lumeng JC. Behavioral Associations with Overweight in Low-Income Children. Obesity (Silver Spring, Md.) 2017 doi: 10.1002/oby.22033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kong KL, Eiden RD, Anzman-Frasca S, Stier CL, Paluch RA, Mendez J, Slominski E, Gengatharan G, Epstein LH. Repeatability of the infant food reinforcement paradigm: Implications of individual and developmental differences. Appetite. 2018;120:123–129. doi: 10.1016/j.appet.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 61.Epstein LH, Salvy SJ, Carr KA, Dearing KK, Bickel WK. Food reinforcement, delay discounting and obesity. Physiology & behavior. 2010;100(5):438–45. doi: 10.1016/j.physbeh.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Epstein LH, Stein JS, Paluch RA, MacKillop J, Bickel WK. Binary components of food reinforcement: Amplitude and persistence. Appetite. 2018;120:67–74. doi: 10.1016/j.appet.2017.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Temple JL. Factors that influence the reinforcing value of foods and beverages. Physiology & behavior. 2014;136:97–103. doi: 10.1016/j.physbeh.2014.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hudson JI, Hiripi E, Pope HG, Jr, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biological psychiatry. 2007;61(3):348–58. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lewinsohn PM, Seeley JR, Moerk KC, Striegel-Moore RH. Gender differences in eating disorder symptoms in young adults. The International journal of eating disorders. 2002;32(4):426–40. doi: 10.1002/eat.10103. [DOI] [PubMed] [Google Scholar]
- 66.Healy K, Conroy RM, Walsh N. The prevalence of binge-eating and bulimia in 1063 college students. Journal of psychiatric research. 1985;19(2–3):161–6. doi: 10.1016/0022-3956(85)90012-3. [DOI] [PubMed] [Google Scholar]
- 67.Field AE, Colditz GA, Peterson KE. Racial/ethnic and gender differences in concern with weight and in bulimic behaviors among adolescents. Obesity research. 1997;5(5):447–54. doi: 10.1002/j.1550-8528.1997.tb00669.x. [DOI] [PubMed] [Google Scholar]
- 68.Field AE, Camargo CA, Jr, Taylor CB, Berkey CS, Frazier AL, Gillman MW, Colditz GA. Overweight, weight concerns, and bulimic behaviors among girls and boys. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38(6):754–60. doi: 10.1097/00004583-199906000-00024. [DOI] [PubMed] [Google Scholar]
- 69.Croll J, Neumark-Sztainer D, Story M, Ireland M. Prevalence and risk and protective factors related to disordered eating behaviors among adolescents: relationship to gender and ethnicity. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2002;31(2):166–75. doi: 10.1016/s1054-139x(02)00368-3. [DOI] [PubMed] [Google Scholar]
- 70.Striegel-Moore RH, Rosselli F, Perrin N, DeBar L, Wilson GT, May A, Kraemer HC. Gender difference in the prevalence of eating disorder symptoms. The International journal of eating disorders. 2009;42(5):471–4. doi: 10.1002/eat.20625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reichborn-Kjennerud T, Bulik CM, Kendler KS, Roysamb E, Maes H, Tambs K, Harris JR. Gender differences in binge-eating: a population-based twin study. Acta psychiatrica Scandinavica. 2003;108(3):196–202. doi: 10.1034/j.1600-0447.2003.00106.x. [DOI] [PubMed] [Google Scholar]
- 72.Babbs RK, Wojnicki FHE, Corwin RLW. Effect of 2-hydroxyestradiol on binge intake in rats. Physiology & behavior. 2011;103(5):508–512. doi: 10.1016/j.physbeh.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sinclair EB, Hildebrandt BA, Culbert KM, Klump KL, Sisk CL. Preliminary evidence of sex differences in behavioral and neural responses to palatable food reward in rats. Physiology & behavior. 2017;176:165–173. doi: 10.1016/j.physbeh.2017.03.042. [DOI] [PubMed] [Google Scholar]
- 74.Zorrilla EP, Sanchez-Alavez M, Sugama S, Brennan M, Fernandez R, Bartfai T, Conti B. Interleukin-18 controls energy homeostasis by suppressing appetite and feed efficiency. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(26):11097–102. doi: 10.1073/pnas.0611523104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kaiyala KJ, Ramsay DS. Direct Animal Calorimetry, the Underused Gold Standard for Quantifying the Fire of Life(). Comparative biochemistry and physiology. Part A, Molecular & integrative physiology. 2011;158(3):252–64. doi: 10.1016/j.cbpa.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Griebeler EM, Werner J. Mass, phylogeny, and temperature are sufficient to explain differences in metabolic scaling across mammalian orders? Ecology and Evolution. 2016;6(23):8352–65. doi: 10.1002/ece3.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yoo W, Mayberry R, Bae S, Singh K, Peter He Q, Lillard JW., Jr A Study of Effects of MultiCollinearity in the Multivariable Analysis. International journal of applied science and technology. 2014;4(5):9–19. [PMC free article] [PubMed] [Google Scholar]
- 78.Greenhouse SW, Geisser S. On methods in the analysis of profile data. Psychometrika. 1959;24(2):95–112. [Google Scholar]
- 79.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86(2):420–8. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 80.Hodos W. Progressive ratio as a measure of reward strength. Science (New York, N.Y.) 1961;134(3483):943–4. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- 81.Wong T, Harber V. Lower excess postexercise oxygen consumption and altered growth hormone and cortisol responses to exercise in obese men. The Journal of clinical endocrinology and metabolism. 2006;91(2):678–86. doi: 10.1210/jc.2005-1148. [DOI] [PubMed] [Google Scholar]
- 82.Sartor F, Jackson MJ, Squillace C, Shepherd A, Moore JP, Ayer DE, Kubis HP. Adaptive metabolic response to 4 weeks of sugar-sweetened beverage consumption in healthy, lightly active individuals and chronic high glucose availability in primary human myotubes. European journal of nutrition. 2013;52(3):937–48. doi: 10.1007/s00394-012-0401-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Simonson DC, DeFronzo RA. Indirect calorimetry: methodological and interpretative problems. The American journal of physiology. 1990;258(3 Pt 1):E399–412. doi: 10.1152/ajpendo.1990.258.3.E399. [DOI] [PubMed] [Google Scholar]
- 84.Lynch WJ. Sex differences in vulnerability to drug self-administration. Experimental and clinical psychopharmacology. 2006;14(1):34–41. doi: 10.1037/1064-1297.14.1.34. [DOI] [PubMed] [Google Scholar]
- 85.Becker JB, Koob GF. Sex Differences in Animal Models: Focus on Addiction. Pharmacological reviews. 2016;68(2):242–63. doi: 10.1124/pr.115.011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pursey KM, Stanwell P, Gearhardt AN, Collins CE, Burrows TL. The prevalence of food addiction as assessed by the Yale Food Addiction Scale: a systematic review. Nutrients. 2014;6(10):4552–90. doi: 10.3390/nu6104552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science (New York, N.Y.) 2004;305(5686):1014–7. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- 88.Ghitza UE, Gray SM, Epstein DH, Rice KC, Shaham Y. The anxiogenic drug yohimbine reinstates palatable food seeking in a rat relapse model: a role of CRF1 receptors. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2006;31(10):2188–96. doi: 10.1038/sj.npp.1300964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Meule A, Gearhardt AN. Food addiction in the light of DSM-5. Nutrients. 2014;6(9):3653–71. doi: 10.3390/nu6093653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.American Psychiatric Association. 5. Washington, DC: 2013. [Google Scholar]
- 91.Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW, Jr, Logrip ML, Rivier C, Repunte-Canonigo V, Zorrilla EP, Sanna PP, Heilig M, Koob GF. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(22):7563–71. doi: 10.1523/JNEUROSCI.0069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wade CL, Vendruscolo LF, Schlosburg JE, Hernandez DO, Koob GF. Compulsive-like responding for opioid analgesics in rats with extended access. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2015;40(2):421–8. doi: 10.1038/npp.2014.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Spierling SRKAD, Zorrilla EP. In Intermittent access to palatable food leads to compulsive self-administration and hyperirritability during withdrawal in rats, The Society for the Study of Ingestive Behavior, Porto, Portugal, Porto, Portugal. 2016 [Google Scholar]
- 94.Lovejoy JC, Sainsbury A. Sex differences in obesity and the regulation of energy homeostasis. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2009;10(2):154–67. doi: 10.1111/j.1467-789X.2008.00529.x. [DOI] [PubMed] [Google Scholar]
- 95.Lovejoy JC. The influence of sex hormones on obesity across the female life span. Journal of women’s health. 1998;7(10):1247–56. doi: 10.1089/jwh.1998.7.1247. [DOI] [PubMed] [Google Scholar]
- 96.Perry AC, Martin L. Race differences in obesity and its relationship to the sex hormone milieu. Hormone molecular biology and clinical investigation. 2014;19(3):151–61. doi: 10.1515/hmbci-2014-0004. [DOI] [PubMed] [Google Scholar]
- 97.Palmer BF, Clegg DJ. The sexual dimorphism of obesity. Molecular and cellular endocrinology. 2015;402:113–9. doi: 10.1016/j.mce.2014.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stock MJ, Rothwell NJ. Factors influencing brown fat and the capacity for diet-induced thermogenesis. International journal of obesity. 1985;9(Suppl 2):9–15. [PubMed] [Google Scholar]
- 99.Rothwell NJ, Stock MJ. The development of obesity in animals: the role of dietary factors. Clinics in endocrinology and metabolism. 1984;13(3):437–49. doi: 10.1016/s0300-595x(84)80032-8. [DOI] [PubMed] [Google Scholar]
- 100.Epstein LH, Temple JL, Neaderhiser BJ, Salis RJ, Erbe RW, Leddy JJ. Food Reinforcement, the Dopamine D(2) Receptor Genotype, and Energy Intake in Obese and Nonobese Humans. Behavioral neuroscience. 2007;121(5):877–886. doi: 10.1037/0735-7044.121.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Epstein LH, Leddy JJ, Temple JL, Faith MS. Food Reinforcement and Eating: A Multilevel Analysis. Psychological bulletin. 2007;133(5):884–906. doi: 10.1037/0033-2909.133.5.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Swithers SE. Not-so-healthy sugar substitutes? Current Opinion in Behavioral Sciences. 2016;9(Supplement C):106–110. doi: 10.1016/j.cobeha.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ohara I, Naruse MM, Itokawa Y. The effect of palatability and feeding conditions on digestive functions in rats. Journal of the American College of Nutrition. 1996;15(2):186–191. doi: 10.1080/07315724.1996.10718587. [DOI] [PubMed] [Google Scholar]
- 104.Inui-Yamamoto C, Furudono Y, Yamamoto T. Hedonics of taste influence the gastric emptying in rats. Physiology & behavior. 2009;96(4):717–722. doi: 10.1016/j.physbeh.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 105.Tsuji T, Tanaka S, Kida K, Bakhshishayan S, Kogo M, Yamamoto T. Disrupted normal ingestion during glucose intake modulates glucose kinetics in humans. SpringerPlus. 2015;4:621. doi: 10.1186/s40064-015-1419-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Saitou K, Lees JN, Tordoff MG. Taste hedonics influence the disposition of fat by modulating gastric emptying in rats. PloS one. 2014;9(3):e90717. doi: 10.1371/journal.pone.0090717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Glendinning JI, Stano S, Holter M, Azenkot T, Goldman O, Margolskee RF, Vasselli JR, Sclafani A. Sugar-induced cephalic-phase insulin release is mediated by a T1r2+T1r3-independent taste transduction pathway in mice. American journal of physiology. Regulatory, integrative and comparative physiology. 2015;309(5):R552–60. doi: 10.1152/ajpregu.00056.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Just T, Pau HW, Engel U, Hummel T. Cephalic phase insulin release in healthy humans after taste stimulation? Appetite. 2008;51(3):622–627. doi: 10.1016/j.appet.2008.04.271. [DOI] [PubMed] [Google Scholar]
- 109.Castro DC, Cole SL, Berridge KC. Lateral hypothalamus, nucleus accumbens, and ventral pallidum roles in eating and hunger: interactions between homeostatic and reward circuitry. Frontiers in systems neuroscience. 2015;9:90. doi: 10.3389/fnsys.2015.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.