Abstract
Introduction
Averaging at 13.4%, current literature reports widely varying prevalence rates of hallucinations in patients with probable Alzheimer's disease (AD), and is still inconclusive on contributive factors to hallucinations in AD.
Methods
This study assessed prevalence, associated factors and clinical characteristics of hallucinations in 1227 patients with probable AD, derived from a tertiary memory clinic specialized in early diagnosis of dementia. Hallucinations were assessed with the Neuropsychiatric Inventory.
Results
Hallucination prevalence was very low, with only 4.5% (n = 55/1227) affected patients. Hallucinations were mostly visual (n = 40/55) or auditory (n = 12/55). Comorbid delusions were present in over one-third of cases (n = 23/55).
Hallucinations were associated with increased dementia severity, neuropsychiatric symptoms, and a lifetime history of hallucination-evoking disease (such as depression and sensory impairment), but not with age or gender.
Discussion
In the largest sample thus far, we report a low prevalence of hallucinations in probable AD patients, comparable to rates in non-demented elderly. Our results suggest that hallucinations are uncommon in early stage AD. Clinicians that encounter hallucinations in patients with early AD should be sensitive to hallucination-evoking comorbidity.
Keywords: Alzheimer's disease, Hallucinations, Low prevalence, Comorbidity, Dementia severity
1. Introduction
Hallucinations occur in a variety of psychiatric, neurologic, and somatic disorders, as well as in the general population [1]. Their presence can induce distress and impair daily functioning toward a stage that professional help is necessary [2]. Better understanding of hallucinations can improve both clinical assessment and treatment [1], [2].
Reported prevalence rates of hallucinations in patients with probable Alzheimer's disease (AD) vary widely from 7% to 35% [3], averaging at 13.4% (“Research in context”; Supplementary Fig. 1, Supplementary Tables 1ab). Their presence has been repeatedly associated with more severe cognitive and functional decline, earlier institutionalization, higher burden of disease, and increased mortality [4]. It is therefore essential to better understand hallucinations in AD.
However, heterogeneity between studies on hallucinations in probable AD is large and complicates comparability of study results [3]. As such, current literature is not conclusive on potentially contributive factors, such as dementia severity [3]. Also, the possibility of other diagnoses and medication use as alternative contributing factors to hallucinations in patients with probable AD is often underexposed.
The present study tries to improve the understanding of these uncertainties by studying hallucinations in a large sample of patients with probable AD, derived from a tertiary research memory clinic specialized in early detection of dementia [5]. We assessed the prevalence and phenomenology of hallucinations and studied potentially associated factors by comparing hallucinating and nonhallucinating participants on demographics, dementia stage and severity, other neuropsychiatric symptoms, and medical history and use of medication that can trigger hallucinations.
2. Methods
We retrospectively included all patients with probable AD from the Amsterdam Dementia Cohort [5], who were seen between January 2005 and January 2018, and studied with the Neuropsychiatric Inventory (NPI) [6] during baseline diagnostic assessment of cognitive complaints. All participants fulfilled criteria for probable AD as formulated by the National Institute for Neurological and Communicative Disorders/Alzheimer's Disease and Related Disorders Association [7] and had been diagnosed within 30 days from their initial visit. Diagnosis was based on standardized multidisciplinary assessment, including patients' history, neurological examination, vital functions, neuropsychological assessment, whole-brain magnetic resonance imaging, electroencephalography and routine serum laboratory, and cerebrospinal fluid sampling in a subsample [5].
NPI assessment was conducted in patients' caregivers, by a specialized dementia research nurse during the study day. A participant was considered “hallucinating” if he/she had a frequency score of ≥1 on the NPI hallucination subscale. Further details on hallucination phenomenology were retrieved with hallucination items of the NPI, and, if necessary, by reviewing patients' charts. The overall presence and severity of neuropsychiatric symptoms were based on total NPI scores.
Subjects' medical history was dichotomously marked as relevant if one or more diagnoses had ever been present, in which hallucinations are reportedly part of the associated symptomatology, as stated by recent overview articles [1], [8] (listed in Table 1). Similar dichotomization was applied if patients used one or more drugs with hallucinations listed as a side effect [9], referred to as hallucination-inducing medication (Table 1). Ranking of relevant history and medication was performed independently by two authors (M.D. and M.M.J.L.); discrepancies were solved by consensus. Dementia severity was based on scores from the Mini-Mental State Examination (MMSE) (27–30 no dementia, 20–26 mild dementia, 10–19 moderate, and 0–9 severe) [10] and the Clinical Dementia Rating (CDR) [11].
Table 1.
Comparison of demographic and clinical characteristics between AD patients with (+) and without (−) hallucinations (n = 1227)
| Factor | Hall (+) n = 55 |
Hall (−) n = 1172 |
Statistics |
||
|---|---|---|---|---|---|
| Median (IQR) | Median (IQR) | P | Z | U | |
| Age (yrs) | 67.2 (62.5–72.6) | 66.7 (60.5–72.3) | .44 | .78 | 34,226.5 |
| MMSE score∗,‡ | 19 (13–22) | 21 (17–24) | <.001 | −3.7 | 20,674.0 |
| CDR∗,‡ | 1 (1–2) | 1 (0.5–1) | .003 | 3.0 | 31,829.5 |
| Total NPI score∗ | 24 (13–34) | 8 (3–16) | <.001 | 7.1 | 48,802.0 |
| Total NPI score (excl. hallucination items)∗ |
22 (10–29.5) |
8 (3–16) |
<.001 |
5.8 |
45,475.5 |
| n (%) |
n (%) |
P |
χ2 |
df |
|
| Female gender | 27 (49.1) | 602 (51.4) | .74 | .11 | 1 |
| Presence of comorbid delusions (NPI)∗ | 22 (40.0) | 84 (7.2) | <.001 | 72 | 1 |
| History of hallucination-associated disease∗,‡,§ | 21 (38.2) | 299 (25.2) | .036 | 4.4 | 1 |
| Use of hallucination-inducing medication†,‡,¶ | 31 (56.4) | 517 (44.1) | .074 | 3.2 | 1 |
Abbreviations: AD, Alzheimer’s disease; CDR, clinical dementia rating; IQR, interquartile range; MMSE, Mini-Mental State Examination; NPI, Neuropsychiatric Inventory.
NOTE. Results that are statistically significant (P < .05) are listed in bold.
Statistically significant (P < .05).
Trend level of statistical significance (P < .1).
Missing data in MMSE (n = 15, of which 4 in Hall (+) group) and CDR (n = 118, of which 6 in Hall (+) group). Missing data on medical history (n = 3) and medication use (n = 6) were supplemented by reviewing patient's charts.
≥1 relevant diagnosis in medical history (diagnosis considered “relevant” if hallucinations have been reported to occur as a comorbid symptom [1], [8]): Schizophrenia spectrum disorder; Mood disorder; Anxiety disorder; Personality disorder; Posttraumatic stress disorder; Substance abuse; Hearing impairment; Visual impairment; Epilepsy; Systemic lupus erythematosus; Autism spectrum disorder; Delirium.
Use of ≥1 hallucination-inducing medication: Antidepressants (selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, tricyclic antidepressants, monoamine oxidase inhibitors); Benzodiazepines; Oral anticholinergic drugs; Dopaminergic drugs (dopamine agonists, levodopa); Oral beta-blockers; Opiates; Lithium; Methylphenidate; Modafinil; Memantine; Betahistine; Oral antihistaminergic drugs; Antimigrainous drugs; Proton pump inhibitors; Clonidine; Baclofen; Disulfiram.
Confidence intervals (95%) for prevalence rates of hallucinations were calculated using Clopper-Pearson's exact method in R, version 3.2.0, package PropCIs. Hallucinating and nonhallucinating subjects were compared using chi-square tests for categorical variables and Mann-Whitney U-tests for continuous variables, using IBM SPSS Statistics, version 22. The level of two-tailed significance was set at P < .05.
3. Results
Out of 1545 patients diagnosed with probable AD during baseline screening between January 2005 and January 2018, 1227 subjects (79.4%) had NPI data available, with a mean age of 66.6 (standard deviation 7.9) (Supplementary Fig. 2). Supplementary Table 3 shows basic characteristics of the included sample (n = 1227). There were no substantial differences between the group with and without NPI data (Supplementary Table 2).
Hallucinations occurred in 55 out of 1227 participants (4.5%; 95% confidence interval 3.4%–5.8%).
The 55 hallucinating subjects mainly reported experiences in the visual (n = 40; 73%) or auditory modality (n = 12; 22%). A smaller group reported olfactory (n = 5; 9%) and tactile hallucinations (n = 3; 5%); hallucination modality was unknown in 10 participants (18%). According to the NPI, delusions were present in 23 hallucinating participants (42%), of which paranoia (n = 9), home intruders (n = 10) and theft (n = 12) were reported most frequently.
3.1. Associated factors
Hallucinating subjects showed significantly higher percentages of comorbid delusions than nonhallucinating subjects and had higher total NPI scores (Table 1). The percentage of subjects with a history of hallucination-associated disease was higher in those with hallucinations (Table 1). At trend level significance, the percentage of hallucination-inducing medication use appeared higher in the hallucinating group.
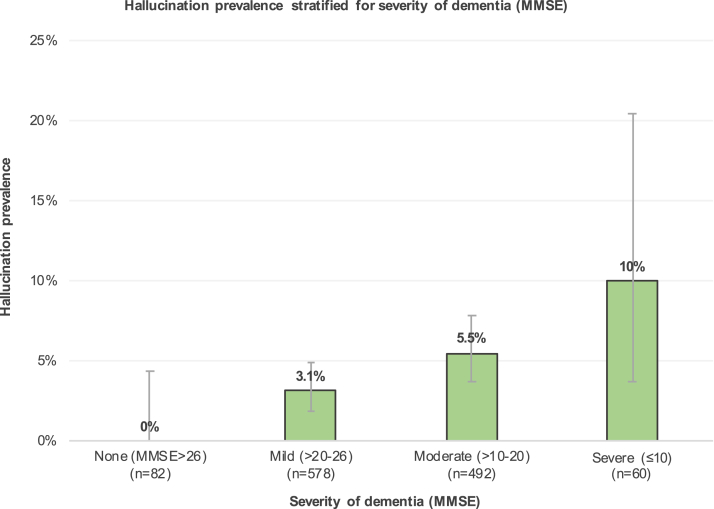
Hallucinating subjects had significantly lower MMSE scores and a significantly increased CDR in comparison with the nonhallucinating subjects (Table 1). Stratification for severity of dementia resulted in statistically significant distributions for both MMSE (χ2 12.3, P .006, df 3) and CDR (χ2 11.7, P .020, df 4) and an increasing percentage of hallucination prevalence with dementia severity (Fig. 1, Supplementary Fig. 3). No differences were observed with regard to age or gender (Table 1).
Fig. 1.
Prevalence of hallucinations, as stratified for dementia severity based on MMSE scores (total n = 1227). Error bars indicate lower and upper borders of 95% confidence intervals. MMSE data were missing in 15 subjects, 4 of which reported hallucinations. Distribution was statistically significant (χ2 12.3, P .006, df 3). Abbreviation: MMSE, Mini-Mental State Examination.
4. Discussion
In the largest sample of patients with probable AD to date, consisting predominantly of patients with early stage disease and relatively young age, we observed a remarkably low prevalence of hallucinations (4.5%) in comparison with existing literature (Supplementary Fig. 1). In studies from comparable research clinics, even the lowest reported prevalence (7.0%) [12] exceeded the upper bound of our 95% confidence interval (5.8%). In 188 subjects with mild probable AD, Wadsworth et al. [13] described a similar prevalence to ours (5.3%) but excluded subjects with comorbid psychiatric or neurological disorders.
The hallucination prevalence in this sample is comparable to the NPI-based prevalence of hallucinations in a nondemented population sample aged ≥65 years (4.5%; n = 80/1781) [14]. Because our sample is derived from a research clinic specialized in diagnosis of early stages of dementia [5], the finding of a near-normal prevalence of hallucinations within this sample suggests that hallucinations should not be considered a common symptom in early stage AD (in contrast to dementia with Lewy bodies).
Indeed, we observed significant associations between the presence of hallucinations and both decreased MMSE scores and an increased CDR. Hallucination prevalence rates increased with intensifying categories of dementia severity, with percentages up to 10% in the group with MMSE scores of 10 or less. These observations correspond with previous studies suggesting the uncommonness of hallucinations in early stage AD [15], [16], [17] and an increase in cumulative hallucination prevalence with disease progression [17]. The mean age of our sample was younger than that of other cohorts [3], but, in our sample, hallucinating and nonhallucinating subjects' age did not differ significantly.
Thirty-eight percent of hallucinating subjects reported a lifetime history of hallucination-evoking diagnoses; significantly higher than the nonhallucinating control group (25%). A similar trend was observed with regard to the use of hallucination-inducing medication. Also, hallucinating subjects had a significantly elevated NPI score regardless of the hallucination score, indicating an increased overall presence and severity of neuropsychiatric symptoms. These observations imply that the presence of hallucinations in our sample does not necessarily have to be attributed to a diagnosis of AD alone but may also be evoked by other diagnoses or medication use. This implication stresses the importance of proper hallucination assessment in patients with AD. Clinicians who encounter hallucinations in patients with AD should consider the broad diagnostic spectrum in which hallucinations occur, such as dementia with Lewy bodies, delirium, psychotic or affective disorders, and sensory impairment, so that treatment options can be properly adjusted [1]. As such, we recommend clinicians who encounter hallucinations in early stage AD patients to actively approach them within the context of medication use and current and prior disease.
4.1. Limitations
Most included subjects were not seen for follow-up visitations. Hence, we were not able to study the course and occurrence of hallucinations and AD diagnosis longitudinally.
As hallucinations are based on subjective experiences, using caregiver-based assessment may have led to underreporting of hallucinations. It would be of added value to replicate hallucination assessment in a similar sample with an alternative but equally valid patient-based questionnaire and compare this to caregiver-based results. The screener version of the Questionnaire for Psychotic Experiences may be a promising alternative for this purpose [18].
NPI data were not available for all patients seen during the inclusion period. Although the included sample remains large, this may have influenced generalizability.
Finally, due to the retrospective study design, assessment of hallucination-associated diagnoses was limited to incorporation of lifetime medical history. As a result, we cannot attribute any time-related associations to the occurrence of hallucinations and potentially relevant diagnoses in our sample. Ideally, future studies on hallucinations in probable AD should incorporate current comorbidity to assess its influence more extensively.
5. Conclusion
In the largest sample of patients with probable AD thus far, predominantly in early stages, we found hallucinations in only 4.5%, similar to rates in nondemented elderly. Our findings substantiate that hallucinations are not common in early stage AD, but their prevalence increases with higher severity of dementia. In early stage AD, hallucinations may have different etiologies and should prompt accurate differential diagnosis, including sensory impairment, psychiatric diagnoses, and medication use.
Research in Context.
-
1.
Systematic review: In extension to a recent meta-analysis by Zhao et al. (2016), we performed a meta-analysis on hallucination prevalence in patients with probable Alzheimer’s disease (AD) by searching a common scientific database (PubMed/Medline) on October 4, 2017. The average prevalence was 13.4% (95% confidence interval 10.5–16.9). Included studies and calculations are presented in Supplementary Fig. 1 and Supplementary Tables 1ab.
-
2.
Interpretation: At 4.5%, we observed a substantially low prevalence of hallucinations in probable AD in comparison with existing literature and the outcome of the meta-analysis. We attribute this to the uncommon appearance of hallucinations in early stages of AD, which is consistent with earlier findings.
-
3.
Future directions: In patients with hallucinations in early stages of probable AD, we suggest to include the following factors in future studies and clinical assessment (a) the presence of other diagnoses (i.e., sensory impairment or dementia with Lewy bodies) and population-based factors that may evoke hallucinations, and (b) the use of hallucination-inducing medication.
Acknowledgments
This study was partly supported by ZONMW TOP grant 40-00812-98-13009.
Footnotes
The authors have declared that no conflict of interest exists.
The authors A.W.L. and M.D. have contributed equally and share second authorship.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.dadm.2018.03.005.
Supplementary data
References
- 1.Sommer I.E.C., Koops S., Blom J.D. Comparison of auditory hallucinations across different disorders and syndromes. Neuropsychiatry (London) 2012;2:57–68. [Google Scholar]
- 2.Johns L.C., Kompus K., Connell M., Humpston C., Lincoln T.M., Longden E. Auditory verbal hallucinations in persons with and without a need for care. Schizophr Bull. 2014;40:255–264. doi: 10.1093/schbul/sbu005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao Q.-F., Tan L., Wang H.-F., Jiang T., Tan M.-S., Tan L. The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: systematic review and meta-analysis. J Affect Disord. 2016;190:264–271. doi: 10.1016/j.jad.2015.09.069. [DOI] [PubMed] [Google Scholar]
- 4.El Haj M., Roche J., Jardri R., Kapogiannis D., Gallouj K., Antoine P. Clinical and neurocognitive aspects of hallucinations in Alzheimer’s disease. Neurosci Biobehav Rev. 2017;83:713–720. doi: 10.1016/j.neubiorev.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Der Flier W.M., Pijnenburg Y.A.L., Prins N., Lemstra A.W., Bouwman F.H., Teunissen C.E. Optimizing patient care and research: the Amsterdam dementia cohort. J Alzheimer’s Dis. 2014;41:313–327. doi: 10.3233/JAD-132306. [DOI] [PubMed] [Google Scholar]
- 6.Cummings J.L. The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology. 1997;48:S10–S16. doi: 10.1212/wnl.48.5_suppl_6.10s. [DOI] [PubMed] [Google Scholar]
- 7.McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Kawas C.H. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sommer I.E., Kahn R.S. Psychosis susceptibility syndrome: an alternative name for schizophrenia. Lancet Psychiatry. 2014;1:111. doi: 10.1016/S2215-0366(14)70288-3. [DOI] [PubMed] [Google Scholar]
- 9.Farmacotherapeutisch Kompas n.d. Available at: https://farmacotherapeutischkompas.nl. Accessed February 15, 2018.
- 10.Perneczky R., Wagenpfeil S., Komossa K., Grimmer T., Diehl J., Kurz A. Mapping scores onto stages: mini-mental state examination and clinical dementia rating. Am J Geriatr Psychiatry. 2006;14:139–144. doi: 10.1097/01.JGP.0000192478.82189.a8. [DOI] [PubMed] [Google Scholar]
- 11.Hughes C.P., Berg L., Danziger W.L., Coben L.A.M.R. A new clinical scale for the stating of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 12.Mizrahi R., Starkstein S.E., Jorge R., Robinson R.G. Phenomenology and clinical correlates of delusions in Alzheimer disease. Am J Geriatr Psychiatry. 2006;14:573–581. doi: 10.1097/01.JGP.0000214559.61700.1c. [DOI] [PubMed] [Google Scholar]
- 13.Wadsworth L.P., Lorius N., Donovan N.J., Locascio J.J., Rentz D.M., Johnson K.A. Neuropsychiatric symptoms and global functional impairment along the Alzheimer’s continuum. Dement Geriatr Cogn Disord. 2012;34:96–111. doi: 10.1159/000342119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Linde R., Stephan B.C.M., Matthews F.E., Brayne C., Savva G.M. Behavioural and psychological symptoms in the older population without dementia - relationship with socio-demographics, health and cognition. BMC Geriatr. 2010;10:87. doi: 10.1186/1471-2318-10-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jost B.C., Grossberg G.T. The evolution of psychiatric symptoms in Alzheimer’s disease: a natural history study. J Am Geriatr Soc. 1996;44:1078–1081. doi: 10.1111/j.1532-5415.1996.tb02942.x. [DOI] [PubMed] [Google Scholar]
- 16.Bassiony M.M., Lyketsos C.G. Delusions and hallucinations in Alzheimer’s disease: review of the brain decade. Psychosomatics. 2003;44:388–401. doi: 10.1176/appi.psy.44.5.388. [DOI] [PubMed] [Google Scholar]
- 17.Devanand D., Brockington C., Moody B., Brown R., Mayeaux R., Endicott H. Behavioral syndromes in Alzheimer’s disease. Int Psychogeriatrics. 1992;4:161–184. [PubMed] [Google Scholar]
- 18.Sommer I.E., Kleijer H., Hugdahl K. Toward personalized treatment of hallucinations. Curr Opin Psychiatry. 2018;31:237–245. doi: 10.1097/YCO.0000000000000416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.