Abstract
Chronic pulmonary hypertension (PH) is associated with right ventricular failure and high mortality regardless of the underlying disease. Currently, therapies can improve clinical outcomes in specific subsets of patients, but have little impact on the progression of pulmonary vascular remodeling. Upon new advances in vector development and delivery techniques, gene therapy is a novel strategy in this field with the potential of overcoming the main limitations of approved drug therapies: modulation of novel anti-remodeling targets and selective pulmonary vasculature targeting with minimal systemic effects. In the recent years, several reports have shown that gene transfer to the pulmonary vascular system is feasible in rodent models of PH. Our group has focused on the translation of airway delivery of viral vectors in small and large animals. Here, we describe a procedure to achieve vector transduction at the distal vasculature in animal models of PH and the methods to evaluate the outcomes of this intervention as a promising new approach in pulmonary vascular diseases.
Keywords: Airway delivery, Gene therapy, Pulmonary hypertension, Large animal model, Adeno-associated virus, Right ventricular failure, Vascular remodeling, Pulmonary vascular disease
1 Introduction
Pulmonary vascular disease (PVD) is defined by the development of vessel wall remodeling changes in the distal pulmonary vasculature, and is a consequence of a heterogeneous group of clinical conditions [1]. In the clinical setting, PVD is characterized by progressive dyspnea and exercise intolerance, and diagnosis relies on the detection of pulmonary hypertension (PH) upon right heart catheterization. PH is defined by a mean pulmonary artery (PA) pressure above 25 mmHg [1], and based on available clinical studies, underlying PVD is suspected by additional hemodynamic abnormalities in the pulmonary circulation, such as increased pulmonary vascular resistance (PVR), transpulmonary gradient or diastolic to PA wedge pressure differences [1–3]. The main determinant of prognosis in patients suffering PH is the impact of sustained high afterload on right ventricular function, leading to premature heart failure and death [4].
Current therapeutic options for chronic PH are largely dependent on the clinical classification for each patient undergoing the diagnostic process [1]. Advances in the cellular and molecular mechanisms involved in Group 1 PH (also designated as pulmonary arterial hypertension, PAH) have led to novel drug developments targeting the main pathways including endothelin receptor antagonists, prostacyclin analogs and activators of the soluble guanylate cyclase (sGC)/cGMP axis (phosphodiesterase five inhibitors, and more recently, sGC activators) [1]. Clinical trials for these drugs have focused on Group 1 PH, while some benefit may be present in other groups with novel agents [5, 6], in particular groups 2 (PH due to left heart disease) and 4 (chronic thromboembolic PH).
Limitations for the widespread use of current vasodilator drugs include the frequency of systemic, undesired side effects, lack of long-term sustained clinical benefits, as well as a high economic cost of these treatments [1]. In addition, during the past few years, the unraveling of novel molecular mechanisms involved in PH have set growing interest in developing more specific, target-driven therapeutic strategies. In this regard, gene therapy may overcome some of the limitations of current treatments, by selectively modulating novel pathways that are not targeted by any drug at present [7]. Recently, several studies by independent groups have shown the potential therapeutic benefit of modulating a variety of molecular targets using gene therapy [7, 8]. For instance, endothelial NOS, prostacyclin synthase or BMPR2 have been successfully modulated in the pulmonary vasculature leading to improved hemodynamics in animal models of PH [9–11].
In order to develop gene therapy strategies for PH, vectors that efficiently target the pulmonary vasculature and provide sustained expression of the gene of interest are needed. In this regard, advances in viral vector technology have made available the recombinant adeno-associated viruses (AAV)s that allow different tissue tropisms based on the capsid proteins composition, while eliciting minimal immune response. In addition, delivery methods that preferentially transduce the lung vasculature with minimal exposure of the vector to off-target tissues are essential to guarantee the feasibility, safety, and translatability of this strategy for PH patients [12].
The purpose of this protocol is to describe a novel airway delivery method of vectors in large animal models of PH that efficiently transduces the distal pulmonary circulation and elicits improvements in vascular remodeling and hemodynamics.
2 Materials
2.1 Animal Preparation and PH Model Creation
For Anesthesia Induction and Maintenance
-
1
Telazol (tiletamine/zolazepam).
-
2
Isoflurane.
-
3
Propofol.
-
4
Fentanyl patch.
-
5
Prophylactic antibiotics.
-
6
Respirator suitable for swine with adjustable inspiratory oxygen concentration.
-
7
ECG and pulse oxymetry monitor.
For Surgical PH Model Creation in Swine
-
8
Surgical suite and sterile drapes.
-
9
Standard surgical tools: Scissors, forceps, scalpel. Bioabsorbable and Nylon sutures, Silicone Thoracic Drain 20Fr., Gauzes.
-
10
Cotton Umbilical Tape 1/8″×18″.
-
11
A 3.5-mm diameter plastic cylinder.
-
12
Furosemide.
2.2 Functional Evaluation of PH in Large Animals: Hemodynamic and Echocardiography Assessments
Procedure room equipped with a fluoroscopy system (C-arm).
Standard cath pack for sterile percutaneous angiographies (syringes, towels, bowls, gauze).
Sheath introducer 8 French.
Swan-Ganz Catheter 7 French.
Capnograph.
Blood gas analyzer.
Pressure transducers.
2.3 Airway Gene Delivery
Procedure room equipped with a fluoroscopy system (C-arm).
MicroSprayer® Aerosolizer and accompanying syringes (Model IA-1B, Penn-Century, Inc.) customized for large animal experiments (in 20–40 kg Yorkshire swine, a 50 cm-length tip is optimal, but needs to be designed according to the animal species and the proximal airway to carina distance).
Multipurpose coronary diagnostic catheter shortened to fit the Sprayer (7 Fr).
Viral vector encoding reporter gene (lacZ or GFP) or the therapeutic gene of interest.
Ambu bag.
Airway filter with high filtration efficiency.
Personal protective equipment (PPE) including gloves, masks, gown, and eye protection.
2.4 Evaluation of the Transduction Efficiency Using β-Galactosidase Expression
O.C.T. Compound.
Glass slides.
Cryotome.
X-Gal staining kit.
Neutral buffered 10 % formalin solution.
Primary antibodies against reporter gene or gene of interest.
Brightfield and confocal microscope.
3 Methods
3.1 Animal Preparation and PH Model Creation
Fast the animals overnight. Administer prophylactic antibiotics prior to surgery.
Anesthesia is induced with intramuscular administration of 6.0 mg/kg Telazol (tiletamine/zolazepam). Orotracheal intubation is performed first by trained personnel, and peripheral oxygen saturation and heart rate are continuously monitored (see Note 1). A peripheral ear vein access is subsequently obtained.
For surgical procedures (surgical PH model creation), inhaled isoflurane (1–3 %) is adjusted according to animal sedation status. Analgesia after the procedure is provided using postoperative 25–50 μg/h fentanyl patch. During the procedure, oxygen saturation, heart rate, and systemic blood pressure are continuously monitored. Animals are given prophylactic antibiotics twice daily for 5 days after the thoracotomy.
Surgical creation of the PH model in swine. A left lateral thoracotomy at the fifth intercostal space is performed under sterile conditions (see Note 2). Remove the lung from surgical site by applying a wet gauze and squeeze (see Note 3). Obtain a good view of the left atrial posterior wall where pulmonary veins enter. Widen the incision if necessary.
The superior left pulmonary vein and the common inferior pulmonary vein are carefully dissected in the extrapericardial space close to the left atrium. Consistent degree of venous stenoses are achieved by placing a cotton umbilical tape around a 3.5-mm diameter plastic cylinder that is subsequently removed once the tape is tightly secured (see Note 4).
Close the chest in layers, making sure that all air is evacuated using a drainage chest tube. Furosemide 4 mg/kg is given after the procedure to prevent acute pulmonary edema [13, 14].
3.2 Functional Evaluation of PH in Large Animals: Hemodynamic and Echocardiography Assessments
Follow the same anesthesia induction as in Subheading 2.2. Use intravenous propofol 8–10 mg/kg/h for hemodynamic evaluation (see Note 5).
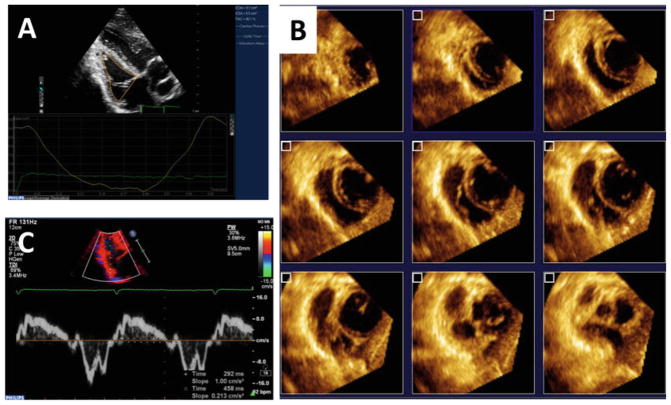
Transthoracic echocardiography can be used for right ventricle (RV) noninvasive imaging and is convenient to perform within the animal facility. Apical views of the RV can be obtained with the animal on right lateral recumbency by placing the probe in the sub-xiphoid position. Modified apical views of the RV and 3D datasets can be acquired with ECG gating (Fig. 1). Offline analyses provides quantitative information of RV dimensions and performance [13].
Under sterile conditions, a femoral vascular access is obtained using the Seldinger technique. For right heart catheterization with a 7 French Swan-Ganz catheter, an 8-Fr sheath is placed in the femoral vein (see Note 6). As an alternative, the jugular veins can also be accessed in swine but care must be taken to avoid undesired carotid artery punctures.
Positioning of the Swan-Ganz catheter to obtain right side hemodynamics is achieved using fluoroscopic guidance (C-arm). Calibrate pressure sensor carefully. This is particularly important when measuring right side pressures as they are often much lower than left side pressures. Before measurements are obtained, hemodynamic stability must be obtained and the catheter locations should be confirmed for every measurement (see Note 7).
Fig. 1.
Right ventricular noninvasive characterization using echocardiography allows evaluation of the PH model and the changes induced by therapeutic gene transfer. Two- (a) and three (b) dimensional datasets can be obtained from modified apical views in swine models, as well as the Doppler spectral signal (c ) for time intervals and Tei index
3.3 Airway Gene Delivery
Therapies should be preceded by hemodynamic measurements to obtain baseline values. Gene delivery can be performed subsequently. Connect an airway filter to the tracheal tube.
Research personell in the operating room should take appropriate precautions for vectors (see Notes 8 and 9).
The vector is prepared in the injection syringes (see Note 10).
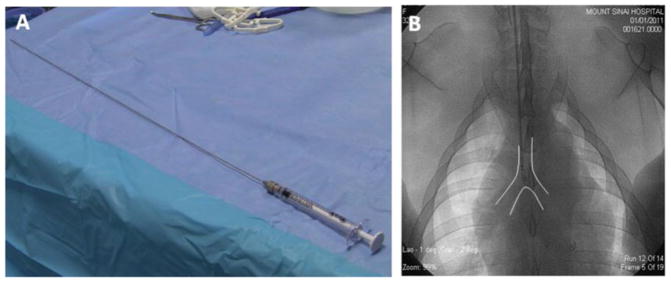
The aerosolizer device is inserted carefully through the endotracheal tube and the position of the tip determined using fluoroscopic guidance (see Note 11). By inserting the aerosolizer tip in a 7 Fr multipurpose coronary diagnostic catheter, cut to fit the Sprayer inside, the device can be advanced in the trachea while avoiding undesired damage in the tracheal mucosa. Once the tip of the aerosolizer is 2–3 cm proximal to the tracheal bifurcation, the multipurpose coronary diagnostic catheter is gently pulled back a few cm (Fig. 2).
The total vector dose is split into three equal aliquots that will be injected in the dorsal, right, and left lateral recumbent positions, allowing at least 5 min between each injection (see Note 12).
Injection of the vector solution should be coordinated to the inspiration phase. Also, pre-injection alveolar recruitment maneuvers using the Ambu will facilitate a more even and distal distribution of the vector.
After vector delivery is completed, mechanically ventilate for an additional 20–30 min with continuous monitoring of the EKG, hemodynamics, and respiratory parameters.
Once the observation period is finished without complications, the animal is recovered. The vector leak from the airway is minimal; however, keep the precaution materials on throughout the procedure.
Fig. 2.
Intratracheal gene delivery requires a MicroSprayer device (a ) that is placed near the carina guided by fluoroscopy (b)
3.4 Evaluation of the Transduction Efficiency
Humanly euthanize the animals after an appropriate time post-gene delivery to allow transgene expression. Through a median sternotomy, lung tissue from both side of the lungs at different lobes are collected. Remove blood from the tissue specimens by perfusing the vessels with PBS (see Note 13).
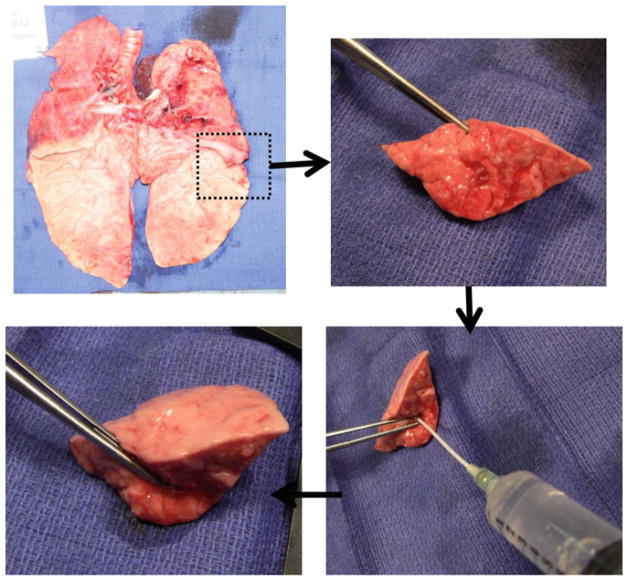
For lung tissue fixation, gently insufflate the airway with 50 % OCT in PBS, embed in labeled OCT molds and freeze the blocks. Using a cryotome, sections (8–10 μm) are prepared on glass slides for subsequent staining (see Note 14) (Fig. 3).
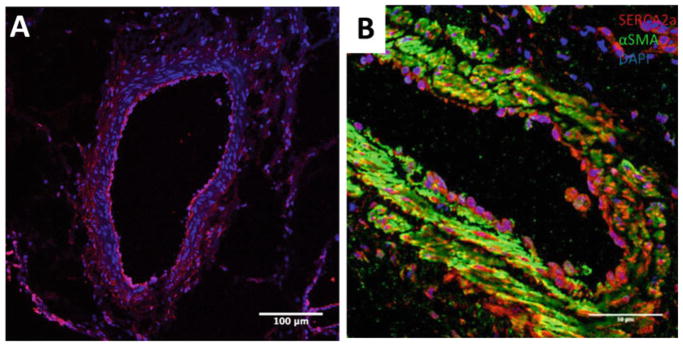
The staining technique to detect transgene expression depends on the reporter or protein of interest. For vectors with the lacZ reporter, the product of β-galactosidase activity can be detected in brightfield microscopy using the X-Gal staining kit. However, detection of β-galactosidase using specific primary antibodies is more sensitive and can be more precisely localized using confocal microscopy. For specific proteins of interest, primary antibodies that are well validated in swine tissue are needed. Colocalization with specific cell type markers such as alpha-smooth muscle actin (for smooth muscle cells) or endothelial NOS (endothelial cells) allows a more clear identification of preferential cell type of gene expression (see Note 15) (Fig. 4).
Fig. 3.
Lung tissue is inflated with OCT for easier handling and ulterior staining techniques
Fig. 4.
Assessment of transgene expression can be performed using immunofluorescent staining. Four weeks after the delivery procedure, β-galactosidase protein is found in the pulmonary vasculature (pink, a). Upon delivery of the AAV1.CMV.SERCA2a vector, overexpression of SERCA2a protein (red, b) colocalizes with smooth muscle cells (green, b)
Acknowledgments
This work is supported by NIH P50 HL112324, R01 HL119046, R01 HL117505, R01 HL128099, R01 HL129814, R01HL131404, & T32 HL007824 (R. J. H.), and a Transatlantic Leducq Foundation grant. We would like to acknowledge the Gene Therapy Resource Program (GTRP) of the National Heart, Lung, and Blood Institute, National Institutes of Health for providing some of the gene vectors used in these studies. J.A. was supported by the Fundacion Alfonso Martin-Escudero. N.H. was supported by the French Federation of Cardiology.
Footnotes
In animals with moderate to severe PH, hypoxia can significantly worsen the hemodynamics and animals can easily die from brief hypoxia. Oxygen should be supplied continuously during the preparation, and rapid intubation and immediate ventilation is necessary to prevent prolonged hypoxemia that may influence the stability of the hemodynamic evaluation.
When entering the pleural cavity, use caution not to injure the lung. Cutting the pleural membrane during the expiration will reduce the risk of accidental injury.
Lungs can be inflated using an Ambu bag after banding the pulmonary veins.
The optimal degree of vein stenosis depends of the animal growth rate. Applying too tight of a stenosis can cause subacute lung edema that develops 2–3 h after surgery. The data provided applies to female Yorkshire swine with BW 10–13 kg.
Some anesthesia drugs such as isoflurane have a strong vasodilatory effect and can mask mild PH.
Guidance of the percutaneous puncture using vascular echography minimizes the number of attempts and vascular injury, and is advisable if repeated right heart catheterization procedures to monitor hemodynamics overtime are planned.
Appropriate ventilation parameters are set depending on the investigators needs. The following parameters provide stable and reproducible conditions under general anesthesia in our experience: oxygen inspiratory fraction 40 %, 10 ml/kg tidal volume at 15 respirations per minute to maintain an end-tidal CO2 between 35 and 45 mmHg as determined by capnography. A portable blood gas analyzer provides a detailed blood gas profile that can be particularly informative in diseased animals. Cardiac output is determined by thermodilution.
When testing vectors that can affect research personnel, personal protective equipment including gloves, gowns, shoe covers, respirators, face shields and safety glasses must be worn during the viral vector delivery procedures and animal necropsies. The risk of infection by exposure to an infectious aerosol must be minimized by primary containment and multiple secondary barriers such as specialized ventilation systems, air treatment systems to decontaminate or remove agents from exhaust air, and controlled access zones.
We have previously used AAV vectors using this delivery method [12]. According to the NIH Guidelines, recombinant AAVs in which the transgene does not encode either a potentially oncogenic gene product or toxins, and are produced in the absence of a helper virus can in most cases be handled at Biosafety Level 1. Decontamination of working areas is recommended. Experiments must be performed in accordance with the Guidelines for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee for the use of AAV in these animals.
If the vector should be kept cold to maintain its activity, vector preparation should be done by a second operator after placing the Sprayer in the appropriate position.
An L-shape connector with a hole for inserting the sprayer will facilitate the procedure and reduce vector leak from the animal.
Raising the head by 20 cm will facilitate a more distal deposition of the injected solution.
Lung adhesions at the site of surgical manipulation is frequent in these animal models. This may limit the integrity of certain regions of the lung parenchyma.
Cryosectioning of the lung OCT block may be challenging especially when incomplete airway insufflation is present. As an alternative, prior fixation in 10 % formalin can be used. In formalin-fixed paraffin blocks, deparaffination and the antigen retrieval step with sodium citrate pH 6.0 may yield good immunostaining results for many of the antibodies.
Due to cellular turnover, the gene expression signal detected using different techniques may fade overtime.
References
- 1.Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS, Gomez-Sanchez MA, Jondeau G, Klepetko W, Opitz C, Peacock A, Rubin L, Zellweger M, Simonneau G. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT) Eur Heart J. 2009;30(20):2493–2537. doi: 10.1093/eurheartj/ehp297. ehp297 [pii] [DOI] [PubMed] [Google Scholar]
- 2.Lau EMT, Manes A, Celermajer DS, Galiè N. Early detection of pulmonary vascular disease in pulmonary arterial hypertension: time to move forward. Eur Heart J. 2011;32:2489. doi: 10.1093/eurheartj/ehr160. [DOI] [PubMed] [Google Scholar]
- 3.Vachiery JL, Adir Y, Barbera JA, Champion H, Coghlan JG, Cottin V, De Marco T, Galie N, Ghio S, Gibbs JS, Martinez F, Semigran M, Simonneau G, Wells A, Seeger W. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol. 2013;62(25 Suppl):D100–D108. doi: 10.1016/j.jacc.2013.10.033. S0735-1097(13)05876-2 [pii] [DOI] [PubMed] [Google Scholar]
- 4.Sutendra G, Michelakis ED. Pulmonary arterial hypertension: challenges in translational research and a vision for change. Sci Transl Med. 2013;5(208):208sr205. doi: 10.1126/scitranslmed.3005428. 5/208/208sr5 [pii] [DOI] [PubMed] [Google Scholar]
- 5.Ghofrani H-A, D’Armini AM, Grimminger F, Hoeper MM, Jansa P, Kim NH, Mayer E, Simonneau G, Wilkins MR, Fritsch A, Neuser D, Weimann G, Wang C. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013;369(4):319–329. doi: 10.1056/NEJMoa1209657. [DOI] [PubMed] [Google Scholar]
- 6.Gheorghiade M, Greene SJ, Butler J, et al. Effect of vericiguat, a soluble guanylate cyclase stimulator, on natriuretic peptide levels in patients with worsening chronic heart failure and reduced ejection fraction: the socrates-reduced randomized trial. JAMA. 2015;314(21):2251–2262. doi: 10.1001/jama.2015.15734. [DOI] [PubMed] [Google Scholar]
- 7.Reynolds PN. Gene therapy for pulmonary hypertension: prospects and challenges. Expert Opin Biol Ther. 2011;11(2):133–143. doi: 10.1517/14712598.2011.542139. [DOI] [PubMed] [Google Scholar]
- 8.Reynolds AM, Holmes MD, Danilov SM, Reynolds PN. Targeted gene delivery of BMPR2 attenuates pulmonary hypertension. Eur Respir J. 2012;39(2):329–343. doi: 10.1183/09031936.00187310. [DOI] [PubMed] [Google Scholar]
- 9.Meng L-K, Liu C-G. Gene therapies for pulmonary hypertension—from experimental trials to bedside aspects. Eur J Cardiothorac Surg. 2010;37(2):407–419. doi: 10.1016/j.ejcts.2009.06.045. [DOI] [PubMed] [Google Scholar]
- 10.Hadri L, Kratlian RG, Benard L, Maron BA, Dorfmuller P, Ladage D, Guignabert C, Ishikawa K, Aguero J, Ibanez B, Turnbull IC, Kohlbrenner E, Liang L, Zsebo K, Humbert M, Hulot JS, Kawase Y, Hajjar RJ, Leopold JA. Therapeutic efficacy of AAV1.SERCA2a in monocrotaline-induced pulmonary arterial hypertension. Circulation. 2013;128(5):512–523. doi: 10.1161/CIRCULATIONAHA.113.001585. CIRCULATIONAHA.113.001585 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Granton J, Langleben D, Kutryk MJ, Camack N, Galipeau J, Courtman D, Stewart DJ. Endothelial NO-synthase gene-enhanced progenitor cell therapy for pulmonary arterial hypertension: the PHACeT trial. Circ Res. 2015;117:645. doi: 10.1161/circresaha.114.305951. [DOI] [PubMed] [Google Scholar]
- 12.Aguero J, Ishikawa K, Hadri L, Santos-Gallego CG, Fish KM, Kohlbrenner E, Hammoudi N, Kho C, Lee A, Ibáñez B, García-Alvarez A, Zsebo K, Maron BA, Plataki M, Fuster V, Leopold JA, Hajjar RJ. Intratracheal Gene Delivery of SERCA2a Ameliorates Chronic Post-Capillary Pulmonary Hypertension: A Large Animal Model. J Am Coll Cardiol. 2016;67(17):2032–46. doi: 10.1016/j.jacc.2016.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aguero J, Ishikawa K, Hadri L, Santos-Gallego C, Fish K, Hammoudi N, Chaanine A, Torquato S, Naim C, Ibanez B, Pereda D, Garcia-Alvarez A, Fuster V, Sengupta PP, Leopold JA, Hajjar RJ. Characterization of right ventricular remodeling and failure in a chronic pulmonary hypertension model. Am J Physiol Heart Circ Physiol. 2014;307(8):H1204–H1215. doi: 10.1152/ajpheart.00246.2014. ajpheart.00246.2014 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pereda D, Garcia-Alvarez A, Sanchez-Quintana D, Nuno M, Fernandez-Friera L, Fernandez-Jimenez R, Garcia-Ruiz JM, Sandoval E, Aguero J, Castella M, Hajjar RJ, Fuster V, Ibanez B. Swine model of chronic postcapillary pulmonary hypertension with right ventricular remodeling: long-term characterization by cardiac catheterization, magnetic resonance, and pathology. J Cardiovasc Transl Res. 2014;7(5):494–506. doi: 10.1007/s12265-014-9564-6. [DOI] [PubMed] [Google Scholar]