Abstract
Chemokines are small secreted proteins with chemoattractant properties that play a key role in inflammation. One such chemokine, Stromal cell-derived factor-1 (SDF-1) also known as CXCL12, and its receptor, CXCR4, are expressed and functional in cardiac myocytes. SDF-1 both stimulates and enhances the cellular signal which attracts potentially beneficial stem cells for tissue repair within the ischemic heart. Paradoxically however, this chemokine is known to act in concert with the inflammatory cytokines of the innate immune response which contributes to cellular injury through the recruitment of inflammatory cells during ischemia. In the present study, we have demonstrated that SDF-1 has dose dependent effects on freshly isolated cardiomyocytes. Using Tunnel and caspase 3-activation assays, we have demonstrated that the treatment of isolated adult rat cardiac myocyte with SDF-1 at higher concentrations (pathological concentrations) induced apoptosis. Furthermore, ELISA data demonstrated that the treatment of isolated adult rat cardiac myocyte with SDF-1 at higher concentrations upregulated TNF-α protein expression which directly correlated with subsequent apoptosis. There was a significant reduction in SDF-1 mediated apoptosis when TNF-α expression was neutralized which suggests that SDF-1 mediated apoptosis is TNF-α-dependent. The fact that certain stimuli are capable of driving cardiomyocytes into apoptosis indicates that these cells are susceptible to clinically relevant apoptotic triggers. Our findings suggest that the elevated SDF-1 levels seen in a variety of clinical conditions, including ischemic myocardial infarction, may either directly or indirectly contribute to cardiac cell death via a TNF-α mediated pathway. This highlights the importance of this receptor/ligand in regulating the cardiomyocyte response to stress conditions.
Keywords: Chemokines, Apoptosis, Stromal derived factor-1 (SDF-1), Tumor necrosis factor (TNF)
Introduction
The CXC chemokine, SDF-1 is a potent chemotatic protein with a well-characterized role in the mobilization and homing of progenitor cells to hematopoietic tissues and works through interaction with its specific receptor ligand, CXCR4 [1]. CXCR4 is expressed on a variety of hematopoietic cells, inflammatory cells, and non-hematopoietic progenitor cell types. More recently, it has been shown by us and others that SDF-1 levels are also elevated in infarcted myocardium, areas of ischemia, and tissue injury [2–4]. Given SDF-1’s essential role in bone marrow (BM) homing and recruitment [5, 6], SDF-1 has been tested in gene therapy as a recruiter of BM-derived progenitor cells to the myocardium as a treatment for ventricular dysfunction and myocardial repair. However, to date, several human clinical trials of stem cell therapy have shown limited cardiac benefit to individuals suffering from myocardial infarction [7–9]. Our work suggests that the ectopic expression of SDF-1’s receptor, CXCR4, worsens the hemodynamic and structural parameters in a murine model of ischemic reperfusion injury; CXCR4 over expression was associated with: (1) presence of inflammatory cells; (2) presence of inflammatory mediators i.e. cytokines such as tumor necrosis factor-alpha (TNF-α) and; (3) inflammation-mediated injuries i.e., cardiomyocyte apoptosis and necrosis. Our data suggest that SDF-1-CXCR4 over activation can be toxic rather than beneficial [4]. In the present study, we hypothesize that the deleterious effects of SDF-1 on cardiac cells, including apoptosis, could be mediated by SDF-1-induced TNF-α production.
TNF-α is a proinflammatory cytokine that produces negative inotropic effects in the heart [10]. In addition to mast cells, which are a notable source of TNF-α, the heart is also a TNF-producing organ [11, 12]. According to Ferrari and Feldman et al., the normal heart does not express TNF-α, however, the failing heart produces robust quantities. Besides inducing apoptosis, TNF-α has been implicated in the modulation of cardiac contractility and peripheral resistance, the two most important hemodynamic determinants of cardiac function [13, 14]. It was assumed that TNF-α is deleterious to myocardial function in humans because it induces a negative inotropic state in patients who have not undergone heart transplants [15]. Accumulating evidence indicates that myocardial TNF-α is an autocrine contributor to myocardial dysfunction and cardiomyocyte death in ischemia–reperfusion (I/R) injury, sepsis, chronic heart failure, viral myocarditis, and cardiac allograft rejection [12]. As a matter of fact, it was postulated that high levels of TNF-α could cause severe cardiac pathologies and participate in changes such as remodeling, fibrosis, and apoptosis [16].
Cardiac dysfunction has also been associated with a systemically elevated chemokine level, both in animals and humans [17–19]. Most studies in this area have focused on chemokine expression as a prominent feature of the post-infarction inflammatory response. Such studies have investigated the role of chemokines in inflammatory leukocyte recruitment [20]. They have not, however, addressed the possibility of an autocrine/paracrine effect wherein the chemokine receptors, present on the cardiomyocyte surface, modulate functional responses to stress. Interestingly, as a strategy for repairing damaged cardiac tissue following myocardial infarction, one potentially promising approach involves the use of cell therapy [6, 21–24] to prevent or reverse heart failure [25]. Another related strategy for cardiac repair involves stem cells (SCs) mobilization with factors such as cytokines [26]. Several laboratories have shown that the cells can be mobilized and can home to areas of injury, in part by SDF-1-CXCR4 interaction [27–30].
Given SDF-1’s essential role in BM homing and recruitment [5, 6], SDF-1 has been tested in gene therapy as a recruiter of BM-derived SCs to the myocardium in a murine infarct model [7, 31, 32]. Indeed, a current clinical trial is examining the effects of injecting SDF-1 directly into the myocardium of patients with ischemic heart disease. SDF-1 acting through CXCR4 could indeed recruit BM-derived SCs to the infarcted heart, and increase homing following injury, supporting the notion that this chemokine might have therapeutic potential. However, as evidenced by the published literature, controversies exist over the protective versus apoptotic effects of SDF-1/CXCR4 axis in injury models [33–37]. Many studies have suggested that SDF-1/CXCR4 activation can exert pathological effects. Han et al., has previously demonstrated that binding of SDF-1 to its receptor induces neuronal apoptosis in vitro. The pathological roles of SDF-1 in increasing the severity of neurological impairment, is contributed to increased astrocyte cell death [38]. Moreover, it was shown that SDF-1 could induce TNF-α production, which provides a course of soluble cytotoxic factors that mediate neuronal cell death [38]. In our I/R model, CXCR4-infected hearts showed both increased TNF-α expression and elevated apoptosis post I/R [4], suggesting that myocardial cells may produce TNF-α. Since TNF-α receptors are also expressed by cardiomyocytes and TNF-α can trigger apoptosis in many cell types, it is likely that endogenous TNF-α may contribute to apoptosis in cardiac cells as well. It is then likely that the concentration of TNF-α is much higher in cardiac tissue relative to serum levels where serum levels of TNF-α are shown to be elevated in many human cardiac-related pathogenic conditions, including heart failure. Whether SDF-1/CXCR4 activation of apoptotic pathways result, in part, from TNF-α production and/or whether SDF-1 induces apoptosis independently of TNF-α needs to be clarified and will therefore be addressed in this study. Our findings suggest a concentration-dependent effect of SDF-1 on cardiomyocytes: SDF-1 at increased, pathological, concentrations induces TNF-α and subsequently cardiomyocyte death. This implication suggests an important role of the SDF-1/CXCR4 axis in regulating the expression of a proinflammatory marker in cardiomyocyte which may contribute to the pathological changes and myocardial dysfunction observed in chronic heart disease.
Materials and methods
Isolation of adult rat ventricular myocytes
Cardiomyocytes were prepared from Sprague–Dawley adult rat hearts as previously described [39]. Briefly, rat hearts were excised and the aorta was quickly cannulated. The hearts were first perfused with a low calcium Tyrode’s buffer and then with an enzyme solution containing collagenase and protease. They were minced, filtered, and suspended in Tyrode’s solution, and cultured in medium M199 containing appropriate supplements.
Apoptosis
Apoptosis was detected by using TUNEL (Terminal deoxy-nucleotidyl Transferase Biotin-dUTP Nick End Labeling) Fluorescein kit (Roche Diagnostics, Indianapolis, IN) and confirmed with the Cell Death Detection ELISAPLUS (Roche Diagnostics, Indianapolis, IN) by quantization of the cytoplasmic histone-associated DNA fragments.
ELISAPLUS
An ELISA for histone-associated DNA fragments. Briefly, the photometric enzyme immunoassay is used for the quantitative in vitro determination of cytoplasmic histone-associated DNA fragments (mono- and oligonucleosomes) after induced cell death [40]. ELISA assay was performed according to the manufacturer’s protocol (Roche Diagnostics, Indianapolis, IN).
TUNEL
In situ labeling of fragmented DNA was performed with tdt UTP nick end-labeling according to manufacturer’s protocol as previously described [4]. This technique was performed on cardiomyocytes that had been plated on laminin-coated coverslips and cultured in DMEM-F12/10% FCS in the presence or absence of the test agent. Briefly, cardiomyocytes were grown on coverslips, incubated with or without SDF-1 (at 1, 10, 100, 200, 500 ng/ml) for 24–48 h. Cells were washed and fixed with 3.7% formaldehyde. TUNEL assay was performed with the in Situ Cell Death Detection Kit (Fluorescein) from Roche, for which labeled nucleotides, incorporated in nucleotide polymers, are detected and quantified by fluorescence microscopy. Cell numbers were the mean of five representative high power fields per coverslip. Three independent experiments were performed, in each experiment, each condition was performed in triplicate (i.e., coverslips from three separate dishes were counted per treatment condition, a total of 15 fields).
Protein preparation and immunoblot analysis
Membrane and tissue homogenates were prepared as previously described [4, 39]. Briefly, cells were incubated in the presence or absence of SDF-1 and/or MAPK signaling inhibitors; e.g., the p38 (SB203580; 10 μM), for 24 h. Cells were solubilized in lysate buffer (Cell Signaling Technology, Beverly, MA), supplemented with protease inhibitor cocktail (Sigma, St. Louis, MO). The homogenates were centrifuged and protein concentrations in the supernatants were determined by the Bio-Rad Protein Assay (Bio-Rad Laboratories, Hercules, CA). Equal amounts of protein were loaded onto wells and separated by 12% SDS-PAGE and transferred to Immobilon-P transfer membranes (Millipore, Bedford, MA). The membranes were incubated in blocking buffer for 1 h and incubated with antibodies to caspase 3, BAX, P38, JNK/SAPK, phospho-P38, phospho-JNK/SAPK, GAPDH (Cell Signaling Technology, Beverly, MA), Phospho-MAPKAPK2 and MAPKAPK2 (Abcam, Cambridge, MA) overnight at 4 °C. The membranes were washed and incubated in secondary HRP conjugated antibodies, 1:2000 dilutions (Cell Signaling Technology Beverly, MA). Immunocomplexes were visualized with enhanced chemiluminescence and autoradiographs were analyzed by laser densitometry. Three independent experiments were performed; the groups represent the mean of three separate experiments.
Caspase 3 activity assay
Freshly isolated adult rat cardiomyocytes were treated with SDF-1 with or without neutralizing antibody to TNF-α for 24 h and apoptosis was quantified in the form of caspase-3 activation using the Apo-One fluorometric assay system (Promega Corporation, Madison, WI) according to the manufacturer’s protocol [41].
Cell fractionation-mitochondria isolation assay
Subcellular fractionation of cultured cells was performed using commercially available kit (Thermo Fisher Scientific, Waltham, MA) according to the manufacturer’s protocol. The mitochondria isolation kit uses a non-mechanical, reagent based method to separate intact mitochondrial from cytosolic fraction [42].
Enzyme linked immunosorbent assay (ELISA)
The amounts of TNF-α protein in the cell lysates and/or cell culture supernatant were determined by sandwich ELISA. Quantitation of the levels of immunoreactive TNF-α was performed using a kit purchased from R&D systems (Min-neapolis, MN, USA). ELISA was performed according to manufacturer’s instructions as previously described [43].
Statistical analysis
The statistical significance between experimental and control groups was determined by Student’s t test or by one-way ANOVA followed by the Bonferroni post hoc test or two-way ANOVA followed by Tukey’s test t using Prism software (Graphpad, San Diego, CA). A P value of < 0.05 was considered statistically significant (*P < 0.05, **P < 0.01, #P < 0.001).
Results
SDF-1 at high doses (≥ 300 ng/ml) induces apoptosis in cardiomyocytes
In this study, we investigate the effects of different SDF-1 concentrations on cardiomyocyte viability and survival. We hypothesize that SDF-1 is capable of driving isolated cardiomyocytes into apoptosis at high concentrations. It has been previously reported that cytokines such as TNF-α have concentration-dependent bimodal effects on cell survival. Previous studies by Poznansky et al. also suggested that SDF-1 has concentration dependent effects on T-cells in which low concentrations are chemotactic but higher concentrations have opposite effects and actually initiate the movement of T-cells away from the chemoattractant [44]. Thus, we set this study to investigate the dose dependent effects of SDF-1 in cardiomyocytes death/survival.
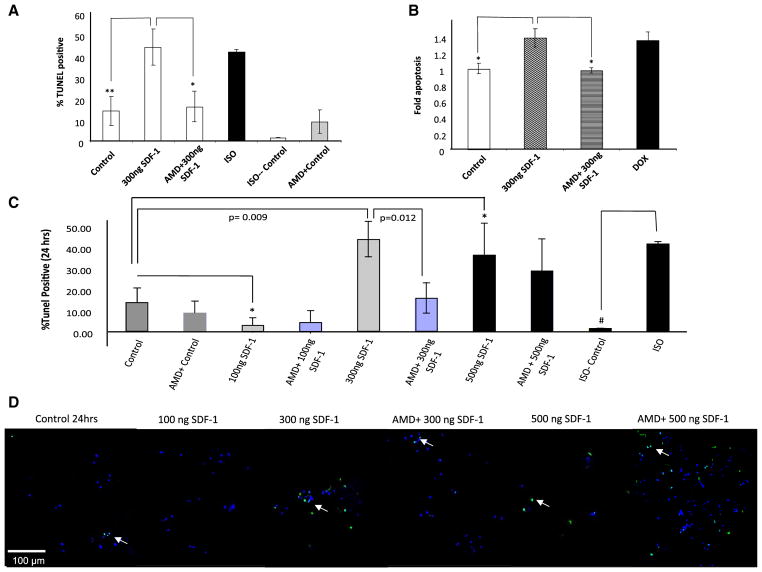
We have taken the approach of using several techniques in parallel to evaluate apoptosis in cardiomyocytes in vitro. Freshly isolated adult rat cardiomyocytes were treated for 24 h with either 300 ng/ml SDF-1, 1 μM doxorubicin (DOX), 100 μM isoproterenol (ISO) or diluent (ISO control). Both doxorubicin and isoproterenol are known to induce cardiomyocyte apoptosis thus they were used as positive controls for the assay. AMD3100, a small bicyclam molecule, is a CXCR4 antagonist, which was utilized to assess whether the observed apoptosis was induced through the binding of SDF-1 to its receptor. We pretreated cardiomyocytes with 10 mM AMD3100 for 1 h followed by a 24 h treatment with 300 ng/ml SDF-1 and 10 mM AMD3100. Apoptosis was detected by TUNEL assay (Fig. 1a) and confirmed with the Cell Death Detection ELISAPLUS kit by quantitation of cytoplasmic histone-associated DNA fragments (Fig. 1b). As shown in Fig. 1a, b, SDF-1 treatment at 300 ng/ml induces cardiomyocyte apoptosis as assessed using two independent assays: TUNEL staining (Fig. 1a) and ELISAPLUS, an ELISA for histone-associated DNA fragments (Fig. 1b). To address whether SDF-1 mediated apoptosis is concentration dependent, different concentration of SDF-1 ranging from 100 ng (physiological concentrations) to 500 ng/ml (pathological concentrations) were used to stimulate cardiomyocytes over 24 h. A double-staining technique was used i.e., TUNEL staining through use of an in situ Cell Death Detection Kit for apoptotic cell nuclei and DAPI staining for all cell nuclei. TUNEL positive cells were visualized as indicated by green fluorescence staining (Fig. 1d) and the percentage of TUNEL positive cells was determined (i.e., number of TUNEL-positive myocytes/total number of myocytes × 100) (Fig. 1c). Assays were performed in a blinded manner. Representative images show a significant increase in TUNEL positive cells (green signals) at 300 ng/ml SDF-1 (**p < 0.01 vs. control) after 24 h of treatment (Fig. 1d). AMD3100 blocks 300 ng/ml SDF-1 induced apoptosis (*p < 0.05 vs. 300 ng/ml SDF-1 + AMD3100) suggesting the observed apoptosis is CXCR4-mediated (Fig. 1a–d). With the 500 ng/ml SDF-1 treatment, many cells are already dead at this time point. Similar results were published by Colamussi et al. who showed that SDF-1 induces apoptosis in CD4+ T cell [36]. Kinetic assays demonstrate that SDF-1 at higher concentrations can induce apoptosis, which is not apparent at lower concentrations (p < 0.05 compared 100 ng/ml SDF-1 vs. control) (Fig. 1c). Indeed SDF-1 at lower concentration (100 ng/ml) seems to be protective against basal cell death observed in control group (*p < 0.05 vs. control). We should note that there is a basal rate of cell death in the cultured myocytes. We contribute these deaths in this primary cell line to the subset of myocytes that were under stress during the isolation process. Cell death in our control group is the data that represents this phenomenon.
Fig. 1.
SDF-1 concentration dependent effects on cardiomyocytes survival. a, b SDF-1 induces apoptosis in cardiomyocytes. Freshly isolated rat cardiomyocytes were treated for 24 h with either 300 ng/ml SDF-1, 1 μM DOX, 100 μM ISO or diluent (ISO control). Pre-treatment with 10 mM AMD for 1 h was followed by a 24 h treatment with 300 ng/ml SDF-1 plus 10 mM AMD. a Apoptosis was detected by using TUNEL and b confirmed with the Cell Death Detection ELISAPLUS by quantization the cytoplasmic histone-associated DNA fragments (n = 3; *p < 0.05, **p < 0.01 vs. 300 ng/ml SDF-1). c, d To assess the SDF-1 dose response curve, freshly isolated adult rat cardiomyocyte were treated for 24 h with either SDF-1 (100, 300 or 500 ng/ml) with or without pretreatment with 10 mM AMD3100, specific CXCR4 antagonist for 1 h prior to the treatment. ISO (100 μM) was used as a positive control to induced apoptosis in cardiomyocytes. Apoptosis was detected by using TUNEL assay. A double-staining technique was used i.e., TUNEL staining by using an In Situ Cell Death Detection Kit (Fluorescein) for apoptotic cell nuclei and DAPI (Blue) staining for all cell nuclei. TUNEL positive cells were visualized as indicated by green fluorescence staining and percentage (%) of TUNEL positive cells was determined (i.e., number of TUNEL-positive myocytes/total number of myocytes × 100). Assays were performed in a blinded manner. Three independent experiments were performed, in each experiment, each condition was performed in triplicate (i.e., coverslips from three separate dishes were counted per treatment condition, a total of 15 fields) (n = 15; *p < 0.05, **p < 0.01, #p < 0.001). d Selective images demonstrating TUNEL positive cells (green signal) for each group are shown
Collectively the data supports our hypothesis that SDF-1 can induce apoptosis in adult rat cardiomyocytes and that this effect is dose dependent. Since CXCR4 is constitutively expressed and the ligand is constitutively available, this might explain why SDF-1 shows its deleterious effects only when the expression of ligand is dysregulated. This rationale is consistent with the elevated SDF-1 levels seen in a variety of clinical conditions, including ischemic myocardial disorders and heart failure where the pathophysiologic processes of disease further contribute to SDF-1-induced cardiac cell death [45, 46].
SDF-1 induces caspase 3-like activation
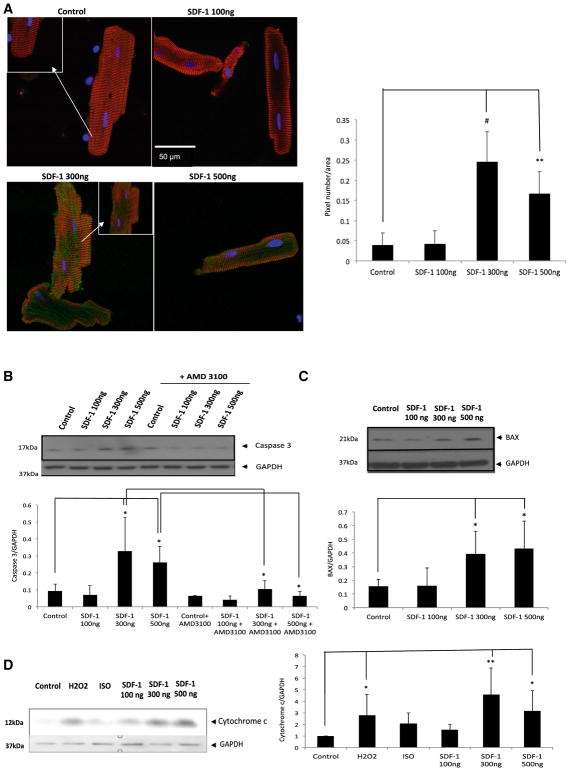
Caspases are important apoptotic signaling molecules. Activation of caspases occurs through both a mitochondria-dependent and -independent pathway. Both of these pathways activate downstream execution caspases, including caspase 3. To assess whether changes in caspase activity play a role in SDF-1-induced cell death, we determined the activation of caspase 3 using two independent assays: (1) detection of caspase 3-like protease activity (ApoAlert Caspase 3 Fluorescent Assay Kit) and (2) immunoblotting for the activated form of caspase 3 [41, 47]. Freshly isolated adult rat cardiomyocytes were treated for 24 h with either 100 or 300 or 500 ng/ml of SDF-1. Treatment with SDF-1 at higher concentrations (300 and 500 ng/ml) significantly increased caspase 3-like protease activity as compared to myocytes incubated with 100 ng/ml SDF-1 as detected by green fluorescence staining (**p < 0.01, #P < 0.001 vs. control) (Fig. 2a left panel). The amount of green fluorescence was quantified using the ImageJ program (Fig. 2a right panel). The number of green fluorescence pixels per cell was measured and normalized to its cell area. To confirm our data, we used an immunoblotting protocol in which we detected a significant increase in active caspase 3 protein (cleaved protein) expression at 300 and 500 ng/ml SDF-1 treatment (*p < 0.05 vs. control), thus implicating an increase in active caspase 3 activity (Fig. 2b). This activity decreased significantly in cultures co-treated with AMD 3100 the commercially available SDF-1 antagonist (*p < 0.05 compared 300 ng/ml SDF-1 vs. 300 ng/ml SDF-1 + AMD3100; *p < 0.05 compared 500 ng/ml SDF-1 vs. 500 ng/ml SDF-1 + AMD3100) suggesting the observed caspase 3 activation is CXCR4-mediated (Fig. 2b).
Fig. 2.
SDF-1 at high concentrations activates caspase 3 and induces cell death. Freshly isolated adult rat cardiomyocytes were treated for 24 h with either 100 or 300 or 500 ng/ml SDF-1, Apoptosis was detected by using three independent assays: a cardiomyocytes were fixed, stained with anti-caspase 3 using detection of caspase 3-like protease activity (ApoAlert Caspase 3 Fluorescent Assay Kit) and visualized with FITC conjugate (green), α-actinin antibody visualized by Cy3 conjugate (red) and nuclei were stained with DAPI (blue). Significant caspase 3 activation (green signal) was detected at ≥ 300 ng/ml. The images are representative of n = 3 experiments (left panel). The amount of green fluorescence was quantified using image j program (right panel). Each cell green fluorescence pixels was measured and normalized to its cell area. Three different experiments were chosen, in each experiment, five cells were measured; graph represents a total of 15 cells per condition (n = 15; **p < 0.01, #p < 0.001 vs. control). b Immunoblotting was performed for the activated form of caspase 3 (Asp175) (upper panel) using cardiomyocytes lysates in which cells were treated in present or absence of AMD3100 (10 mM). Pretreatment with 10 mM AMD for 1 h was followed by a 24 h treatment with 100 or 300 or 500 ng/ml SDF-1 plus 10 mM AMD. Densitometric analysis of data is shown (lower panel) (n = 3; *p < 0.05). c Immunoblotting was performed for the pro-apoptotic protein, BAX (upper panel) using cardiomyocytes lysates in which cells were treated with either 100 or 300 or 500 ng/ml SDF-1 for 24 h. Densitometric analysis of data is shown (lower panel) (n = 3; *p < 0.05). d Subcellular fractionation of cultured cells was performed using commercially available kit and cytosolic fraction was used to perform western blot analysis. Immunoblotting was performed using the cytochrome c primary antibody followed by corresponding secondary antibody. The blot was visualized using a Bio-Rad imager (left panel). Densitometric analysis of data from three different experiments is shown (right panel) (n = 3; *p < 0.05, **p < 0.01 vs. control)
In the mitochondria-dependent mechanism of caspase 3 activation, mitochondria release cytochrome c into the cytosol early in the process which mainly activates caspase 9, among others, the end result of which is the activation of caspase 3. These downstream players in turn cleave key substrates and coordinate the process of apoptotic cell death. Interestingly, BAX is a pro-apoptotic Bcl-2-family protein that resides in the cytosol, been shown to induce cytochrome c release and caspase activation in vivo and in vitro [48, 49]. We looked at BAX protein expression in our system (Fig. 2c). There is a significant increase in BAX protein expression in cardiomyocytes treated with higher concentration of SDF-1 (300–500 ng/ml) (*p < 0.05 vs. control). To detect potential cytochrome c release, cultured cardiomyocytes were treated with SDF-1 at different doses for 24 h at which point the cells were fractionated. Upon processing, the mitochondrial and the cytosolic portions of the cells were separated. The cytosolic fraction was separated on the acrylamide gel and immunoblotted for cytochrome c protein expression. Our data demonstrate a significant increase in cytochrome c protein expression in the cytosolic fraction of cardiomyocytes treated with higher concentrations of SDF-1 (300 and 500 ng/ml) (**p < 0.01, *p < 0.05 vs. control) for 24 h as compared to cells that were treated with the physiologic concentration of SDF-1 (100 ng/ml) (Fig. 2d), suggesting cytochrome c release from the mitochondria as a potential underlying mechanism. ISO and H2O2 were used as negative and positive controls (*p < 0.05 H2O2 vs. control). It is well demonstrated that H2O2-mediated apoptosis is mitochondria dependent mechanism but ISO-induced apoptosis is suggested to be through other pathways [50–52].
Inhibition of the P38 MAPK pathway did not reverse the apoptotic effect of SDF-1
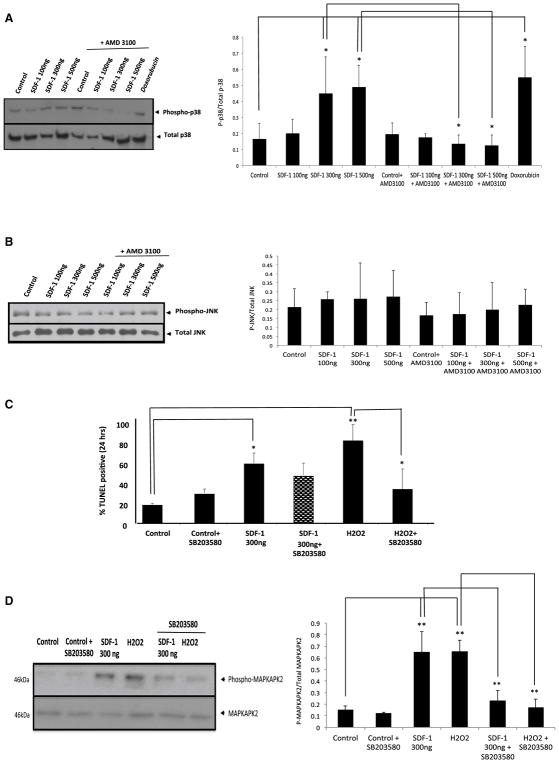
MAPK pathways are implicated in regulating cell death/survival pathway. For instance, P38 MAPK can be induced by various insults, and attenuation of p38 MAPK activation during ischemia helps protect against ischemia-mediated necrosis and apoptosis [53]. To determine whether MAPK signaling pathways participate in SDF-1 induced cell death, we examined MAPK phosphorylation by Western blot analysis. Briefly, freshly isolated adult rat cardiomyocyte were treated for 24 h with either 100, 300 or 500 ng/ml SDF-1, with or without pretreatment with 10 mM AMD3100 (a specific CXCR4 blocking compound) for 1 h. 1 μM DOX treatment was used as a positive control. Western blot analysis was performed for detection of Phospho-P38 (Fig. 3a left panel). Treatment of cardiomyocytes with SDF-1 at higher concentrations (300–500 ng/ml) resulted in significantly elevated levels of p38 phosphorylation as compared to cells exposed to SDF-1 at lower concentrations (100 ng/ml) (*p < 0.05 compared 300 ng to 500 ng/ml SDF-1 vs. control) (Fig. 3a right panel). This activity decreased significantly in cultures co-treated with AMD 3100 (*p < 0.05 compared 300 ng/ml SDF-1 vs. 300 ng/ml SDF-1 + AMD3100; *p < 0.05 compared 500 ng/ml SDF-1 vs. 500 ng/ml SDF-1 + AMD3100) suggesting that the observed increase in p38 phosphorylation is CXCR4-mediated (Fig. 3a right panel). These data suggest that activation and signaling of p38 pathway may play a role in the apoptotic effects of this chemokine. Thus, an inhibitor of the p38 MAPK (SB203580; 10 μM) was included in treated cell cultures. Cardiomyocytes were pretreated with 10 μM SB203580 for 1 h followed by a 24 h treatment with SDF-1 (300 ng/ml) or H2O2 (positive control to induce apoptosis) and apoptosis was detected by TUNEL assay as described before (Fig. 3c). Although the number of apoptotic cells in cardiomyocyte cultures co-treated with SDF-1 (300 ng/ml) and SB203580 was somewhat reduced when compared to cultures treated with SDF-1 (300 ng/ml) only, this was not statistically significant (Fig. 3c). However, p38 inhibition had significant effects on H2O2-mediated cell death (positive control) (*p < 0.05 compared to H2O2), suggesting that oxidative stress does not underlie SDF-1-mediated apoptosis.
Fig. 3.
Inhibition of the P38 MAPK pathway did not reverses the apoptotic effect of SDF-1. a, b Freshly isolated adult rat cardiomyocyte were treated for 24 h with either 100 or 200 or 500 ng/ml SDF-1, with or without pretreatment with 10 mM AMD3100 (a specific CXCR4 blocking compound) for 1 h, or 1 μM DOX (positive control). Western blot analysis was performed for detection of Phospho-P38 and Phospho-JNK/SAPK primary antibodies. Representative gels; left (phospho-p38) and right (Phospho-JNk/SAPK) panels are shown here. Densitometric analysis of data from three different experiments is shown (lower panels). c To determine the contribution of P38 MAPK on SDF-1-mediated apoptosis, inhibitor of the p38 MAPK (SB203580; 10 μM) were included in the treatment of cultures of adult rat cardiomyocytes. Cardiomyocytes were pretreated with 10 μM SB203580, p38 inhibitor, for 1 h followed by a 24 h treatment with SDF-1 (300 ng/ml) or H2O2 (positive control to induce apoptosis). Apoptosis was detected by TUNEL assay as described before. Percentage (%) of TUNEL positive cells was determined (i.e., number of TUNEL-positive myocytes/total number of myocytes × 100). Assays were performed in a blinded manner. Three independent experiments were performed, in each experiment, each condition was performed in triplicate (i.e., coverslips from three separate dishes were counted per treatment condition, a total of 15 fields) (n = 15; *p < 0.05, **p < 0.01). d Freshly isolated cardiomyocytes were treated as described before and we used immunoblotting assay for detection of Phospho-MAPKAPK2 and MAPKAPK antibodies (left panel). Densitometric analysis of data from three different experiments is shown (right panel) (n = 3; **p < 0.01 vs. control)
To assess whether 10 μM SB203580 has indeed inhibited the p38 down-stream signaling pathway, we performed Western blots for anti-MAPKAP Kinase 2 (phospho T334). When p38 is activated, it subsequently phosphorylates MAPKAP Kinase 2 (MAPKAPK2) therefore phosphorylation was monitored as an index of p38 activation [54, 55]. Cardiomyocytes were treated as described before and we used an immunoblotting assay to detect MAPKAPK2 phosphorylation (Fig. 3d left panel). Our data indicates that there is a significant increase in MAPKAPK2 phosphorylation upon treatment with 300 ng/ml SDF-1 and H2O2 (positive control) (**p < 0.01 vs. control). As anticipated, we detected a significant decrease in MAPKAPK2 phosphorylation when SB203580 pretreated cells were treated with either SDF-1 300 ng/ml or H2O2 (*p < 0.05 compared 300 ng/ml SDF-1 vs. SDF-1 + SB203580; *p < 0.05 compared H2O2 vs. H2O2 + SB203580) demonstrating that p38 MAPK inhibitor SB203580 had indeed been inhibiting the downstream pathway, however, it did not significantly reduced SDF-1 mediated apoptosis but indeed reduced H2O2-mediated cardiomyocytes apoptosis (Fig. 3d right panel).
We performed a complementary Western blot analysis for phosphorylated JNK/SAPK, another MAPK pathway that has been shown to induce cell death and is believed to be involved in mitochondria-dependent apoptosis [56, 57]. It has been suggested that JNK/SAPK plays a role in the translocation of pro-apoptotic BAX to the mitochondria and subsequently, the activation of the intrinsic cell death pathway. However, in our system, we did not see any significant change in JNK/SAPK phosphorylation upon SDF-1 treatment (Fig. 3b) thus, it is unlikely that SDF-1 mediated apoptosis is JNK/SAPK dependent.
SDF-1 induced-apoptosis is TNF-α-dependent
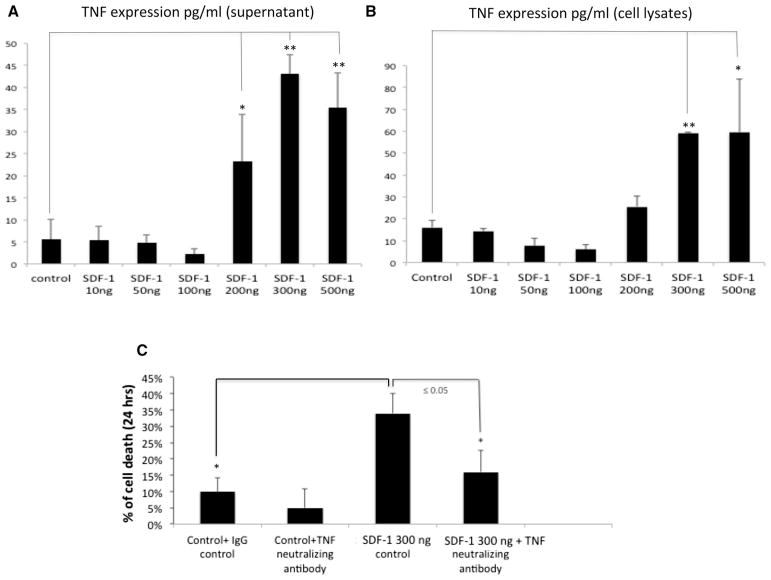
The apoptosis seen in SDF-1 at high concentrations might be the result of other, unexplained factors, exclusive of a direct mechanism. SDF-1 has been shown to stimulate TNF-α and subsequent apoptosis in astrocytes [38]. Therefore, to investigate whether SDF-1/CXCR4 activation can also affect TNF-α expression, freshly isolated adult rat cardiomyocytes were treated with SDF-1 at various doses (dose response curve) and the TNF-α protein expression was assessed in both cell culture supernatants (Fig. 4a) and cell lysates (Fig. 4b) using ELISA assay. Our data indicated that TNF-α protein is highly expressed in cardiomyocytes in response to increasing SDF-1 concentrations (≥ 300 ng/ml) (*p < 0.05, **p < 0.01 compared to control) suggesting SDF-1 regulation of TNF-α production is concentration dependent (Fig. 4a, b). To confirm whether SDF-1 mediated apoptosis is TNF-α dependent, cardiomyocytes were pretreated with neutralizing antibody against TNF-α (5 μg/ml of anti-murine TNF-α) and cell death was assessed using TUNEL at 24 h post treatment with SDF-1 (300 ng/ml). IgG isotype match was used as negative control. We choose the 300 ng/ml concentration of SDF-1 because it resulted in maximum TNF-α production based on our ELISA data (Fig. 4a, b). There was a significant reduction in SDF-1 mediated apoptosis when TNF-α expression was neutralized (*p < 0.05) suggesting that SDF-1 mediated apoptosis is partially TNF-α mediated (Fig. 4c). This is the first report demonstrating that cardiac SDF-1 can induce TNF-α from cardiomyocytes at pathological concentrations.
Fig. 4.
SDF-1 induced-TNF-α production. a, b To address whether SDF-1 induces TNF-α production, freshly isolated adult rat cardiomyocyte were treated for 24 h with either 10, 50,100, 200, 300 or 500 ng/ml SDF-1 and the TNF-α protein expression was assessed in either cell culture supernatant (a) or cell lysates (b) using ELISA assay. c To conform whether SDF-1 mediated apoptosis is TNF-α-dependent, cardiomyocytes were pretreated with neutralizing antibody against TNF-α (5 μg/ml of anti-murine TNF-α) followed by SDF-1 treatment (300 ng/ml) for 24 h. IgG isotype match was used as negative control. Apoptosis was detected by using TUNEL assay and percentage (%) of TUNEL positive cells was determined (i.e., number of TUNEL-positive myocytes/total number of myocytes × 100). Three independent experiments were performed, in each experiment, each condition was performed in triplicate (i.e., coverslips from three separate dishes were counted per treatment condition, a total of 15 fields) (n = 15; *p < 0.05)
Collectively, our findings suggest that the effect of SDF-1 on cardiomyocytes is concentration dependent: at physiological levels, SDF-1 is necessary for heart homeostasis, however at an increased pathological level, SDF-1 induces TNF-α and subsequent apoptotic events which ultimately and may contribute to the pathological changes observed in chronic heart failure.
Discussion
We set this study to investigate the dose dependent effects of SDF-1 in cardiomyocytes death and survival. We hypothesize that SDF-1 mediated TNF-α is capable of driving rat cardiomyocytes into apoptosis. The SDF-1 concentrations used in this study are based on our own previous publications as well as literature [39, 44, 58]. Previous studies by Poznansky et al. suggested that SDF-1 have concentration dependent effects on T-cells in which low concentration of SDF-1 (< 10 nM) are chemotactic but higher concentrations of SDF (> 10–100 nM) have anti-inflammatory effects i.e., initiates the movement of T-cells away from SDF-1 [44]; this demonstrated dual and paradoxical role in inflammatory regulation now has a corollary in the myocardium as demonstrated by our recent data. Cardiomyocytes express and release SDF-1 into their microenvironment which binds CXCR4 on the cell surface to elicit biological responses in the originating cell as well as the neighboring cells within the heart muscle. This autocrine/paracrine function is likely under measured given that SDF-1’s half-life in vivo is only a few minutes [59]. Furthermore, SDF-1 is cleaved by dipeptidyl-peptidase IV (CD26/DPP IV) which is present in the blood in both a soluble and membrane-bound form [59] and thus the amount of SDF-1 that is measured in patients’ plasma is not necessarily represents the actual local concentrations of SDF-1 in the heart and it is likely to be much higher [60].
We present results from in vitro studies using cultured adult rat cardiomyocytes. Our data suggest that SDF-1 has dose dependent effects: treatment of adult rat cardiomyocytes with higher concentrations of SDF-1 induces TNF-α secretion, and subsequently leads to cardiomyocyte apoptosis. Herein, these data define an important role for the SDF-1/CXCR4 axis in the regulation of a proinflammatory marker and its potential contribution to the progression of chronic heart disease. Our data suggests that chronic SDF-1 upregulation during ischemic reperfusion injury, and chronic heart failure, might actually act as an autocrine contributor to myocardial dysfunction and cardiomyocyte death. This is the first report to demonstrate the dose dependence effects of SDF-1-induced apoptosis in cultured cardiomyocytes in vitro.
The protective role for SDF-1 at lower concentration has been previously reported. Rakish et al. has demonstrated SDF-1 (100 ng/ml) significantly decreased the hypoxia-induced cardiomyocyte cell death. Others, including our group, have reported that SDF-1 at concentration of 100 ng/ml can negatively modulate β-adrenergic receptor activity and cardiomyocyte contractility [39, 58]. The relationship between survival signaling pathways and the regulation of apoptosis is rather complex. Activation of many signaling molecules such as MAPK pathways have been implicated in the survival and death of cardiomyocytes [56, 57].
We have previously shown in our in vivo model of ischemic reperfusion injury, that there is an increase in SDF-1 and CXCR4 expression. Here, our in vitro model demonstrates that SDF-1 appears to have a context-dependent response in cardiac myocytes.
Apoptosis is a major cause of myocyte cell death during ischemic reperfusion injury and chronic heart failure; SDF-1 seems to be contributing to this process through various pathways. MAPK pathways, p38 and JNK/SAPK can be induced by various insults including hypoxia and they been known to play a role in mitochondria-dependent apoptosis [56, 57, 61]. The exact mechanism(s) involved in the pro-apoptotic action of this MAPK signaling pathway is not known. However, treatment of cardiac myocytes with SDF-1 at higher concentrations (300–500 ng/ml) results in significantly elevated levels of p38 phosphorylation as compared to cells exposed to SDF-1 at lower concentrations (100 ng/ml). These data suggest that enhanced p38 phosphorylation in SDF-1 treated cardiomyocyte cultures may play a role in the apoptotic effects of this chemokine. To further refine our model, an inhibitor of p38 MAPK (SB203580; 10 μM) was included in treated cell cultures. Although the number of apoptotic cells in cardiomyocytes cultures co-treated with SDF-1 (300 ng//ml) and SB203580 was somewhat reduced when compared to cultures treated with SDF-1 (300 ng//ml) only, the result was not statistically significant. Interestingly, even though p38 inhibition did not abrogate SDF-1-mediated cell death, there were significant effects on H2O2-mediated cell death, suggesting that oxidative stress does not underlie SDF-1-mediated apoptosis. However oxidative stress has been demonstrated to enhance SDF-1 and likely CXCR4 expression and most likely potentiates and thus contributes to their deleterious effects [4].
JNK/SAPK has also been shown to be involved in the inactivation of the anti-apoptotic Bcl-2 and Bcl-xL proteins, which they may play a role in BAX translocation to the mitochondria [62]. However, we did not see significant changes in the levels of JNK/SAPK phosphorylation in SDF-1 treated cultures, thus it is less likely that JNK/SAPK is directly involved in SDF-1 mediated cell death mechanism. The indirect contributions and activation of this signaling molecule require further clarification.
Moreover, we have previously published that SDF-1 and CXCR4 expression is regulated on the cardiomyocytes [4, 63]. For example, both CXCR4 and its ligand, SDF-1, are upregulated in the heart following myocardial ischemia; this is correlated with the up-regulation of TNF-α and increase in apoptotic markers contributing to myocardial damage and dysfunction [4]. It is often assumed that TNF-mediated myocardial damage is secondary to TNF-α-mediated inflammation. Since cardiomyocytes constitutively express SDF-1 and its receptor CXCR4, next we investigated the following questions: (1) how TNF-α production is regulated by SDF-1; (2) whether SDF-1-mediated TNF-α expression affected cell death; and (3) whether TNF-α is secreted from cardiomyocytes within the heart. Our data suggests that SDF-1 can regulate TNF-α expression within cardiomyocytes. Moreover, we saw a significant reduction in SDF-1 mediated apoptosis when TNF-α expression was neutralized suggesting that SDF-1 mediated apoptosis is in part TNF-α mediated. Collectively, we have demonstrated a potential role for the chemokine, SDF-1, in regulating myocardial TNF-α-induced apoptosis. This data will shed light on some of the pressing controversies that exist over the protective and apoptotic effects of SDF-1-CXCR4 in injury models [33–37].
Our results reveal a novel signaling mechanism of SDF-1/CXCR4 axis and its concentration-dependent regulation of cardiac myocyte cell death. To our knowledge, there has not been any study evaluating the biphasic effects of SDF-1 on cardiac myocytes. These findings suggest that the elevated SDF-1 levels seen in a variety of clinical conditions, including ischemic myocardial disorders and heart failure may contribute to TNF-α-induced cardiac cell death. This research will help develop novel targeted therapies that can be safely downregulate TNF-α in the context of chronic heart failure. In addition, our research may shed light on why numerous clinical trials of anti-TNF-α therapies have failed to show any therapeutic benefit. Since myocardial TNF-α production and its potential contribution to myocardial damage have been overlooked, SDF-1-induced myocardial TNF-α may ultimately contribute to changes observed in chronic heart failure including myocardial dysfunction and cardiomyocyte death. This highlights the importance of this receptor/ligand in regulating cardiomyocytes apoptosis and could potentially aid in the understanding and treatment of a wide variety of cardiac disorders.
Acknowledgments
This work was supported, in part by, (1) American Heart Association (GRNT4180006) and (2) National Institute of Minority Health and Health Disparities of the National Institutes of Health (G12MD007597). We would like to acknowledge the Howard University RCMI (Research Centers in Minority Institutions) and the HU-Advance-it society for providing helpful programs to address minority health and health disparities.
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, Nagler A, Ben-Hur H, Many A, Shultz L, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 2.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 3.Mieno S, Ramlawi B, Boodhwani M, Clements RT, Minamimura K, Maki T, Xu SH, Bianchi C, Li J, Sellke FW. Role of stromal-derived factor-1alpha in the induction of circulating CD34 + CXCR4 + progenitor cells after cardiac surgery. Circulation. 2006;114:I186–I192. doi: 10.1161/circulationaha.105.001610. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Chemaly E, Liang L, Kho C, Lee A, Park J, Altman P, Schecter AD, Hajjar RJ, Tarzami ST. Effects of CXCR4 gene transfer on cardiac function after ischemia-reperfusion injury. Am J Pathol. 2010;176:1705–1715. doi: 10.2353/ajpath.2010.090451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jo DY, Rafii S, Hamada T, Moore MA. Chemotaxis of primitive hematopoietic cells in response to stromal cell-derived factor-1. J Clin Invest. 2000;105:101–111. doi: 10.1172/JCI7954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes S. Cardiac stem cells. J Pathol. 2002;197:468–478. doi: 10.1002/path.1159. [DOI] [PubMed] [Google Scholar]
- 7.Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1α plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110:3300–3305. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- 8.Zhang G, Nakamura Y, Wang X, Hu Q, Suggs LJ, Zhang J. Controlled release of stromal cell-derived factor-1 alpha in situ increases c-kit + cell homing to the infarcted heart. Tissue Eng. 2007;13:2063–2071. doi: 10.1089/ten.2006.0013. [DOI] [PubMed] [Google Scholar]
- 9.Elmadbouh I, Haider H, Jiang S, Idris NM, Lu G, Ashraf M. Ex vivo delivered stromal cell-derived factor-1α promotes stem cell homing and induces angiomyogenesis in the infarcted myocardium. J Mol Cell Cardiol. 2007;42:792–803. doi: 10.1016/j.yjmcc.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torre-Amione G, Kapadia S, Lee J, Durand JB, Bies RD, Young JB, Mann DL. Tumor necrosis factor-alpha and tumor necrosis factor receptors in the failing human heart. Circulation. 1996;93:704–711. doi: 10.1161/01.cir.93.4.704. [DOI] [PubMed] [Google Scholar]
- 11.Gordon JR, Galli SJ. Mast cells as a source of both pre-formed and immunologically inducible TNF-alpha/cachectin. Nature. 1990;346:274–276. doi: 10.1038/346274a0. [DOI] [PubMed] [Google Scholar]
- 12.Meldrum DR. Tumor necrosis factor in the heart. Am J Physiol. 1998;274:R577–R595. doi: 10.1152/ajpregu.1998.274.3.R577. [DOI] [PubMed] [Google Scholar]
- 13.Ferrari R. The role of TNF in cardiovascular disease. Pharmacol Res. 1999;40:97–105. doi: 10.1006/phrs.1998.0463. [DOI] [PubMed] [Google Scholar]
- 14.Feldman AM, Combes A, Wagner D, Kadakomi T, Kubota T, Li YY, McTiernan C. The role of tumor necrosis factor in the pathophysiology of heart failure. J Am Coll Cardiol. 2000;35:537–544. doi: 10.1016/s0735-1097(99)00600-2. [DOI] [PubMed] [Google Scholar]
- 15.Garza EHH, Garza JLH, Gonzalez HR, Trevino AT, Flores MI, Amione GT. Importance of tumor necrosis factor-alpha in the pathogenesis of heart failure. Rev Esp Cardiol. 2002;55:61–66. [PubMed] [Google Scholar]
- 16.Bryant D, Becker L, Richardson J, Shelton J, Franco F, Peshock R, Thompson M, Giroir B. Cardiac failure in transgenic mice with myocardial expression of tumor necrosis factor-alpha. Circulation. 1998;97:1375–1381. doi: 10.1161/01.cir.97.14.1375. [DOI] [PubMed] [Google Scholar]
- 17.Seino Y, Ikeda U, Sekiguchi H, Morita M, Konishi K, Kasahara T, Shimada K. Expression of leukocyte chemotactic cytokines in myocardial tissue. Cytokine. 1995;7:301–304. doi: 10.1006/cyto.1995.0037. [DOI] [PubMed] [Google Scholar]
- 18.Shioi T, Matsumori A, Kihara Y, Inoko M, Ono K, Iwanaga Y, Yamada T, Iwasaki A, Matsushima K, Sasayama S. Increased expression of interleukin-1 beta and monocyte chemotactic and activating factor/monocyte chemoattractant protein-1 in the hypertrophied and failing heart with pressure overload. Circ Res. 1997;81:664–671. doi: 10.1161/01.res.81.5.664. [DOI] [PubMed] [Google Scholar]
- 19.Behr TM, Wang X, Aiyar N, Coatney RW, Li X, Koster P, Angermann CE, Ohlstein E, Feuerstein GZ, Winaver J. Monocyte chemoattractant protein-1 is upregulated in rats with volume-overload congestive heart failure. Circulation. 2000;102:1315–1322. doi: 10.1161/01.cir.102.11.1315. [DOI] [PubMed] [Google Scholar]
- 20.Frangogiannis NG, Entman ML. Targeting the chemokines in myocardial inflammation. Circulation. 2004;110:1341–1342. doi: 10.1161/01.CIR.0000141560.18364.63. [DOI] [PubMed] [Google Scholar]
- 21.Limbourg FP, Ringes-Lichtenberg S, Schaefer A, Jacoby C, Mehraein Y, Jager MD, Limbourg A, Fuchs M, Klein G, Ballmaier M, et al. Haematopoietic stem cells improve cardiac function after infarction without permanent cardiac engraftment. Eur J Heart Fail. 2005;7:722–729. doi: 10.1016/j.ejheart.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 23.Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 24.Britten MB, Abolmaali ND, Assmus B, Lehmann R, Honold J, Schmitt J, Vogl TJ, Martin H, Schachinger V, Dimmeler S, et al. Infarct remodeling after intracoronary progenitor cell treatment in patients with acute myocardial infarction (TOPCARE-AMI): mechanistic insights from serial contrast-enhanced magnetic resonance imaging. Circulation. 2003;108:2212–2218. doi: 10.1161/01.CIR.0000095788.78169.AF. [DOI] [PubMed] [Google Scholar]
- 25.Laflamme MA, Zbinden S, Epstein SE, Murry CE. Cell-based therapy for myocardial ischemia and infarction: pathophysiological mechanisms. Annu Rev Pathol. 2007;2:307–339. doi: 10.1146/annurev.pathol.2.010506.092038. [DOI] [PubMed] [Google Scholar]
- 26.Kang HJ, Kim HS, Zhang SY, Park KW, Cho HJ, Koo BK, Kim YJ, Soo Lee D, Sohn DW, Han KS, et al. Effects of intracoronary infusion of peripheral blood stem-cells mobilised with granulocyte-colony stimulating factor on left ventricular systolic function and restenosis after coronary stenting in myocardial infarction: the MAGIC cell randomised clinical trial. Lancet. 2004;363:751–756. doi: 10.1016/S0140-6736(04)15689-4. [DOI] [PubMed] [Google Scholar]
- 27.Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, Ponomaryov T, Taichman RS, Arenzana-Seisdedos F, Fujii N, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 28.Broxmeyer HE. Chemokines in hematopoiesis. Curr Opin Hematol. 2008;15:49–58. doi: 10.1097/MOH.0b013e3282f29012. [DOI] [PubMed] [Google Scholar]
- 29.Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci USA. 1998;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 31.Moore MA, Hattori K, Heissig B, Shieh JH, Dias S, Crystal RG, Rafii S. Mobilization of endothelial and hematopoietic stem and progenitor cells by adenovector-mediated elevation of serum levels of SDF-1, VEGF, and angiopoietin-1. Ann N Y Acad Sci. 2001;938:36–45. doi: 10.1111/j.1749-6632.2001.tb03572.x. (discussion 45–37) [DOI] [PubMed] [Google Scholar]
- 32.Hiasa K, Ishibashi M, Ohtani K, Inoue S, Zhao Q, Kitamoto S, Sata M, Ichiki T, Takeshita A, Egashira K. Gene transfer of stromal cell-derived factor-1alpha enhances ischemic vasculogenesis and angiogenesis via vascular endothelial growth factor/endothelial nitric oxide synthase-related pathway: nextgeneration chemokine therapy for therapeutic neovascularization. Circulation. 2004;109:2454–2461. doi: 10.1161/01.CIR.0000128213.96779.61. [DOI] [PubMed] [Google Scholar]
- 33.Khan MZ, Brandimarti R, Patel JP, Huynh N, Wang J, Huang Z, Fatatis A, Meucci O. Apoptotic and antiapoptotic effects of CXCR4: is it a matter of intrinsic efficacy? Implications for HIV neuropathogenesis. AIDS Res Hum Retroviruses. 2004;20:1063–1071. doi: 10.1089/aid.2004.20.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu X, Dai S, Wu WJ, Tan W, Zhu X, Mu J, Guo Y, Bolli R, Rokosh G. Stromal cell derived factor-1 alpha confers protection against myocardial ischemia/reperfusion injury: role of the cardiac stromal cell derived factor-1 alpha CXCR4 axis. Circulation. 2007;116:654–663. doi: 10.1161/CIRCULATIONAHA.106.672451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Penn MS, Zhang M, Deglurkar I, Topol EJ. Role of stem cell homing in myocardial regeneration. Int J Cardiol. 2004;95(Suppl 1):S23–S25. doi: 10.1016/s0167-5273(04)90007-1. [DOI] [PubMed] [Google Scholar]
- 36.Colamussi ML, Secchiero P, Zella D, Curreli S, Mirandola P, Capitani S, Zauli G. Stromal derived factor-1 alpha induces apoptosis in activated primary CD4+ T cells. Aids. 2000;14:748–750. doi: 10.1097/00002030-200004140-00017. [DOI] [PubMed] [Google Scholar]
- 37.Ilhan A, Nabokikh A, Maj M, Vidakovic M, Nielsen JH, Prikoszovich T, Niederle B, Base W, Luger A, Wagner L. CXCL12/SDF-1 over-expression in human insulinomas and its biological relevance. Mol Cell Endocrinol. 2009;298:1–10. doi: 10.1016/j.mce.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 38.Han Y, He T, Huang DR, Pardo CA, Ransohoff RM. TNF-alpha mediates SDF-1 alpha-induced NF-kappa B activation and cytotoxic effects in primary astrocytes. J Clin Invest. 2001;108:425–435. doi: 10.1172/JCI12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.LaRocca TJ, Schwarzkopf M, Altman P, Zhang S, Gupta A, Gomes I, Alvin Z, Champion HC, Haddad G, Hajjar RJ, et al. β2-Adrenergic receptor signaling in the cardiac myocyte is modulated by interactions with CXCR4. J Cardiovasc Pharmacol. 2010;56:548–559. doi: 10.1097/FJC.0b013e3181f713fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang C, Soori M, Miles FL, Sikes RA, Carson DD, Chung LW, Farach-Carson MC. Paracrine factors produced by bone marrow stromal cells induce apoptosis and neuroendocrine differentiation in prostate cancer cells. Prostate. 2011;71:157–167. doi: 10.1002/pros.21231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharlow ER, Leimgruber S, Murray S, Lira A, Sciotti RJ, Hickman M, Hudson T, Leed S, Caridha D, Barrios AM, et al. Auranofin is an apoptosis-simulating agent with in vitro and in vivo anti-leishmanial activity. ACS Chem Biol. 2014;9:663–672. doi: 10.1021/cb400800q. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tarzami ST, Calderon TM, Deguzman A, Lopez L, Kitsis RN, Berman JW. MCP-1/CCL2 protects cardiac myocytes from hypoxia-induced apoptosis by a G(alphai)-independent pathway. Biochem Biophys Res Commun. 2005;335:1008–1016. doi: 10.1016/j.bbrc.2005.07.168. [DOI] [PubMed] [Google Scholar]
- 44.Poznansky MC, Olszak IT, Foxall R, Evans RH, Luster AD, Scadden DT. Active movement of T cells away from a chemokine. Nat Med. 2000;6:543–548. doi: 10.1038/75022. [DOI] [PubMed] [Google Scholar]
- 45.Damas JK, Eiken HG, Oie E, Bjerkeli V, Yndestad A, Ueland T, Tonnessen T, Geiran OR, Aass H, Simonsen S, et al. Myocardial expression of CC- and CXC-chemokines and their receptors in human end-stage heart failure. Cardiovasc Res. 2000;47:778–787. doi: 10.1016/s0008-6363(00)00142-5. [DOI] [PubMed] [Google Scholar]
- 46.Damas JK, Gullestad L, Ueland T, Solum NO, Simonsen S, Froland SS, Aukrust P. CXC-chemokines, a new group of cytokines in congestive heart failure—possible role of platelets and monocytes. Cardiovasc Res. 2000;45:428–436. doi: 10.1016/s0008-6363(99)00262-x. [DOI] [PubMed] [Google Scholar]
- 47.Rossiter JP, Anderson LL, Yang F, Cole GM. Caspase-3 activation and caspase-like proteolytic activity in human perinatal hypoxic-ischemic brain injury. Acta Neuropathol. 2002;103:66–73. doi: 10.1007/s004010100432. [DOI] [PubMed] [Google Scholar]
- 48.Jurgensmeier JM, Xie Z, Deveraux Q, Ellerby L, Bredesen D, Reed JC. Bax directly induces release of cytochrome c from isolated mitochondria. Proc Natl Acad Sci USA. 1998;95:4997–5002. doi: 10.1073/pnas.95.9.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Finucane DM, Bossy-Wetzel E, Waterhouse NJ, Cotter TG, Green DR. Bax-induced caspase activation and apoptosis via cytochrome c release from mitochondria is inhibitable by Bcl-xL. J Biol Chem. 1999;274:2225–2233. doi: 10.1074/jbc.274.4.2225. [DOI] [PubMed] [Google Scholar]
- 50.Chen P, Hu YF, Wang L, Xiao WF, Bao XY, Pan C, Yi HS, Chen XY, Pan MH, Lu C. Mitochondrial apoptotic pathway is activated by H2O2-mediated oxidative stress in BmN-SWU1 cells from Bombyx mori ovary. PLoS ONE. 2015;10:e0134694. doi: 10.1371/journal.pone.0134694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saito S, Hiroi Y, Zou Y, Aikawa R, Toko H, Shibasaki F, Yazaki Y, Nagai R, Komuro I. Beta-adrenergic pathway induces apoptosis through calcineurin activation in cardiac myocytes. J Biol Chem. 2000;275:34528–34533. doi: 10.1074/jbc.M002844200. [DOI] [PubMed] [Google Scholar]
- 52.Shin SY, Kim T, Lee HS, Kang JH, Lee JY, Cho KH, Kim DH. The switching role of beta-adrenergic receptor signalling in cell survival or death decision of cardiomyocytes. Nat Commun. 2014;5:5777. doi: 10.1038/ncomms6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steenbergen C. The role of p38 mitogen-activated protein kinase in myocardial ischemia/reperfusion injury; relationship to ischemic preconditioning. Basic Res Cardiol. 2002;97:276–285. doi: 10.1007/s00395-002-0364-9. [DOI] [PubMed] [Google Scholar]
- 54.Streicher JM, Ren S, Herschman H, Wang Y. MAPK-activated protein kinase-2 in cardiac hypertrophy and cyclooxygenase-2 regulation in heart. Circ Res. 2010;106:1434–1443. doi: 10.1161/CIRCRESAHA.109.213199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marber MS, Rose B, Wang Y. The p38 mitogen-activated protein kinase pathway—a potential target for intervention in infarction, hypertrophy, and heart failure. J Mol Cell Cardiol. 2011;51:485–490. doi: 10.1016/j.yjmcc.2010.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tournier C, Hess P, Yang DD, Xu J, Turner TK, Nimnual A, Bar-Sagi D, Jones SN, Flavell RA, Davis RJ. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science. 2000;288:870–874. doi: 10.1126/science.288.5467.870. [DOI] [PubMed] [Google Scholar]
- 57.Kunz M, Ibrahim S, Koczan D, Thiesen HJ, Kohler HJ, Acker T, Plate KH, Ludwig S, Rapp UR, Brocker EB, et al. Activation of c-Jun NH2-terminal kinase/stress-activated protein kinase (JNK/SAPK) is critical for hypoxia-induced apoptosis of human malignant melanoma. Cell Growth Differ. 2001;12:137–145. [PubMed] [Google Scholar]
- 58.Pyo RT, Sui J, Dhume A, Palomeque J, Blaxall BC, Diaz G, Tunstead J, Logothetis DE, Hajjar RJ, Schecter AD. CXCR4 modulates contractility in adult cardiac myocytes. J Mol Cell Cardiol. 2006;41:834–844. doi: 10.1016/j.yjmcc.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Proost P, Struyf S, Schols D, Durinx C, Wuyts A, Lenaerts JP, De Clercq E, De Meester I, Van Damme J. Processing by CD26/dipeptidyl-peptidase IV reduces the chemotactic and anti-HIV-1 activity of stromal-cell-derived factor-1alpha. FEBS Lett. 1998;432:73–76. doi: 10.1016/s0014-5793(98)00830-8. [DOI] [PubMed] [Google Scholar]
- 60.Abi-Younes S, Sauty A, Mach F, Sukhova GK, Libby P, Luster AD. The stromal cell-derived factor-1 chemokine is a potent platelet agonist highly expressed in atherosclerotic plaques. Circ Res. 2000;86:131–138. doi: 10.1161/01.res.86.2.131. [DOI] [PubMed] [Google Scholar]
- 61.Liu J, Wu N, Ma LN, Zhong JT, Liu G, Zheng LH, Lin XK. p38 MAPK signaling mediates mitochondrial apoptosis in cancer cells induced by oleanolic acid. Asian Pac J Cancer Prev. 2014;15:4519–4525. doi: 10.7314/apjcp.2014.15.11.4519. [DOI] [PubMed] [Google Scholar]
- 62.Kharbanda S, Saxena S, Yoshida K, Pandey P, Kaneki M, Wang Q, Cheng K, Chen YN, Campbell A, Sudha T, et al. Translocation of SAPK/JNK to mitochondria and interaction with Bcl-x(L) in response to DNA damage. J Biol Chem. 2000;275:322–327. doi: 10.1074/jbc.275.1.322. [DOI] [PubMed] [Google Scholar]
- 63.Larocca TJ, Jeong D, Kohlbrenner E, Lee A, Chen J, Hajjar RJ, Tarzami ST. CXCR4 gene transfer prevents pressure overload induced heart failure. J Mol Cell Cardiol. 2012;53:223–232. doi: 10.1016/j.yjmcc.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]