Abstract
BACKGROUND
The current U.S. priority ranking for heart candidates is based on treatment intensity, not objective markers of severity of illness. This system may encourage centers to overtreat candidates.
OBJECTIVES
This study sought to describe national variation in the intensity of treatment of adult heart transplantation candidates and identify center-level predictors of potential overtreatment.
METHODS
The registrations of all U.S. adult heart transplantation candidates from 2010 to 2015 were collected from the SRTR (Scientific Registry of Transplant Recipients). “Potential overtreatment” was defined as treatment of a candidate who did not meet American Heart Association cardiogenic shock criteria with either high-dose inotropes or an intra-aortic balloon pump. Multilevel logistic regression and propensity score models were used to adjust for candidate variability at each center. Center-level variables associated with potential overtreatment were identified.
RESULTS
From 2010 to 2015, 108 centers listed 12,762 adult candidates who were not in cardiogenic shock for heart transplantation. Of these, 1,471 (11.6%) were potentially overtreated with high-dose inotropes or intra-aortic balloon pumps. In the bottom quartile of centers, only 2.1% of candidates were potentially overtreated compared with 27.6% at top quartile centers, an interquartile difference of 25.5% (95% confidence interval: 21% to 30%). Adjusting for candidate differences did not significantly alter the interquartile difference. Local competition with 2 or more centers increased the odds of potential overtreatment by 50% (adjusted odds ratio: 1.50; 95% confidence interval: 1.07 to 2.11).
CONCLUSIONS
There is wide variation in the treatment practices of adult heart transplantation centers. Competition for transplantable donor hearts is associated with the potential overtreatment of hemodynamically stable candidates. Overtreatment may compromise the fair and efficient allocation of scarce deceased donor hearts.
Keywords: ethics, heart transplantation, organ allocation
Heart transplantation is a definitive, life-saving treatment for end-stage heart failure, a devastating disease that kills >250,000 Americans each year (1). Unfortunately, the supply of deceased donor hearts cannot meet the demand for transplantation. In the United States, more than one-third of candidates will die or be delisted without transplant (2,3). Under the current allocation system, candidates are given priority for scarce deceased donor hearts based on “status,” a 3-tiered ranking system intended to prioritize medically urgent candidates (4,5). Status is determined by treatment intensity, based on the premise that candidates nearer death will require escalation of life-sustaining therapies.
There were expected to be relatively few patients waiting at the highest priority “Status 1A,” as these candidates who are receiving the most intense forms of cardiac life-support therapy should have short life expectancies without heart transplantation (4). However, the proportion of Status 1A candidates has doubled in the past 10 years and now >40% of candidates wait at this highest priority designation, decreasing the likelihood that lower priority candidates are allocated a donor heart (2). Because status is based on therapy and not objective markers of illness, it has been suggested that this trend could be explained in part by transplantation centers “gaming the waitlist” by overtreating less urgent candidates with medically unnecessary therapy to elevate their statuses to the level needed to receive a transplant (6–8). In response to these concerns, the Organ Procurement and Transplant Network (OPTN) has proposed a new requirement that high-priority candidates meet American Heart Association (AHA) hemodynamic criteria for cardiogenic shock, “rather than qualifying [for status] based on the presence of the therapy alone” (9,10). With these criteria, the OPTN is specifically targeting the use of intense Status 1A qualifying therapy on candidates who do not physiologically require it. Recently, it has been shown that many candidates listed at Status 1A with high-dose inotropes and intra-aortic balloon pumps (IABP) are not in cardiogenic shock by AHA criteria and therefore are potentially overtreated (11,12).
However, heart transplantation centers may have legitimate nonhemodynamic reasons to treat candidates with intensive support therapies, such as severely impaired functional status or impending end-organ damage from heart failure. Analysis of the geographic variation in the treatment practices of heart transplantation centers provides an opportunity to explore this potentially inappropriate practice more fully. Intercenter variation in transplantation practices could be explained by geographic variation in candidate characteristics, which would exonerate centers that use this practice frequently. Donor hearts are allocated first to local Status 1A candidates in the same geographic region (through an organ procurement organization [OPO]) as the donor (13). Therefore, some of the variation in transplantation center listing practices may be explainable by differences in competitive forces that transplantation centers experience, such as the number of competing centers in an OPO.
This study uses a complete national registry to describe the center-level variation in the treatment of adult heart transplantation candidates, accounting for differences in candidate characteristics with risk standardization and propensity score methods. Secondarily, this study identifies the center-level predictors of potential overtreatment of heart transplantation candidates.
METHODS
DATA SOURCE AND STUDY PERIOD
This study used data from the SRTR (Scientific Registry of Transplant Recipients). The SRTR data system includes data on all donors, wait-listed candidates, and transplant recipients in the United States, submitted by the members of the OPTN. The Health Resources and Services Administration, U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. Initial registration data of all U.S. adult heart transplantation candidates added to the waitlist between January 1, 2010, and December 31, 2015, the same time period as of this work, quantified the impact of the shock requirement on the contemporary U.S. heart candidate population (11). Initial registration is a standard reference point in the care of advanced heart failure patients when complete candidate information is collected.
STUDY POPULATION AND OUTCOME VARIABLE: CANDIDATES AT RISK FOR POTENTIAL OVERTREATMENT
Hemodynamics on heart transplantation candidates are required to be recorded at the time of initial listing and note what supportive therapy the candidate was receiving at the time of measurement. Potentially overtreated candidates were defined as those candidates who were listed as Status 1A via treatment with high-dose inotropes with invasive hemodynamic monitoring or IABP in an intensive care setting, despite not meeting the hemodynamic requirements for cardiogenic shock. Cardiogenic shock was defined using the methodology published by the OPTN, which is based on AHA guidelines and minimum inotrope dose requirements (Figure 1, Online Table 1) (9–11). The study population was all heart transplantation candidates who were not in cardiogenic shock and therefore at risk for potential overtreatment. Candidates in cardiogenic shock were excluded from the principal analysis, as these candidates were not at risk for potential overtreatment. Similarly, candidates not subject to the shock requirement, specifically those with surgically placed mechanical circulatory support devices or a board-reviewed Status 1A exception, were excluded. Finally, candidates listed as Status 1A with veno-arterial extracorpeal membrane oxygenation, percutaneous ventricular assist devices, or who had missing hemodynamic data were conservatively considered to be in cardiogenic shock and excluded (Figure 2).
FIGURE 1. Visual Summary of the New OPTN/UNOS Shock Criteria for Adult Heart Transplant Candidates.
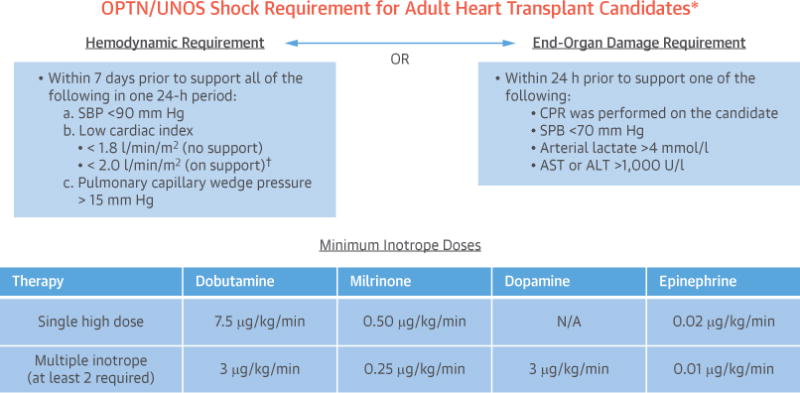
Under the new adult heart allocation system, candidates listed with veno-arterial extracorporeal membrane oxygenation, percutaneous ventricular assist devices, intra-aortic balloon pump, and high-dose inotropes will be subject to cardiogenic shock criteria (based on American Heart Association definitions) to qualify for high-priority listing. The criteria include either meeting hemodynamic requirements and minimum inotrope doses (left) or having evidence of poor end-organ perfusion (right). *Candidates supported with surgically placed mechanical circulatory support devices (such as continuous-flow left ventricular assist devices) are exempt from the requirement. †Candidates supported with high-dose inotropes are allowed to have a cardiac index up to 2.2 l/min/m2 while in support. ALT = alanine transaminase; AST = aspartate transaminase; CPR = cardiopulmonary resuscitation; N/A = not available; OPTN = Organ Procurement and Transplant Network; SBP = systolic blood pressure; UNOS = United Network for Organ Sharing.
FIGURE 2. Flow Diagram Identifying Study Population From SRTR Dataset.
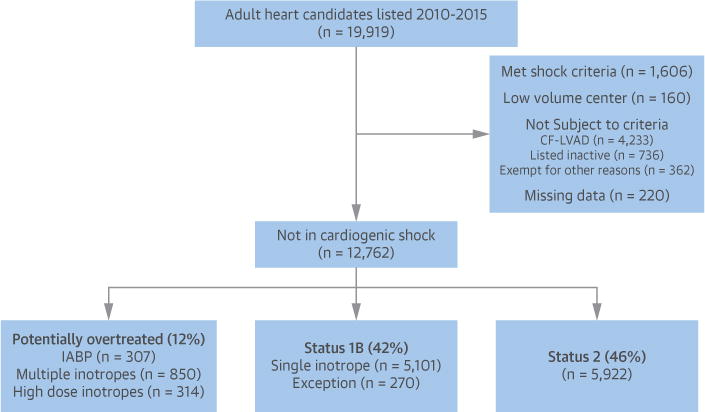
Flow diagram constructed according to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines for observational studies. IABP = intra-aortic balloon pump; CF-LVAD = continuous-flow left ventricular assist device; SRTR = Scientific Registry of Transplant Recipients. Other reasons for exclusion include Status 1A exception or support with veno-arterial extracorpeal membrane oxygenation or percutaneous ventricular assist devices.
For candidates in the study population, all available nonhemodynamic data from the transplantation candidate registration was collected. Medical data included age, weight, height, sex, body mass index, cardiac diagnosis, blood type, renal function, history of diabetes, cerebrovascular disease, malignancy, cardiac surgery, smoking, and defibrillator placement. Functional status was recorded on the Karnofsky 11-point performance status scale, which has been validated in heart failure patients (14,15). Socioeconomic variables included citizenship, race, insurance type, education, and work history.
CENTER- AND OPO-LEVEL VARIABLES
For active heart transplantation centers during the study period, the yearly listing volume, transplantation volume, time to transplantation, and listing practices (initial status and therapy support) were calculated. To measure the local competition for transplantable hearts in each OPO area, the number of candidates listed, transplantations performed, supply of donor hearts, proportion of transplantations performed using local hearts, recipient status, and number of centers were calculated. The transplantation market share of each center and the OPO Herfindahl–Hirschman index (16) were also calculated. All variables were calculated individually for each candidate using data from the prior year’s listing to account for variation over time. Centers with very low listing volume, defined as <5 adult listings per year, were excluded.
STATISTICAL ANALYSIS
Using unadjusted rates of potential overtreatment, we identified centers that fell in the top and bottom quartiles of potential overtreatment. The interquartile differences for all candidate, center-level, and OPO-level variables were compared. To account for correlations of candidates among centers, robust standard errors clustered by center were used. Time to transplantation and survival from time of candidate listing (including post-transplantation period for transplanted candidates) were estimated using Cox proportional hazard models with shared frailty by center.
ADJUSTMENT FOR CANDIDATE CHARACTERISTICS
There are legitimate nonhemodynamic reasons, such as very poor functional status, end-organ jeopardy, or the inability to use durable mechanical circulatory support, to treat a candidate with intensive therapies not strictly indicated by hemodynamic measurements. Therefore, we accounted for differences in nonhemodynamic candidate characteristics using 2 different methods.
First, because the data were clustered with candidates listed at centers located within OPO, we estimated a multivariate multilevel logistic regression model with random intercepts at the OPO and center levels (17,18). We used the model results to calculate risk-standardized potential overtreatment rates using methodology developed for public reporting of outcomes (19–23). This standardized rate provides an index to compare different centers, accounting for differences in candidate mix at each center.
Second, we performed a propensity score nearest-neighbor matching analysis to minimize bias from candidate-level differences, a recommended sensitivity analysis when performing risk-standardization (23). A propensity score was used to match candidates listed at top quartile centers to similar candidates listed in the bottom quartile based on all available candidate factors. After matching, recommended balancing diagnostics were performed to ensure adequate covariate balance in the propensity score–matched sample (24). The matched groups were compared to estimate the average effect of being listed at a top quartile center versus a bottom quartile center.
IDENTIFYING CENTER- AND OPO-LEVEL PREDICTORS OF POTENTIAL OVERTREATMENT
We then developed multilevel logistic regression models to identify OPO- and center-level variables associated with potential overtreatment. Because certain variables (e.g., OPO listing and transplantation volume) were highly collinear, we performed initial variable selection by calculating variance inflation factors and removing variables with variance inflation factors >10. For remaining variables with correlations of >0.7, we removed the variable(s) with the higher variance inflation factors. We then entered all remaining variables into the multilevel logistic regression model and performed backward selection with an exclusion criterion of p >0.1, forcing the retention of all controlling candidate-level variables. After OPO-level variable selection, we repeated the same process with center-level variables.
Analysis performed with Stata version 15 (StataCorp, College Station, Texas). A p value of <0.05 was considered significant and was calculated for all comparisons as a way to characterize the results even in situations where the entire population was included.
RESULTS
From 2010 to 2015, there were 19,919 adult heart-only candidate listings with 12,726 noncardiogenic shock candidates at risk for potential overtreatment listed at 108 centers within 51 OPO. Of at-risk candidates, 1,471 (11.6%) were potentially overtreated with high-dose inotropes or an IABP and listed as Status 1A, 5,369 (42.1%) were listed as Status 1B, and 5,922 (46.4%) were listed as Status 2 (Figure 2). The geographic variation in the unadjusted rate of potential overtreatment by OPO is displayed in the Central Illustration. There is substantial intraregional variation, with neighboring OPO often having dramatically different potential overtreatment rates. Of note, the 3 largest urban areas in the United States (New York, Chicago, and Los Angles) have high rates of potential overtreatment.
CENTRAL ILLUSTRATION. Geographic Variation in the Rate of Potential Overtreatment of U.S. Heart Transplant Candidates.
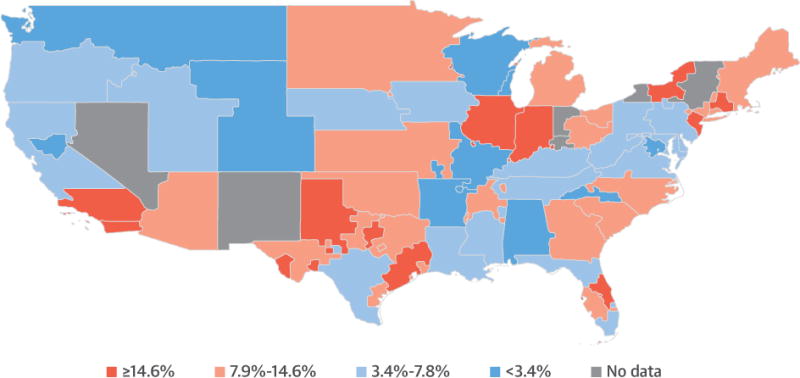
National variation in the unadjusted rates of treatment of heart transplant candidates with balloon pumps or high-dose inotropes despite the absence of cardiogenic shock are displayed. Rates are aggregated at the organ procurement organization level, the first local level of organ allocation in the United States. Colors correspond to quartiles of potential overtreatment.
There was wide center-level variation in potential overtreatment, with center rates ranging from 0% to 68% (Figure 3). The distribution was right-skewed with the top quartile of centers responsible for 60% of all potentially overtreated candidates. In the bottom quartile, there were 27 centers who potentially overtreated only 2.1% (n = 50) of 2,345 at-risk candidates. In contrast, the 28 centers in the top quartile potentially overtreated 27.6% (n = 884) of 3,203 at-risk candidates, interquartile difference of 25.5% (95% confidence interval [CI]: 21% to 30%). The odds of potential overtreatment were 1,650% higher in the top quartile than in the bottom (odds ratio [OR]: 17.5; 95% CI: 13.1 to 23.4).
FIGURE 3. Unadjusted and Risk-Standardized Rates of Potential Overtreatment by Center.
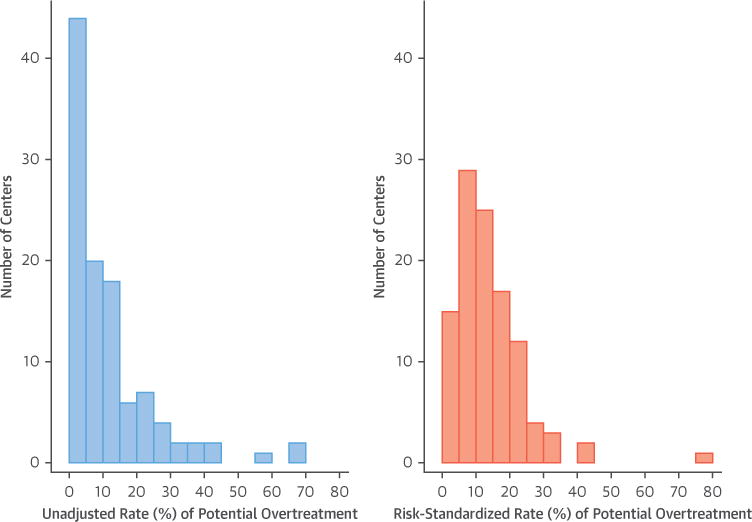
Rates are center averages during the 2010 to 2015 period. Adjusted rates calculated with multilevel regression results are shown in Online Table 2 using the methodology described in the Online Appendix. Only 7 centers had a >10% decrease in potential overtreatment rate after risk standardization. Ninety-four (87%) centers had a <10% change in overtreatment rate after risk standardization.
The top and bottom quartile centers were of similar size with no significant differences in transplantation volume and listed a similar proportion of noncardiogenic shock candidates at risk for overtreatment (64% vs. 64%; p = 0.951) (Table 1). Top quartile centers operated in more competitive OPO environments with more centers per OPO (3.5 vs. 2.4; p = 0.014) and a higher percentage of recipients who were Status 1A at transplantation (71% vs. 58%; p = 0.009). Top quartile centers had significantly different practice patterns than bottom quartile centers. Top quartile centers were more likely to initially list candidates as Status 1A (39% vs. 15%; p < 0.001) and use Status 1A–qualifying high-dose inotrope therapy (20% vs. 2%; p < 0.001). Top and bottom quartile centers listed similar proportions of candidates supported with durable left ventricular assist devices (28% vs. 23%; p = 0.218).
TABLE 1.
Center-Level Differences by Rate of Potential Overtreatment
| All Centers 108 Centers (N = 19,539) | Bottom Quartile <3.4% Overtreatment 27 Centers (n = 3,658) | Top Quartile ≥14.6% Overtreatment 28 Centers (n = 5,020) | p Value | |
|---|---|---|---|---|
| Candidates at risk | 12,762 (65) | 2,345 (64) | 3,203 (64) | 0.951 |
|
| ||||
| OPO-level variables | ||||
| OPO listing volume | 101 ± 63 | 91 ± 68 | 149 ± 72 | 0.033 |
| OPO transplant volume | 63 ± 43 | 55 ± 42 | 97 ± 53 | 0.029 |
| OPO Status 1A transplant proportion | 0.62 ± 0.18 | 0.58 ± 0.20 | 0.71 ± 0.13 | 0.009 |
| OPO Status 1B transplant proportion | 0.34 ± 0.16 | 0.36 ± 0.18 | 0.23 ± 0.12 | 0.004 |
| OPO donor heart supply | 53 ± 29 | 40 ± 23 | 71 ± 35 | 0.004 |
| OPO HHI | 0.58 ± 0.26 | 0.63 ± 0.29 | 0.45 ± 0.20 | 0.041 |
| Number of centers in OPO | 2.7 ± 1.3 | 2.4 ± 1.3 | 3.5 ± 1.2 | 0.014 |
|
| ||||
| Center-level variables | ||||
| Center listing volume | 47 ± 30 | 42 ± 30 | 52 ± 41 | 0.551 |
| Center transplant volume | 29 ± 23 | 24 ± 17 | 36 ± 33 | 0.313 |
| Market share of center | 0.57 ± 0.31 | 0.58 ± 0.34 | 0.40 ± 0.27 | 0.054 |
| Proportion of center transplantations using OPO hearts | 0.63 ± 0.20 | 0.60 ± 0.23 | 0.59 ± 0.18 | 0.792 |
| Time to transplantation | 189 ±249 | 246 ± 273 | 144 ± 214 | <0.001 |
| 90-day transplantation rate | 0.31 ± 0.17 | 0.21 ± 0.13 | 0.40 ± 0.18 | <0.001 |
| 365-day transplantation rate | 0.55 ± 0.18 | 0.47 ± 0.17 | 0.62 ± 0.16 | <0.001 |
|
| ||||
| Listing status | <0.001 | |||
| Status 1A | 4,777 (24) | 537 (15) | 1,970 (39) | |
| Status 1B | 8,110 (42) | 1,815 (50) | 1,629 (32) | |
| Status 2 | 5,922 (30) | 1,184 (32) | 1,320 (26) | |
| Inactive | 730 (4) | 122 (3) | 101 (2) | |
|
| ||||
| Listing therapy | <0.001 | |||
| None | 6,252 (32) | 1,227 (34) | 1,386 (28) | |
| High-dose inotropes | 1,770 (9) | 78 (2) | 999 (20) | |
| IABP | 647 (3) | 60 (2) | 239 (5) | |
| ECMO | 212 (1) | 28 (1) | 60 (1) | |
| CF-LVAD | 4,599 (24) | 1,034 (28) | 1,134 (23) | |
| Low-dose single IV inotropes | 4,936 (25) | 1,028 (28) | 915 (18) | |
| Other | 647 (3) | 119 (3) | 211 (4) | |
Values are n (%) or mean±SD. All values calculated by year, excluding candidates with missing data or at low volume centers (n = 380). The p values for comparisons for between centers in the bottom and top quartile of overtreatment were calculated using generalized linear models with robust SE clustered by center.
CF-LVAD = continuous flow left ventricular assist device; ECMO = extracorporeal membrane oxygenation; HHI = Herfindahl-Hirschman index; IABP = intra-aortic balloon pump; IV = intravenous; OPO = organ procurement organization.
Noncardiogenic shock candidates at risk for potential overtreatment listed at top and bottom quartile centers had only a few clinically meaningful differences in characteristics (Table 2). Top quartile at-risk candidates had higher cardiac indices (2.25 vs. 2.12 ml/min/m2, p = 0.014) despite worse functional status (37% vs. 26% with severe impairment requiring hospitalization, p < 0.001). Top quartile noncardiogenic shock candidates were transplanted faster (median days to transplantation: 146 vs. 412, p = 0.002) and had higher survival from listing (hazard ratio for death/deterioration: 0.78; 95% CI: 0.65 to 0.93) (Online Figure 1) compared with candidates listed at bottom quartile centers. The higher survival from listing at top quartile centers was similar for all initial listing statuses of non-cardiogenic shock candidates (test for a differential effect by status: p = 0.73) (Online Figure 1).
TABLE 2.
Characteristics of Candidates at Risk for Potential Overtreatment by Center of Listing
| All At-Risk Candidates 108 Centers (N = 12,762) | Bottom Quartile 27 Centers (n = 2,345) | Top Quartile 28 Centers (n = 3,203) | p Value | |
|---|---|---|---|---|
| Potentially overtreated | 1,471 (11.6) | 50 (2.1) | 884 (27.6) | <0.001 |
|
| ||||
| Median time to transplantation | 260 [67–844] | 412 [138–1,124] | 146 [38–539] | 0.002 |
|
| ||||
| Survival from listing | 0.006 | |||
| 1 yr | 84.5 (83.8–85.2) | 82.6 (80.9–84.1) | 85.5 (84.2–86.7) | |
| 3 yrs | 73.3 (72.4–74.2) | 69.6 (67.3–71.8) | 76.3 (74.4–78.0) | |
|
| ||||
| Post-transplantation survival | 0.083 | |||
| 1 yr | 91.0 (90.2–91.6) | 88.4 (86.3–90.2) | 91.9 (90.6–93.0) | |
| 3 yrs | 84.7 (83.7–85.7) | 82.7 (80.1–85.0) | 86.5 (84.7–88.2) | |
|
| ||||
| Age at listing, yrs | 53±12 | 53±13 | 54±12 | 0.039 |
|
| ||||
| Height, cm | 174±10 | 174±10 | 173±10 | 0.189 |
|
| ||||
| Weight, kg | 83±18 | 84±18 | 81±18 | 0.098 |
|
| ||||
| Cardiac index, ml/kg/m2 | 2.17±0.63 | 2.12±0.62 | 2.25±0.63 | 0.014 |
|
| ||||
| Mean PAP, mm Hg | 30±10 | 31±10 | 30±10 | 0.221 |
|
| ||||
| Mean PCWP, mm Hg | 20±8 | 20±8 | 20±9 | 0.333 |
|
| ||||
| Diagnosis | 0.455 | |||
| Dilated cardiomyopathy | 5,174 (41) | 960 (41) | 1,340 (42) | |
| Ischemic cardiomyopathy | 4,335 (34) | 761 (32) | 1,144 (36) | |
| Restrictive cardiomyopathy | 1,579 (12) | 265 (11) | 321 (10) | |
| Other | 1,674 (13) | 359 (15) | 398 (12) | |
|
| ||||
| Blood type O | 5,448 (43) | 990 (42) | 1,365 (43) | 0.928 |
|
| ||||
| Female | 3,550 (28) | 621 (26) | 906 (28) | 0.275 |
|
| ||||
| Karnofsky performance status | ||||
| Limited impairment, 10%–30% | 4,292 (34) | 972 (41) | 748 (23) | 0.003 |
| Moderate impairment, 40%–60% | 4,905 (38) | 741 (32) | 1,135 (35) | |
| Severe impairment, 70%–100% | 3,191 (25) | 616 (26) | 1,172 (37) | |
| Unknown/missing | 374 (3) | 16 (1) | 148 (5) | |
|
| ||||
| Working for income | 1,696 (13) | 312 (13) | 325 (10) | 0.073 |
|
| ||||
| Race | 0.986 | |||
| White | 8,448 (66) | 1,478 (63) | 1,988 (62) | |
| Black | 2,716 (21) | 515 (22) | 697 (22) | |
| Hispanic | 1,058 (8) | 233 (10) | 337 (11) | |
| Other | 540 (4) | 119 (5) | 181 (6) | |
|
| ||||
| College or higher education | 6,987 (55) | 1,292 (55) | 1,626 (51) | 0.181 |
|
| ||||
| Insurance type | 0.419 | |||
| Private | 6,733 (53) | 1,083 (46) | 1,675 (52) | |
| Medicaid | 1,336 (10) | 330 (14) | 372 (12) | |
| Medicare | 4,177 (33) | 830 (35) | 1,027 (32) | |
| Other | 516 (4) | 102 (4) | 129 (4) | |
|
| ||||
| BMI, kg/m2 | 0.088 | |||
| <25 | 3,812 (30) | 689 (29) | 1,061 (33) | |
| 25–29 | 4,629 (36) | 823 (35) | 1,176 (37) | |
| 30–34 | 3,289 (26) | 617 (26) | 731 (23) | |
| ≥35 | 1,032 (8) | 216 (9) | 235 (7) | |
|
| ||||
| Diabetes | 3,653 (29) | 667 (28) | 949 (30) | 0.619 |
|
| ||||
| Renal function | 0.352 | |||
| ≥60 ml/min/1.73 m2 | 6,693 (52) | 1,215 (52) | 1,699 (53) | |
| 30–59 ml/min/1.73 m2 | 5,118 (40) | 971 (41) | 1,247 (39) | |
| <30 ml/min/1.73 m2 | 579 (5) | 96 (4) | 147 (5) | |
| On dialysis | 372 (3) | 63 (3) | 110 (3) | |
|
| ||||
| Smoking history | 5,795 (45) | 1,124 (48) | 1,355 (42) | 0.215 |
|
| ||||
| CVA history | 688 (5) | 112 (5) | 142 (4) | 0.713 |
|
| ||||
| History of malignancy | 1,071 (8) | 200 (9) | 237 (7) | 0.357 |
|
| ||||
| History of cardiac surgery | 4,210 (33) | 840 (36) | 1,136 (35) | 0.907 |
|
| ||||
| Defibrillator in place | 10,137 (79) | 1,950 (83) | 2,459 (77) | 0.016 |
Values n (%), median [interquartile range], % (95% CI), or mean±SD. Candidates not at risk of potential overtreatment or with missing data (n = 7,157) were excluded (see Figure 1). The p values for comparisons for between top and bottom quartiles of overtreatment were calculated with robust SE clustered by center.
BMI = body mass index; CI = confidence interval; CVA = cerebrovascular accident; PAP = pulmonary artery pressure; PCWP = pulmonary capillary wedge pressure.
The geographic distribution of unadjusted and risk-standardized center rates was similar with a standardized interquartile difference of 23.2% (95% CI: 19.4% to 27.0%) (Figure 3, Online Figure 2, Online Table 2 for full model used during risk standardization). Of the 28 top quartile centers by unadjusted rate of potential overtreatment, 21 (75%) remained in the top quartile of standardized potential overtreatment rate (Online Table 3). In well-balanced propensity score matched candidate cohorts (Online Figure 3, Online Table 4 for balancing tests), the interquartile difference in potential overtreatment was 24.9% (95% CI: 22.9% to 26.8%) and was not significantly different from the unadjusted interquartile difference.
The OPO- and center-level variables with significant associations to potential overtreatment after adjusting for candidate level variables are displayed in Table 3 (Online Tables 5 and 6 for full models). For every 10% increase in the percentage of recipients who were Status 1A at transplant, the odds of potential overtreatment increased 6% (adjusted odds ratio [aOR]: 1.06; 95% CI: 1.00 to 1.14). If there were 3 or more centers in the OPO, the odds of potential overtreatment were 50% higher (aOR: 1.50; 95% CI: 1.07 to 2.11). Finally, the shortest quartile of median time from Status 1A listing to heart transplantation (<19 days) was associated with a 43% increase in odds of potential overtreatment (aOR: 1.43; 95% CI: 1.19 to 1.72) relative to the middle second and third quartiles (19 to 63 days). In the full model adjusting for candidate- and OPO-level differences, the significant center-level practice variables associated with potential overtreatment were the percentage of all candidates (not just those at risk for overtreatment) listed as Status 1A and the center’s 30-day transplantation rate for all candidates. For every 10% increase in the percentage of candidates a center initially listed as Status 1A, the odds of potential overtreatment increased 20% (aOR: 1.20; 95% CI: 1.11 to 1.29). For every 10% increase in a center’s 30-day transplantation rate (percentage of candidates transplanted within 30 days of listing), the odds of potential overtreatment increased 19% (aOR: 1.09 to 1.29).
TABLE 3.
OPO- and Center-Level Predictors of Potential Overtreatment in a Multilevel Logistic Regression
| Model 2 | Model 3 | |
|---|---|---|
| OPO competition variables | ||
| Percentage of recipients who were Status 1A at transplant (per 10%) | 1.06* (1.00–1.14) | 1.02 (0.96–1.10) |
| 3+ centers in OPO, base 1–2 centers | 1.50* (1.07–2.11) | 1.45* (1.07–1.97) |
| Median time from Status 1A listing to heart transplantation by quartile, base middle quartiles, 19–63 days | ||
| <19 days | 1.43* (1.19–1.72) | 1.25* (1.04–1.51) |
| ≥64 days | 1.22† (0.98–1.52) | 1.31* (1.05–1.63) |
|
| ||
| Center practice variables | ||
| Percentage of candidates listed as Status 1A, per 10% | 1.20* (1.11–1.29) | |
| 30-day transplantation rate, per 10% | 1.19* (1.09–1.29) | |
|
| ||
| Candidates | 12,762 | 12,762 |
|
| ||
| Centers | 108 | 108 |
Values are odds ratios (95% CI) or n.
p < 0.05.
p < 0.1. Candidate-level variable coefficients and full model results can be found in Online Tables 5 and 6. Model 2: Adjusted for candidate and OPO-level competition variables. Model 3: Adjusted for candidate, OPO-level competition, and center practice variables. The “30-day transplantation rate” is the percentage of candidates transplanted within 30 days of listing at a particular center.
DISCUSSION
In this study of 12,762 adult heart transplant candidates listed in the United States between 2010 and 2015, we found wide center-level variation in treatment and listing practices that could not be explained by variation in candidate characteristics. The odds that a candidate was treated with high-dose inotropes or an IABP despite not being in cardiogenic shock was 17.5 × higher in the top quartile of centers than in the bottom quartile. Accounting for candidate-level differences with either risk standardization or propensity score matching did not reduce the magnitude of the center-level variation. Competitive local organ markets with more centers and higher percentages of top priority Status 1A candidates had higher rates of potential overtreatment.
Because the cardiogenic shock criteria are based on well-established major society guidelines (10), our null hypothesis for this study was that treatment of adult heart transplant candidates who were not in cardiogenic shock with high-dose inotropes or IABP would be rare. Furthermore, we hypothesized that what little overtreatment did occur could be explained by nonhemodynamic candidate variables. Instead, we found wide center-level variation in potential overtreatment with a more than 17-fold difference in the odds of potential overtreatment between the top and bottom quartile centers. After accounting for candidate variability with 2 different methods, the large variation in heart transplantation center treatment practices was unchanged.
The current U.S. geographic sharing system distributes donor hearts to local Status 1A candidates first. In this context, the center- and OPO-level variables associated with potential overtreatment have intuitive explanations. Centers located in more competitive OPO environments, with more Status 1A candidates and higher numbers of centers per OPO, were more likely to potentially overtreat candidates. In the pre-1999 heart transplant allocation system, which had only 2 status tiers, a similar relationship between competition and overtreatment was found (8). The relationship between median days from Status 1A listing to transplant with potential overtreatment can also be explained as a center response to the local transplantation environment. In OPO with good organ supply and very short Status 1A wait times (<19 days), admission to the intensive care unit (ICU) and treatment with IABP or high-dose inotropes may be used by centers with the goal of transplanting candidates quickly.
Overtreatment is not a new phenomenon in organ transplantation. Prior to the development of the Model for End-Stage Liver Disease (MELD) system in liver allocation, liver candidates with chronic liver disease who were physically in the ICU received higher priority for transplantation (25). After MELD was implemented and the ICU priority bump eliminated, the probability of ICU admission dropped 45% despite an increase in average recipient MELD score (26). This suggests that before MELD was implemented, unnecessary ICU admissions were frequent.
From the perspective of a heart transplantation physician, who is responsible for the care of an individual patient, potential overtreatment can be understood. There may be compelling subjective factors, such as severely impaired functional status, which make the use of high-dose inotropes or an IABP appropriate even if the patient is not technically in cardiogenic shock. Furthermore, for an individual patient, the risks of a long wait at lower status may outweigh the harms of overtreatment (6). In our study, we found that centers with top quartile rates of potential overtreatment transplanted their non-cardiogenic shock candidates much faster (median: 146 days vs. 412 days) and had better overall survival outcomes, regardless of initial listing status. These results imply that centers may be using escalation of medical therapies strategically to get their candidates transplanted earlier and achieve better overall results. These positive outcomes likely re-enforce aggressive treatment practices.
However, widespread overtreatment is problematic for multiple reasons. First, it could lead to excess cost and unnecessary risk of therapy-related complications. Chronic inotrope therapy increases mortality in stable advanced heart failure and high-dose inotrope therapy is only indicated in cardiogenic shock (27–29). More importantly, overtreatment in heart transplantation unfairly elevates the status of less urgent candidates while truly urgent candidates die waiting (or perhaps are never listed). The federal final rule governing U.S. organ transplantation states that candidates should be offered organs “from most to least medically urgent” (5). Because the median time from Status 1A listing to transplantation has ballooned to >3 months (2) and the sickest candidates benefit the most from heart transplantation (30), misallocation of hearts is ethically unacceptable. Further research is required to estimate how many of the >600 heart transplant candidates who die or become too sick to undergo transplantation each year could have been saved with a better allocation system (3).
It may be argued that the problem of overtreatment will be effectively solved by using the shock criteria to bar less urgent candidates from high priority listing status (31). However, as stated by the OPTN, the expected effect of the shock requirement is “based on current behavior and practices, and [this has raised concern] that the proposal would influence practitioners to behave differently than they currently do” (31). We believe it unlikely that transplantation programs that currently utilize IABP or multiple inotropes in aggressive treatment strategies will feel comfortable listing the majority of their candidates at low-priority status. Centers may be tempted to “game” hemodynamic measurements, perhaps by taking the lower of thermodilution or Fick cardiac indices. We also anticipate that centers will shift practices and use more surgically placed mechanical circulatory support devices, which would be exempt from the shock requirement (9). Finally, we believe therapy-based allocation systems will always be susceptible to manipulation and recommend the development of an objective scoring system for heart allocation analogous to MELD or the Lung Allocation System.
STUDY LIMITATIONS
We have been careful in this paper to use the term “potential” overtreatment to describe our principal outcome for a reason. Though based on major society guidelines, the criteria we used to define potential overtreatment were only announced in December 2016 and are not yet implemented as OPTN policy (9). Indeed, the criteria as written would penalize a center for using high levels of inotropic or mechanical support that ended up raising a candidate’s cardiac index above arbitrary cutoffs, even if the treatment team believed that strategy to be clinically necessary. Also, the AHA cardiogenic shock criteria have not been prospectively validated in the heart transplant candidate population and may not be the best way to risk stratify candidates (11,32,33). However, because the criteria are based on objective, audited hemodynamic measures that are routinely employed in the management of heart failure patients, we believe that the potential overtreatment outcome was adequate to measure variation in treatment practices.
While we used all of the available candidate characteristics and 2 different methods to account for candidate variability, it is possible that key unmeasured candidate variables could justify some of the center-level variation in treatment. However, it is exceedingly unlikely that additional candidate variables could explain the large interquartile difference observed.
CONCLUSIONS
There is meaningful variation in the treatment practices of adult heart transplantation centers. Heart transplantation centers that overtreat candidates have shorter waiting times and improved survival. Competition for transplantable hearts may drive overtreatment of hemodynamically stable heart transplant candidates. Overtreatment may compromise the fair and efficient allocation of scarce deceased donor hearts.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN SYSTEMS-BASED PRACTICE
The current heart allocation system, which ranks candidates according to treatment intensity, has resulted in wide variation in transplantation center practice for similar candidates, and competition for available donor hearts is associated with overtreatment of hemodynamically stable transplant candidates.
TRANSLATIONAL OUTLOOK
An improved allocation system that is not based on intensity of treatment may reduce overtreatment, promote improve allocation of donor hearts, and save lives.
Acknowledgments
The authors would like to acknowledge Nikhil Narang for his assistance with the project by providing relevant clinical information, and Andrew Borasso for his assistance with the study design.
Supported in part by institutional Clinical and Translational Science Award grant UL1 RR024999. Dr. Parker is supported by National Institutes of Health T32 Training grant 5T32HL007605-32. Dr. Anderson has received consulting fees from GE HealthCare; and has served on the speakers bureau for Novartis and Relypsa. Dr. Churpek is supported by National Institutes of Health grants K08 HL121080-03 and R01 GM 123193; and has a patent pending (ARCD.P053US.P2) for risk stratification algorithms. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. The data reported here have been supplied by the Minneapolis Medical Research Foundation as the contractor for the SRTR (Scientific Registry of Transplant Recipients). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. government.
ABBREVIATIONS AND ACRONYMS
- AHA
American Heart Association
- IABP
intra-aortic balloon pump
- ICU
intensive care unit
- MELD
Model for End-Stage Liver Disease
- OPO
organ procurement organization(s)
- OPTN
Organ Procurement and Transplant Network
APPENDIX
For a supplemental Methods section as well as figures and tables, please see the online version of this paper.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, et al. for the American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colvin M, Smith JM, Skeans MA, et al. OPTN/SRTR 2015 Annual Data Report: Heart. Am J Transplant. 2017;17(Suppl 1):286–356. doi: 10.1111/ajt.14128. [DOI] [PubMed] [Google Scholar]
- 3.U.S. Department of Health and Human Services. Organ Procurement and Transplantation Network: National Data. Available at: https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/#. Accessed June 27, 2017.
- 4.OPTN. Policy 6: Allocation of Hearts and Heart-Lungs. Organ Procurement and Transplant Network: Policies. Available at: https://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf#nameddest=Policy_06. Accessed December 10, 2016.
- 5.Health Resources and Services Administration (HRSA), Department of Health and Human Services (HHS) Organ procurement and transplantation network: final rule. Fed Regist. 2013;78:40033–42. [PubMed] [Google Scholar]
- 6.Movsesian M. Should Doctors Game the Transplant Wait List To Help Their Patients? NPR website. 2016 Jul 24; Available at: http://www.npr.org/sections/health-shots/2016/07/24/486787474/should-doctors-game-the-transplant-wait-list-to-help-their-patients. Accessed September 27, 2016.
- 7.Stevenson LW. The urgent priority for transplantation is to trim the waiting list. J Heart Lung Transplant. 2013;32:861–7. doi: 10.1016/j.healun.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 8.Scanlon DP, Hollenbeak CS, Lee W, Loh E, Ubel PA. Does competition for transplantable hearts encourage ‘gaming’ of the waiting list? Health Aff (Millwood) 2004;23:191–8. doi: 10.1377/hlthaff.23.2.191. [DOI] [PubMed] [Google Scholar]
- 9.U.S. Department of Health and Human Services. Organ Procurement and Transplantation Network: Modify Adult Heart Allocation 2016 2nd Round. Available at: https://optn.transplant.hrsa.gov/governance/public-comment/modify-adult-heart-allocation-2016-2nd-round/. Accessed December 15, 2016.
- 10.Reynolds HR, Hochman JS. Cardiogenic shock: current concepts and improving outcomes. Circulation. 2008;117:686–97. doi: 10.1161/CIRCULATIONAHA.106.613596. [DOI] [PubMed] [Google Scholar]
- 11.Parker WF, Garrity ER, Fedson S, Churpek MM. Potential impact of a shock requirement on adult heart allocation. J Heart Lung Transplant. 2017;36:1013–6. doi: 10.1016/j.healun.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parker WF, Garrity ER, Fedson S, Churpek MM. Trends in the use of inotropes to list adult heart transplant candidates at status 1A. Circ Heart Fail. 2017;10:e004483. doi: 10.1161/CIRCHEARTFAILURE.117.004483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatch OS. 2048—National Organ Transplant Act. 98th Congress (1983-1984) 1984 Oct 19; Available at: https://www.congress.gov/bill/98th-congress/senate-bill/2048. Accessed December 23, 2015.
- 14.Johnson MJ, Bland JM, Davidson PM, Newton PJ, Oxberry SG, Abernethy AP, Currow DC. The relationship between two performance scales: New York Heart Association Classification and Karnofsky Performance Status Scale. J Pain Symptom Manage. 2014;47:652–8. doi: 10.1016/j.jpainsymman.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Karnofsky DA, Burchenal JH. The clinical evaluation of chemotherapeutic agents in cancer. In: MacLeod CM, editor. Evaluation of chemotherapeutic agents. New York: Columbia University Press; 1949. pp. 191–205. [Google Scholar]
- 16.Hirschman AO. National Power and the Structure of Foreign Trade. Berkeley, CA: University of California Press; 1945. [Google Scholar]
- 17.Hedeker D. Multilevel models for ordinal and nominal variables. In: de Leeuw J, Meijer E, editors. Handbook of Multilevel Analysis. New York, NY: Springer; 2008. pp. 237–74. Available at: https://link.springer.com/chapter/10.1007/978-0-387-73186-5_6. Accessed July 28, 2017. [Google Scholar]
- 18.Snijders T, Bosker R. Multilevel Analysis: An Introduction to Basic and Advanced Multilevel Modeling. 2nd. Los Angeles, CA: SAGE Publications; 2011. [Google Scholar]
- 19.Krumholz HM, Wang Y, Mattera JA, et al. An administrative claims model suitable for profiling hospital performance based on 30-day mortality rates among patients with heart failure. Circulation. 2006;113:1693–701. doi: 10.1161/CIRCULATIONAHA.105.611194. [DOI] [PubMed] [Google Scholar]
- 20.Asch DA, Nicholson S, Srinivas S, Herrin J, Epstein AJ. Evaluating obstetrical residency programs using patient outcomes. JAMA. 2009;302:1277–83. doi: 10.1001/jama.2009.1356. [DOI] [PubMed] [Google Scholar]
- 21.Krumholz HM, Lin Z, Keenan PS, et al. Relationship between hospital readmission and mortality rates for patients hospitalized with acute myocardial infarction, heart failure, or pneumonia. JAMA. 2013;309:587–93. doi: 10.1001/jama.2013.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shahian DM, Torchiana DF, Shemin RJ, Rawn JD, Normand S-LT. Massachusetts cardiac surgery report card: implications of statistical methodology. Ann Thorac Surg. 2005;80:2106–13. doi: 10.1016/j.athoracsur.2005.06.078. [DOI] [PubMed] [Google Scholar]
- 23.Shahian DM, Normand S-LT. Comparison of “risk-adjusted” hospital outcomes. Circulation. 2008;117:1955–63. doi: 10.1161/CIRCULATIONAHA.107.747873. [DOI] [PubMed] [Google Scholar]
- 24.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiesner R, Edwards E, Freeman R, et al. for the United Network for Organ Sharing Liver Disease Severity Score Committee Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91–6. doi: 10.1053/gast.2003.50016. [DOI] [PubMed] [Google Scholar]
- 26.Snyder J. Gaming the liver transplant market. J Law Econ Organ. 2010;26:546–68. [Google Scholar]
- 27.Cohn JN, Goldstein SO, Greenberg BH, et al. for the Vesnarinone Trial Investigators A dose-dependent increase in mortality with vesnarinone among patients with severe heart failure. N Engl J Med. 1998;339:1810–6. doi: 10.1056/NEJM199812173392503. [DOI] [PubMed] [Google Scholar]
- 28.Packer M, Carver JR, Rodeheffer RJ, et al. for the PROMISE Study Research Group Effect of oral milrinone on mortality in severe chronic heart failure. N Engl J Med. 1991;325:1468–75. doi: 10.1056/NEJM199111213252103. [DOI] [PubMed] [Google Scholar]
- 29.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 30.Singh TP, Milliren CE, Almond CS, Graham D. Survival benefit from transplantation in patients listed for heart transplantation in the United States. J Am Coll Cardiol. 2014;63:1169–78. doi: 10.1016/j.jacc.2013.11.045. [DOI] [PubMed] [Google Scholar]
- 31.Uccellini K, OPTN/UNOS Thoracic Organ Transplantation Committee, editor. Proposal to Modify the Adult Heart Allocation System. Briefing paper. 2016 Available at: https://optn.transplant.hrsa.gov/media/2006/thoracic_brief_201612.pdf. Accessed March 12, 2016.
- 32.Narang N, Thibodeau JT, Levine BD, et al. Inaccuracy of estimated resting oxygen uptake in the clinical setting. Circulation. 2014;129:203–10. doi: 10.1161/CIRCULATIONAHA.113.003334. [DOI] [PubMed] [Google Scholar]
- 33.Balik M, Pachl J, Hendl J, Martin B, Jan P, Jan H. Effect of the degree of tricuspid regurgitation on cardiac output measurements by thermodilution. Intensive Care Med. 2002;28:1117–21. doi: 10.1007/s00134-002-1352-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


