Abstract
Despite the great progress in cardiovascular health and clinical care along with marked decline in morbidity and mortality, cardiovascular diseases remain the leading causes of death and disability in the developed world. New therapeutic approaches, targeting not only systematic but also causal dysfunction, are ultimately needed to provide a valuable alternative for treatment of complex cardiovascular diseases. In heart failure, there are currently a number of trials that have been either completed or are ongoing targeting the sarcoplasmic reticulum calcium ATPase pump (SERCA2a) gene transfer in the context of heart failure. Recently, a phase 2 trial was completed, demonstrating safety and suggested benefit of adeno-associated virus type 1/SERCA2a gene transfer in advanced heart failure, supporting larger confirmatory trials. The experimental and clinical data suggest that, when administrated through perfusion, virus vector carrying SERCA2a can also transduce vascular endothelial and smooth muscle cells (EC and SMC) thereby improving the clinical benefit of gene therapy. Indeed, recent advances in understanding the molecular basis of vascular dysfunction point towards a reduction of sarcoplasmic reticulum Ca2+ uptake and an impairment of Ca2+ cycling in vascular EC and SMC from patients and preclinical models with cardiac diseases or with cardiovascular risk factors such as diabetes, hypercho-lesterolemia, coronary artery diseases, as well as other conditions such as pulmonary hypertension. In recent years, several studies have established that SERCA2a gene-based therapy could be an efficient option to treat vascular dysfunction. This review focuses on the recent finding showing the beneficial effects of SERCA2a gene transfer in vascular EC and SMC.
Keywords: Cardiovascular disease, endothelial cells, gene therapy, SERCA2a, vascular smooth muscle cells
INTRODUCTION
Cardiovascular diseases and heart failure (HF) remain a major cause of morbidity and mortality in the developed world. During the last ten years, significant advance has been achieved in understanding the different intracellular and molecular mechanisms that become abnormal during HF [1]. One of the key abnormalities in both human HF and experimental animal model of HF is an abnormal intracellular calcium ion (Ca2+) handling [2, 3].
Cytosolic Ca2+ concentration is more than 1000 time lower ([Ca2+]i ~ 100 nM) compared to the high Ca2+ concentration found in both extracellular environment (in mM range) and intracellular pool (Sarco/Endoplasmic Reticulum (SR/ER)). This gradient allows Ca2+ to be an omnipresent second messenger which concentration changes becoming critical signals for a variety of cellular physiological functions. Among the proteins involved in controlling of calcium signaling, the sarco/endoplasmic reticulum Ca2+ATPase pumps (SERCA) serve a dual function [3, 4].
Alterations of Ca2+ handling, leading to aberrant calcium signal transduction, have been reported in numerous cardiovascular diseases, affecting all types of cardiovascular cells, including cardiomyocytes, vascular smooth muscle cells (SMC) and endothelial cells (EC). In failing cardiomyocytes, deficient SR Ca2+ uptake is associated with a decrease in both expression and activity of SERCA type 2a isoform (SERCA2a) [1, 2]. Unregulated Ca2+, in turn, regulates the effectors of SR Ca2+ release and Ca2+ influx channels, further worsening the abnormal Ca2+ distribution. Normalization of SERCA2a functions has been shown to increase contractility in failing human cardiomyocytes and to improve hemodynamics along with survival in rodent and large animal models of HF [1, 5]. Beyond its enhancing effect on contractility, SERCA2a gene transfer has been shown to restore the energetic state of the heart, both in terms of energy supply and utilization, and to decrease ventricular arrhythmias [6–8]. Following a long line of investigation in animal models, SERCA2a gene transfer has shown therapeutic promise and a number of clinical trials using SERCA2a as a target have been initiated [9]. The recent successful and safe completion of a phase 2 trial targeting SERCA2a, along with the start of more recent phase 1 trials, opens a new era for gene therapy for the treatment of HF. SERCA2a gene transfer into cardiomyocytes for the treatment of HF is intensively reviewed [1, 10].
Interestingly, the same therapeutic molecule, SERCA2a, revealed significant benefits when administrated to vascular EC and SMC. Taking account the possibility of transduction of vascular cells and the heart at the same time through coronary perfusion, the therapeutic SERCA2a administration can be considered as a universal approach correcting defected Ca2+ signal transduction in numerous cardiovascular cells. Furthermore, recent data suggest that in several pathologies, SERCA2a can be locally administrated exclusively to vascular cells, limiting the cost of treatment and virus exposure. This review focuses on the Ca2+ handling in vascular SMC and EC, its alteration during different cardiovascular pathologies, and discusses the potential interest of therapeutic SERCA2a gene transfer to vascular cells.
1. CALCIUM SIGNALING IN VASCULAR CELLS
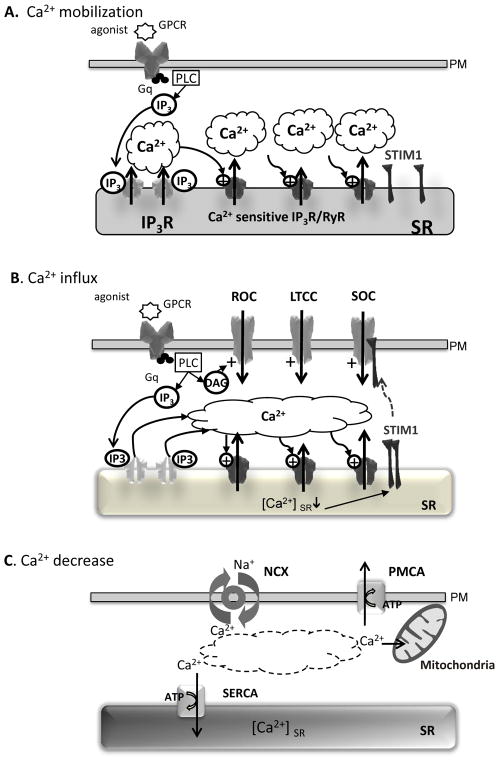
The fact that Ca2+ is involved in multiple signaling pathways also imposes its coordinated regulation in both time and space to ensure reliability and specificity. A classical view of Ca2+ signaling can be dissected in three steps; Ca2+ mobilization, Ca2+ influx and cytosolic [Ca2+] decrease to basal levels. Of course the Ca2+ signaling in vascular cells involves cross-talk between Ca2+ influx through the plasma membrane, signaling molecules in the sarcolemmal membrane and Ca2+ release machinery in the intracellular organelles.
1.a. Calcium Signaling
Elevation of [Ca2+]i in response to agonist binding to plasma membrane receptors or depolarization involves Ca2+ release from intracellular SR stores that can be followed by Ca2+ influx through the plasma membrane (Fig. 1A & 1B). For instance, G-protein coupled receptors will lead to the activation of phospholipase and inositol 1,4,5 triphosphate (IP3) and diacylglycerol (DAG) production. IP3 diffuses in the cytosol to reach its receptor (IP3R) located in the membrane of the reticulum and triggers the opening of the Ca2+ channel allowing the release of Ca2+. In many cell types this first increase in cytosolic Ca2+ activates the ryanodine receptor-operated channels (RyR) also located in the SR membrane, resulting in a further increase of cytosolic Ca2+. This process termed calcium-induced calcium release (CICR) has been involved in the propagation and the emphasis of a local and initial Ca2+ event along the cell. Although different Ca2+ influx pathways exist, depending on the cell type, including voltage gated Ca2+ channels and receptor-operated Ca2+ channels (activated by DAG), a special importance has been given to the store-operated Ca2+ channels (SOC). SOC channels are triggered by the decrease in stored Ca2+ concentration ([Ca2+]er) during Ca2+ mobilization (Fig. 1B). SOC mechanism was firstly proposed by Putney in 1986 [11], and later confirmed by the identification, in 2005 and 2006, of crucial actors that participated to the creation of the Ca2+ current. Meanly, SOC is composed of the Ca2+ sensor located in the ER; STIM1 (stromal interaction molecule 1) and the Ca2+ channel itself that is formed with units Orai1- 3 [12–15]. When [Ca2+]er decrease (<500μM), Ca2+ dissociates from STIM1, which oligomerizes and translocates to specialized cortical reticulum compartments adjacent to the plasma membrane [16, 17]. There its cytosolic activating domain binds to the Ca2+ channels of the Orai family; this induces their clustering into an opened Ca2+ channel. Furthermore, transient receptor potential canonical (TRPC) channels have also been suggested to participate to SOC and interaction have been described with STIM1 and the scaffolding protein Homer1 [18].
Fig. (1). General aspects of calcium cycling in vascular cells.
A. Calcium mobilization. B. Calcium influx. C. Calcium decrease. Abbreviations: Ca2+ - calcium ion; DAG – diacylglycerol; GPCR – G-protein coupled receptor; IP3 - inositol-1,4,5-trisphosphate, IP3R - inositol-1,4,5-trisphosphate receptor; LTCC – L-type Ca2+ channel; NCX – Na2+/Ca2+ exchanger; PLC - phospholipase C; PM – plasma membrane; PMCA – plasmic membrane Ca2+ ATPase; ROC- receptor activated channel; RyR - the sarco(endo)plasmic reticulum (SR/ER) Ca2+ channel, the ryanodine receptor; SERCA – the sarco(endo)plasmic reticulum Ca2+ATPase; SOC – store-operated Ca2+ channel; SR – sarcoplasmic reticulum; STIM1 - Stromal Interaction Molecule 1.
As SOC induction is directly dependent on the store Ca2+ content, the third player in its regulation has to be SERCA, which pumps the Ca2+ back into these stores, although mitochondria and other proteins such Plasma Membrane Ca2+ATPases (PMCA) and Na2+/Ca2+ exchanger (NCX) also contribute to the decrease in cytosolic Ca2+ (Fig. 1C). Different investigations have established that in some cellular types, SERCA are organized into the same cluster as STIM1 and Orai1 [19, 20]. SOC can also be observed when the Ca2+ leak from those stores is not compensated by calcium uptake calcium uptake by SERCA. Therefore, thapsigargin, a SERCA inhibitor, is commonly used to chemically create SOC currents.
1.b. Sarco/Endoplasmic Reticulum Ca2+ (SERCA)-pumps System
As SERCA is the only Ca2+ transporter in the SR, regulation of its functions by the cell constitutes a key mechanism to adjust Ca2+ homeostasis in the reticulum depending on the cell type and its state of differentiation. It is therefore not surprising that a particular effort has been made to target these Ca2+ handling proteins among the others to restore Ca2+ homeostasis in cardiac pathologies where Ca2+ defects radically affect cell function.
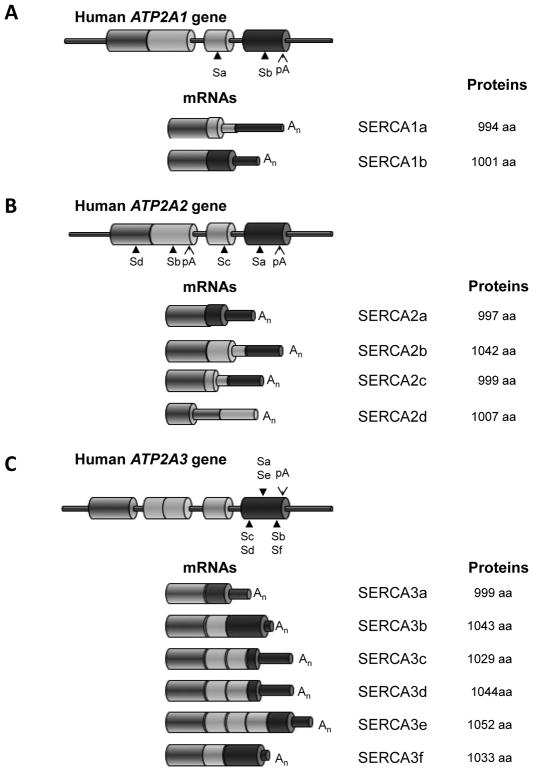
There are 3 tissue-specific genes of the SERCA family, ATP2A1, ATP2A2 and ATP2A3 encoding for SERCA1, SERCA2 and SERCA3 isoforms respectively (Fig. 2) [21]. Each gene gives rise to different SERCA isoforms by alternative splicing characterised by tissue-specific expression (Figs. 2 & 3A).
Fig. (2). Alternative splicing of the human ATP2A genes.
Representation of the 3′-end of the human ATP2A genes coding for SERCA1 (A), SERCA2 (B) and SERCA3 (C) proteins. Cylinders represent exons. Sa-Sf: position of stop codons for the corresponding isoforms, pA: position of polyadenylation sites. Representation of the 3′-end of the different mRNA variants and the sizes of the corresponding proteins. Large cylinders are translated segments; the narrow cylinders are untranslated segments.
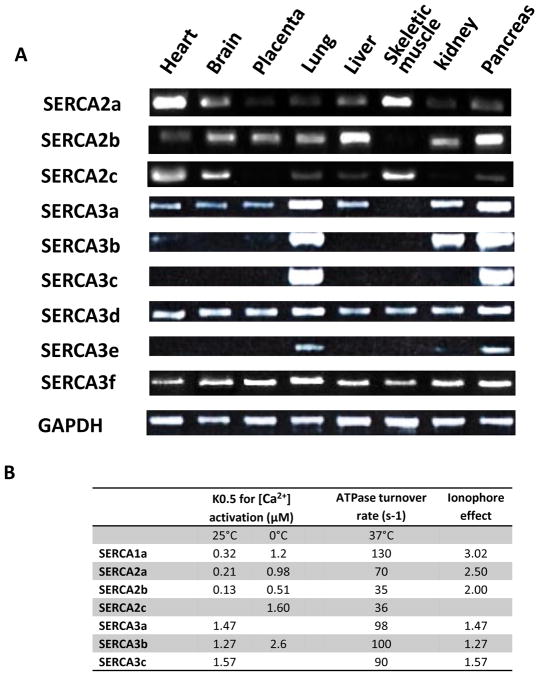
Fig. (3). Distinction of the different SERCA isoformes. A.
Typical RT-PCR analysis (n = 5) showing differential expression of human, SERCA2 and SERCA3 mRNA in normal human tissues (MTC; multiple tissue cDNAs, Clontech Laboratories, Inc.). B. Table summarizing functional characteristic of human SERCA recombinant proteins (from [22, 25, 33, 43, 116]). SERCA affinity for Ca2+ is displays as K0.5 for [Ca2+] activation (μM) at 37 or 0°C; the rate of the different SERCA turnover cycle at 37°C; and the effect of store depletion on the SERCA activity.
The ATP2A1 gene gives rise to two isoforms SERCA1a and −1b (Fig. 2A); SERCA1b is the fetal isoform and its expression normally switches to the mature isoform, SERCA1 [22]. SERCA1 was initially described as the fast twitch skeletal muscle isoform [23]. However, following studies revealed relatively high expressions of SERCA1 mRNA also in the heart, similar to the levels observed in skeletal muscle, and lower expressions of SERCA1 mRNA was also detected in the liver, kidney, brain and pancreas [22].
Regarding the human ATP2A2 gene, currently four alternative variants, termed SERCA2a, −2b, −2c and −2d, have been identified (Fig. 2B). The SERCA2b isoform is expressed ubiquitously, while the SERCA2a is preferentially expressed in cardiac, skeletal and vascular smooth muscle. The SERCA2c isoform was only described recently and expression of its mRNA was observed in the heart, brain and skeletal muscle (Fig. 3A). The corresponding protein was also described in cardiomyocytes and in haematopoietic cells [24, 25]. The SERCA2d variant and recombinant protein were also described recently although its existence as an endogenous protein remains to be established [26]. The functional characterization of the first three SERCA2 isoforms (Fig. 3B) performed by Dally et al. [25] in HEK-293 cells revealed that recombinant SERCA2 display distinct intrinsic functionalities: SERCA2a and 2b showed different affinity for Ca2+ and turnover rate (K0.5 = 0.985 μM,; 70s-1) and (K0.5 = 0.508 μM; 35 s-1), respectively. In the same conditions, SERCA2c showed an even lower affinity and turnover rate similar to SERCA2b (K0.5 = 1.604 μM; 36 s−1). The reference for these experiments was SERCA1a which displayed an affinity similar to SERCA2a and an ATP hydrolysis rate similar to SERCA2b or −2c (K0.5 = 1.0.3 μM; 36 s−1) (Fig. 3B).
The larger SERCA family is ATP2A3 which codes for 6 human isoforms from SERCA3a to SERCA3f [27–33]. The splicing pattern of SERCA3 displayed in (Fig. 2C) has recently been reviewed in detail [34]. Several SERCA3 iso-forms (SERCA3a, −3d & −3f) can been found at the mRNA level in nearly every tissue (Fig. 3A). In other words, this means that SERCA3 is co-expressed with SERCA2b in a selected set of cell types such as colonic and gastric epithelium, vascular EC, pancreaticβ-cells and Purkinje neurons of the cerebellum [35–42]. However, even if mRNA distribution of some SERCA3 is detectable in every tested tissue, their level of protein expression might be very low to undetectable. Indeed, the level of SERCA3 expression is heterogeneous within human tissues, and human blood platelets appear to be the richest source [34]. SERCA3 isoforms are characterized by a lower Ca2+ affinity (10 times lower than SERCA1a) and a rapid turnover (around 100 s−1) [43]. They preferentially work at high levels of [Ca2+]i [32, 44]. Additionally, steady-state and transient-kinetic studies showed that SERCA3’s affinity for cytosolic Ca2+ is insensitive to the luminal Ca2+ level [33, 43] (Fig. 3B). This suggests that at normal resting levels SERCA3 isoforms are essentially inactive, but play a role when the cytosolic Ca2+ increases and can modulate the Ca2+ signal independently of the intra-cellular store Ca2+ content.
The diversity of SERCA isoforms suggests that these proteins could control different physiological functions in the cell. Indeed, several SERCA isoforms can be co-expressed within the same cell; expression of certain isoforms (SERCA2b) is constant and ubiquitous, expression of others is associated with the acquisition of some particular differentiation state or specialized function.
2. CALCIUM CYCLING IN ENDOTHELIAL CELLS
2.a. Role of Vascular Endothelial Cells
The vascular endothelium is the internal lining of blood vessels and acts as an important autocrine and paracrine organ. It maintains vascular homeostasis by modulating blood vessel tone, by regulating local cellular growth and extracellular matrix deposition, and by controlling homeostatic as well as inflammatory responses. Endothelial cells (ECs) respond to various agonists and shear stress by producing a variety of vasculoregulatory and vasculotropic molecules able to act locally or at distant sites. Of note, one of the most potent relaxing factors, first called endothelium-derived relaxing factor [45], was then identified as nitric oxide (NO) (Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical [46].
Reduced bioactivity of NO is the current benchmark for recognizing endothelial cell dysfunction [47]. Endothelial dysfunction significantly contributes to the increase of platelet-vessel wall interaction, vasoconstriction, pro-inflammation, and proliferation. Under these conditions, endothelium-dependent vasodilation is reduced, and endothelium-dependent constrictor responses are augmented. Upon vessel wall injury, platelets rapidly adhere to the exposed sub-endothelial matrix, through several cellular receptors present on platelets or endothelial cells and various adhesive proteins. Subsequent platelet activation results in the recruitment of additional platelets and the generation of platelet aggregates, forming a stable platelet plug, a primary event of atherosclerosis lesion formation. Thus, alteration of the vascular endothelium is a primary event in the pathogenesis of the atherosclerotic process and is well described in patients with cardiovascular diseases and diabetes [48–50].
Strategies capable of assessing changes in the vascular endothelium at the preclinical stage hold potential to refine cardiovascular risk. For these reasons, the study of the human endothelium has become central in cardiovascular research and clinical research has focused on elucidating the role of endothelial dysfunction in influencing vascular disease progression [51]. Therapeutic strategies aimed at improving or preserving endothelial function therefore may be promising in terms of preventing and treating coronary artery disease.
2.b. Calcium Cycling and Signalling in Endothelial Cells
In endothelial cells, NO is synthesized by endothelial NO synthase (eNOS, endothelial NOS isoform) through the cleavage of L-arginin (Fig. 4A). Calcium plays a key role in the control of NO synthesis. Physiological stimuli such as shear stress or endothelial receptor agonists lead to a cytosolic Ca2+ rise. This increase in intracellular Ca2+ originates from mobilization of the IP3- and ryanodine-sensitive stores [52], followed by a sustained plateau dependent on the presence of extracellular Ca2+ [53]. Free Ca2+ in the cytosol reversibly bind with calmodulin (CaM); the resulting Ca2+/CaM complexes activate both the disassociation of eNOS from calveolin and NO production.
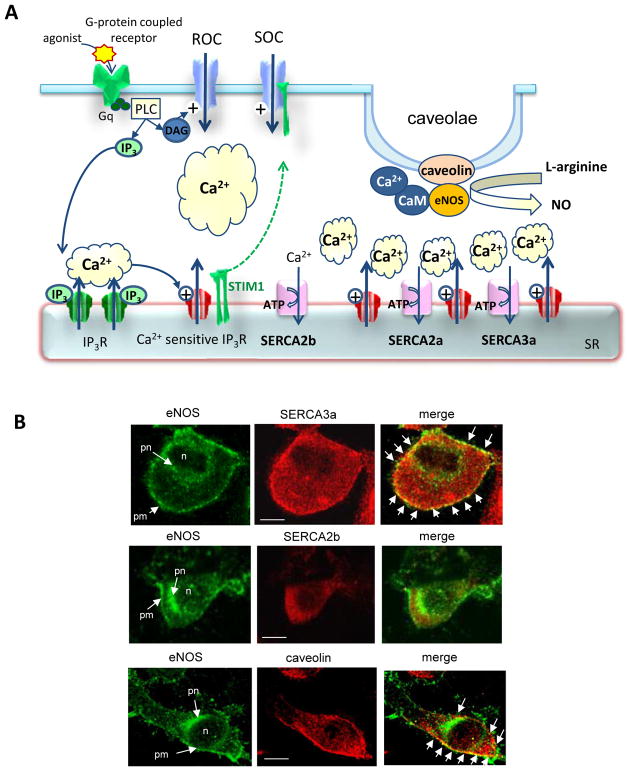
Fig. (4). Involvement of SERCA2a in the control calcium transient in endothelial cells.
A. Schematic representation of the calcium signaling ECs. Abbreviations: CaM - Calmodulin; DAG - Diacylglycerol; GPCR - G-Protein Coupled Receptor; PLC - Phospholipase C; IP3 - Inositol-1,4,5-trisphosphate, IP3R - IP3 Receptor; NO, Nitric Oxyde; eNOS – endothelial NO Synthase; SR/ER - Sarco/Endoplasmic Reticulum; SERCA - SR/ER Ca2+ATPase; STIM1 - Stromal Interaction Molecule 1.
B. Colocalization of eNOS with caveolin and SERCA3a in ECs (EAhy926 cell line). Confocale immunofluorescence with a-eNOS (Trans-duction laboratories), a-caveolin (Transduction laboratories), α-SERCA2b and α-SERCA3a (generated by R. Bobe). Abbreviations: n – nuclei; pn- perinuclear reticulum; pm – sub-plasmamembrane reticulum. Arrows in “merge” indicated colocalization (yellow) of eNOS with SERCA3a and caveolin. Bar – 5 μM.
The initial calcium mobilization through IP3R was described to be insufficient to maintain eNOS activity. Several agonists inducing NO synthesis and vasorelaxation, such as histamine or acetylcholine, trigger in ECs a repetitive increase of free calcium (oscillations of free Ca2+) which takes the form of waves [54–56]. The mode of Ca2+ cycling is determined by the rapidity of ER Ca2+ uptake and the capacity of ER Ca2+ storage (75% of EC intracellular Ca2+ [57]). Indeed, an important determinant of the sensitivity of ER release channels (IP3R and RyR) is the luminal concentration of Ca2+; as this builds up, it increase the sensitivity of the ER release channels that participate to its amplification and progression in process named CICR [58]. The calcium uptake is mainly achieved by SERCA. Requirement of high SERCA activity for NO synthesis was proven by ablation of the SERCA activator S100A1 in EC [59]. This modification diminished agonist-induced [Ca2+]i transients and basal acetylcholine-induced endothelial NO release, leading to impaired endothelium-dependent vascular relaxation and hypertension. By contrast, increasing SERCA activity by S100A1 overexpression in ECs, amplified agonist-induced [Ca2+]i transients and enhanced NO generation.
2.c. Physiological Role of SERCA Isoforms in Endothelial Cells
Endothelial cells express SERCA2b, the fast “cardiac” SERCA2a isoform and numerous SERCA3 isoforms. Of note, SERCA3a, activated only by high concentrations of free cytosolic Ca2+, was described as the major endothelial-specific isoform [60, 61]. Concerning the control of eNOS activity, it appears that both isoforms SERCA3a and SERCA2a are involved. First, SERCA3a, but not SERCA2b is colocalized with eNOS and caveolin in sub-plasmamembrane reticulum in EC (Fig. 4B). Second, genetic ablation of SERCA3 in transgenic mice results in defective endothelium-dependent relaxation indicating a deficit of NO production by EC [62, 63]. Calcium imaging of cultured aortic EC demonstrated that the acetylcholine-induced intracellular Ca2+ signal was sharply diminished in SERCA3-deficient cells and that replenishment of the acetylcholine-responsive Ca2+ stores is severely impaired [62]. Regarding SERCA2a, its physiological function in EC was discovered more recently by Hadri et al. [64]. The authors [64] reported that SERCA2a is normally expressed in human EC and is associated with eNOS in functional protein-protein complex indicating that both proteins are localized in the same calcium environment. Overexpression of SERCA2a in human EC results in increased ER Ca2+ storage and mobilization but does not affect store-operated calcium entry. In line with this, SERCA2a overexpression increased the sensitivity of cells to agonist (histamine) and the amplitude and frequency of histamine-induced Ca2+ oscillations (Fig. 5). Consequently, eNOS activity and cGMP production were significantly increased in SERCA2a overexpressing EC [64]. Moreover, modifying the Ca2+ signal in EC, SERCA2a promotes the expression of eNOS in ECs, further favoring NO production. To summarize: the role of SERCA2a in EC lies in the control of agonist-induced cytosolic Ca2+ oscillations, thereby the Ca2+ dependent processes regulating NOS activity and NO synthesis (Figs. 4 & 5).
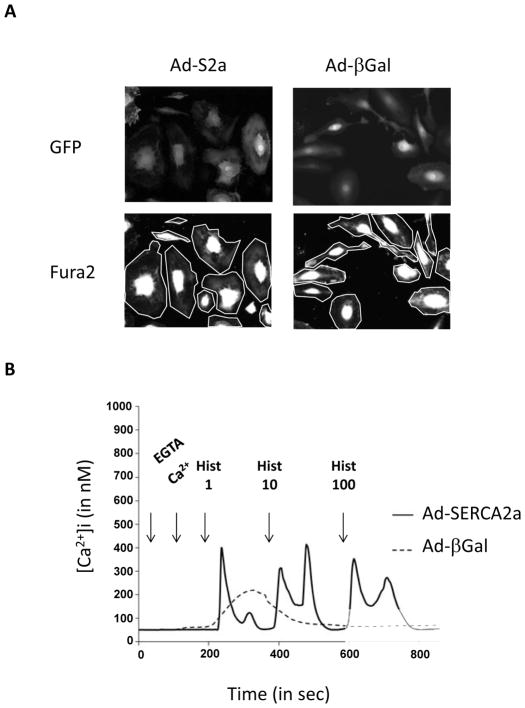
Fig. (5). Effect of SERCA2a overexpression in Histamine-induced calcium transient in endothelial cells.
A. Human coronary artery ECs infected by either Ad-β galactosidase or Ad-SERCA2a were identified by GFP fluorescence (upper). FURA-2 fluorescence (lower) Two area delimited by cell perimeter were monitored for FURA-2 fluorescence recording. B. Calcium responses to increasing histamine doses (1–10 μmol/l) in presence of extracellular calcium (100 μmol/l EGTA plus 300 μmol/l CaCl2) recorded in single (a) control β-gal-infected and (b) for SERCA2a-infected EC.
3. CALCIUM CYCLING AND SIGNALLING IN VASCULAR SMOOTH MUSCLE CELLS
In VSMCs, two SERCA genes (ATP2A2 and ATP2A3) are simultaneously expressed. While expression levels of SERCA3, the lower Ca2+ affinity Ca2+ pumps, remains a minority, SERCA2 isoforms seem to be widely expressed and might participate to the basal control of Ca2+ uptake.
3.a. Diversity of Vascular Smooth Muscle Cell Phenotype
Within blood vessels, SMCs are heterogeneous as they maintain a considerable plasticity along their life allowing vascular SMCs (VSMCs) to display several phenotypes depending of local environment [65, 66]. Accordingly, the current classification of VSMC phenotypes is the dichotomy: synthetic/proliferating/migratory/inflammatory phenotype vs contractile/quiescent/differentiated ones. In mature vessels, the quiescent/contractile phenotype is predominant and in charge of vascular tone control.
Human coronary arteries possess both phenotype and in the media are found contractile VSMCs, while the subendothelial intima contains synthetic VSMCs.
In response to injury, contractile VSMCs changed to a synthetic phenotype. This process plays a vital part in vascular repair, but is also the primary patho-physiological mechanism leading to vascular remodeling occurring in vascular proliferative diseases including atherosclerosis and post-angioplasty restenosis [66]. A similar progression is observed during in-vitro VSMC culture; primary cells rapidly change their differentiated markers to present synthetic/proliferating phenotypes.
VSMCs can also be classified based on their contractile properties being phasic vs. tonic contracting smooth muscle [67]. Phasic smooth muscle displays rhythmic contractile activity, while tonic smooth muscle can be constantly contracted [67]. The tonic contraction is characteristic of the large arteries and veins with the exception of the portal vein. Phasic contraction is observed in small resistance arteries (SRA, 20–50 μm diameters) that regulate vascular function i.e. flow and pressure. Both tonic and phasic contractile activity (termed vasomotion) are observed, in-vivo, in small arteries [68–71] and conducted vasomotor responses [72]. The contractile VSMC phenotypes are controlled by the expression of distinct contractile proteins. The fast isoform of smooth muscle myosin heavy chain (MHC) is only expressed in phasic VSMCs (small arteries of the heart, lung, muscular femoral artery, small mesenteric arteries and renal afferent arterioles), while the slow isoform of smooth muscle myosin, termed “non muscular myosin” (NM-B) is found in all types of VSMCs (meaning that synthetic VSMC only expressed this isoform) [66]. It is though that MHC is involved in the velocity of shortening during vasomotion while NM-B acts in force maintenance during tonic contraction.
As the Ca2+ signalling pathway appears to be responsible for the regulation of both contraction and transcriptional signalling, it is not surprising to observe different types of Ca2+ signal in synthetic and contractile tonic or phasic VSMCs, depending of distinct calcium handling proteins (For review see [65, 73, 74]).
3.b. Calcium Signalling in Synthetic/Proliferating VSMC
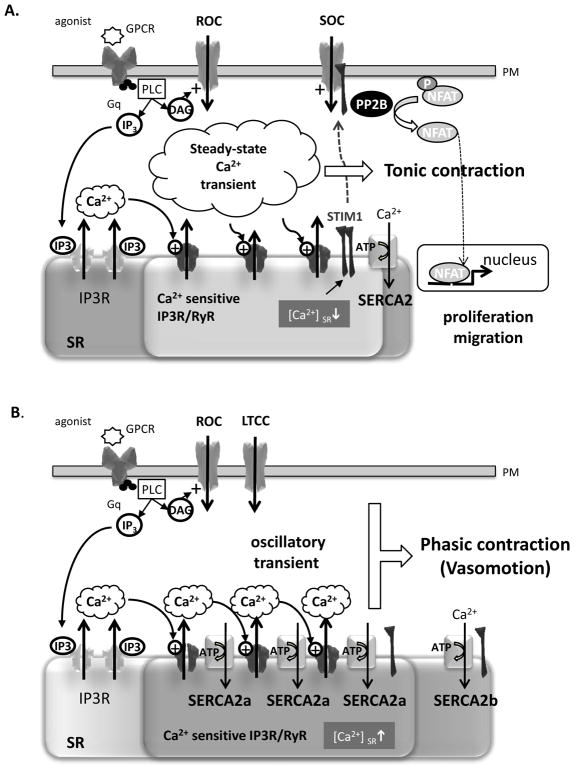
The knowledge of calcium signalling in VSMC is dominated by experiments in cultured in vitro synthetic VSMCs, which do not specifically represent native vessels. In synthetic VSMCs, a general decrease of proteins, such as voltage activated L-type calcium channel (LTCC), SR calcium release channel RyR and the fast isoform of SR calcium pump SERCA2a, that shape the contractile response is observed (Fig. 6A). On the contrary, expression of proteins interfering with SOC channel function (ORAI1-3 units, STIM1 and IP3R) [58, 75] [76, 77] is increased. Therefore, agonist stimulation that results to PLC activation leads to large cytosolic Ca2+ mobilization through IP3R that is poorly re-uptake by SERCA2b in absence of SERCA2a. Additionally, depleted stores trigger STIM1 translocation towards the plasma membrane and activate SOC Ca2+ influx. The resulting long lasting increase in [Ca2+]i is involved in the activation transcription factor NFAT (nuclear factor of activated T lymphocytes) that is thought to play an important part in proliferation [78]. Of note, contractile proteins, such as NM-B, are still expressed in these cells meaning that tonic contraction during steady-state Ca2+ increase can still produce [66].
Fig. (6). Involvement of SERCA2a in the physiological control of SOCE in VSMC.
Schematic representation of the calcium signaling in control synthetic (A) or overexpressing SERCA2a (B) VSMC. GPCR - G-protein coupled receptor; IP3 - inositol-1,4,5-trisphosphate, IP3R - IP3 receptor; NFAT - nuclear factor of activated T lymphocytes; PLC - phospholipase C; RyR, Ryanodine receptor; SR/ER sarco/endoplasmic reticulum; ORAI1 - the pore forming unit; SERCA - SE/ER Ca2+ATPase; STIM1 - Stromal Interaction Molecule 1.
When SERCA2a expression by gene transfer in synthetic VSMCs is restored, both proliferation and migration in the presence of serum were inhibited in association with the inhibition of transcription factor NFAT [79, 80]. Expression of SERCA2a seems to increase Ca2+ uptake, preventing the association between STIM1 and Orai1 and suppressing the store-operated calcium influx [80]. Of note, we did not observe SOC influx in response to agonist stimulation in contractile VSMCs that naturally express SERCA2a (Bobe & Lipskaia, unpublished data). Worthy of mentioning, elevation of cytosolic Ca2+ in response to agonist stimulation is preserved in SERCA2a expressed synthetic VSMCs [80], giving the possibility of tonic contractile response.
3.c. Calcium Signalling in Contractile Tonic and Phasic VSMC
Ca2+ flux in contractile VSMC is predominantly mediated by the plasmalemmal L-type calcium channel (Fig. 6B). LTCC may open in response to membrane depolarization, controlled by a diverse array of potassium and sodium channels and nonselective transient receptor potential ion channels (TRPC), or in response to receptor signaling (receptor operated Ca2+ channels, ROC). In these conditions, the contractile response is dependent on extracellular Ca2+ influx that will initiate Ca2+ mobilization through IP3R (puff) or RyR2 (spark) [58, 81]. This initial increase in cytosolic Ca2+ triggers activation of IP3R and /or RyR clusters (a process termed CICR) resulting in a larger SR Ca2+ mobilization that greatly increases the cytosolic concentration of Ca2+ that will enable VSMC contractions [56, 68]. The nature of intracellular Ca2+ transient controls the type of the contraction. A steady-state increase in Ca2+ creates a tonic contraction, whereas oscillatory Ca2+ transient forms phasic contraction.
Accumulating evidences suggest that the nature of Ca2+ transient in VSMCs depends solely on the SR Ca2+ATPase functioning: i) Phasic VSMCs have a more peripherally located SR compared to centrally located SR in tonic VSMCs, influencing the velocity of Ca2+ uptake (rev in [67, 82]; ii) Drugs interfering with intracellular stores or IP3 pathway, abolish spontaneous vasomotion [83, 84]; iii) inhibition of SERCA activity blocks the Ca2+ oscillations, proving their dependence on Ca2+ release from the SR. Such a paradigm is also supported by the fact that Ca2+ oscillations are preserved in the absence of extracellular Ca2+[71, 80, 85, 86].
A consistent characteristic of Ca2+ oscillations during vasomotion is that they do not represent simple diffusion of released Ca2+, but involve a regenerative release of Ca2+ mediated by CICR through IP3R or RyR. According to Berridge’s model (referred to as “cytosolic oscillator”) the speed with which the SR internal store is loaded plays a critical role in Ca2+ oscillation frequencies by setting the sensitivity of the IP3R or RyR, which determines the next Ca2+ spike[58, 87]. During the activation phase of the membrane receptor and Ca2+channels, Ca2+ spikes are dependent on the sensitivity SR calcium channels that are controlled by the Ca2+ concentration in the SR lumen. Faster the calcium store is refilled, faster the channel re-opened, resulting in the oscillatory mode of Ca2+ cycling required for VSMC phasic contraction.
SERCA2a, the fast Ca2+ pump, expressed in contractile VSMCs, might play an important part in the creation of this “cytosolic oscillator”. Several evidence argue for this proposal: i) compared to SERCA2b, SERCA2a displays a faster catalytic turnover partly due to a faster rate of dephosphorylation and a lower affinity to Ca2+ (Fig. 3B); ii) SERCA2a is not expressed in vein VSMCs that only exhibit tonic contraction tonic contraction (Hadri et al. unpublished data); iii) finally, Bobe et al. (2010) [80] reported direct evidence of SERCA2a involvement in the control of the “cytosolic oscillator”: as its expression into synthetic cultured VSMCs changes the agonist-induced calcium transient from the steady-state to the oscillatory mode, that is characteristic of phasic contractile cells (Fig. 7).
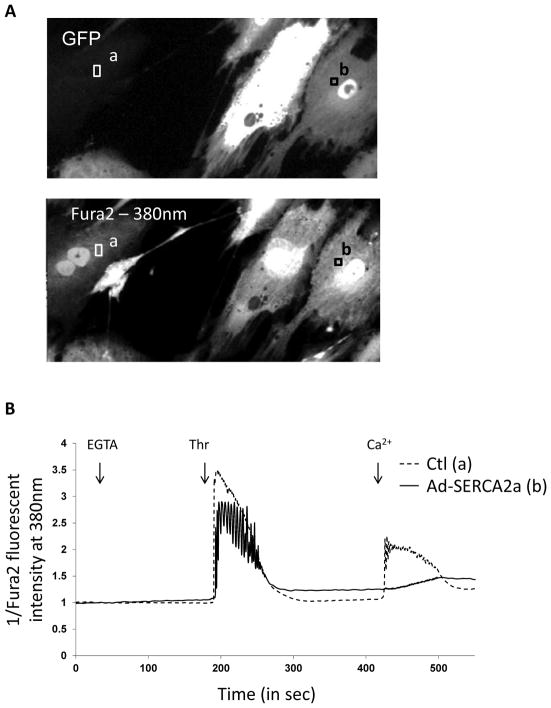
Fig. (7). Effect of SERCA2a expression in VSMC.
A. Ad-SERCA2a-infected cells were identified by GFP fluorescence (upper); FURA-2 fluorescence (lower) was similar in both infected and non-infected cells. Two areas were monitored for FURA-2 fluorescence recording: (a) for non-infected control cells and (b) for SERCA2a-infected cells. B. Typical traces (representative of the [Ca2+]i) recorded in single non-infected (a) or infected cell (b). Cells were treated, in the absence of calcium (EGTA, 100 μM), with 1 U/mL of thrombin and Ca2+ (CaCl2, 300 μM). In order to detect [Ca2+]i oscillations, fluorescence intensity was only recorded in response to one excitation wavelength (380 nm) in order to increase the rate of recording up to 7 images per second.
In conclusion, SERCA2a is acting as a trigger of the “cytosolic oscillator” in VSMCs thus controlling the type of smooth muscle contraction (phasic vs tonic). Furthermore, by increasing SR Ca2+ upload and content, SERCA2a prevents SOC influx and consequently, NFAT dependent proliferation and migration.
4. INTERACTION BETWEEN ENDOTHELIAL AND VASCULAR SMOOTH MUSCLE CELLS: IMPACT ON VASCULAR FUNCTION
In blood vessels, rhythmical contractions occur in small resistance vessels of the microcirculation, as well in the larger arteries [69]. Vasomotion is expected to increase flow as its amplitude increases, decreasing in turn vascular resistance [88]. Vasomotion is viewed as beneficial and its up-regulation during pathological conditions, such as hypertension, may be considered as protective [68].
The control of force production in VSMCs is critical for normal regulation of blood flow and pressure. The phenotypic state of VSMCs influences the contractile response of numerous vasoconstrictor and vasodilator signals, such as thrombin, angiotensin, norepinephrine or nitric oxide, the most important vasodilator source. It is admitted that NO/cGMP may relax VSMCs through reduction in Ca2+ sensitivity or Ca2+ flux [89]. To date, studies have predominantly examined tonic contractions and it is possible that in physically contracting VSMCs, NO/cGMP signaling activates vasomotion [90, 91]. Indeed, in the rat mesenteric artery, removal of the endothelium has been shown to desynchronize oscillations in [Ca2+]i and to abolish vasomotion [71]. This effect has been proposed to be due to the loss of NO and the subsequent activation of a cGMP-dependent depolarizing current in SMCs. In line with this, inhibition of NO with L-NAME abolishes vasomotion and cGMP, and activates vasomotion in endothelium-denuded arteries [92–94]. The fact that NO/cGMP may relax tonic VSMCs and induce force (vasomotion) in phasic VSMCs, argues that this signaling has a strong determinant effect on smooth muscle phenotype.
In intact arteries, oscillations of endothelial [Ca2+]i [95] and membrane potential [96, 97] have been reported in association with vasomotion. The role of these oscillations for vasomotion has not been investigated. As oscillations of cytosolic Ca2+ in ECs are related to NO/cGMP production, we can speculate that the role of the endothelium could be to provide a certain level of cGMP, which is necessary for coordination of the oscillations in the SMCs. Indeed, in the absence of the endothelium, addition of cGMP will lead to a synchronization of the Ca2+ transients in the vascular SMCs and vasomotion. Thus, the EC may therefore have the capacity to pace directly the vascular SMCs and be responsible for vasomotion.
The physiological role of SERCA2a in both vascular ECs and SMCs consists of controling the “cytosolic oscillator” responsible for establishing the oscillatory type of calcium cycling. We propose that SERCA2a gene transfer to the native arteries could promote vasomotion trough simultaneous actions on EC (NO/cGMP production) and SMC (phasic contraction), thereby enhancing blood flow and tissue oxygenation. This effect could be beneficial when SERCA2a is administrated in the context of different cardiovascular pathologies.
5. SERCA2A GENE TRANSFER THERAPY AS GLOBAL STRATEGY FOR CARDIOVASCULAR DISEASES
5.1. Vascular Dysfunction in Cardiovascular Diseases
Microvascular dysfunction is described in a number of pathological conditions, including HF, hypertension, ischemia, coronary artery disease, diabetes, obesity [98–101]. Vascular smooth muscle dysfunction could result from a change in the signal or VSMC function and response; some preliminary data suggest that intrinsic contractility changes in VSMC (reduced frequency, shorten velocity of phasic contractions) are involved in these contexts [102, 103].
Endothelial dysfunction also occurs in a number of cardiovascular diseases, including hypertension, ischemia, diabetes, atherosclerosis [104] and thus results in reduced vasodilatory response in tonic contractile vessels and in reduced vasomotion in phasic contractile vessels. Reduced bioavailability of NO in ECs can result either from the reduction of eNOS expression [64], or defected calcium cycling due to the decrease of SERCA2a activity and/or expression [53, 64, 105].
Results from animal models demonstrated that microvascular dysfunction can be corrected by SERCA2a gene transfer. Sakata et al. has shown that transcoronary gene transfer of SERCA2a increases coronary blood flow in a type 2 diabetic rat model [106]. More recently, Hadri et al. (2010) demonstrated in the porcine HF model, that SERCA2a and eNOS protein expressions were downregulated in the coronary arteries [64]. The authors reported that perfusion of coronary arteries with adeno-associated virus 1 encoding human SERCA2a gene (AAV1.SERCA2a) resulted in i) significant virus transduction of coronary artery EC; ii) restitution of both SERCA2a and eNOS protein expressions to normal levels; iii) increase of coronary blood flow in a swine HF model. Furthermore, in human coronary artery ECs, increasing SERCA2a activity through gene transfer also increased eNOS protein level via transcriptional activation of eNOS promoter [80], increased eNOS phosphorylation, ER Ca2+ storage and Ca2+ mobilization without any changes in SOC entry. Increases in ER Ca2+ content and mobilization can contribute to the increase of the frequency of histamine-induced Ca2+ oscillations and thus to an increase in eNOS activity and cGMP production in EC [64]. These results suggest that increased coronary flow occurring after intracoronary SERCA2a gene transfer in a swine HF model might be due to increased eNOS expression and activity in coronary artery ECs.
Finally, the phase 2 clinical trials evaluating the effects of AAV1.SERCA2a intracoronary administration in patients with advanced HF showed that the mid- and low-dose treated patients groups had clinical effects only during the first six months while the high dose group performed well for the duration of the study. In addition, ventricular tissue from patients in the low and mid dose groups at the time of transplantation or ventricular assist device implantation showed little expression of exogenous SERCA2a. Therefore the transitory beneficial effects of SERCA2a overexpression may be explained by infection of the coronary ECs which would lead to enhanced eNOS expression and improved blood flow. But due to the lifecycle of ECs, this effect would be diluted in a few months [107]. Whether VSMCs from cardiac SRA can also be transduced during intracoronary AAV injection, and whether SERCA2a transduction of small arteries promote vasomotion and tissue oxygenation have yet to be defined.
In conclusion, overexpression of SERCA2a by in vivo AAV1-mediated gene transfer (via intracoronary delivery), in addition to its beneficial effects on contractile function, may also play an important role in the regulation of endothelial function, modulating agonist-induced NO production in ECs.
5.2. Vascular Proliferative Diseases
Extensive proliferation of VSMC is a principal component of vascular occlusive diseases [108]. Antiproliferative approaches used so far to the treat cardiovascular diseases have been focused on restenosis and graft atherosclerosis, during which neointimal hyperplasia is rapid and localized. When atherosclerosis is symptomatic, the principal technique used for restoring blood flow is percutaneous transluminal coronary angioplasty (PCTA), known as balloon angioplasty, followed by coronary stenting. Nonetheless, 10 to 20 % of patients present post-interventional in -stent restenosis due to the re-narrowing of arteries caused by VSMCs proliferation. If this can be reduced using drug-eluting stents (stents eluting antiproliferative drugs), these stents impair the re-endothelialization and can afterward lead to late thrombosis [109, 110]. In humans, contractile VSMC trans-differentiation towards a synthetic phenotype after PTCA appears to be a fundamental process of vascular healing [111]. Conversely, redifferentiation of neointimal VSMCs after stent implantation has been described to be concomitant to lower platelet activation, a decline in inflammation and of EC layer [112]. Therefore establishing novel molecular target(s) of DESs that can simultaneously avoid VSMCs proliferation, antagonize vascular remodeling but facilitating re-endothelialization, is necessary.
SERCA2a can be considered as a promising molecule for preventing in-stent restenosis. We have indeed reported that SERCA2a gene transfer inhibits in a rat carotid injury model intimal thickening, neointimal proliferation, and VSMC transdifferentiation in injured segments while enabling re-endothelialization [79]. Furthermore, SERCA2a gene transfer prevents VSMC transdifferentiation in injured segments while allowing re-endothelialization [79]. Thus, SERCA2a can be considered as a promising molecule for preventing in-stent restenosis. Furthermore, since for the treatment of HF, SERCA2a virus vector is administrated through balloon catheter to coronary arteries, coronary occlusion can be treated at the same time.
Endothelial cells can also be transduced during systemic AAV1.SERCA2a virus vector administration. Endothelial dysfunction and lack of NO associated with an attenuation of endothelium-dependent vasodilatation represent an early marker of vascular regions susceptible to intimal wall thickening [113]. Thus, the endothelium is considered as a target for correctional intervention [114]. SERCA2a transduction of ECs could increase NO synthesis and improve vasodilatation in atherosclerotic vessels. In addition to its function as a vasodilator, NO, released from ECs, reduces vascular permeability, inhibits platelet aggregation and adhesion to the vascular wall, controls the expression of proteins involved in atheroma formation, and decreases the expression of monocyte chemoattractant protein (MCP-1) and of the surface adhesion molecules such as CD11/CD18, VCAM-1, ICAM-1 and P-selectin. In addition, NO decreases oxidation of LDL and inhibits proliferation of VSMCs, producing anti-atherogenic effects [115]. Therapeutic benefits of SERCA2a transduction on development of atherosclerotic disease are yet to be defined.
6. CONCLUSION
Based on recent evidences demonstrating that both amplitude and duration of the Ca2+ signal influence physiological cell function, SERCA regulating the spatiotemporal pattern of Ca2+ transient, has emerged as a critical component of Ca2+ -dependent processes. In the case of VSMCs, oscillatory and steady-state modes of cytosolic Ca2+ cycling related to different modes of vascular functioning: tonic or phasing contraction respectively. Furthermore, the decrease of SERCA function favors activation of SOC entry, thereby inducing proliferation and migration of VSMCs through activation of the transcription factor NFAT. In the case of ECs, the oscillatory mode of Ca2+ cycling is associated with an increase of NOS activity and NO production. Of note, Ca2+ oscillations in ECs are important for vasomotion function. SERCA2a gene transfer switches Ca2+ cycling to oscillatory modes in both VSMCs and ECs. Thus, SERCA2a gene transfer could increase vascular flow and tissue oxygenation and prevent vascular remodeling and atherosclerotic thickening in cardiovascular diseases.
Recent advances in understanding the molecular basis of cardiovascular dysfunction, along with the evolution of increasingly efficient gene transfer technology, have placed cardiovascular diseases within reach of gene based therapy. The recent successful and safe completion of a phase 2 trial targeting SERCA2a in HF opens a new perspective for HF gene therapy. Along with discovery of new Ca2+ sensitive molecular mechanisms in VSMCs and ECs associated with vascular dysfunction in numerous cardiovascular diseases, SERCA2a appears to be a promising therapeutic molecule, able to correct simultaneously several aspects of disease. In the coming years, with a better understanding of the molecular mechanisms of vascular dysfunction associated with cardiovascular diseases, gene therapy of VSMCs and ECs will be considered as a viable adjunctive treatment to mechanical (PCTA; vessel bypass) and pharmacological therapy for vascular diseases.
Acknowledgments
We thank Zela Keuylian for editing.
LIST OF ABBREVIATIONS
- CaM
Calmodulin
- CICR
Calcium-induced calcium release
- DAG
Diacylglycerol
- EC
Endothelial cells
- eNOS
Endothelial nitric oxide synthase
- HF
Heart failure
- IP3
Inositol 1,4,5 phosphate
- LTCC
L-type calcium channels
- MHC
Smooth muscle myosin heavy chain
- NFAT
Nuclear factor of activated T lymphocytes
- NM-B
Non muscular myosin
- NO
Nitric oxide
- IP3R
IP3 receptor
- ROC
Receptor Operated Ca2+ channels
- RyR
Ryanodine receptor-operated channels
- SERCA
Sarco/Endoplasmic Reticulum ATPase
- SOC
Store-operated Ca2+ channels
- STIM1
Stromal interaction molecule 1
- TRPC
Transient receptor potential canonical
- VSMC
Vascular smooth muscle cells
Footnotes
CONFLICT OF INTEREST
LL is supported by AHA SDG 0930116N, LH by NIH (1K01HL103176); RB is supported by Association Française Contre les Myopathies (AFM); JJL is supported by MEC-FEDER BFU2010-C02-01 and by a postdoctoral fellowship from the Junta de Extremadura (POS0922). RJH is supported by NIH grants HL088434, HL080498, HL100396 and NIH/NHLBI Contract HHSN268201000045C.
References
- 1.Lipskaia L, Chemaly ER, Hadri L, Lompre AM, Hajjar RJ. Sarcoplasmic reticulum Ca(2+) ATPase as a therapeutic target for heart failure. Expert Opin Biol Ther. 2010;10(1):29–41. doi: 10.1517/14712590903321462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Del Monte F, Hajjar RJ. Intracellular devastation in heart failure. Heart Fail Rev. 2008;13(2):151–62. doi: 10.1007/s10741-007-9071-9. [DOI] [PubMed] [Google Scholar]
- 3.Bers DM. Calcium cycling and signaling in cardiac myocytes. Ann Rev Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 4.Lipskaia L, Hulot JS, Lompre AM. Role of sarco/endoplasmic reticulum calcium content and calcium ATPase activity in the control of cell growth and proliferation. Pflugers Arch. 2009;457(3):673–85. doi: 10.1007/s00424-007-0428-7. [DOI] [PubMed] [Google Scholar]
- 5.Kawase Y, Hajjar RJ. The cardiac sarcoplasmic/endoplasmic reticulum calcium ATPase: a potent target for cardiovascular diseases. Nat Clin Pract Cardiovasc Med. 2008;5(9):554–65. doi: 10.1038/ncpcardio1301. [DOI] [PubMed] [Google Scholar]
- 6.del Monte F, Williams E, Lebeche D, et al. Improvement in survival and cardiac metabolism after gene transfer of sarcoplasmic reticulum Ca2+ATPase in a rat model of heart failure. Circulation. 2001;104(12):1424–9. doi: 10.1161/hc3601.095574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prunier F, Kawase Y, Gianni D, et al. Prevention of ventricular arrhythmias with sarcoplasmic reticulum Ca2+ ATPase pump overexpression in a porcine model of ischemia reperfusion. Circulation. 2008;118(6):614–24. doi: 10.1161/CIRCULATIONAHA.108.770883. [DOI] [PubMed] [Google Scholar]
- 8.Sakata S, Lebeche D, Sakata N, et al. Restoration of mechanical and energetic function in failing aortic-banded rat hearts by gene transfer of calcium cycling proteins. J Mol Cell Cardiol. 2007;42(4):852–61. doi: 10.1016/j.yjmcc.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kratlian RG, Hajjar RJ. Cardiac gene therapy: from concept to reality. Curr Heart Fail Rep. 2012;9(1):33–9. doi: 10.1007/s11897-011-0077-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tilemann L, Ishikawa K, Weber T, Hajjar RJ. Gene therapy for heart failure. Circulation Res. 2012;110(5):777–93. doi: 10.1161/CIRCRESAHA.111.252981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Putney JW. A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 12.Liou J, Kim ML, Heo WD, et al. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15(13):1235–41. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roos J, DiGregorio PJ, Yeromin AV, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169(3):435–45. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peinelt C, Vig M, Koomoa DL, et al. Amplification of CRAC current by STIM1 and CRACM1 (Orai1) Nat Cell Biol. 2006;8(7):771–3. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soboloff J, Spassova MA, Tang XD, Hewavitharana T, Xu W, Gill DL. Orai1 and STIM reconstitute store-operated calcium channel function. J Biol Chem. 2006;281(30):20661–5. doi: 10.1074/jbc.C600126200. [DOI] [PubMed] [Google Scholar]
- 16.Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol. 2006;174(6):803–13. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen WW, Demaurex N. Morphological and functional aspects of STIM1-dependent assembly and disassembly of store-operated calcium entry complexes. Biochem Soc Transact. 2012;40(1):112–8. doi: 10.1042/BST20110620. [DOI] [PubMed] [Google Scholar]
- 18.Yuan JP, Lee KP, Hong JH, Muallem S. The closing and opening of TRPC channels by Homer1 and STIM1. Acta Physiol (Oxf) 2012;204(2):238–47. doi: 10.1111/j.1748-1716.2011.02319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sampieri A, Zepeda A, Asanov A, Vaca L. Visualizing the store-operated channel complex assembly in real time: Identification of SERCA2 as a new member. Cell Calcium. 2009;45(5):439–46. doi: 10.1016/j.ceca.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 20.Manjarres IM, Rodriguez-Garcia A, Alonso MT, Garcia-Sancho J. The sarco/endoplasmic reticulum Ca(2+) ATPase (SERCA) is the third element in capacitative calcium entry. Cell Calcium. 2010;47(5):412–8. doi: 10.1016/j.ceca.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Burk SE, Lytton J, MacLennan DH, Shull GE. cDNA cloning, functional expression, and mRNA tissue distribution of a third organellar Ca2+ pump. J Biol Chem. 1989;264(31):18561–8. [PubMed] [Google Scholar]
- 22.Chami M, Gozuacik D, Lagorce D, et al. SERCA1 truncated proteins unable to pump calcium reduce the endoplasmic reticulum calcium concentration and induce apoptosis. J Cell Biol. 2001;153(6):1301–14. doi: 10.1083/jcb.153.6.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brandl CJ, Green NM, Korczak B, MacLennan DH. Two Ca2+ ATPases Genes: Homologies and Mechanistic Implications of Deduced Amino Acid Sequences. Cell. 1986;44:597–607. doi: 10.1016/0092-8674(86)90269-2. [DOI] [PubMed] [Google Scholar]
- 24.Gélèbart P, Martin V, Enouf J, Papp B. Identification of a new SERCA2 splice variant regulated during monocytic differentiation. Biochem Biophys Res Commun. 2003;303(2):676–84. doi: 10.1016/s0006-291x(03)00405-4. [DOI] [PubMed] [Google Scholar]
- 25.Dally S, Bredoux R, Corvazier E, et al. Ca2+ATPases in non-failing and failing heart: evidence for a novel cardiac sarco/endoplasmic reticulum Ca2+ATPase 2 isoform (SERCA2c) Biochem J. 2006;395(2):249–58. doi: 10.1042/BJ20051427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimura T, Nakamori M, Lueck JD, et al. Altered mRNA splicing of the skeletal muscle ryanodine receptor and sarcoplasmic/endoplasmic reticulum Ca2+-ATPase in myotonic dystrophy type 1. Hum Mol Genet. 2005;14(15):2189–200. doi: 10.1093/hmg/ddi223. [DOI] [PubMed] [Google Scholar]
- 27.Bobe R, Bredoux R, Wuytack F, et al. The rat platelet 97-kDa Ca2+ATPase isoform is the sarcoendoplasmic reticulum Ca2+ATPase 3 protein. J Biol Chem. 1994;269(2):1417–24. [PubMed] [Google Scholar]
- 28.Wuytack F, Papp B, Verboomen H, et al. A sarco/endoplasmic reticulum Ca2+ATPase 3-type Ca2+ pump is expressed in platelets, in lymphoid cells, and in mast cells. J Biol Chem. 1994;269(2):1410–6. [PubMed] [Google Scholar]
- 29.Bobe R, Lacabaratz-Porret C, Bredoux R, et al. Expression of two isoforms of the third sarco/endoplasmic reticulum Ca2+ATPase (SERCA3) in platelets. Possible recognition of the SERCA3b isoform by the PL/IM430 monoclonal antibody. FEBS Lett. 1998;423(2):259–64. doi: 10.1016/s0014-5793(98)00106-9. [DOI] [PubMed] [Google Scholar]
- 30.Dode L, De Greef C, Mountian I, et al. Structure of the human sarco/endoplasmic reticulum Ca2+ATPase 3 gene. Promoter analysis and alternative splicing of the SERCA3 pre-mRNA. J Biol Chem. 1998;273(22):13982–94. doi: 10.1074/jbc.273.22.13982. [DOI] [PubMed] [Google Scholar]
- 31.Kovacs T, Felfoldi F, Papp B, et al. All three splice variants of the human sarco/endoplasmic reticulum Ca2+ATPase 3 gene are translated to proteins: a study of their co-expression in platelets and lymphoid cells. Biochem J. 2001;358(Pt 3):559–68. doi: 10.1042/0264-6021:3580559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin V, Bredoux R, Corvazier E, et al. Three novel sarco/endoplasmic reticulum Ca2+ATPase (SERCA) 3 isoforms. Expression, regulation, and function of the membres of the SERCA3 family. J Biol Chem. 2002;277(27):24442–52. doi: 10.1074/jbc.M202011200. [DOI] [PubMed] [Google Scholar]
- 33.Bobe R, Bredoux R, Corvazier E, et al. Identification, expression, function, and localization of a novel (sixth) isoform of the human sarco/endoplasmic reticulum Ca2+ATPase 3 gene. J Biol Chem. 2004;279(23):24297–306. doi: 10.1074/jbc.M314286200. [DOI] [PubMed] [Google Scholar]
- 34.Bobe R, Bredoux R, Corvazier E, et al. How many Ca2+ATPase isoforms are expressed in a cell type? A growing family of membrane proteins illustrated by studies in platelets. Platelets. 2005;16(3–4):133–50. doi: 10.1080/09537100400016847. [DOI] [PubMed] [Google Scholar]
- 35.Wu KD, Lee WS, Wey J, Bungard D, Lytton J. Localization and quantification of endoplasmic reticulum Ca(2+)-ATPase isoform transcripts. Am J Physiol. 1995;269(3 Pt 1):C775–84. doi: 10.1152/ajpcell.1995.269.3.C775. [DOI] [PubMed] [Google Scholar]
- 36.Wuytack F, Dode L, Baba-Aissa F, Raeymaekers L. The SERCA3-type of organellar Ca2+ pumps. Biosci Rep. 1995;15(5):299–306. doi: 10.1007/BF01788362. [DOI] [PubMed] [Google Scholar]
- 37.Poch E, Leach S, Snape S, Cacic T, MacLennan DH, Lytton J. Functional characterization of alternatively spliced human SERCA3 transcripts. Am J Physiol. 1998;275:C1449–C58. doi: 10.1152/ajpcell.1998.275.6.C1449. [DOI] [PubMed] [Google Scholar]
- 38.Ozog A, Pouzet B, Bobe R, Lompre AM. Characterization of the 3′ end of the mouse SERCA 3 gene and tissue distribution of mRNA spliced variants. FEBS Lett. 1998;427(3):349–52. doi: 10.1016/s0014-5793(98)00464-5. [DOI] [PubMed] [Google Scholar]
- 39.Baba-Aissa F, Raeymaekers L, Wuytack F, Dode L, Casteels R. Distribution and isoform diversity of the organellar Ca2+ pumps in the brain. Mol Chem Neuropathol. 1998;33(3):199–208. doi: 10.1007/BF02815182. [DOI] [PubMed] [Google Scholar]
- 40.Anger M, Samuel JL, Marotte F, Wuytack F, Rappaport L, Lompré AM. In situ mRNA distribution of sarco(endo)plasmic reticulum Ca2+ATPase isoforms during ontogeny in the rat. J Mol Cell Cardiol. 1994;26:539–50. doi: 10.1006/jmcc.1994.1064. [DOI] [PubMed] [Google Scholar]
- 41.Anger M, Samuel J-L, Marotte F, Wuytack F, Rappaport L, Lompré A-M. The sarco(endo)plasmic reticulum Ca2+ATPase mRNA isoform, SERCA 3, is expressed in endothelial and epithelial cells in various organs. FEBS Lett. 1993;334:45–8. doi: 10.1016/0014-5793(93)81677-r. [DOI] [PubMed] [Google Scholar]
- 42.Arredouani A, Guiot Y, Jonas JC, et al. SERCA3 ablation does not impair insulin secretion but suggests distinct roles of different sarcoendoplasmic reticulum Ca2+ pumps for Ca2+ homeostasis in pancreatic beta-cells. Diabetes. 2002;51(11):3245–53. doi: 10.2337/diabetes.51.11.3245. [DOI] [PubMed] [Google Scholar]
- 43.Dode L, Vilsen B, Van Baelen K, Wuytack F, Clausen JD, Andersen JP. Dissection of the functional differences between sarco(endo)plasmic reticulum Ca2+ATPase (SERCA) 1 and 3 isoforms by steady-state and transient kinetic analyses. J Biol Chem. 2002;277(47):45579–91. doi: 10.1074/jbc.M207778200. [DOI] [PubMed] [Google Scholar]
- 44.Lytton J, Westlin M, Burk SE, Shull GE, MacLennan DH. Functional comparisons between isoforms of the sarcoplasmic or endoplasmic reticulum family of calcium pumps. J Biol Chem. 1992;267:14483–9. [PubMed] [Google Scholar]
- 45.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288(5789):373–6. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 46.Ignarro LJ, Byrns RE, Buga GM, Wood KS. Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circ Res. 1987;61(6):866–79. doi: 10.1161/01.res.61.6.866. [DOI] [PubMed] [Google Scholar]
- 47.Esper RJ, Nordaby RA, Vilarino JO, Paragano A, Cacharron JL, Machado RA. Endothelial dysfunction: a comprehensive appraisal. Cardiovasc Diabetol. 2006;5:4. doi: 10.1186/1475-2840-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schalkwijk CG, Stehouwer CD. Vascular complications in diabetes mellitus: the role of endothelial dysfunction. Clin Sci. 2005;109(2):143–59. doi: 10.1042/CS20050025. [DOI] [PubMed] [Google Scholar]
- 49.Son SM. Role of vascular reactive oxygen species in development of vascular abnormalities in diabetes. Diabetes Res Clin Pract. 2007;77(Suppl 1):S65–70. doi: 10.1016/j.diabres.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 50.Packard RR, Libby P. Inflammation in atherosclerosis: from vascular biology to biomarker discovery and risk prediction. Clin Chem. 2008;54(1):24–38. doi: 10.1373/clinchem.2007.097360. [DOI] [PubMed] [Google Scholar]
- 51.Ramli J, CalderonArtero P, Block RC, Mousa SA. Novel therapeutic targets for preserving a healthy endothelium: strategies for reducing the risk of vascular and cardiovascular disease. Cardiol J. 2011;18(4):352–63. [PMC free article] [PubMed] [Google Scholar]
- 52.Wang XD, Lau F, Li L, Yoshikawa A, Vanbreemen C. Acetylcholine-sensitive intracellular Ca2+ store in fresh endothelial cells and evidence for ryanodine receptors. Circ Res. 1995;77(1):37–42. doi: 10.1161/01.res.77.1.37. [DOI] [PubMed] [Google Scholar]
- 53.Lin S, Fagan KA, Li KX, Shaul PW, Cooper DM, Rodman DM. Sustained endothelial nitric-oxide synthase activation requires capacitative Ca2+ entry. J Biol Chem. 2000;275(24):17979–85. doi: 10.1074/jbc.275.24.17979. [DOI] [PubMed] [Google Scholar]
- 54.Kasai Y, Yamazawa T, Sakurai T, Taketani Y, Iino M. Endothelium-dependent frequency modulation of Ca2+ signalling in individual vascular smooth muscle cells of the rat. J Physiol. 1997;504(Pt 2):349–57. doi: 10.1111/j.1469-7793.1997.349be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paltauf-Doburzynska J, Frieden M, Spitaler M, Graier WF. Histamine-induced Ca2+ oscillations in a human endothelial cell line depend on transmembrane ion flux, ryanodine receptors and endoplasmic reticulum Ca2+-ATPase. J Physiol. 2000;524(Pt 3):701–13. doi: 10.1111/j.1469-7793.2000.00701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aalkjaer C, Nilsson H. Vasomotion: cellular background for the oscillator and for the synchronization of smooth muscle cells. Br J Pharmacol. 2005;144(5):605–16. doi: 10.1038/sj.bjp.0706084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tran QK, Ohashi K, Watanabe H. Calcium signalling in endothelial cells. Cardiovasc Res. 2000;48(1):13–22. doi: 10.1016/s0008-6363(00)00172-3. [DOI] [PubMed] [Google Scholar]
- 58.Berridge MJ. Inositol trisphosphate and calcium signalling mechanisms. Biochim et Biophys Acta. 2009;1793(6):933–40. doi: 10.1016/j.bbamcr.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 59.Pleger ST, Harris DM, Shan C, et al. Endothelial S100A1 modulates vascular function via nitric oxide. Circ Res. 2008;102(7):786–94. doi: 10.1161/CIRCRESAHA.108.172031. [DOI] [PubMed] [Google Scholar]
- 60.Mountian II, Baba-Aissa F, Jonas JC, Humbert De S, Wuytack F, Parys JB. Expression of Ca(2+) Transport Genes in Platelets and Endothelial Cells in Hypertension. Hypertension. 2001;37(1):135–41. doi: 10.1161/01.hyp.37.1.135. [DOI] [PubMed] [Google Scholar]
- 61.Lipskaia L, Pourci ML, Delomenie C, et al. Phosphatidylinositol 3-kinase and calcium-activated transcription pathways are required for VLDL-induced smooth muscle cell proliferation. Circ Res. 2003;92(10):1115–22. doi: 10.1161/01.RES.0000074880.25540.D0. [DOI] [PubMed] [Google Scholar]
- 62.Liu LH, Paul RJ, Sutliff RL, et al. Defective endothelium-dependent relaxation of vascular smooth muscle and endothelial cell Ca2+ signaling in mice lacking sarco(endo)plasmic reticulum Ca2+ATPase isoform 3. J Biol Chem. 1997;272(48):30538–45. doi: 10.1074/jbc.272.48.30538. [DOI] [PubMed] [Google Scholar]
- 63.Kao J, Fortner CN, Liu LH, Shull GE, Paul RJ. Ablation of the SERCA3 gene alters epithelium-dependent relaxation in mouse tracheal smooth muscle. Am J Physiol. 1999;277(2 Pt 1):L264–70. doi: 10.1152/ajplung.1999.277.2.L264. [DOI] [PubMed] [Google Scholar]
- 64.Hadri L, Bobe R, Kawase Y, et al. SERCA2a gene transfer enhances eNOS expression and activity in endothelial cells. Mol Ther. 2010;18(7):1284–92. doi: 10.1038/mt.2010.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wamhoff BR, Bowles DK, Owens GK. Excitation-transcription coupling in arterial smooth muscle. Circ Res. 2006;98(7):868–78. doi: 10.1161/01.RES.0000216596.73005.3c. [DOI] [PubMed] [Google Scholar]
- 66.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84(3):767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 67.Fisher SA. Vascular smooth muscle phenotypic diversity and function. Physiol Genomics. 2010;42A(3):169–87. doi: 10.1152/physiolgenomics.00111.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haddock RE, Hill CE. Rhythmicity in arterial smooth muscle. J Physiol. 2005;566(Pt 3):645–56. doi: 10.1113/jphysiol.2005.086405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nilsson H, Aalkjaer C. Vasomotion: mechanisms and physiological importance. Mol Interv. 2003;3(2):79–89. 51. doi: 10.1124/mi.3.2.79. [DOI] [PubMed] [Google Scholar]
- 70.Parthimos D, Haddock RE, Hill CE, Griffith TM. Dynamics of a three-variable nonlinear model of vasomotion: comparison of theory and experiment. Biophys J. 2007;93(5):1534–56. doi: 10.1529/biophysj.107.106278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peng H, Matchkov V, Ivarsen A, Aalkjaer C, Nilsson H. Hypothesis for the initiation of vasomotion. Circ Res. 2001;88(8):810–5. doi: 10.1161/hh0801.089603. [DOI] [PubMed] [Google Scholar]
- 72.Figueroa XF, Isakson BE, Duling BR. Connexins: gaps in our knowledge of vascular function. Physiology (Bethesda) 2004;19:277–84. doi: 10.1152/physiol.00008.2004. [DOI] [PubMed] [Google Scholar]
- 73.House SJ, Potier M, Bisaillon J, Singer HA, Trebak M. The non-excitable smooth muscle: calcium signaling and phenotypic switching during vascular disease. Pflugers Archiv: Eur J Physiol. 2008;456(5):769–85. doi: 10.1007/s00424-008-0491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thorneloe KS, Nelson MT. Ion channels in smooth muscle: regulators of intracellular calcium and contractility. Can J Physiol Pharmacol. 2005;83(3):215–42. doi: 10.1139/y05-016. [DOI] [PubMed] [Google Scholar]
- 75.Lewis RS. The molecular choreography of a store-operated calcium channel. Nature. 2007;446(7133):284–7. doi: 10.1038/nature05637. [DOI] [PubMed] [Google Scholar]
- 76.Berra-Romani R, Mazzocco-Spezzia A, Pulina MV, Golovina VA. Ca2+ handling is altered when arterial myocytes progress from a contractile to a proliferative phenotype in culture. Am J Physiol Cell Physiol. 2008;295(3):C779–90. doi: 10.1152/ajpcell.00173.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Potier M, Gonzalez JC, Motiani RK, et al. Evidence for STIM1- and Orai1-dependent store-operated calcium influx through ICRAC in vascular smooth muscle cells: role in proliferation and migration. FASEB J. 2009;23(8):2425–37. doi: 10.1096/fj.09-131128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386(6627):855–8. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- 79.Lipskaia L, del Monte F, Capiod T, et al. Sarco/endoplasmic reticulum Ca2+-ATPase gene transfer reduces vascular smooth muscle cell proliferation and neointima formation in the rat. Circ Res. 2005;97(5):488–95. doi: 10.1161/01.RES.0000180663.42594.aa. [DOI] [PubMed] [Google Scholar]
- 80.Bobe R, Hadri L, Lopez JJ, et al. SERCA2a controls the mode of agonist-induced intracellular Ca2+ signal, transcription factor NFAT and proliferation in human vascular smooth muscle cells. J Mol Cell Cardiol. 2011;50(4):621–33. doi: 10.1016/j.yjmcc.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4(7):517–29. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 82.Wray S, Burdyga T. Sarcoplasmic reticulum function in smooth muscle. Physiol Rev. 2010;90(1):113–78. doi: 10.1152/physrev.00018.2008. [DOI] [PubMed] [Google Scholar]
- 83.Bartlett IS, Crane GJ, Neild TO, Segal SS. Electrophysiological basis of arteriolar vasomotion in vivo. J Vasc Res. 2000;37(6):568–75. doi: 10.1159/000054090. [DOI] [PubMed] [Google Scholar]
- 84.Haddock RE, Hill CE. Differential activation of ion channels by inositol 1,4,5-trisphosphate (IP3)- and ryanodine-sensitive calcium stores in rat basilar artery vasomotion. J Physiol. 2002;545(Pt 2):615–27. doi: 10.1113/jphysiol.2002.027904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lino M. Biphasic Ca2+ dependence of inositol 1,4,5-trisphosphate-induced Ca2+ release in smooth muscle cells of the guinea pig taenia caeci. J Gen Physiol. 1990;95:1103–22. doi: 10.1085/jgp.95.6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ruehlmann DO, Lee CH, Poburko D, van Breemen C. Asynchronous Ca(2+) waves in intact venous smooth muscle. Circ Res. 2000;86(4):E72–9. doi: 10.1161/01.res.86.4.e72. [DOI] [PubMed] [Google Scholar]
- 87.Berridge MJ. Smooth muscle cell calcium activation mechanisms. J Physiol. 2008;586(Pt 21):5047–61. doi: 10.1113/jphysiol.2008.160440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Meyer C, de Vries G, Davidge ST, Mayes DC. Reassessing the mathematical modeling of the contribution of vasomotion to vascular resistance. J Appl Physiol. 2002;92(2):888–9. doi: 10.1152/jappl.2002.92.2.888. [DOI] [PubMed] [Google Scholar]
- 89.Lincoln TM, Dey N, Sellak H. Invited review: cGMP-dependent protein kinase signaling mechanisms in smooth muscle: from the regulation of tone to gene expression. J Appl Physiol. 2001;91(3):1421–30. doi: 10.1152/jappl.2001.91.3.1421. [DOI] [PubMed] [Google Scholar]
- 90.Wang W, Nepiyushchikh Z, Zawieja DC, et al. Inhibition of myosin light chain phosphorylation decreases rat mesenteric lymphatic contractile activity. AmJ PhysiolHeart Circ Physiol. 2009;297(2):H726–34. doi: 10.1152/ajpheart.00312.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Seppey D, Sauser R, Koenigsberger M, Beny JL, Meister JJ. Does the endothelium abolish or promote arterial vasomotion in rat mesenteric arteries? Explanations for the seemingly contradictory effects. J Vas Res. 2008;45(5):416–26. doi: 10.1159/000124283. [DOI] [PubMed] [Google Scholar]
- 92.Jackson WF, Busse R. Elevated guanosine 3′:5′-cyclic monophosphate mediates the depression of nitrovasodilator reactivity in endothelium-intact blood vessels. Naunyn Schmiedebergs Arch Pharmacol. 1991;344(3):345–50. doi: 10.1007/BF00183010. [DOI] [PubMed] [Google Scholar]
- 93.Gustafsson H. Vasomotion and underlying mechanisms in small arteries. An in vitro study of rat blood vessels. Acta Physiol Scand Suppl. 1993;614:1–44. [PubMed] [Google Scholar]
- 94.Matchkov VV, Aalkjaer C, Nilsson H. A cyclic GMP-dependent calcium-activated chloride current in smooth-muscle cells from rat mesenteric resistance arteries. J Gen Physiol. 2004;123(2):121–34. doi: 10.1085/jgp.200308972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schuster A, Oishi H, Beny JL, Stergiopulos N, Meister JJ. Simultaneous arterial calcium dynamics and diameter measurements: application to myoendothelial communication. A J Physiol Heart Circ Physiol. 2001;280(3):H1088–96. doi: 10.1152/ajpheart.2001.280.3.H1088. [DOI] [PubMed] [Google Scholar]
- 96.Segal SS, Beny JL. Intracellular recording and dye transfer in arterioles during blood flow control. Am J Physiol. 1992;263(1 Pt 2):H1–7. doi: 10.1152/ajpheart.1992.263.1.H1. [DOI] [PubMed] [Google Scholar]
- 97.Muraki K, Watanabe M, Imaizumi Y. Nifedipine and nisoldipine modulate membrane potential of vascular endothelium via a myoendothelial pathway. Life Sci. 2000;67(26):3163–70. doi: 10.1016/s0024-3205(00)00908-5. [DOI] [PubMed] [Google Scholar]
- 98.Hong H, Aksenov S, Guan X, Fallon JT, Waters D, Chen C. Remodeling of small intramyocardial coronary arteries distal to a severe epicardial coronary artery stenosis. Arteriosclerosis, Thrombosis Vasc Biol. 2002;22(12):2059–65. doi: 10.1161/01.atv.0000041844.54849.7e. [DOI] [PubMed] [Google Scholar]
- 99.Sorop O, Merkus D, de Beer VJ, et al. Functional and structural adaptations of coronary microvessels distal to a chronic coronary artery stenosis. Circulation Res. 2008;102(7):795–803. doi: 10.1161/CIRCRESAHA.108.172528. [DOI] [PubMed] [Google Scholar]
- 100.Werner GS, Ferrari M, Richartz BM, Gastmann O, Figulla HR. Microvascular dysfunction in chronic total coronary occlusions. Circulation. 2001;104(10):1129–34. doi: 10.1161/hc3401.095098. [DOI] [PubMed] [Google Scholar]
- 101.Stepp DW, Belin De Chantemele EJ. Structural remodeling in the limb circulation: impact of obesity and diabetes. Microcirculation. 2007;14(4–5):311–6. doi: 10.1080/10739680701285609. [DOI] [PubMed] [Google Scholar]
- 102.Zhang H, Fisher SA. Conditioning effect of blood flow on resistance artery smooth muscle myosin phosphatase. Circulation Res. 2007;100(5):730–7. doi: 10.1161/01.RES.0000260189.38975.35. [DOI] [PubMed] [Google Scholar]
- 103.Payne MC, Zhang HY, Shirasawa Y, et al. Dynamic changes in expression of myosin phosphatase in a model of portal hypertension. AmJ Physiol Heart Circ Physiol. 2004;286(5):H1801–10. doi: 10.1152/ajpheart.00696.2003. [DOI] [PubMed] [Google Scholar]
- 104.Forstermann U, Mugge A, Alheid U, Haverich A, Frolich JC. Selective attenuation of endothelium-mediated vasodilation in atherosclerotic human coronary arteries. Circ Res. 1988;62(2):185–90. doi: 10.1161/01.res.62.2.185. [DOI] [PubMed] [Google Scholar]
- 105.Adachi T. Modulation of vascular sarco/endoplasmic reticulum calcium ATPase in cardiovascular pathophysiology. Adv Pharmacol. 2010;59:165–95. doi: 10.1016/S1054-3589(10)59006-9. [DOI] [PubMed] [Google Scholar]
- 106.Sakata S, Lebeche D, Sakata Y, et al. Transcoronary gene transfer of SERCA2a increases coronary blood flow and decreases cardiomyocyte size in a type 2 diabetic rat model. Am J Physiol Heart Circ Physiol. 2007;292(2):H1204–7. doi: 10.1152/ajpheart.00892.2006. [DOI] [PubMed] [Google Scholar]
- 107.Jessup M, Greenberg B, Mancini D, et al. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure. Circulation. 2011;124(3):304–13. doi: 10.1161/CIRCULATIONAHA.111.022889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Andres V, Castro C. Antiproliferative strategies for the treatment of vascular proliferative disease. Curr Vasc Pharmacol. 2003;1(1):85–98. doi: 10.2174/1570161033386763. [DOI] [PubMed] [Google Scholar]
- 109.Luscher TF, Steffel J, Eberli FR, et al. Drug-eluting stent and coronary thrombosis: biological mechanisms and clinical implications. Circulation. 2007;115(8):1051–8. doi: 10.1161/CIRCULATIONAHA.106.675934. [DOI] [PubMed] [Google Scholar]
- 110.Fukuda D, Sata M, Tanaka K, Nagai R. Potent inhibitory effect of sirolimus on circulating vascular progenitor cells. Circulation. 2005;111(7):926–31. doi: 10.1161/01.CIR.0000155612.47040.17. [DOI] [PubMed] [Google Scholar]
- 111.Ueda M, Becker AE, Naruko T, Kojima A. Smooth muscle cell de-differentiation is a fundamental change preceding wound healing after percutaneous transluminal coronary angioplasty in humans. Coron Artery Dis. 1995;6(1):71–81. doi: 10.1097/00019501-199501000-00011. [DOI] [PubMed] [Google Scholar]
- 112.Nakagawa M, Naruko T, Ikura Y, et al. A decline in platelet activation and inflammatory cell infiltration is associated with the phenotypic redifferentiation of neointimal smooth muscle cells after bare-metal stent implantation in acute coronary syndrome. J Atheroscler Thromb. 2010;17(7):675–87. doi: 10.5551/jat.3426. [DOI] [PubMed] [Google Scholar]
- 113.Halcox JP, Schenke WH, Zalos G, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106(6):653–8. doi: 10.1161/01.cir.0000025404.78001.d8. [DOI] [PubMed] [Google Scholar]
- 114.Ding H, Triggle CR. Endothelial cell dysfunction and the vascular complications associated with type 2 diabetes: assessing the health of the endothelium. Vasc Health Risk Manag. 2005;1(1):55–71. doi: 10.2147/vhrm.1.1.55.58939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li H, Föstermann U. Nitric oxide in the pathogenesis of vascular disease. J Pathol. 2000;190:244–54. doi: 10.1002/(SICI)1096-9896(200002)190:3<244::AID-PATH575>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 116.Dode L, Andersen JP, Leslie N, Dhitavat J, Vilsen B, Hovnanian A. Dissection of the functional differences between sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) 1 and 2 isoforms and characterization of Darier disease (SERCA2) mutants by steady-state and transient kinetic analyses. J Biol Chem. 2003;278(48):47877–89. doi: 10.1074/jbc.M306784200. [DOI] [PubMed] [Google Scholar]