Abstract
In a previous study, we reported that human milk oligosaccharides (HMOs) isolated from five donor milk samples possessed antimicrobial and antibiofilm activity against Streptococcus agalactiae, also known as Group B Streptococcus or GBS. Herein, we present a broader evaluation of the antimicrobial and antibiofilm activity by screening HMOs from 14 new donors against three strains of GBS and two of the ESKAPE pathogens of particular interest to child health, Staphylococcus aureus and Acinetobacter baumannii. Growth and biofilm assays showed that HMOs from these new donors possessed antimicrobial and antibiofilm activity against all three strains of GBS, antibiofilm activity against methicillin-resistant S. aureus strain USA300, and antimicrobial activity against A. baumannii strain ATCC 19606.
Keywords: S. agalactiae, GBS, S. aureus, A. baumannii, antimicrobial, antibiofilm
Graphical abstract

When antibiotics were first introduced in the 1930s, they were considered the most important advancement in modern medicine. Deaths attributed to communicable diseases were drastically reduced leading to the belief that infectious diseases were conquerable. Bacteria, however, counter antibiotic chemotherapy with resistance mechanisms that result in the emergence of infections untreatable by the current artillery of therapeutics.
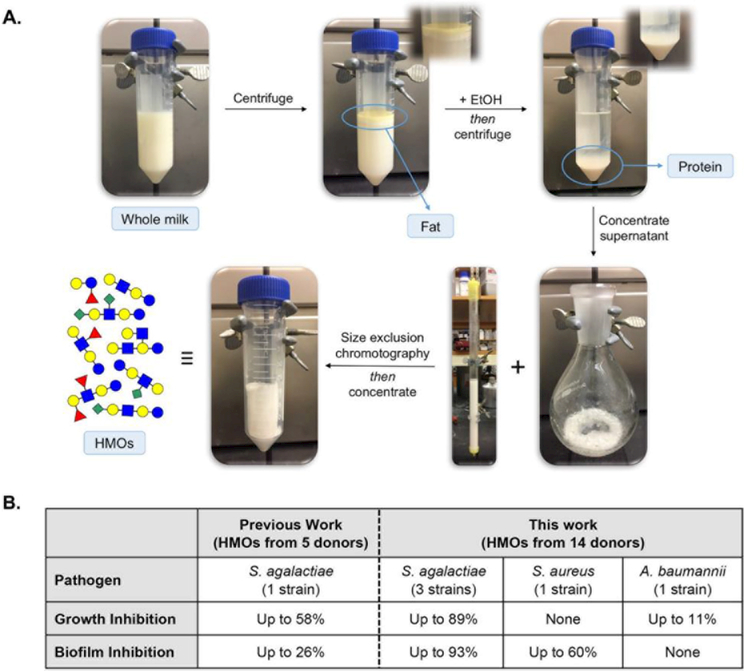
Efforts to develop new antimicrobial agents with unique structural motifs and novel modes of action to fight multidrug resistant pathogens are ongoing.1–3 On the basis of the volume of studies detailing the ability of human milk oligosaccharides (HMOs) to combat enteric gut pathogens, we hypothesized these molecules could function as antimicrobial and antivirulence agents against bacterial pathogens.4–8 This hypothesis was validated in a preliminary study which revealed that HMOs isolated from five donor breast milk samples inhibited the growth and biofilm production of Streptococcus agalactiae (Group B Streptococcus, GBS) (Figure 1). In the presence of one specific sample, the biofilm architecture was also altered.9 Herein, we report the results of a broader evaluation of HMO antimicrobial and antibiofilm activity.
Figure 1.
(A) Schematic illustration of HMO isolation from whole milk. (B) HMO inhibition of growth and biofilm production presented in a previous study and this work.
RESULTS AND DISCUSSION
At the onset of this program, one goal was to expand the number of HMO samples studied in order to investigate a potential relationship between Lewis blood group, secretor status, and biological activity. Prior to bacterial assays, Lewis blood groups for the 14 new donors were assigned using the high throughput mass fingerprinting technique developed by Kunz and co-workers (Tables 2–5). 10 Using this method, Lewis blood group can be determined through analysis of the HMO mass spectrum and specific fragment ions.
Table 2. Antimicrobial Activity of HMOs against Three Strains of S. agalactiae (GBS)a.
| Change in biomass from control (%) | |||||||
|---|---|---|---|---|---|---|---|
|
S. agalactiae CNCTC 10/84 |
S. agalactiae GB590 |
S. agalactiae GB2 |
|||||
| Donor # | Lewis blood group |
THB | THB + 1% glc |
THB | THB + 1% glc |
THB | THB + 1% glc |
| 0 | a−b+ | −4 ± 2 | +11 ± 2 | +14 ± 3 | +11 ± 3 | +5 ± 2 | +9 ± 2 |
| 5 | a−b+ | −26 ± 1 | −12 ± 2 | −31 ± 6 | −9 ± 2 | −22 ± 1 | −5 ± 1 |
| 7 | a−b+ | −3 ± 1 | +13 ± 4 | +6 ± 3 | +8 ± 2 | −1 ± 2 | −3 ± 2 |
| 8 | a−b+ | −80 ± 6 | −5 ± 2 | −75 ± 9 | −8 ± 5 | −89 ± 4 | −6 ± 2 |
| 14 | a−b+ | +3 ± 1 | +43 ± 1 | +8 ± 4 | +50 ± 2 | +14 ± 2 | +57 ± 1 |
| 19 | a−b+ | −8 ± 2 | +7 ± 3 | +13 ± 1 | +28 ± 2 | +11 ± 2 | +14 ± 3 |
| 24 | a−b+ | −11 ± 3 | +8 ± 1 | +11 ± 3 | +20 ± 2 | +9 ± 3 | −3 ± 1 |
| 32 | a−b+ | −14 ± 1 | −16 ± 1 | +10 ± 2 | +15 ± 3 | +14 ± 2 | +6 ± 2 |
| 34 | a−b+ | +2 ± 1 | +2 ± 3 | +21 ± 3 | +25 ± 4 | +15 ± 2 | +19 ± 5 |
| 37 | a−b+ | −1 ± 2 | −17 ± 3 | +23 ± 3 | +24 ± 3 | 0 ± 2 | +19 ± 3 |
| 17 | a+b− | −2 ± 1 | +4 ± 4 | +7 ± 2 | +17 ± 3 | +7 ± 2 | +17 ± 4 |
| 18 | a+b− | −13 ± 3 | +11 ± 1 | −11 ± 3 | +14 ± 2 | −1 ± 2 | −6 ± 2 |
| 29 | a+b− | −42 ± 1 | −17 ± 2 | −35 ± 11 | −22 ± 6 | −15 ± 1 | −6 ± 1 |
| 31 | a+b− | −6 ± 2 | +18 ± 2 | +3 ± 2 | +33 ± 4 | +7 ± 2 | +24 ± 3 |
Significant growth inhibition (p ≤ 0.05, one-way ANOVA) compared to control is boldfaced.
Table 5. Antibiofilm Activity of HMOs against S. aureus and A. baumanniia.
| Change in biofilm/biomass from control (%) | |||||
|---|---|---|---|---|---|
|
S. aureus USA300 |
A. baumannii ATCC 19606 |
||||
| Donor # |
Lewis blood group |
THB |
THB + 1% glc |
THB |
THB + 1% glc |
| 0 | a−b+ | +40 ± 7 | −46 ± 7 | +82 ± 8 | +58 ± 12 |
| 5 | a−b+ | +446 ± 119 | −25 ± 6 | +197 ± 37 | +79 ± 17 |
| 7 | a−b+ | +90 ± 19 | −21 ± 11 | +87 ± 51 | +114 ± 20 |
| 8 | a−b+ | +325 ± 169 | −33 ± 8 | +153 ± 71 | +117 ± 19 |
| 14 | a−b+ | +215 ± 81 | −39 ± 9 | +128 ± 6 | −48 ± 7 |
| 19 | a−b+ | +59 +± 39 | −40 ± 9 | +96 ± 54 | +83 ± 29 |
| 24 | a−b+ | +89 ± 56 | −60 ± 11 | +72 ± 55 | +56 ± 14 |
| 32 | a−b+ | +113 ± 56 | −22 ± 12 | +26 ± 33 | +73 ± 33 |
| 34 | a−b+ | +104 ± 57 | −23 ± 12 | +26 ± 33 | +48 ± 23 |
| 37 | a−b+ | +80 ± 51 | −35 ± 10 | +80 ± 48 | +71 ± 32 |
| 17 | a+b− | +126 ± 51 | −20 ± 11 | +71 ± 48 | +70 ± 22 |
| 18 | a+b− | +160 ± 69 | −8 ± 17 | +90 ± 55 | +95 ± 21 |
| 29 | a+b− | +342 ± 139 | +5 ± 12 | +321 ± 24 | +114 ± 41 |
| 31 | a+b− | +68 ± 42 | −25 ± 9 | +198 ± 29 | +62 ± 21 |
Significant biofilm inhibition (p ≤ 0.05, one-way ANOVA) compared to control is bold.
Blood groups vary by patterns of oligosaccharide fucosylation. These patterns are based on the secretor (Se) and Lewis (Le) blood group systems. Secretor mothers possess an active Se gene locus encoding for the α-1,2 fucosyltransferase FUT2. The presence of this glycosyltransferase results in the production of milk rich in α-1,2 fucosylated HMOs. Non-secretors lack an active Se locus and do not produce HMOs with this glycosidic linkage. Lewis positive mothers have an active Le gene locus encoding for the α-1,3 and α-1,4 fucosyltransferase FUT3 which installs α-1,4 fucosylation. Because Lewis negative mothers do not have an active Le locus, their milk lacks α-1,4 fucosylated HMOs. For a,b blood group classifications, Le-positive secretors are Le (a−b+), Le-positive nonsecretors are Le (a+b−), and Le-negative secretors and Le-negative nonsecretors are both Le (a−b−). The distribution of Lewis blood groups for the mothers in this study, as well as the previous study, tracks well with distributions reported previously for larger populations.11-13
After assigning a blood group to each HMO sample, we next moved to test the hypothesis that HMOs are broad spectrum antimicrobial and antibiofilm agents. Thus, we expanded the scope of bacterial pathogens examined to include three strains of S. agalactiae of varying serotypes (CNCTC 10/84, GB590, and GB2). GBS serotypes are characterized according to the structure of their capsular polysaccharides (CPS). CNCTC 10/84 is a serotype V strain, whereas GB590 is a serotype III, and GB2 is a serotype Ia strain. Of the ten identified serotypes, serotypes Ia, Ib, II, III, and V account for greater than 85% of cases of invasive GBS disease worldwide.14,15 In addition to GBS, we investigated anti-bacterial activity against two additional bacteria known as members of the “ESKAPE” group of pathogens, Staphylococcus aureus (USA300, MRSA) and Acinetobacter baumannii (ATCC 19606). The ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) are the leading cause of multidrug resistant (MDR) nosocomial infections throughout the world and are resistant to many first-line antibiotic therapies thus highlighting the need for new therapeutic strategies.16–18
S. aureus and A. baumannii were specifically selected for this expanded study due to the urgent need to develop therapeutics for infectious diseases that affect pediatric populations (Table 1).19–22 With the incidence of both community- and hospitalacquired staph infections on the rise, S. aureus is one of the most important bacterial pathogens related to human health. S. aureus is a natural colonizer of mammalian skin and mucous membranes while also being the leading cause of skin and soft tissue infections. Although most staph infections are not lifethreatening, S. aureus can cause severe infections such as sepsis, pneumonia, bone and joint infections, and infective endocarditis. In terms of transmission, S. aureus readily spreads through contaminated surfaces and is carried by children and adults alike via dirty hands or fingernails.23–26
Table 1. Important Pathogens Responsible for Infection During Pediatric Age Period.
| age | common pathogens |
|---|---|
| <2 days | Group B Streptococcus |
| 2 days to 2 weeks | Group B Streptococcus |
| 14 to 60 days | Group B Streptococcus |
| Staphylococcus aureus | |
| Escherichia coli | |
| Klebsiella pneumonia | |
| Enterobacteriaceae | |
| Listeria monocytogenes | |
| 2 months to 5 years | Group B Streptococcus |
| Streptococcus pneumoniae | |
| Staphylococcus aureus | |
| Staphylococcus epidermidis | |
| Candida albicans | |
| Haemophilus influenza | |
| Enterobacteriaceae | |
| Acinetobacter baumannii | |
| 60 days to 5 years | Haemophilus influenza |
| Streptococcus pneumonia | |
| Acinetobacter baumannii | |
| 5 to 10 years | Group A Streptococcus |
| Streptococcus pneumoniae | |
| Acinetobacter baumannii | |
| 10 to 21 years | Group A Streptococcus |
| Haemophilus influenza | |
| Streptococcus pneumonia | |
| Mycoplasma pneumoniae | |
| Chlamydia pneumonia |
As for A. baumannii, this pathogen has become a major source of nosocomial infections worldwide. It is particularly known for its ability to survive on hospital equipment and surfaces for long intervals of time as well as for its high incidence of infection among immunocompromised individuals. 27–29 Extending its reach beyond the hospital, A. baumannii has more recently gained notoriety for its increased association with infection among military personnel in combat regions. Its prevalence in the conflict in Iraq has even garnered it colloquial names like “Iraqibacter” and “iraqi-baumannii.” The infection does not, however, necessarily stay contained to the combat zones. As such, the children and relatives of veterans and active duty servicemen and women may face increased risk of infection when the servicemen and women return home.27,28,30 In children, invasive Acinetobacter infections typically present as bacteremia, meningitis, or sepsis, and infections primarily affect newborns or toddlers with underlying medical conditions.31
Antimicrobial and antibacterial activities were evaluated at 24 h. using a plate-based biofilm assay which allows for spectrophotometric quantification of both bacterial growth and biofilm production. For each screen, we examined the effects of HMOs in Todd Hewitt Broth (THB) and THB supplemented with 1% glucose as glucose supplementation has been shown to augment bacterial biofilm production.32,33 All assays were performed using an HMO concentration of ca. 5 mg/mL as this value approximates the low end of physiological concentrations; HMOs are typically found in breast milk at 5–20 mg/mL.34
First, to determine antimicrobial activity, we compared the biomass of bacteria grown in the presence of HMOs to that of bacteria grown in the absence of HMOs. Several HMO samples were found to significantly inhibit bacterial growth for the three GBS strains in both growth conditions (p ≤ 0.05 by one-way ANOVA with posthoc Dunnett’s multiple comparison test) (Table 2 and Figures S17, S21, S24, S28, S31, and S35). The results are presented as the average percent change ± SEM from the control (bacteria grown in unsupplemented media) of three independent experiments each with three technical replicates where negative numbers represent an overall decrease in bacterial growth and positive numbers represent an overall increase in bacterial growth. Additionally, the results are organized such that the data are divided by Lewis blood group. Notably, HMOs from Donor 8 reduced growth by an average of over 70% for all GBS strains when grown in glucosefree media. Interestingly, when GBS was grown in media supplemented with 1% glucose, Donor 8 HMOs decreased growth by less than 10% for all strains.
The profound antimicrobial activity of Donor 8’s HMOs, particularly when compared to that of the other donors tested, may, in part, be a result of when in the lactation period the sample was collected. The time of collection can be important as HMO concentration and expression change over the course of lactation. For example, HMO concentration is highest in colostrum and several reports have shown higher concentrations of α-1,2 fucosylated HMOs, such as 2′-FL, in this early milk.35–37 It is possible that milk from Donor 8 was collected at an earlier lactation stage than the other samples and thus has larger quantities of certain HMOs that are particularly protective against GBS. Due to deidentification, it is difficult to confidently assign reasons for the marked effects of this sample.
While no HMOs demonstrated growth inhibition against S. aureus in either growth medium, HMOs from four samples significantly decreased bacterial growth of A. baumannii in media supplemented with 1% glucose (Table 3 and Figures S38, S41, S44, and S47). Decreases in bacterial growth for these samples ranged from 6% to 11% compared to the control. This result is notable as it reverses the trend for HMO antimicrobial activity seen against GBS. More specifically, against GBS, greater HMO antimicrobial activity was seen in THB than THB + 1% glucose, whereas against A. baumannii, greater activity was seen in THB + 1% glucose over THB.
Table 3. Antimicrobial Activity of HMOs against S. aureus and A. baumanniia.
| Change in biomass from control (%) | |||||
|---|---|---|---|---|---|
|
S. aureus USA300 |
A. baumannii ATCC 19606 |
||||
| Donor # |
Lewis Blood Group |
THB |
THB + 1% glc |
THB | THB + 1% glc |
| 0 | a−b+ | +8 ± 2 | +6 ± 3 | +4 ± 2 | −1 ± 2 |
| 5 | a−b+ | +9 ± 2 | +44 ± 2 | +5 ± 2 | 1 ± 1 |
| 7 | a−b+ | −2 ± 2 | +0 ± 3 | 0 ± 2 | −4 ± 1 |
| 8 | a−b+ | +2 ± 3 | +22 ± 1 | −5 ± 1 | −10 ± 2 |
| 14 | a−b+ | + 1 ± 2 | −7 ± 2 | +6 ± 4 | −2 ± 2 |
| 19 | a−b+ | +10 ± 2 | +11 ± 4 | +2 ± 2 | 0 ± 1 |
| 24 | a−b+ | +4 ± 2 | −3 ± 4 | +2 ± 2 | −6 ± 2 |
| 32 | a−b+ | +3 ± 2 | −4 ± 4 | +7 ± 1 | 0 ± 1 |
| 34 | a−b+ | +6 ± 2 | +1 ± 3 | +8 ± 2 | +4 ± 2 |
| 37 | a−b+ | +8 ± 2 | +5 ± 3 | +8 ± 2 | +2 ± 1 |
| 17 | a+b− | +4 ± 2 | −2 ± 3 | +8 ± 2 | 1 ± 1 |
| 18 | a+b− | −2 ± 2 | −8 ± 5 | −2 ± 2 | −5 ± 1 |
| 29 | a+b− | +5 ± 2 | +12 ± 3 | −7 ± 2 | −11 ± 2 |
| 31 | a+b− | +3 ± 3 | −5 ± 3 | −2 ± 1 | −6 ± 1 |
Significant growth inhibition (p ≤ 0.05, one-way ANOVA) compared to control is boldfaced.
Carbohydrate catabolism has been implicated as a critical step in the pathogenesis of streptococcal disease as a number of mechanisms (i.e., initiation of virulence factors) are closely associated with the ability of streptococci to use glucose.38 We hypothesize that, in the case of GBS, glucose supplementation increases bacterial proliferation thereby assisting the bacteria in averting exposure to HMOs. Conversely, A. baumannii is a member of the glucose nonfermenting class of bacteria which cannot catabolize glucose and thus cannot use glucose oxidatively.39 While glucose catabolism is not possible, glucose does enhance A. baumannii anabolism. Interestingly, it has been demonstrated that glucose availability enhances lipopolysaccharide (LPS) production in A. baumannii.40 In theory, as LPS is a major component of the outer membrane of Gram-negative pathogens, one would anticipate the presence of glucose would enhance the growth of A. baumannii. Thus, more research is needed to explain the observed reversal in selectivity.
To determine changes in biofilm production, we compared biofilm/biomass ratios of bacteria grown in the presence of HMOs to those grown in the absence of HMOs. This ratio accounts for antimicrobial activity and permits analysis of changes in biofilm production relative to the number of bacterial cells. Using this standard, all HMO samples were found to significantly reduce biofilm formation in at least one GBS strain (Table 4 and Figures S18, S19, S22, S25, S26, S29, S32, S33, and S36). In several cases, biofilm inhibition reached as high as 70–80% relative to the control. It is important to note that in order to determine significant reductions in biofilm production when GBS was grown in THB, the results from Donor 8 were omitted from analysis. Results from Donor 8 were confirmed to be outliers by both ROUT (Q = 1%) and Grubbs (a = 0.05) outlier tests. It is likely the exceptionally high biofilm/biomass ratios seen for Donor 8 HMOs are attributable to the extreme reduction in bacterial growth when bacteria were grown in THB. When in THB, HMOs from this donor caused at least a 75% reduction in biomass compared to the control across the three strains. With the less dramatic antimicrobial activity of Donor 8 HMOs observed in THB + 1% glucose, the biofilm/biomass ratios return to more reasonable values in this growth medium.
Table 4. Antibiofilm Activity of HMOs against Three Strains of S. agalactiae (GBS)a, b.
| Change in biomass from control (%) | |||||||
|---|---|---|---|---|---|---|---|
|
S. agalactiae
CNCTC 10/84 |
S. agalactiae
GB590 |
S. agalactiae
GB2 |
|||||
| Donor # |
Lewis
blood group |
THB |
THB + 1% glc |
THB |
THB + 1% glc |
THB |
THB + 1% glc |
| 0 | a−b+ | −67 ± 11b | − 32 ± 13 | −40 ± 28 | −26 ± 6 | −28 ± 1bc | −45 ± 3 |
| 5 | a−b+ | −80a ± 7 | −1 ± 8 | −17 ± 35 | −19 ± 8 | −51 ± 6b | −45 ± 3 |
| 7 | a−b+ | −33 ± 13 | −36 ± 11 | −23 ± 22 | −24 ± 5 | +10 ± 37 | −6 ± 4 |
| 8 | a−b+ | +346 ± 229 | −5 ± 17 | +178 ± 115 | −21 ± 7 | +273 ± 71 | −49 ± 5 |
| 14 | a−b+ | −63 ± 13b | −38 ± 11 | −46 ± 18 | −58 ± 5 | −93 ± 4b | −83 ± 1 |
| 19 | a−b+ | −71 ± 7b | −23 ± 16 | −10 ± 54 | −28 ± 5 | −40 ± 10b | −51 ± 2 |
| 24 | a−b+ | −70 ± 8b | −81 ± 3 | 0 ± 46 | −42 ± 10 | −70 ± 9b | −33 ± 4 |
| 32 | a−b+ | −79 ± 6b | −21 ± 12 | −13 ± 44 | −20 ± 6 | +31 ± 25 | −6 ± 3 |
| 34 | a−b+ | −37 ± 16 | −20 ± 8 | 11 ± 32 | 5 ± 7 | +8 ± 24 | −13 ± 3 |
| 37 | a−b+ | −53 ± 11b | +34 ± 14 | 22 ± 35 | −5 ± 3 | +39 ± 28 | −10 ± 3 |
| 17 | a+b− | −65 ± 7b | −20 ± 8 | −35 ± 17 | −11 ± 3 | +11 ± 24 | −19 ± 3 |
| 18 | a+b− | −38 ± 18 | −40 ± 12 | −18 ± 40 | −18 ± 3 | −53 ± 21b | +7 ± 5 |
| 29 | a+b− | −60 ± 8b | −27 ± 12 | −3 ± 52 | +80 ± 31 | −37 ± 12b | −23 ± 5 |
| 31 | a+b− | −33 ± 15 | −43 ± 9 | −23 ± 25 | −54 ± 5 | −43 ± 10b | −69 ± 2 |
Significant biofilm inhibition (p ≤ 0.05, one-way ANOVA) compared to control is bold.
Statistically significant activity when results from Donor 8 were omitted; Donor 8 was determined to be an outlier by both ROUT and Grubbs tests.
Overall, GBS strain GB2 appeared to be the most susceptible strain as 11 HMO samples significantly reduced biofilm formation. While GB590 also seemed particularly susceptible, due to large fluctuations in biofilm measurements attributable to variations in plate workup, no decreases in biofilm formation when GB590 was grown in THB were deemed significant. No biofilm inhibition was observed for any HMOs against A. baumannii (Table 5 and Figures S45 and S48). We did observe, however, that HMOs from several donors significantly reduced biofilm production in S. aureus (Table 5 and Figures S39 and S42). These reductions ranged from 30% to 60% relative to the control. Interestingly, these results were unique to the THB + 1% glucose growth condition.
While the limited antimicrobial activity of HMOs against the Gram-negative species A. baumannii was not surprising, we were intrigued by the lack of antimicrobial activity seen against S. aureus. An earlier report from the McGuire and Bode laboratories showed that HMO extracts stimulated the growth of S. aureus (isolated from human milk) over 24 h but that this growth-stimulating effect was not attributable to bacterial HMO catabolism. Additionally, they found that the extent of growth stimulation was dependent on the nutritional components of the growth medium.41 While we observed very limited significant bacterial growth increases when S. aureus was grown in the presence of HMOs (only 2 samples caused significant growth increases in either growth medium), this lack of growth does provide additional evidence that S. aureus may not metabolize HMOs (Figures S38 and S41).
In addition to the lack of antimicrobial activity observed against S. aureus, the differing effects of HMOs on S. aureus biofilm production in THB compared to THB + 1% glucose were striking. Moreover, the increase in biofilm production but not biomass when S. aureus was grown in the presence of HMOs in glucose-free THB growth medium was particularly interesting. The potential for HMOs to serve as stimulants for biofilm production was not, however, addressed in the McGuire and Bode study. As a result, we elected to investigate the effects of HMOs on S. aureus growth and biofilm production when the bacteria were treated with a combination of HMOs and a known S. aureus biofilm inhibitor, N-acetylcysteine (Ac-Cys-OH, NAC).42–44 Initial screens were performed to determine both minimum inhibitory concentrations (MICs) of NAC against S. aureus and patterns of biofilm formation in the presence of sub-MIC concentrations of NAC. In both THB and THB + 1% glucose, the MIC was determined to be 8 mg/mL (Figures S49 and S51). For biofilm production in THB, the only significant effect was seen at 2 mg/mL NAC. Despite being a reported biofilm inhibitor, at a concentration 4-fold lower than the MIC, NAC was found to significantly increase biofilm production (Figure S50). This result is not wholly surprising, however, as numerous reports have shown increased bacterial biofilm production for bacterial species, such as S. aureus, when bacteria are grown in the presence of sub-MIC antimicrobial compound concentrations. 45–47 In contrast, no concentration of NAC was found to significantly increase biofilm production when THB + 1% glucose was used. Furthermore, at 4 mg/mL, NAC significantly decreased biofilm formation without completely inhibiting bacterial growth (Figure S52).
For the combined NAC and HMO treatment, we elected to assay 4 HMO samples. Samples 1 and 2 featured HMOs from Donor 5 and Donor 7. These samples were chosen due to their contrasting effects on S. aureus biofilm production (Table 5). For the remaining two samples, HMO cocktails were created on the basis of antimicrobial and antibiofilm activities. Five HMO samples that generally exhibited greater than 10% growth inhibition across the three GBS strains were combined to create an antimicrobial cocktail (am-HMO). Seven HMO samples that generally exhibited greater than 20% reduction in biofilm formation across the GBS strains were similarly combined to create an antibiofilm cocktail (ab-HMO).
We observed that the combined treatment of HMOs (ca. 5 mg/mL) and NAC (at varying concentrations) generally did not result in greater growth inhibition than treatment with NAC alone in either growth medium. In THB, the combinations of 2 mg/mL NAC and HMOs from Donor 5, Donor 7, or the ab-HMO cocktail resulted in a modestly significant reduction in bacterial growth compared to treatment with NAC alone (Figure 2). However, no growth inhibition was observed for any other combination of HMO and NAC in either growth condition. Furthermore, several combinations actually resulted in increased bacterial growth compared to treatment with NAC alone (Figures 2 and S54).
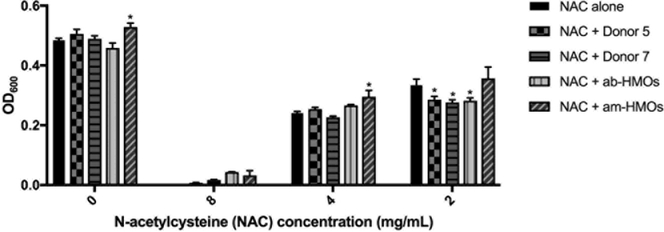
Figure 2.
Biomass for S. aureus strain USA300 after 24 h of growth in THB media alone, media supplemented with ca. 5 mg/mL of HMOs from various samples, media supplemented with varying concentrations of NAC, or media supplemented with combinations of NAC at various concentrations and HMO samples at ca. 5 mg/mL. Data expressed as mean biomass measurements (OD600) ± SEM of 3 separate experiments, each with 3 technical replicates. ** represents p = 0.0028 by two-way ANOVA, F(12 166) = 2.654 with posthoc Dunnett’s multiple comparison test comparing each NAC and HMO combination at a given NAC concentration to NAC alone at the same NAC concentration. When NAC concentration = 0 mg/mL, growth in media alone is compared to growth in media supplemented with HMOs. Any negative biomass values were assigned a value of 0.
For biofilm production at sub-MIC NAC concentrations, only the combination of 2 mg/mL NAC and the am-HMO cocktail in THB + 1% glucose caused a significant reduction in biofilm production compared to treatment with NAC alone. Interestingly, however, this combined treatment did not reduce biofilm levels to a greater extent than treatment with the am-HMO cocktail alone. Moreover, multiple HMO samples were actually found to increase biofilm production relative to treatment with NAC alone in either THB or THB + 1% glucose (Figures 3 and S53). Taken together, the results of these combination studies appear to further demonstrate that HMOs have the potential to act as both growth and biofilm production stimulants for S. aureus.
Figure 3.
Biofilm to biomass ratio for S. aureus USA300 after 24 h of growth in THB + 1% glucose alone, media supplemented with ca. 5 mg/mL of HMOs from various samples, media supplemented with varying concentrations of NAC, or media supplemented with combinations of NAC at various concentrations and HMO samples at ca. 5 mg/mL. Data expressed as mean biofilm/biomass ratio measurements (OD560/600) ± SEM of 3 separate experiments, each with 3 technical replicates. ∗∗∗∗ represents p < 0.0001 by two-way ANOVA, F(12 165) = 4.92 with posthoc Dunnett’s multiple comparison test comparing each NAC and HMO combination at a given NAC concentration to NAC alone at the same NAC concentration. When NAC concentration = 0 mg/mL, growth of S. aureus USA300 in media alone is compared to growth of S. aureus USA300 in media supplemented with ca. 5 mg/mL of the various HMO samples. With all treatments, biofilm/biomass ratios at the MIC concentrations were recorded as 0. Treatment with NAC alone, NAC + Donor 5, and NAC + am-HMOs had an MIC value of 8 mg/mL NAC.
Assaying three GBS strains against 14 donor samples has not revealed a relationship between biological activity and Lewis blood group or secretor status. In fact, the data suggests that HMOs from secretors and nonsecretors generally demonstrate comparable levels of biological activity. While HMOs did not reveal significant antimicrobial activity against MRSA, they were active against A. baumannii demonstrating that the activity is not limited to GBS or Gram-positive pathogens. On the basis of the activity we have observed, it appears as though HMOs generally have narrow-spectrum antimicrobial activity against GBS. In addition to determining the mechanism(s) for this activity, future efforts will focus on detailing whether this activity is specific to S. agalactiae or is general to all streptococci species. Additionally, our results lend support to the claim that S. aureus is unable to catabolize HMOs but that HMOs may serve alternatively as growth stimulants for this bacteria.41
In terms of antibiofilm activity, it appears as though HMOs may possess broader-spectrum antibiofilm activity across Gram-positive species. We observed up to 93% biofilm inhibition against GBS and up to 60% biofilm inhibition against S. aureus relative to biofilm production in the absence of any HMOs. Although significant biofilm inhibition was observed against S. aureus, significant increases in biofilm production were also observed as a result of HMO supplementation. Furthermore, these effects appeared to be largely affected by the nutritional composition of the growth medium. Thus, the ability of HMOs to serve as antibiofilm agents as opposed to biofilm production stimulants will require further study.
From the standpoint of bacterial pathogenesis, the ability to construct and maintain a structured multicellular bacterial community depends on the production of extracellular matrix components. Microbes produce complex biofilm matrices consisting of proteins, extracellular DNA, and polysaccharides. Polysaccharide overproduction can alter the morphology of a colony.48–50 We hypothesize the antibiofilm activity of HMOs is related to the presence of complex, long-chain human milk oligosaccharides. To test this hypothesis, future work is focused on completing HMO fractionation to study the antibiofilm activity of high molecular weight oligosaccharides. In theory, the bacteria could recognize these oligosaccharides as surrogates for “wild-type” bacterial polysaccharides as they are structurally similar.49–52 This mechanism could coerce the bacteria into decreasing production of other biofilm forming components.
In summary, we have shown that HMOs demonstrate the ability to modulate both bacterial growth and biofilm production on a species-dependent basis. It appears as though HMOs possess narrow-spectrum activity against GBS as characterized by the excellent antimicrobial and antibiofilm profiles we have observed thus far. In terms of antibiotic development, narrow-spectrum antibiotics are highly valuable and advantageous over broad-spectrum antibiotics due to their lower susceptibility to resistance development, decreased collateral damage to the host microbiome, and decreased development of antibiotic-associated colitis.53 To compliment these advantages, HMOs are themselves nontoxic at any concentration and are well-known to aid in proper neonate microbiome development. As such, we feel that HMOs possessing narrow-spectrum antibacterial activity represent particularly exciting tools to combat infectious disease.54
Given the growing need for narrow-spectrum antimicrobial agents and the growing potential of HMOs to serve as antibacterials, future efforts are focused on assaying HMOs against additional species of Streptococcus as well as both Gram-positive and Gram-negative pathogens of varying cellular morphologies in order to more fully define the activity spectrum. Further studies will also be undertaken to investigate the mechanisms underlying the growth and biofilm modulating properties we have observed. Results in these regards will be reported in due course.
METHODS
HMO Isolation
Human milk samples were obtained from 14 healthy, lactating women between 3 days and 3 months postnatal under a collection protocol approved by the Vanderbilt University Institutional Review Board (IRB#100897)and were stored at −20 °C. The deidentified milk was provided by Dr. J.-H. Weitkamp from the Vanderbilt Department of Pediatrics under a collection protocol approved by the Vanderbilt University Institutional Review Board (IRB#100897). Milk samples were first thawed then centrifuged for 30 min at 4 °C. Following centrifugation, the resultant top lipid layer was removed. The proteins were then removed by diluting the remaining sample with roughly 1:1 v/v 180 or 200 proof ethanol and centrifuging the samples for 30 min at 4 °C followed by removal of the resulting HMO-containing supernatant. The supernatant was then concentrated in vacuo, and the remaining salts were removed by P-2 Gel (H2O elutant). The oligosaccharides were then dried by lyopholization.9
MS and MS/MS Analysis of HMO Samples
Dried HMO samples were prepared and processed for evaluation by reconstitution in water to approximately 1 mg/mL. These solutions were deposited on a matrix-assisted laser desorption/ionization (MALDI) target plate as follows: 1 μL of HMO was spotted followed by 0.2 μL of 10 mM NaCl and 1 μL of DHB matrix (60 mg/mL in 50% methanol). The spots were allowed to air-dry and then were analyzed in positive ion mode on a 9.4T Fourier transform ion cyclotron resonance (FT-ICR) mass spectrometer (MS) (Bruker Solarix). Mass spectra were acquired in positive ion mode from m/z 300 to 2500. Sodium ion adducts of HMOs were detected with a mass accuracy of >2 ppm. MS/MS analysis was performed for selected ions with a linear ion trap mass spectrometer equipped with a MALDI source (LTQ XL, Thermo Scientific). Selected sodium adduct ions of interest were isolated with a 1 amu window and fragmented via CID using a collision energy of 35 eV.9
Bacterial Strains and Culture Conditions
Bacterial strains used in this study are shown in Table S1. All strains were grown on tryptic soy agar plates supplemented with 5% sheep blood (blood agar plates) at 37 °C in ambient air overnight. Strains were subcultured from blood agar plates into 5 mL of Todd-Hewitt broth (THB) and incubated under shaking conditions at 180 rpm at 37 °C in ambient air overnight. Following overnight incubation, bacterial density was quantified through absorbance readings at 600 nm (OD600) using a Promega GloMax-Multi Detection System plate reader. Bacterial numbers were determined using the predetermined coeffcient of 1 OD600 = 109 CFU/mL.
HMO Bacterial Biofilm Assays
All bacterial strains were grown overnight as described above and used to inoculate fresh THB or THB + 1% glucose at a multiplicity of infection (MOI) of 106 colony forming units per 200 μL of growth medium in 96 well tissue culture treated, sterile polystyrene plates. HMOs isolated from the 14 human milk samples were then added to achieve a final carbohydrate concentration of ca. 5 mg/mL. Bacteria grown in THB or THB + 1% glucose in the absence of any HMOs served as the controls. Biofilm assays were conducted as previously described.9 Briefly, cultures were incubated under static conditions at 37 °C in ambient air for 24 h. Bacterial growth was quantified through absorbance readings at an optical density of 600 nm (OD600). Results were analyzed compared to controls in the absence of HMOs and were expressed as the percent change in biomass with negative numbers indicating a net decrease in biomass and positive numbers indicating a net increase in biomass. Then, culture medium was removed, and wells were washed gently with phosphate buffered saline (PBS, pH 7.4) to remove non-adherent cells; the remaining biofilms were stained with a 10% crystal violet solution for 5–10 min for Gram-positive bacteria and 15–20 min for Gram-negative bacteria. Following staining, wells were washed with PBS and allowed to dry at room temperature for at least 30 min. After drying, the remaining crystal violet stain was solubilized via addition of 200 μL of 80% ethanol/20% acetone solution. Biofilm formation was quantified through absorbance readings (OD560). Results were analyzed compared to controls in the absence of HMOs and expressed as the percent change in biofilm/biomass ratio with negative numbers indicating a net decrease in biofilm production and positive numbers indicating a net increase in biofilm production.
Broth Microdilution Method for Determination of Minimum Inhibitory Concentrations and Biofilm Production Patterns
S. aureus cultures were grown overnight as described above and used to inoculate fresh THB or THB + 1% glucose to achieve 5 × 105 CFU/mL. To 96 well tissue culture treated, sterile polystyrene plates was added the inoculated media in the presence of increasing concentrations of N-acetylcysteine (NAC) to achieve a final volume of 100 μL per well. Bacteria grown in THB or THB + 1% glucose in the absence of NAC served as the controls. The plates were incubated under static conditions at 37 °C in ambient air for 24 h. Bacterial growth was quantified through absorbance readings (OD600). The minimum inhibitory concentrations (MICs) were assigned at the lowest concentration of compound at which no visible growth of bacteria was observed. Biofilm production patterns were then determined using the procedure described above with the exception that the final step of solubilizing the remaining crystal violet stain was done via addition of 100 μL of 80% ethanol/20% acetone.
HMO and NAC Combined Bacterial Biofilm Assays
S. aureus cultures were grown overnight as described above and used to inoculate fresh THB or THB + 1% glucose to achieve 5 × 105 CFU/mL. To the inoculated media was added HMOs from Donor 5, Donor 7, am-HMO cocktail, or ab-HMO cocktail to achieve an HMO concentration of ca. 5 mg/mL. To 96 well tissue culture treated, sterile polystyrene plates was added the HMO-containing inoculated media in the presence of increasing concentrations of N-acetylcysteine (NAC) to achieve a final volume of 100 μL per well. MICs and biofilm production patterns were determined as previously described.
Statistical Analysis
The data shown represent at least 3 independent experiments. Data are expressed as the mean of three technical replicates ± SEM. Statistical analyses were performed in GraphPad Prism Software v. 7.0c. Statistical significance for the individual HMO sample assays and the NAC treatment assays was determined using one-way ANOVA with posthoc Dunnett’s multiple comparison test comparing growth and/or biofilm production in the presence of HMOs or NAC to growth and/or biofilm production in media alone. Statistical significance for the combined NAC and HMO treatment assays was determined using two-way ANOVA with posthoc Dunnett’s multiple comparison test comparing growth and/or biofilm production for each NAC and HMO combination at a given NAC concentration to treatment with NAC alone at the same NAC concentration.
Supplementary Material
ACKNOWLEDGMENTS
S.D.T. would like to acknowledge Vanderbilt University, the Department of Pediatrics at Vanderbilt University Medical Center, and the Institute of Chemical Biology for financial support. J.A.G. is supported by the Department of Veterans Affairs CDA-2 1IK2BX001701. D.L.A. acknowledges a travel grant from the Amgen Foundation and Vanderbilt Pre3 Initiative (Preventing adverse Pregnancy outcomes & Prematurity, a Transinstitutional Program of Vanderbilt University) and has been supported by the Mitchum E. Warren, Jr. Graduate Research Fellowship. K.M.C. acknowledges support from the Vanderbilt Chemical Biology Interface (CBI) training program (T32 GM065086), the Vanderbilt Pre3 Initiative for a travel grant, and a Mitchum E. Warren, Jr. Graduate Research Fellowship. Dr. Michelle Reyzer and Prof. Richard Caprioli are acknowledged for assistance with mass spectral analysis. Clinical strains of GBS (GBS590 and GBS2) were generously provided by Dr. Shannon Manning at Michigan State University. Donor mothers are acknowledged for their generous contribution.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsinfecdis.7b00183.
HMO isolation procedure and schematic representation; 1H and 13C NMR of Donor 14 HMO isolate; MALDI MS and MS/MS spectra for HMO isolates; bacterial strains; HMO biomass, biofilm, and biofilm/biomass figures; NAC biomass and biofilm/biomass figures; NAC and HMO combined treatment biomass and biofilm/biomass figures (PDF)
Author Contributions
D.L.A. completed assays with S. agalactiae. K.M.C. completed assays with S. aureus and A. baumannii. R.S.D. cultured the bacteria. K.M.C. wrote the paper with input from all authors. Each author analyzed the data. J.-H.W. collected the milk samples. S.D.T., J.A.G., and D.M.A. oversaw the research program.
Notes
The authors declare no competing financial interest.
REFERENCES
- (1).Melander RJ, and Melander C (2017) The Challenge of Overcoming Antibiotic Resistance: An Adjuvant Approach? ACS Infect. Dis 3 (8), 559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Rossiter SE, Fletcher MH, and Wuest WM (2017) Natural Products as Platforms To Overcome Antibiotic Resistance. Chem. Rev 117 (19), 12415–12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Wencewicz TA (2016) New antibiotics from Nature’s chemical inventory. Bioorg. Med. Chem 24 (24), 6227–6252. [DOI] [PubMed] [Google Scholar]
- (4).Musilova S, Rada V, Vlkova E, and Bunesova V (2014) Beneficial effects of human milk oligosaccharides on gut microbiota. Benefic. Microbes 5 (3), 273–83. [DOI] [PubMed] [Google Scholar]
- (5).Newburg DS (2013) Glycobiology of human milk. Biochemistry (Moscow) 78 (7), 771–85. [DOI] [PubMed] [Google Scholar]
- (6).Liu B, and Newburg DS (2013) Human milk glycoproteins protect infants against human pathogens. Breastfeed Med 8 (4), 354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Newburg DS (2009) Neonatal protection by an innate immune system of human milk consisting of oligosaccharides and glycans. J. Anim. Sci 87 (13 Suppl), 26–34. [DOI] [PubMed] [Google Scholar]
- (8).Morrow AL, Ruiz-Palacios GM, Jiang X, and Newburg DS (2005) Human-milk glycans that inhibit pathogen binding protect breast-feeding infants against infectious diarrhea. J. Nutr 135 (5), 1304–1307. [DOI] [PubMed] [Google Scholar]
- (9).Ackerman DL, Doster RS, Weitkamp JH, Aronoff DM, Gaddy JA, and Townsend SD (2017) Human Milk Oligosaccharides Exhibit Antimicrobial and Antibiofilm Properties against Group B Streptococcus. ACS Infect. Dis 3, 595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Blank D, Gebhardt S, Maass K, Lochnit G, Dotz V, Blank J, Geyer R, and Kunz C (2011) High-throughput mass finger printing and Lewis blood group assignment of human milk oligosaccharides. Anal. Bioanal. Chem 401 (8), 2495–510. [DOI] [PubMed] [Google Scholar]
- (11).Arifuzzaman M, Ahmed T, Rahman MA, Chowdhury F, Rashu R, Khan AI, LaRocque RC, Harris JB, Bhuiyan TR, Ryan ET, Calderwood SB, and Qadri F (2011) Individuals with Le(a+b−) blood group have increased susceptibility to symptomatic vibrio cholerae O1 infection. PLoS Neglected Trop. Dis 5 (12), e1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Jaff MS (2010) Higher frequency of secretor phenotype in O blood group - its benefits in prevention and/or treatment of some diseases. Int. J. Nanomed 5, 901–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Vague P, Melis C, Mercier P, Vialettes B, and Lassmann V (1978) The increased frequency of the Lewis negative blood group in a diabetic population. Diabetologia 15 (1), 33–6. [DOI] [PubMed] [Google Scholar]
- (14).Melin P, and Efstratiou A (2013) Group B streptococcal epidemiology and vaccine needs in developed countries. Vaccine 31 (Suppl 4), D31–D42. [DOI] [PubMed] [Google Scholar]
- (15).Xia FD, Mallet A, Caliot E, Gao C, Trieu-Cuot P, and Dramsi S (2015) Capsular polysaccharide of Group B Streptococcus mediates biofilm formation in the presence of human plasma. Microbes Infect 17 (1), 71–6. [DOI] [PubMed] [Google Scholar]
- (16).Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, and Bartlett J (2009) Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis 48 (1), 1–12. [DOI] [PubMed] [Google Scholar]
- (17).Pendleton JN, Gorman SP, and Gilmore BF (2013) Clinical relevance of the ESKAPE pathogens. Expert Rev. Anti-Infect. Ther 11 (3), 297–308. [DOI] [PubMed] [Google Scholar]
- (18).Bassetti M, Merelli M, Temperoni C, and Astilean A (2013) New antibiotics for bad bugs: where are we? Ann. Clin. Microbiol. Antimicrob 12, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Alter SJ, Vidwan NK, Sobande PO, Omoloja A, and Bennett JS (2011) Common childhood bacterial infections. Curr. Probl Pediatr Adolesc Health Care 41 (10), 256–83. [DOI] [PubMed] [Google Scholar]
- (20).Van den Bruel A, Bruyninckx R, Vermeire E, Aerssens P, Aertgeerts B, and Buntinx F (2005) Signs and symptoms in children with a serious infection: a qualitative study. BMC Fam. Pract 6, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Rajaratnam JK, Marcus JR, Flaxman AD, Wang H, Levin-Rector A, Dwyer L, Costa M, Lopez AD, and Murray CJ (2010) Neonatal, postneonatal, childhood, and under-5 mortality for 187 countries, 1970–2010: a systematic analysis of progress towards Millennium Development Goal 4. Lancet 375 (9730), 1988–2008. [DOI] [PubMed] [Google Scholar]
- (22).Galetto-Lacour A, and Gervaix A (2010) Identifying severe bacterial infection in children with fever without source. Expert Rev. Anti-Infect. Ther 8 (11), 1231–7. [DOI] [PubMed] [Google Scholar]
- (23).Tong SY, Davis JS, Eichenberger E, Holland TL, and Fowler VG Jr. (2015) Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and manage-ment. Clin. Microbiol. Rev 28 (3), 603–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Cotton JL, Tao J, and Balibar CJ (2009) Identification and characterization of the Staphylococcus aureus gene cluster coding for staphyloferrin A. Biochemistry 48 (5), 1025–35. [DOI] [PubMed] [Google Scholar]
- (25).Lindsay JA, and Holden MT (2004) Staphylococcus aureus: superbug, super genome? Trends Microbiol 12 (8), 378–85. [DOI] [PubMed] [Google Scholar]
- (26).Otto M (2010) Staphylococcus colonization of the skin and antimicrobial peptides. Expert Rev. Dermatol 5 (2), 183–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Howard A, O’Donoghue M, Feeney A, and Sleator RD (2012) Acinetobacter baumannii: an emerging opportunistic pathogen. Virulence 3 (3), 243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Garnacho-Montero J, and Amaya-Villar R (2010) Multi-resistant Acinetobacter baumannii infections: epidemiology and management. Curr. Opin. Infect. Dis 23 (4), 332–9. [DOI] [PubMed] [Google Scholar]
- (29).Sunenshine RH, Wright MO, Maragakis LL, Harris AD, Song X, Hebden J, Cosgrove SE, Anderson A, Carnell J, Jernigan DB, Kleinbaum DG, Perl TM, Standiford HC, and Srinivasan A (2007) Multidrug-resistant Acinetobacter infection mortality rate and length of hospitalization. Emerging Infect. Dis 13 (1), 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).O’Shea MK (2012) Acinetobacter in modern warfare. Int. J. Antimicrob. Agents 39 (5), 363–75. [DOI] [PubMed] [Google Scholar]
- (31).Hu J, and Robinson JL (2010) Systematic Review of Invasive Acinetobacter Infections in Children. Can. J. Infect Dis Med. Microbiol 21 (2), 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Rinaudo CD, Rosini R, Galeotti CL, Berti F, Necchi F, Reguzzi V, Ghezzo C, Telford JL, Grandi G, and Maione D (2010) Specific involvement of pilus type 2a in biofilm formation in group B Streptococcus. PLoS One 5 (2), e9216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Rosini R, and Margarit I (2015) Biofilm formation by Streptococcus agalactiae: influence of environmental conditions and implicated virulence factors. Front. Cell. Infect. Microbiol 5, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Bode L (2012) Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology 22 (9), 1147–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Asakuma S, Urashima T, Akahori M, Obayashi H, Nakamura T, Kimura K, Watanabe Y, Arai I, and Sanai Y (2008) Variation of major neutral oligosaccharides levels in human colostrum. Eur. J. Clin. Nutr 62 (4), 488–94. [DOI] [PubMed] [Google Scholar]
- (36).Kunz C, Meyer C, Collado MC, Geiger L, Garcia-Mantrana I, Bertua-Rios B, Martinez-Costa C, Borsch C, and Rudloff S (2017) Influence of Gestational Age, Secretor, and Lewis Blood Group Status on the Oligosaccharide Content of Human Milk. J. Pediatr. Gastroenterol. Nutr 64 (5), 789–798. [DOI] [PubMed] [Google Scholar]
- (37).Urashima T, Asakuma S, Leo F, Fukuda K, Messer M, and Oftedal OT (2012) The predominance of type I oligosaccharides is a feature specific to human breast milk. Adv. Nutr 3 (3), 473S–82S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Almengor AC, Kinkel TL, Day SJ, and McIver KS (2007) The catabolite control protein CcpA binds to Pmga and influences expression of the virulence regulator Mga in the Group A streptococcus. J. Bacteriol 189 (23), 8405–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Winn W Jr, Allen S, Janda W, Koneman E, Procop G, Schreckenberger P, and Woods G, Eds. (2005) Nonfermenting Gram negative bacilli In Koneman’s Color Atlas and Textbook of Diagnostic Microbiology, 6th ed, pp 305–391, LWW, Baltimore, MD. [Google Scholar]
- (40).Rossi E, Longo F, Barbagallo M, Peano C, Consolandi C, Pietrelli A, Jaillon S, Garlanda C, and Landini P (2016) Glucose availability enhances lipopolysaccharide production and immunogenicity in the opportunistic pathogen Acinetobacter baumannii. Future Microbiol 11 (3), 335–49. [DOI] [PubMed] [Google Scholar]
- (41).Hunt KM, Preuss J, Nissan C, Davlin CA, Williams JE, Shafii B, Richardson AD, McGuire MK, Bode L, and McGuire MA (2012) Human milk oligosaccharides promote the growth of staphylococci. Applied and environmental microbiology 78 (14), 4763–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Perez-Giraldo C, Rodriguez-Benito A, Moran FJ, Hurtado C, Blanco MT, and Gomez-Garcia AC (1997) Influence of N-acetylcysteine on the formation of biofilm by Staphylococcus epidermidis. J. Antimicrob. Chemother 39 (5), 643–646. [DOI] [PubMed] [Google Scholar]
- (43).Leite B, Gomes F, Melo P, Souza C, Teixeira P, Oliveira R, and Pizzolitto E (2013) N-acetylcysteine and vancomycin alone and in combination against staphylococci biofilm. Rev. Bras. Eng. Biomed 29 (2), 184–192. [Google Scholar]
- (44).Aslam S, Trautner BW, Ramanathan V, and Darouiche RO (2007) Combination of tigecycline and N-acetylcysteine reduces biofilm-embedded bacteria on vascular catheters. Antimicrob. Agents Chemother 51 (4), 1556–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Charlebois A, Jacques M, and Archambault M (2014) Biofilm formation of Clostridium perfringens and its exposure to low-dose antimicrobials. Front. Microbiol 5, 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Kaplan JB (2011) Antibiotic-induced biofilm formation. Int. J. Artif Organs 34 (9), 737–51. [DOI] [PubMed] [Google Scholar]
- (47).Lazaro-Diez M, Remuzgo-Martinez S, Rodriguez-Mirones C, Acosta F, Icardo JM, Martinez-Martinez L, and Ramos-Vivas J (2016) Effects of Subinhibitory Concentrations of Ceftaroline on Methicillin-Resistant Staphylococcus aureus (MRSA) Biofilms. PLoS One 11 (1), e0147569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Limoli DH, Jones CJ, and Wozniak DJ (2015) Bacterial Extracellular Polysaccharides in Biofilm Formation and Function. Microbiology Spectrum 3 (3), 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Arciola CR, Campoccia D, Ravaioli S, and Montanaro L (2015) Polysaccharide intercellular adhesin in biofilm: structural and regulatory aspects. Front. Cell. Infect. Microbiol 5, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Xia FD, Mallet A, Caliot E, Gao C, Trieu-Cuot P, and Dramsi S (2015) Capsular polysaccharide of Group B Streptococcus mediates biofilm formation in the presence of human plasma. Microbes Infect 17 (1), 71–76. [DOI] [PubMed] [Google Scholar]
- (51).Zdorovenko EL, Shashkov AS, Zhurina MV, Plakunov VK, and Knirel YA (2015) Structure of the O-specific polysaccharides from planktonic and biofilm cultures of Pseudomonas chlororaphis 449. Carbohydr. Res 404, 93–97. [DOI] [PubMed] [Google Scholar]
- (52).Murphy K, Park AJ, Hao Y, Brewer D, Lam JS, and Khursigara CM (2014) Influence of O Polysaccharides on Biofilm Development and Outer Membrane Vesicle Biogenesis in Pseudomonas aeruginosa PAO1. J. Bacteriol 196 (7), 1306–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Maxson T, and Mitchell DA (2016) Targeted Treatment for Bacterial Infections: Prospects for Pathogen-Specific Antibiotics Coupled with Rapid Diagnostics. Tetrahedron 72 (25), 3609–3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Craft KM, and Townsend SD (2017) The Human Milk Glycome as a Defense Against Infectious Diseases: Rationale, Challenges, and Opportunities. ACS Infect. Dis, DOI: 10.1021/acsinfecdis.7b00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.