CONSPECTUS
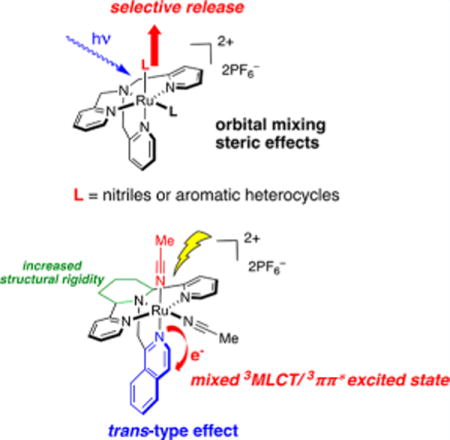
Metal complexes have many proven applications in the caging and photochemical release of biologically active compounds. Photocaging groups derived from Ru(II) traditionally have been composed of ancillary ligands that are planar and bi- or tridentate, such as 2,2′-bipyridine (bpy), 2,2′:6′,2″-terpyridine (tpy), and 1,10-phenanthroline (phen). Complexes bearing ancillary ligands with denticities higher than three represent a new class of Ru(II)-based photocaging groups that are grossly underdeveloped. Because high-denticity ancillary ligands provide the ability to increase the structural rigidity and control the stereochemistry, our groups initiated a research program to explore the applications of such ligands in Ru(II)-based photocaging. Ru(TPA), bearing the tetradentate ancillary ligand tris(2-pyridylmethyl)amine (TPA), has been successfully utilized to effectively cage nitriles and aromatic heterocycles. Nitriles and aromatic heterocycles caged by the Ru(TPA) group show excellent stability in aqueous solutions in the dark, and the complexes can selectively release the caged molecules upon irradiation with light. Ru(TPA) is applicable as a photochemical agent to offer precise spatiotemporal control over biological activity without undesired toxicity. In addition, Ru(II) polypyridyl complexes with desired photochemical properties can be synthesized and identified by solid-phase synthesis, and the resulting complexes show properties to similar to those of complexes obtained by solution-phase synthesis. Density functional theory (DFT) calculations reveal that orbital mixing between the π* orbitals of the ancillary ligand and the Ru−N dσ* orbital is essential for ligand photodissociation in these complexes. Furthermore, the introduction of steric bulk enhances the photoliability of the caged molecules, validating that steric effects can largely influence the quantum efficiency of photoinduced ligand exchange in Ru(II) polypyridyl complexes. Recently, two new photocaging groups, Ru(cyTPA) and Ru(1-isocyTPQA), have been designed and synthesized for caging of nitriles and aromatic heterocycles, and these complexes exhibit unique photochemical properties distinct from those derived from Ru(TPA). Notably, the unusually greater quantum efficiency for the ligand exchange in [Ru(1-isocyTPQA)(MeCN)2](PF6)2, Φ400 = 0.033(3), uncovers a trans-type effect in the triplet metal-to-ligand charge transfer (3MLCT) state that enhances photoinduced ligand exchange in a new manner. DFT calculations and ultrafast transient spectroscopy reveal that the lowest-energy triplet state in [Ru(1-isocyTPQA)(MeCN)2](PF6)2 is a highly mixed 3MLCT/3ππ* excited state rather than a triplet metal-centered ligand-field (3LF) excited state; the latter is generally accepted for ligand photodissociation. In addition, Mulliken spin density calculations indicate that a majority of the spin density in [Ru(1-isocyTPQA)(MeCN)2](PF6)2 is localized on the isoquinoline arm, which is opposite to the cis MeCN, rather than on the ruthenium center. This significantly weakens the Ru−N6 (cis MeCN) bond, which then promotes the ligand photodissociation. This newly discovered effect gives a clearer perception of the interplay between the 3MLCT and 3LF excited states of Ru(II) polypyridyl complexes, which may be useful in the design and applications of ruthenium complexes in the areas of photoactivated drug delivery and photosensitizers.
Graphical abstract
1. INTRODUCTION
Selective release of bioactive molecules can achieve spatiotemporal control over activity, which offers researchers a convenient method to investigate biological systems.1–5 Because of the success of transition metal complexes in photoinduced ligand release with low-energy light, ruthenium(II)-based systems have been developed for caging in a manner that is inherently different from traditional organic chromophores that photorelease substrates developed over the past decade.6–17
Ruthenium(II) polypyridyl complexes have emerged and functioned as photocaging groups for many bioactive molecules.8,12,18–42 Most Ru(II) polypyridyl complexes exhibit lowest-lying triplet metal-to-ligand charge transfer (3MLCT) states with thermally accessible low-lying triplet ligand-field (3LF) state(s) with Ru−L(σ*) antibonding character (L = ligand). Thus, it is generally accepted that ligands coordinated to the metal center can be easily dissociated upon irradiation with light at room temperature through population of the 3LF state(s).27,33,43 In addition, caged molecules are released as a primary photochemical step, thus avoiding undesirable photo-damage that commonly occurs from organic photocages arising from dark reactions.1,43–46 The three-dimensional geometry and tunable photophysical properties also make Ru(II) polypyridyl complexes attractive photocaging agents.27,46–48
[Ru(bpy)3]2+ (bpy = 2,2′-bipyridine) and its derivatives [Ru(bpy)2(L)2]2+ (L = leaving ligand) exhibit strong singlet MLCT (1MLCT) absorption in the visible range. Bpy and related planar heteroaromatic ligands (Figure 1) have been largely employed to protect many bioactive molecules.12,18,19,36,38,41,49 For example, Ru(bpy)2-caged neurotransmitters are able to achieve high levels of spatial and temporal control over receptor activity in live neuronal cells.12,33,49 The Ru(bpy)2 fragment has also been used extensively to cage enzyme inhibitors and small-molecule drugs in live-cell assays.21,28,34,36,50,51 Apart from Ru(bpy)2, Ru(II)-based photocaging groups are frequently composed of bi- or tridentate ancillary ligands, including 2,2′:6′,2″-terpyridine (tpy) and 1,10-phenanthroline (phen).8,21,28,35,39,52,53 These imine-based photocages have been effectively applied to release bioactive molecules bearing nitriles, thioethers, and aromatic heterocycles, which generally cannot be protected by traditional organic photocages.
Figure 1.
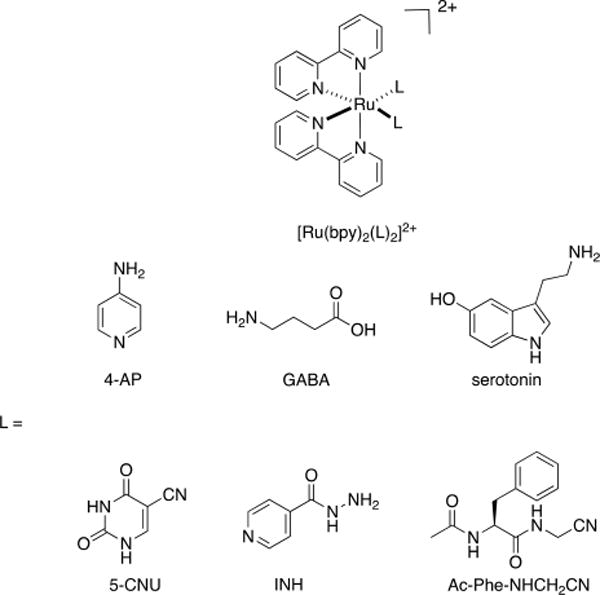
Structures of [Ru(bpy)2(L)2]2+, where L = 4-aminopyridine (4-AP), γ-aminobutyric acid (GABA), serotonin, 5-cyanouracil (5-CNU), isoniazid (INH), and the cathepsin inhibitor Ac-Phe-NHCH2CN.
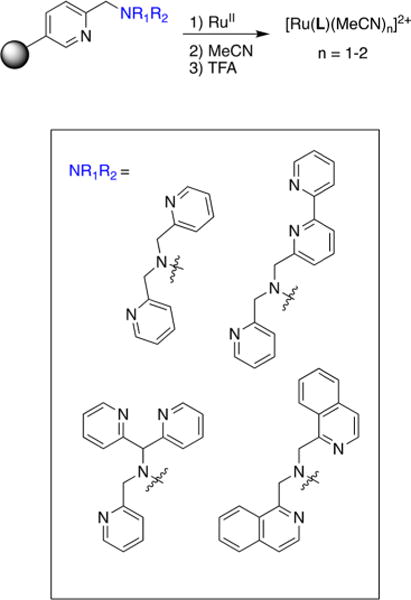
Although Ru(II) polypyridyl complexes bearing these planar bi- or tridentate ancillary ligands have been successfully employed for caging of bioactive molecules, those derived from ancillary ligands with denticities of four or higher have been largely unexplored. Notably, ancillary ligands with higher denticities not only increase the stability of the coordination sphere of Ru(II) polypyridyl complexes but also provide rigidity that reduces nonradiative decay pathways that lead to fast, unproductive excited-state deactivation to the ground state. In this Account, we present the recent development of a new class of Ru(II) photocaging groups based on the structure of the nonplanar tetradentate ligand tris(2-pyridylmethyl)amine (TPA). The TPA ligand, along with its derivatives 2,2′-((2R,6S)-1-(pyridin-2-ylmethyl)piperidine-2,6-diyl)dipyridine (cyTPA) and 1-(((2R,6S)-2,6-bis(pyridin-2-yl)piperidin-1-yl)-methyl)isoquinoline (1-isocyTPQA), were shown to be effective ancillary ligands to Ru(II) for caging applications. The corresponding complexes [Ru(L′)(L)2]+ (L′ = TPA, cyTPA, 1-isocyTPQA; L = caged ligand) are able to cage groups found in bioactive molecules, including nitriles and aromatic heterocycles. The caged complexes are highly stable in the dark but are able to selectively release the molecules of interest upon irradiation with light. In an effort to obtain Ru(II) polypyridyl complexes that possess the desired photoreactivity, a solid-phase synthetic method was introduced to rapidly synthesize and identify complexes that exhibit strong reactivity with visible light. In addition, we validated that orbital mixing and steric effects strongly influence the quantum efficiency of photoinduced ligand exchange. Finally, we discovered a trans-type effect that also plays a critical role in promoting ligand photodissociation from the 3MLCT state induced by 3MLCT/3ππ* mixing instead of from the expected 3LF state(s).
2. THE DEVELOPMENT OF Ru(TPA) FOR PHOTOCAGING
Ru(TPA) is distinct from traditional photocages in terms of structure and photoreactivity. As a result of the nonplanar ligand geometry, Ru(TPA) exhibits markedly different photophysical and photochemical properties compared with traditional complexes containing Ru(bpy)2 and Ru(tpy).26,29,42,43,48,54 In addition, the tertiary amine scaffold of TPA is highly amenable to derivatization,48 making Ru(TPA) an attractive lead for the design of novel Ru(II)-based photocages.
Functionalities such as nitriles and aromatic heterocycles can be effectively protected and liberated from the Ru(TPA) fragment.26,29,42,48 Two complexes containing nitrile ligands for photorelease and three with aromatic heterocycles were prepared, having the general formula [Ru(TPA)(L)2](PF6)2, where L = MeCN (1), the cathepsin K inhibitor Cbz-Leu-NHCH2CN (2), pyridine (py) (3), nicotinamide (NA) (4), and imidazole (Im) (5) (Figure 2). These complexes are highly stable in the dark and are capable of selectively releasing the caged molecules upon irradiation with light. Because the 1MLCT bands in 3–5 are observed at λ > 400 nm, the uncaging process for these complexes can be initiated with visible light instead of the UV light required for nitrile release in 1 and 2. By occupying four coordination sites on the ruthenium center, the tetradentate TPA ligand increases structural rigidity, which results in enhanced dark stability of the complexes compared with the Ru(bpy)2 and Ru(tpy) cages. The quantum yields (Φ350) for the exchange of the nitrile positioned cis to the amine N atom (the cis nitrile) were measured to be Φ350 = 0.012(1) and Φ350 = 0.011(1) for 1 and 2, respectively, in H2O (λirr = 350 nm), which are ∼20-fold lower than that of [Ru(bpy)2(MeCN)2]2+ (Φ400 = 0.21 with λirr = 400 nm). The quantum yields for the ligand exchange of the cis aromatic heterocycles in 3–5 were lower than those of the related nitrile complexes 1 and 2, with values of 9.7(8) × 10−3 for 3, 9.1(8) × 10−3 for 4, and 5.3(3) × 10−4 for 5 in H2O (λirr = 400 nm). This decrease in photodissociation efficiency in the latter is attributed to the stronger σ donation from aromatic heterocycles compared with nitriles along with changes in the electronic structures of the complexes.
Figure 2.
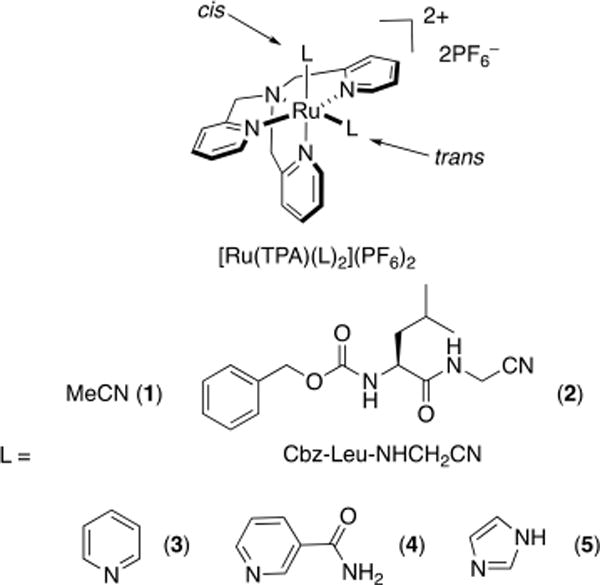
Structures of [Ru(TPA)(L)2](PF6)2, where L = MeCN (1), Cbz-Leu-NHCH2CN (2), py (3), NA (4), and Im (5).
Importantly, complex 2 functions as a potent inhibitor of human cathepsin K upon irradiation with light, exhibiting similar potency to the free inhibitor Cbz-Leu-NHCH2CN under similar experimental conditions. Enzyme inhibition by 2 was enhanced by a factor of ∼89 upon exposure to light, with IC50 values of 63 nM under irradiation and 5.6 μM when 2 was kept in the dark (Figure 3). In addition, cell viability measurements for 3–5 against PC-3 cells showed that the caged complexes and the resulting photochemical byproducts are well-tolerated by PC-3 cells, indicating that Ru(TPA) is applicable as a photochemical tool toward the development of Ru(II) polypyridyl complexes for biological research applications.26 Our data also suggest that the complexes do not generate 1O2 in addition to the release of the caged nitriles.
Figure 3.
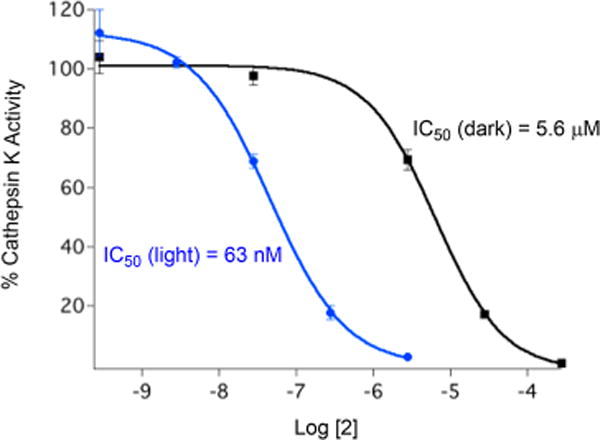
IC50 curves for 2 under light (blue) and dark (black) conditions against human cathepsin K. The reported data are based on three independent experiments. Conditions: 0.4 M acetate buffer (1% DMSO), pH 5.5; [DTT] = 8 mM, [cathepsin K] = 2 nM, and [Z-Gly-Pro-Arg-AMC] = 100 μM; irradiation time (λirr = 365 nm) = 15 min; 8 W light source. The enzyme activity was determined with the fluorogenic substrate Z-Gly-Pro-Arg-AMC. Adapted from ref 29. Copyright 2014 American Chemical Society.
Next, in an effort to achieve spectral tuning of Ru(II) polypyridyl complexes into the visible range, Ru(II)-caged nitriles bearing tetra- and pentadentate ancillary ligands were designed and synthesized by a solid-phase method.48 Briefly, Ru(II)-caged MeCN complexes were synthesized using a library of TPA derivatives obtained on polystyrene resin, which were then metalated using fac-[Ru(DMSO)3(O2CCCF3)2(H2O)], converted into caged nitriles upon treatment with MeCN/H2O, and cleaved from the resin for photochemical analysis (Figure 4). The data show a wide range of spectral tuning and reactivity with visible light for these complexes. Lead complexes exhibiting strong absorption in the visible range were synthesized by solution-phase methods for comparison, and the results showed that complexes obtained by solid-phase synthesis have similar photochemical behavior as those prepared by traditional solution-phase synthesis. However, we noticed that although some complexes show absorption in the visible range, they do not undergo ligand exchange upon irradiation with visible light. Density functional theory (DFT) calculations revealed that apart from absorbing in the desired wavelength range, orbital mixing is essential for effective ligand photodissociation in Ru(II) polypyridyl complexes. For example, in the lead complex [Ru(TQA)(MeCN)2](PF6)2 (6) (TQA = tris(1-isoquinolylmethyl)amine) (Figure 5), the photorelease of the cis MeCN can be attributed to the appropriate orbital mixing between the π* orbitals of the isoquinolyl rings of TQA and the Ru−N (cis MeCN) dσ* orbital in the excited state (Figure 5). However, such an orbital overlap is not preferable for the trans MeCN, causing the trans MeCN to remain bound to the ruthenium center.
Figure 4.
Solid-phase synthesis of Ru(II)-caged MeCN complexes.
Figure 5.
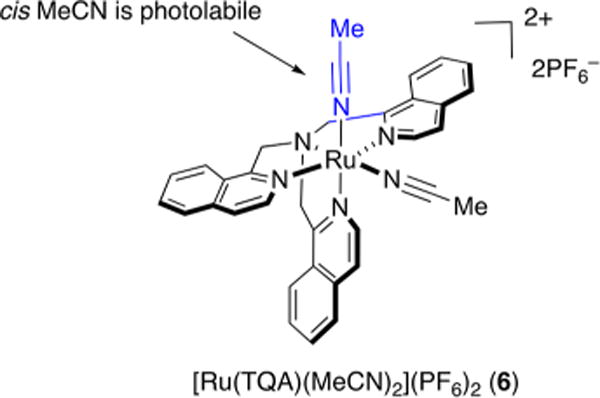
Structure of [Ru(TQA)(MeCN)2(PF6)2 (6).
The introduction of steric bulk in Ru(II) polypyridyl complexes is known to enhance the quantum efficiencies of photoinduced ligand exchange.27,32,35,39 The steric bulk distorts the pseudo-octahedral geometry around the ruthenium center, lowering the energy of the metal-centered 3LF state(s), thus bringing these state(s) closer to the 3MLCT states.32,39 Accordingly, we surmised that steric effects may also influence ligand photodissociation in the Ru(TPA) series. Therefore, in order to investigate steric effects in the Ru(TPA) system, methyl groups were consecutively introduced to the pyridyl arms of TPA, generating three new complexes having the general formula [Ru(L)(MeCN)2](PF6)2, where L = MeTPA (7), Me2TPA (8) and Me3TPA (9) (Figure 6).54 The data confirmed that 7–9 are less stable in the dark than 1 under similar conditions, where the caged MeCN ligands exchange more readily with solvent without irradiation. For example, 8 and 9 undergo thermal ligand exchange in organic solvents and water in short time periods (<1 h) in the absence of light. In addition, our results indicate that the addition of methyl groups into the structure of TPA facilitates the photodissociation of the trans MeCNs in 7–9. Therefore, steric bulk surrounding the coordination sphere must be balanced to ensure dark stability while enhancing photodissociation, showing the profound effect of steric effects on photoactivated ligand dissociation.
Figure 6.
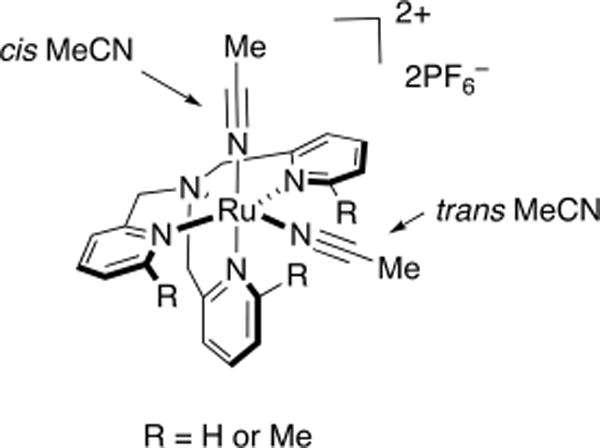
Structures of [Ru(L)(MeCN)2](PF6)2, where L = MeTPA (7), Me2TPA (8), and Me3TPA (9).
3. THE DEVELOPMENT OF Ru(CYTPA) FOR PHOTOCAGING
Tetradentate ligands based on the structure of TPA, namely, cyTPA (10) and 1-isocyTPQA (11) (Figure 7), were recently developed in our group.55 Each ancillary ligand possesses a piperidine ring that not only increases the structural rigidity of the complexes but also avoids the formation of stereoisomers by locking the two pyridyl donors into the trans configuration. In order to examine the photophysical and photochemical properties that arise from Ru(II) complexation of 10 and 11, we prepared [Ru(cyTPA)(MeCN)2]+ (12) and [Ru(1-isocyTPQA)(MeCN)2]+ (13), both of which cage MeCN ligands, as well as the pyridine-caged complexes [Ru(cyTPA)-(py)2]+ (14) and [Ru(1-isocyTPQA)(py)2]+ (15) (Figure 7). Electronic absorption spectra of 12–15 are shown in Figure 8.
Figure 7.
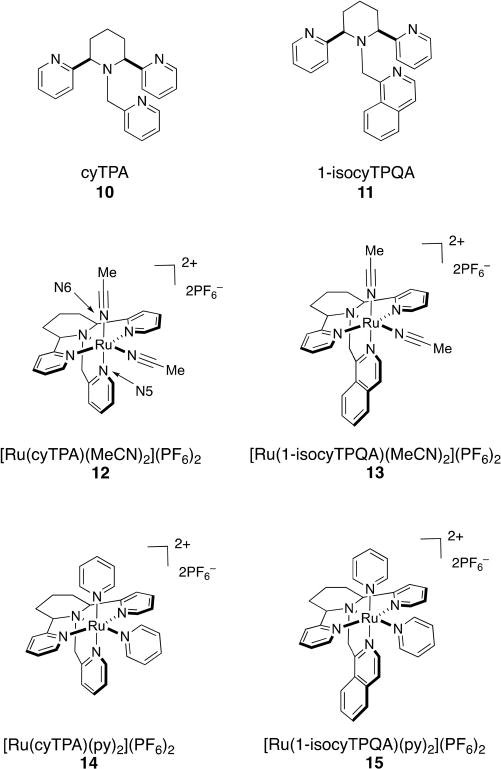
Structures of cyTPA (10), 1-isocyTPQA (11), Ru−MeCN complexes (12) and (13), and Ru−py complexes (14) and (15).
Figure 8.
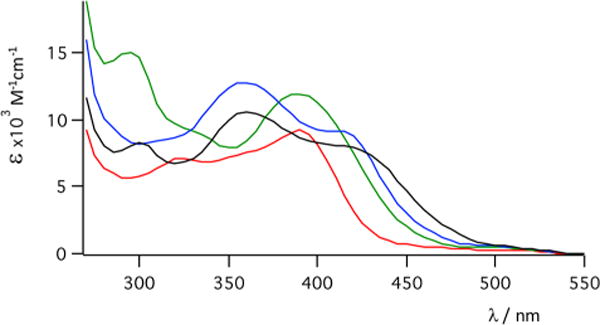
Electronic absorption spectra of 12 (red), 13 (green), 14 (blue), and 15 (black).
All of the complexes derived from TPA preferentially release the MeCN or py ligand positioned cis to the tertiary N atom of the piperidine upon irradiation with light, consistent with the data for 1–5. The introduction of the piperidine ring into the TPA scaffold rigidifies the resulting complexes, and the photoinduced ligand exchange quantum yield of photoinduced ligand exchange in 12 is lower than that of 1, while those of 14 and 15 are lower than that of 3 (Table 1).26,29 Surprisingly, complex 13 has a 5-fold greater quantum yield for ligand exchange relative to 12 and, as expected, a significantly greater quantum yield than the two py-releasing complexes 14 and 15. DFT calculations indicated that the lowest-energy triplet state in 13 is a highly mixed 3MLCT/3ππ* excited state, rather than a 3LF state as is the case in 12, 14, and 15.27 This result was confirmed by Mulliken spin density calculations, which show that unlike 12, 14, and 15, in which the spin density in the lowest-energy triplet state is localized on the Ru(II) center, consistent with a 3LF state, the majority of the spin density in 13 is localized on the isoquinoline ring, which is positioned trans to the leaving cis MeCN ligand (Figure 9). Such an unusual phenomenon likely weakens the Ru−N6 (cis MeCN) bond in 13 in the excited state, leading to the enhancement of the quantum efficiency for the nitrile release. In addition, Mayer bond order (MBO) analyses confirmed this trans-type effect, where in the triplet state the bond strength of Ru−N5 (N in the isoquinoline ring) increases and the bond strength of Ru−N6 (cis MeCN) decreases (Table 2). Ultrafast transient absorption spectra further indicated that ligand dissociation occurs from the 3MLCT/3ππ state in 13 and that higher activation barriers must be overcome in complexes 12, 14, and 15 in order to populate the dissociative 3LF states from their 3MLCT states, consistent with the lower quantum yields observed for the latter. Taken together, these results demonstrate that this newly discovered trans-type effect significantly promotes ligand photodissociation in Ru(II) polypyridyl complexes, which will be useful in the design and applications of these complexes in the areas of photoactivated drug delivery and photosensitizers.
Table 1.
Photophysical Characterization Data for Complexes 1, 3, and 12–15
| complex | Φ | λmax (nm) | ε × 103 (M−1 cm−1)c | |
|---|---|---|---|---|
| L = MeCN | 1 | 0.012(1)a | 380 | 11200 |
| 12 | 0.0066(3)b | 390 | 9200 | |
| 13 | 0.033(3)b | 390 | 11900 | |
| L = py | 3 | 0.0097(8)b | 355 | 10800 |
| 14 | 0.0012(1)b | 360 | 12800 | |
| 15 | 0.0013(1)b | 360 | 10600 |
Quantum yields (Φ) for the first ligand exchange process of 1 in H2O (<5% acetone) at 298 K (λirr = 350 nm).
Quantum yields (Φ) for the first ligand exchange process of 3 and 12–15 in H2O (<5% acetone) at 298 K (λirr = 400 nm).
Extinction coefficients of 1, 3 and 12–15 in DMSO.
Figure 9.
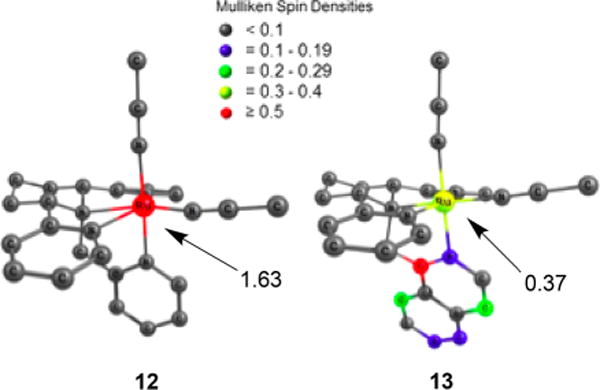
Mulliken spin densities of the lowest-energy triplet state calculated for 12 and 13. Adapted from ref 55. Copyright 2017 American Chemical Society.
Table 2.
MBO Analyses of 12–15 in the Singlet Ground State and Lowest-Energy Triplet State
| complex | Ru−N6a
|
Ru−N5b
|
||
|---|---|---|---|---|
| singlet | triplet | singlet | triplet | |
| 12 | 0.611 | 0.612 | 0.434 | 0.446 |
| 13 | 0.599 | 0.518 | 0.415 | 0.652 |
| 14 | 0.164 | 0.168 | 0.397 | 0.421 |
| 15 | 0.148 | 0.144 | 0.369 | 0.380 |
N6 is the N atom of the cis MeCN in 12 and 13 and cis py in 14 and 15.
N5 is the N atom of the py arm of cyTPA in 12 and 14 and the isoquinoline arm of 1-isocyTPQA in 13 and 15.
4. CONCLUSION
In this Account, a new class of Ru(II)-based photocages bearing the nonplanar tetradentate ancillary ligands TPA and cyTPA have been discussed. Their photoreactivity was evaluated by making Ru(TPA)- and Ru(cyTPA)-caged nitriles and aromatic heterocycles. We have demonstrated that the caged molecules can be selectively released upon irradiation with light. In addition, solid-phase synthesis was introduced for the preparation of Ru(II) polypyridyl complexes possessing the desired photochemical properties, from which the absorptions of the complexes were able to be tuned into the visible range by altering the structure of TPA. Furthermore, we validated that orbital mixing and steric effects can have a strong influence on photoinduced ligand exchange. Finally, a trans-type effect that greatly enhances the quantum efficiency of photodissociation was discovered in the Ru(1-isocyTPQA) system, providing a new mechanism for achieving efficient ligand photodissociation.
It is noteworthy that complexes derived from Ru(cyTPA) and Ru(1-isocyTPQA) exhibit lower quantum yields than those derived from Ru(TPA) under similar experimental conditions. This may result from the increased structural rigidity introduced by the piperidine ring, decreasing the role of orbital mixing between the π* orbitals of the ancillary ligand and the Ru−N6 dσ* orbital. Extending the π conjugation system of the N5 donor or replacing that with fluorophores may reduce the 3MLCT−3LF energy gap, which may in turn promote the quantum efficiency of the complexes in these series.
Our research strongly supports the development of Ru(II) polypyridyl complexes bearing ancillary ligands with high denticities. In the future, we will continue our efforts to design new Ru(II)-based photocages and will also evaluate the properties of these new ligand-release complexes in various biological environments in order to apply them as effective photochemical tools for research applications.
Acknowledgments
We gratefully acknowledge the National Institutes of Health (Grant EB 016072), the National Science Foundation (Grants CHE1212281 and CHE1465067), the Lumigen Instrument Center at Wayne State University, the Center for Chemical and Biophysical Dynamics (CCBD) at The Ohio State University, and the Ohio Supercomputer Center for their generous support of this research.
Biographies
Ao Li graduated from Xiangtan University in China in 2013 with a B.E. in Polymer Materials and Engineering. He received his Ph.D. in Chemistry from Wayne State University in 2018, working under the direction of Prof. Jeremy J. Kodanko. His research focused on the design and synthesis of Ru(II) polypyridyl complexes for effective caging of nitriles and aromatic heterocycles.
Claudia Turro is originally from Argentina and attended Michigan State University, where she earned both her B.S. and Ph.D. in Chemistry. Under the guidance of Profs. Daniel Nocera and George Leroi, her research focused on ultrafast proton-coupled electron transfer and excited states of inorganic complexes. She then investigated the interactions of metal complexes with DNA and other supramolecular systems at Columbia University with Prof. Nicholas Turro as a Jane Coffin Childs Memorial Fellow. She began her independent career as a faculty member at The Ohio State University in 1996.
Jeremy J. Kodanko grew up in northeastern Wisconsin and attended the University of Wisconsin, where he received a B.S. in Chemistry, performing undergraduate research on protease inhibition in the laboratory of Daniel H. Rich. He earned a Ph.D. from the University of California-Irvine under the guidance of Larry E. Overman, where his research focused on natural product synthesis and methods development. He was an NIH postdoctoral fellow at the Massachusetts Institute of Technology in the laboratory of Stephen J. Lippard, focusing on bioinorganic and coordination chemistry. He started his independent career in 2006 at Wayne State University, where his laboratory focuses on the development of metal compounds for biological applications.
Footnotes
ORCID
Jeremy J. Kodanko: 0000-0001-5196-7463
Author Contributions
The manuscript was written through contributions of all authors. All authors have approved the final version of the manuscript. All authors contributed equally.
Notes
The authors declare no competing financial interest.
References
- 1.Klán P, Šolomek T, Bochet CG, Blanc A, Givens R, Rubina M, Popik V, Kostikov A, Wirz J. Photoremovable Protecting Groups in Chemistry and Biology: Reaction Mechanisms and Efficacy. Chem Rev. 2013;113:119–191. doi: 10.1021/cr300177k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ciesienski KL, Franz KJ. Keys for Unlocking Photolabile Metal-Containing Cages. Angew Chem, Int Ed. 2011;50:814–824. doi: 10.1002/anie.201002542. [DOI] [PubMed] [Google Scholar]
- 3.Lee H-M, Larson DR, Lawrence DS. Illuminating the Chemistry of Life: Design, Synthesis, and Applications of “Caged” and Related Photoresponsive Compounds. ACS Chem Biol. 2009;4:409–427. doi: 10.1021/cb900036s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deiters A. Principles and applications of the photochemical control of cellular processes. ChemBioChem. 2010;11:47–53. doi: 10.1002/cbic.200900529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brieke C, Rohrbach F, Gottschalk A, Mayer G, Heckel A. Light-controlled tools. Angew Chem, Int Ed. 2012;51:8446–8476. doi: 10.1002/anie.201202134. [DOI] [PubMed] [Google Scholar]
- 6.Mayer G, Heckel A. Biologically active molecules with a “light switch”. Angew Chem, Int Ed. 2006;45:4900–4921. doi: 10.1002/anie.200600387. [DOI] [PubMed] [Google Scholar]
- 7.Niesel J, Pinto A, Peindy N’Dongo HW, Merz K, Ott I, Gust R, Schatzschneider U. Photoinduced CO release, cellular uptake and cytotoxicity of a tris(pyrazolyl)methane (tpm) manganese tricarbonyl complex. Chem Commun. 2008:1798–1800. doi: 10.1039/b719075a. [DOI] [PubMed] [Google Scholar]
- 8.Bahreman A, Limburg B, Siegler MA, Bouwman E, Bonnet S. Spontaneous Formation in the Dark, and Visible Light-Induced Cleavage, of a Ru–S Bond in Water: A Thermodynamic and Kinetic Study. Inorg Chem. 2013;52:9456–9469. doi: 10.1021/ic401105v. [DOI] [PubMed] [Google Scholar]
- 9.Ford PC. Polychromophoric metal complexes for generating the bioregulatory agent nitric oxide by single-and two-photon excitation. Acc Chem Res. 2008;41:190–200. doi: 10.1021/ar700128y. [DOI] [PubMed] [Google Scholar]
- 10.Eroy-Reveles AA, Leung Y, Mascharak PK. Release of nitric oxide from a sol–gel hybrid material containing a photoactive manganese nitrosyl upon illumination with visible light. J Am Chem Soc. 2006;128:7166–7167. doi: 10.1021/ja061852n. [DOI] [PubMed] [Google Scholar]
- 11.Chakraborty I, Carrington SJ, Mascharak PK. Photodelivery of CO by Designed PhotoCORMs: Correlation between Absorption in the Visible Region and Metal–CO Bond Labilization in Carbonyl Complexes. ChemMedChem. 2014;9:1266–1274. doi: 10.1002/cmdc.201402007. [DOI] [PubMed] [Google Scholar]
- 12.Zayat L, Salierno M, Etchenique R. Ruthenium(II) Bipyridyl Complexes as Photolabile Caging Groups for Amines. Inorg Chem. 2006;45:1728–1731. doi: 10.1021/ic0512983. [DOI] [PubMed] [Google Scholar]
- 13.Basa PN, Antala S, Dempski RE, Burdette SC. A zinc(II) photocage based on a decarboxylation metal ion release mechanism for investigating homeostasis and biological signaling. Angew Chem. 2015;127:13219–13223. doi: 10.1002/anie.201505778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patra AK, Mascharak PK. A ruthenium nitrosyl that rapidly delivers NO to proteins in aqueous solution upon short exposure to UV light. Inorg Chem. 2003;42:7363–7365. doi: 10.1021/ic030110h. [DOI] [PubMed] [Google Scholar]
- 15.Ford P, Bourassa J, Miranda K, Lee B, Lorkovic I, Boggs S, Kudo S, Laverman L. Photochemistry of metal nitrosyl complexes. Delivery of nitric oxide to biological targets. Coord Chem Rev. 1998;171:185–202. [Google Scholar]
- 16.Farrer NJ, Woods JA, Salassa L, Zhao Y, Robinson KS, Clarkson G, Mackay FS, Sadler PJ. A Potent Trans-Diimine Platinum Anticancer Complex Photoactivated by Visible Light. Angew Chem, Int Ed. 2010;49:8905–8908. doi: 10.1002/anie.201003399. [DOI] [PubMed] [Google Scholar]
- 17.Knoll JD, Turro C. Control and utilization of ruthenium and rhodium metal complex excited states for photoactivated cancer therapy. Coord Chem Rev. 2015;282–283:110–126. doi: 10.1016/j.ccr.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zamora A, Denning CA, Heidary DK, Wachter E, Nease LA, Ruiz J, Glazer EC. Ruthenium-containing P450 inhibitors for dual enzyme inhibition and DNA damage. Dalton Trans. 2017;46:2165–2173. doi: 10.1039/c6dt04405k. [DOI] [PubMed] [Google Scholar]
- 19.Smith NA, Zhang P, Greenough SE, Horbury MD, Clarkson GJ, McFeely D, Habtemariam A, Salassa L, Stavros VG, Dowson CG, Sadler PJ. Combatting AMR: photoactivatable ruthenium(II)–isoniazid complex exhibits rapid selective antimyco-bacterial activity. Chem Sci. 2017;8:395–404. doi: 10.1039/c6sc03028a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li A, Yadav R, White JK, Herroon MK, Callahan BP, Podgorski I, Turro C, Scott EE, Kodanko JJ. Illuminating cytochrome P450 binding: Ru(II)-caged inhibitors of CYP17A1. Chem Commun. 2017;53:3673–3676. doi: 10.1039/c7cc01459g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lameijer LN, Ernst D, Hopkins SL, Meijer MS, Askes SH, Le Dévédec SE, Bonnet S. A red light-activated ruthenium-caged NAMPT inhibitor remains phototoxic in hypoxic cancer cells. Angew Chem, Int Ed. 2017;56:11549–11553. doi: 10.1002/anie.201703890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cuello-Garibo J-A, Meijer MS, Bonnet S. To Cage or To Be Caged? The Cytotoxic Species in Ruthenium-Based PhotoActivated Chemotherapy Is Not Always the Metal. Chem Commun. 2017;53:6768–6771. doi: 10.1039/c7cc03469e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loftus LM, White JK, Albani BA, Kohler L, Kodanko JJ, Thummel RP, Dunbar KR, Turro C. New RuII Complex for Dual Activity: Photoinduced Ligand Release and 1O2 Production. Chem - Eur J. 2016;22:3704–3708. doi: 10.1002/chem.201504800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huisman M, White JK, Lewalski VG, Podgorski I, Turro C, Kodanko JJ. Caging the uncageable: using metal complex release for photochemical control over irreversible inhibition. Chem Commun. 2016;52:12590–12593. doi: 10.1039/c6cc07083c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herroon MK, Sharma R, Rajagurubandara E, Turro C, Kodanko JJ, Podgorski I. Photoactivated inhibition of cathepsin K in a 3D tumor model. Biol Chem. 2016;397:571–582. doi: 10.1515/hsz-2015-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li A, White JK, Arora K, Herroon MK, Martin PD, Schlegel HB, Podgorski I, Turro C, Kodanko JJ. Selective Release of Aromatic Heterocycles from Ruthenium Tris(2-pyridylmethyl)amine with Visible Light. Inorg Chem. 2016;55:10–12. doi: 10.1021/acs.inorgchem.5b02600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knoll JD, Albani BA, Turro C. New Ru(II) Complexes for Dual Photoreactivity: Ligand Exchange and 1O2 Generation. Acc Chem Res. 2015;48:2280–2287. doi: 10.1021/acs.accounts.5b00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karaoun N, Renfrew AK. A luminescent ruthenium(II) complex for light-triggered drug release and live cell imaging. Chem Commun. 2015;51:14038–14041. doi: 10.1039/c5cc05172j. [DOI] [PubMed] [Google Scholar]
- 29.Sharma R, Knoll JD, Martin PD, Podgorski I, Turro C, Kodanko JJ. Ruthenium tris(2-pyridylmethyl)amine as an effective photocaging group for nitriles. Inorg Chem. 2014;53:3272–3274. doi: 10.1021/ic500299s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Respondek T, Sharma R, Herroon MK, Garner RN, Knoll JD, Cueny E, Turro C, Podgorski I, Kodanko JJ. Inhibition of Cathepsin Activity in a Cell-Based Assay by a Light-Activated Ruthenium Compound. ChemMedChem. 2014;9:1306–1315. doi: 10.1002/cmdc.201400081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Renfrew AK. Transition metal complexes with bioactive ligands: mechanisms for selective ligand release and applications for drug delivery. Metallomics. 2014;6:1324–1335. doi: 10.1039/c4mt00069b. [DOI] [PubMed] [Google Scholar]
- 32.Knoll JD, Albani BA, Durr CB, Turro C. Unusually Efficient Pyridine Photodissociation from Ru(II) Complexes with Sterically Bulky Bidentate Ancillary Ligands. J Phys Chem A. 2014;118:10603–10610. doi: 10.1021/jp5057732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zayat L, Filevich O, Baraldo LM, Etchenique R. Ruthenium polypyridyl phototriggers: from beginnings to perspectives. Philos Trans R Soc, A. 2013;371:20120330. doi: 10.1098/rsta.2012.0330. [DOI] [PubMed] [Google Scholar]
- 34.Respondek T, Garner RN, Herroon MK, Podgorski I, Turro C, Kodanko JJ. Light Activation of a Cysteine Protease Inhibitor: Caging of a Peptidomimetic Nitrile with RuII(bpy)2. J Am Chem Soc. 2011;133:17164–17167. doi: 10.1021/ja208084s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldbach RE, Rodriguez-Garcia I, van Lenthe JH, Siegler MA, Bonnet S. N-Acetylmethionine and Biotin as Photocleavable Protective Groups for Ruthenium Polypyridyl Complexes. Chem - Eur J. 2011;17:9924–9929. doi: 10.1002/chem.201101541. [DOI] [PubMed] [Google Scholar]
- 36.Garner RN, Gallucci JC, Dunbar KR, Turro C. [Ru(bpy)2(5-cyanouracil)2]2+ as a potential light-activated dual-action therapeutic agent. Inorg Chem. 2011;50:9213–9215. doi: 10.1021/ic201615u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salierno M, Marceca E, Peterka DS, Yuste R, Etchenique R. A fast ruthenium polypyridine cage complex photoreleases glutamate with visible or IR light in one and two photon regimes. J Inorg Biochem. 2010;104:418–422. doi: 10.1016/j.jinorgbio.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Filevich O, Salierno M, Etchenique R. A caged nicotine with nanosecond range kinetics and visible light sensitivity. J Inorg Biochem. 2010;104:1248–1251. doi: 10.1016/j.jinorgbio.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 39.Bonnet S, Limburg B, Meeldijk JD, Gebbink RJK, Killian JA. Ruthenium-decorated lipid vesicles: light-induced release of [Ru(terpy)(bpy)(OH2)]2+ and thermal back coordination. J Am Chem Soc. 2011;133:252–261. doi: 10.1021/ja105025m. [DOI] [PubMed] [Google Scholar]
- 40.Rial Verde EM, Zayat L, Etchenique R, Yuste R. Photorelease of GABA with visible light using an inorganic caging group. Front Neural Circuits. 2008;2:2. doi: 10.3389/neuro.04.002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mosquera J, Sánchez MI, Mascareñas JL, Vázquez ME. Synthetic peptides caged on histidine residues with a bisbipyridyl ruthenium(II) complex that can be photolyzed by visible light. Chem Commun. 2015;51:5501–5504. doi: 10.1039/c4cc08049a. [DOI] [PubMed] [Google Scholar]
- 42.Li A, Turro C, Kodanko JJ. Ru(II) polypyridyl complexes as photocages for bioactive compounds containing nitriles and aromatic heterocycles. Chem Commun. 2018;54:1280–1290. doi: 10.1039/c7cc09000e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tu Y-J, Mazumder S, Endicott JF, Turro C, Kodanko JJ, Schlegel HB. Selective Photodissociation of Acetonitrile Ligands in Ruthenium Polypyridyl Complexes Studied by Density Functional Theory. Inorg Chem. 2015;54:8003–8011. doi: 10.1021/acs.inorgchem.5b01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Furuta T, Wang SS-H, Dantzker JL, Dore TM, Bybee WJ, Callaway EM, Denk W, Tsien RY. Brominated 7-hydroxycoumarin-4-ylmethyls: photolabile protecting groups with biologically useful cross-sections for two photon photolysis. Proc Natl Acad Sci U S A. 1999;96:1193–1200. doi: 10.1073/pnas.96.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Olson JP, Kwon H-B, Takasaki KT, Chiu CQ, Higley MJ, Sabatini BL, Ellis-Davies GC. Optically selective two-photon uncaging of glutamate at 900 nm. J Am Chem Soc. 2013;135:5954–5957. doi: 10.1021/ja4019379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salassa L, Garino C, Salassa G, Gobetto R, Nervi C. Mechanism of ligand photodissociation in photoactivable [Ru-(bpy)2L2]2+ complexes: a density functional theory study. J Am Chem Soc. 2008;130:9590–9597. doi: 10.1021/ja8025906. [DOI] [PubMed] [Google Scholar]
- 47.Mari C, Pierroz V, Ferrari S, Gasser G. Combination of Ru(II) complexes and light: new frontiers in cancer therapy. Chem Sci. 2015;6:2660–2686. doi: 10.1039/c4sc03759f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharma R, Knoll JD, Ancona N, Martin PD, Turro C, Kodanko JJ. Solid-Phase Synthesis as a Platform for the Discovery of New Ruthenium Complexes for Efficient Release of Photocaged Ligands with Visible Light. Inorg Chem. 2015;54:1901–1911. doi: 10.1021/ic502791y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zayat L, Calero C, Alborés P, Baraldo L, Etchenique R. A New Strategy for Neurochemical Photodelivery: Metal–Ligand Heterolytic Cleavage. J Am Chem Soc. 2003;125:882–883. doi: 10.1021/ja0278943. [DOI] [PubMed] [Google Scholar]
- 50.Sgambellone MA, David A, Garner RN, Dunbar KR, Turro C. Cellular toxicity induced by the photorelease of a caged bioactive molecule: design of a potential dual-action Ru(II) complex. J Am Chem Soc. 2013;135:11274–11282. doi: 10.1021/ja4045604. [DOI] [PubMed] [Google Scholar]
- 51.Ramalho SD, Sharma R, White JK, Aggarwal N, Chalasani A, Sameni M, Moin K, Vieira PC, Turro C, Kodanko JJ, Sloane BF. Imaging sites of inhibition of proteolysis in pathomimetic human breast cancer cultures by light-activated ruthenium compound. PLoS One. 2015;10:e0142527. doi: 10.1371/journal.pone.0142527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Göttle AJ, Alary F, Boggio-Pasqua M, Dixon IM, Heully J-L, Bahreman A, Askes SH, Bonnet S. Pivotal Role of a Pentacoordinate 3MC State on the Photocleavage Efficiency of a Thioether Ligand in Ruthenium(II) Complexes: A Theoretical Mechanistic Study. Inorg Chem. 2016;55:4448–4456. doi: 10.1021/acs.inorgchem.6b00268. [DOI] [PubMed] [Google Scholar]
- 53.Bahreman A, Limburg B, Siegler MA, Koning R, Koster AJ, Bonnet S. Ruthenium Polypyridyl Complexes Hopping at Anionic Lipid Bilayers through a Supramolecular Bond Sensitive to Visible Light. Chem - Eur J. 2012;18:10271–10280. doi: 10.1002/chem.201200624. [DOI] [PubMed] [Google Scholar]
- 54.Arora K, White JK, Sharma R, Mazumder S, Martin PD, Schlegel HB, Turro C, Kodanko JJ. Effects of Methyl Substitution in Ruthenium Tris(2-pyridylmethyl)amine Photocaging Groups for Nitriles. Inorg Chem. 2016;55:6968–6979. doi: 10.1021/acs.inorgchem.6b00650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Loftus LM, Li A, Fillman KL, Martin PD, Kodanko JJ, Turro C. Unusual Role of Excited State Mixing in the Enhancement of Photoinduced Ligand Exchange in Ru(II) Complexes. J Am Chem Soc. 2017;139:18295–18306. doi: 10.1021/jacs.7b09937. [DOI] [PMC free article] [PubMed] [Google Scholar]


