Abstract
Sepsis is an often fatal condition that arises when the immune response to an infection causes widespread systemic organ injury. A critical unmet need in combating sepsis is the lack of accurate early biomarkers that produce actionable results in busy clinical settings. Here, we report the development of a point-of-care platform for rapid sepsis detection. Termed IBS (integrated biosensor for sepsis), our approach leverages i) the newly-found pathophysiological role of cytokine interleukin-3 (IL-3) in early sepsis, and ii) a hybrid magneto-electrochemical sensor for IL-3 detection. The developed platform produces test results within 1 hour from native blood samples, and detects IL-3 at a sensitivity of <10 pg/mL; this performance is >5-times faster and >10-times more sensitive than a current gold standard, enzyme-linked immunoadsorbent assay. Using clinical samples, we show that high plasma IL-3 levels are associated with high organ failure rate and thus greater risk of mortality, confirming the potential of IL-3 as an early diagnostic biomarker. Compact and fast, the IBS platform can be readily integrated into clinical workflows, enabling timely diagnosis and proactive treatment of sepsis.
Keywords: sepsis, organ failure, IL-3, point-of-care, electrochemical sensing
Graphical abstract
INTRODUCTION
Sepsis is the leading cause of death from infection, especially if not identified and treated promptly.1–4 In the US alone, sepsis is responsible for more than 250,000 deaths annually.5, 6 with the mortality rate reaching up to 50%. It also incurs a considerable financial burden, accounting for > $20 billion of total US hospital costs.1, 7 Incidences continue to rise, because of the emergence of drug-resistant pathogens, growing elderly population, and the increased use of immunosuppression.8 Sepsis requires immediate medical treatment, ideally within 24 hours of the disease onset, because of its dynamic and acute nature.9–11 Unfortunately, current diagnostic methods (e.g., measurement of vital signs or scores (qSOFA) to determine onset of the systemic inflammatory response syndrome) often fail to meet the required speed or accuracy.12 Certain serum biomarkers such as calcitonin and uPAR have been evaluated but lack the necessary sensitivity.13–15 Since most patients have an underlying infection (esp. lung, urinary tract, skin), blood cultures are necessary but positive results are not diagnostic since they do not discriminate between local infection and progression to sepsis. It is generally accepted that earlier recognition and focused management improves the outcomes in sepsis.
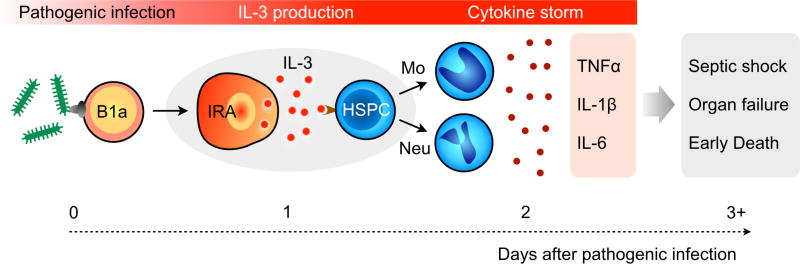
In prior studies, we had identified a previously unknown contributor to sepsis.16–18 Particularly, we have identified the inflammatory factor interleukin-3 (IL-3) as an independent predictor of septic shock and death being produced by IRA B cells following TLR activation. IL-3 operates upstream of key cytokines including TNFα, IL-1β and IL-6 (Scheme 1). In a proof-of-concept study, we showed that high IL-3 levels in septic patients are associated with high mortality.17 A recent study further confirmed the role of IL-3 in the immune regulation and potential response to corticosteroids during sepsis.19 Translating this discovery into a clinically viable test, however, requires a fast, easy-to-use platform that could overcome the long assay time (5–8 hours) and complexity of a standard enzyme-linked immunosorbent assay (ELISA).
Scheme 1. Newly discovered mechanism of sepsis.
Peritoneal B1a cells are activated by pathogens and give rise to IL-3+ B cells (IRA, innate response activator). IL-3 acts on hematopoietic stem progenitor cells (HSPC) to promote the emergency generation of inflammatory leukocytes that are released into the circulation. This leads to an uncontrolled cytokine storm, multiple organ failure, and septic shock that may result in death.
Here, we report the development of a point-of-care (POC) approach, termed IBS (integrated biosensor for sepsis), for rapid and accurate sepsis diagnosis. Specifically, we advanced a portable biosensor consisting of a disposable kit for blood processing and an electrical detection system. The kit is used to capture IL-3 on magnetic beads and label it for electrochemical reaction; the detector then measures electrical currents for IL-3 quantification. This strategy has practical advantages: i) target protein (IL-3) can be enriched directly from blood; ii) the assay achieves high detection sensitivity through magnetic enrichment and enzymatic signal amplification; iii) based on the electrical measurements, the sensor can be easily miniaturized and easy-to-use. When benchmarked against ELISA, IBS was 10-fold more sensitive and 5-fold faster, producing diagnostic results in an actionable time frame (<1 hour). Further testing with human clinical samples (n = 62) affirmed IL-3 as a potent diagnostic and prognostic biomarker; detection sensitivity and specificity were 91.3% and 82.4% respectively, and high IL-3 levels at sepsis onset were significantly correlated with patient survival.
RESULTS
IBS platform
The IBS assay starts by extracting the target protein IL-3 through immunomagnetic enrichment. The captured protein is then subsequently labeled with detection antibodies and oxidizing enzyme (horseradish peroxidase, HRP). Finally, the beads are mixed with chromogenic electron mediators (3,3′,5,5′-tetramethylbenzidine, TMB); the enzyme catalyzes the oxidation of TMB and the reduction of H2O2. The oxidized TMB is then reduced by receiving electrons from the electrode, which generates electrical current as an analytical read out. Using magnetic beads allowed for direct and fast extraction of IL-3 from blood samples. Signal can also be amplified by concentrating magnetic beads underneath the electrodes.
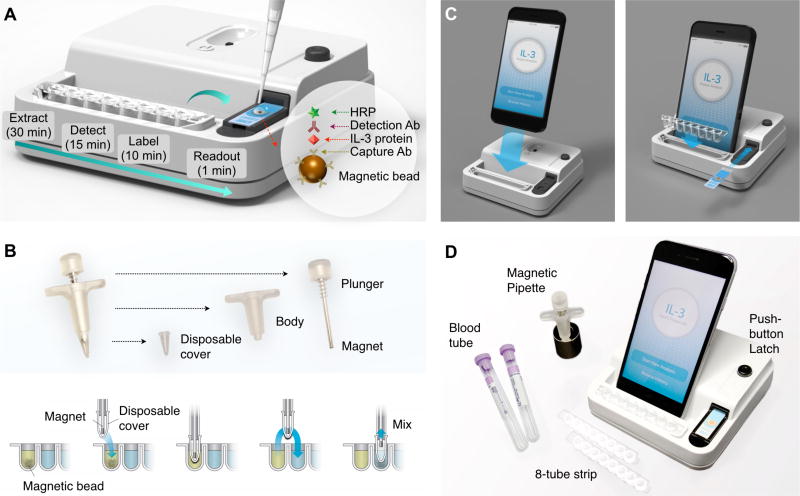
To streamline sample processing, we designed a magnetic assay tool that consisted of a magnetic pipette and a tray of linear wells. The pipette had a sheathed magnet attached to a mechanical plunger (Fig. 1B). This device allowed for quick collection and release magnetic beads. The linear tray was preloaded with reagents and washing buffers, reflecting the assay procedure. We loaded blood samples, mixed with immunomagnetic beads, to the first well, and sequentially transferred magnetic beads using the magnetic pipette. This unit rendered the assay simple, cost-effective and self-contained; no additional equipment (e.g., centrifuge) was needed for sample preparation.
Figure 1. Integrated biosensor for sepsis (IBS).
(A) Assay schematic. IL-3 is captured on magnetic beads directly in plasma or whole blood and labeled antibodies. Electrical signal is generated through enzymatic reaction. The entire assay is performed using a portable device, and complete within <1 hour. HRP, horseradish peroxidase. Ab, antibody. (B) Magnetic kit for sample processing. A magnetic pipette was custom-built to facilitate the sample handling. The plunger mechanism allows for easy collection and dispension of magnetic beads. (C) The base-station, housing measurement electronics, docks with a smartphone and a multi-well tray. An electrode slides in to the system for electrical connection. A smartphone app provides an user-interface for the system control (via Bluetooth) and data upload to a cloud server. (D) Photo of the whole assay system.
We further combined sample processing and signal detection into a single monolithic device (Fig. 1C, and Fig. S1) to promote portable, user-friendly operations. The integrated IBS device had the following features. i) A miniaturized electronics was designed (Fig. S2), comprising potentiostats capable of measuring a wide range of current (± 7.5 µA), a microcontroller unit for signal processing, and a bluetooth module for wireless communication. ii) A latch mechanism was developed to connect an electrode to the system. Compared to commonly-used card-edge connectors that require forceful insertion, this new method enabled a quick slip-loading of electrodes. iii) A smartphone was docked to the system, and functioned as a touch-screen interface. We also designed a smartphone app for system control and data storage (Fig. S3). The resulting IBS device was a standalone handheld unit, measuring electrical currents and displaying IL-3 concentrations. The overall device cost was about $50 US dollars (Table S1).
Assay optimization
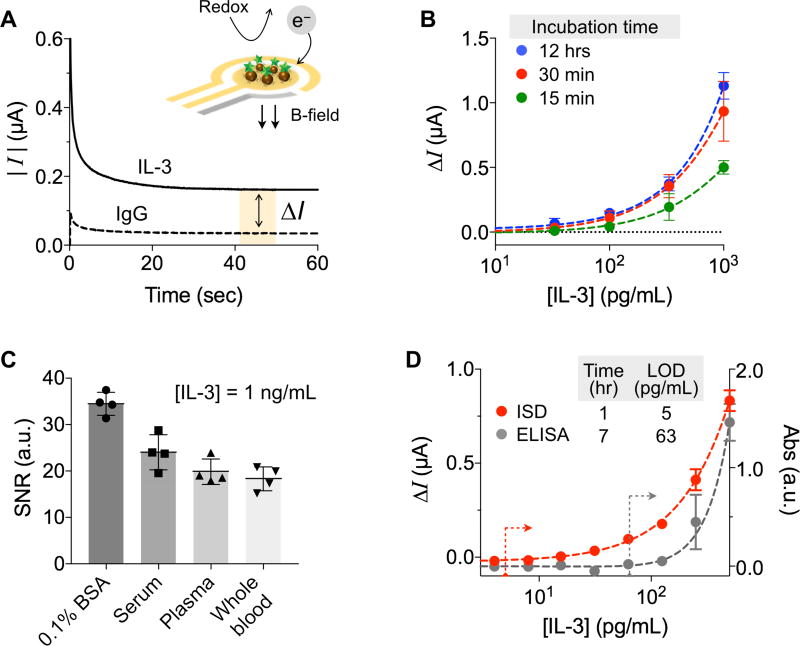
We applied the chronoamperometry method for signal detection. The electrical current generated from TMB reduction was monitored, while a reduction potential was applied to a working electrode (Fig. 2A). The current level (I) reached a plateau within 1 min after the reduction potential was applied. We averaged the current level (I) from 40 to 45 seconds as a representative value.
Figure 2. Assay characteristics and optimization.
(A) Electrical currents are generated on or near the electrode via TMB redox reaction catalyzed by HRP-coated magnetic beads. With 100 mV reduction potential, the current level reached a plateau within 1 min. The current difference between the IL-3 and IgG control samples (ΔI) was used as an analytical metric. (B) IL-3 extraction yields were compared under different incubation conditions: 15 min, 30 min, and 12 hrs. Thirty minute incubation was sufficient. (C) Signal-to-noise ratios (SNR) were measured in different media spiked with IL-3 of 1 ng/mL. SNR values were statistically identical among serum, plasma, and whole blood (P = 0.07, one-way ANOVA). (D) Varying concentrations of IL-3 were spiked into human plasma and assayed by IBS and ELISA. The IBS performed 7-times faster and 12-times more sensitive than ELISA. All measurements were performed in triplicate, and the data are displayed as mean ± SD.
We prepared a pair of magnetic beads: one conjugated with antibodies against IL-3 (IL-3 beads) and the other with antibodies against isotype matched IgG (IgG beads). The analytical measure was the net current difference ΔI = IIL-3 − IIgG, where IIL-3 and IIgG were the current levels with IL-3 and IgG beads, respectively. We also compared signal levels with differently sized beads (diameters of 2.7 and 4.5 µm), and found that the measured signal levels were similar when the total surface area of beads was matched. We opted to use 2.7 µm beads, as the bigger ones tended to sediment and required longer time for magnetic isolation. The optimal bead concentration for high signal was ~5.0×107 beads/mL (Fig. S4).
We further optimized the assay protocol with the goal of minimizing the sampling time while maximizing sensitivity. Samples were prepared by spiking IL-3 into human plasma. We generated standard curves, varying the IL-3 extraction time with magnetic beads (Fig. 2B). We observed similar results between 30 min and 12 hour incubation. For a given incubation time, the extraction at room temperature was as efficient as at 37 °C. Further testing showed that subsequent assay steps (i.e., labeling of detection antibodies and HRP enzyme) were efficient for 10 min incubation each. The sample-to-detection time, measured from the IL-3 extraction from patient sample to the sensor readouts, was ~50 min, which is >7-times faster than ELISA (7 hours).
Assay characterization
We next examined the assay robustness. We spiked IL-3 (1 ng/mL) in different biological fluids (PBS with 1% BSA, human plasma, serum, and whole blood) and performed the IBS assay (Fig. 2C). The signal-to-noise ratio (SNR) was the highest with the pure buffer. SNR values were lower in the rest of media, while their differences were statistically insignificant (P = 0.07, one-way ANOVA). The sample handling thus can be simplified by using plasma or whole blood; no additional process is needed to obtain serum (Fig. S5). We further characterize the IBS assay using IL-3 spiked plasma samples. Titration experiments established the limit of detection (LOD) of ~5 pg/mL (Fig. 2D), while similar measurements with ELISA required more than 100 pg/mL for reliable detection. In addition, the dynamic ranges of the IBS span an order of magnitude larger than ELISA. Using matched controls (IgG beads) was important to compensate for background signals from sample-dependent, nonspecific binding.
Clinical study
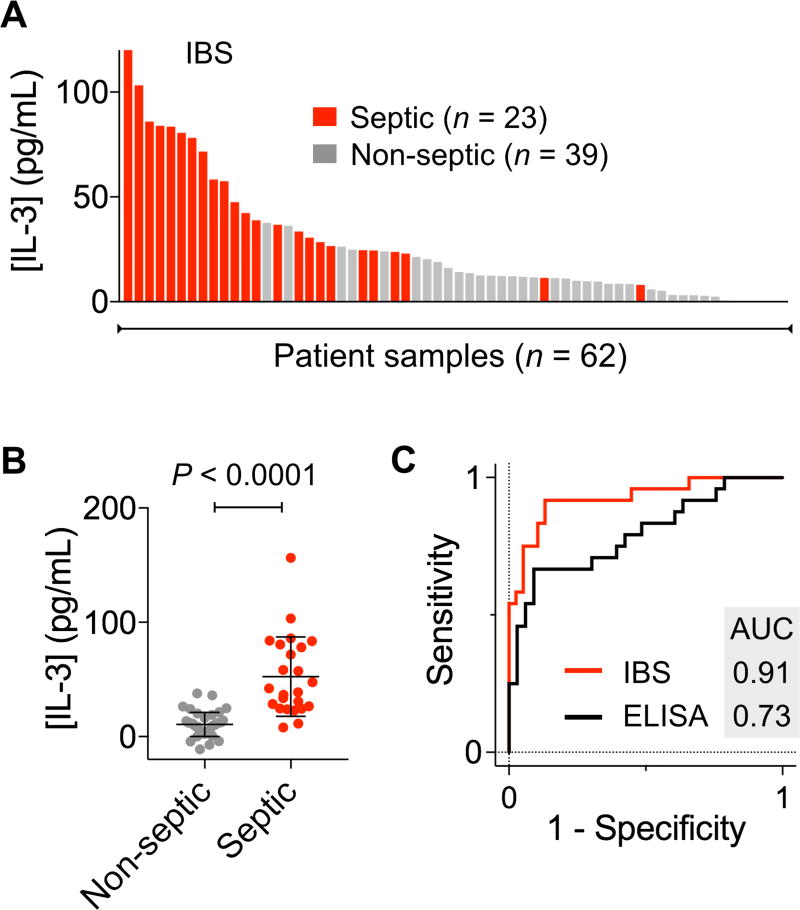
We finally applied IBS to detect IL-3 in clinical samples. Plasma or serum samples were collected from patients with symptoms of systemic infection, inflammation, or both. We obtained 23 samples from septic patients and another 39 from non-septic patients (Table S2). The onset of sepsis was defined as circulation failure, thereby the beginning of catecholamine therapy. We split each sample into two aliquots, and used them for IBS and ELISA, respectively. Overall, we observed higher IL-3 level in septic patient samples (Fig. 3A and Fig. S6). IBS, however, better differentiated septic from non-septic patient groups (Fig. 3B), which can be attributed to its higher sensitivity than ELISA.
Figure 3. IL-3 as a biomarker for sepsis detection.
Patient samples (23 from septic and 39 from non-septic patients) were analyzed with the IBS assay. (A) A waterfall plot shows the IL-3 levels measured by the IBS system. Each column represents a different sample (red, septic; grey, non-septic). (B) IL-3 level was higher in septic patients than in non-septic controls (P < 0.0001, unpaired t-test). (C) A receiver-operation characteristic curve was constructed. The area under the curve (AUC) was 0.91 with IBS and 0.73 with ELISA. The optimum threshold value that maximized both sensitivity and specificity was [IL-3] = 21.7 pg/mL.
We next constructed a receiver operating characteristic (ROC) curve using IL-3 level as a predictor (Fig. 3C). The optimal [IL-3] cutoff that maximizes both sensitivity and specificity was 21.7 pg/mL. With this cutoff, the detection sensitivity was 91.3% and the specificity 82.4%. We applied the cross validation method to estimate the area under the curve (AUC). The AUC value was 0.91± 0.08 (95% confidence interval) for IBS, whereas ELISA yielded smaller AUC of 0.73 ± 0.15 (95% confidence interval). We also evaluated the accuracy of the diagnostic test with or without including other host factors (e.g., sex, age). Adding these factors, along with IL-3 level, did not improve the detection accuracy, and the AUC value remained statistically identical (P = 0.52).
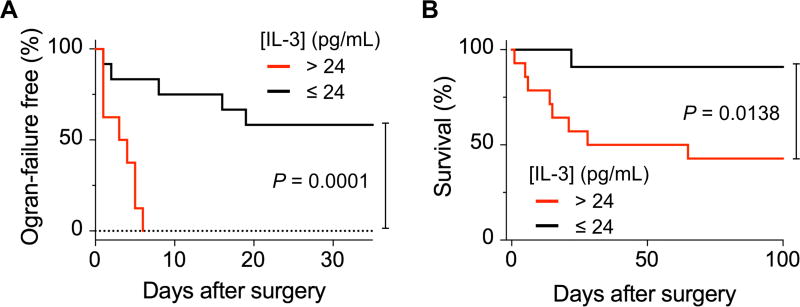
We further performed the survival analysis, testing the impact of IL-3 on organ failures. We hypothesized that IL-3 could be used as a biomarker to identify “patients at risk”. These patients may develop a systemic inflammatory reaction that often results in multiple organ failures and even death. We used a cohort of 25 admitted patients who had at least one surgery. Kaplan-Meier analysis showed that patients with IL-3 > 24 pg/mL are more prone to organ failures, thus confirming the significant association of IL-3 with organ failures (P = 0.0001 for Log-Rank test; hazard ratio, 8.092) (Fig. 4A). Patients with high blood levels of IL-3 (> 24 pg/mL) showed about 9-fold greater hazard of death than those with low levels (P = 0.0138 for Log-Rank test; hazard ratio: 8.783) (Fig. 4B).
Figure 4. Organ failure and mortality analyses.
IL-3 level could serve as a predictive marker for organ failure free (A) and overall patient survival (B). Log-Rank test was used to compare times-to-event (times-to-organ failure or death) between low and high IL-3 groups. Kaplan-Meier method was used to calculate the survival function with a minimal p-value approach for cutoff optimization.
DISCUSSION
We developed the point-of-care IBS system for fast, sensitive identification of early sepsis in busy clinical settings. Our approach is based on emerging understanding on host response to infection. IL-3, a cytokine that activates proliferation of hematopoietic stem cells and progenitors,20 has recently been identified as a key regulator during early sepsis.17 IL-3 operates upstream of key cytokines including TNFα, IL-1β and IL-6; high IL-3 level and can trigger a detrimental cytokine storm. It is thus a direct measure of early immune response and may serve well as a productive serum biomarker. Up to date, alternative serum biomarkers of sepsis have had fairly low accuracy (e.g., calcitonin, soluble uPAR), one of the reasons why there has been intense interest in identifying alternative, causally related markers.
The magneto-electrochemical sensing strategy developed here enables POC diagnostics: i) magnetic beads provides large surface area (~230 mm2) for target capture and an easy way to perform assay steps; ii) the analytical signal is read out by compact, rugged electronics. Applying the IBS prototype, we achieved rapid (<1 hr), highly sensitive (<10 pg/mL) IL-3 detection in human plasma samples. Whole blood can also be used without preprocessing, which would better position the assay into most clinical workflows. The pilot clinical study supported the potential of IL-3 as a surrogate biomarker of sepsis: the sensitivity was 91.3%, and the specificity 82.4%. In comparison, procalcitonin (PCT) has been shown to have accuracies below 80%.21 Because IBS is fast and consumes small amount of samples (100 µL), it can be readily adopted to track temporal changes of biomarkers. This capacity would aid in not only the timely diagnosis of acute septic shock, but also reliable prognostication assessment of the risk. Also, using the small amount of samples would be especially advantageous for detection of sepsis in new borns/infants as blood samples from preterm infants (with a blood volume of <50 mL) are limited in volume.22–24
Several aspects of the current study could be improved. First, we envision creating a sepsis marker panel and expanding IBS for multiplexed detection. Sepsis has complex pathophysiology of systemic nature, not limited to single organs.25, 26 Measuring multiple markers (e.g., IL-3, TNFα, IL-1β, PCT, IL-6) would potentially enable and even more robust characterization of host response in an effort to further improve diagnostic accuracy. As a platform technology, IBS would be easily scaled up for such measurements. Second, sample preparation steps will have to be automated to realize a “sample-in and answer-out” system. We expect that the use of magnetic beads, thereby magnetic actuation, would facilitate replacing current manual operations with automation. Fully integrated, easy-to-use IBS will promote assay reproducibility and help collect large data set to rigorously validate biomarkers with clinical samples. With these developments, IBS would be a powerful clinical tool to enable early sepsis diagnosis and improve treatment outcomes.
MATERIALS AND METHODS
Construction of the IBS system
The integrated device (10 × 10 × 2.5 cm3) is composed of two parts: sensor unit and sample processing unit. The sensor unit consists of a custom-designed potentiostat, a microcontroller (Atmega328, Atmel Corporation), a digital-to-analog converter (DAC8552, Texas Instruments), and an analog-to-digital converter (ADC161S626, Texas Instruments) (Fig. S3b and Table S1). There are two operational amplifiers (AD8606, Analog Devices) in the potentiostat: one for maintaining the potential difference between the working electrode and the reference, and the other for converting low-level current to voltage. Other sensor components include a communication module (Bluefruit EZLink) for Bluetooth connection with external devices (i.e., smartphones or laptops), disposable screen-printed gold electrodes (220AT, Metrohm), and a 9V battery. The device runs continuously for 3–4 hr with a fully charged battery. The estimated energy consumption of the reader is ~0.05 mW, and the averaged noise level is ~0.3 nA. The sample processing unit consists of a custom-built magnetic pipette, and disposable 0.2 mL 8-tube strips (USA Scientific).
Smartphone Application
Using Xcode, we created an iOS application to facilitate system operation and data recording. The application allows users to record patient information and measurement details (time stamps, current value, and estimated analyte concentration). The data is stored with cloud storage.
Magnetic bead preparation
Five milligrams of magnetic beads coated with epoxy groups (Dynabeads M-270 Epoxy, Invitrogen) was suspended in 1 mL of 0.1 M sodium phosphate solution at room temperature for 10 min. The magnetic beads were separated from the solution with a permanent magnet and resuspended in 100 µL of the same solution. One hundred micrograms of antibodies against IL-3 (R&D systems) or control IgG (R&D systems) was added and mixed thoroughly. One hundred microliters of 3 M ammonium sulfate solution was added, and the whole mixture was incubated overnight at 4 °C with slow tilt rotation. The beads were washed twice with PBS solution and finally resuspended in 0.25 mL of PBS with 1% bovine serum albumin (BSA) to have the final bead concentration of ~109/mL.
IBS assay
Hundred microliters of IL-3-spiked PBS solution (or plasma) was mixed with 5 µL of the antibody conjugated bead solution for 30 min at room temperature. Magnetic beads were separated from the solution with a permanent magnet and washed twice with PBS solution with 0.05% Tween-20 (PBS-T). Fifty microliters of biotinylated antibodies (R&D systems) diluted in 0.1% BSA solution was mixed with the beads for 10 min at room temperature. The beads were separated and washed as described before. Fifty microliters of high sensitivity streptavidin-conjugated HRP enzymes (Thermo-Fisher Scientific, 1:1000 diluted in 0.1% BSA solution) was mixed with the beads for 5 min at room temperature. The beads were separated and washed as described before, and then resuspended in 15 µL of PBS. Seven micrometers of the prepared bead solution and 20 µL of UltraTMB substrate solution (Thermo-Fisher Scientific) were loaded on top of the electrode. After 3 min, chronoamperometry measurement was started with the electrochemical sensor. The current levels in the range of 40–45 s were averaged.
Enzyme-linked immunosorbent assay
IL-3 antibody (R&D systems) was diluted to 2 µg/mL concentration in PBS and added to the Maxisorp 96-well plate (Nunc) for overnight incubation at 4 °C. After being washed three times with PBS-T, 2% BSA in PBS blocking solution was added to the plate for 2 h incubation at room temperature. Subsequently, serial dilute standard or samples were added to each well for at least 2 h incubation at room temperature. After the samples were discarded and washed three times with PBS-T, biotinylated detection antibodies (0.4 µg/mL, diluted in 0.1% BSA solution) were added to each well and incubated at room temperature for 2 h. Unbound antibodies were washed with PBS-T three times. High sensitivity streptavidin–HRP molecules (1:5000 diluted in 0.1% BSA solution) were added to the each well for 30 min at room temperature. After being washed out with PBS, TMB solution to each well was added and incubate for 20 min, added equal volume of stopping solution (2 M H2SO4), and read the optical density at 450 nm. Along with TMB substrate, SuperSignal® ELISA Femto Maximum Sensitivity Substrate (Thermo-Fisher Scientific) was also used.
Clinical samples
This clinical SEPIL-3 study was approved by the local ethics committee (Trial-Code-Nr.: EK 308082013) on September, 19th 2013. Blood samples were collected in the interdisciplinary operative intensive care unit of the University Hospital of Erlangen, Germany. Study patients or their legal designees signed written informed consent. After blood collection, plasma of all study participants was immediately obtained by centrifugation, transferred into cryotubes, and stored at −80°C until further processing. Quantification of IL-3 in human plasma samples was performed using an enzyme linked immunosorbent assay kit (R&D Systems). Hundred microliters of patient sample was used for each IBS analysis.
Statistics
The Spearman correlation coefficient was used to quantify the correlations. Group differences were tested using unpaired t-test. All tests were two-sided, and a P value of <0.05 was considered statistically significant. The ROC curve was constructed using the IL-3 level as a predictor for sepsis. The level cutoff was selected using Youden’s index, which maximizes the sum of sensitivity and specificity. For survival analysis, Log-Rank test was used to compare times-to-event (times-to-organ failure or death) between low and high IL-3 groups. Kaplan-Meier method was used to calculate the survival function with a minimal p-value approach for cutoff optimization. Hazard ratio was estimated using Cox Proportional Hazards model.
Supplementary Material
Acknowledgments
The authors were supported in part by NIH grants R21-CA205322 (H.L.), R01-HL113156 (H.L.), K99-CA201248 (H.I.), R01-CA204019 (R.W.), R01-EB010011 (R.W.), R01-EB00462605A1 (R.W.), P01-CA069246 (R.W.), Liz Tilberis Award Fund (C.M.C); MGH scholar fund (H.L. and F.K.S.); Andrew L. Warshaw, M.D. Institute for Pancreatic Cancer Research (H.I.), Lustgarten Foundation (R.W.), and the National Research Foundation of Korea (NRF-2017M3A9B4025699, NRF-2017M3A9B4025709; H.L.).
References
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, Angus DC, Reinhart K International, F. O. A. C. T. Assessment of Global Incidence and Mortality of Hospital-Treated Sepsis. Current Estimates and Limitations. Am J Respir Crit Care Med. 2016;193:259–272. doi: 10.1164/rccm.201504-0781OC. [DOI] [PubMed] [Google Scholar]
- 3.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-Term Cognitive Impairment and Functional Disability Among Survivors of Severe Sepsis. JAMA. 2010;304:1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Poll T, Opal SM. Host-Pathogen Interactions in Sepsis. Lancet Infect Dis. 2008;8:32–43. doi: 10.1016/S1473-3099(07)70265-7. [DOI] [PubMed] [Google Scholar]
- 5.Hotchkiss RS, Monneret G, Payen D. Sepsis-Induced Immunosuppression: From Cellular Dysfunctions to Immunotherapy. Nat Rev Immunol. 2013;13:862–874. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leentjens J, Kox M, van der Hoeven JG, Netea MG, Pickkers P. Immunotherapy for the Adjunctive Treatment of Sepsis: From Immunosuppression to Immunostimulation. Time for a Paradigm Change. Am J Respir Crit Care Med. 2013;187:1287–1293. doi: 10.1164/rccm.201301-0036CP. [DOI] [PubMed] [Google Scholar]
- 7.Torio CM, Andrews RM. Europe PMC. Healthcare Cost and Utilization Project; 2006. National Inpatient Hospital Costs: The Most Expensive Conditions By Payer, 2011: Statistical Brief #160. [PubMed] [Google Scholar]
- 8.Martin GS, Mannino DM, Moss M. The Effect of Age on the Development and Outcome of Adult Sepsis. Crit Care Med. 2006;34:15–21. doi: 10.1097/01.ccm.0000194535.82812.ba. [DOI] [PubMed] [Google Scholar]
- 9.Seymour CW, Gesten F, Prescott HC, Friedrich ME, Iwashyna TJ, Phillips GS, Lemeshow S, Osborn T, Terry KM, Levy MM. Time to Treatment and Mortality During Mandated Emergency Care for Sepsis. N Engl J Med. 2017;376:2235–2244. doi: 10.1056/NEJMoa1703058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whiles BB, Deis AS, Simpson SQ. Increased Time to Initial Antimicrobial Administration is Associated With Progression to Septic Shock in Severe Sepsis Patients. Crit Care Med. 2017;45:623–629. doi: 10.1097/CCM.0000000000002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garnacho-Montero J, Gutiérrez-Pizarraya A, Escoresca-Ortega A, Fernández-Delgado E, López-Sánchez JM. Adequate Antibiotic Therapy Prior to Icu Admission in Patients With Severe Sepsis and Septic Shock Reduces Hospital Mortality. Crit Care. 2015;19:302. doi: 10.1186/s13054-015-1000-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haydar S, Spanier M, Weems P, Wood S, Strout T. Comparison of Qsofa Score and Sirs Criteria as Screening Mechanisms for Emergency Department Sepsis. Am J Emerg Med. 2017;35:1730–1733. doi: 10.1016/j.ajem.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Donadello K, Scolletta S, Covajes C, Vincent JL. Supar as a Prognostic Biomarker in Sepsis. BMC Med. 2012;10:2. doi: 10.1186/1741-7015-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kibe S, Adams K, Barlow G. Diagnostic and Prognostic Biomarkers of Sepsis in Critical Care. J Antimicrob Chemother. 2011;66(Suppl 2):ii33–40. doi: 10.1093/jac/dkq523. [DOI] [PubMed] [Google Scholar]
- 15.Reinhart K, Bauer M, Riedemann NC, Hartog CS. New Approaches to Sepsis: Molecular Diagnostics and Biomarkers. Clin Microbiol Rev. 2012;25:609–634. doi: 10.1128/CMR.00016-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rauch PJ, Chudnovskiy A, Robbins CS, Weber GF, Etzrodt M, Hilgendorf I, Tiglao E, Figueiredo JL, Iwamoto Y, Theurl I, Gorbatov R, Waring MT, Chicoine AT, Mouded M, Pittet MJ, Nahrendorf M, Weissleder R, Swirski FK. Innate Response Activator B Cells Protect Against Microbial Sepsis. Science. 2012;335:597–601. doi: 10.1126/science.1215173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weber GF, Chousterman BG, He S, Fenn AM, Nairz M, Anzai A, Brenner T, Uhle F, Iwamoto Y, Robbins CS, Noiret L, Maier SL, Zönnchen T, Rahbari NN, Schölch S, Klotzsche-von Ameln A, Chavakis T, Weitz J, Hofer S, Weigand MA, Nahrendorf M, Weissleder R, Swirski FK. Interleukin-3 Amplifies Acute Inflammation and is a Potential Therapeutic Target in Sepsis. Science. 2015;347:1260–1265. doi: 10.1126/science.aaa4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weber GF, Chousterman BG, Hilgendorf I, Robbins CS, Theurl I, Gerhardt LM, Iwamoto Y, Quach TD, Ali M, Chen JW, Rothstein TL, Nahrendorf M, Weissleder R, Swirski FK. Pleural Innate Response Activator B Cells Protect Against Pneumonia Via a Gm-Csf-igm Axis. J Exp Med. 2014;211:1243–1256. doi: 10.1084/jem.20131471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bentzer P, Fjell C, Walley KR, Boyd J, Russell JA. Plasma Cytokine Levels Predict Response to Corticosteroids in Septic Shock. Intensive Care Med. 2016;42:1970–1979. doi: 10.1007/s00134-016-4338-z. [DOI] [PubMed] [Google Scholar]
- 20.Chousterman BG, Swirski FK, Weber GF. Cytokine Storm and Sepsis Disease Pathogenesis. Semin Immunopathol. 2017;39:517–528. doi: 10.1007/s00281-017-0639-8. [DOI] [PubMed] [Google Scholar]
- 21.Wacker C, Prkno A, Brunkhorst FM, Schlattmann P. Procalcitonin as a Diagnostic Marker for Sepsis: A Systematic Review and Meta-Analysis. Lancet Infectious Disease. 2013;13:426–435. doi: 10.1016/S1473-3099(12)70323-7. [DOI] [PubMed] [Google Scholar]
- 22.Howie SR. Blood Sample Volumes in Child Health Research: Review of Safe Limits. Bull World Health Organ. 2011;89:46–53. doi: 10.2471/BLT.10.080010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prosser A, Hibbert J, Strunk T, Kok CH, Simmer K, Richmond P, Burgner D, Currie A. Phagocytosis of Neonatal Pathogens By Peripheral Blood Neutrophils and Monocytes From Newborn Preterm and Term Infants. Pediatr Res. 2013;74:503–510. doi: 10.1038/pr.2013.145. [DOI] [PubMed] [Google Scholar]
- 24.Sankar MJ, Agarwal R, Deorari AK, Paul VK. Sepsis in the Newborn. Indian journal of pediatrics. 2008;75:261–266. doi: 10.1007/s12098-008-0056-z. [DOI] [PubMed] [Google Scholar]
- 25.Pierrakos C, Vincent JL. Sepsis Biomarkers: A Review. Crit Care. 2010;14:R15. doi: 10.1186/cc8872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marshall JC, Vincent JL, Fink MP, Cook DJ, Rubenfeld G, Foster D, Fisher CJ, Faist E, Reinhart K. Measures, Markers, and Mediators: Toward a Staging System for Clinical Sepsis. A Report of the Fifth Toronto Sepsis Roundtable, Toronto, Ontario, Canada, October 25–26, 2000. Critical care Medicine. 2003;31:1560–1567. doi: 10.1097/01.CCM.0000065186.67848.3A. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.