Abstract
Scaffolding proteins represent an evolutionary solution to controlling the specificity of information transfer in intracellular networks. They are highly concentrated in complexes located in specific subcellular locations. One of these complexes is the postsynaptic density of the excitatory synapses. There, scaffolding proteins regulate various processes related to synaptic plasticity, such as glutamate receptor trafficking and signalling, and dendritic structure and function. Most scaffolding proteins can be grouped into 4 main families: discs large (DLG), discs-large-associated protein (DLGAP), Shank and Homer. Owing to the importance of scaffolding proteins in postsynaptic density architecture, it is not surprising that variants in the genes that code for these proteins have been associated with neuropsychiatric diagnoses, including schizophrenia and autism-spectrum disorders. Such evidence, together with the clinical, neurobiological and genetic overlap described between schizophrenia and autism-spectrum disorders, suggest that alteration of scaffolding protein dynamics could be part of the pathophysiology of both. However, despite the potential importance of scaffolding proteins in these psychiatric conditions, no systematic review has integrated the genetic and molecular data from studies conducted in the last decade. This review has the following goals: to systematically analyze the literature in which common and/or rare genetic variants (single nucleotide polymorphisms, single nucleotide variants and copy number variants) in the scaffolding family genes are associated with the risk for either schizophrenia or autism-spectrum disorders; to explore the implications of the reported genetic variants for gene expression and/or protein function; and to discuss the relationship of these genetic variants to the shared genetic, clinical and cognitive traits of schizophrenia and autism-spectrum disorders.
Introduction
Schizophrenia and autism-spectrum disorders are neurodevelopmental psychiatric disorders that have a prevalence of approximately 1% and 2.5% worldwide, respectively,1,2 and have profound human and economic consequences.
Schizophrenia and autism-spectrum disorders were nosologically separated in the Diagnostic and Statistical Manual Mental Disorders, third edition (1980).3 However, evidence has been accumulating to suggest that they may partially overlap in their clinical, neurobiological, behavioural and cognitive features, and that they may have some common etiological roots.4 Regarding their clinical expression, some authors have proposed that the negative symptoms of schizophrenia can be construed more broadly as deficits in social communication and motivation, which are also found in people with autism-spectrum disorders.5 Similarly, the grossly disorganized or abnormal motor behaviour described in schizophrenia includes a number of signs and symptoms consistent with those of autism-spectrum disorders, such as repeated stereotyped movements, echolalia, unpredictable agitation and decreased interaction with or interest in one’s environment.5,6 The disorders also share some cognitive deficits7–9; in particular, deficits in social cognition have received much attention.10–15 As well, there are brain structural similarities between these disorders. For instance, lower grey matter volume in the limbic–striato–thalamic circuitry is common to schizophrenia and autism-spectrum disorders,16 and reduced volume and thickness of the insula have been found in patients with first-episode psychosis and in high-functioning patients with autism-spectrum disorders.17 Similar alterations to the white matter integrity of the left fronto-occipital fasciculus have recently been found in patients with schizophrenia and in patients with autism-spectrum disorders.18
In recent years, the field of molecular genetics has been uncovering evidence of an overlapping and complex polygenetic architecture for these disorders. Evidence suggests that studying pathways common to both may shed light on their pathophysiology and clinical heterogeneity.
Robust longitudinal and epidemiological studies have shown that 25% of people with childhood-onset schizophrenia have a history of a premorbid autism-spectrum disorder19; that the adult outcomes of children with atypical autism include psychotic disorders20; that autistic traits in infancy increase the risk for psychotic experiences later in life21; and that there is some co-occurrence of autism-spectrum disorders and psychotic disorders.22,23 This overlap is further supported by family studies, which have reported that the presence of one of these diagnoses in a first-degree relative increases the risk of the other.24–27 Similarly, schizophrenia is more common in parents of patients with autism than in parents of healthy controls.24
Twin studies have also recognized the important contribution of genetic factors to both schizophrenia and autism-spectrum disorders, with heritability estimates of h2 = 64%–80%28,29 and h2 = 64%–91%,30 respectively.
Over the last decade, molecular studies have contributed to our initial understanding of the complex genetic architecture of schizophrenia and autism-spectrum disorders, and later to identifying genes that are involved in both disorders. In this sense, it is currently accepted that an individual’s genetic risk of schizophrenia or an autism-spectrum disorder can be attributed to either many common variants with a frequency of > 1% (single nucleotide polymorphisms [SNPs]), each conferring a modest level of risk (odds ratio = 1.1–1.5); or rare mutations with a frequency of < 1% (single nucleotide variants [SNVs] and copy number variants [CNVs]) that are usually associated with a larger penetrance on the phenotype (odds ratio > 2).31,32
The most recent studies to examine genome-wide SNPs contributing to these disorders have estimated that genetic variation from SNPs accounts for 23% and 17% of the variance in risk of schizophrenia and autism-spectrum disorders, respectively.33 Based on the significant but small correlation between SNP heritability estimates in both disorders, the coheritability between them has been quantified at around 4%.33 In this regard, genome-wide association studies have identified several SNPs associated with schizophrenia and/or autism-spectrum disorders.34–37
Meanwhile, genome-wide and microarray-based comparative genomic hybridizations have found that the CNV burden is also increased in patients with schizophrenia or autism-spectrum disorders compared with healthy controls.38–41 For example, microduplications of 1q21.1 or 16p11.2 and deletions at 2p16.3, 15q11.2 or 22q11.21 have been reported in patients with schizophrenia and autism-spectrum disorders.42 De novo gene-disrupting SNVs have also been found to occur at higher rates in patients with autism-spectrum disorders than in controls.43–46 In schizophrenia, the initially reported increased rates of putatively functional mutations47,48 have not been replicated in 2 larger studies,49,50 but those later studies found that the enrichment of loss-of-function de novo mutations was relatively concentrated in genes that overlapped with those affected by de novo mutations in autism-spectrum disorders. In addition, an excess of de novo mutation was confirmed in an independent sample of patients with schizophrenia.51
With regard to the identification of specific genes involved in both schizophrenia and autism-spectrum disorders, findings from CNV and SNV studies have shown a notable consistency in some functionally enriched sets of genes. Genetic studies assessing common or rare variants show a certain convergence on reporting genes involved in glutamatergic synapse plasticity.49,52–55 A structure located in glutamatergic synapses that has been associated with both disorders is the postsynaptic density (PSD).50,56–61 For example, Bayés and colleagues found that mutations in 199 human PSD genes were involved in more than 200 diseases, half being nervous-system disorders.61 That study suggested that impairments in PSD proteins might underlie psychiatric disorders and their associated cognitive, behavioural and clinical phenotypes, but no systematic review based on this hypothesis has integrated the molecular data generated across studies in the last decade. Another example has been provided by Purcell and colleagues, who, after analyzing the exome sequences of 2536 patients with schizophrenia and 2543 controls, reported that SNVs were significantly more frequent in cases than controls, and that these SNVs were especially enriched in the activity-regulated cytoskeleton-associated (ARC) complex of PSD.50
PSD proteins and pathophysiological hypotheses
The PSD is a specialized matrix located at the excitatory postsynaptic terminals with a dish-shaped aspect, a surface area of 0.07 μm2 and a thickness of 30 to 40 nm on electron microscopy (Fig. 1A).62 The PSD can also be described as a highly organized and dynamic macromolecular complex consisting of several hundred proteins that process, integrate and converge the excitatory glutamatergic synaptic signals on the nucleus. As a point of convergence for the glutamatergic signalling pathways with other neurotransmitter systems, the composition and regulation of the PSD is essential for ensuring normal synaptic neurotransmission and plasticity.63,64
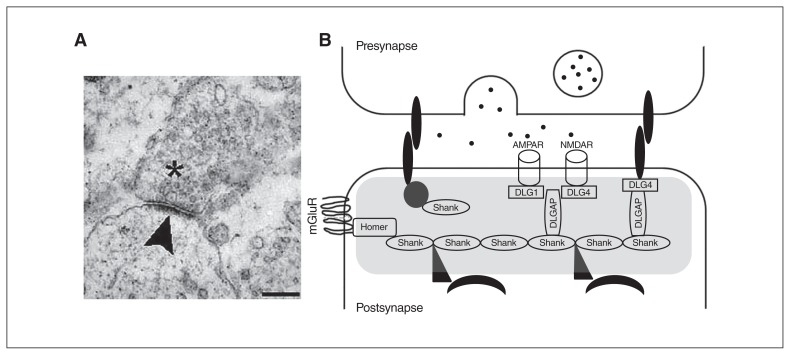
Fig. 1.
Image of the postsynaptic density (PSD) and scheme of the scaffolding proteins at the PSD that have been analyzed in the present review. (A) An electronic microscope image of a synapse; vesicles can be observed in the presynaptic neuron (asterisk). The electron-dense structure observed in the postsynaptic element is the PSD (arrowhead). Scale bar, 250 nm. Image retrieved under the Creative Commons Attribution License from Heupel et al. Neural Devel 2008, https://doi.org/10.1186/1749-8104-3-25. (B) A scheme of the PSD (grey shading). Multimerization of Shank1 to 3 proteins generate a network that links numerous proteins to the postsynaptic receptors. Homer proteins, including Homer1b/c, Homer2 and Homer3, also act as adaptors and interact with several PSD proteins, such as type I-mGluRs. The DLGAP1 to 4 proteins interact with DLG proteins, including the DLG1/SAP-97, DLG2/PSD-93, DLG3/SAP-102 and DLG4/PSD-95, to coregulate different ion channels, such as the NMDAR and AMPAR. AMPAR = α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; DLG = discs large; DLGAP = discs-large-associated protein; mGluR = metabotropic glutamate receptor; NMDAR = N-methyl-d-aspartate receptor; SAP = synapse-associated protein.
The PSD is enriched with different membrane components, such as glutamate receptors, tyrosine kinases, G protein–coupled receptors, ion channels or cell adhesion molecules, which are assembled by cytoplasmatic scaffolding proteins.65 Among the proteins that make up the PSD, studies have reported associations between genes coding for scaffolding proteins and both schizophrenia and autism-spectrum disorders, suggesting that variants in these genes might increase the risk of these disorders. For instance, recent studies have found that SNPs and CNVs in autism-spectrum disorders and schizophrenia are particularly concentrated in scaffolding genes and other PSD-related genes.66,67 Other studies have indicated changes in the expression of scaffolding genes in schizophrenia and autism-spectrum disorders compared with healthy controls.68,69 A recent study reported that gene-disrupting ultra-rare variants were more abundant in schizophrenia cases than in controls, and that these mutations were particularly enriched in scaffolding genes and other PSD genes.70
Scaffolding proteins can be defined as molecular circuit boards that can organize a wide variety of circuit relationships between signalling proteins. More specifically, the main function of scaffolding proteins is to bring together 2 or more proteins to facilitate their interaction and functions, linked to critical roles in cellular signalling. This is possible because scaffolding proteins are composed of several protein–protein interaction modules, most notably the PSD-95/discs large/zona occludens-1 (PDZ) and Src homology 3 (SH3) domains.71 Since scaffolding protein complexes are dynamic, they have the ability to change specific protein interactions to rapidly adapt to changing environmental requirements or diverse signalling cues.72 This versatility is related to their modularity, which allows for recombination of protein interaction domains to generate variability in signalling pathways. Such properties are seen as a simple evolutionary solution to controlling the specificity of information flow in intracellular networks, generating precise signalling behaviours.73
Owing to their dynamic configuration, postsynaptic scaffolding molecules not only establish the internal organization of the PSD, allowing neurons to respond efficiently to stimuli, but they also regulate processes related to synaptic plasticity, such as glutamate receptor trafficking and signalling, and dendritic structure and function,74,75 which critically determine the characteristics of excitatory synaptic transmission (Fig. 1B).
Disruption of scaffolding genes might alter the homeostasis of the PSD and contribute to the synaptic dysfunctions associated with schizophrenia and autism-spectrum disorders.76 However, despite the potential importance of scaffolding proteins in these psychiatric conditions, no systematic review has addressed the integration of genetic and molecular data generated across studies.
The nature and function of the different families of scaffolding proteins included in this review, and the characteristics of the genes encoding them, are shown in Figure 1 and briefly summarized below.
The discs large protein subfamily
The discs large (DLG) subfamily is a group of proteins in the membrane-associated guanylate kinase (MAGUK) family, and consists of DLG1, DLG2, DLG3 and DLG4. These proteins have 3 PDZ domains in their N-terminus, which allow them to interact with a variety of binding partners in the PSD, such as glutamatergic receptors, as well as other cytoplasmic scaffolding proteins. The DLG proteins control the transmission of extracellular signals to downstream signalling molecules of the PSD and regulate the localization of glutamatergic receptors N-methyl-d-aspartate (NMDA) and α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) at neuronal synapses and dendrites.77 Moreover, they regulate the trafficking and clustering of ionic channels and the excitability of the presynaptic terminals, affecting the amount of neurotransmitter released.78 Since their temporal and spatial expression differ, it is believed that DLG members complement each other in performing these functions from embryonic to adult stages.79
The DLG1 gene (also known as SAP-97) maps on chromosome 3q29 and encodes the synapse-associated protein 97 (SAP-97 or DLG1), which is thought to play a role in synaptogenesis80 and glutamatergic receptor trafficking during development.77
The DLG2 gene (also known as PSD-93) is located on chromosome 11q14.1. Different studies have suggested that the protein it encodes (PSD-93, DLG2) plays a role in the regulation of synaptic plasticity. The DLG2 protein interacts with the tyrosine kinase Fyn, which is involved in the phosphorylation-based regulation of NMDA receptors that is required for the induction of NMDA-receptor-dependent long-term potentiation.81
The DLG3 gene (also known as SAP-102) is located on Xq13.1, and the protein it encodes (DLG3) is the first protein related to intellectual disability that has been directly linked to glutamate receptor signalling and trafficking.82 Later studies have replicated the association of this gene with intellectual disability,83,84 suggesting that DLG3 somehow modulates cognition. This is consistent with the observed embryonic expression of this protein and its role in the regulation of synaptic formation and plasticity during brain development.85
The DLG4 gene is located on chromosome 17p13.1, and the protein it encodes (PSD-95, DLG4) is involved in the maturation of synapse formation and the NMDA receptor signalling pathway. It participates in the clustering and trafficking of NMDA and AMPA receptors in the PSD.63,79 Moreover, DLG4 interacts with the dopamine receptor D1 (DRD1) and the NMDA receptor, and regulates positive feedback between them.86 The degradation of DLG4 is regulated by other proteins that have also been associated with autism-spectrum disorders.87
The discs-large-associated protein family
The discs-large-associated protein (DLGAP) family is made up of 4 proteins encoded by different homonymous genes: DLGAP1 (18p11), DLGAP2 (8p23), DLGAP3 (1p34), and DLGAP4 (20q11). All proteins have 5 repeats of 14 amino acids in the middle region, followed by a proline-rich sequence and a C-terminal PDZ-binding motif that mediate interactions with other PSD proteins.88
Although the differential roles of each family member are unknown, all DLGAP proteins play an important role in organizing the postsynaptic signalling complex in glutamatergic synapses,89 and are especially involved in the stabilization of synaptic junctions and regulation of neurotransmission.90 In addition, DLGAP proteins clearly have a central role in the regulation of synaptic ion channels, including both NMDA and AMPA receptors.91
DLGAP2 (also known as SAPAP2 or GKAP) is the most studied of the proteins in this family. It interacts directly with DLG4 and Shank proteins to form a complex that plays critical roles in synaptic morphogenesis and function.90,92
It has been proposed that SAPAP proteins provide a link between the PSD-95 family of proteins and the actincytoskeleton through interactions with the Shank/ProSAP proteins, which in turn bind the actin-binding protein cortactin.93–97 Additionally, Shank/ProSAP binds to Homer, which interacts with metabotropic glutamate receptors.98 Therefore, in the current scaffolding model, PSD-95/SAPAP/Shank interactions play an important role in organizing the large postsynaptic signalling complex at glutamatergic synapses.96,97,99,100
The Shank protein family
Another PSD scaffolding protein group is the SH3 and multiple ankyrin repeat domains (Shank) family, which consists of 3 proteins encoded by different genes that are differently expressed in the brain97,101: SHANK1 (19q13.33), SHANK2 (11q13.3) and SHANK3 (22q13.3). So far, it is unclear whether individual Shank family proteins fulfill unique physiologic functions, but the structural similarity between Shank forms has led to the observation that many interaction partners of Shank proteins in the synapse are recognized equally by all 3 family members.93
In this regard, Shank proteins crosslink Homer, DLGAP2 and DLG4 proteins in the PSD and participate in glutamatergic downstream signalling by assembling glutamate receptors with other scaffolding proteins, cytoskeleton factors and intracellular effectors.102 Multimerization of Shank1–3 proteins can generate a network in the PSD that links numerous proteins to the postsynaptic receptors. In addition, Shank proteins promote the formation, maturation and enlargement of dendritic spines.103
The Homer protein family
Homer proteins include 3 different members that are encoded by 3 different homonymous genes: HOMER1 (5q14), HOMER2 (15q25) and HOMER3 (19p13). Homer proteins can also be classified into constitutively expressed isoforms (i.e., Homer1b/c, Homer2 and Homer3), which are bimodal proteins with an N-terminal domain that mediates the interaction with other PSD proteins, and a C-terminal coiled-coil domain that enables self-assembly, as well as short splice variants (Homer1a and Ania-3) that lack the C-terminal domain and cannot self-assemble.104 The various protein forms are differentially expressed over time and place.105
Homer proteins act as multimodal adaptors by interacting with several PSD proteins, such as type I metabotropic glutamate receptors (mGLuR1–5), Shank proteins or synaptic signalling molecules such as inositol 1,4,5-triphosphate receptors (IP3Rs), and binding them to the cytoskeleton.102 Homer proteins are also involved in glutamatergic synapses by regulating glutamatergic receptor trafficking, the function of plasma membrane ion channels and intracellular messenger systems.106 For these reasons, Homer proteins are important for cell signalling, cell excitability, synaptic neurotransmission and neuronal plasticity.107,108
Objectives
The objectives of this review were as follows: to conduct a systematic review of the literature in which variations within the above-mentioned scaffolding genes are associated with either schizophrenia or autism-spectrum disorders, and to describe the degree of overlap between both diagnoses; to explore whether the reported genetic variants putatively associated with schizophrenia or autism-spectrum disorders are involved in changes of gene expression or protein functionality according to basic research data; and to consider the implications of the reported associations for the development of these disorders and their associated phenotypes.
Methods
We conducted a systematic search of the PubMed, PsycINFO and Web of Science databases. The search terms were “Schizophrenia or autism” and “postsynaptic density proteins or PSD or scaffold* proteins” without date restrictions. Inclusion criteria were original articles that reported i) the association of genetic variants (SNPs, SNVs and CNVs) of the genes in the DLG protein subfamily or the DLGAP, Shank or Homer protein families with schizophrenia or autism-spectrum disorders; and ii) genetic variations in these genes and their functional consequences, based on animal-model or in vitro studies.
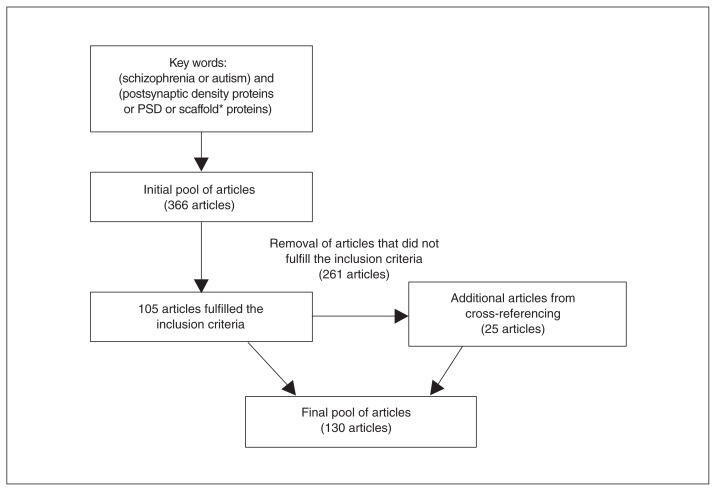
This search initially retrieved 366 articles. After evaluating whether they fulfilled the inclusion criteria, we excluded 261 articles. Another 25 studies and reviews that were relevant for the topic were found from cross-referencing and included in this review. The final pool of articles comprised 130 papers (Fig. 2).
Fig. 2.
Flow diagram of the literature search. PSD = postsynaptic density.
Results
Table 1 describes SNPs in scaffolding genes that have been associated with schizophrenia or autism-spectrum disorders. Table 2 describes SNVs, and Table 3 describes CNVs. Table 4 describes expression and functional information on scaffolding genes obtained from basic studies. Table 5 describes the SNPs, SNVs and CNVs of risk that are shared by both schizophrenia and autism-spectrum disorders.
Table 1.
Single nucleotide polymorphisms in scaffolding genes associated with schizophrenia, autism-spectrum disorders and other clinical phenotypes of interest
| Gene | Single nucleotide polymorphisms | Sources | |||
|---|---|---|---|---|---|
|
| |||||
| Schizophrenia | Austism-spectrum disorders | Schizophrenia and autism-spectrum disorders | Other associated phenotypes | ||
| DLG1 | rs382579, rs2122824, rs7616588, rs7638423, rs6805929, rs2044862, rs4916461, rs338187, rs10489880109 rs9843659109,110 |
— | — | — | Sato et al.109 Uezato et al.110 |
| DLG3 | — | ss104807047, rs28391150, rs1886890, rs3215810, rs41303736, ss104807048111 | — | rs28391150 (associated with intellectual disability)83 ss104807047 (associated with intellectual disability)84 |
Zanni et al.83 Philips et al.84 Kantojärvi et al.111 |
| DLG4 | rs2230178, rs6145976, rs2017365, rs739669, rs13333112 rs222837113 rs390200, rs222853, rs17203281112,113 |
— | — | — | Cheng et al.112 Balan et al.113 |
| DLGAP1 | rs145691437, rs3786431, rs201567254, rs3745051, rs11662259114 | — | — | — | Li et al.114 |
| DLGAP2 | rs2906568, rs2293909116 | — | rs2906569, rs2301963115,116 | rs2301963 (associated with decreased orbitofrontal cortex white matter volume)117 | Chien et al.115 Li et al.116 Wu et al.117 |
| SHANK3 | — | rs9616915118,119 Independent studies did not find association with autism spectrum disorder120,121 |
— | — | Shao et al.118 Mashayekhi119 Sykes et al.120 Qin et al.121 |
| HOMER1 | rs4704560, rs2290639122 rs9293785123 |
— | — | rs4704560 C allele (associated with hallucinations in patients with Parkinson’s disease)124 rs2290639 (associated with substance abuse)125 |
Spellmann et al.122 Zhao et al.123 De Luca et al.124 Strauss et al.125 |
| HOMER2 | rs2306428, rs86949, SNP20126 | — | — | — | Gilks et al.126 |
SNP = single nucleotide polymorphism.
Table 2.
Single nucleotide variants in scaffolding genes associated with schizophrenia, autism-spectrum disorders and other clinical phenotypes of interest
| Gene | Single nucleotide variants | Sources | |||
|---|---|---|---|---|---|
|
| |||||
| Schizophrenia | Autism-spectrum disorders | Schizophrenia and autism-spectrum disorders | Other associated phenotypes | ||
| DLG1 | g.196863463C>T49 g.196812562A>T, g.196812614A>T, g.196842947T>C, g.196867096C>A50 g.196863502G>C*127 |
g.196817764T>C59 | — | — | Fromer et al.49 Purcell et al.50 Iossifov et al.59 Xing et al.127 |
| DLG2 | g.83194295C>T49 g.83180351C>T, g.83180371T>G, g.83243821T>G, g.83497759G>A50 |
— | — | — | Fromer et al.49 Purcell et al.50 |
| DLG4 | g.7096826C>T50 | g.7106562G>Aa127 | — | — | Purcell et al.50 Xing et al.127 |
| DLGAP1 | c.1922A>G114 | — | — | — | Li et al.114 |
| DLGAP2 | g.1497520G>A50 c.−69+9C>T, c.−69+13C>T, c.−69+47C>T, c.−69+55C>T, c.−32A>G, c.341A>G,* c.438C>T, c.990+60T>C, c.1192G>A, c.1920+37A>G, c.1920+94T>A, c.1927G>A, c.2493G>C, c.2634C>T, c.2797G>A,* c.2884G>A,* c.2663G>A116 |
c.44C>T,* c.277C>A,* c.545G>A,* c.574G>T, c.1516T>C,* c.2392G>C,* c.970A>T115 g.1616734C>T*127 g.1626547G>C59 |
c.841C>G,*† c.2135C>T,†‡ c.2750C>T*†‡115,116 |
— | Purcell et al.50 Iossifov et al.59 Chien et al.115 Li et al.116 Xing et al.127 |
| DLGAP3 | c.1141G>A, c.1759G>C, c.2309G>T, c.2578−11C>T128 | 35365700G>A59 | — | — | Iossifov et al.59 Li et al.128 |
| SHANK1 | g.51205733T>A50 | g.51220161C>T, g.51206952G>A,* g.51205840C>T,* g.51170826G>A, g.51170775G>A, g.51165632C>T129 g.51220076C>T, g.51219998C>T, g.51215287C>T,* g.51206988G<T, g.51205886C>T,* g.51191281C>T,* g.51172180G>A, g.51171270C>T,* g.51170856C>T, g.51170854C>T,* g.51170779C>T, g.51170674C>A,* g.51170418G>A, g.51170407G>T, g.51170362A>T,* g.51170359T>C,* g.51170046A>T,* g.51169830C>T,* g.51165932C>T, g.51165929C>T,* g.51165767G>A,* g.51165574C>A*130 |
— | — | Purcell et al.50 Leblond et al.129 Sato et al.130 |
| SHANK2 | g.70666649G>A, g.70666499C>A, g.70544817G>T, g.70349029T>C, g.70333526G>T, g.70333043G>T, g.70333967G>A, g.70331576C>T, g.70331462G>T, g.70319333C>A131 g.70644595G>A*132 |
c.76C>T, c.622C>T, c.3380C>T, c.4048G>A, c.467A>G, c.492C>T, c.527−18C>A, c.640+11C>T, c.80033C>T, c.942+19G>A, c.924+133G>C, c.1061−81C>T, c.1141+49G>A, c.1148−109C>T, c.1201A>C, c.1264G>A, c.1302+35G>A, c.1303−54C>T, g.70336411G>A,* c.1392G>T, c.1923G>A, c.2052G>A, c.2823C>T, c.3135C>T, c.3843−12C>T, g.70666749G>A,* g.70644566G>A,* g.70331881G>A,* g.70319339C>T133 g.70666635G>A,* g.70544853C>A,* g.70348949C>A,* g.70348913C>T,* g.70332914C>T,* g.70332890C>T,* g.70332272C>T,* g.70331795C>T,* g.70319359A>G*134 g.70821018C>G g.70858273A>C59 |
— | c.76C>T, c.467A>G, c.942+19G>A, c.924+133G>C, c.1141+49G>A, c.1148−109C>T (associated with intellectual disability)133 | Iossifov et al.59 Peykov et al.131 Homann et al.132 Berkel et al.133 Leblond et al.134 |
| SHANK3 | g.49484091C>T g.49506476C>T46,135 g.51117040G/A, g.51117200G/T, g.51117489C/T, g.51117580C/T, g.51117585G/A, g.51137217A/G, g.51143287C/T, g.51144513C/G, g.51153371G/A, g.51159735C/T, g.51159798A/G, g.51159802C/T, g.51159828G/A, g.51160154G/A, g.51169180A/G51 |
g.51117341C>G, g.51159953G>A, g.51169240A>G136 g.51159293G>T*129,136 c.670G>A136,138 g.51121780C>T,* g.51159458G>T,* g.51113103C>T136,140 g.51117094C>G,* g.51160615G>T137 g.51142357C>T, g.51153464G>A,* g.51158686G>T,* g.51158945T>C,* g.51159965C>A,* g.51159988C>T,* g.51160086A>T,* g.51160057G>A129 c.1527G>A138 g.51113615T>C*139 g.51121844A>G,* g.51123071C>T,* g.51159169G>T,* g.51160477C>G, g.51169213G>A,* g.51160589T>C141 g.51159778G>A,* g.51160049C>T,* § 141,142 c.1563G>A, c.1967G>A, c.4908C>T142 g.1159884G>A,* g.51160018A>T, g.51169259C>T* (p.1572A>V), 51169364C>T,* 51169442G>A,* 51169459C>T, 51169463C>T,* 51169480G>A, g.51169207C>T* g.51169499G>A143 c.612C>A, c.763C>T, c.898C>T, c.920C>G, c.1315C>T, c.1337G>T, c. 3761C>T, c.3764C>T, c.3836C>T, c.4025C>T, c.4405G>C, c.4406G>T, c.4490G>A, c.4720G>A144 c.5008A>T145 |
g.49506159G>T129,135,136 | g.49506476C>T, c.5008A>T, c.1527G>A (associated with intellectual disability)46,138,145 | Awadalla et al.46 Girard et al.51 Leblond et al.129 Gauthier et al.135 Durand et al.136 Boccuto et al.137 Soorya et al.138 Gauthier et al.139 Durand et al.140 Moessner et al.141 Waga et al.142 Schaaf et al.143 Kelleher et al.144 Cochoy et al.145 |
| HOMER1 | IVS4 þ146 | c.195G>T, c.290C>T, c.425C>T, c.968G>A, c.1090C>T144 | Kelleher et al.144 Norton et al.146 |
||
Table 3.
Copy number variants in scaffolding genes associated with schizophrenia, autism-spectrum disorders and other clinical phenotypes of interest
| Gene | Copy number variants | Sources | |||
|---|---|---|---|---|---|
|
| |||||
| Schizophrenia | Autism-spectrum disorders | Schizophrenia and autism-spectrum disorders | Other associated phenotypes | ||
| DLG1 | — | del:195971510_197675831147 | del:3q2945,50,57,148–156 | Deletion of 3q29 has been associated with intellectual disability,148,149,154–156 developmental delay,152 impaired social skills and repetitive behaviour150,152 | Sanders et al.45 Purcell et al.50 Kirov et al.57 Pinto et al.147 Levinson et al.148 Mulle et al.149 Magri et al.150 Szatkiewicz et al.151 Quintero-Rivera et al.152 Levy et al.153 Willatt et al.154 Ballif et al.155 Sagar et al.156 |
| DLG2 | del:83472750_83842973 del:84006106_8422606457 del:83680969_83943977158 |
del:84032216_84276593157 dup:83108466_83378706159 |
— | — | Kirov et al.57 Cuscó et al.157 Walsh et al.158 Egger et al.159 |
| DLG4 | — | dup:17p13.1_p13.2153,160 | — | — | Levy et al.153 Sanders et al.160 |
| DLGAP2 | dup:1436299_164283740 | dup:704383_152191060,147 del:1130900_6780950162 |
del:8p23.3161,163–165 | Duplication of 1436299_1642837 has been associated with intellectual disability40 Deletion of 8p23.3 has been associated with intellectual disability161 | Guilmatre et al.40 Pinto et al.60 Pinto et al.147 Chien et al.161 Szatmari et al.162 Costain et al.163 Marshall et al.164 Ozgen et al.165 |
| SHANK1 | — | del:55872189_55935995, del:55808307_55871709130 del:19q13.33166 |
— | Deletion of 55872189_55935995 has been associated with impaired social communication skills, repetitive behaviours and higher functioning130 Deletion of 55808307_55871709 has been associated with developmental abnormalities and disrupted social skills130 |
Sato et al.130 Prasad et al.166 |
| SHANK2 | — | del:70154458_7022063260 del:70077507_70506315, del:70119917_70187872, del: 70154458_70220632147 del:70220882_70154208133 dup:70520567_71017315167 del:70332092(2)CTG > C59 |
— | Deletion of 70154458_70220632, 70077507_70506315 and 70119917_70187872 has been associated with language delay60,147 Deletion of 70220882_70154208, 70077507_70506315 and 70119917_70187872 has been associated with intellectual disability133,147 |
Iossifov et al.59 Pinto et al.60 Berkel et al.133 Pinto et al.147 Gai et al.167 |
| SHANK3 | — | del:c.1320_1338, dup:c.1497+910bp/ins, del:c.1497+910bp/del142 del:c.3259168 del:48927548_51224208, del:48444959_51224208, del:49114430_51224208, del:44321641_51224208, del:46143471_51224208, del:44427703_51224208, del:46905533_51224208, del:49028732_51224208, del:49028732_51224208, del:43745129_51224208, del:50267252_51224208, del:45902119_51224208, del:42918711_51224208, del:45583935_51224208, del:48551989_51206201, del:51083118_51224208, del:45428606_51224208, del:44800014_51224208, del:44023173_51224208, del:43218614_51224208, del:46787434_51224208, del:49460840_51224208, del:51115526_51234443, del:45705241_51224208, del:49004395_51224208, del:42822943_51224208118, del:45159185_49582267169 del:45159185_49582267, del:47996161_49512530, del:49468716_49485255, del:49470371_49567383, del:49470371_49480446147 |
del:22q13.340,60,136,137,141,164,170–174 | Deletion of 22q13.3 has been associated with intellectual disability, language alteration,60,137,139,170,174 developmental delay and impaired social interaction140 Deletion of 45159185_49582267 has been associated with intellectual disability, hyperactivity, attention deficits and agressiveness147 Deletion of 47996161_49512530 has been associated with severe intellectual disability and cortical atrophy147 Deletion of 49470371_49567383 and 49470371_49480446 have been associated with language delay147 |
Guilmatre et al.40 Pinto et al.60 Durand et al.136 Boccuto et al.137 Gauthier et al.139 Moessner et al.141 Waga et al.142 Pinto et al.147 Marshall et al.164 Nemirovsky et al.168 Yuen et al.169 Failla et al.170 Crespi et al.171 Sebat et al.172 Wang et al.173 Bonaglia et al.174 |
| HOMER1 | dup:78375511_78797532167 | — | — | — | Gai et al.167 |
del = deletion; dup = duplication.
Table 4.
Expression, animal model and pharmacological studies on the reviewed scaffolding genes
| Gene | Expression studies | Functional studies | Sources |
|---|---|---|---|
| DLG1 | Reduced expression of DLG1 mRNA in PFC of schizophrenia patients68 Reduced expression of DLG1 protein in PFC of schizophrenia patients175 |
Administration of the NMDA receptor antagonist PCP caused an upregulation of DLG1 gene transcription in the neocortex of rats176 | Dracheva et al.68 Toyooka et al.175 Hiraoka et al.176 |
| DLG2 | Increased DLG2 mRNA and decreased protein expression in prefrontal cortex and anterior cingulate cortex of schizophrenia patients177 | PSD-93 mutant mice exhibited deficits in LTP178 PSD-93 mutant mice showed cognitive abnormalities179 PSD-93 mutant mice did not show any abnormality of synaptic structure or function in cerebellum180 |
Kristiansen et al.177 Carlisle et al.178 Nithianantharajah et al.179 McGee et al.180 |
| DLG3 | Increased DLG3 mRNA and protein expression in the thalamus of schizophrenia patients181 Decreased DLG3 protein expression in the thalamus of schizophrenia patients182 |
Mice lacking DLG3 exhibited impairments of spatial learning183 | Clinton et al.181 Clinton et al.182 Cuthbert et al.183 |
| DLG4 | Increased DLG4 mRNA and decreased protein expression in ACC of schizophrenia patients177 Increased DLG4 mRNA and protein expression in thalamus of schizophrenia patients182,185 Increased DLG4 mRNA expression in the occipital cortex of schizophrenia patients186 Decreased DLG4 mRNA expression in the PFC of schizophrenia patients69 Decreased DLG4 mRNA and protein expression in the DLPFC of schizophrenia patients187 Decreased DLG4 protein expression in thalamus of schizophrenia patients182 Decreased DLG4 protein expression in hippocampus188,189 Decreased mRNA expression in the striatum190 No changes in either DLG4 mRNA or protein expression in PFC of schizophrenia patients175,186 No changes in either DLG4 mRNA or protein expression in the hippocampus of schizophrenia patients69,182 |
DLG4 mutant mice displayed schizophrenia and autism-spectrum disorder–like phenotypes184 DLG4 mutant mice displayed aberrant AMPA receptor-mediated transmission178,191 DLG4 mutant mice exhibited enhancement in LTP and deficit in LTD178,192–194 DLG4 mutant mice exhibited disrupted synaptic plasticity and impaired learning192 Ketamine reduced DLG4 mRNA in cortical regions of rats195 |
Ohnuma et al.69 Toyooka et al.175 Kristiansen et al.177 Carlisle et al.178 Clinton et al.179 Clinton et al.182 Feyder et al.184 Clinton et al.185 Dracheva et al.186 Funk et al.187 Toro et al.188 Matosin et al.189 Kristiansen et al.190 Nakagawa et al.191 Migaud et al.192 Ehrlich et al.193 Xu et al.194 de Bartolomeis et al.195 |
| DLGAP2 | — | DLGAP2 knockout mice displayed abnormal social behaviour196 | Jiang-Xie et al.196 |
| SHANK1 | — |
SHANK1 mutant mice showed alterations in motor system and social behaviour197–199 SHANK1 mutant mice showed social communication deficits200 |
Silverman et al.197 Hung et al.198 Wöhr et al.199 Sungur et al.200 |
| SHANK2 | — |
SHANK2(−/−) mutant mice were hyperactive and displayed autistim-like behaviours, including social interaction and repetitive jumping201,202,203 SHANK2(−/−) mutant mice exhibited fewer dendritic spines, reduced basal synaptic transmission and reduced frequency of miniature excitatory postsynaptic currents201,203 SHANK2(−/−) mutant mice showed a decrease in NMDA receptor function. Direct stimulation of the NMDA receptor with a partial agonist normalized its function and improved social interaction.202 |
Schmeisset et al.201 Won et al.202 Ha et al.203 |
| SHANK3 | — |
SHANK3 mutant mice exhibited self-injurious repetitive grooming behaviours204,206,207 and social interaction,204,205,207,209,210 learning and memory205 deficits. They also showed anxiety and motor deficits206,208,209 SHANK3 mutant mice showed deficits in glutamatergic transmission and synaptic plasticity and reduced synaptic concentrations of scaffolding proteins (e.g., DLGAP3, Homer1).204,208–210 Re-expression of the SHANK3 gene in adults led to improvements in synaptic protein composition, spine density and neural function, as well as selective rescue in autism-related phenotypes.208 Insulin-like growth factor-1 reversed synaptic and behavioural deficits in SHANK3 mutant mice206 and phenotypic changes in human neuronal models of Rett syndrome.211 SHANK3B knockout mice exhibited early hyperactivation and precocious maturation of corticostriatal circuits212 |
Arons et al.204 Wang et al.205 Bozdagi et al.206 Peça et al.207 Mei et al.208 Bozdagi et al.209 Yang et al.210 Marchetto et al.211 Peixoto et al.212 |
| HOMER1 | Increased Homer1a protein expression in hippocampal interneurons of schizophrenia patients213 Increased Homer1a and decreased Homer1b protein expression in hippocampus of schizophrenia patients189 |
HOMER1 knockout mice displayed impaired fear memory formation214 and impaired LTP215 HOMER1 knockout mice showed abnormalities in motivational, emotional, cognitive and sensorimotor processing216 HOMER1 knockout mice also showed somatic growth retardation, poor motor coordination, enhanced sensory reactivity, learning deficits and increased aggression in social interaction218 Overexpression of HOMER1 in knockout mice reverted the cognitive and behavioural impairments217 Exposure to novel environments upregulated HOMER1 mRNA in the hippocampus of rats219 Methamphetamine or cocaine administration upregulated HOMER1 mRNA in the neocortex of rats220 LSD or PCP administration upregulated HOMER1 mRNA in the PFC of rats221,222 10Ketamine increased HOMER1 mRNA in the cortical regions, striatum and nucleus accumbens of rats195,223 Antipsychotics (haloperidol, olanzapine or clozapine) induced an increment of Homer1 protein expression in the cortex, the striatum, the caudate-putamen or nucleus accumbens of rats107,224–228 |
de Bartolomeis et al.107 Matosin et al.189 de Bartolomeis et al.195 Leber et al.213 Inoue et al.214 Gerstein et al.215 Szumlinski et al.216 Lominac et al.217 Jaubert et al.218 Vazdarjanova et al.219 Fujiyama et al.220 Cochran et al.221 Nichols et al.222 Iasevoli et al.223 Iasevoli et al.224 Iasevoli et al.225 Ambesi-Impiombato et al.226 Polese et al.227 Tomasetti et al.228 |
ACC = anterior cingulate cortex; AMPA = α-amino-3-hydroxy-5-methylisoxazole-4 propionic acid; LSD = lysergic acid; LTD = long-term depression; LTP = long-term potentiation; NMDA = N-methyl-d-aspartate; PCP = phencyclidine; PFC = prefrontal cortex; PSD = postsynaptic density.
Table 5.
Summary of variants in scaffolding genes associated with both schizophrenia and autism-spectrum disorders
| Genes | SNPs | SNVs | CNVs | Sources |
|---|---|---|---|---|
| DLG1 | — | — | del:3q2945,50,57,148–156 | Sanders et al.45 Purcell et al.50 Kirov et al.57 Levinson et al.148 Mulle et al.149 Magri et al.150 Szatkiewicz et al.151 Quintero-Rivera et al.152 Levy et al.153 Willatt et al.154 Ballif et al.155 Sagar et al.156 |
| DLGAP2 | rs2906569, rs2301963115,116 | c.841C>G, c.2135C>T c.2750C>T115,116 |
del:8p23.3161,163–165 | Chien et al.115 Li et al.116 Chien et al.161 Costain et al.163 Marshall et al.164 Ozgen et al.165 |
| SHANK3 | — | g.49506159G>T129, 135,136 | del:22q13.340,60,136,137,141,164,170–174 | Guilmatre et al.40 Pinto et al.60 Leblond et al.129 Gauthier et al.135 Durand et al.136 Boccuto et al.137 Moessner et al.141 Marshall et al.164 Failla et al.170 Crespi et al.171 Sebat et al.172 Wang et al.173 Bonaglia et al.174 |
CNV = copy number variant; del = deletion; SNP = single nucleotide polymorphism; SNV = single nucleotide variant.
DLG protein subfamily
Protein DLG1
Both SNPs in DLG1 have been associated with schizophrenia (Table 1). An SNV has been associated with autism-spectrum disorders (Table 2). Furthermore, deletions of the chromosomal region 3q29 have been related to schizophrenia and autism-spectrum disorders (Table 3) and have been associated with impaired cognition and social dysfunction (Table 3).
Two studies reported reduced expression of DLG1 in the prefrontal cortex of postmortem brain samples of patients with schizophrenia (Table 4). To the best of our knowledge, there are no expression studies in autism-spectrum disorders samples.
An animal-model study reported that glutamate-receptor NMDA antagonists upregulate DLG1 mRNA expression in the cerebral cortex of mice (Table 4).
Protein DLG2
Different studies have identified rare mutations in the DLG2 gene in schizophrenia and autism-spectrum disorders. While SNVs have been detected only in schizophrenia (Table 2), CNVs have been identified in both disorders (Table 3). Interestingly, a deletion identified in intron 6 of the gene in patients with autism-spectrum disorders157 partially overlaps with another deletion spanning from intron 2 to intron 6 in patients with schizophrenia.57
Although still not tested in patients with autism-spectrum disorders, alterations in mRNA and protein expression have been reported in the prefrontal cortex of postmortem brain samples of patients with schizophrenia (Table 4).
Animal-model studies seem to support the function of DLG2 as a regulator of synaptic plasticity; they have shown that DLG2 mutant mice display cognitive abnormalities and long-term potentiation deficits (Table 4).
Protein DLG3
To our knowledge, only 1 genetic study has associated 6 intronic SNPs in DLG3 with autism-spectrum disorders (Table 1), although their consequences for protein function or expression are unknown.
Despite conflicting results in postmortem brain expression studies, it seems that alterations in DLG3 could underlie the neurobiology of schizophrenia. In this regard, both increased and decreased DLG3 mRNA and protein expression have been reported in the thalamus of schizophrenia patients (Table 4).
Animal-model studies support a role for this gene in cognition; mice lacking DLG3 exhibit impaired learning (Table 4).
Protein DLG4
For the DLG4 gene, SNPs (Table 1) and CNVs (Table 3) have been identified only in patients with schizophrenia and with autism-spectrum disorders, respectively. In contrast, SNVs have been associated with both disorders (Table 2).
Among these variants, the SNP rs13331 (T/C), located at the 3′UTR of the gene, is especially interesting because the T allele was first associated with schizophrenia, and a posterior reporter gene assay indicated that subjects carrying this allele had decreased DLG4 protein activity. Based on these results, the authors suggested that reduced DLG4 activity or expression may increase the risk of developing schizophrenia.112
Alterations in mRNA and protein expression have been reported in postmortem brain samples of patients with schizophrenia (Table 4), although the direction of the results is inconsistent. Up to now, no expression studies have been performed in patients with autism-spectrum disorders.
Animal-model studies also appear to support an important role for DLG4 in regulating excitatory synapses and synaptic plasticity, while mutant mice also displayed clinical phenotypes related to schizophrenia and autism-spectrum disorders, such as impaired learning, abnormal communication, altered motor coordination or other abnormal behaviour (Table 4).
Summary
These findings suggest that mutations in DLG genes might increase the risk of developing schizophrenia and autism-spectrum disorders, as well as related cognitive deficits, by contributing to the disruption of glutamatergic synapses. Although the neurobiological mechanisms underlying these disruptions are still unknown, some pathways can be inferred. For instance, mutations affecting DLG2 might modify the tyrosine phosphorylation-based regulation of NMDA receptors, altering NMDA-receptor-related signalling. Similarly, mutations in DLG4 might dysregulate NMDA receptor activity, because this protein also anchors different protein tyrosine kinases.229 Other mechanisms might explain the association between DLG4 and both schizophrenia and autism-spectrum disorders. It has been reported that DLG4 inhibits the interaction between dopamine receptor D1 and the NMDA receptor, preventing a reciprocal damaging overactivation of both receptors.86 This suggests that reduced expression or dysfunction of this protein might dysregulate glutamatergic and dopaminergic homeostasis. Finally, since DLG4 enhances the expression of NMDA receptor subunits NR2A and NR2B230 and the traffic of the NMDA receptor to synapses,231 diminished expression or alterations in protein function might also compromise NMDA-receptor-mediated signalling transduction.
The DLGAP protein family
Some SNPs (Table 1) and SNVs (Table 2) in the DLGAP1 gene and SNVs (Table 2) in the DLGAP3 gene have been associated with schizophrenia. No studies have assessed the association between DLGAP4 and schizophrenia or autism-spectrum disorders.
There is more evidence for an association between the DLGAP2 gene and both disorders. The SNPs (Table 1), SNVs (Table 2) and CNVs (Table 3) in this gene have been associated with both schizophrenia and autism-spectrum disorders. Interestingly, some variants were coincident in both disorders (Table 1, Table 2 and Table 3).
On one hand, the SNP rs2906569 (A>G) in intron 1 and the missense SNP rs2301963 (C>A; P384Q) in exon 3 have been associated with both autism-spectrum disorders115 and schizophrenia.116 Although the functional significance of rs2906569 is difficult to infer, it could affect either the final protein function or the regulation of gene expression by altering different processes, such as splicing, translation regulation or mRNA polyadenylation.232 The missense variant rs2301963, in which CC homozygotes were overrepresented in patients with schizophrenia and patients with autism-spectrum disorders, could affect final protein activity according to bioinformatics analyses.115
On the other hand, 3 nonsynonymous exonic de novo variants (c.841C>G, c.2135C>T and c.2750C>T) have been identified in both schizophrenia116 and autism spectrum disorders.115 The c.841C>G and c.2750C>T mutations were predicted to damage protein function using PolyPhen-2 or Pmut.
Moreover, deletion of the chromosomic region 8p23.3 has been detected in patients with schizophrenia and patients with autism-spectrum disorders. This deletion and other CNVs spanning this gene have been found in patients with schizophrenia and patients with autism-spectrum disorders who have intellectual disability.40,161
To the best of our knowledge, there are no studies of the expression of DLGAP2 in either autism-spectrum disorders or schizophrenia. Animal-model studies have suggested that alterations in this gene might lead to disadaptative social behaviour (Table 4).
Taken together, these findings suggest that disruptions of this gene might alter the function or expression of DLGAP2 and ultimately dysregulate its interplay with other PSD proteins, which could underlie the development of both schizophrenia and autism-spectrum disorders, as well as the manifestation of related clinical phenotypes, such as abnormal social behaviour. Interestingly, animal studies have found that DLGAP2 is vital for normal synaptic structure and function of the orbitofrontal cortex, a brain region that is implicated in the self-regulation of social-emotional behaviour.233 There is also evidence that the orbitofrontal cortex is disrupted in patients with autism, and animal studies have indicated that a lesion of the orbitofrontal cortex may cause aggressive behaviour.234
The Shank protein family
Protein Shank1
Up to now, most of the SNVs in SHANK1 have been associated with autism-spectrum disorders and not schizophrenia (Table 2). Deletions in SHANK1 have also been associated with autism-spectrum disorders (Table 3), with some detected in patients with pronounced social dysfunction.
No expression studies have been carried out for either schizophrenia or autism-spectrum disorders, but animal-model studies seem to indicate that alterations in SHANK1 could lead to impaired social skills (Table 4).
Protein Shank2
While SNVs in this gene have been identified in patients with schizophrenia and patients with autism-spectrum disorders (Table 2), CNVs have been found only in people with autismspectrum disorders, particularly with respect to intellectual disability or language delay (Table 3).
Among these mutations, the SNV (A1731S) detected in patients with schizophrenia is noteworthy. The authors of a study involving a functional assay in HEK293 cells reported that this variant has a significant effect on the F/G-actin ratio and concluded that diminished actin polymerization could lead to impairments in synapse formation and maintenance by reducing the presynaptic contacts.131
To the best of our knowledge, no expression studies have been performed for this gene. However, animal-model studies suggest that disruption of this gene could lead to the cognitive and social dysfunction associated with schizophrenia or autism-spectrum disorders by altering NMDA receptor function (Table 4).
Protein Shank3
There is accumulating evidence that SHANK3 mutations contribute to the pathology of neurodevelopment disorders. Two SNVs in the SHANK3 gene have been identified in patients with schizophrenia (Table 2), and different variants (SNPs, SNVs and CNVs) have been associated with autismspectrum disorders (Table 1, Table 2 and Table 3). Among them, the missense variant G1011V (g.49506159G>T) located in exon 21 has been identified in patients with schizophrenia and patients with autism-spectrum disorders (Table 2). Further studies are needed to test whether this variant has any consequence for the protein function.
The R1117X nonsense mutation (g.49484091C>T) has been identified in exon 21 of the SHANK3 gene in patients with schizophrenia and intellectual disability. This amino acid change resulted in a truncated form of the Shank3 protein that lacked the Homer-binding site, causing its loss of function.46,135
The A198G (g.51117341C>G) identified in people with autism-spectrum disorders generated a frameshift mutation that introduced a premature STOP codon at position 1227, leading to a truncated form of Shank3 that also caused its loss of function. This mutation disrupted actin polymerization, the regulation of spine formation and the molecular organization of the PSD.136
Two frameshift mutations causing premature STOP codons (g.51117094C>G and g.51160615G>T) resulted in the loss of protein function by losing the C-terminal region of the protein, which is crucial for interactions with other PSD proteins.137
Regarding CNVs, different studies have identified deletions in the SHANK3 gene in patients with schizophrenia and patients with autism-spectrum disorders with intellectual disability, developmental delay, language alterations or impaired social interaction (Table 3). Similarly, Phelan McDermid Syndrome (22q13.3 deletion syndrome), which includes deletion of the SHANK3 gene, is characterized by neonatal hypotonia, global developmental delay, absence of speech, autistic behaviour and intellectual disability.235
Animal-model studies also appear to support a role for this gene in the cognitive and behavioural clinical phenotypes related to schizophrenia and autism-spectrum disorders. Shank3 mutant mice display reduced social interaction, affiliation behaviour, repetitive behaviour and communicative deficits (Table 4).
Summary
In summary, results for Shank proteins suggest that their disruption might underlie some of the cognitive and social dysfunction present in schizophrenia and autism-spectrum disorders. This is in line with the latest data showing that the prevalence of SHANK3 mutations in people with autismspectrum disorders is 0.5% to 0.7%, and that a SHANK3 mutation is present in approximately 2% of people with both an autism-spectrum disorder and intellectual disability.129,138,236 More specifically, from animal-model studies it has been suggested that these dysfunctions might be caused by alterations in the NMDA-receptor-related signalling pathway. There is evidence that SHANK2 (−/−) mutant mice display abnormal NMDA receptor function and show alterations in behaviour and social skills.201 Interestingly, when mutant mice were stimulated with NMDA receptor agonists, NMDA receptor function was normalized and their social interactions improved.202 Regarding the pathophysiological mechanisms underlying the social deficits in SHANK3 mutation, it has been reported that knockdown of the SHANK3 gene in rat cortical cultures causes a loss of NMDA receptor function and alterations in its membrane trafficking through the disruption of the actin cytoskeleton.237 Furthermore, Arons and colleagues showed that the loss of function of the SHANK3 gene resulted in reduced glutamatergic synaptic transmission, whereas overexpression of this gene increased the number and size of excitatory synapses and the expression levels of other PSD proteins, such as DLG4 and Homer1.204 Therefore, the behavioural and cognitive alterations present in patients carrying mutations in SHANK genes might be related to dysfunctions in NMDA-receptor-related glutamatergic signalling, which in turn might be caused by abnormalities in the interactions between Shank and other PSD proteins or anomalies in the actin polymerization processes.
The Homer protein family
Although 1 study reported a putative role for the HOMER2 gene in schizophrenia (Table 1), it is principally the HOMER1 gene that has been associated with schizophrenia and autism-spectrum disorders.
Three SNPs in HOMER1 have been associated with schizophrenia (Table 1). Among them, rs4704560 has also been associated with the risk of developing psychotic symptoms in Parkinson disease (Table 1). In addition, 1 SNV and 1 CNV have been found in patients with schizophrenia (Table 2 and Table 3). As well, SNVs (Table 2) and CNVs (Table 3) have been detected in people with autism-spectrum disorders.
Expression studies have reported increases in Homer1a protein and decreases in Homer1b in the hippocampus of postmortem brain samples of schizophrenia (Table 4).
Animal-model studies suggest that HOMER1 transcripts might control cognitive and behaviour functions (Table 4). It has also been suggested that mutations in HOMER1 might increase the risk of developing schizophrenia by dysregulating NMDA receptors and their associated signalling pathway. Pharmacological studies have shown that the NMDR inhibitor phencyclidine (PCP) and the NMDA receptor antagonist ketamine increase HOMER1 mRNA in rat prefrontal cortex and ventral striatum and nucleus accumbens, respectively. Other studies show that HOMER1 mRNA and/or related protein expression levels are modified by psychotomimetic drugs and the antipsychotic haloperidol (Table 3).
Summary
Overall, although HOMER1 has been associated with both schizophrenia and autism-spectrum disorders, genetic, expression and animal model studies do not provide conclusive results. This could be because different Homer1 isoforms have different functions. It has been suggested that the long isoform Homer1c is implicated in the regulation of working memory and sensorimotor function, whereas Homer1a could modulate the behavioural and emotional area.217 Additionally, some studies report that the balance between long and short Homer forms determines the normal functioning of the synaptic architecture and function and influences synaptic plasticity dynamics238; therefore, an alteration of this balance could dysregulate synaptic signalling, leading to neurochemical, structural and behavioural changes.217,218
Discussion
Accumulating evidence supporting biological overlap between schizophrenia and autism-spectrum disorders has fuelled research into common underlying mechanisms to provide a better understanding of the etiology of these disorders, their diagnosis and treatment. One such mechanism involves the synaptic plasticity in which the PSD structure plays a key role. This review has summarized genetic variants in the main scaffolding genes of the PSD that have been associated with schizophrenia and/or autism-spectrum disorders to date. Moreover, evidence coming from genetic, brain expression and animal model studies suggests that genetic variants in scaffolding genes could contribute to the deregulation of the glutamate receptor signalling pathways of the PSD, which may be involved in the pathophysiology of schizophrenia and autism-spectrum disorders, and the development of related shared phenotypes, such as cognitive or social dysfunction.
Such a cross-disorder effect of scaffolding gene and protein dysregulation seems consistent with their role as a dynamic complex that regulates cell signalling pathways and determines the specificity of information flow in intracellular networks.72 Because scaffolding proteins coordinate the excitatory synaptic transmission and mediate functional changes at the synapse, thus regulating synaptic plasticity among other processes, 73 they can be seen as crucial pieces of the complex puzzle of synaptic homeostasis maintenance. The fact that common (SNPs) and rare (SNVs and CNVs) variants have been identified in scaffolding genes in both schizophrenia and autism-spectrum disorders is in agreement with the view that both kinds of variants complementarily and heterogeneously underlie the shared genetic susceptibility to these disorders (Table 5) by generating synaptic instability.
From the present review, it is possible to infer that patients with schizophrenia or autism-spectrum disorders primarily share CNVs that include the complete length of one or more genes. In this regard, CNVs in the PSD genes seem to increase the risk of developing either schizophrenia or autism-spectrum disorders. For instance, deletions of the chromosomal region 3q29, which includes the DLG1 gene, have been related to both schizophrenia and autism-spectrum disorders.45,49 Taking into account the usually large effect size of rare variants on a phenotype, we should not be surprised that alterations in the number of copies (deletions or duplications) of these genes have an impact on PSD functioning and plasticity. In addition to being associated with specific disorders, these variants have been associated with certain phenotypes in these or other disorders, or even in healthy controls. Pocklington and colleagues showed that rare CNV burden may be relevant to cognitive dysfunction in patients with schizophrenia,239 and Stefansson and colleagues found that CNVs conferring risk of either schizophrenia or autism-spectrum disorders, including CNVs in the DLG1 and DLG2 genes, also affect cognitive function in healthy controls.240 Other studies have similarly detected that mutations in PSD genes, including some of the scaffolding genes reviewed here, such as DLG383 or SHANK3,241 are present in patients with intellectual disability.
This review has also provided evidence that, although several SNPs and SNVs in the scaffolding genes have been associated with schizophrenia or autism-spectrum disorders, only a few have been reported in both: 2 SNPs and 3 SNVs in the DLGAP2 gene and 1 SNV in the SHANK3 gene (Table 5). Variants that occur in both diagnoses might be targets of special interest for our understanding of common pathophysiological mechanisms and shared clinical features. Although it is difficult to infer the functional significance of these variants, bioinformatic analyses have indicated that some of the DLGAP2 gene variants (rs2301963, c.841C>G and c.2750C>T) might affect final protein function or expression. In relation to SHANK3, to our knowledge, there is no available information about the functionality of the missense SNV (G1011V) that has also been found associated with both disorders.
Nevertheless, the general lack of specificity observed here can be explained in terms of the pleiotropic nature of scaffolding genes. Variants in different scaffolding genes, either at the allelic or the gene level, may dysregulate the homeostasis of the PSD, which is finally expressed as features associated with different neurodevelopment disorders. In addition to this pleiotropy, the polygenic nature of psychiatric disorders and the polygenic nature of the intermediate molecular pathways known to underlie at least part of the autism-spectrum disorders/schizophrenia pathology (such as the proper functioning of the PSD) should also be considered. This directly links with additional genetic phenomena, such as gene–gene interactions. In recent years, the gene-pathways methodology has been developed to study whether different genes with similar functions are jointly associated with a single phenotype. So far, only a few studies have assessed the effect of common variance in scaffolding genes as a functional gene set or the epistatic effects of other related PSD functional gene sets on the risk of schizophrenia or autism-spectrum disorders. One recent study explored the enrichment of schizophrenia-associated ultra-rare variants and found a significant enrichment of disrupting ultrarare variants among genes defined as encoding interactors with DLG4 and ARC and NMDA receptors.70 Another study observed an enrichment of SNPs associated with autismspectrum disorders in gene sets related to synaptic structure and function, including genes related to scaffolding proteins, β-catenin nuclear pathways, glutamate receptor activity and adherents junctions.67 In addition, although not significant after correction, a nominal association between a PSD protein defined gene set (including ARC and NMDA receptor complexes) with schizophrenia has been reported.36 In all, the effect of common and rare variants in scaffolding genes on schizophrenia and autism-spectrum disorders reflects the complex and heterogeneous genetic architecture of these disorders, and further analyses of gene sets could facilitate the untangling of this complexity.
In addition to genetic data, expression and animal-model studies have indicated the importance of scaffolding genes in schizophrenia and autism-spectrum disorders. There is evidence that patients with schizophrenia or autism-spectrum disorders display deviations from normal scaffolding protein brain expression levels, supporting the hypothesis that the deregulation of these genes might underlie the neurobiology of both disorders. However, to our knowledge, there are no brain expression studies of the 2 genes (DLGAP2 and SHANK3) in which overlapping variants that predispose individuals to schizophrenia and/or autismspectrum disorders were found.52,115,116,135,242 Further research is required to test whether these coincident genetic variants contribute to modifying protein expression levels. In contrast, studies with animal models have shown the importance of scaffolding genes in ensuring cognitive and social function. We have reviewed different studies in which mice with scaffolding protein mutations show schizophrenia and autistim-like phenotypes.184,205 The use of animal models is extremely useful for understanding how changes in the gene sequence can affect phenotypes. As an example of the potential importance of animal models, there are SHANK3-deficient mice in which synaptic deficits were reversed with insulin-like growth factor-1.206 In a recent pilot study, insulin-like growth factor-1 has been used to treat 9 children with autism, and preliminary results have shown a reduction in social deficits.243
Limitations
Some limitations of this review should be acknowledged. First, since the reviewed association studies do not always include the same genetic regions or variants, coincident variants can be linked to shared genetic variability between schizophrenia and autism-spectrum disorders, but noncoincidence cannot be interpreted as a lack of it. Second, patients with low IQ scores are generally excluded from association studies. Since the scaffolding genes reviewed here seem to contribute to cognitive phenotypes, it is plausible to hypothesize that the effects of SNPs and SNVs in these genes were detected less often than they actually occur. Third, the relationship between the effect size associated with common and rare variants, the statistical power needed to detect these effects and the sample sizes in the studies reported in the different articles reviewed should be considered. The odds ratios associated with schizophrenia risk SNPs are typically about 1.10 to 1.50, whereas schizophrenia-associated CNVs confer a significantly increased risk of illness (odds ratios for several CNVs exceed 8).244 Therefore, rare variants can more easily create significant genome-wide associations than common variants.245
Conclusion
Advances in genetic technologies, together with the assembly of large patient cohorts, have made it possible to identify some genes and biological pathways involved in both schizophrenia and autism-spectrum disorders. Among them, scaffolding genes implicated in the PSD have been repeatedly associated with schizophrenia and autism-spectrum disorders, pointing toward these genes’ common involvement in the neurobiology of these disorders and in some shared clinical phenotypes, such as cognitive and social impairment. This review summarizes evidence that many different variants could introduce numerous slight alterations in the PSD pathway, leading to its inappropriate development or insufficiently robust response to environmental insults.
Acknowledgements
This study was supported by: the Network of European Funding for Neuroscience Research, ERA-NET NEURON (PiM2010ERN-00642); Instituto de Salud Carlos III through the project PI15/01420 (co-funded by European Regional Development Fund /European Social Fund, “Investing in your future”); and SAF 2015-71526-REDT; and Ajuts de Personal Investigador predoctoral en Formació (APIF) to J Soler, 2017-2018. Thanks to the Comissionat per a Universitats i Recerca del DIUE (2014SGR1636).
Footnotes
Competing interests: M. Parellada reports personal fees from Fundación Orange, outside the submitted work. M.-O. Krebs reports personal fees or travel grant from Janssen and Otsuka-Lundbeck, outside the submitted work. No other competing interests declared.
Contributors: J. Soler and M. Fatjó-Vilas designed the study and acquired the data, which all authors analzyed. J. Soler and M. FatjóVilas wrote the article, which all authors reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
References
- 1.McGrath J, Saha S, Chant D, et al. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev. 2008;30:67–76. doi: 10.1093/epirev/mxn001. [DOI] [PubMed] [Google Scholar]
- 2.Elsabbagh M, Divan G, Koh YJ, et al. Global prevalence of autism and other pervasive developmental disorders. Autism Res. 2012;5:160–79. doi: 10.1002/aur.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Psychiatric Association. Diagnostic and statistical manual of mental disorders III. Washington (DC): APA; 1980. [Google Scholar]
- 4.Stone WS, Iguchi L. Do apparent overlaps between schizophrenia and autistic spectrum disorders reflect superficial similarities or etiological commonalities? N Am J Med Sci (Boston) 2011;4:124–33. doi: 10.7156/v4i3p124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hommer RE, Swedo SE. Schizophrenia and autism-related disorders. Schizophr Bull. 2015;41:313–4. doi: 10.1093/schbul/sbu188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5) Arlington (VA): APA; 2013. [Google Scholar]
- 7.Eack SM, Bahorik AL, McKnight SA, et al. Commonalities in social and non-social cognitive impairments in adults with autism spectrum disorder and schizophrenia. Schizophr Res. 2013;148:24–8. doi: 10.1016/j.schres.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai MC, Lombardo MV, Baron-Cohen S. Autism. Lancet. 2014;383:896–910. doi: 10.1016/S0140-6736(13)61539-1. [DOI] [PubMed] [Google Scholar]
- 9.Green MF. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J Clin Psychiatry. 2006;67:36–42. [PubMed] [Google Scholar]
- 10.Fett A-KJ, Viechtbauer W, Dominguez MD, et al. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci Biobehav Rev. 2011;35:573–88. doi: 10.1016/j.neubiorev.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Horan WP, Kern RS, Shokat-Fadai K, et al. Social cognitive skills training in schizophrenia: an initial efficacy study of stabilized outpatients. Schizophr Res. 2009;107:47–54. doi: 10.1016/j.schres.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalton KM, Nacewicz BM, Johnstone T, et al. Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci. 2005;8:519–26. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spezio ML, Adolphs R, Hurley RSE, et al. Analysis of face gaze in autism using ‘Bubbles’. Neuropsychologia. 2007;45:144–51. doi: 10.1016/j.neuropsychologia.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 14.Chung YS, Barch D, Strube M. A meta-analysis of mentalizing impairments in adults with schizophrenia and autism spectrum disorder. Schizophr Bull. 2014;40:602–16. doi: 10.1093/schbul/sbt048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bora E, Yucel M, Pantelis C. Theory of mind impairment in schizophrenia: meta-analysis. Schizophr Res. 2009;109:1–9. doi: 10.1016/j.schres.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 16.Cheung C, Yu K, Fung G, et al. Autistic disorders and schizophrenia: related or remote? an anatomical likelihood estimation. PLoS One. 2010;5:e12233. doi: 10.1371/journal.pone.0012233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parellada M, et al. Insular pathology in young people with high-functioning autism and first-episode psychosis. Psychol Med. 2017;47:2472–82. doi: 10.1017/S0033291717000988. [DOI] [PubMed] [Google Scholar]
- 18.Katz J, et al. Similar white matter but opposite grey matter changes in schizophrenia and high-functioning autism. Acta Psychiatr Scand. 2016;134:31–9. doi: 10.1111/acps.12579. [DOI] [PubMed] [Google Scholar]
- 19.Rapoport J, Chavez A, Greenstein D, et al. Autism spectrum disorders and childhood-onset schizophrenia: clinical and biological contributions to a relation revisited. J Am Acad Child Adolesc Psychiatry. 2009;48:10–8. doi: 10.1097/CHI.0b013e31818b1c63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mouridsen SE, Rich B, Isager T, et al. Psychiatric disorders in individuals diagnosed with infantile autism as children: a case control study. J Psychiatr Pract. 2008;14:5–12. doi: 10.1097/01.pra.0000308490.47262.e0. [DOI] [PubMed] [Google Scholar]
- 21.Bevan Jones R, Thapar A, Lewis G, Zammit S. The association between early autistic traits and psychotic experiences in adolescence. Schizophr Res. 2012;135:164–9. doi: 10.1016/j.schres.2011.11.037. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan S, Rai D, Golding J, et al. The association between autism spectrum disorder and psychotic experiences in the Avon Longitudinal Study of Parents and Children (ALSPAC) birth cohort. J Am Acad Child Adolesc Psychiatry. 2013;52:806–814.e2. doi: 10.1016/j.jaac.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Stahlberg O, Soderstrom H, Rastam M, et al. Bipolar disorder, schizophrenia, and other psychotic disorders in adults with childhood onset AD/HD and/or autism spectrum disorders. J Neural Transm. 2004;111:891–902. doi: 10.1007/s00702-004-0115-1. [DOI] [PubMed] [Google Scholar]
- 24.Daniels JL, et al. Parental psychiatric disorders associated with autism spectrum disorders in the offspring. Pediatrics. 2008;121:e1357–62. doi: 10.1542/peds.2007-2296. [DOI] [PubMed] [Google Scholar]
- 25.Mortensen PB, Pedersen MG, Pedersen CB. Psychiatric family history and schizophrenia risk in Denmark: Which mental disorders are relevant? Psychol Med. 2010;40:201–10. doi: 10.1017/S0033291709990419. [DOI] [PubMed] [Google Scholar]
- 26.Larsson HJ, Eaton WW, Madsen KM, et al. Risk factors for autism: perinatal factors, parental psychiatric history, and socioeconomic status. Am J Epidemiol. 2005;161:916–25. doi: 10.1093/aje/kwi123. [DOI] [PubMed] [Google Scholar]
- 27.Sullivan PF, Magnusson C, Reichenberg A, et al. Family history of schizophrenia and bipolar disorder as risk factors for autism. Arch Gen Psychiatry. 2012;69:1099–103. doi: 10.1001/archgenpsychiatry.2012.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lichtenstein P, Yip BH, Björk C, et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–9. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry. 2003;60:1187–92. doi: 10.1001/archpsyc.60.12.1187. [DOI] [PubMed] [Google Scholar]
- 30.Tick B, Bolton P, Happé F, et al. Heritability of autism spectrum disorders: a meta-analysis of twin studies. J Child Psychol Psychiatry. 2016;57:585–95. doi: 10.1111/jcpp.12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bodmer W, Bonilla C. Common and rare variants in multifactorial susceptibility to common diseases. Nat Genet. 2008;40:695–701. doi: 10.1038/ng.f.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geschwind DH, Flint J. Genetics and genomics of psychiatric disease. Science. 2015;349:1489–94. doi: 10.1126/science.aaa8954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee SH, Ripke S, Neale BM, et al. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet. 2013;45:984–94. doi: 10.1038/ng.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Purcell SM, Wray NR, Stone JL, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–52. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang K, Zhang H, Ma D, et al. Common genetic variants on 5p14.1 associate with autism spectrum disorders. Nature. 2009;459:528–33. doi: 10.1038/nature07999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ripke S, Neale BM, Corvin A, et al. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–7. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiss LA, Arking DE, Daly MJ, et al. A genome-wide linkage and association scan reveals novel loci for autism. Nature. 2009;461:802–8. doi: 10.1038/nature08490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.International Schizophrenia Consortium. Rare chromosomal deletions and duplications increase risk of schizophrenia. Nature. 2008;455:237–41. doi: 10.1038/nature07239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu B, Roos JL, Levy S, et al. Strong association of de novo copy number mutations with sporadic schizophrenia. Nat Genet. 2008;40:880–5. doi: 10.1038/ng.162. [DOI] [PubMed] [Google Scholar]
- 40.Guilmatre A, Dubourg C, Mosca AL, et al. Recurrent rearrangements in synaptic and neurodevelopmental genes and shared biologic pathways in schizophrenia, autism, and mental retardation. Arch Gen Psychiatry. 2009;66:947–56. doi: 10.1001/archgenpsychiatry.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poultney CS, Goldberg AP, Drapeau E, et al. Identification of small exonic CNV from whole-exome sequence data and application to autism spectrum disorder. Am J Hum Genet. 2013;93:607–19. doi: 10.1016/j.ajhg.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malhotra D, Sebat J. CNVs: harbingers of a rare variant revolution in psychiatric genetics. Cell. 2012;148:1223–41. doi: 10.1016/j.cell.2012.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Roak BJ, Deriziotis P, Lee C, et al. Exome sequencing in sporadic autism spectrum disorders identifies severe de novo mutations. Nat Genet. 2011;43:585–9. doi: 10.1038/ng.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neale BM, Kou Y, Liu L, et al. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature. 2012;485:242–5. doi: 10.1038/nature11011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanders SJ, Murtha MT, Gupta AR, et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature. 2012;485:237–41. doi: 10.1038/nature10945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Awadalla P, Gauthier J, Myers RA, et al. Direct measure of the de novo mutation rate in autism and schizophrenia cohorts. Am J Hum Genet. 2010;87:316–24. doi: 10.1016/j.ajhg.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Girard SL, Gauthier J, Noreau A, et al. Increased exonic de novo mutation rate in individuals with schizophrenia. Nat Genet. 2011;43:860–3. doi: 10.1038/ng.886. [DOI] [PubMed] [Google Scholar]
- 48.Xu B, Roos JL, Dexheimer P, et al. Exome sequencing supports a de novo mutational paradigm for schizophrenia. Nat Genet. 2011;43:864–8. doi: 10.1038/ng.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fromer M, Pocklington AJ, Kavanagh DH, et al. De novo mutations in schizophrenia implicate synaptic networks. Nature. 2014;506:179–84. doi: 10.1038/nature12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Purcell SM, Moran JL, Fromer M, et al. A polygenic burden of rare disruptive mutations in schizophrenia. Nature. 2014;506:185–90. doi: 10.1038/nature12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Girard SL, Dion PA, Bourassa CV, et al. Mutation burden of rare variants in schizophrenia candidate genes. PLoS One. 2015;10:e0128988. doi: 10.1371/journal.pone.0128988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iossifov I, Ronemus M, Levy D, et al. De novo gene disruptions in children on the autistic spectrum. Neuron. 2012;74:285–99. doi: 10.1016/j.neuron.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kenny EM, Cormican P, Furlong S, et al. Excess of rare novel loss-of-function variants in synaptic genes in schizophrenia and autism spectrum disorders. Mol Psychiatry. 2014;19:872–9. doi: 10.1038/mp.2013.127. [DOI] [PubMed] [Google Scholar]
- 54.Leblond CS, Nava C, Polge A, et al. Meta-analysis of SHANK mutations in autism spectrum disorders: a gradient of severity in cognitive impairments. PLoS Genet. 2014;10:e1004580. doi: 10.1371/journal.pgen.1004580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krumm N, O’Roak BJ, Shendure J, et al. A de novo convergence of autism genetics and molecular neuroscience. Trends Neurosci. 2014;37:95–105. doi: 10.1016/j.tins.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Föcking M, Lopez LM, English JA, et al. Proteomic and genomic evidence implicates the postsynaptic density in schizophrenia. Mol Psychiatry. 2015;20:424–32. doi: 10.1038/mp.2014.63. [DOI] [PubMed] [Google Scholar]
- 57.Kirov G, Pocklington AJ, Holmans P, et al. De novo CNV analysis implicates specific abnormalities of postsynaptic signalling complexes in the pathogenesis of schizophrenia. Mol Psychiatry. 2012;17:142–53. doi: 10.1038/mp.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen J, Yu S, Fu Y, et al. Synaptic proteins and receptors defects in autism spectrum disorders. Front Cell Neurosci. 2014;8:276. doi: 10.3389/fncel.2014.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Iossifov I, O’Roak BJ, Sanders SJ, et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature. 2014;515:216–21. doi: 10.1038/nature13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pinto D, Pagnamenta AT, Klei L, et al. Functional impact of global rare copy number variation in autism spectrum disorders. Nature. 2010;466:368–72. doi: 10.1038/nature09146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bayés A, Van de Lagemaat LN, Collins MO, et al. Characterization of the proteome, diseases and evolution of the human postsynaptic density. Nat Neurosci. 2011;14:19–21. doi: 10.1038/nn.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sheng M, Hoogenraad CC. The postsynaptic architecture of excitatory synapses: a more quantitative view. Annu Rev Biochem. 2007;76:823–47. doi: 10.1146/annurev.biochem.76.060805.160029. [DOI] [PubMed] [Google Scholar]
- 63.de Bartolomeis A, Latte G, Tomasetti C, et al. Glutamatergic postsynaptic density protein dysfunctions in synaptic plasticity and dendritic spines morphology: relevance to schizophrenia and other behavioral disorders pathophysiology, and implications for novel therapeutic approaches. Mol Neurobiol. 2014;49:484–511. doi: 10.1007/s12035-013-8534-3. [DOI] [PubMed] [Google Scholar]
- 64.MacGillvary HD, Song Y, Raghavachari S, et al. Nanoscale scaffolding domains within the postsynaptic density concentrate synaptic AMPA receptors. Neuron. 2013;78:615–22. doi: 10.1016/j.neuron.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carlin RK, Grab DJ, Cohen RS, et al. Isolation and characterization of postsynaptic densities from various brain regions: enrichment of different types of postsynaptic densities. J Cell Biol. 1980;86:831–45. doi: 10.1083/jcb.86.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marshall CR, Howrigan DP, Merico D, et al. Contribution of copy number variants to schizophrenia from a genome-wide study of 41,321 subjects. Nat Genet. 2016;49:27–35. doi: 10.1038/ng.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Autism Spectrum Disorders Working Group of the Psychiatric Genomics Consortium. Meta-analysis of GWAS of over 16,000 individuals with autism spectrum disorder highlights a novel locus at 10q24.32 and a significant overlap with schizophrenia. Mol Autism. 2017;8:21. doi: 10.1186/s13229-017-0137-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dracheva S, McGurk SR, Haroutunian V. mRNA expression of AMPA receptors and AMPA receptor binding proteins in the cerebral cortex of elderly schizophrenics. J Neurosci Res. 2005;79:868–78. doi: 10.1002/jnr.20423. [DOI] [PubMed] [Google Scholar]
- 69.Ohnuma T, Kato H, Arai H, et al. Gene expression of PSD95 in prefrontal cortex and hippocampus in schizophrenia. Neuroreport. 2000;11:3133–7. doi: 10.1097/00001756-200009280-00019. [DOI] [PubMed] [Google Scholar]
- 70.Genovese G, Fromer M, Stahl EA, et al. Increased burden of ultrarare protein-altering variants among 4,877 individuals with schizophrenia. Nat Neurosci. 2016;19:1433–41. doi: 10.1038/nn.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pawson T, Nash P. Assembly of cell regulatory systems through protein interaction domains. Science. 2003;300:445–52. doi: 10.1126/science.1083653. [DOI] [PubMed] [Google Scholar]
- 72.Garbett D, Bretscher A. The surprising dynamics of scaffolding proteins. Mol Biol Cell. 2014;25:2315–9. doi: 10.1091/mbc.E14-04-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Good MC, Zalatan JG, Lim WA. Scaffold proteins: hubs for controlling the flow of cellular information. Science. 2011;332:680–6. doi: 10.1126/science.1198701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sheng M, Kim E. The postsynaptic organization of synapses. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a005678. pii: a005678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gao C, Tronson NC, Radulovic J. Modulation of behavior by scaffolding proteins of the post-synaptic density. Neurobiol Learn Mem. 2013;105:3–12. doi: 10.1016/j.nlm.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Verpelli C, Schmeisser MJ, Sala C, et al. Scaffold proteins at the postsynaptic density. Adv Exp Med Biol. 2012;970:29–61. doi: 10.1007/978-3-7091-0932-8_2. [DOI] [PubMed] [Google Scholar]
- 77.Howard MA, Elias GM, Elias LAB, et al. The role of SAP97 in synaptic glutamate receptor dynamics. Proc Natl Acad Sci U S A. 2010;107:3805–10. doi: 10.1073/pnas.0914422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Montgomery JM, Zamorano PL, Garner CC. MAGUKs in synapse assembly and function: an emerging view. Cell Mol Life Sci. 2004;61:911–29. doi: 10.1007/s00018-003-3364-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Oliva C, Escobedo P, Astorga C, et al. Role of the MAGUK protein family in synapse formation and function. Dev Neurobiol. 2012;72:57–72. doi: 10.1002/dneu.20949. [DOI] [PubMed] [Google Scholar]
- 80.Poglia L, Muller D, Nikonenko I. Ultrastructural modifications of spine and synapse morphology by SAP97. Hippocampus. 2011;21:990–8. doi: 10.1002/hipo.20811. [DOI] [PubMed] [Google Scholar]
- 81.Sato Y, Tao Y-X, Su Q, et al. Post-synaptic density-93 mediates tyrosine-phosphorylation of the N-methyl-D-aspartate receptors. Neuroscience. 2008;153:700–8. doi: 10.1016/j.neuroscience.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tarpey P, Parnau J, Blow M, et al. Mutations in the DLG3 gene cause nonsyndromic X-linked mental retardation. Am J Hum Genet. 2004;75:318–24. doi: 10.1086/422703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zanni G, van Esch H, Bensalem A, et al. A novel mutation in the DLG3 gene encoding the synapse-associated protein 102 (SAP102) causes non-syndromic mental retardation. Neurogenetics. 2010;11:251–5. doi: 10.1007/s10048-009-0224-y. [DOI] [PubMed] [Google Scholar]
- 84.Philips AK, et al. X-exome sequencing in Finnish families with intellectual disability–four novel mutations and two novel syndromic phenotypes. Orphanet J Rare Dis. 2014;9:49. doi: 10.1186/1750-1172-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Murata Y, Constantine-Paton M. Postsynaptic density scaffold SAP102 regulates cortical synapse development through EphB and PAK signaling pathway. J Neurosci. 2013;33:5040–52. doi: 10.1523/JNEUROSCI.2896-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang J, Xu TX, Hallett PJ, et al. PSD-95 uncouples dopamine-glutamate interaction in the D1/PSD-95/NMDA receptor complex. J Neurosci. 2009;29:2948–60. doi: 10.1523/JNEUROSCI.4424-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tsai N-P, Wilkerson JR, Guo W, et al. Multiple autism-linked genes mediate synapse elimination via proteasomal degradation of a synaptic scaffold PSD-95. Cell. 2012;151:1581–94. doi: 10.1016/j.cell.2012.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hirao K, Hata Y, Ide N, et al. A novel multiple PDZ domain-containing molecule interacting with N-methyl-D-aspartate receptors and neuronal cell adhesion proteins. J Biol Chem. 1998;273:21105–10. doi: 10.1074/jbc.273.33.21105. [DOI] [PubMed] [Google Scholar]
- 89.Welch JM, Wang D, Feng G. Differential mRNA expression and protein localization of the SAP90/PSD-95-associated proteins ( SAPAPs) in the nervous system of the mouse. J Comp Neurol. 2004;472:24–39. doi: 10.1002/cne.20060. [DOI] [PubMed] [Google Scholar]
- 90.Takeuchi M, Hata Y, Hirao K, et al. SAPAPs. A family of PSD-95/SAP90-associated proteins localized at postsynaptic density. J Biol Chem. 1997;272:11943–51. doi: 10.1074/jbc.272.18.11943. [DOI] [PubMed] [Google Scholar]
- 91.Rasmussen AH, Rasmussen HB, Silahtaroglu A. The DLGAP family: neuronal expression, function and role in brain disorders. Mol Brain. 2017;10:43. doi: 10.1186/s13041-017-0324-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ranta S, Zhang Y, Ross B, et al. Positional cloning and characterisation of the human DLGAP2 gene and its exclusion in progressive epilepsy with mental retardation. Eur J Hum Genet. 2000;8:381–4. doi: 10.1038/sj.ejhg.5200440. [DOI] [PubMed] [Google Scholar]
- 93.Boeckers TM, Winter C, Smalla KH, et al. Proline-rich synapse-associated proteins ProSAP1 and ProSAP2 interact with synaptic proteins of the SAPAP/GKAP family. Biochem Biophys Res Commun. 1999;264:247–52. doi: 10.1006/bbrc.1999.1489. [DOI] [PubMed] [Google Scholar]
- 94.Du Y, Weed SA, Xiong WC, et al. Identification of a novel cortactin SH3 domain-binding protein and its localization to growth cones of cultured neurons. Mol Cell Biol. 1998;18:5838–51. doi: 10.1128/mcb.18.10.5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Naisbitt S, Kim E, Tu JC, et al. Shank, a novel family of postsynaptic density proteins that binds to the NMDA receptor/PSD-95/GKAP complex and cortactin. Neuron. 1999;23:569–82. doi: 10.1016/s0896-6273(00)80809-0. [DOI] [PubMed] [Google Scholar]
- 96.Scannevin RH, Huganir RL. Postsynaptic organisation and regulation of excitatory synapses. Nat Rev Neurosci. 2000;1:133–41. doi: 10.1038/35039075. [DOI] [PubMed] [Google Scholar]
- 97.Sheng M, Kim E. The Shank family of scaffold proteins. J Cell Sci. 2000;113:1851–6. doi: 10.1242/jcs.113.11.1851. [DOI] [PubMed] [Google Scholar]
- 98.Tu JC, Xiao B, Naisbitt S, et al. Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron. 1999;23:583–92. doi: 10.1016/s0896-6273(00)80810-7. [DOI] [PubMed] [Google Scholar]
- 99.Kennedy MB. Signal-processing machines at the postsynaptic density. Science. 2000;290:750–4. doi: 10.1126/science.290.5492.750. [DOI] [PubMed] [Google Scholar]
- 100.McGee AW, Bredt DS. Assembly and plasticity of the glutamatergic postsynaptic specialization. Curr Opin Neurobiol. 2003;13:111–8. doi: 10.1016/s0959-4388(03)00008-4. [DOI] [PubMed] [Google Scholar]
- 101.Lim S, Naisbitt S, Yoon J, et al. Characterization of the Shank family of synaptic proteins. Multiple genes, alternative splicing, and differential expression in brain and development. J Biol Chem. 1999;274:29510–8. doi: 10.1074/jbc.274.41.29510. [DOI] [PubMed] [Google Scholar]
- 102.Hayashi MK, Tang C, Verpelli C, et al. The postsynaptic density proteins Homer and Shank form a polymeric network structure. Cell. 2009;137:159–71. doi: 10.1016/j.cell.2009.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Roussignol G, Ango F, Romorini S, et al. Shank expression is sufficient to induce functional dendritic spine synapses in aspiny neurons. J Neurosci. 2005;25:3560–70. doi: 10.1523/JNEUROSCI.4354-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shiraishi-Yamaguchi Y, Furuichi T. The Homer family proteins. Genome Biol. 2007;8:206. doi: 10.1186/gb-2007-8-2-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Foa L, Gasperini R. Developmental roles for Homer: more than just a pretty scaffold. J Neurochem. 2009;108:1–10. doi: 10.1111/j.1471-4159.2008.05726.x. [DOI] [PubMed] [Google Scholar]
- 106.Thomas U. Modulation of synaptic signalling complexes by Homer proteins. J Neurochem. 2002;81:407–13. doi: 10.1046/j.1471-4159.2002.00869.x. [DOI] [PubMed] [Google Scholar]
- 107.de Bartolomeis A, Aloj L, Ambesi-Impiombato A, et al. Acute administration of antipsychotics modulates Homer striatal gene expression differentially. Brain Res Mol Brain Res. 2002;98:124–9. doi: 10.1016/s0169-328x(01)00327-8. [DOI] [PubMed] [Google Scholar]
- 108.Hennou S, Kato A, Schneider EM, et al. Homer-1a/Vesl-1S enhances hippocampal synaptic transmission. Eur J Neurosci. 2003;18:811–9. doi: 10.1046/j.1460-9568.2003.02812.x. [DOI] [PubMed] [Google Scholar]
- 109.Sato J, Shimazu D, Yamamoto N, et al. An association analysis of synapse-associated protein 97 (SAP97) gene in schizophrenia. J Neural Transm. 2008;115:1355–65. doi: 10.1007/s00702-008-0085-9. [DOI] [PubMed] [Google Scholar]
- 110.Uezato A, Kimura-Sato J, Yamamoto N, et al. Further evidence for a male-selective genetic association of synapse-associated protein 97 (SAP97) gene with schizophrenia. Behav Brain Funct. 2012;8:2. doi: 10.1186/1744-9081-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kantojärvi K, Kotala I, Rehnström K, et al. Fine mapping of Xq11.1-q21.33 and mutation screening of RPS6KA6, ZNF711, ACSL4, DLG3, and IL1RAPL2 for autism spectrum disorders (ASD) Autism Res. 2011;4:228–33. doi: 10.1002/aur.187. [DOI] [PubMed] [Google Scholar]
- 112.Cheng M-C, Lu CL, Luu SU, et al. Genetic and functional analysis of the DLG4 gene encoding the post-synaptic density protein 95 in schizophrenia. PLoS One. 2010;5:e15107. doi: 10.1371/journal.pone.0015107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Balan S, Yamada K, Hattori E, et al. Population-specific haplotype association of the postsynaptic density gene DLG4 with schizophrenia, in family-based association studies. PLoS One. 2013;8:e70302. doi: 10.1371/journal.pone.0070302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li J-M, Lu CL, Cheng MC, et al. Genetic analysis of the DLGAP1 gene as a candidate gene for schizophrenia. Psychiatry Res. 2013;205:13–7. doi: 10.1016/j.psychres.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 115.Chien WH, Gau SSF, Liao HM, et al. Deep exon resequencing of DLGAP2 as a candidate gene of autism spectrum disorders. Mol Autism. 2013;4:26. doi: 10.1186/2040-2392-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li J-M, Lu CL, Cheng MC, et al. Role of the DLGAP2 gene encoding the SAP90/PSD-95-associated protein 2 in schizophrenia. PLoS One. 2014;9:e85373. doi: 10.1371/journal.pone.0085373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wu K, Hanna GL, Easter P, et al. Glutamate system genes and brain volume alterations in pediatric obsessive-compulsive disorder: a preliminary study. Psychiatry Res. 2013;211:214–20. doi: 10.1016/j.pscychresns.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shao S, Xu S, Yang J, et al. A commonly carried genetic variant, rs9616915, in SHANK3 gene is associated with a reduced risk of autism spectrum disorder: replication in a Chinese population. Mol Biol Rep. 2014;41:1591–5. doi: 10.1007/s11033-013-3005-5. [DOI] [PubMed] [Google Scholar]
- 119.Mashayekhi F, Mizban N, Bidabadi E, et al. The association of SHANK3 gene polymorphism and autism. Minerva Pediatr. 2016 doi: 10.23736/S2724-5276.16.04539-4. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 120.Sykes NH, Toma C, Wilson N, et al. Copy number variation and association analysis of SHANK3 as a candidate gene for autism in the IMGSAC collection. Eur J Hum Genet. 2009;17:1347–53. doi: 10.1038/ejhg.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Qin J, Jia M, Wang L, et al. Association study of SHANK3 gene polymorphisms with autism in Chinese Han population. BMC Med Genet. 2009;10:61. doi: 10.1186/1471-2350-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Spellmann I, Rujescu D, Musil R, et al. Homer-1 polymorphisms are associated with psychopathology and response to treatment in schizophrenic patients. J Psychiatr Res. 2011;45:234–41. doi: 10.1016/j.jpsychires.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 123.Zhao Z, Webb BT, Jia P, et al. Association study of 167 candidate genes for schizophrenia selected by a multi-domain evidence-based prioritization algorithm and neurodevelopmental hypothesis. PLoS One. 2013;8:e67776. doi: 10.1371/journal.pone.0067776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.De Luca V, Annesi G, De Marco EV, et al. HOMER1 promoter analysis in Parkinson’s disease: association study with psychotic symptoms. Neuropsychobiology. 2009;59:239–45. doi: 10.1159/000230689. [DOI] [PubMed] [Google Scholar]
- 125.Strauss J, McGregor S, Freeman N, et al. Association study of early-immediate genes in childhood-onset mood disorders and suicide attempt. Psychiatry Res. 2012;197:49–54. doi: 10.1016/j.psychres.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gilks WP, Allott EH, Donohoe G, et al. Replicated genetic evidence supports a role for HOMER2 in schizophrenia. Neurosci Lett. 2010;468:229–33. doi: 10.1016/j.neulet.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 127.Xing J, Kimura H, Wang C, et al. Resequencing and association analysis of six PSD-95-related genes as possible susceptibility genes for schizophrenia and autism spectrum disorders. Sci Rep. 2016;6:27491. doi: 10.1038/srep27491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li J-M, Lu CL, Cheng MC, et al. Exonic resequencing of the DLGAP3 gene as a candidate gene for schizophrenia. Psychiatry Res. 2013;208:84–7. doi: 10.1016/j.psychres.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 129.Leblond CS, Nava C, Polge A, et al. Meta-analysis of SHANK mutations in autism spectrum disorders: a gradient of severity in cognitive impairments. PLoS Genet. 2014;10:e1004580. doi: 10.1371/journal.pgen.1004580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sato D, Lionel AC, Leblond CS, et al. SHANK1 deletions in males with autism spectrum disorder. Am J Hum Genet. 2012;90:879–87. doi: 10.1016/j.ajhg.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Peykov S, Berkel S, Schoen M, et al. Identification and functional characterization of rare SHANK2 variants in schizophrenia. Mol Psychiatry. 2015;20:1489–98. doi: 10.1038/mp.2014.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Homann OR, Misura K, Lamas E, et al. Whole-genome sequencing in multiplex families with psychoses reveals mutations in the SHANK2 and SMARCA1 genes segregating with illness. Mol Psychiatry. 2016;21:1690–5. doi: 10.1038/mp.2016.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Berkel S, et al. Mutations in the SHANK2 synaptic scaffolding gene in autism spectrum disorder and mental retardation. Nat Genet. 2010;42:489–91. doi: 10.1038/ng.589. [DOI] [PubMed] [Google Scholar]
- 134.Leblond CS, Marshall CR, Weiss B, et al. Genetic and functional analyses of SHANK2 mutations suggest a multiple hit model of autism spectrum disorders. PLoS Genet. 2012;8:e1002521. doi: 10.1371/journal.pgen.1002521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gauthier J, Champagne N, Lafrenière RG, et al. De novo mutations in the gene encoding the synaptic scaffolding protein SHANK3 in patients ascertained for schizophrenia. Proc Natl Acad Sci U S A. 2010;107:7863–8. doi: 10.1073/pnas.0906232107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Durand CM, Betancur C, Boeckers TM, et al. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet. 2007;39:25–7. doi: 10.1038/ng1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Boccuto L, Lauri M, Sarasua SM, et al. Prevalence of SHANK3 variants in patients with different subtypes of autism spectrum disorders. Eur J Hum Genet. 2013;21:310–6. doi: 10.1038/ejhg.2012.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Soorya L, Kolevzon A, Zweifach J, et al. Prospective investigation of autism and genotype-phenotype correlations in 22q13 deletion syndrome and SHANK3 deficiency. Mol Autism. 2013;4:18. doi: 10.1186/2040-2392-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gauthier J, Spiegelman D, Piton A, et al. Novel de novo SHANK3 mutation in autistic patients. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:421–4. doi: 10.1002/ajmg.b.30822. [DOI] [PubMed] [Google Scholar]
- 140.Durand CM, Perroy J, Loll F, et al. SHANK3 mutations identified in autism lead to modification of dendritic spine morphology via an actin-dependent mechanism. Mol Psychiatry. 2012;17:71–84. doi: 10.1038/mp.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Moessner R, Marshall CR, Sutcliffe JS, et al. Contribution of SHANK3 mutations to autism spectrum disorder. Am J Hum Genet. 2007;81:1289–97. doi: 10.1086/522590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Waga C, Okamoto N, Ondo Y, et al. Novel variants of the SHANK3 gene in Japanese autistic patients with severe delayed speech development. Psychiatr Genet. 2011;21:208–11. doi: 10.1097/YPG.0b013e328341e069. [DOI] [PubMed] [Google Scholar]
- 143.Schaaf CP, Sabo A, Sakai Y, et al. Oligogenic heterozygosity in individuals with high-functioning autism spectrum disorders. Hum Mol Genet. 2011;20:3366–75. doi: 10.1093/hmg/ddr243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kelleher RJ, Geigenmüller U, Hovhannisyan H, et al. High-throughput sequencing of mGluR signaling pathway genes reveals enrichment of rare variants in autism. PLoS One. 2012;7:e35003. doi: 10.1371/journal.pone.0035003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cochoy DM, Kolevzon A, Kajiwara Y, et al. Phenotypic and functional analysis of SHANK3 stop mutations identified in individuals with ASD and/or ID. Mol Autism. 2015;6:23. doi: 10.1186/s13229-015-0020-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Norton N, Williams HJ, Williams NM, et al. Mutation screening of the Homer gene family and association analysis in schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2003;120B:18–21. doi: 10.1002/ajmg.b.20032. [DOI] [PubMed] [Google Scholar]
- 147.Pinto D, Delaby E, Meirco D, et al. Convergence of genes and cellular pathways dysregulated in autism spectrum disorders. Am J Hum Genet. 2014;94:677–94. doi: 10.1016/j.ajhg.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Levinson DF, Duan J, Oh S, et al. Copy number variants in schizophrenia: confirmation of five previous findings and new evidence for 3q29 microdeletions and VIPR2 duplications. Am J Psychiatry. 2011;168:302–16. doi: 10.1176/appi.ajp.2010.10060876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mulle JG, Dodd AF, McGrath JA, et al. Microdeletions of 3q29 confer high risk for schizophrenia. Am J Hum Genet. 2010;87:229–36. doi: 10.1016/j.ajhg.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Magri C, Sacchetti E, Traversa M, et al. New copy number variations in schizophrenia. PLoS One. 2010;5:e13422. doi: 10.1371/journal.pone.0013422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Szatkiewicz JP, O’Dushlaine C, Chen G, et al. Copy number variation in schizophrenia in Sweden. Mol Psychiatry. 2014;19:762–73. doi: 10.1038/mp.2014.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Quintero-Rivera F, Sharifi-Hannauer P, Martinez-Agosto JA. Autistic and psychiatric findings associated with the 3q29 microdeletion syndrome: case report and review. Am J Med Genet A. 2010;152A:2459–67. doi: 10.1002/ajmg.a.33573. [DOI] [PubMed] [Google Scholar]
- 153.Levy D, Ronemus M, Yamrom B, et al. Rare de novo and transmitted copy-number variation in autistic spectrum disorders. Neuron. 2011;70:886–97. doi: 10.1016/j.neuron.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 154.Willatt L, Cox J, Barber J, et al. 3q29 microdeletion syndrome: clinical and molecular characterization of a new syndrome. Am J Hum Genet. 2005;77:154–60. doi: 10.1086/431653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ballif BC, Theisen A, Coppinger J, et al. Expanding the clinical phenotype of the 3q29 microdeletion syndrome and characterization of the reciprocal microduplication. Mol Cytogenet. 2008;1:8. doi: 10.1186/1755-8166-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sagar A, Bishop JR, Tessman DC, et al. Co-occurrence of autism, childhood psychosis, and intellectual disability associated with a de novo 3q29 microdeletion. Am J Med Genet A. 2013;161A:845–9. doi: 10.1002/ajmg.a.35754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cuscó I, Medrano A, Gener B, et al. Autism-specific copy number variants further implicate the phosphatidylinositol signaling pathway and the glutamatergic synapse in the etiology of the disorder. Hum Mol Genet. 2009;18:1795–804. doi: 10.1093/hmg/ddp092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Walsh T, McClellan JM, McCarthy SE, et al. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320:539–43. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- 159.Egger G, Roetzer KM, Noor A, et al. Identification of risk genes for autism spectrum disorder through copy number variation analysis in Austrian families. Neurogenetics. 2014;15:117–27. doi: 10.1007/s10048-014-0394-0. [DOI] [PubMed] [Google Scholar]
- 160.Sanders SJ, Ercan-Sencicek AG, Hus V, et al. Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron. 2011;70:863–85. doi: 10.1016/j.neuron.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chien WH, Gau SS, Wu YY, et al. Identification and molecular characterization of two novel chromosomal deletions associated with autism. Clin Genet. 2010;78:449–56. doi: 10.1111/j.1399-0004.2010.01395.x. [DOI] [PubMed] [Google Scholar]
- 162.Szatmari P, Paterson AD, Zwaigenbaum L, et al. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet. 2007;39:319–28. doi: 10.1038/ng1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Costain G, Lionel AC, Merico D, et al. Pathogenic rare copy number variants in community-based schizophrenia suggest a potential role for clinical microarrays. Hum Mol Genet. 2013;22:4485–501. doi: 10.1093/hmg/ddt297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Marshall CR, Noor A, Vincent JB, et al. Structural variation of chromosomes in autism spectrum disorder. Am J Hum Genet. 2008;82:477–88. doi: 10.1016/j.ajhg.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ozgen HM, van Daalen E, Bolton PF, et al. Copy number changes of the microcephalin 1 gene (MCPH1) in patients with autism spectrum disorders. Clin Genet. 2009;76:348–56. doi: 10.1111/j.1399-0004.2009.01254.x. [DOI] [PubMed] [Google Scholar]
- 166.Prasad A, Merico D, Thiruvahindrapuram B, et al. A discovery resource of rare copy number variations in individuals with autism spectrum disorder. G3 (Bethesda) 2012;2:1665–85. doi: 10.1534/g3.112.004689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gai X, Xie HM, Perin JC, et al. Rare structural variation of synapse and neurotransmission genes in autism. Mol Psychiatry. 2012;17:402–11. doi: 10.1038/mp.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Nemirovsky SI, Córdoba M, Zaiat JJ, et al. Whole genome sequencing reveals a de novo SHANK3 mutation in familial autism spectrum disorder. PLoS One. 2015;10:e0116358. doi: 10.1371/journal.pone.0116358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yuen RKC, Thiruvahindrapuram B, Merico D, et al. Whole-genome sequencing of quartet families with autism spectrum disorder. Nat Med. 2015;21:185–91. doi: 10.1038/nm.3792. [DOI] [PubMed] [Google Scholar]
- 170.Failla P, Romano C, Alberti A, et al. Schizophrenia in a patient with subtelomeric duplication of chromosome 22q. Clin Genet. 2007;71:599–601. doi: 10.1111/j.1399-0004.2007.00819.x. [DOI] [PubMed] [Google Scholar]
- 171.Crespi B, Stead P, Elliot M. Evolution in health and medicine Sackler colloquium: comparative genomics of autism and schizophrenia. Proc Natl Acad Sci U S A. 2010;107(Suppl):1736–41. doi: 10.1073/pnas.0906080106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sebat J, Lakshmi B, Malhotra D, et al. Strong association of de novo copy number mutations with autism. Science. 2007;316:445–9. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wang L-S, Hranilovic D, Wang K, et al. Population-based study of genetic variation in individuals with autism spectrum disorders from Croatia. BMC Med Genet. 2010;11:134. doi: 10.1186/1471-2350-11-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bonaglia MC, Giorda R, Mani E, et al. Identification of a recurrent breakpoint within the SHANK3 gene in the 22q13.3 deletion syndrome. J Med Genet. 2006;43:822–8. doi: 10.1136/jmg.2005.038604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Toyooka K, Iritani S, Makifuchi T, et al. Selective reduction of a PDZ protein, SAP-97, in the prefrontal cortex of patients with chronic schizophrenia. J Neurochem. 2002;83:797–806. doi: 10.1046/j.1471-4159.2002.01181.x. [DOI] [PubMed] [Google Scholar]
- 176.Hiraoka S, Kajii Y, Kuroda Y, et al. The development- and phencyclidine-regulated induction of synapse-associated protein-97 gene in the rat neocortex. Eur Neuropsychopharmacol. 2010;20:176–86. doi: 10.1016/j.euroneuro.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 177.Kristiansen LV, Beneyto M, Haroutunian V, et al. Changes in NMDA receptor subunits and interacting PSD proteins in dorsolateral prefrontal and anterior cingulate cortex indicate abnormal regional expression in schizophrenia. Mol Psychiatry. 2006;11:737–47. doi: 10.1038/sj.mp.4001844. [DOI] [PubMed] [Google Scholar]
- 178.Carlisle HJ, Fink AE, Grant SGN, et al. Opposing effects of PSD-93 and PSD-95 on long-term potentiation and spike timing-dependent plasticity. J Physiol. 2008;586:5885–900. doi: 10.1113/jphysiol.2008.163469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Nithianantharajah J. Synaptic scaffold evolution generated components of vertebrate cognitive complexity. Nat Neurosci. 2013;16:16–24. doi: 10.1038/nn.3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.McGee AW, Topinka JR, Hashimoto K, et al. PSD-93 knock-out mice reveal that neuronal MAGUKs are not required for development or function of parallel fiber synapses in cerebellum. J Neurosci. 2001;21:3085–91. doi: 10.1523/JNEUROSCI.21-09-03085.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Clinton SM, Haroutunian V, Davis KL, et al. Altered transcript expression of NMDA receptor-associated postsynaptic proteins in the thalamus of subjects with schizophrenia. Am J Psychiatry. 2003;160:1100–9. doi: 10.1176/appi.ajp.160.6.1100. [DOI] [PubMed] [Google Scholar]
- 182.Clinton SM, Meador-Woodruff JH. Abnormalities of the NMDA receptor and associated intracellular molecules in the thalamus in schizophrenia and bipolar disorder. Neuropsychopharmacology. 2004;29:1353–62. doi: 10.1038/sj.npp.1300451. [DOI] [PubMed] [Google Scholar]
- 183.Cuthbert PC, Stanford LE, Coba MP, et al. Synapse-associated protein 102/dlgh3 couples the NMDA receptor to specific plasticity pathways and learning strategies. J Neurosci. 2007;27:2673–82. doi: 10.1523/JNEUROSCI.4457-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Feyder M, Karlsson RM, Mathur P, et al. Association of mouse Dlg4 (PSD-95) gene deletion and human DLG4 gene variation with phenotypes relevant to autism spectrum disorders and Williams’ syndrome. Am J Psychiatry. 2010;167:1508–17. doi: 10.1176/appi.ajp.2010.10040484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Clinton SM, Haroutunian V, Meador-Woodruff JH. Up-regulation of NMDA receptor subunit and post-synaptic density protein expression in the thalamus of elderly patients with schizophrenia. J Neurochem. 2006;98:1114–25. doi: 10.1111/j.1471-4159.2006.03954.x. [DOI] [PubMed] [Google Scholar]
- 186.Dracheva S, Marras SA, Elhakem SL, et al. N-methyl-D-aspartic acid receptor expression in the dorsolateral prefrontal cortex of elderly patients with schizophrenia. Am J Psychiatry. 2001;158:1400–10. doi: 10.1176/appi.ajp.158.9.1400. [DOI] [PubMed] [Google Scholar]
- 187.Funk AJ, Mielnik CA, Koene R, et al. Postsynaptic density-95 isoform abnormalities in schizophrenia. Schizophr Bull. 2017;45:sbw173. doi: 10.1093/schbul/sbw173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Toro C, Deakin JFW. NMDA receptor subunit NRI and postsynaptic protein PSD-95 in hippocampus and orbitofrontal cortex in schizophrenia and mood disorder. Schizophr Res. 2005;80:323–30. doi: 10.1016/j.schres.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 189.Matosin N, Fernandez-Enright F, Lum JS, et al. Molecular evidence of synaptic pathology in the CA1 region in schizophrenia. NPJ Schizophr. 2016;2:16022. doi: 10.1038/npjschz.2016.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kristiansen LV, Meador-Woodruff JH. Abnormal striatal expression of transcripts encoding NMDA interacting PSD proteins in schizophrenia, bipolar disorder and major depression. Schizophr Res. 2005;78:87–93. doi: 10.1016/j.schres.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 191.Nakagawa T, Futai K, Lashuel HA, et al. Quaternary structure, protein dynamics, and synaptic function of SAP97 controlled by L27 domain interactions. Neuron. 2004;44:453–67. doi: 10.1016/j.neuron.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 192.Migaud M, Charlesworth P, Dempster M, et al. Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature. 1998;396:433–9. doi: 10.1038/24790. [DOI] [PubMed] [Google Scholar]
- 193.Ehrlich I, Klein M, Rumpel S, et al. PSD-95 is required for activity-driven synapse stabilization. Proc Natl Acad Sci U S A. 2007;104:4176–81. doi: 10.1073/pnas.0609307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Xu W, Schlüter OM, Steiner P, et al. Molecular dissociation of the role of PSD-95 in regulating synaptic strength and LTD. Neuron. 2008;57:248–62. doi: 10.1016/j.neuron.2007.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.De Bartolomeis A, Sarappa C, Buonaguro EF, et al. Different effects of the NMDA receptor antagonists ketamine, MK-801, and memantine on postsynaptic density transcripts and their topography: role of Homer signaling, and implications for novel antipsychotic and pro-cognitive targets in psychosis. Prog Neuropsychopharmacol Biol Psychiatry. 2013;46:1–12. doi: 10.1016/j.pnpbp.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 196.Jiang-Xie L-F, Liao HM, Chen CH, et al. Autism-associated gene Dlgap2 mutant mice demonstrate exacerbated aggressive behaviors and orbitofrontal cortex deficits. Mol Autism. 2014;5:32. doi: 10.1186/2040-2392-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Silverman JL, Turner SM, Barkan CL, et al. Sociability and motor functions in Shank1 mutant mice. Brain Res. 2011;1380:120–37. doi: 10.1016/j.brainres.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hung AY, Futai K, Sala C, et al. Smaller dendritic spines, weaker synaptic transmission, but enhanced spatial learning in mice lacking Shank1. J Neurosci. 2008;28:1697–708. doi: 10.1523/JNEUROSCI.3032-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wöhr M, Roullet FI, Hung AY, et al. Communication impairments in mice lacking Shank1: reduced levels of ultrasonic vocalizations and scent marking behavior. PLoS One. 2011;6:e20631. doi: 10.1371/journal.pone.0020631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sungur AÖ, Schwarting RKW, Wöhr M. Early communication deficits in the Shank1 knockout mouse model for autism spectrum disorder: Developmental aspects and effects of social context. Autism Res. 2016;9:696–709. doi: 10.1002/aur.1564. [DOI] [PubMed] [Google Scholar]
- 201.Schmeisser MJ, Ey E, Wegener S, et al. Autistic-like behaviours and hyperactivity in mice lacking ProSAP1/Shank2. Nature. 2012;486:256–60. doi: 10.1038/nature11015. [DOI] [PubMed] [Google Scholar]
- 202.Won H, Lee HR, Gee HY, et al. Autistic-like social behaviour in Shank2-mutant mice improved by restoring NMDA receptor function. Nature. 2012;486:261–5. doi: 10.1038/nature11208. [DOI] [PubMed] [Google Scholar]
- 203.Ha S, Lee D, Cho YS, et al. Cellular/molecular cerebellar Shank2 regulates excitatory synapse density, motor coordination, and specific repetitive and anxiety-like behaviors. J Neurosci. 2016;36:12129–43. doi: 10.1523/JNEUROSCI.1849-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Arons MH, Thynne CJ, Grabrucker AM, et al. Autism-associated mutations in ProSAP2/Shank3 impair synaptic transmission and neurexin-neuroligin-mediated transsynaptic signaling. J Neurosci. 2012;32:14966–78. doi: 10.1523/JNEUROSCI.2215-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wang X, McCoy PA, Rodriguiz RM, et al. Synaptic dysfunction and abnormal behaviors in mice lacking major isoforms of Shank3. Hum Mol Genet. 2011;20:3093–108. doi: 10.1093/hmg/ddr212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Bozdagi O, Tavassoli T, Buxbaum JD. Insulin-like growth factor-1 rescues synaptic and motor deficits in a mouse model of autism and developmental delay. Mol Autism. 2013;4:9. doi: 10.1186/2040-2392-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Peça J, Feliciano C, Ting JT, et al. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472:437–42. doi: 10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Mei Y, Monteiro P, Zhou Y, et al. Adult restoration of Shank3 expression rescues selective autistic-like phenotypes. Nature. 2016;530:481–4. doi: 10.1038/nature16971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Bozdagi O, Sakurai T, Papapetrou D, et al. Haploinsufficiency of the autism-associated Shank3 gene leads to deficits in synaptic function, social interaction, and social communication. Mol Autism. 2010;1:15. doi: 10.1186/2040-2392-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Yang M, Bozdagi O, Scattoni ML, et al. Reduced excitatory neurotransmission and mild autism-relevant phenotypes in adolescent Shank3 null mutant mice. J Neurosci. 2012;32:6525–41. doi: 10.1523/JNEUROSCI.6107-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Marchetto MCN, Carromeu C, Acab A, et al. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell. 2010;143:527–39. doi: 10.1016/j.cell.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Peixoto RT, Wang W, Croney DM, et al. Early hyperactivity and precocious maturation of corticostriatal circuits in Shank3B −/− mice. Nat Neurosci. 2016;19:716–24. doi: 10.1038/nn.4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Leber SL, Llenos IC, Miller CL, et al. Homer1a protein expression in schizophrenia, bipolar disorder, and major depression. J Neural Transm. 2017;124:1261–73. doi: 10.1007/s00702-017-1776-x. [DOI] [PubMed] [Google Scholar]
- 214.Inoue N, Nakao H, Migishima R, et al. Requirement of the immediate early gene vesl-1S/homer-1a for fear memory formation. Mol Brain. 2009;2:7. doi: 10.1186/1756-6606-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Gerstein H, O’Riordan K, Osting S, et al. Rescue of synaptic plasticity and spatial learning deficits in the hippocampus of Homer1 knockout mice by recombinant Adeno-associated viral gene delivery of Homer1c. Neurobiol Learn Mem. 2012;97:17–29. doi: 10.1016/j.nlm.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Szumlinski KK, Lominac KD, Kleschen MJ, et al. Behavioral and neurochemical phenotyping of Homer1 mutant mice: possible relevance to schizophrenia. Genes Brain Behav. 2005;4:273–88. doi: 10.1111/j.1601-183X.2005.00120.x. [DOI] [PubMed] [Google Scholar]
- 217.Lominac KD, Oleson EB, Pava M, et al. Distinct roles for different Homer1 isoforms in behaviors and associated prefrontal cortex function. J Neurosci. 2005;25:11586–94. doi: 10.1523/JNEUROSCI.3764-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Jaubert PJ, Golub MS, Lo YY, et al. Complex, multimodal behavioral profile of the Homer1 knockout mouse. Genes Brain Behav. 2007;6:141–54. doi: 10.1111/j.1601-183X.2006.00240.x. [DOI] [PubMed] [Google Scholar]
- 219.Vazdarjanova A, McNaughton BL, Barnes CA, et al. Experience-dependent coincident expression of the effector immediate-early genes arc and Homer 1a in hippocampal and neocortical neuronal networks. J Neurosci. 2002;22:10067–71. doi: 10.1523/JNEUROSCI.22-23-10067.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Fujiyama K, Kajii Y, Hiraoka S, et al. Differential regulation by stimulants of neocortical expression of mrt1, arc, and homer1a mRNA in the rats treated with repeated methamphetamine. Synapse. 2003;49:143–9. doi: 10.1002/syn.10220. [DOI] [PubMed] [Google Scholar]
- 221.Cochran SM, Fujimura M, Morris BJ, et al. Acute and delayed effects of phencyclidine upon mRNA levels of markers of glutamatergic and GABAergic neurotransmitter function in the rat brain. Synapse. 2002;46:206–14. doi: 10.1002/syn.10126. [DOI] [PubMed] [Google Scholar]
- 222.Nichols CD, Sanders-Bush E. A single dose of lysergic acid diethylamide influences gene expression patterns within the mammalian brain. Neuropsychopharmacology. 2002;26:634–42. doi: 10.1016/S0893-133X(01)00405-5. [DOI] [PubMed] [Google Scholar]
- 223.Iasevoli F, Polese D, Ambesi-Impiombato A, et al. Ketamine-related expression of glutamatergic postsynaptic density genes: possible implications in psychosis. Neurosci Lett. 2007;416:1–5. doi: 10.1016/j.neulet.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 224.Iasevoli F, Fiore G, Cicale M, et al. Haloperidol induces higher Homer1a expression than risperidone, olanzapine and sulpiride in striatal sub-regions. Psychiatry Res. 2010;177:255–60. doi: 10.1016/j.psychres.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 225.Iasevoli F, Tomasetti C, Marmo F, et al. Divergent acute and chronic modulation of glutamatergic postsynaptic density genes expression by the antipsychotics haloperidol and sertindole. Psychopharmacology (Berl) 2010;212:329–44. doi: 10.1007/s00213-010-1954-0. [DOI] [PubMed] [Google Scholar]
- 226.Ambesi-Impiombato A, Panariello F, Dell’aversano C, et al. Differential expression of Homer 1 gene by acute and chronic administration of antipsychotics and dopamine transporter inhibitors in the rat forebrain. Synapse. 2007;61:429–39. doi: 10.1002/syn.20385. [DOI] [PubMed] [Google Scholar]
- 227.Polese D, de Serpis AA, Ambesi-Impiombato A, et al. Homer 1a gene expression modulation by antipsychotic drugs: involvement of the glutamate metabotropic system and effects of D-cycloserine. Neuropsychopharmacology. 2002;27:906–13. doi: 10.1016/S0893-133X(02)00371-8. [DOI] [PubMed] [Google Scholar]
- 228.Tomasetti C, Dell’Aversano C, Iasevoli F, et al. Homer splice variants modulation within cortico-subcortical regions by dopamine D2 antagonists, a partial agonist, and an indirect agonist: implication for glutamatergic postsynaptic density in antipsychotics action. Neuroscience. 2007;150:144–58. doi: 10.1016/j.neuroscience.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 229.Tezuka T, Umemori H, Akiyama T, et al. PSD-95 promotes Fyn-mediated tyrosine phosphorylation of the N-methyl-D-aspartate receptor subunit NR2A. Proc Natl Acad Sci U S A. 1999;96:435–40. doi: 10.1073/pnas.96.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Cousins SL, Papadakis M, Rutter AR, et al. Differential interaction of NMDA receptor subtypes with the post-synaptic density-95 family of membrane associated guanylate kinase proteins. J Neurochem. 2008;104:903–13. doi: 10.1111/j.1471-4159.2007.05067.x. [DOI] [PubMed] [Google Scholar]
- 231.Sans N, Prybylowski K, Petralia RS, et al. NMDA receptor trafficking through an interaction between PDZ proteins and the exocyst complex. Nat Cell Biol. 2003;5:520–30. doi: 10.1038/ncb990. [DOI] [PubMed] [Google Scholar]
- 232.Dunham I, Kundaje A, Aldred SF, et al. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Bachevalier J, Loveland KA. The orbitofrontal–amygdala circuit and self-regulation of social–emotional behavior in autism. Neurosci Biobehav Rev. 2006;30:97–117. doi: 10.1016/j.neubiorev.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 234.Nelson RJ, Trainor BC. Neural mechanisms of aggression. Nat Rev Neurosci. 2007;8:536–46. doi: 10.1038/nrn2174. [DOI] [PubMed] [Google Scholar]
- 235.Phelan MC. Deletion 22q13.3 syndrome. Orphanet J Rare Dis. 2008;3:14. doi: 10.1186/1750-1172-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Betancur C, Buxbaum JD. SHANK3 haploinsufficiency: a ‘common’ but underdiagnosed highly penetrant monogenic cause of autism spectrum disorders. Mol Autism. 2013;4:17. doi: 10.1186/2040-2392-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Duffney LJ, Wei J, Cheng J, et al. Shank3 deficiency induces NMDA receptor hypofunction via an actin-dependent mechanism. J Neurosci. 2013;33:15767–78. doi: 10.1523/JNEUROSCI.1175-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Van Keuren-Jensen K, Cline HT. Visual experience regulates metabotropic glutamate receptor-mediated plasticity of AMPA receptor synaptic transmission by Homer1a Induction. J Neurosci. 2006;26:7575–80. doi: 10.1523/JNEUROSCI.5083-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Pocklington AJ, O’Donovan M, Owen MJ. The synapse in schizophrenia. Eur J Neurosci. 2014;39:1059–67. doi: 10.1111/ejn.12489. [DOI] [PubMed] [Google Scholar]
- 240.Stefansson H, Meyer-Lindenberg A, Steinberg S, et al. CNVs conferring risk of autism or schizophrenia affect cognition in controls. Nature. 2014;505:361–6. doi: 10.1038/nature12818. [DOI] [PubMed] [Google Scholar]
- 241.Hamdan FF, Gauthier J, Araki Y, et al. Excess of de novo deleterious mutations in genes associated with glutamatergic systems in nonsyndromic intellectual disability. Am J Hum Genet. 2011;88:306–16. doi: 10.1016/j.ajhg.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Durand CM, Betancur C, Boeckers TM, et al. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet. 2007;39:25–7. doi: 10.1038/ng1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Kolevzon A, Bush L, Wang AT, et al. A pilot controlled trial of insulin-like growth factor-1 in children with Phelan-McDermid syndrome. Mol Autism. 2014;5:54. doi: 10.1186/2040-2392-5-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Harrison PJ. Recent genetic findings in schizophrenia and their therapeutic relevance. J Psychopharmacol. 2015;29:85–96. doi: 10.1177/0269881114553647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Cirulli ET, Goldstein DB. Uncovering the roles of rare variants in common disease through whole-genome sequencing. Nat Rev Genet. 2010;11:415–25. doi: 10.1038/nrg2779. [DOI] [PubMed] [Google Scholar]