Abstract
Background
Heightened response to drug-related cues is a hallmark of addiction. Extended-release naltrexone (XR-NTX) is a US Food and Drug Administration–approved pharmacotherapy for relapse prevention in patients with opioid use disorder (OUD). In these patients, XR-NTX has been shown to reduce brain responses to opioid-related visual stimuli. To assess the biomarker potential of this phenomenon, it is necessary to determine whether this effect is limited to opioid-related stimuli and whether it is associated with key OUD symptoms.
Methods
Using functional MRI (fMRI), we measured the brain responses to opioid-related and control (i.e., sexual and aversive) images in detoxified patients with OUD before, during and after XR-NTX treatment. Craving and withdrawal severity were evaluated using clinician- and self-administered instruments during each session.
Results
We included 24 patients with OUD in our analysis. During XR-NTX treatment, we found reduced responses to opioid-related stimuli in the nucleus accumbens (NAcc) and medial orbitofrontal cortex (mOFC). The reduction in mOFC response was specific to the opioid-related stimuli. The reduced NAcc and mOFC opioid cue reactivity was correlated with reduction in clinician-assessed and self-reported withdrawal symptoms, respectively.
Limitations
The study was not placebo-controlled owing to ethical, safety and feasibility concerns.
Conclusion
Extended-release naltrexone reduces the NAcc and mOFC cue reactivity in patients with OUD. This effect is specific to opioid-related stimuli in the mOFC only. The reduction in neural response to opioid-related stimuli is more robust in patients with greater decline in withdrawal severity. Our results support the clinical utility of mesocorticolimbic cue reactivity in monitoring the XR-NTX treatment outcomes and highlight the link between opioid withdrawal symptomatology and neural opioid cue reactivity.
Introduction
Opioid use disorder (OUD) is an acute and growing public health problem. In 2013, OUD caused about 50 000 deaths worldwide and accounted for more than 40% of all substance abuse–related deaths.1 Opioid use disorder is particularly prevalent in the United States, where the number of fatalities involving opioid overdose more than quadrupled from 5990 in 1999 to 29 467 in 2014,2 despite the greater variety and availability of treatment options. These statistics suggest that urgent action is needed to increase the effectiveness of the available OUD treatments.
Opioid use disorder is driven by the strongly reinforcing nature of opioid agonists, mediated predominantly by the μ-opioid receptor (MOR).3 A major mechanism of MOR-mediated reinforcement is inhibition of the GABAergic input into the ventral tegmental area (VTA), leading to dopamine release in the nucleus accumbens (NAcc).4–6 Naltrexone (NTX) is a nonselective opioid antagonist that competitively blocks the effects of opioid agonists on the MOR, presenting a theoretically attractive means of relapse prevention in detoxified patients with OUD.7 However, the oral formulation of NTX is characterized by poor compliance.8 To overcome this obstacle, a once-a-month, injectable, extended-release naltrexone (XR-NTX) was approved by the US Food and Drug Administration in 2010.9,10 Despite its demonstrated clinical effectiveness and broad insurance coverage, XR-NTX use in the United States has been limited.11 Motivational and neural mechanisms underlying XR-NTX action may be one of the factors responsible for the gap between effectiveness and acceptance. These mechanisms are not well understood, although their importance in the overall effectiveness of an addiction treatment is well recognized.12 For example, it is still unknown how XR-NTX modulates the neurocognitive processing of drug-related stimuli in patients with OUD, whether the effect of XR-NTX on neural activity is associated with negative motivational states (e.g., withdrawal),13 and how long the neurocognitive XR-NTX effects endure after the treatment is discontinued.14
After repeatedly signalling drug arrival, drug-related visual and olfactory stimuli can become conditional stimuli or “cues” that elicit conditioned responses.15 Such responses include appetitive drug motivation (craving) and, especially in patients with OUD, conditioned withdrawal-like symptoms.16–18 These cue-triggered responses are thought to play an important role in perpetuating drug use and relapse.16,17,19 Drug cues have been shown to activate the dopaminergic mesocorticolimbic (MCL) pathway that includes the VTA, NAcc and medial orbitofrontal cortex (mOFC).20,21 Therefore, MCL drug cue reactivity has the potential to serve as a biomarker of addiction.12,18,22 Extended-release naltrexone has been shown to modulate both neurophysiological and behavioural responses to drug cues in heroin-addicted individuals, including decreased cue reactivity in the caudate and medial prefrontal cortex, and reduction in craving.12,23 In addition, XR-NTX also decreases responses to appetitive natural stimuli, such as sweet taste24 and palatable food,25 while tending to increase aversive responses.25 However, prior studies have not directly compared the effects of XR-NTX on the neural and behavioural responses to drug-related stimuli versus nondrug appetitive and aversive stimuli. Such a comparison would help determine to what extent XR-NTX-related modulation differs between drug-related and nondrug stimuli. This is crucial if neural responses to drug stimuli are to be used for clinical treatment monitoring.
Opioid addiction has been conceptualized as a progression from impulsive seeking of reward to compulsive avoidance of withdrawal and is characterized by a high rate of relapse after abstinence is achieved.26 Withdrawal symptoms play a central role in the maintenance of ongoing drug use in patients with OUD,13,27 either directly or by increasing the positive reinforcing effects of opioids. Increasing the positive reinforcing effects in turn drives future drug-seeking behaviour when the patient is again in withdrawal.27 Withdrawal-based motivation may coexist with motivation related to the powerful positive reinforcement effects mediated by the MOR.3 The positive reinforcing effects are especially important for the initiation of drug use and cue-triggered relapse after pharmacologic withdrawal has ceased. At the neuropharmacological level, withdrawal induces a reduction in the extracellular tonic dopamine concentration in the MCL that elevates the sensitivity to phasic dopamine release. This results in a heightened MCL reactivity to both the drug and drug-related cues.28
The present study aimed to test the differential effect of XR-NTX on the MCL response to opioid-related versus nonopioid evocative visual stimuli in detoxified individuals with OUD. We hypothesized that XR-NTX reduces the brain response to opioid-related but not sexual or aversive stimuli in patients with OUD, and that this effect is associated with the concurrent changes in the severity of withdrawal symptoms. To test this hypothesis, we studied the brain response to visual stimuli in patients with OUD before, during and after XR-NTX treatment using functional MRI (fMRI) and a cue-reactivity paradigm comprising drug, sexual and aversive stimuli. Given the positive incentive value of drug-related stimuli27 and the repeated recruitment of the MCL reward circuit (e.g., NAcc and mOFC) in processing rewarding stimuli,29–31 we focused on these regions. We examined whether XR-NTX differentially modulates their neural responses to opioid-related versus opioid-unrelated stimuli and whether such modulation is associated with opioid withdrawal.
Methods
Participants
We recruited participants through newspaper advertisements in Philadelphia, Pa., between 2012 and 2014. Benefits of participation included free, medically supervised, 3-month treatment for OUD, referral to community providers after study completion, and compensation for the time and travel expenses related to participation. All participants gave written informed consent to participate in the protocol, which was approved by the University of Pennsylvania Institutional Review Board.
Inclusion and exclusion criteria
The DSM-IV-TR diagnosis of opioid dependence was established using the best-estimate format based on all available sources of information, including history, the Structured Clinical Interview for DSM-IV32 and the Addiction Severity Index 5th Edition.33
Inclusion criteria were age between 18 and 59 years; a DSM-IV-TR diagnosis of opioid dependence confirmed by self-report and medical records documenting daily opioid use for more than 2 weeks in the past 3 months; evidence of detoxification from opioids before XR-NTX injections, established by urine drug screen (Redwood Toxicology Laboratory) and a negative naloxone challenge test; and good physical health ascertained by history and physical examination, blood chemistry and urinalysis.
Exclusion criteria were current use of medications that could confound blood oxygen level–dependent fMRI response, such as antidopaminergic agents, anticonvulsants, and β-blockers; current psychosis, dementia, intellectual disability, or lifetime history of schizophrenia; clinically significant cardiovascular, hematologic, hepatic, renal, pulmonary, metabolic, gastrointestinal, neurologic, or endocrine abnormalities; pregnancy or breastfeeding; history of clinically significant head trauma; contraindications for XR-NTX treatment, including medical conditions requiring opioid analgesics such as chronic pain disorder, planned surgery, obesity, elevated liver enzymes more than 3 times the upper limit of normal, or failure to complete opioid detoxification; contraindications for MRI, such as indwelling magnetically active foreign bodies, or fear of enclosed spaces; and current use of illicit drugs (e.g., cocaine) except marijuana.
Functional MRI task
During the fMRI sessions, participants viewed 4 categories of visual stimuli (cues) in a pseudorandom order: drug, sexual, aversive and neutral. Each stimulus category included 24 unique images that were presented twice, resulting in a total of 192 trials (see Appendix 1, available at jpn.ca/170036-a1, “Stimuli” section, for more details).
Each trial of the fMRI cue-reactivity task consisted of a stimulus displayed for 500 ms followed by a crosshair displayed for 1500 ms. The stimulus trials were interspersed with 48 baseline periods during which crosshairs were displayed for 2000 ms. Pseudorandom order of the stimuli trials and baseline periods was generated using optseq2 (https://surfer.nmr.mgh.harvard.edu/optseq). The task duration was 8 minutes, 28 seconds.
Behavioural assessments
Before the fMRI cue-reactivity task, physical symptoms of opioid withdrawal were measured using the Clinical Opiate Withdrawal Scale (COWS),34 which is a clinician-administered scale that assesses 11 common opioid withdrawal symptoms. After COWS assessment, self-reported opioid craving and withdrawal were recorded using a 10-point scale (0 = none; 9 = extremely). Following the cue-reactivity task, the self-reported opioid craving and withdrawal and the COWS assessments were repeated (see Appendix 1, “Additional behavioural assessments” section, for more details).
Procedure
After providing informed consent, participants completed baseline assessments and were offered up to 3 monthly intra-muscular injections of XR-NTX (380 mg gradually released from dissolvable polymer microspheres over a period of 1 month; manufactured by Alkermes Inc., under the brand name Vivitrol; see Appendix 1, “Study medication” section, for more details).
About 3 days (mean 3.13 ± 8.35, range 0–36 d) before the first XR-NTX injection, participants underwent the first fMRI session (i.e., the pretreatment session). The second fMRI session (i.e., the on-treatment session) was completed 10.17 ± 2.44 (range 7–14) days after the first XR-NTX injection. A third optional posttreatment fMRI session was completed 41.64 ± 9.98 (range 28–64) days after the last XR-NTX injection; however, the posttreatment session was not the main focus of the present study. The methods and results pertaining to the posttreatment session are included in Appendix 1 (“Procedure and analyses of the posttreatment session” and “Results from the posttreatment session” sections). During each fMRI session, the COWS, self-reported craving and self-reported withdrawal were assessed before the fMRI cue-reactivity task and immediately after the task.
Behavioural data analysis
Because of the high participant attrition rate, the present analyses focused primarily on the pre- and on-treatment sessions. We analyzed participants’ COWS and self-reported craving and withdrawal scores using 2-way repeated-measures analysis of variance (ANOVA), which tested the main effect of cue exposure (pre- v. post-fMRI), the main effect of session (pre- v. on-treatment) and their interaction. Exploratory analyses involving the optional posttreatment fMRI session are reported in Appendix 1 (“Procedure and analyses of the posttreatment session” section).
MRI data acquisition and analysis
We acquired the MRI data using a Siemens Tim Trio 3 T system and analyzed the data using SPM 8 (Wellcome Trust Centre for Neuroimaging). Images were preprocessed and subjected to individual-level statistical analyses by modelling the effects of drug, sexual and aversive stimuli compared with the neutral stimuli. At the group level, the bilateral NAcc and mOFC were defined as regions of interest (ROIs) based on their consistent involvement in the processing of positive incentive value in general29–31 and in response to drug cues in particular.20 For each ROI, the contrast values for drug, sexual and aversive stimuli during the pre- and on-treatment sessions were subjected to 2 × 3 repeated-measures ANOVAs with session (pre- v. on-treatment) and stimulus (drug v. sexual v. aversive) as within-subjects variables. We explored the session × stimulus interaction in other brain regions by conducting whole-brain ANOVA. Significant activation was identified at a corrected p < 0.05 threshold (voxel-level p < 0.005, cluster extent > 137 voxels). See Appendix 1, “MRI data acquisition” and “MRI data analyses” sections, for more details.
We tested the Pearson correlation between the reduction in drug-related neural activity and the reduction in opioid craving and withdrawal due to the XR-NTX treatment (i.e., pre- minus on-treatment). Pre-fMRI opioid craving and withdrawal measures were used for correlation analyses so that they were not influenced by exposure to drug cues.
Results
Participants
Twenty-five individuals with OUD were enrolled in the study. One participant was excluded because of concurrent use of cocaine, leaving 24 participants (15 men, 9 women, mean age 30.21 ± 8.47 [range 20–47] yr) for the final analysis. Twenty-one participants were right-handed, and three were left-handed. Mean education level was 13.88 ± 2.42 (range 19–24) years. Four participants used heroin exclusively, 7 used prescription opioids exclusively, and 13 used both with expressed preference for one or the other drug category.
Participant attrition and missing data
All 24 participants received the first XR-NTX injection, 17 (70.83%) received both the first and second XR-NTX injections, and 15 (62.50%) received all 3 injections. All 24 participants completed the pre- and on-treatment fMRI sessions. Four participants were missing 1 or more COWS scores. Eleven (45.83%) completed the posttreatment COWS and self-reported craving and withdrawal assessments. Nine (37.50%) completed the posttreatment fMRI session. See Appendix 1, ”Participant attrition and missing data” section, for more details.
Behavioural results
There were main effects of session on opioid withdrawal symptoms as measured by COWS (F1,19 = 6.40, p = 0.020), on self-reported craving (F1,23 = 34.64, p < 0.001) and on self-reported withdrawal (F1,23 = 9.74, p = 0.005), such that the COWS scores, self-reported craving and self-reported withdrawal significantly declined from the pretreatment to the on-treatment sessions. We also observed a significant main effect of cue exposure on craving (F1,23 = 7.38, p = 0.012), such that the fMRI cue-reactivity task increased participants’ craving for opioids. Cue exposure did not have a main effect on the COWS scores (F1,19 = 1.36, p = 0.36) or on the self-reported withdrawal (F1,23 = 0.49, p = 0.83). There was no interaction between cue exposure and session (COWS: F1,19 = 0.44, p = 0.52; self-reported craving: F1,23 = 1.30, p = 0.27; self-reported withdrawal: F1,23 = 1.11, p = 0.30; Table 1).
Table 1.
Clinical Opiate Withdrawal Scale and self-reported craving and withdrawal scores
| Measure | Session; mean ± SD | |
|---|---|---|
|
| ||
| Pretreatment | On-treatment | |
| COWS (pre-fMRI) | 2.73 ± 2.41 | 1.87 ± 1.32 |
| COWS (post-fMRI) | 2.81 ± 2.44 | 1.50 ± 1.47 |
| Self-report craving (pre-fMRI) | 3.38 ± 2.16 | 1.08 ± 1.47 |
| Self-report craving (post-fMRI) | 4.54 ± 2.73 | 1.75 ± 2.15 |
| Self-report withdrawal (pre-fMRI) | 1.79 ± 2.34 | 0.25 ± 0.61 |
| Self-report withdrawal (post-fMRI) | 1.58 ± 2.34 | 0.38 ± 0.82 |
COWS = Clinical Opiate Withdrawal Scale; fMRI = functional magnetic resonance imaging; SD = standard deviation.
Exploratory analyses of 11 participants showed that both the COWS scores and the self-reported craving and withdrawal at the posttreatment session were lower than at the pretreatment session. These scores were either lower than, or comparable to those at the on-treatment session (see Appendix 1, “Results from the posttreatment session” section, for more details).
Functional MRI results
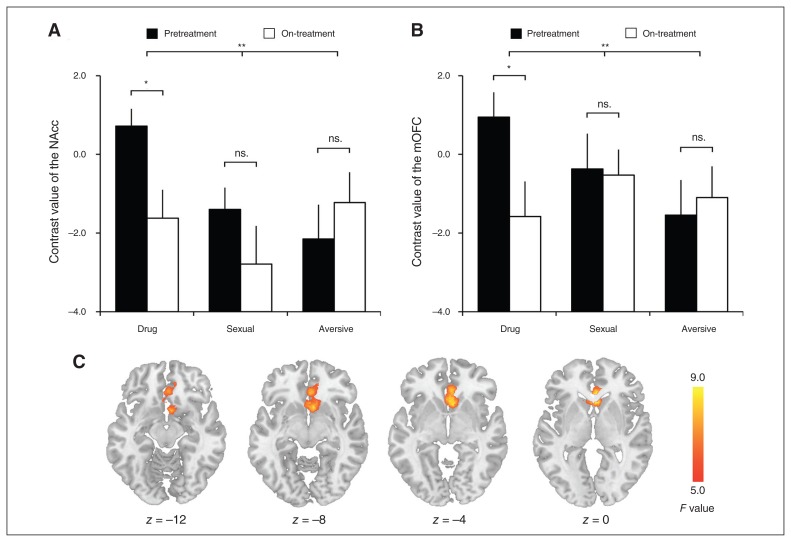
Significant session × stimulus interaction was observed in the NAcc (F2,46 = 5.29, p = 0.009; Fig. 1A) and the mOFC (F2,46 = 5.47, p = 0.007; Fig. 1B). Post hoc analysis showed that the neural response to drug cues in these regions during the pretreatment session was greater than during the on-treatment session (NAcc: 0.72 ± 2.17 v. −1.62 ± 3.54, t23 = 2.62, p = 0.015; mOFC: 0.94 ± 3.11 v. 1.58 ± 4.34, t23 = 2.31, p = 0.030). The neural response to sexual or aversive cues did not differ between the pre- and on-treatment sessions (NAcc, sexual: t23 = 1.12, p = 0.27; NAcc, aversive: t23 = −1.09, p = 0.29; mOFC, sexual: t23 = 0.13, p = 0.90; mOFC, aversive: t23 = −0.45, p = 0.66). We also performed pairwise comparisons of the change in neural response to different stimuli. The change scores were calculated as pretreatment minus on-treatment. We found that the reduction in NAcc response to drug stimuli (mean 2.34 ± 4.38) did not significantly differ from that to sexual stimuli (1.39 ± 6.05, t23 = 0.99, p = 0.33), but was significantly greater than that to aversive stimuli (−0.93 ± 4.14, t23 = 3.06, p = 0.006). The reduction in NAcc response to sexual stimuli was significantly greater than that to aversive stimuli (t23 = 2.17, p = 0.041). We also found that the reduction in mOFC response to drug stimuli (2.52 ± 5.35) was greater than that to sexual stimuli (0.16 ± 6.00, t23 = 2.45, p = 0.022) and that to aversive stimuli (−0.45 ± 4.86, t23 = 3.42, p = 0.002). The change in mOFC response to sexual and aversive stimuli did not significantly differ (t23 = 0.60, p = 0.55).
Fig. 1.
(A) The nucleus accumbens (NAcc) response to cues during the pretreatment and on-treatment sessions. (B) The medial orbitofrontal cortex (mOFC) response to cues during the pre- and on-treatment sessions. (C) Results of whole-brain analysis of variance showing significant session × stimulus interaction in the NAcc and mOFC. The error bar represents standard error of mean; *p < 0.05; **p < 0.01.
We conducted a whole-brain analysis to explore the effect of session × stimulus interaction on the neural activity in other brain regions. We found that the interaction was associated with a single cluster in the ventral striatum that extended to the mOFC (k = 679, Z = 3.45, Montreal Neurological Institute [MNI] coordinates: x, y, z = 4, 18, −2; Fig. 1C).
Exploratory analyses of the 9 participants with available posttreatment data showed that the NAcc response to drug stimuli at the posttreatment session was comparable to that at the pretreatment session and greater than that at the on-treatment session. A similar trend was observed for the mOFC (see Appendix 1, “Results from the posttreatment session” section, for more details).
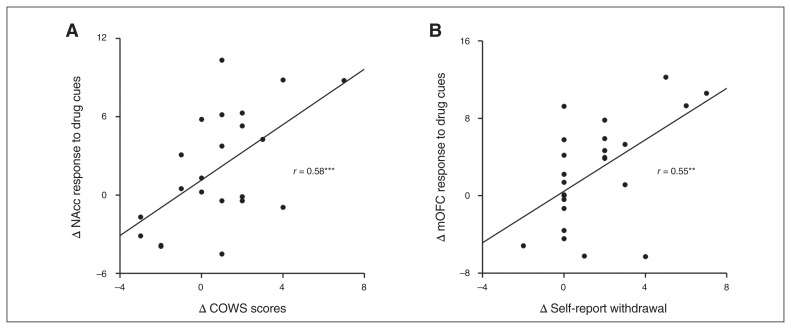
Correlation analysis
The reduction in drug-related neural activity between the pre- and on-treatment sessions in the NAcc was significantly correlated with the decline in withdrawal symptoms indexed by the COWS score (r = 0.58, p = 0.005; Fig. 2A). Reduced response to drug cues in the mOFC and the decline in COWS scores were not significantly correlated (r = 0.32, p = 0.15). Conversely, the decline in self-reported subjective withdrawal symptom severity was significantly correlated with the reduction in drug-related neural activity in the mOFC (r = 0.55, p = 0.005; Fig. 2B), but not in the NAcc (r = 0.25, p = 0.25). No correlation was found between self-reported craving and NAcc response (r = −0.12, p = 0.57) or between craving and mOFC response (r = 0.08, p = 0.70).
Fig. 2.
(A) Correlation between the change in Clinical Opiate Withdrawal Scale (COWS) scores and the change in nucleus accumbens (NAcc) response to opioid drug cues. (B) Correlation between the change in self-reported opioid withdrawal and the change in medial orbitofrontal cortex (mOFC) response to opioid drug cues. All change calculations reflect the difference between pretreatment and on-treatment assessments. Δ = pretreatment minus on-treatment; **p < 0.01; ***p < 0.005.
Discussion
Using fMRI, we compared the effects of XR-NTX on the brain response to drug-related, sexual and aversive visual stimuli in detoxified patients with OUD. We found that the NAcc and mOFC brain responses to opioid-related cues were significantly reduced after 2 weeks of XR-NTX treatment, whereas their responses to nondrug stimuli did not significantly change. Moreover, the reduction in NAcc response was positively correlated with a decline in the objectively measured symptoms of opioid withdrawal, whereas the reduction in mOFC response was positively correlated with a decline in self-reported severity of withdrawal. The NAcc and mOFC are the key components of the MCL approach and reward system. Our findings suggest a differential effect of XR-NTX on the MCL responses to opioid-related and normally evocative stimuli in abstinent patients with OUD. In addition, the individual difference in the decline of opioid withdrawal was associated with XR-NTX’s blunting of MCL cue reactivity.
The MCL system plays an important role in the processing of pathologically rewarding stimuli across a range of addictive substances and behaviours, such as alcohol,35 marijuana, 36 cocaine,37 opioids12,18,23 and gambling.38 Meta-analyses have shown that the NAcc and the mOFC are among the core MCL regions engaged in drug cue reactivity.20 Consistent with this literature, our data show heightened NAcc and mOFC responses to drug-related visual cues in heroin and prescription opioid abusers before XR-NTX treatment. Drug cue reactivity has been a candidate biomarker and therapeutic target22 because of its important role in triggering relapse. Extended-release naltrexone is an effective treatment of OUD that significantly improves treatment retention, reduces relapse and reduces subjective reports of craving.39 Recent studies have also found that XR-NTX reduces the brain response to visual heroin cues among heroin-dependent patients. 12,21 The present study extends previous findings by showing that the effect of XR-NTX on the NAcc and mOFC responses to opioid-related cues is selective. The fact that XR-NTX has little effect on the MCL responses to naturally evocative stimuli regardless of valence (i.e., sexual and aversive pictures) provides neurobiological evidence in support of prior observational studies showing no change in pleasurable activities during treatment.40 These findings also raise the possibility that in patients with OUD, opioid-related but not naturally evocative visual stimuli induce opioid neurotransmission that has been blunted by XR-NTX.
An exploratory whole-brain analysis revealed that no regions showed a significant interaction other than our a priori MCL ROIs. This finding is unlikely to be a false negative since we used a whole-brain threshold similar to the one that has been shown to produce balanced type I and type II errors. 41 Whether or not this threshold produces an excessive type I error rate remains a subject of continued discussion. 42–45 This finding confirms that the NAcc and mOFC are the only regions that change their sensitivity to drug cues in response to XR-NTX. A similar fMRI study that examined the cue reactivity in a group of methadone maintenance patients with OUD showed that a regular daily dose of methadone reduced the MCL neural response to opioid cues in the amygdala and insula, but not in the NAcc and mOFC.18 Therefore, while both opioid agonist and antagonist treatments have been proven to be effective for OUD, their effectiveness may be achieved by acting on different subcircuits of the MCL system. It would be interesting for future studies to test whether and how the distinct patterns of reduction in MCL cue reactivity account for the fundamental motivational differences between agonist and antagonist treatments.
The reduction in the MCL response to drug cues induced by XR-NTX was correlated with a decline in withdrawal symptom severity. The correlation suggests that XR-NTX has a greater impact on the cue-triggered MCL activation in individuals with lower levels of ongoing withdrawal. One possible explanation could be that these individuals had greater recovery of endogenous opioid function, making them more responsive to the effects of naltrexone.46 Moreover, given the role of the MCL system in negative reinforcement,47 it is also possible that these individuals were less susceptible to the negative reinforcing effects of drug cues during XR-NTX treatment. Specifically, the reduction in the NAcc and mOFC cue reactivity was associated with a decline in objective and self-report basal withdrawal symptomatology, respectively. This association requires further study to determine whether withdrawal symptoms have a predictive value for XR-NTX outcomes, such as adherence23 and relapse, as well as whether withdrawal symptoms causally influence these outcomes. The distinct correlation patterns of NAcc and mOFC with clinician-determined and self-reported withdrawal symptoms, respectively, is consistent with the notion that compared with the NAcc, the mOFC is more responsive to subjective reward-related experience.30 In addition, we found that whereas self-reported opioid craving decreased from pre- to on-treatment sessions, such a decrease was not associated with the XR-NTX effect on MCL cue reactivity. The lack of correlation with craving does not preclude the possibility that XR-NTX reduces the positive reinforcing effects of opioids.27,48 Rather, it may reflect the fact that craving is more difficult to report (undermining correlations) than withdrawal symptoms, or that craving has a closer association with brain regions outside the examined ROIs (e.g., the anterior cingulate cortex and the temporal lobe49).
We found that XR-NTX had little effect on the brain response to the normally evocative aversive and appetitive stimuli categories. This observation extends the findings of prior studies in alcohol-dependent patients that reported a reduction in the brain response in several cortical areas specific to alcohol-related cues50 but did not find reduced pleasurable activities during XR-NTX treatment.40 Nonetheless, the literature on the effect of opioid antagonism on the neural and behavioural responses to normally evocative stimuli remains mixed. For example, acute administration of naltrexone reduces sexual behaviour in previously sexually active male monkeys51 and diminishes lambs’ preference for their own mothers (compared with an unknown ewe).52 In healthy humans, naltrexone attenuated the positive feelings associated with social connection,53 increased brain response (in the amygdala and insula) to aversive stimuli,25 and reduced brain response (in the caudate and the anterior cingulate cortex) to appetitive food stimuli.25 In individuals with opioid addiction, XR-NTX treatment was associated with a decline in their liking of the sweet taste24 and their perception of cuteness of baby portraits.54 The divergence between these reports and our findings could stem from several factors. First, there are significant methodological differences, including the much higher naltrexone plasma levels achieved by acute doses of oral naltrexone in contrast with an extended-release preparation such as XR-NTX. Second, none of the studies was performed in patients with OUD receiving XR-NTX. In the study methodologically closest to ours,25 the brain regions showing decreased response to normally appetitive stimuli did not overlap with the brain regions that showed session × stimulus interactions in our study. Finally, if XR-NTX effects on naturally evocative stimuli are subtle relative to its effects on drug-related stimuli, it is possible that our sample size was insufficient to detect them. Future work is needed to address these possibilities.
Limitations
Our results should be interpreted with a number of caveats. First, the small size of prescription opioids versus heroin subgroups did not allow us to directly compare them. Such a comparison would be important to conduct in future studies, given the growing prevalence of prescription opioid abuse.55,56 Second, the study was not placebo-controlled. Such a control condition would be challenging in the context of XR-NTX treatment. Participants almost invariably test the opioid blockade in the early stages of treatment57 and are able to quickly discover whether they are in the XR-NTX or placebo group. Moreover, testing opioid blockade by a patient on placebo who may try a higher than usual dose to achieve desired effects could increase the risk of opioid overdose. Third, our stimuli were not matched on pleasantness (see Appendix 1, “Stimuli” section, for more details) and were not assessed on other dimensions, including arousal, dominance,58 or effort expenditure to view.59 Finally, the limited number of brain regions of interest (i.e., NAcc and mOFC), the focus on the session × stimulus interaction instead of pairwise comparison between sessions across stimulus categories, and a relatively small sample size may have limited our ability to fully unravel the effects of XR-NTX on brain function (e.g., neural responses to sexual and aversive stimuli). It is our hope that our study sets the stage for filling these gaps in future research.
Conclusion
Extended-release naltrexone reduces the NAcc and mOFC response to opioid-related visual stimuli in detoxified patients with OUD. This effect is specific to opioid-related stimuli in the mOFC but not in the NAcc. The reduction in the MCL response to opioid-related cues is associated with reduction in opioid withdrawal, but not with craving symptomatology. Together, these findings support the potential for clinical application of drug cue-reactivity paradigms paired with neuroimaging to monitor XR-NTX treatment.
Footnotes
Competing interests: C. O’Brien declares having received consulting fees from Alkermes plc. D. Langleben received honoraria from Alkermes plc in 2016 and 2017 for participation on its scientific advisory board. No other competing interests declared.
Funding: The study was supported by the Commonwealth of Pennsylvania CURE grant SAP#4100055577 (PI: A. Childress) and the following NIH grants: T32 Translational Addiction Research postdoctoral fellowship DA028874 to Zhenhao Shi (PI: A. Childress and R. Pierce); DA024553 (PI: C. O’Brien); DA036028 (PI: D. Langleben); N01DA-14-7788 (PI: D.E. Moody); and HD084746 (PI: A. Wang). Study medicine was donated by the manufacturer (Alkermes plc).
Contributors: C. O’Brien, A. Childress and D. Langleben designed the study. A Childress and D. Langleben acquired the data, which all authors analyzed. Z. Shi, A. Childress and D. Langleben wrote the article, which all authors reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
References
- 1.Naghavi M, Wang H, Lozano R, et al. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–71. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Institute on Drug Abuse. Overdose death rates. North Bethesda (MD): the Institute; 2015. [Google Scholar]
- 3.Wise RA, Koob GF. The development and maintenance of drug addiction. Neuropsychopharmacology. 2014;39:254–62. doi: 10.1038/npp.2013.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson SW, North RA. Opioids excite dopamine neurons by hyper-polarization of local interneurons. J Neurosci. 1992;12:483–8. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–98. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 6.Fields HL, Margolis EB. Understanding opioid reward. Trends Neurosci. 2015;38:217–25. doi: 10.1016/j.tins.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mannelli P, Peindl KS, Wu LT. Pharmacological enhancement of naltrexone treatment for opioid dependence: a review. Subst Abuse Rehabil. 2011;2:113–23. doi: 10.2147/SAR.S15853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rothenberg JL, Sullivan MA, Church SH, et al. Behavioral naltrexone therapy: an integrated treatment for opiate dependence. J Subst Abuse Treat. 2002;23:351–60. doi: 10.1016/s0740-5472(02)00301-x. [DOI] [PubMed] [Google Scholar]
- 9.Nunes EV, Rothenberg JL, Sullivan MA, et al. Behavioral therapy to augment oral naltrexone for opioid dependence: A ceiling on effectiveness? Am J Drug Alcohol Abuse. 2006;32:503–17. doi: 10.1080/00952990600918973. [DOI] [PubMed] [Google Scholar]
- 10.Krupitsky E, Zvartau E, Woody G. Use of naltrexone to treat opioid addiction in a country in which methadone and buprenorphine are not available. Curr Psychiatry Rep. 2010;12:448–53. doi: 10.1007/s11920-010-0135-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aletraris L, Roman PM. Adoption of injectable naltrexone in US substance use disorder treatment programs. J Stud Alcohol Drugs. 2015;76:143–51. [PMC free article] [PubMed] [Google Scholar]
- 12.Langleben DD, Ruparel K, Elman I, et al. Extended-release naltrexone modulates brain response to drug cues in abstinent heroin-dependent patients. Addict Biol. 2014;19:262–71. doi: 10.1111/j.1369-1600.2012.00462.x. [DOI] [PubMed] [Google Scholar]
- 13.Koob GF. The dark side of emotion: the addiction perspective. Eur J Pharmacol. 2015;753:73–87. doi: 10.1016/j.ejphar.2014.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon MS, Kinlock TW, Vocci FJ, et al. A phase 4, pilot, open-label study of VIVITROL® (extended-release naltrexone XR-NTX) for prisoners. J Subst Abuse Treat. 2015;59:52–8. doi: 10.1016/j.jsat.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Wikler A. Recent progress in research on the neurophysiologic basis of morphine addiction. Am J Psychiatry. 1948;105:329–38. doi: 10.1176/ajp.105.5.329. [DOI] [PubMed] [Google Scholar]
- 16.O’Brien CP, Testa T, O’Brien TJ, et al. Conditioned narcotic withdrawal in humans. Science. 1977;195:1000–2. doi: 10.1126/science.841320. [DOI] [PubMed] [Google Scholar]
- 17.Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–40. [PubMed] [Google Scholar]
- 18.Langleben DD, Ruparel K, Elman I, et al. Acute effect of methadone maintenance dose on brain FMRI response to heroin-related cues. Am J Psychiatry. 2008;165:390–4. doi: 10.1176/appi.ajp.2007.07010070. [DOI] [PubMed] [Google Scholar]
- 19.Childress AR, Hole AV, Ehrman RN, et al. Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogr. 1993;137:73–95. [PubMed] [Google Scholar]
- 20.Chase HW, Eickhoff SB, Laird AR, et al. The neural basis of drug stimulus processing and craving: an activation likelihood estimation meta-analysis. Biol Psychiatry. 2011;70:785–93. doi: 10.1016/j.biopsych.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zijlstra F, Veltman DJ, Booij J, et al. Neurobiological substrates of cue-elicited craving and anhedonia in recently abstinent opioid-dependent males. Drug Alcohol Depend. 2009;99:183–92. doi: 10.1016/j.drugalcdep.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Courtney KE, Schacht JP, Hutchison K, et al. Neural substrates of cue reactivity: association with treatment outcomes and relapse. Addict Biol. 2016;21:3–22. doi: 10.1111/adb.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang AL, Elman I, Lowen SB, et al. Neural correlates of adherence to extended-release naltrexone pharmacotherapy in heroin dependence. Transl Psychiatry. 2015;5:e531. doi: 10.1038/tp.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langleben DD, Busch EL, O’Brien CP, et al. Depot naltrexone decreases rewarding properties of sugar in patients with opioid dependence. Psychopharmacology (Berl) 2012;220:559–64. doi: 10.1007/s00213-011-2503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murray E, Brouwer S, McCutcheon R, et al. Opposing neural effects of naltrexone on food reward and aversion: implications for the treatment of obesity. Psychopharmacology (Berl) 2014;231:4323–35. doi: 10.1007/s00213-014-3573-7. [DOI] [PubMed] [Google Scholar]
- 26.Koob GF, Rocio M, Carrera A, et al. Substance dependence as a compulsive behavior. J Psychopharmacol. 1998;12:39–48. doi: 10.1177/026988119801200106. [DOI] [PubMed] [Google Scholar]
- 27.Hutcheson DM, Everitt BJ, Robbins TW, et al. The role of withdrawal in heroin addiction: enhances reward or promotes avoidance? Nat Neurosci. 2001;4:943–7. doi: 10.1038/nn0901-943. [DOI] [PubMed] [Google Scholar]
- 28.Grace AA. The tonic/phasic model of dopamine system regulation: its relevance for understanding how stimulant abuse can alter basal ganglia function. Drug Alcohol Depend. 1995;37:111–29. doi: 10.1016/0376-8716(94)01066-t. [DOI] [PubMed] [Google Scholar]
- 29.Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27:765–76. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 30.Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72:341–72. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 31.Schoenbaum G, Roesch MR, Stalnaker TA. Orbitofrontal cortex, decision-making and drug addiction. Trends Neurosci. 2006;29:116–24. doi: 10.1016/j.tins.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision (DSM-IV-TR) Washington, DC: APA; 2000. [Google Scholar]
- 33.McLellan AT, Kushner H, Metzger D, et al. The fifth edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 34.Wesson DR, Ling W. The clinical opiate withdrawal scale (COWS) J Psychoactive Drugs. 2003;35:253–9. doi: 10.1080/02791072.2003.10400007. [DOI] [PubMed] [Google Scholar]
- 35.Filbey FM, Claus E, Audette AR, et al. Exposure to the taste of alcohol elicits activation of the mesocorticolimbic neurocircuitry. Neuropsychopharmacology. 2008;33:1391–401. doi: 10.1038/sj.npp.1301513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Filbey FM, Schacht JP, Myers US, et al. Marijuana craving in the brain. Proc Natl Acad Sci U S A. 2009;106:13016–21. doi: 10.1073/pnas.0903863106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Volkow ND, Wang GJ, Telang F, et al. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–8. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crockford DN, Goodyear B, Edwards J, et al. Cue-induced brain activity in pathological gamblers. Biol Psychiatry. 2005;58:787–95. doi: 10.1016/j.biopsych.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 39.Krupitsky E, Nunes EV, Ling W, et al. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377:1506–13. doi: 10.1016/S0140-6736(11)60358-9. [DOI] [PubMed] [Google Scholar]
- 40.O’Brien CP, Gastfriend DR, Forman RF, et al. Long-term opioid blockade and hedonic response: preliminary data from two open-label extension studies with extended-release naltrexone. Am J Addict. 2011;20:106–12. doi: 10.1111/j.1521-0391.2010.00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Soc Cogn Affect Neurosci. 2009;4:423–8. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nichols TE, Eklund A, Knutsson H. A defense of using resting-state fMRI as null data for estimating false positive rates. Cogn Neurosci. 2017;8:144–9. doi: 10.1080/17588928.2017.1287069. [DOI] [PubMed] [Google Scholar]
- 43.Slotnick SD. Cluster success: fMRI inferences for spatial extent have acceptable false-positive rates. Cogn Neurosci. 2017;8:150–5. doi: 10.1080/17588928.2017.1319350. [DOI] [PubMed] [Google Scholar]
- 44.Slotnick SD. Resting-state fMRI data reflects default network activity rather than null data: a defense of commonly employed methods to correct for multiple comparisons. Cogn Neurosci. 2017;8:141–3. doi: 10.1080/17588928.2016.1273892. [DOI] [PubMed] [Google Scholar]
- 45.Eklund A, Nichols TE, Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A. 2016;113:7900–5. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boecker H, Sprenger T, Spilker ME, et al. The runner’s high: opioidergic mechanisms in the human brain. Cereb Cortex. 2008;18:2523–31. doi: 10.1093/cercor/bhn013. [DOI] [PubMed] [Google Scholar]
- 47.Navratilova E, Atcherley CW, Porreca F. Brain circuits encoding reward from pain relief. Trends Neurosci. 2015;38:741–50. doi: 10.1016/j.tins.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95(Suppl 2):S91–117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- 49.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–9. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 50.Lukas SE, Lowen SB, Lindsey KP, et al. Extended-release naltrexone (XR-NTX) attenuates brain responses to alcohol cues in alcohol-dependent volunteers: a bold FMRI study. Neuroimage. 2013;78:176–85. doi: 10.1016/j.neuroimage.2013.03.055. [DOI] [PubMed] [Google Scholar]
- 51.Meller RE, Keverne EB, Herbert J. Behavioural and endocrine effects of naltrexone in male talapoin monkeys. Pharmacol Biochem Behav. 1980;13:663–72. doi: 10.1016/0091-3057(80)90010-6. [DOI] [PubMed] [Google Scholar]
- 52.Shayit M, Nowak R, Keller M, et al. Establishment of a preference by the newborn lamb for its mother: the role of opioids. Behav Neurosci. 2003;117:446–54. doi: 10.1037/0735-7044.117.3.446. [DOI] [PubMed] [Google Scholar]
- 53.Inagaki TK, Ray LA, Irwin MR, et al. Opioids and social bonding: naltrexone reduces feelings of social connection. Soc Cogn Affect Neurosci. 2016;11:728–35. doi: 10.1093/scan/nsw006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang AL, Lowen SB, Elman I, et al. Sustained opioid antagonism increases striatal sensitivity to baby schema in opioid dependent women. Eur Neuropsychopharmacol. 2016;26:S695–6. [Google Scholar]
- 55.United Nations Office on Drugs and Crime. World Drug Report 2014. Vienna, Austria: United Nations; 2014. [Google Scholar]
- 56.Cicero TJ, Ellis MS, Surratt HL, et al. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71:821–6. doi: 10.1001/jamapsychiatry.2014.366. [DOI] [PubMed] [Google Scholar]
- 57.Sullivan MA, Bisaga A, Mariani JJ, et al. Naltrexone treatment for opioid dependence: Does its effectiveness depend on testing the blockade? Drug Alcohol Depend. 2013;133:80–5. doi: 10.1016/j.drugalcdep.2013.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bradley MM, Lang PJ. Measuring emotion: the self-assessment manikin and the semantic differential. J Behav Ther Exp Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 59.Kim BW, Kennedy DN, Lehar J, et al. Recurrent, robust and scalable patterns underlie human approach and avoidance. PLoS ONE. 2010;5:e10613. doi: 10.1371/journal.pone.0010613. [DOI] [PMC free article] [PubMed] [Google Scholar]