Abstract
Background
Cognitive impairments are well-established features of schizophrenia, but there is ongoing debate about the nature and degree of cognitive impairment in patients with schizoaffective disorder and bipolar disorder. We hypothesized that there is a spectrum of increasing impairment from bipolar disorder to schizoaffective disorder bipolar type, to schizoaffective disorder depressive type and schizophrenia.
Methods
We compared performance on the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery between participants with schizophrenia (n = 558), schizoaffective disorder depressive type (n = 112), schizoaffective disorder type (n = 76), bipolar disorder (n = 78) and healthy participants (n = 103) using analysis of covariance with post hoc comparisons. We conducted an ordinal logistic regression to examine whether cognitive impairments followed the hypothesized spectrum from bipolar disorder (least severe) to schizophrenia (most severe). In addition to categorical diagnoses, we addressed the influence of symptom domains, examining the association between cognition and mania, depression and psychosis.
Results
Cognitive impairments increased in severity from bipolar disorder to schizoaffective disorder bipolar type, to schizophrenia and schizoaffective disorder depressive type. Participants with schizophrenia and schizoaffective disorder depressive type showed equivalent performance (d = 0.07, p = 0.90). The results of the ordinal logistic regression were consistent with a spectrum of deficits from bipolar disorder to schizoaffective disorder bipolar type, to schizophrenia/schizoaffective disorder depressive type (odds ratio = 1.98, p < 0.001). In analyses of the associations between symptom dimensions and cognition, higher scores on the psychosis dimension were associated with poorer performance (B = 0.015, standard error = 0.002, p < 0.001).
Limitations
There were fewer participants with schizoaffective disorder and bipolar disorder than schizophrenia. Despite this, our analyses were robust to differences in group sizes, and we were able to detect differences between groups.
Conclusion
Cognitive impairments represent a symptom dimension that cuts across traditional diagnostic boundaries.
Introduction
Current diagnostic approaches view schizophrenia and bipolar disorder as distinct psychiatric conditions, despite emerging evidence of significant genetic and phenotypic overlap between them.1 One of the most obvious challenges to the simple dichotomous view is the existence of the intermediate condition, schizoaffective disorder.2 The relationship between schizoaffective disorder, schizophrenia and bipolar disorder is uncertain, and it has been variously suggested that schizoaffective disorder is a subtype of schizophrenia or bipolar disorder, that it reflects comorbidity of schizophrenia and a mood disorder, that it is an independent disorder, and, finally, that it lies in the middle of a spectrum that ranges from a predominantly affective disorder to a predominantly psychotic disorder.3 The latter hypothesis suggests that prototypical bipolar disorder and schizophrenia lie on the extreme ends of a diagnostic spectrum, and that schizoaffective disorder represents patients who have features of both disorders.4 Support for this concept comes from evidence that symptomatic and functional outcomes for schizoaffective disorder are intermediate between schizophrenia and bipolar disorder.5,6 More recently, it has been proposed that schizophrenia and bipolar disorder lie on a gradient of neurodevelopmental impairment indexed by the extent of cognitive dysfunction, with schizoaffective disorders occupying an intermediate position.1,7,8
Neuropsychological studies that provide support for a diagnostic spectrum have demonstrated increasing severity of impairment from bipolar disorder to schizoaffective disorder to schizophrenia, although these differences were not always significant.9–11 In one of the largest studies to date, Hill and colleagues10 showed an association between ratings on the Schizo-Bipolar Scale12 and composite cognition scores, with more severe impairments among those with prominent psychosis and fewer affective symptoms. However, findings from neuropsychological studies of these 3 disorders have been inconsistent, some indicating that performance in schizoaffective disorder is similar to schizophrenia13 and others indicating no differences between diagnostic groups.14–17
There are a number of potential explanations for the conflicting findings between studies, including differences in the use of covariates and study participants’ phase of illness. Studies of symptomatic participants with schizophrenia, schizoaffective disorder and bipolar disorder have reported similar levels of impairment.15,16 It has been argued that cognitive impairments are state-dependent in bipolar disorder and improve during periods of remission. However, more recent research has demonstrated that cognitive impairments are present in euthymic bipolar disorder.18 Lifetime history of psychosis in bipolar disorder has been identified as another important factor that may influence cognitive function. Studies do not consistently report the proportion of participants with bipolar disorder who have a lifetime history of psychosis, despite evidence that its presence or absence differentiates participants with cognitive impairments from those without impairments.17 Finally, studies often consider people with schizoaffective disorder as a single group, but data indicating whether differences exist between the subtypes of schizoaffective disorder (depressive or bipolar) are scarce. The study by Hill and colleagues10 showed greater overall impairment in participants with the depressive subtype of schizoaffective disorder than the bipolar subtype, although the differences were not significant. Two smaller studies found no differences between participants with the depressive subtype and participants with schizophrenia, but neither considered the bipolar subtype.14,19 This suggests that considering both subtypes of schizoaffective disorder as a single group may obscure findings. To our knowledge, no published studies have compared the subtypes of schizoaffective disorder individually to schizophrenia and bipolar disorder.
The aim of this study was to test the hypothesis that there is a spectrum of increasing cognitive impairment from bipolar disorder, through schizoaffective disorder bipolar subtype (schizoaffective bipolar), to schizoaffective disorder depressive subtype (schizoaffective depressive) and schizophrenia. We also hypothesized that lifetime frequency and severity of psychotic symptoms (across and within diagnostic boundaries) would be associated with cognitive impairment. We tested these hypotheses in 3 ways. First, we compared cognitive performance between diagnostic groups. Second, we examined whether cognition could be considered a continuous measure across disorders. For this analysis, we combined the schizophrenia and schizoaffective depressive groups into a single group based on pre-existing data suggesting that performance between these groups is equivalent.10,14,19 Third, we examined whether cognitive performance was associated with symptom domains across diagnostic groups.
Methods
Participants
We recruited participants as part of the Cognition in Mood, Psychosis and Schizophrenia Study (CoMPaSS), a study based in the United Kingdom that recruits from outpatient clinics. This sample includes participants previously referred to as the Cardiff Cognition in Schizophrenia sample (described elsewhere by Rees and colleagues20). All patient groups were recruited as part of a single study, and all aspects of recruitment, response rates, phenotyping and determining diagnosis were equivalent across groups. Participants were interviewed using the Schedules for Clinical Assessment in Neuropsychiatry.21 Trained raters reviewed this interview, along with available clinical records, to determine a consensus lifetime DSM-IV diagnosis22 (inter-rater reliability κ statistics: schizophrenia = 0.83, schizoaffective depressive = 0.63, schizoaffective bipolar = 0.72, bipolar disorder = 0.85). Participants were excluded if they had a neurologic condition that was likely to affect their ability to participate in the study, or if they had a current substance dependence disorder.
We recruited control participants from the community and completed the Mini International Neuropsychiatric Interview23 as a screen for mental disorders. Controls were excluded if they met criteria for schizophrenia or bipolar disorder or had a family history of these conditions. All participants provided written informed consent and were reimbursed for their participation. Participants were assessed for capacity to provide informed consent by their clinical team and an appropriately trained researcher.
The study had UK multi-site NHS ethics approval granted by South East Wales Research Ethics Committee Panel (REC reference number: 07/WSE03/110; full study title: Genetic susceptibility to cognitive deficits across the schizophrenia/bipolar disorder diagnostic divide).
Neuropsychological assessment
We assessed cognitive ability using the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery (MCCB).24 This battery was designed specifically for use in schizophrenia research but has also been shown to be a valid and reliable cognitive measure in bipolar disorder.25–27 The MCCB measures 7 domains of cognition using 10 tasks:
Speed of processing (Brief Assessment of Cognition in Schizophrenia: Symbol Coding; Category Fluency: Animal Naming; Trail Making Test: Part A)
Working memory (Wechsler Memory Scale III: Spatial Span; Letter-Number Span)
Attention/vigilance (Continuous Performance Test: Identical Pairs)
Verbal learning (Hopkins Verbal Learning Test–Revised)
Visual learning (Brief Visuospatial Memory Test–Revised)
Reasoning and problem-solving (Neuropsychological Assessment Battery: Mazes)
Social cognition (Mayer-Salovey-Caruso Emotional Intelligence Test: Managing Emotions).
For each task, z scores were derived using the mean and standard deviation of the control group (50% men, mean age 41.7 years). We calculated domain and composite scores following the MCCB manual procedures. Composite scores were calculated only if a participant had completed 5 or more domains. It was possible to calculate composite scores for 926 of the 927 participants.
Demographic and clinical variables
We rated lifetime mood disorder and psychosis history using the Bipolar Affective Disorder Dimension Scale (BADDS).28 The BADDS consists of 4 dimensions: mania, depression, psychosis and incongruence. The first 3 dimensions were included and reflected the severity and frequency of these symptom domains. We rated current symptoms as the total of the global scores for the Scale for the Assessment of Negative Symptoms (SANS)29 and the Scale for the Assessment of Positive Symptoms (SAPS).30 We measured global functioning using the Global Assessment Scale (GAS).31 We estimated premorbid IQ using the National Adult Reading Test.32 Doses of antipsychotic medication at time of assessment were calculated as olanzapine equivalents,33 and lifetime antipsychotic exposure was calculated from interview and notes data in number of months. Intraclass correlation coefficients for the clinical variables ranged from 0.71 to 0.95.
Statistical analysis
Comparing cognition between diagnostic groups
We performed statistical analyses to compare the groups using R version 3.1.2. For each cognitive domain and across diagnostic groups, we compared performance using analysis of covariance with age and sex as covariates and followed up with Tukey’s HSD for pairwise comparisons. We used Bonferroni correction to adjust for multiple comparisons, resulting in an α of 0.00625 (0.05/8, 7 domains and composite score). We did not correct the α further for the number of pairwise comparisons, because Tukey’s HSD is already a conservative test that corrects for family-wise error rate. We calculated Cohen’s d by dividing mean group difference by the pooled standard deviation and used it as a measure of effect size.34 We used repeated-measures analysis of variance to compare profiles of cognitive performance between groups. The within-subject factor was cognitive domain. We investigated the effects of medication and symptoms as potential confounding variables by including olanzapine equivalent dose, duration of antipsychotic exposure, SAPS total scores, SANS total scores, BADDS lifetime depression, educational attainment and parental occupation as covariates.
Examining cognition as a dimension across diagnostic groups
To test our hypothesis that cognition can be considered a dimensional phenotype showing increasing impairment from bipolar disorder to schizoaffective bipolar to schizophrenia and schizoaffective depressive combined, we conducted an ordinal regression using SPSS version 22, with diagnosis as the outcome, composite cognition score as the predictor, and age and sex as covariates. We combined schizophrenia and schizoaffective depressive, given pre-existing data indicating that their degree of impairment is comparable.10,14,19 Diagnosis was coded on an ordinal scale: 0 = schizoaffective depressive and schizophrenia, 1 = schizoaffective bipolar, 2 = bipolar disorder.
Exploring cross-disorder symptom dimensions and cognitive performance
We entered each BADDS dimension into separate linear regressions as predictors, with composite cognition as the outcome, using R version 3.1.2. This was initially done across the whole sample and then separately for bipolar disorder/schizoaffective bipolar and schizophrenia/schizoaffective depressive.
Results
Demographic and clinical variables
The final sample included 824 participants with a diagnosis of schizophrenia (n = 558), schizoaffective depressive (n = 112), schizoaffective bipolar (n = 76) or bipolar disorder (n = 78), as well as 103 control participants. The bipolar disorder group included all participants who met criteria for a diagnosis of bipolar disorder type I (n = 68) or type II (n = 10), of whom 59 had a lifetime history of psychosis. Demographic and clinical variables for each diagnostic group are displayed in Table 1. Groups differed in proportion of men (χ2 = 61.39, p < 0.001), with more men in the schizophrenia group. Therefore, we used sex as a covariate in all analyses. We also observed differences in estimated premorbid IQ (F = 22.64, p < 0.001) and years of education (F = 14.19, p < 0.001), which were lower for those with schizophrenia and schizoaffective depressive than for those with bipolar disorder and schizoaffective bipolar. Groups differed with respect to current positive and negative symptoms (SAPS: F = 65.96, p < 0.001; SANS: F = 64.16, p < 0.001) with lower scores in those with bipolar disorder than in those in all other groups. Measures of current global functioning (GAS) differed between groups (F = 4.99, p = 0.002), with higher scores observed in the bipolar disorder group.
Table 1.
Demographic and clinical variables
| DSM-IV diagnosis | Bipolar disorder (n = 78) | Schizoaffective disorder bipolar type (n = 76) | Schizoaffective disorder depressive type (n = 112) | Schizophrenia (n = 558) |
|---|---|---|---|---|
| Age, yr, mean ± SD | 45.8 ± 10.6 | 43.8 ± 10.6 | 44.1 ± 10.1 | 43.3 ± 11.9 |
| Sex, % male | 40 | 46 | 40 | 69 |
| Estimated premorbid IQ, mean ± SD | 97.5 ± 22.4 | 94.0 ± 21.5 | 85.3 ± 20.2 | 81.7 ± 23.7 |
| Education, yr, mean ± SD | 14.6 ± 3.3 | 13.7 ± 3.0 | 12.3 ± 2.3 | 12.7 ± 2.7 |
| Taking antipsychotic, % | 63.2 | 74.7 | 77.7 | 85.5 |
| Olanzapine equivalent dose, mg, median (IQR) | 8 (4–16) | 15 (10–20) | 15 (6.5–20) | 13.7 (6.7–20.3) |
| Antipsychotic exposure, mo, median (IQR) | 60 (18–120) | 153 (72–253.5) | 168 (84–247) | 170 (96–264) |
| Current SAPS score, median (IQR)* | 0 (0–0) | 2 (0–5) | 2 (0–5) | 3 (0–6) |
| Current SANS score, median (IQR)* | 0.5 (0–3) | 4 (2–7) | 6 (2–9) | 5.5 (2–9) |
| GAS score past week, mean ± SD | 70.8 ± 14.2 | 60.1 ± 16.8 | 58.6 ± 15.8 | 60.2 ± 15.1 |
GAS = Global Assessment Scale; IQR = interquartile range; SANS = Scale for the Assessment of Negative Symptoms; SAPS = Scale for the Assessment of Positive Symptoms; SD = standard deviation.
Current SAPS and SANS scores represent the sum of the global scores.
Comparing cognition between diagnostic groups
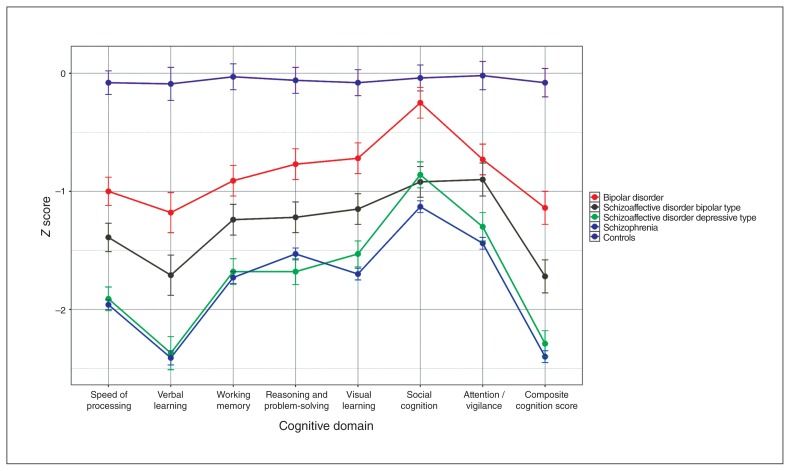
There was a significant main effect of diagnosis for all domains of cognition in the analysis of covariance (for example, composite cognition: F4,921 = 94.12, p = 0.006; see Appendix 1, Table S1, available at jpn.ca/170076-a1). Figure 1 displays the z scores (marginal means) observed for each group, demonstrating increasing severity of cognitive impairment from controls to bipolar disorder, to schizoaffective bipolar, to schizophrenia and schizoaffective depressive.
Fig. 1.
Neuropsychological performance for participants with bipolar disorder, schizoaffective disorder bipolar type, schizoaffective disorder depressive type and schizophrenia.
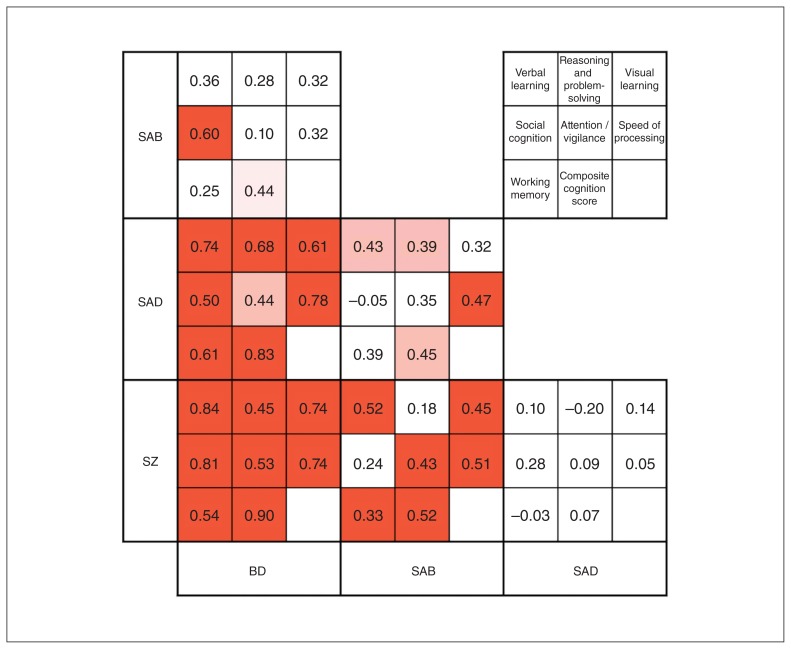
Effect sizes for each pairwise comparison between diagnoses for all domains are displayed in Figure 2. All diagnostic groups were impaired compared with controls across cognitive domains, with the exception of social cognition in those with bipolar disorder. The bipolar disorder group was the least impaired of the diagnostic groups, performing 0.5 to 1.25 standard deviations below the mean of the control group across domains (composite cognition: d = 1.12, p < 0.001). Although the groups were small, we compared bipolar disorder type I (n = 68) and bipolar disorder type II (n = 10) and found no significant differences between them (composite cognition: d = −0.07, p = 0.83; see Appendix 1, Table S2, for comparisons between domains). The results remained consistent when analysis was restricted to bipolar disorder type I (Appendix 1, Table S3). We also compared bipolar disorder with and without psychosis and found no significant differences between these groups (composite cognition: d = 0.34, p = 0.2; see Appendix 1, Table S4, for comparisons between domains). Caution should be applied in interpretation of the results comparing bipolar disorder subgroups, given the small samples of participants without psychosis (n = 19) and with bipolar disorder type II (n = 10). The schizoaffective bipolar group was more impaired than the bipolar disorder group, although this did not withstand correction for multiple testing (composite cognition: d = 0.44, p = 0.02). The schizophrenia and schizoaffective depressive groups were the most cognitively impaired and did not differ on any cognitive variable (composite cognition: d = 0.07, p = 0.90), corroborating our a priori decision to amalgamate these groups for subsequent analyses. These participants were more impaired than those with schizoaffective bipolar (schizophrenia: d = 0.52, p < 0.001; schizoaffective depressive: d = 0.45, p = 0.01) and those with bipolar disorder (schizophrenia: d = 0.90, p < 0.001; schizoaffective depressive: d = 0.83, p < 0.001). In contrast to other domains, levels of impairment in social cognition between schizoaffective bipolar, schizoaffective depressive and schizophrenia did not differ (Cohen’s d for pairwise comparisons between these groups ranged from 0.05 to 0.28). All 3 of these groups were more impaired than the bipolar disorder group with respect to social cognition (Cohen’s d ranged from 0.50 to 0.81).
Fig. 2.
Pairwise comparisons. Each 3 × 3 section displays the Cohen’s d effect sizes for the difference between 2 diagnostic groups for each domain of cognition. Lighter shade p < 0.05, darker shade p < 0.006. BD = bipolar disorder; SAB = schizoaffective disorder bipolar type; SAD = schizoactive disorder depressive type; SZ = schizophrenia.
To test whether between-group differences were qualitative or merely quantitative, we compared cognitive profiles between diagnostic groups using repeated-measures analysis of variance, including cognitive domain as the within-subject factor. Mauchly’s test indicated that the assumption of sphericity had been violated (χ220 = 360.23, p < 0.001), so degrees of freedom were corrected using Huynh-Feldt estimates of sphericity. The diagnosis × domain interaction was not significant (F15.50,3051.33 = 1.62, p = 0.06). We repeated the analysis excluding social cognition (given the quantitative differences in this domain), and the diagnosis × domain interaction was not significant (F1.60,2680.70 = 1.604, p = 0.07), indicating that patterns of cognitive ability did not differ by diagnostic group, but rather differed quantitatively.
We went on to investigate the effects of the potential confounding variables: olanzapine equivalent dose, duration of antipsychotic exposure, total SANS scores and total SAPS scores. The main effect of diagnostic group on composite cognitive scores remained significant after controlling for duration of antipsychotic exposure (F3,765 = 16.18, p < 0.001), olanzapine equivalent dose at time of testing (F3,773 = 21.42, p < 0.001), total SAPS score (F3,807 = 24.52, p < 0.001) and total SANS score (F3,805 = 16.71, p < 0.001; see Appendix 1, Tables S5 to S8, for full data). Olanzapine equivalent dose at time of testing, duration of antipsychotic exposure and negative symptoms were associated with cognitive performance in all domains. Current psychotic symptoms (SAPS score) were not associated with performance across domains, other than social cognition. We repeated the analyses including educational attainment and parental occupations (as measures of socioeconomic status), and the effect of diagnosis on cognition remained significant (Appendix 1, Table S9). Finally, we added diagnosis, olanzapine equivalent dose, duration of antipsychotic exposure, total SANS scores, total SAPS scores and lifetime depression (as measured by the BADDS depression scale) as predictors into a single model. The main effect of diagnostic group on composite cognition remained significant (F3,694 = 8.33, p < 0.001; see Appendix 1, Table S10, for individual domains). After correction for multiple testing, we observed significant differences in composite cognition scores between schizoaffective depressive and bipolar disorder (d = 0.65, p < 0.001) and schizophrenia and bipolar disorder (d = 0.58, p < 0.001). The relative contributions of each covariate can be found in Appendix 1, Table S11.
Examining cognition as a dimension across diagnostic groups
We used ordinal regression to test whether cognition could be considered a dimensional phenotype across the diagnostic spectrum. This analysis indicated that higher cognitive scores were associated with higher scores on the diagnostic scale (0 = schizoaffective depressive/schizophrenia, 1 = schizoaffective bipolar and 2 = bipolar disorder; see Appendix 1, Table S12, for the full model), supporting a spectrum of increasing impairment from bipolar disorder to schizoaffective bipolar to schizophrenia/schizoaffective depressive. An alternative way of interpreting this result is that among our clinical cases, participants with a score 1 standard deviation higher in composite cognition were almost twice as likely to be diagnosed with schizoaffective bipolar or bipolar disorder than schizophrenia (odds ratio = 1.98, p < 0.001). Ordinal regression outputs a single odds ratio for the effect of the explanatory variable across all levels of the dependent variable, because there is an assumption that the coefficients must be equal across all levels (assumption of proportional odds). We confirmed this assumption using the test of parallel lines in SPSS (χ23 = 4.97, p = 0.17) and by comparing the coefficients for binary regressions for each cut-off point in the scale. The results of the ordinal regression did not change after adjustment for olanzapine equivalent dose, antipsychotic exposure in months and current negative symptoms (odds ratio = 1.63, p < 0.001), although we interpret this result with caution given that the proportional odds assumption was violated in this model (χ2 = 26.98, p < 0.001).
The analysis was followed up with binary regressions between the diagnostic groups (model 1: bipolar disorder and schizoaffective bipolar; model 2: schizoaffective bipolar and schizoaffective depressive/schizophrenia) to compare the gradients from one diagnosis to the next on the scale (Appendix 1, Table S12). The resulting coefficients were equivalent for models 1 and 2. This confirmed that there is a gradient of increasing impairment from bipolar disorder to schizoaffective bipolar, to schizophrenia/schizoaffective depressive.
Exploring cross-disorder symptom dimensions and cognitive performance
Median BADDS dimension scores for each diagnostic group are presented in Appendix 1, Table S13. Higher scores on the lifetime mania and depression dimensions were associated with better cognitive performance (mania: B = 0.010, SE = 0.001, p < 0.001; depression: B = 0.004, SE = 0.001, p = 0.012). Higher scores on the lifetime psychosis dimension predicted poorer cognitive performance (psychosis: B = −0.015, SE = 0.002, p < 0.001). In the subgroup analyses (bipolar disorder and schizoaffective bipolar only, schizophrenia and schizoaffective depressive only), neither mania nor depression scores predicted performance, but higher psychosis scores were associated with lower cognitive scores (schizoaffective bipolar/bipolar disorder: B = −0.010, SE = 0.003, p < 0.001; schizoaffective depressive/schizophrenia: B = −0.011, SE = 0.003, p < 0.001). All analyses were repeated adjusting for age, sex, antipsychotic exposure in months, olanzapine equivalent dose and current negative symptoms. This did not change the results (Appendix 1, Table S14), although the association between BADDS psychosis scores and cognition in the schizoaffective depressive and schizophrenia subgroup did not survive correction for multiple testing.
Discussion
We set out to test the hypothesis that there is a spectrum of increasing cognitive impairment from bipolar disorder to schizophrenia and schizoaffective depressive. We report that while cognitive profiles were similar across disorders, impairments increased in severity from bipolar disorder to schizoaffective bipolar, to schizophrenia and schizoaffective depressive. We found no differences between schizophrenia and schizoaffective depressive with respect to severity of cognitive impairments. Differences between groups were not explained by differences in antipsychotic medication or current positive and negative symptoms. In accordance with our hypothesis, ordinal regression modelling provided support for a gradient of increasing cognitive impairment across disorders. Finally, we found that higher scores on the BADDS psychosis dimension, a measure of the severity and frequency of lifetime psychosis, were associated with lower cognitive scores.
Performance across the cognitive domains was equivalent in the schizophrenia and schizoaffective depressive groups. These results suggest that from a cognitive perspective, there is questionable validity in the nosological distinction between schizophrenia and schizoaffective depressive. Therapies developed to improve cognition in schizophrenia should also be targeted at patients with schizoaffective depressive type. These findings also highlight the importance of considering the subtypes of schizoaffective disorder separately, because these groups differed in severity of cognitive impairment.
Differences in overall cognition between schizoaffective bipolar and bipolar disorder were not significant after correction for multiple testing. However, the effect size between these groups (d = 0.44) was larger than that observed between schizophrenia and schizoaffective depressive (d = 0.07). This finding may explain why we still observed a linear trend from bipolar disorder to schizoaffective bipolar to schizophrenia and schizoaffective depressive in the ordinal regression analysis. We used a conservative Bonferroni-corrected α value to control the type-I error rate, but at the cost of loss of power, which could explain the lack of significant difference. However, it should be noted that there were smaller differences between schizoaffective bipolar and bipolar disorder on individual domains, which were not significant even at α = 0.05.
Diagnostic groups were differentiated on the basis of severity of cognitive impairments, but the overall pattern of impairment was similar between groups. This finding suggests that cognitive impairment can be considered a dimensional phenotype that cuts across diagnostic boundaries. These results were consistent with those of previous studies showing that multiple domains of cognition are affected, and that these impairments increase in severity from bipolar disorder to schizophrenia.9–12 Similarities between the cognitive profiles of these disorders are consistent with a shared underlying neurobiology that differs quantitatively rather than qualitatively across the diagnostic groups.1,7,8 Indeed, previous studies have indicated overlap in regions of grey-matter reduction (although less consistently in bipolar disorder)35–38 and genetic susceptibility.39–42
While neurocognitive impairments were evident across all diagnoses, impairments in social cognition were not present in bipolar disorder but were observed in schizophrenia and schizoaffective disorder. The largest difference between participants with schizoaffective bipolar and bipolar disorder was observed in social cognition, suggesting there may be some distinction in the cognitive processes underlying these disorders, despite similar neurocognitive profiles. Social cognition was the only domain associated with current positive symptoms. Previous studies have demonstrated associations between domains of social cognition, particularly theory of mind deficits, and psychotic symptoms in schizophrenia.43–45 These results suggest that certain social cognitive tasks may differentiate bipolar disorder from other disorders in the bipolar disorder/schizophrenia spectrum. The association between social cognitive impairment and psychosis provides support for cognitive models of psychosis that posit a role for social interpretations in the development of psychotic thinking.46
Lifetime history of psychosis, as measured by the BADDS psychosis dimension, was associated with cognitive performance in our cross-diagnostic analysis. The BADDS psychosis dimension measures the prominence of psychotic symptoms over the course of illness and considers both duration and number of psychotic episodes. Lifetime history of psychosis has been associated with poorer cognition.17 Our results expand on these findings by using a dimensional approach to show that lifetime frequency and severity of psychosis predicts severity of cognitive impairments.
This study had several strengths. It is one of the largest samples to date and was of sufficient size to allow us to separate the subtypes of schizoaffective disorder. The sample was well characterized, with consensus lifetime diagnoses based on semistructured interview and medical records. The clinical characterization of the sample allowed us to adjust for the effects of current symptoms and antipsychotic medication, including both current and lifetime antipsychotic exposure.
Limitations
A number of limitations should be noted. The sizes of the diagnostic groups were uneven, and there was a larger sample of participants with schizophrenia than the other disorders. Despite this, our analyses were robust to differences in the group sizes and we were able to detect differences between groups. Our bipolar disorder group consisted of a mixture of patients with and without a lifetime history of psychosis. Given the small number of participants without psychosis, it was not possible to subdivide the bipolar group into those with and without a history of psychosis to examine differences between these groups and schizophrenia or schizoaffective disorder. The MCCB was designed for use with participants who have schizophrenia. Previous studies of bipolar disorder have failed to find deficits in executive functioning using the Neuropsychological Assessment Battery Mazes task.25,27,47 The authors of these studies noted that more complex measures of executive function, such as the Wisconsin Card Sorting Task, may be more sensitive to detecting deficits in bipolar disorder. Although our bipolar group was impaired on the Neuropsychological Assessment Battery Mazes relative to controls, this task may not have been sufficiently complex to differentiate between bipolar disorder and schizoaffective bipolar. Furthermore, our bipolar disorder group was not impaired on the social cognition task (Mayer-Salovey-Caruso Emotional Intelligence Test), but previous studies have identified deficits in theory of mind and emotion recognition, suggesting that patients with bipolar disorder do have impairments in specific domains of social cognition.48,49
Conclusion
Using a large and well-characterized sample, we have demonstrated a gradient of increasing cognitive impairment from bipolar disorder to schizoaffective bipolar, to schizophrenia and schizoaffective depressive. Differences in cognitive profiles between diagnoses were quantitative rather than qualitative. Our findings comparing cognition between diagnostic groups confirmed our a priori decision to combine participants with schizophrenia and schizoaffective depressive in the subsequent analyses. This argues against separating schizophrenia and schizoaffective depressive for such analyses. This study was also the first to use a regression model to demonstrate a gradient of cognitive impairment and show that a dimensional measure of lifetime psychotic episodes is linearly associated with cognition. These results provide support for a model of psychotic and affective disorders in which diagnostic criteria focus on dimensional measures of symptoms rather than on traditional diagnostic categories.
Acknowledgements
This work was supported by a Medical Research Council (MRC) PhD studentship to A. Lynham. The work at Cardiff University was funded by MRC Centre (MR/L010305/1) and Program Grant (G0500509). The authors thank Sophie Bishop for her assistance with data collection and participant recruitment. The authors also thank the participants and clinicians who took part in the CoMPaSS study.
Footnotes
Competing interests: None declared.
Contributors: A. Lynham, M. Owen and J. Walters designed the study. A. Lynham, S. Legge and J. Walters acquired the data, which A. Lynham, L. Hubbard, K. Tansey, M. Hamshere, S. Legge, I. Jones and J. Walters analyzed. A. Lynham, M. Hamshere, M. Owen, I. Jones and J. Walters wrote the article, which all authors critically reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
References
- 1.Craddock N, Owen MJ. The Kraepelinian dichotomy — going, going … but still not gone. Br J Psychiatry. 2010;196:92–5. doi: 10.1192/bjp.bp.109.073429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kasanin J. The acute schizoaffective psychoses. Am J Psychiatry. 1933;90:97–126. doi: 10.1176/ajp.151.6.144. [DOI] [PubMed] [Google Scholar]
- 3.Cheniaux E, Landeira-Fernandez J, Lessa Telles L, et al. Does schizoaffective disorder really exist? A systematic review of the studies that compared schizoaffective disorder with schizophrenia or mood disorders. J Affect Disord. 2008;106:209–17. doi: 10.1016/j.jad.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Crow TJ. Nature of the genetic contribution to psychotic illness — a continuum viewpoint. Acta Psychiatr Scand. 1990;81:401–8. doi: 10.1111/j.1600-0447.1990.tb05471.x. [DOI] [PubMed] [Google Scholar]
- 5.Benabarre A, Vieta E, Colom F, et al. Bipolar disorder, schizoaffective disorder and schizophrenia: epidemiologic, clinical and prognostic differences. Eur Psychiatry. 2001;16:167–72. doi: 10.1016/s0924-9338(01)00559-4. [DOI] [PubMed] [Google Scholar]
- 6.Harrow M, Grossman LS, Herbener ES, et al. Ten-year outcome: patients with schizoaffective disorders, schizophrenia, affective disorders and mood-incongruent psychotic symptoms. Br J Psychiatry. 2000;177:421–6. doi: 10.1192/bjp.177.5.421. [DOI] [PubMed] [Google Scholar]
- 7.Owen MJ, O’Donovan MC, Thapar A, et al. Neurodevelopmental hypothesis of schizophrenia. Br J Psychiatry. 2011;198:173–5. doi: 10.1192/bjp.bp.110.084384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Owen MJ. New approaches to psychiatric diagnostic classification. Neuron. 2014;84:564–71. doi: 10.1016/j.neuron.2014.10.028. [DOI] [PubMed] [Google Scholar]
- 9.Szoke A, Meary A, Trandafir A, et al. Executive deficits in psychotic and bipolar disorders—implications for our understanding of schizoaffective disorder. Eur Psychiatry. 2008;23:20–5. doi: 10.1016/j.eurpsy.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Hill SK, Reilly JL, Keefe RSE, et al. Neuropsychological impairments in schizophrenia and psychotic bipolar disorder: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) Study. Am J Psychiatry. 2013;170:1275–84. doi: 10.1176/appi.ajp.2013.12101298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reichenberg A, Harvey PD, Bowie CR, et al. Neuropsychological function and dysfunction in schizophrenia and psychotic affective disorders. Schizophr Bull. 2009;35:1022–9. doi: 10.1093/schbul/sbn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keshavan MS, Morris DW, Sweeney JA, et al. A dimensional approach to the psychosis spectrum between bipolar disorder and schizophrenia: the Schizo-Bipolar Scale. Schizophr Res. 2011;133:250–4. doi: 10.1016/j.schres.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans JD, Heaton RK, Paulsen JS, et al. Schizoaffective disorder: a form of schizophrenia or affective disorder? J Clin Psychiatry. 1999;60:874–82. [PubMed] [Google Scholar]
- 14.Glahn DC, Bearden CE, Cakir S, et al. Differential working memory impairment in bipolar disorder and schizophrenia: effects of lifetime history of psychosis. Bipolar Disord. 2006;8:117–23. doi: 10.1111/j.1399-5618.2006.00296.x. [DOI] [PubMed] [Google Scholar]
- 15.Amann B, Gomar JJ, Ortiz-Gil J, et al. Executive dysfunction and memory impairment in schizoaffective disorder: a comparison with bipolar disorder, schizophrenia and healthy controls. Psychol Med. 2012;42:2127–35. doi: 10.1017/S0033291712000104. [DOI] [PubMed] [Google Scholar]
- 16.Lewandowski KE, Cohen BM, Keshavan MS, et al. Relationship of neurocognitive deficits to diagnosis and symptoms across affective and non-affective psychoses. Schizophr Res. 2011;133:212–7. doi: 10.1016/j.schres.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simonsen C, Sundet K, Vaskinn A, et al. Neurocognitive dysfunction in bipolar and schizophrenia spectrum disorders depends on history of psychosis rather than diagnostic group. Schizophr Bull. 2009;37:73–83. doi: 10.1093/schbul/sbp034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bora E, Yucel M, Pantelis C. Cognitive endophenotypes of bipolar disorder: a meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. J Affect Disord. 2009;113:1–20. doi: 10.1016/j.jad.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Maj M. Neuropsychological functioning in schizoaffective disorder, depressed type. Acta Psychiatr Scand. 1986;74:524–8. doi: 10.1111/j.1600-0447.1986.tb06278.x. [DOI] [PubMed] [Google Scholar]
- 20.Rees E, Walters JT, Georgieva L, et al. Analysis of copy number variations at 15 schizophrenia-associated loci. Br J Psychiatry. 2014;204:108–17. doi: 10.1192/bjp.bp.113.131052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wing JK, Babor T, Brugha T, et al. SCAN: Schedules for Clinical Assessment in Neuropsychiatry. Arch Gen Psychiatry. 1990;47:589–93. doi: 10.1001/archpsyc.1990.01810180089012. [DOI] [PubMed] [Google Scholar]
- 22.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington (DC): APA; 2000. text rev. [Google Scholar]
- 23.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- 24.Nuechterlein K, Green M, Kern R, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165:203–13. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- 25.Burdick KE, Goldberg TE, Cornblatt BA, et al. The MATRICS Consensus Cognitive Battery in patients with bipolar I disorder. Neuropsychopharmacology. 2011;36:1587–92. doi: 10.1038/npp.2011.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sperry SH, O’Connor LK, Öngür D, et al. Measuring cognition in bipolar disorder with psychosis using the MATRICS Consensus Cognitive Battery. J Int Neuropsychol Soc. 2015;21:1–5. doi: 10.1017/S1355617715000442. [DOI] [PubMed] [Google Scholar]
- 27.Van Rheenen TE, Rossell SL. An empirical evaluation of the MATRICS Consensus Cognitive Battery in bipolar disorder. Bipolar Disord. 2014;16:318–25. doi: 10.1111/bdi.12134. [DOI] [PubMed] [Google Scholar]
- 28.Craddock N, Jones I, Kirov G, et al. The Bipolar Affective Disorder Dimension Scale (BADDS) — a dimensional scale for rating lifetime psychopathology in bipolar spectrum disorders. BMC Psychiatry. 2004;4:19. doi: 10.1186/1471-244X-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andreasen NC. Scale for the assessment of negative symptoms. Iowa City (IA): University of Iowa; 1983. [Google Scholar]
- 30.Andreasen NC. Scale for the assessment of positive symptoms. Iowa City (IA): University of Iowa; 1984. [Google Scholar]
- 31.Endicott J, Spitzer RL, Fleiss JL, et al. The global assessment scale: a procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry. 1976;33:766–71. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- 32.Nelson H, Willison J. NART: National Adult Reading Test. Slough, UK: NFER-Nelson; 1991. [Google Scholar]
- 33.Gardner DM, Murphy AL, O’Donnell H, et al. International consensus study of antipsychotic dosing. Am J Psychiatry. 2010;167:686–93. doi: 10.1176/appi.ajp.2009.09060802. [DOI] [PubMed] [Google Scholar]
- 34.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale (NJ): L Erlbaum; 1988. [Google Scholar]
- 35.Amann BL, Canales-Rodríguez EJ, Madre M, et al. Brain structural changes in schizoaffective disorder compared to schizophrenia and bipolar disorder. Acta Psychiatr Scand. 2016;133:23–33. doi: 10.1111/acps.12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ivleva EI, Bidesi AS, Keshavan MS, et al. Gray matter volume as an intermediate phenotype for psychosis: Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) Am J Psychiatry. 2013;170:1285–96. doi: 10.1176/appi.ajp.2013.13010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ivleva EI, Bidesi AS, Thomas BP, et al. Brain gray matter phenotypes across the psychosis dimension. Psychiatry Res Neuroimaging. 2012;204:13–24. doi: 10.1016/j.pscychresns.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bora E, Fornito A, Yücel M, et al. The effects of gender on grey matter abnormalities in major psychoses: a comparative voxelwise meta-analysis of schizophrenia and bipolar disorder. Psychol Med. 2012;42:295–307. doi: 10.1017/S0033291711001450. [DOI] [PubMed] [Google Scholar]
- 39.Owen MJ, Craddock N, Jablensky A. The genetic deconstruction of psychosis. Schizophr Bull. 2007;33:905–11. doi: 10.1093/schbul/sbm053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lichtenstein P, Yip BH, Björk C, et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–9. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Consortium C-DGotPG. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381:1371–9. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee SH, Ripke S, Neale BM, et al. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet. 2013;45:984–94. doi: 10.1038/ng.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marjoram D, Gardner C, Burns J, et al. Symptomatology and social inference: a theory of mind study of schizophrenia and psychotic affective disorder. Cogn Neuropsychiatry. 2005;10:347–59. doi: 10.1080/13546800444000092. [DOI] [PubMed] [Google Scholar]
- 44.Doody GA, Götz M, Johnstone EC, et al. Theory of mind and psychoses. Psychol Med. 1998;28:397–405. doi: 10.1017/s003329179700648x. [DOI] [PubMed] [Google Scholar]
- 45.Fett A-KJ, Maat A GROUP Investigators. Social cognitive impairments and psychotic symptoms: what is the nature of their association? Schizophr Bull. 2013;39:77–85. doi: 10.1093/schbul/sbr058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garety PA, Freeman D. The past and future of delusions research: from the inexplicable to the treatable. Br J Psychiatry. 2013;203:327. doi: 10.1192/bjp.bp.113.126953. [DOI] [PubMed] [Google Scholar]
- 47.Yatham LN, Torres IJ, Malhi GS, et al. The International Society for Bipolar Disorders — Battery for Assessment of Neurocognition (ISBD-BANC) Bipolar Disord. 2010;12:351–63. doi: 10.1111/j.1399-5618.2010.00830.x. [DOI] [PubMed] [Google Scholar]
- 48.Samamé C, Martino D, Strejilevich S. Social cognition in euthymic bipolar disorder: systematic review and meta-analytic approach. Acta Psychiatr Scand. 2012;125:266–80. doi: 10.1111/j.1600-0447.2011.01808.x. [DOI] [PubMed] [Google Scholar]
- 49.Bora E, Yücel M, Pantelis C. Theory of mind impairment: a distinct trait-marker for schizophrenia spectrum disorders and bipolar disorder? Acta Psychiatr Scand. 2009;120:253–64. doi: 10.1111/j.1600-0447.2009.01414.x. [DOI] [PubMed] [Google Scholar]